Geopolymer Building Materials Based on Fly Ash in Terms of Removing SO2, CO2, and Water Vapor
Abstract
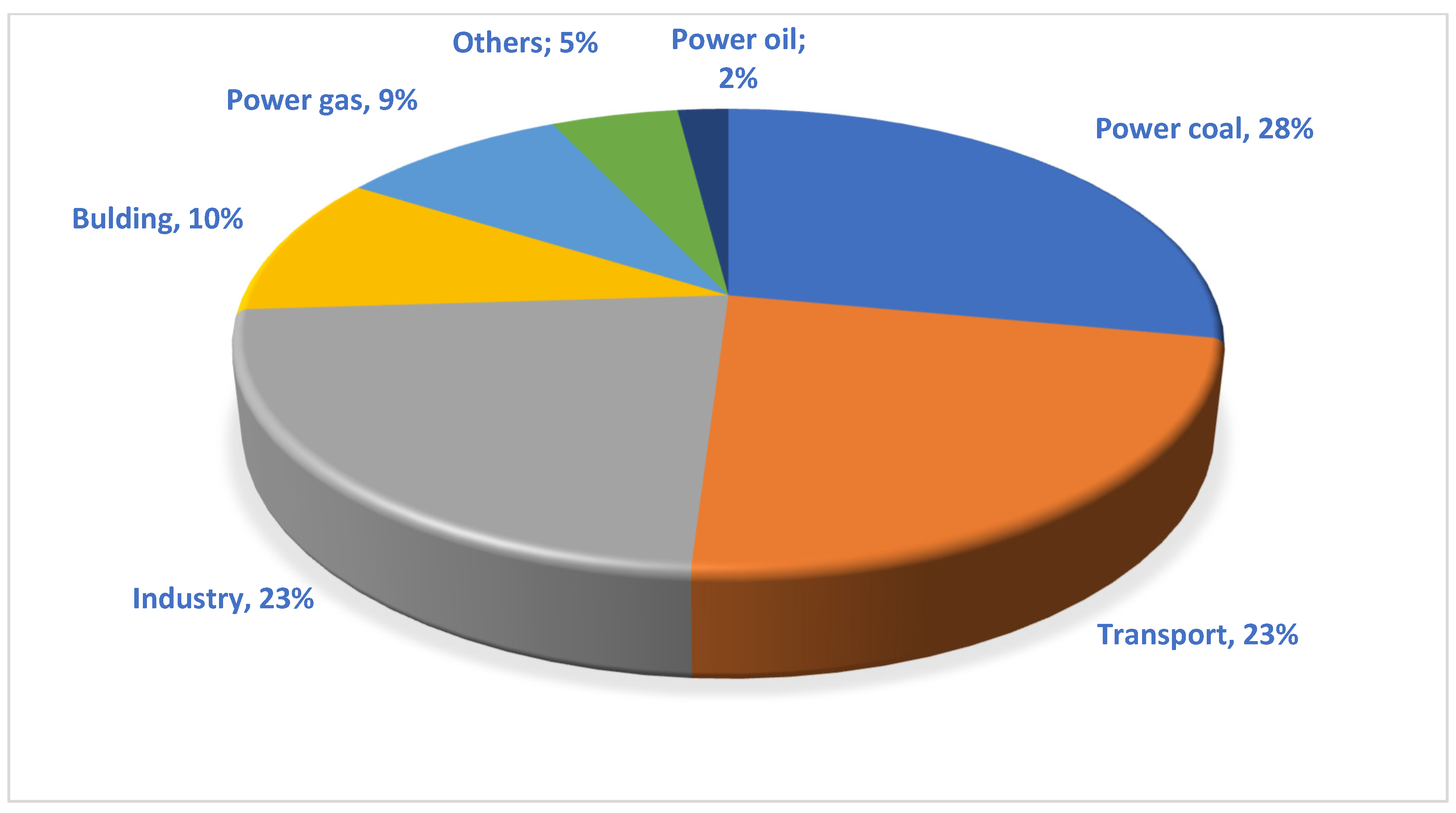
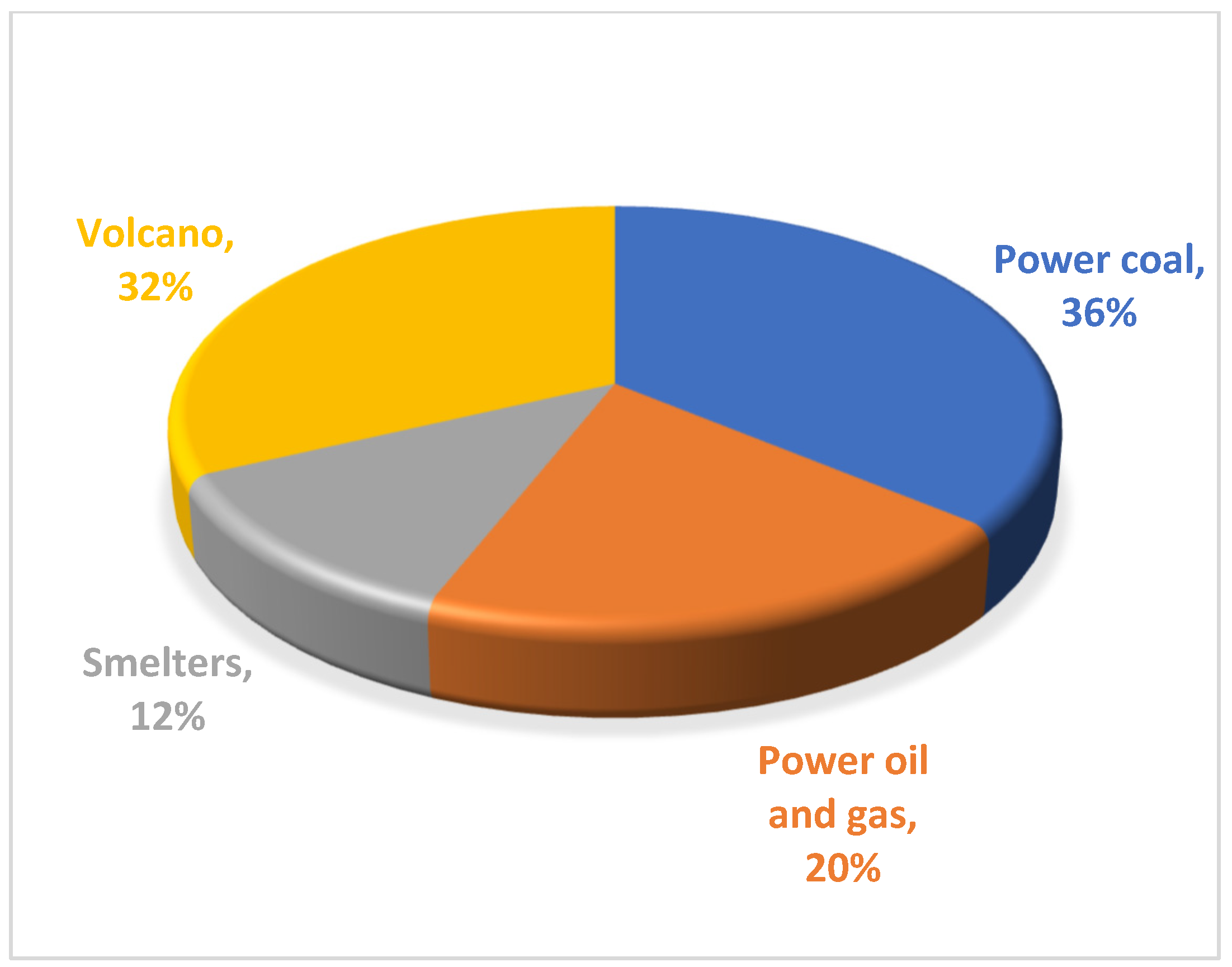
1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Synthesis
2.3. Measurement Methods
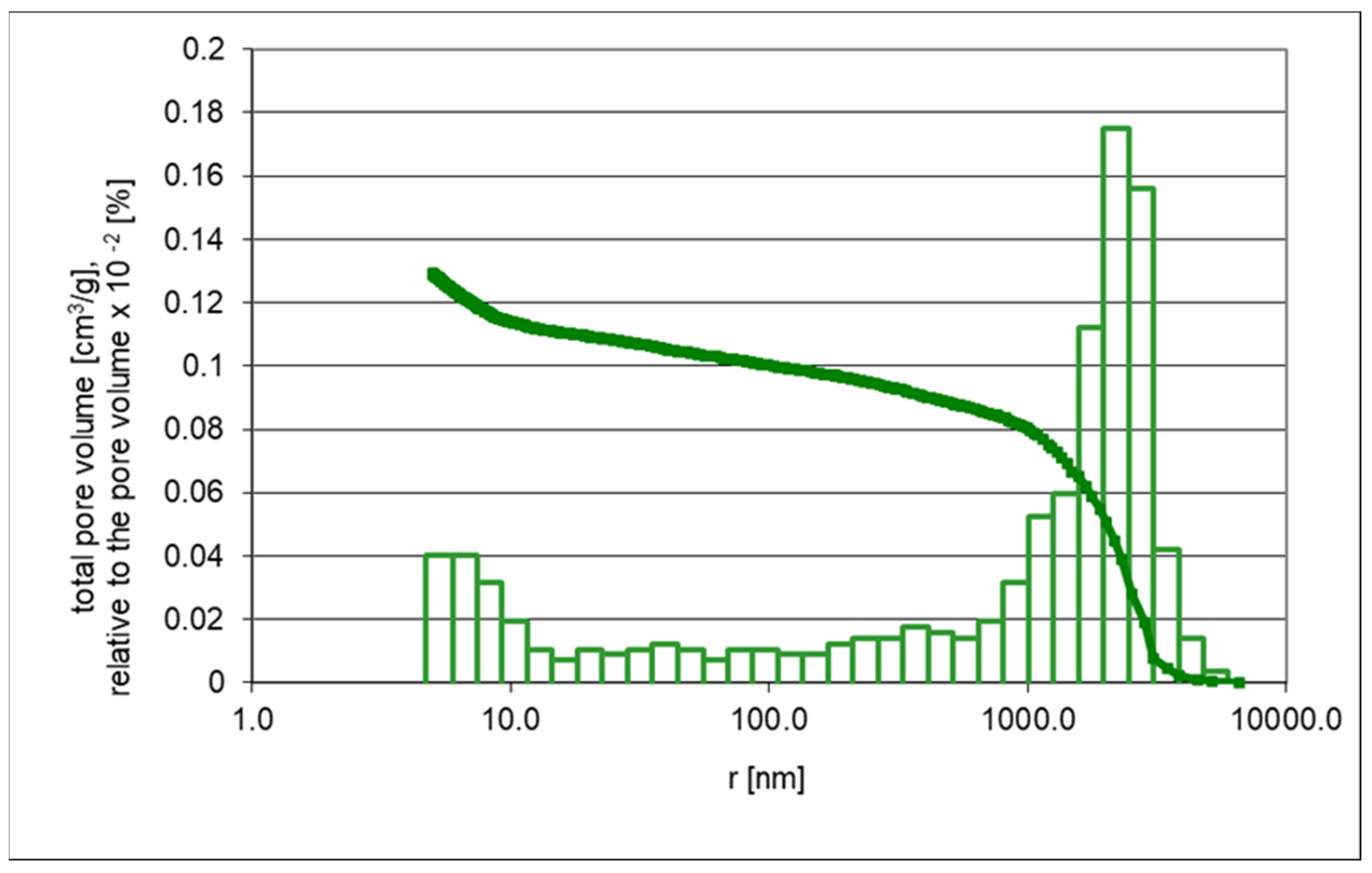
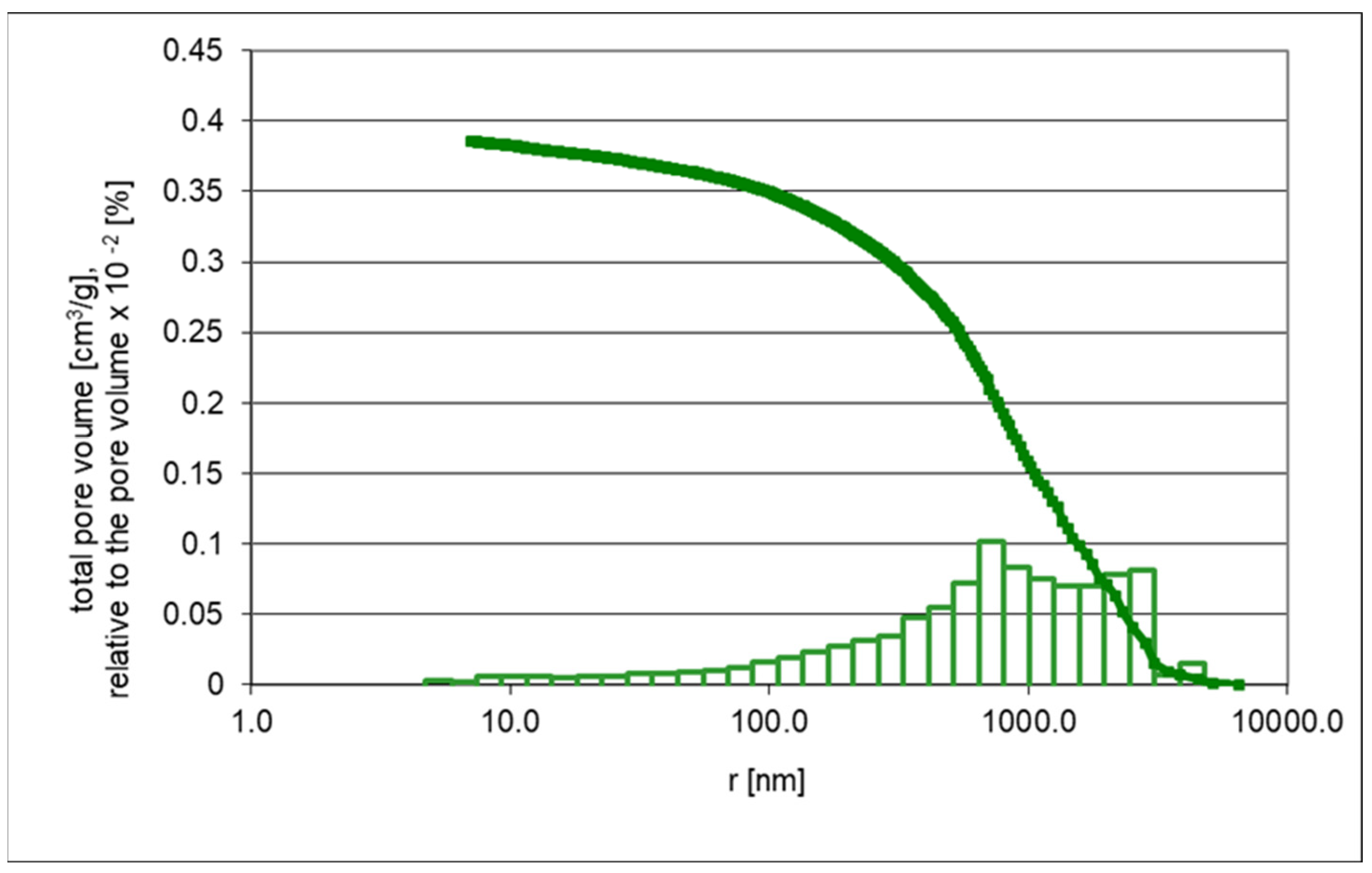
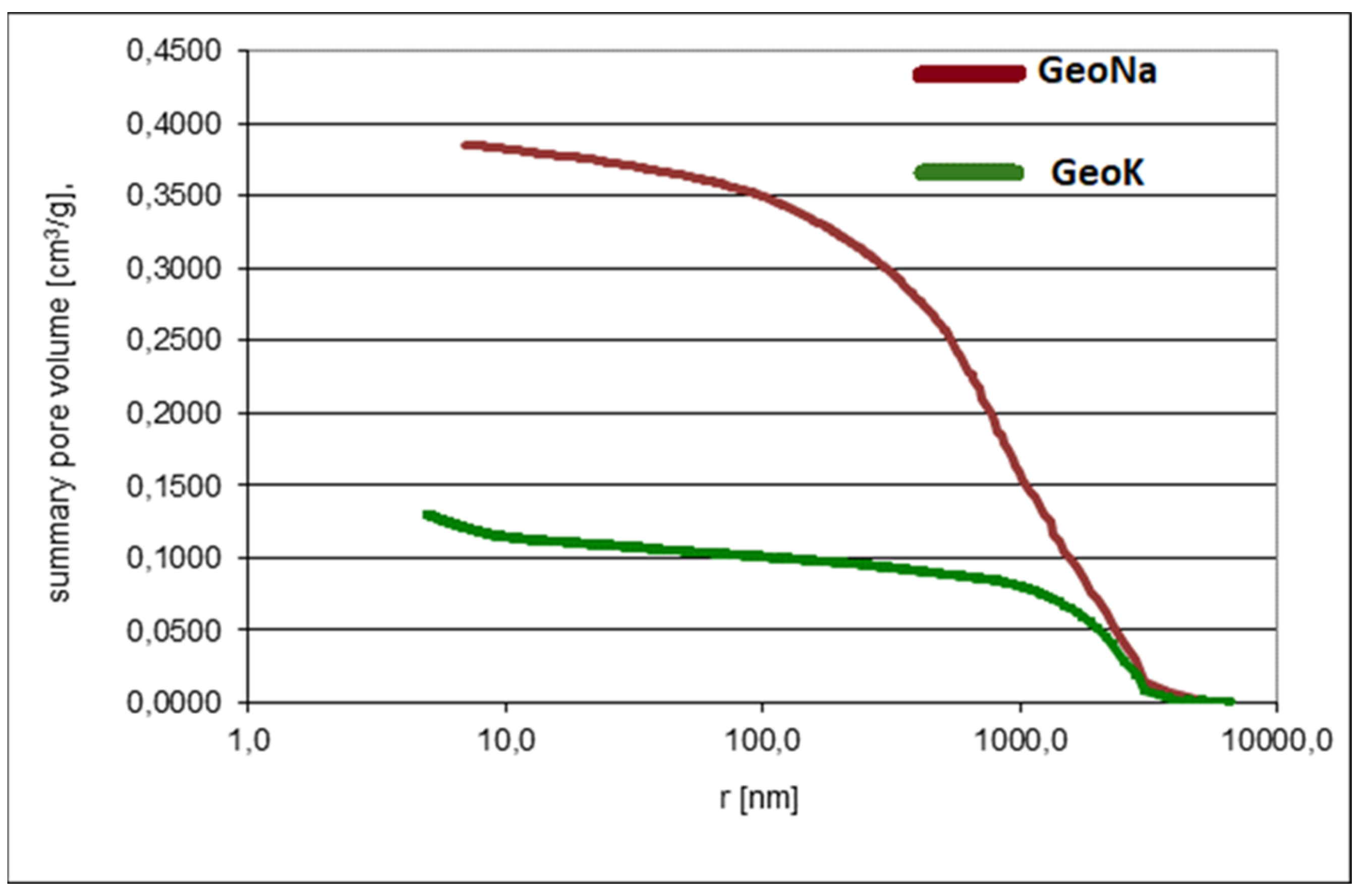
2.3.1. Porosimetry
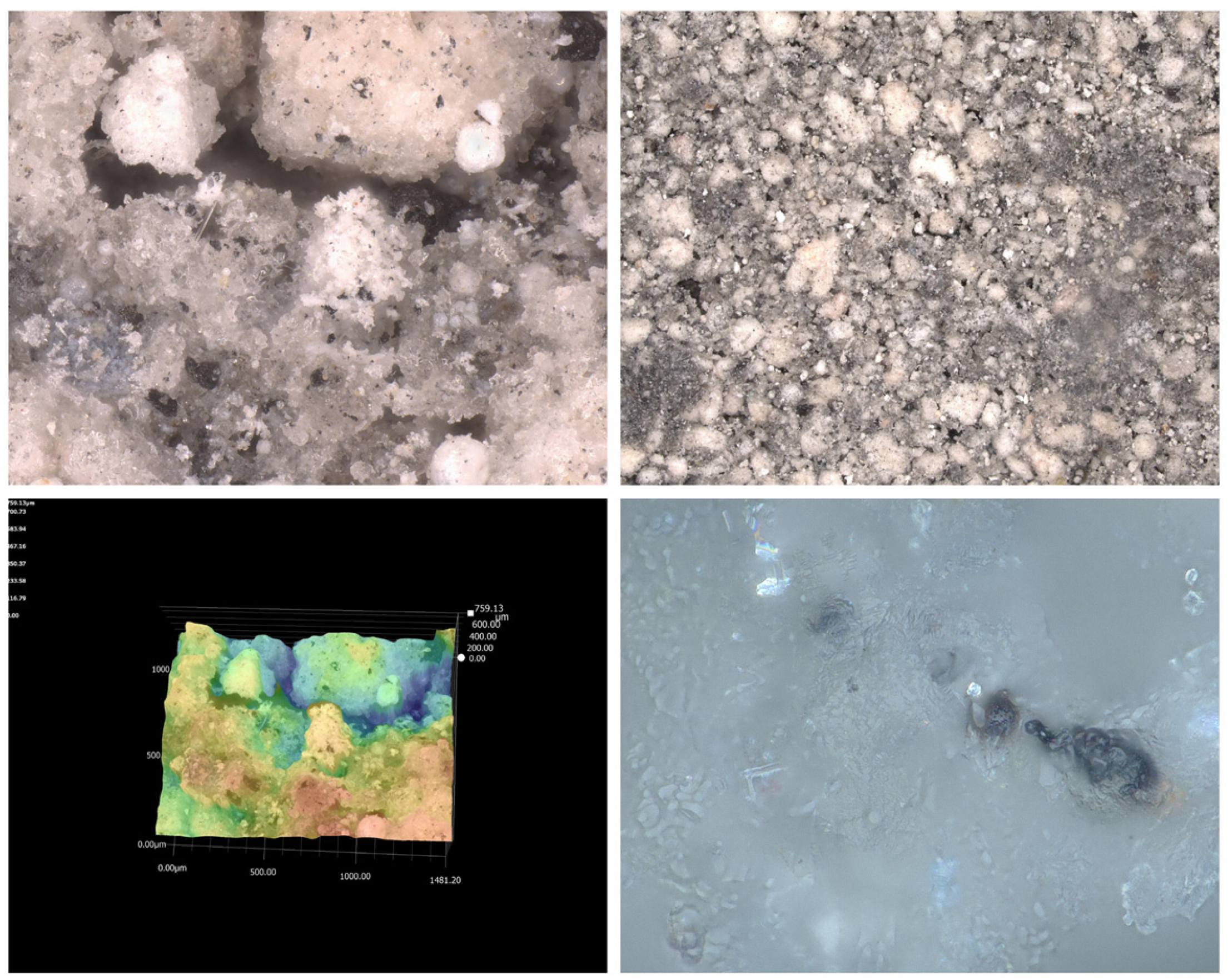
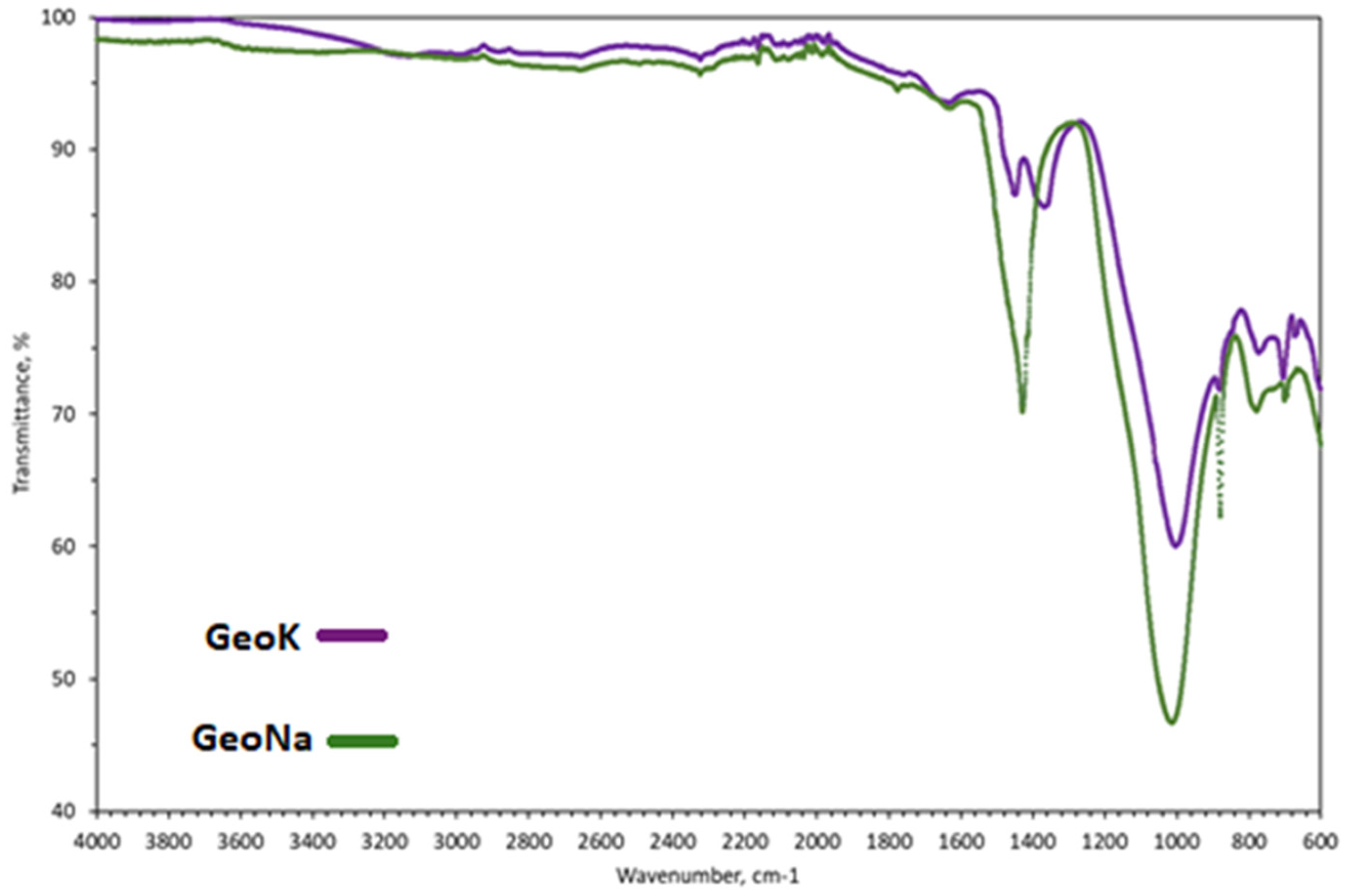
