The State of the Art of Laminar Burning Velocities of H2-Enriched n-C4H10–Air Mixtures
Abstract
1. Introduction
- Introduction;
- Experimental methods used to obtain the laminar burning velocities of n-C4H10–H2–air mixtures;
- Numerical methods used to obtain the laminar burning velocities of n-C4H10–H2–air mixtures;
- Laminar burning velocities of H2-enriched n-C4H10–air mixtures:
- 4.1
- Effect of the mixture equivalence ratio;
- 4.2
- Effect of hydrogen addition;
- 4.3
- Effect of the initial temperature;
- 4.4
- Effect of the initial pressure.
- Challenges and future perspectives;
- Conclusions.
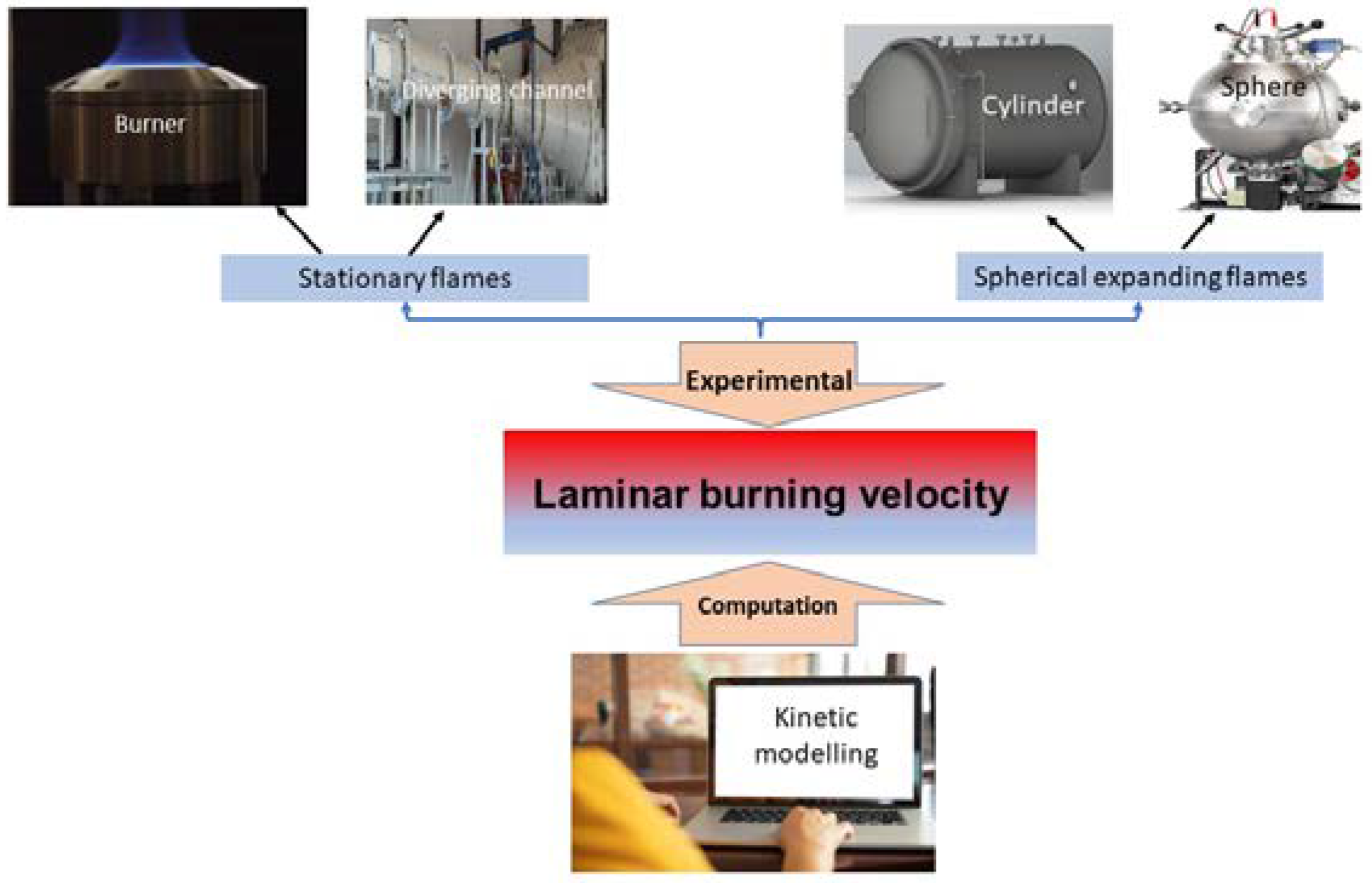
2. Experimental Methods Used in Obtaining the Laminar Burning Velocities of n-C4H10–H2–Air Mixtures
3. Numerical Methods Used in Obtaining the Laminar Burning Velocities of n-C4H10–H2–Air Mixtures
4. Laminar Burning Velocities of H2-Enriched n-C4H10–Air Mixtures
4.1. Effect of the Mixture Equivalence Ratio
4.2. Effect of Hydrogen Addition
4.3. Effect of the Initial Temperature
4.4. Effect of the Initial Pressure
5. Challenges and Future Perspectives
6. Conclusions
- -
- The laminar burning velocity of hydrogen-enriched n-butane–air mixtures has a typical behaviour characteristic for hydrocarbon–air mixtures regarding its variation with the equivalence ratio;
- -
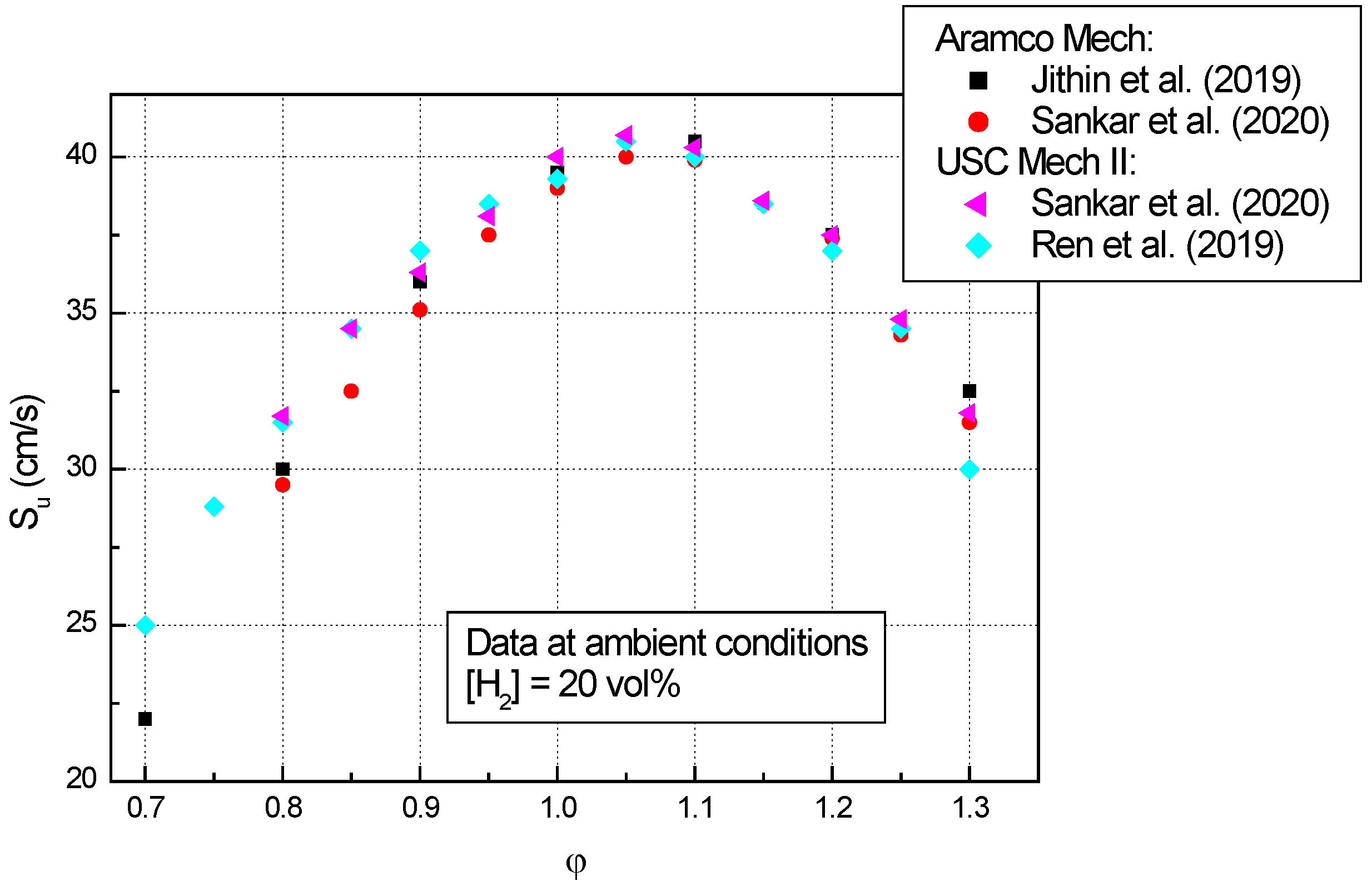
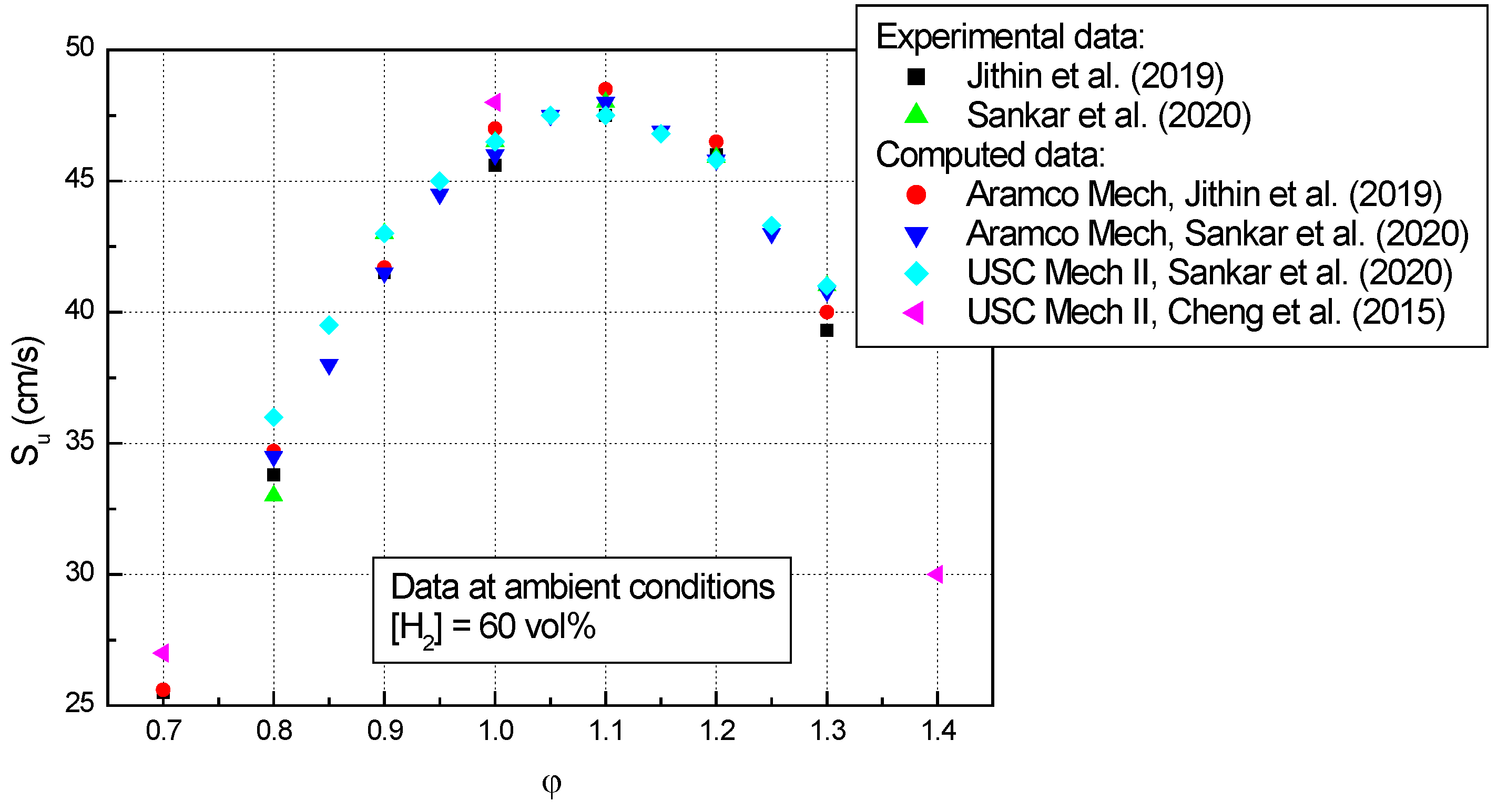
- The experimental laminar burning velocities of n-C4H10–air mixtures having various equivalence ratios (between 0.5–1.5), obtained at ambient initial conditions by various experimental techniques, agree quite well with the computed ones obtained using different kinetic mechanisms;
- -
- At ambient initial conditions and various equivalence ratios, a scattering of the computed data was observed due to the different kinetic mechanisms involved in computations;
- -
- At constant initial temperature, pressure, and n-butane–air equivalence ratio, hydrogen addition leads to an increase in adiabatic flame temperature and in laminar burning velocity; e.g., for a stoichiometric mixture with only 4 vol% H2, the laminar burning velocity increases from 55.0 cm/s at 300 K to 116.6 cm/s at 420 K;
- -
- For stoichiometric mixtures at ambient initial conditions, hydrogen addition increases the laminar burning velocity (either experimental or computed) from around 36 cm/s (mixtures without H2) to around 46 cm/s (mixtures with 60 vol% H2);
- -
- Increases in the laminar burning velocity with increased hydrogen addition is due to the chemical effect, on one hand, and to the thermal effect, on the other hand;
- -
- When hydrogen is added to a mixture, the production of the free radicals O, H, and OH leads to an increase in the heat release rate and, thus, to an increase in laminar burning velocity;
- -
- At constant mixture composition and pressure, the laminar burning velocities of hydrogen-enriched n-butane–air mixtures increase with an increase in the initial temperature;
- -