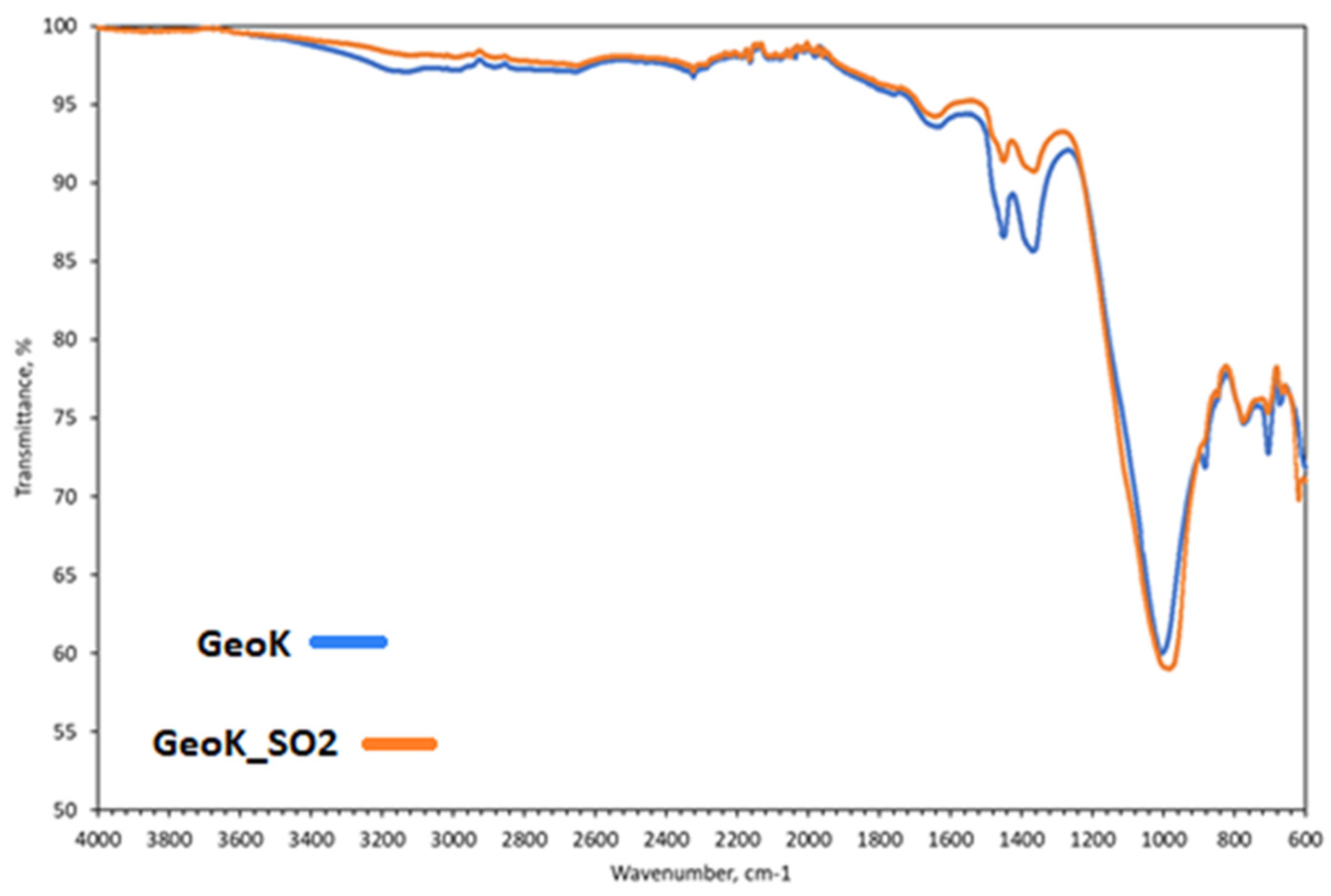
2.3.2. Microscopy
2.3.3. Textural Properties
2.3.4. Sorptions of CO2, SO2 and H2O
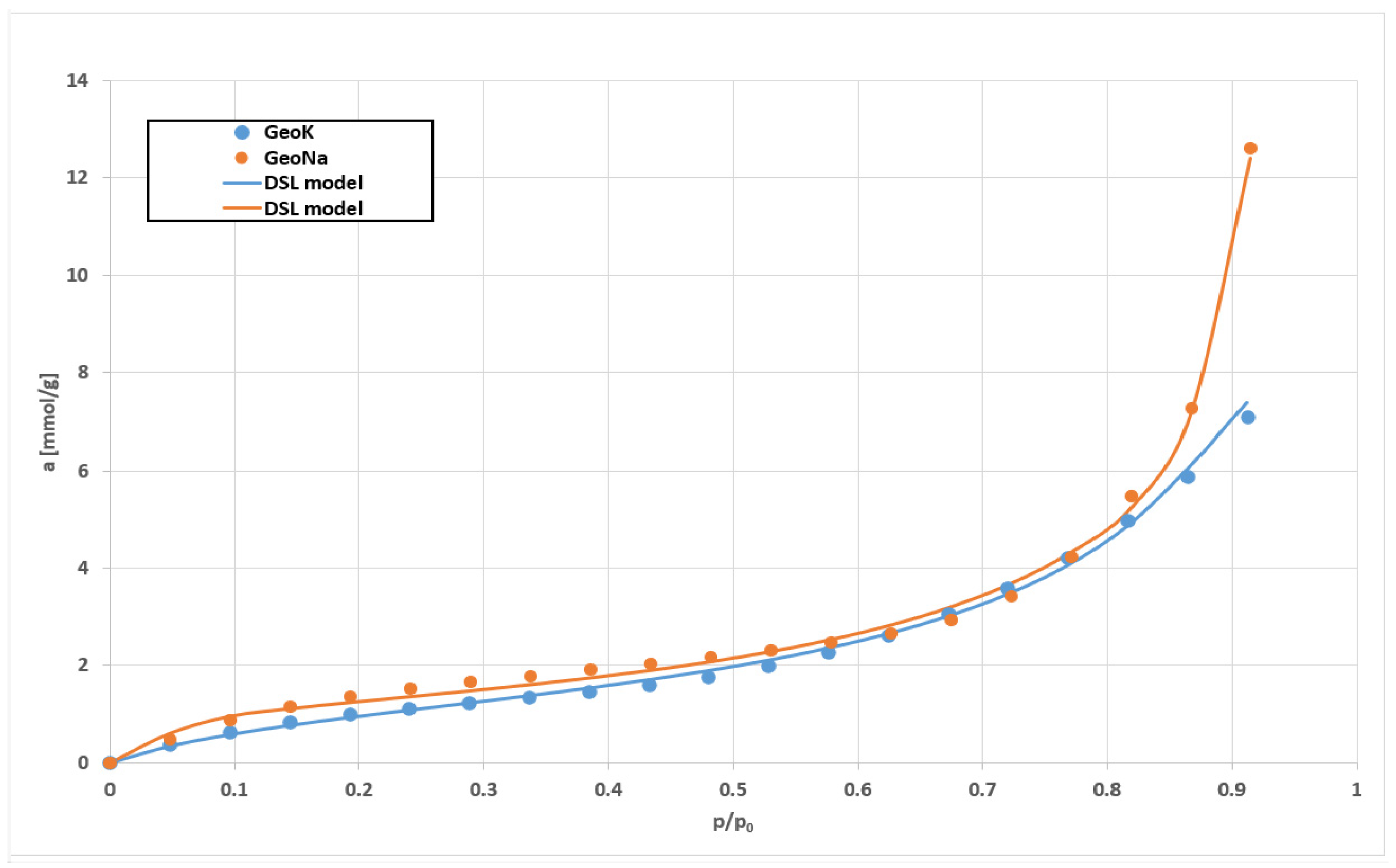
2.4. Research Results
- SSABET, [m2/g]—Specific Surface Area by Brunauer–Emmett–Teller (BET);
- VDR, [cm3/g]—Volume of micropores from Dubinin–Radushkevich equation;
- SDR, [m2/g]—Surface area of micropores from Dubinin–Radushkevich equation;
- VBJH, [cm3/g]—Volume of mesopores from Barrett–Joyner–Halenda (BJH) analysis;
- SBJH, [m2/g]—Surface area of mesopores Barrett–Joyner–Halenda (BJH) analysis;
- Vtotal, [cm3/g]—Total pore volume calculated at p/p0 = 0.995.
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Kowalska, N.; Brodawka, E.; Smoliński, A.; Zarębska, K. The European Education Initiative as a Mitigation Mechanism for Energy Transition. Energies 2022, 15, 6633. [Google Scholar] [CrossRef]
- Coal-Fired Electricity–Analysis. Available online: https://www.iea.org/reports/coal-fired-electricity (accessed on 7 October 2022).
- Ćwik, A.; Casanova, I.; Rausis, K.; Zarębska, K. Utilization of High-Calcium Fly Ashes through Mineral Carbonation: The Cases for Greece, Poland and Spain. J. CO2 Util. 2019, 32, 155–162. [Google Scholar] [CrossRef]
- EU Best Available Techniques Reference Documents (BREFs)—European Environment Agency. Available online: https://www.eea.europa.eu/themes/air/links/guidance-and-tools/eu-best-available-technology-reference (accessed on 31 January 2023).