- The initial temperature influences the laminar burning velocity through the following: (a) the reaction rate and flame temperature; (b) the change in density; (c) the transport property of the mixture;
- -
- The revised data showed a discrepancy between experimental and calculated laminar burning velocities obtained at various initial temperatures between 300 and 420 K;
- -
- The thermal coefficients of stoichiometric n-butane–H2–air mixtures at ambient initial pressure decrease with an increase in hydrogen amount from 1.45 (mixture with 20 vol% H2) to 1.27 (mixture with 60 vol% H2);
- -
- At constant mixture composition and temperature, the laminar burning velocities of hydrogen-enriched n-butane–air mixtures decrease with an increase in the initial pressure; e.g., for a stoichiometric mixture with 5 vol% H2, the laminar burning velocity decreases from 49.0 cm/s at an initial pressure of 1 bar to 18.7 cm/s at an initial pressure of 20 bar;
- -
- An increase in initial pressure significantly reduces the amounts of free radicals (O, H, and OH) within the flame front and, thus, the flame propagation velocity decreases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leppard, W.R.; Rapp, L.A.; Burns, V.R.; Gorse, R.A.; Knepper, J.K.; Koehl, J. Effects of Gasoline Composition on Vehicle Engine-out and Tailpipe Hydrocarbon Emissions-the Auto/Oil Air Quality Improvement Research Program; SAE Paper 920329; SAE International: Warrendale, PA, USA, 1992. [Google Scholar]
- Pekalski, A.A.; Terli, E.; Zevenbergen, J.F.; Lemkowitz, S.M.; Pasman, H.J. Influence of the ignition delay time on the explosion parameters of hydrocarbon-air-oxygen mixtures at elevated pressure and temperature. Proc. Combust. Inst. 2005, 30, 1933–1939. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, L.; Gu, C.; Wang, X.; Yang, W.; Zhao, D. Experimental investigation on ignition and combustion characteristics of n-butane/air mixtures by glow plug in miniature chamber. Fuel 2020, 274, 117857. [Google Scholar] [CrossRef]
- O’Neil, M.J. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th ed.; Royal Society of Chemistry: Cambridge, UK, 2013; p. 268. [Google Scholar]
- Perez Claro, Y.A.; Schoeggl, F.F.; Taylor, S.D.; Yarranton, H.W. Phase behavior of mixtures of bitumen and n-butane. Energy Fuels 2019, 33, 8530–8543. [Google Scholar] [CrossRef]
- Burcat, A.; Scheller, K.; Lifshitz, A. Shock-tube investigation of comparative ignition delay times for C1-C5 alkanes. Combust. Flame 1971, 16, 29–33. [Google Scholar] [CrossRef]
- Healy, D.; Donato, N.S.; Aul, C.J.; Petersen, E.L.; Zinner, C.M.; Bourque, G.; Curran, H.J. n-Butane: Ignition delay measurements at high pressure and detailed chemical kinetic simulations. Combust. Flame 2010, 157, 1526–1539. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Li, Y.; Li, T.; Zhang, Y.; Cao, C.; Zou, J.; Law, C.K. Experimental and kinetic modeling investigation on pyrolysis and combustion of n-butane and i-butane at various pressures. Combust. Flame 2018, 191, 126–141. [Google Scholar] [CrossRef]