- Sauvé, S.; Bernard, S.; Sloan, P. Environmental Sciences, Sustainable Development and Circular Economy: Alternative Concepts for Trans-Disciplinary Research. Environ. Dev. 2016, 17, 48–56. [Google Scholar] [CrossRef]
- Czuma, N.; Samojeden, B.; Zarębska, K.; Motak, M.; Da Costa, P. Modified Fly Ash, a Waste Material from the Energy Industry, as a Catalyst for the CO2 Reduction to Methane. Energy 2022, 243, 122718. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Zhao, D.; Zhong, G.; Sun, Y.; Hu, X.; Sun, J.; Li, X.; Zhu, W.; Li, M.; et al. Research and Application Progress of Geopolymers in Adsorption: A Review. Nanomaterials 2022, 12, 3002. [Google Scholar] [CrossRef] [PubMed]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly Ash-Based Eco-Friendly Geopolymer Concrete: A Critical Review of the Long-Term Durability Properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Luhar, S.; Nicolaides, D.; Luhar, I. Fire Resistance Behaviour of Geopolymer Concrete: An Overview. Buildings 2021, 11, 82. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical Activation of Fly Ash: Effect on Reaction, Structure and Properties of Resulting Geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; He, X.; Su, Y.; Oh, S.-K. Shrinkage Properties and Microstructure of High Volume Ultrafine Phosphorous Slag Blended Cement Mortars with Superabsorbent Polymer. J. Build. Eng. 2020, 29, 101121. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Zhao, F.; Huo, T.; Chen, E.; Tang, S. Comparison between the Influence of Finely Ground Phosphorous Slag and Fly Ash on Frost Resistance, Pore Structures and Fractal Features of Hydraulic Concrete. Fractal Fract. 2022, 6, 598. [Google Scholar] [CrossRef]
- Yong, C.L.; Mo, K.H.; Koting, S. Phosphorus Slag in Supplementary Cementitious and Alkali Activated Materials: A Review on Activation Methods. Constr. Build. Mater. 2022, 352, 129028. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; He, X.; Su, Y.; Zeng, J.; Dai, F.; Guan, S. Effect of Ultrafine Fly Ash and Water Glass Content on the Performance of Phosphorus Slag-Based Geopolymer. Materials 2022, 15, 5395. [Google Scholar] [CrossRef] [PubMed]
- Klima, K.M.; Schollbach, K.; Brouwers, H.J.H.; Yu, Q. Thermal and fire resistance of Class F fly ash based geopolymers–A review. Constr. Build. Mater. 2022, 323, 126529. [Google Scholar] [CrossRef]
- Chen, X.Y.; Klima, K.M.; Brouwers, H.J.H.; Yu, Q. Effect of silica aerogel on thermal insulation and acoustic absorption of geopolymer foam composites: The role of aerogel particle size. Compos. Part B 2022, 242, 110048. [Google Scholar] [CrossRef]
- Babaee, M.; Castel, A. Water vapor sorption isotherms, pore structure, and moisture transport characteristics of alkali-activated and Portland cement-based binders. Cem. Concr. Res. 2018, 113, 99–120. [Google Scholar] [CrossRef]
- Luo, Y.; Brouwers, H.J.H. Document details—Understanding the gel compatibility and thermal behavior of alkali activated Class F fly ash/ladle slag: The underlying role of Ca availability. Cem. Concr. Res. 2023, 170, 107198. [Google Scholar] [CrossRef]
- Saleh, T.A. Protocols for Synthesis of Nanomaterials, Polymers, and Green Materials as Adsorbents for Water Treatment Technologies. Environ. Technol. Innov. 2021, 24, 101821. [Google Scholar] [CrossRef]
- Czuma, N.; Panek, R.; Baran, P.; Zarębska, K. The Influence of Binders for the Pelletization of Fly Ash Zeolites on Sulfur Dioxide Sorption Properties. Clay Miner. 2020, 55, 40–47. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Peralta, R.M.; Peralta, R.A.; Rodríguez-Castellón, E.; de Muniz Moreira, R.F.P. Adding Value to Aluminosilicate Solid Wastes to Produce Adsorbents, Catalysts and Filtration Membranes for Water and Wastewater Treatment. J. Mater. Sci. 2021, 56, 1039–1063. [Google Scholar] [CrossRef]
- Minelli, M.; Medri, V.; Papa, E.; Miccio, F.; Landi, E.; Doghieri, F. Geopolymers as Solid Adsorbent for CO2 Capture. Chem. Eng. Sci. 2016, 148, 267–274. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Global Energy Review: CO2 Emissions in 2021–Analysis. Available online: https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2 (accessed on 25 October 2022).
- Muhammad, B.; Khan, M.K. Foreign Direct Investment Inflow, Economic Growth, Energy Consumption, Globalization, and Carbon Dioxide Emission around the World. Environ. Sci. Pollut. Res. 2021, 28, 55643–55654. [Google Scholar] [CrossRef]
- Quarterly Greenhouse Gas Emissions in the EU. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Quarterly_greenhouse_gas_emissions_in_the_EU (accessed on 26 October 2022).
- KOBIZE. Available online: https://www.kobize.pl/ (accessed on 26 October 2022).