- Holtappels, K. Report on the Experimentally Determined Explosion Limits, Explosion Pressures and Rates of Explosion Pressures Rise, Part 2: Ethane, Ethylene, Propane, N-butane, Ammonia and Carbon Monoxide; SAFEKINEX Deliverable No. 9; Bundesanstalt für Materialforschung und-Prüfung: Berlin, Germany, 2007. [Google Scholar]
- Razus, D.; Mitu, M.; Brinzea, V.; Oancea, D. Pressure evolution during confined deflagration of n-butane/air mixtures. Rev. Chim. 2007, 58, 1170–1175. [Google Scholar]
- Giurcan, V.; Mitu, M.; Razus, D.; Oancea, D. Pressure and temperature influence on propagation indices of n-butane-air gaseous mixtures. Proc. Saf. Environ. Prot. 2017, 111, 94–101. [Google Scholar] [CrossRef]
- Gibbs, G.J.; Calcote, H.F. Effect of molecular structure on burning velocity. J. Chem. Eng. Data 1959, 4, 226–237. [Google Scholar] [CrossRef]
- Günther, R.; Janisch, G. Meßwerte der flammengeschwindigkeit von gasen und gasgemischen. Chem. Ing. Tech. 1971, 43, 975–978. [Google Scholar] [CrossRef]
- Nair, M.R.S.; Gupta, M.C. Burning velocity measurement by bomb method. Combust. Flame 1974, 22, 219–221. [Google Scholar] [CrossRef]
- Sher, E.; Ozdor, N. Laminar burning velocities of n-butane/air mixtures enriched with hydrogen. Combust. Flame 1992, 89, 214–220. [Google Scholar] [CrossRef]
- Davis, S.G.; Law, C.K. Determination of and fuel structure effects on laminar flame speeds of C1 to C8 hydrocarbons. Combust. Sci. Technol. 1998, 140, 427–449. [Google Scholar] [CrossRef]
- Clarke, A.; Stone, R.; Beckwith, P. Measurement of the laminar burning velocity of n-butane and isobutane mixtures under microgravity conditions in a constant volume vessel. J. Inst. Energy 2001, 74, 70–76. [Google Scholar]
- Hirasawa, T.; Sung, C.J.; Joshi, A.; Yang, Z.; Wang, H.; Law, C.K. Determination of laminar flame speeds using digital particle image velocimetry: Binary fuel blends of ethylene, n-butane, and toluene. Proc. Combust. Inst. 2002, 29, 1427–1434. [Google Scholar] [CrossRef]
- Bosschaart, K.J.; De Goey, L.P.H. The laminar burning velocity of flames propagating in mixtures of hydrocarbons and air measured with the heat flux method. Combust. Flame 2004, 136, 261–269. [Google Scholar] [CrossRef]
- Frolov, S.M.; Basevich, V.Y.; Smetanyuk, V.A.; Belyaev, A.A.; Pasman, H.J. Modeling of n-butane ignition, combustion, and preflame oxidation in the 20-l vessel. J. Loss Prev. Proc. Ind. 2007, 20, 562–569. [Google Scholar] [CrossRef]
- Kelley, A.P.; Law, C.K. Nonlinear effects in the extraction of laminar flame speeds from expanding spherical flames. Combust. Flame 2009, 156, 1844–1851. [Google Scholar] [CrossRef]
- Marshall, S.P.; Stone, R.; Hegheş, C.; Davies, T.J.; Cracknell, R.F. High pressure laminar burning velocity measurements and modelling of methane and n-butane. Combust. Theory Model. 2010, 14, 519–540. [Google Scholar] [CrossRef]
- Dirrenberger, P.; Le Gall, H.; Bounaceur, R.; Herbinet, O.; Glaude, P.A.; Konnov, A.; Battin-Leclerc, F. Measurements of laminar flame velocity for components of natural gas. Energy Fuels 2011, 25, 3875–3884. [Google Scholar] [CrossRef]
- Wu, H.; Hu, E.; Yu, H.; Li, Q.; Zhang, Z.; Chen, Y.; Huang, Z. Experimental and numerical study on the laminar flame speed of n-butane/dimethyl ether–air mixtures. Energy Fuels 2014, 28, 3412–3419. [Google Scholar] [CrossRef]
- Prince, J.C.; Treviño, C.; Williams, F.A. A reduced reaction mechanism for the combustion of n-butane. Combust. Flame 2017, 175, 27–33. [Google Scholar] [CrossRef]
- Giurcan, V.; Mitu, M.; Razus, D.; Oancea, D. Experimental study and kinetic modeling of laminar flame propagation in premixed stoichiometric n-butane-air mixture. Rev. Chim. 2019, 70, 1125–1131. [Google Scholar] [CrossRef]
- Jithin, E.V.; Dinesh, K.; Mohammad, A.; Velamati, R.K. Laminar burning velocity of n-butane/Hydrogen/Air mixtures at elevated temperatures. Energy 2019, 176, 410–417. [Google Scholar] [CrossRef]
- Sankar, V.E.V.J.; Mohammad, A.; Velamati, R.K. Effect of hydrogen addition on laminar burning velocity of liquefied petroleum gas blends. Energy Fuels 2020, 34, 798–805. [Google Scholar] [CrossRef]
- Veetil, J.E.; Kumbhakarna, N.; Singh, S.; Velamati, R.K.; Kumar, S. Effect of hydrogen addition on the dynamics of premixed C1–C4 alkane-air flames in a microchannel with a wall temperature gradient. Int. J. Hydrogen Energy 2022, 47, 30660–30670. [Google Scholar] [CrossRef]
- Arafin, F.; Belmont, E. Combustion flame speeds and stability of associated natural gas with high concentrations of C2–C4 alkanes. Energy Fuels 2018, 32, 11821–11830. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Y.; Huang, Z. Progress in combustion investigations of hydrogen enriched hydrocarbons. Renew. Sust. Energ. Rev. 2014, 30, 195–216. [Google Scholar] [CrossRef]
- Costa, M.; Piazzullo, D.; Dolce, A. Hydrogen addition to natural gas in cogeneration engines: Optimization of performances through numerical modelling. Front. Mech. Eng. 2021, 7, 680193. [Google Scholar] [CrossRef]
- Guy, P.; Laszlo, T.; Julien, C. Effects of hydrogen addition on design, maintenance and surveillance of gas networks. Processes 2021, 9, 1219. [Google Scholar] [CrossRef]
- Boodaghi, H.; Etghani, M.M.; Sedighi, K. Numerical study of hydrogen addition on the performance and emission characteristics of compressed natural gas spark-ignition engine using response surface methodology and multi-objective desirability approach. Int. J. Engine Res. 2021, 22, 2575–2596. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.; Zhao, Z. Effect of N2 and CO2 on explosion behavior of H2-liquefied petroleum gas-air mixtures in a confined space. Int. J. Hydrogen Energy 2022, 47, 23887–23897. [Google Scholar] [CrossRef]
- Lee, S.; Oh, S.; Choi, Y.; Kang, K. Effect of n-Butane and propane on performance and emission characteristics of an SI engine operated with DME-blended LPG fuel. Fuel 2011, 90, 1674–1680. [Google Scholar] [CrossRef]
- Chemsafe—Database for Recommended Safety Characteristics, BAM. PTB, DECHEMA, Germany. 2023. Available online: https://www.chemsafe.ptb.de (accessed on 12 March 2023).