- Czuma, N.; Franus, W.; Baran, P.; CWIK, A.; Zarębska, K. SO2 Sorption Properties of Fly Ash Zeolites. Turk. J. Chem. 2020, 44, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Cho, H.; Ahn, Y. Life-Cycle Assessment of SO2 Removal from Flue Gas Using Carbonate Melt. J. Ind. Eng. Chem. 2021, 100, 270–279. [Google Scholar] [CrossRef]
- Hoesly, R.M.; Smith, S.J.; Feng, L.; Klimont, Z.; Janssens-Maenhout, G.; Pitkanen, T.; Seibert, J.J.; Vu, L.; Andres, R.J.; Bolt, R.M.; et al. Historical (1750–2014) Anthropogenic Emissions of Reactive Gases and Aerosols from the Community Emissions Data System (CEDS). Geosci. Model Dev. 2018, 11, 369–408. [Google Scholar] [CrossRef]
- Xu, H.; Liang, W.; Xiang, K. The Environmental Consequences of Place-Based Policies in China: An Empirical Study Based on SO2 Emission Data. China World Econ. 2022, 30, 201–229. [Google Scholar] [CrossRef]
- EUR-Lex-Official Journal of the European Union. Available online: https://eur-lex.europa.eu/TodayOJ/ (accessed on 26 October 2022).
- Garbalińska, H.; Cederholm, L. Przewodność cieplna betonu komórkowego różnych klas gęstości wyznaczana w różnym stanie zawilgocenia. Mater. Bud. 2019, 1, 58–61. [Google Scholar]
- Zarębska, K.; Zabierowski, P.; Gazda-Grzywacz, M.; Czuma, N.; Baran, P. Fly Ash-Based Geopolymers with Refractoriness Properties. Clean Technol. Environ. Policy 2022, 24, 2161–2175. [Google Scholar] [CrossRef]
- Brauneur, S.; Emmet, P.; Teller, E. Adsorption of Gases in Multi Molecular Layer. J. Am Chem. Soc 1938, 60, 309. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET Equation Applicable to Microporous Adsorbents. Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Harkins, W.D.; Jura, G. An Adsorption Method for the Determination of the Area of a Solid without the Assumption of a Molecular Area, and the Area Occupied by Nitrogen Molecules on the Surfaces of Solids. J. Chem. Phys. 1943, 11, 431–432. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1402. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Rockland, L.B. Influences of Hysteresis and Temperature on Moisture Sorption Isotherms. In Water Activity; Routledge: London, UK, 2017; pp. 173–213. [Google Scholar]
- Anderson, R.B. Modification of the Brunauer, Emmett and Teller equations. J. Am. Chem. Soc. 1946, 68, 686–691. [Google Scholar] [CrossRef]