- Muşat, N.G.; Munteanu, V.; Puiu, M.; Razus, D.; Oancea, D. Ignition and quenching of flammable n-butane-air mixture at various initial pressures. Critical properties. Rev. Roum. Chim. 2010, 55, 99–103. [Google Scholar]
- Qi, C.; Yan, X.; Wang, Y.; Ning, Y.; Yu, X.; Hou, Y.; Lv, X.; Ding, J.; Shi, E.; Yu, J. Flammability limits of combustible gases at elevated temperatures and pressures: Recent advances and future perspectives. Energy Fuels 2022, 36, 12896–12916. [Google Scholar] [CrossRef]
- Dinesh, M.H.; Kumar, G.N. Effects of compression and mixing ratio on NH3/H2 fueled Si engine performance, combustion stability, and emission. Energy Convers. Managem. X 2022, 15, 100269. [Google Scholar] [CrossRef]
- Masuk, N.I.; Mostakim, K.; Kanka, S.D. Performance and emission characteristic analysis of a gasoline engine utilizing different types of alternative fuels: A comprehensive review. Energy Fuels 2021, 35, 4644–4669. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Grab Rogaliński, K. Effect of natural gas enrichment with hydrogen on combustion process and emission characteristic of a dual fuel diesel engine. Int. J. Hydrogen Energy 2020, 45, 9088–9097. [Google Scholar] [CrossRef]
- Wei, Z.; Zhen, H.; Fu, J.; Leung, C.; Cheung, C.; Huang, Z. Experimental and numerical study on the laminar burning velocity of hydrogen enriched biogas mixture. Int. J. Hydrogen Energy 2019, 44, 22240–22249. [Google Scholar] [CrossRef]
- Benaissa, S.; Adouane, B.; Ali, S.M.; Mohammad, A. Effect of hydrogen addition on the combustion characteristics of premixed biogas/hydrogen-air mixtures. Int. J. Hydrogen Energy 2021, 46, 18661–18677. [Google Scholar] [CrossRef]
- Kahangamage, U.; Chen, Y.; Leung, C.W.; Ngai, T.Y. Experimental study of lean-burning limits of hydrogen-enriched LPG intended for domestic use. J. Energy Power Technol. 2022, 4, 1–14. [Google Scholar] [CrossRef]
- Wu, X.; Feng, Y.; Xu, G.; Zhu, Y.; Ming, P.; Dai, L. Numerical investigations on charge motion and combustion of natural gas-enhanced ammonia in marine pre-chamber lean-burn engine with dual-fuel combustion system. Int. J. Hydrogen Energy 2023, 48, 11476–11492. [Google Scholar] [CrossRef]
- Wu, X.; Feng, Y.; Gao, Y.; Xia, C.; Zhu, Y.; Shreka, M.; Ming, P. Numerical simulation of lean premixed combustion characteristics and emissions of natural gas-ammonia dual-fuel marine engine with the pre-chamber ignition system. Fuel 2023, 343, 127990. [Google Scholar] [CrossRef]
- Refael, S.; Sher, E. Autoignition of hydrogen-enriched n-butane-air mixture: A theoretical study. Proc. Combust. Inst. 1990, 23, 1789–1796. [Google Scholar] [CrossRef]
- Jiang, X.; Pan, Y.; Sun, W.; Liu, Y.; Huang, Z. Shock-tube study of the autoignition of n-butane/hydrogen mixtures. Energy Fuels 2018, 32, 809–821. [Google Scholar] [CrossRef]
- Le, T.M.H. Flammability Characteristics of Hydrogen and Its Mixtures with Light Hydrocarbons at Atmospheric and Sub-atmospheric Pressures. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2013. [Google Scholar]
- Tang, C.L.; Huang, Z.H.; Law, C.K. Determination, correlation, and mechanistic interpretation of effects of hydrogen addition on laminar flame speeds of hydrocarbon-air mixtures. Proc. Combust. Inst. 2011, 33, 921–928. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, C.; Huang, Z. Kinetic analysis of H2 addition effect on the laminar flame parameters of the C1–C4 n-alkane-air mixtures: From one step overall assumption to detailed reaction mechanism. Int. J. Hydrogen Energy 2015, 40, 703–718. [Google Scholar] [CrossRef]
- Ren, F.; Chu, H.; Xiang, L.; Han, W.; Gu, M. Effect of hydrogen addition on the laminar premixed combustion characteristics the main components of natural gas. J. Energy Inst. 2019, 92, 1178–1190. [Google Scholar] [CrossRef]
- Aravind, B.; Kishore, V.R.; Mohammad, A. Combustion characteristics of the effect of hydrogen addition on LPG–air mixtures. Int. J. Hydrogen Energy 2015, 40, 16605–16617. [Google Scholar] [CrossRef]
- Zitouni, S.E. Combustion Characteristics of Lean Premixed Methane/Higher Hydrocarbon/Hydrogen Flames. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2020. [Google Scholar]
- Westbrook, C.K.; Dryer, F.L. Simplified reaction mechanisms for the oxidation of hydrocarbon fuels in flames. Combust. Sci. Technol. 1981, 27, 31–43. [Google Scholar] [CrossRef]
- Law, C.K.; Sung, C.J.; Wang, H.; Lu, T.F. Development of comprehensive detailed and reduced reaction mechanisms for combustion modelling. AIAA J. 2003, 41, 1629–1646. [Google Scholar] [CrossRef]