- Guggenheim, E.A. Application of Statistical Mechanics; Clarendon Press: Oxford, UK, 1966. [Google Scholar]
- De Boer, J.M. The Dynamic Character of Adsorption; Clarendon Press: Oxford, UK, 1953. [Google Scholar]

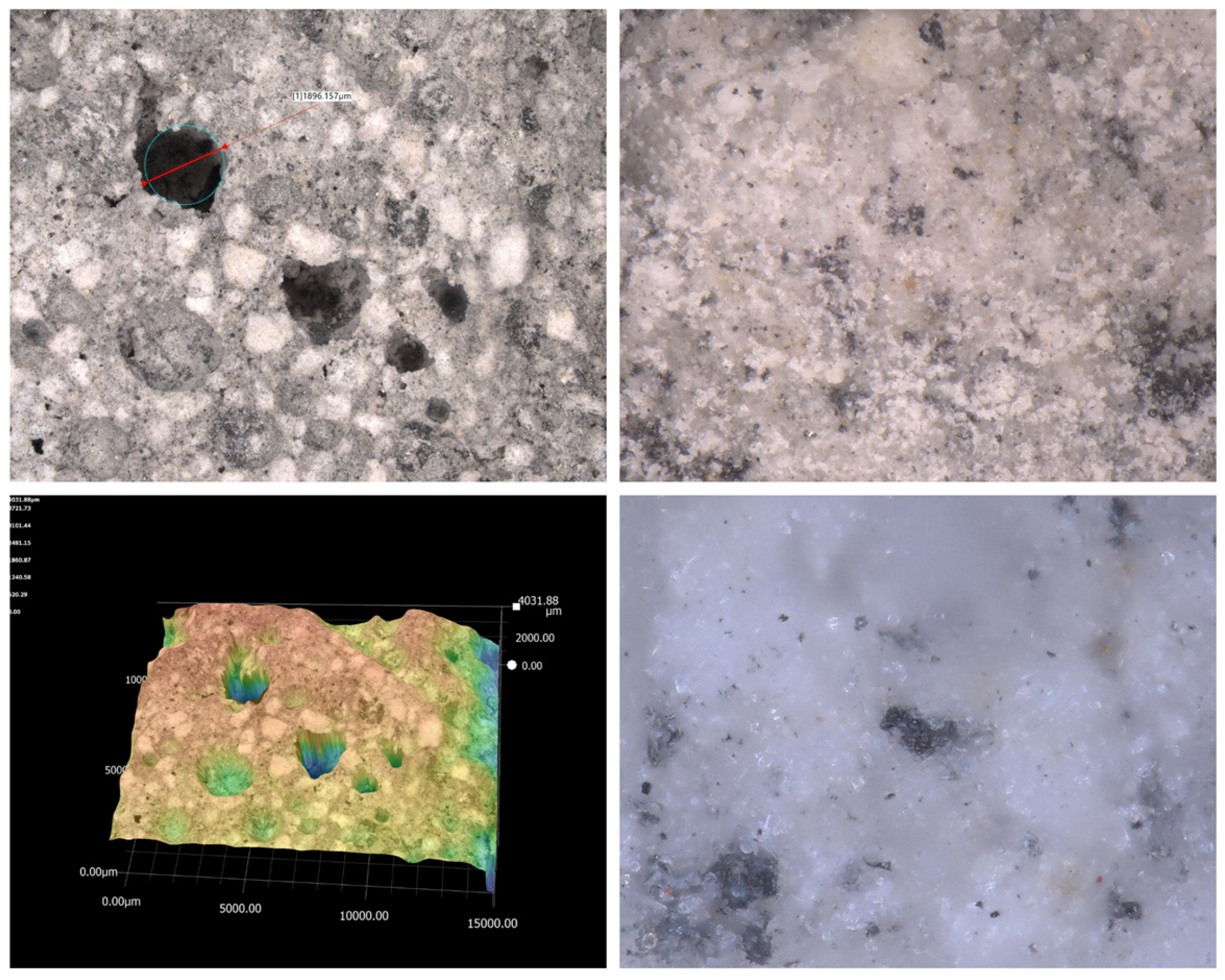
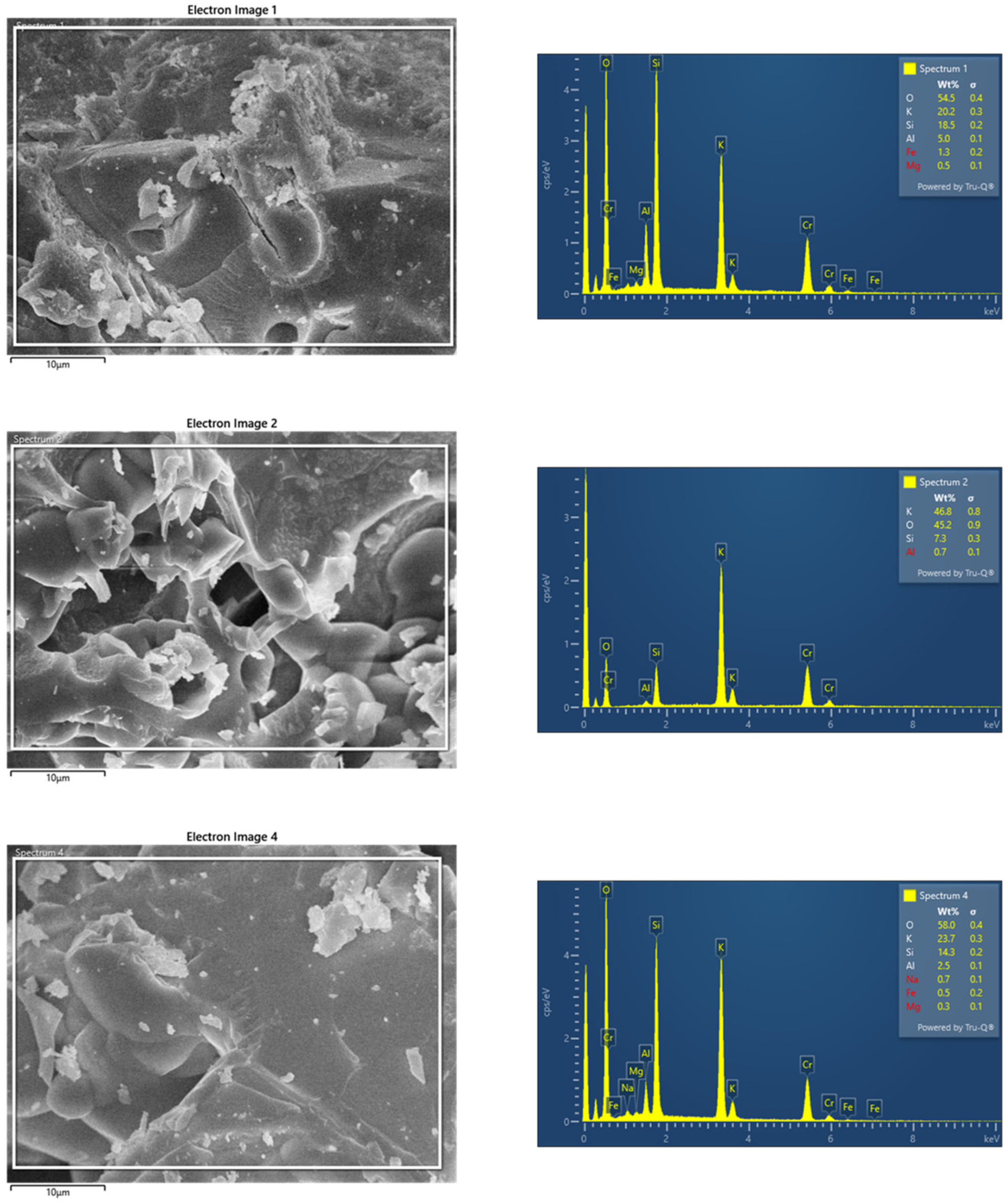
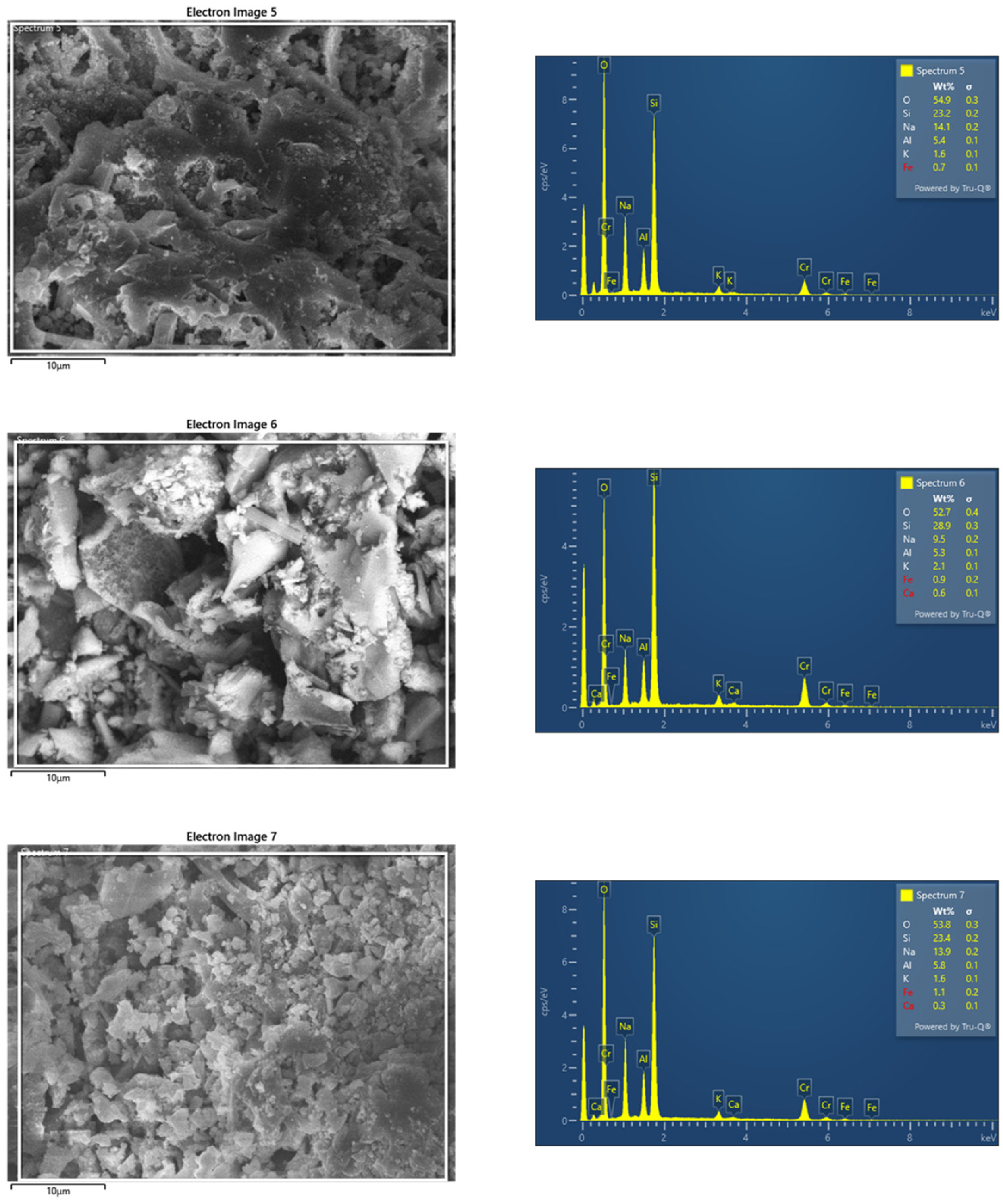
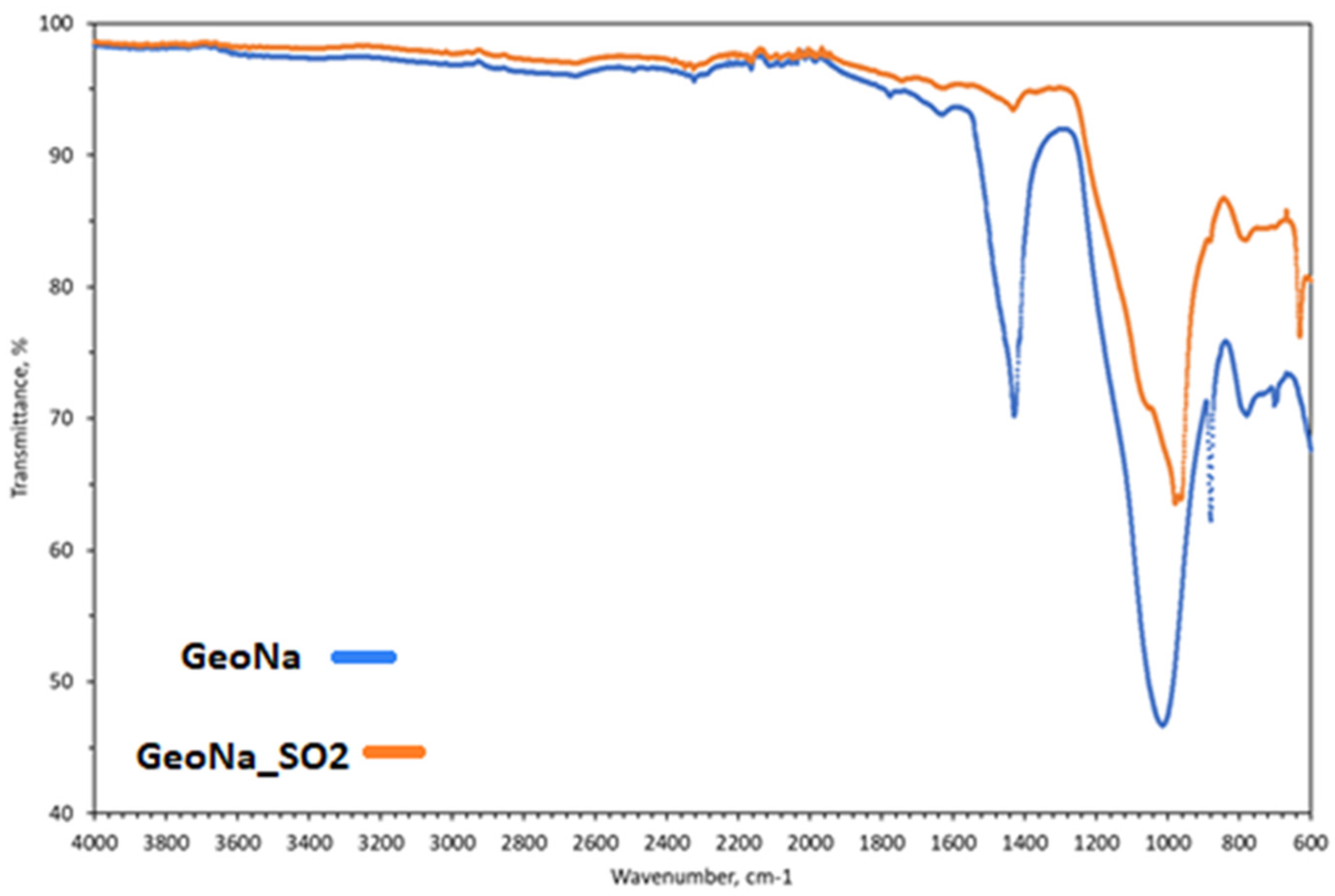
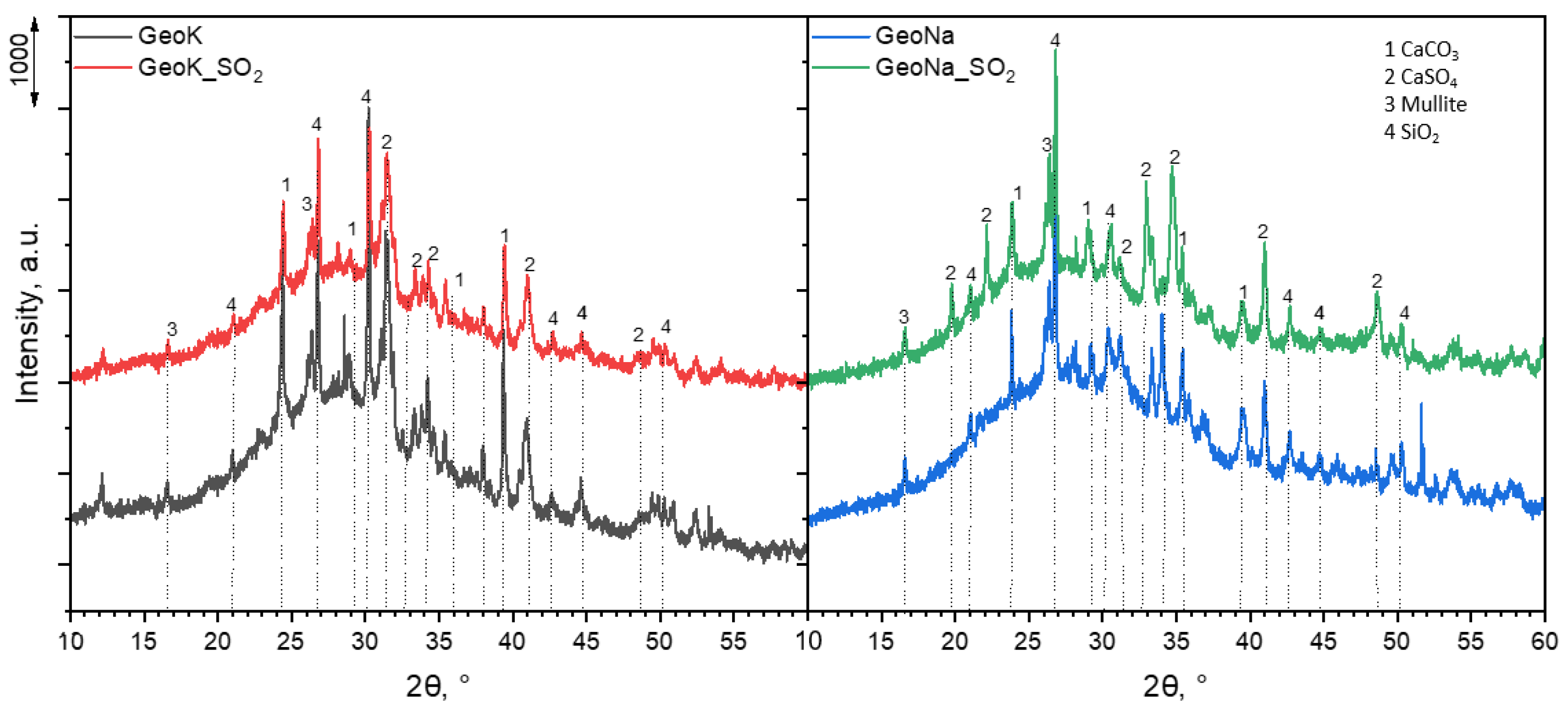
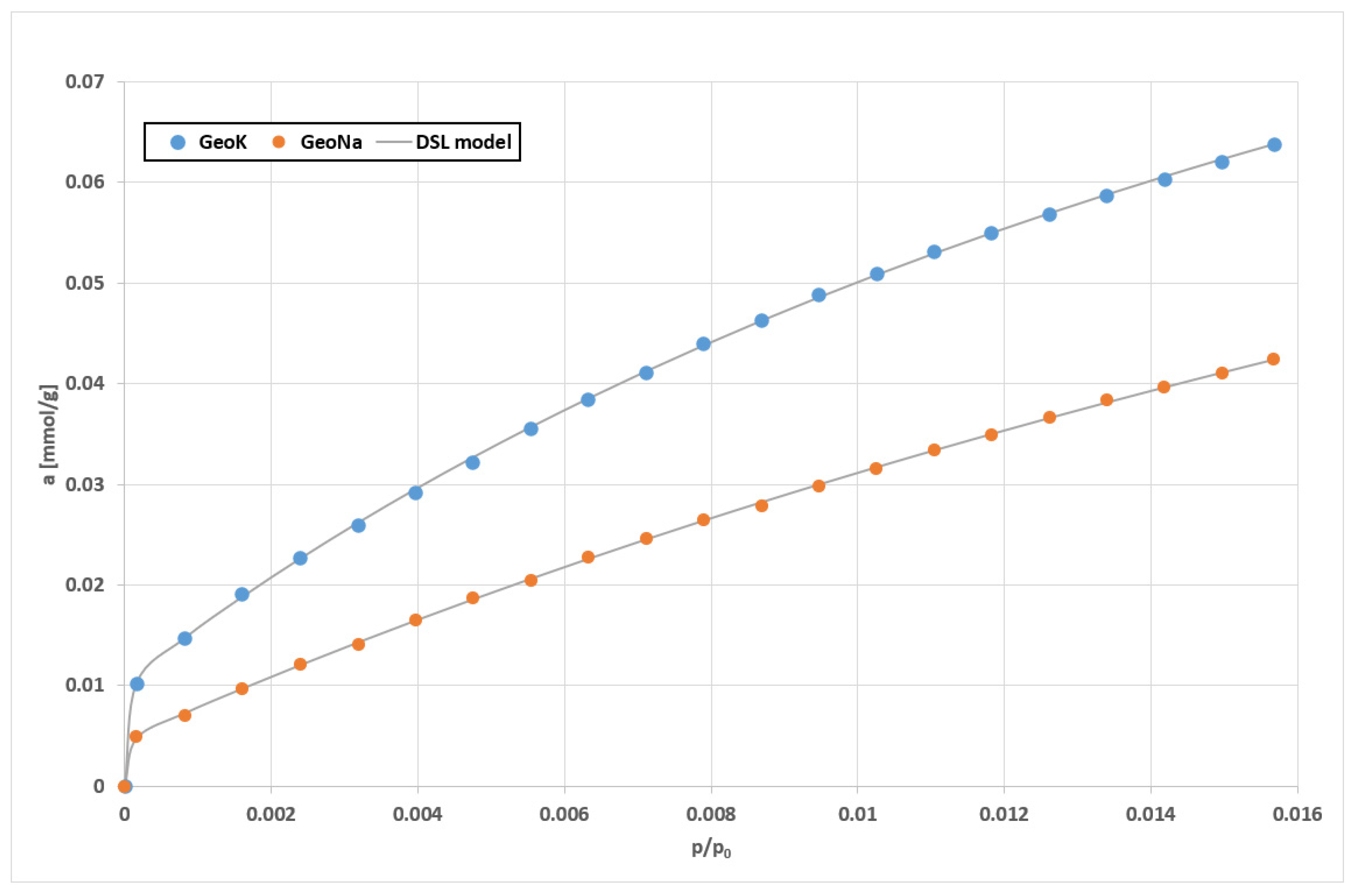
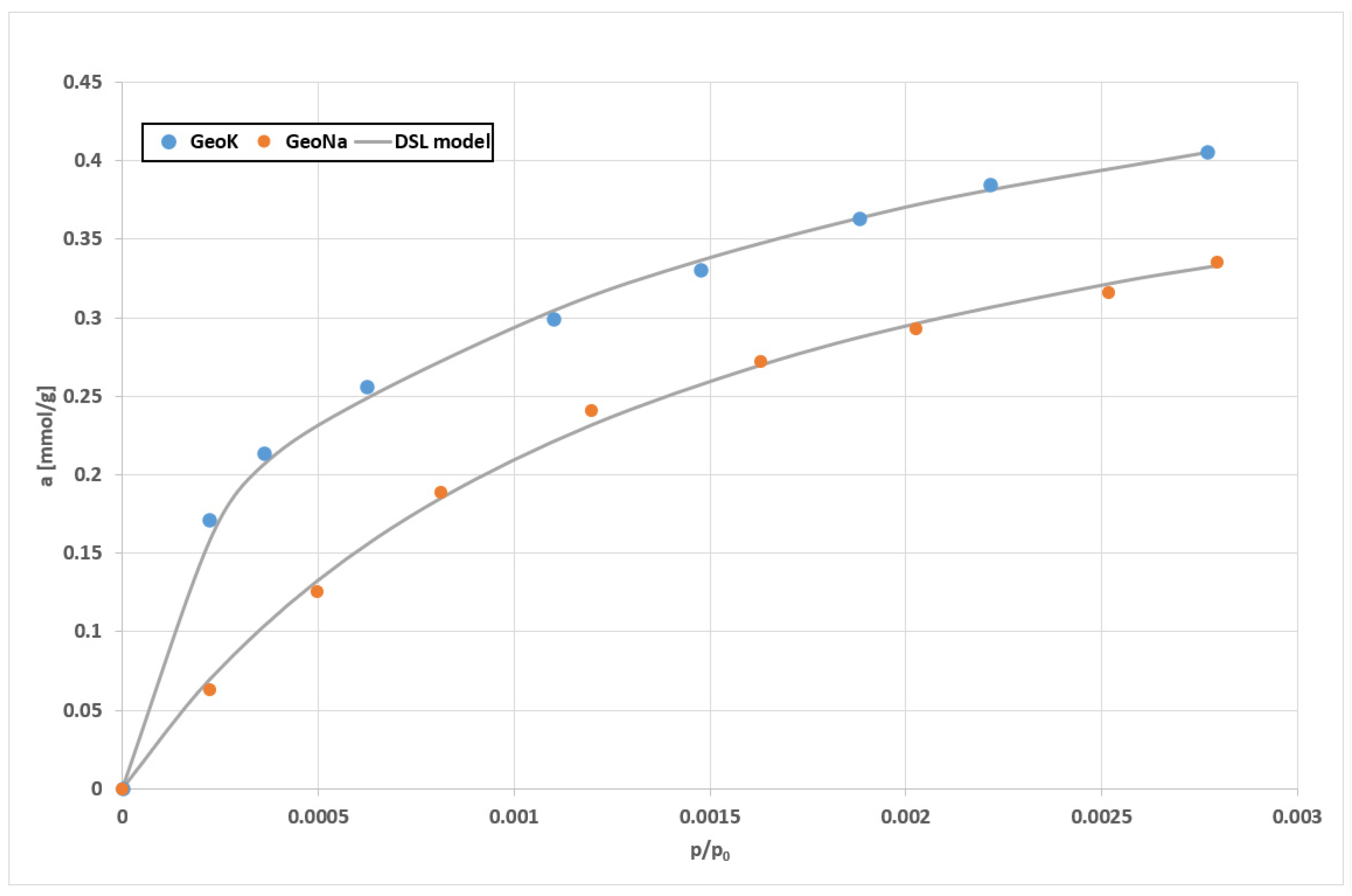
| Sample | SSABET (m2/g) | SDR (m2/g) | VDR (cm3/g) | SBJH (m2/g) | VBJH (cm3/g) | VTotal (cm3/g) |
|---|---|---|---|---|---|---|
| GeoK | 10.1 | 9.2 | 0.003 | 8.7 | 0.058 | 0.081 |
| GeoNa | 3.5 | 3.1 | 0.001 | 3.3 | 0.024 | 0.035 |
| Quantity | GeoK | GeoNa |
|---|---|---|
| Total cumulative volume, TCV (mm3/g) | 129.2 | 387.1 |
| Porosity (%) | 21.23 | 42.97 |
| Density, bulk density (g/cm3) | 1.1441 | 0.9914 |
| Density, apparent density, 101.3 kPa (g/cm3) | 1.5952 | 1.0910 |
| Density, apparent density, 150 MPa (g/cm3) | 2.0867 | 1.9465 |
| Pore volume ˂ 5 nm | 0.1009 | 0.1153 |
| Average pore radius (nm) | 7.0 | 90.6 |
| Quantity | GeoK | GeoNa |
|---|---|---|
| Compressive strength, MPa | 4.901 | 4.984 |
| Refractoriness, °C | 1200 | 1000 |
| Thermal conduction coefficient, W/m·K | 0.207 | 0.232 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarębska, K.; Szczurowski, J.; Gazda-Grzywacz, M.; Wróbel, W.; Bator, J.; Baran, P. Geopolymer Building Materials Based on Fly Ash in Terms of Removing SO2, CO2, and Water Vapor. Energies 2023, 16, 5188. https://doi.org/10.3390/en16135188
Zarębska K, Szczurowski J, Gazda-Grzywacz M, Wróbel W, Bator J, Baran P. Geopolymer Building Materials Based on Fly Ash in Terms of Removing SO2, CO2, and Water Vapor. Energies. 2023; 16(13):5188. https://doi.org/10.3390/en16135188
Chicago/Turabian StyleZarębska, Katarzyna, Jakub Szczurowski, Magdalena Gazda-Grzywacz, Wojciech Wróbel, Jakub Bator, and Paweł Baran. 2023. "Geopolymer Building Materials Based on Fly Ash in Terms of Removing SO2, CO2, and Water Vapor" Energies 16, no. 13: 5188. https://doi.org/10.3390/en16135188
APA StyleZarębska, K., Szczurowski, J., Gazda-Grzywacz, M., Wróbel, W., Bator, J., & Baran, P. (2023). Geopolymer Building Materials Based on Fly Ash in Terms of Removing SO2, CO2, and Water Vapor. Energies, 16(13), 5188. https://doi.org/10.3390/en16135188