- Davis, S.G.; Joshi, A.V.; Wang, H.; Egolfopoulos, F. An optimized kinetic model of H2/CO combustion. Proc. Combust. Inst. 2005, 30, 1283–1292. [Google Scholar] [CrossRef]
- Wang, H.; Sheen, D.A. Combustion kinetic model uncertainty quantification, propagation and minimization. Prog. Energy Combust. Sci. 2015, 47, 1–31. [Google Scholar] [CrossRef]
- Sánchez, A.L.; Williams, F.A. Recent advances in understanding of flammability characteristics of hydrogen. Prog. Energy Combust. Sci. 2014, 41, 1–55. [Google Scholar] [CrossRef]
- Khudhair, O.; Shahad, H.A. A review of laminar burning velocity and flame speed of gases and liquid fuels. Int. J. Curr. Eng. Technol. 2017, 7, 183–197. [Google Scholar]
- Konnov, A.A.; Mohammad, A.; Kishore, V.R.; Kim, N.I.; Prathap, C.; Kumar, S. A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel + air mixtures. Prog. Energy Combust. Sci. 2018, 68, 197–267. [Google Scholar] [CrossRef]
- Xiao, H.; Duan, Q.; Sun, J. Premixed flame propagation in hydrogen explosions. Renew. Sustain. Energy Rev. 2018, 81, 1988–2001. [Google Scholar] [CrossRef]
- Han, W.; Dai, P.; Gou, X.; Chen, Z. A review of laminar flame speeds of hydrogen and syngas measured from propagating spherical flames. App. Energy Combust. Sci. 2020, 1, 100008. [Google Scholar] [CrossRef]
- Pizzuti, L.; Torres, F.A.; Ferreira, R.W.; dos Santos, L.R.; Lacava, P.T.; Martins, C.A. Laminar burning and flammability limits in biogas: A state of the art. In Proceedings of the 10th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, Orlando, FL, USA, 14–16 July 2014. [Google Scholar]
- Pizzuti, L.; Martins, C.A.; Lacava, P.T. Laminar burning velocity and flammability limits in biogas: A literature review. Renew. Sustain. Energy Rev. 2016, 62, 856–865. [Google Scholar] [CrossRef]
- Giurcan, V.; Movileanu, C.; Musuc, A.M.; Mitu, M. Laminar burning velocity of biogas-containing mixtures A literature review. Processes 2021, 9, 996. [Google Scholar]
- Emami, S.D.; Kasmani, R.M.; Hamid, M.D.; Hassan, C.R.C.; Mokhtar, K.M. Kinetic and dynamic analysis of hydrogen-enrichment mixtures in combustor systems-A review paper. Renew. Sustain. Energy Rev. 2016, 62, 1072–1082. [Google Scholar] [CrossRef]
- Abdulraheem, A.A.; Saleh, A.M.; Shahad, H.A. Measurements and data analysis review of laminar burning velocity and flame speed for biofuel/air mixtures. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1094, 012029. [Google Scholar] [CrossRef]
- Goswami, M.; Derks, S.C.; Coumans, K.; Slikker, W.J.; de Andrade Oliveira, M.H.; Bastiaans, R.J.; Luijten, C.M.; de Goey, L.P.H.; Konnov, A.A. The effect of elevated pressures on the laminar burning velocity of methane+ air mixtures. Combust. Flame 2013, 160, 1627–1635. [Google Scholar] [CrossRef]
- Egolfopoulos, F.N.; Hansen, N.; Ju, Y.; Kohse-Höinghaus, K.; Law, C.K.; Qi, F. Advances and challenges in laminar flame experiments and implications for combustion chemistry. Prog. Energy Combust. Sci. 2014, 43, 36–67. [Google Scholar] [CrossRef]
- Faghih, M.; Chen, Z. The constant-volume propagating spherical flame method for laminar flame speed measurement. Sci. Bull. 2016, 61, 1296–1310. [Google Scholar] [CrossRef]
- Sung, C.J.; Huang, Y.; Eng, J.A. Effects of reformer gas addition on the laminar flame speeds and flammability limits of n-butane and iso-butane flames. Combust. Flame 2001, 126, 1699–1713. [Google Scholar] [CrossRef]
- Curran, H.J. Developing detailed chemical kinetic mechanisms for fuel combustion. Proc. Combust. Inst. 2019, 37, 57–81. [Google Scholar] [CrossRef]
- Metghalchi, H.; Keck, J.C. Laminar burning velocity of propane-air mixtures at high temperature and pressure. Combust. Flame 1980, 38, 143–154. [Google Scholar] [CrossRef]
- Law, C.K. Combustion Physics; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Sharma, S.P.; Agrawal, D.D.; Gupta, C.P. The pressure and temperature dependence of burning velocity in a spherical combustion bomb. Proc. Combust. Inst. 1981, 18, 493–501. [Google Scholar] [CrossRef]
- Metghalchi, M.; Keck, J.C. Burning velocities of mixtures of air with methanol, isooctane and indolene at high pressures and temperatures. Combust. Flame 1982, 48, 191–210. [Google Scholar] [CrossRef]
- Clarke, A. Measurement of Laminar Burning Velocity of Air/Fuel/Diluent Mixtures in Zero Gravity. Ph.D. Thesis, Department of Engineering Science, University of Oxford, Oxford, UK, 1994. [Google Scholar]
- Elia, M.; Ulinski, M.; Metghalchi, M. Laminar burning velocity of methane-air-diluent mixtures. J. Eng. Gas Turbines Power 2001, 123, 190–196. [Google Scholar] [CrossRef]
- Marshall, S. Measuring Laminar Burning Velocities. Ph.D. Thesis, Department of Engineering Science, University of Oxford, Oxford, UK, 2010. [Google Scholar]
- Hu, E.; Jiang, X.; Huang, Z.; Iida, N. Numerical study on the effects of diluents on the laminar burning velocity of methane–air mixtures. Energy Fuels 2012, 26, 4242–4252. [Google Scholar] [CrossRef]
- Yuan, Z.; Xie, L.; Sun, X.; Wang, R.; Li, H.; Liu, J.; Duan, D. Effects of water vapor on auto-ignition characteristics and laminar flame speed of methane/air mixture under engine-relevant conditions. Fuel 2022, 315, 123169. [Google Scholar] [CrossRef]
- Ueda, A.; Nisida, K.; Matsumura, Y.; Ichikawa, T.; Nakashimada, Y.; Endo, T.; Kim, W. Effects of hydrogen and carbon dioxide on the laminar burning velocities of methane-air mixtures. J. Energy Inst. 2021, 99, 178–185. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y. Effect of hydrogen on explosion characteristics of liquefied petroleum gas-air mixtures. Int. J. Hydrogen Energy 2022, 47, 4255–4263. [Google Scholar] [CrossRef]
- Boushaki, T.; Dhué, Y.; Selle, L.; Ferret, B.; Poinsot, T. Effects of hydrogen and steam addition on laminar burning velocity of methane–air premixed flame: Experimental and numerical analysis. Int. J. Hydrogen Energy 2012, 37, 9412–9422. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J. Experimental and numerical study on laminar burning characteristics of premixed methane-hydrogen-air flames. Int. J. Hydrogen Energy 2009, 34, 4876–4888. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Lamoureux, N.; Contino, F.; Mounaïm-Rousselle, C. Experimental investigation on laminar burning velocities of ammonia/hydrogen/air mixtures at elevated temperatures. Fuel 2020, 263, 116653. [Google Scholar] [CrossRef]
- Weinberg, F.J. Combustion temperatures: The future? Nature 1971, 233, 239–241. [Google Scholar] [CrossRef]
- Dahoe, A.E. Laminar burning velocities of hydrogen-air mixtures from closed vessel gas explosions. J. Loss Prev. Proc. Ind. 2005, 18, 152–166. [Google Scholar] [CrossRef]
- Glassman, I. Combustion, 3rd ed.; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Zhang, X.; Wang, J.; Chen, Y.; Li, C. Effect of CH4, pressure, and initial temperature on the laminar flame speed of an NH3-air mixture. ACS Omega 2021, 6, 11857–11868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cheung, C.S.; Leung, C.W.; Li, X.; Li, X.; Huang, Z. Effects of fuel composition and initial pressure on laminar flame speed of H2/CO/CH4 bio-syngas. Fuel 2019, 238, 149–158. [Google Scholar] [CrossRef]
- Konnov, A.A. The temperature and pressure dependences of the laminar burning velocity: Experiments and modelling. In Proceedings of the European Combustion Meeting, Budapest, Hungary, 30 March–2 April 2015. [Google Scholar]




| Property | n-C4H10 | H2 | ||
|---|---|---|---|---|
| Value | Reference | Value | Reference | |
| Molecular weight (kg/kmol) | 58.12 | Lee et al. [36] | 2.02 | Qi et al. [39] |
| Lower heating value (MJ/kg) | 45.72 | Lee et al. [36] | 120 | Dinesh et al. [40] |
| Octane number | 92 | Lee et al. [36] | >130 | Masuk et al. [41] |
| Flammability limits in air at ambient pressure and temperature (vol%) | 1.50–8.50 | Chemsafe [37] | 4.1–75.6 | Masuk et al. [41] |
| Autoignition temperature (°C) | 392 | Chemsafe [37] | 560 | Chemsafe [37] |
| Minimum ignition energy (MJ) | 0.900 | Musat et al. [38] | 0.018 | Masuk et al. [41] |
| Molar stoichiometric fraction of combustible in mixture with air | 3.13 | Chemsafe [37] | 29.5 | Chemsafe [37] |
| Adiabatic flame temperature at ambient initial conditions and φ = 1 (K) | 2274 | Giurcan et al. [26] | 2100 | Qi et al. [39] |
| Laminar burning velocity under ambient initial conditions and φ = 1 (m/s) | 0.38 | Giurcan et al. [26] | 3.51 | Dinesh et al. [40] |
| Mixture | Review Title | Year | Reference | |
|---|---|---|---|---|
| H2–air | Recent advances in understanding of flammability characteristics of hydrogen | 2013 | Sanchez et al. [60] | |
| A review of laminar burning velocity and flame speed of gases and liquid fuels | 2017 | Khudhair et al. [61] | ||
| A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel + air mixtures | 2018 | Konnov et al. [62] | ||
| Premixed flame propagation in hydrogen explosions | 2018 | Xiao et al. [63] | ||
| A review of laminar flame speeds of hydrogen and syngas measured from propagating spherical flames | 2020 | Han et al. [64] | ||
| Fuel–air | CH4 | A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel + air mixtures | 2018 | Konnov et al. [62] |
| C2H6 | ||||
| C3H8 | ||||
| C4H10 | ||||
| Biogas | Laminar burning and flammability limits in biogas: A state of the art | 2014 | Pizzuti et al. [65] | |
| Laminar burning velocity and flammability limits in biogas: A literature review | 2016 | Pizzuti et al. [66] | ||
| Laminar burning velocity of biogas-containing mixtures. A literature review | 2021 | Giurcan et al. [67] | ||
| Syngas | A review of laminar flame speeds of hydrogen and syngas measured from propagating spherical flames | 2020 | Han et al. [64] | |
| Fuel–H2–air | CH4 | Progress in combustion investigations of hydrogen enriched hydrocarbons | 2014 | Tang et al. [31] |
| Kinetic and dynamic analysis of hydrogen enrichment mixtures in combustor systems—A review paper | 2016 | Emami et al. [68] | ||
| Measurements and data analysis review of laminar burning velocity and flame speed for biofuel/air mixtures | 2021 | Abdulraheem et al. [69] | ||
| C3H8 | Progress in combustion investigations of hydrogen enriched hydrocarbons | 2014 | Tang et al. [31] | |
| Kinetic and dynamic analysis of hydrogen enrichment mixtures in combustor systems—A review paper | 2016 | Emami et al. [68] | ||
| A review of laminar burning velocity and flame speed of gases and liquid fuels | 2017 | Khudhair et al. [61] | ||
| Biogas | Laminar burning velocity of biogas-containing mixtures. A literature review | 2021 | Giurcan et al. [67] | |
| Stationary flames | Flat burner | Advantages |
|
| Disadvantages |
| ||
| Heat flux | Advantages |
| |
| Disadvantages |
| ||
| Diverging channel | Advantages |
| |
| Disadvantages |
| ||
| Spherical expanding flames | Advantages |
| |
| Disadvantages |
| ||
| Technique | Initial Conditions | Reference | ||
|---|---|---|---|---|
| φ | p0 (bar) | T0 (K) | ||
| Flat-flame burner | 0.5–1.2 | 1.0 | 298–420 | Sher and Ozdor [15] |
| Heat flux method | 0.7–1.3 | 1.0 | 298 | Jithin et al. [27] |
| 0.8–1.3 | 1.0 | 298 | Sankar et al. [28] | |
| Diverging channel method | 0.7–1.3 | 1.0 | 300–450 | Jithin et al. [27] |
| Spherical expanding flames | 0.6–1.5 | 1.0 | 298 | Tang et al. [51] |
| 0.7–0.9 | 1.0 | 298 | Zitouni [55] | |
| Mechanism | Initial Conditions | Reference | ||
|---|---|---|---|---|
| φ | p0 (bar) | T0 (K) | ||
| Aramco 2.0 | 0.7–1.3 | 1.0 | 300–450 | Jithin et al. [27] |
| 0.8–1.3 | 1.0 | 298 | Sankar et al. [28] | |
| USC Mech II | 0.8–1.3 | 1.0 | 298 | Sankar et al. [28] |
| 0.6–1.5 | 1.0 | 298 | Tang et al. [51] | |
| 0.8–1.3 | 1.0 | 298 | Cheng et al. [52] | |
| 0.7–1.3 | 1.0 | 298 | Ren et al. [53] | |
| 0.6–1.3 | 1.0 | 300 | Aravind et al. [54] | |
| 0.7–0.9 | 1.0 | 298 | Zitouni [55] | |
| San Diego | 0.7–0.9 | 1.0 | 298 | Zitouni [55] |
| Modified San Diego | 1.0 | 1.0 | 298 | Veetil et al. [29] |
| GRI Mech 1.2 | 0.7–1.4 | 1.0–20.3 | 300 | Sung et al. [73] |
| [H2] (vol%) | Su (cm/s) | ||
|---|---|---|---|
| T0 = 300 K | T0 = 360 K | T0 = 420 K | |
| 0 | 40.9 | 59.4 | 85.8 |
| 1.4 | 46.2 | 68.2 | 96.8 |
| 2.7 | 50.6 | 77.0 | 105.6 |
| 4.1 | 55.0 | 83.6 | 116.6 |
| [H2] (vol%) | Su (cm/s) | |||||
|---|---|---|---|---|---|---|
| T0 = 300 K | T0 = 360 K | T0 = 420 K | ||||
| Exp. | Comp. | Exp. | Comp. | Exp. | Comp. | |
| 0 | 37.5 | 37.5 | - | 50.0 | 68.0 | 65.5 |
| 40 | 41.6 | 43.0 | 62.0 | 57.5 | - | 72.0 |
| Mixture | α | Reference |
|---|---|---|
| C4H10–air | 1.61 | [26] |
| C4H10–H2–air (20 vol% H2) | 1.45 | [27] |
| C4H10–H2–air (40 vol% H2) | 1.37 | |
| C4H10–H2–air (60 vol% H2) | 1.27 | |
| H2–air | 1.40 | [91] |
| [H2] (vol%) | Su (cm/s) | |
|---|---|---|
| p0 = 1 bar | p0 = 20.3 bar | |
| 0 | 43.0 | 16.3 |
| 1.6 | 44.0 | 17.3 |
| 3.3 | 47.0 | 18.0 |
| 4.9 | 49.0 | 18.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Movileanu, C.; Mitu, M.; Giurcan, V. The State of the Art of Laminar Burning Velocities of H2-Enriched n-C4H10–Air Mixtures. Energies 2023, 16, 5536. https://doi.org/10.3390/en16145536
Movileanu C, Mitu M, Giurcan V. The State of the Art of Laminar Burning Velocities of H2-Enriched n-C4H10–Air Mixtures. Energies. 2023; 16(14):5536. https://doi.org/10.3390/en16145536
Chicago/Turabian StyleMovileanu, Codina, Maria Mitu, and Venera Giurcan. 2023. "The State of the Art of Laminar Burning Velocities of H2-Enriched n-C4H10–Air Mixtures" Energies 16, no. 14: 5536. https://doi.org/10.3390/en16145536
APA StyleMovileanu, C., Mitu, M., & Giurcan, V. (2023). The State of the Art of Laminar Burning Velocities of H2-Enriched n-C4H10–Air Mixtures. Energies, 16(14), 5536. https://doi.org/10.3390/en16145536







