Abstract
All-solid-state lithium batteries (ASSLBs) using argyrodite electrolyte materials have shown promise for applications in electric vehicles (EVs). However, understanding the effects of processing parameters on the ionic conductivity of these electrolytes is crucial for optimizing battery performance and manufacturing methods. This study investigates the influence of electrolyte operating temperature, electrolyte operating pressure, electrolyte pelletization pressure, and electrolyte pelletizing temperature on the ionic conductivity of the Li6PS5Cl0.5Br0.5 argyrodite electrolyte (AmpceraTM, D50 = 10 µm). A specially designed test cell is employed for the experimental measurements, allowing for controlled pelletization and testing within the same tooling. The results demonstrate the significant impact of the four parameters on the ionic conductivity of the argyrodite electrolyte. The electrolyte operating temperature has a more pronounced effect than operating pressure, and pelletizing temperature exerts a greater influence than pelletizing pressure. This study provides graphs that aid in understanding the interplay between these parameters and achieving desired conductivity values. It also establishes a baseline for the maximum pelletizing temperature before undesirable degradation of the electrolyte occurs. By manipulating the pelletizing pressure, operating pressure, and pelletizing temperature, battery engineers can achieve the desired conductivity for specific applications. The findings emphasize the need to consider operating conditions to ensure satisfactory low-temperature performance, particularly for EVs. Overall, this study provides valuable insights into processing and operating conditions for ASSLBs utilizing the Li6PS5Cl0.5Br0.5 argyrodite electrolyte.
1. Introduction
The global transition toward cleaner and more environmentally friendly energy sources has sparked a remarkable surge in the demand for lithium-ion (Li-ion) batteries, which play a pivotal role in energy storage and transportation applications. This surge can be attributed to the growing recognition and adoption of “clean” or “green” energy solutions worldwide. As a result, the research and development endeavors dedicated to advancing battery technology have witnessed an unprecedented upswing, particularly in the realm of batteries that deviate from the traditional composition of lithiated transition-metal-oxide cathodes, graphite anodes, and liquid electrolytes containing lithium salts dissolved in alkyl carbonate solutions [1]. In 2019, the global sales of electric vehicles (EVs), including both battery–electric vehicles (BEVs) and plug-in hybrid electric vehicles (PHEVs), amounted to a modest 2.1 million units, constituting a mere 1.5% of the total vehicle sales worldwide. However, industry projections paint a much more promising picture for the future, estimating that by 2030, EVs will make up a substantial 30% of all vehicle purchases [2]. This anticipated growth in EV adoption is poised to further intensify the demand for Li-ion batteries, reinforcing their status as the primary solution for meeting the burgeoning energy storage requirements. As a result of their relatively high energy and power densities, Li-ion batteries have already established a firm foothold in various applications, including cell phones, laptops, electric vehicles, and stationary electrical-energy storage systems. Nonetheless, the safety concerns surrounding Li-ion batteries have curtailed their widespread utilization and prompted researchers to seek alternative solutions [3]. One of the primary safety concerns associated with traditional Li-ion batteries lies in their liquid electrolytes, which exhibit inadequate thermal stability. Under abusive conditions or insufficient regulation by a reliable battery management system (BMS), these electrolytes can undergo thermal runaway, leading to the potential for combustion or even explosions. To mitigate these risks and expand the potential applications of Li-ion batteries, researchers are actively exploring the development of Li-ion batteries incorporating solid electrolytes [3]. Solid-state electrolytes offer the promise of enhanced safety characteristics, making them an attractive material for integration into Li-ion batteries for electric vehicles and electric aircraft [4,5,6]. Additionally, solid electrolytes bring forth several other advantages. For instance, they can increase the specific energy or energy density of the battery, enabling reductions in both weight and size while maintaining or even enhancing performance levels. However, the path to fully realizing the potential of solid-state electrolyte batteries is fraught with challenges. Addressing these challenges is crucial to unlocking the vast possibilities they hold. One significant hurdle lies in the processing and assembly of solid-state batteries. Developing efficient and scalable manufacturing processes for these batteries remains a complex task, requiring meticulous control over material synthesis, electrode fabrication, and cell assembly. Achieving high-quality, reliable, and cost-effective production methods is essential to meet the burgeoning demand for Li-ion batteries with solid electrolytes. Another key challenge is mitigating the degradation mechanisms that affect the performance and lifespan of solid-state electrolyte batteries. Solid electrolytes are susceptible to various degradation mechanisms, including interface reactions, dendrite formation, and structural changes. Understanding and controlling these degradation processes is vital to ensure the long-term stability and reliability of solid-state electrolyte batteries. Furthermore, reducing the operating pressure of solid-state batteries while maintaining satisfactory performance levels poses a significant challenge. Elevated operating pressures can lead to mechanical stress, compromising the structural integrity of the battery and impeding its overall performance. Developing strategies to reduce the operating pressure without sacrificing performance requires innovative material designs, electrode engineering, and optimization of the cell architecture.
The lithium ionic conductivity of inorganic solid-electrolyte materials typically ranges from 10−5 S/cm to 10−2 S/cm at room temperature [6]. These materials have garnered significant attention and been the focus of much investigation in the field of solid-state batteries due to their potential for overcoming the limitations of traditional liquid electrolytes, such as flammability and stability issues. Among the various solid-electrolyte materials, several have emerged as commonly studied candidates, including sulfide compounds (such as argyrodite, LGPS, LPS), garnet structure oxides (such as LLZO), NASICON-type phosphate glass ceramics (such as LAGP), oxynitrides (such as LIPON), and polymers (such as PEO) [7,8,9]. Argyrodites, particularly Li6PS5X (X = Cl, Br, I), have emerged as highly promising solid-state electrolytes for solid-state batteries [10,11]. Within the Argyrodite family, Li6PS5Cl0.5Br0.5, a crystalline material, has demonstrated exceptional electrochemical stability, good processability, and high ionic conductivity (e.g., 3.5 mS/cm at room temperature) [12]. This combination of properties makes it an attractive candidate for solid-state battery applications. However, it is worth noting that the fabrication processes of sulfide-based lithium batteries present greater challenges compared with other solid-electrolyte materials. Achieving dense and defect-free interfaces between the solid electrolyte and electrodes remains a key hurdle. These challenges arise due to the reactivity of sulfide-based electrolytes with various electrode materials, such as lithium metal, which can result in the formation of unstable interphases and hinder ion conduction. To address these challenges, researchers have focused on understanding the underlying factors that influence the performance of solid-state electrolytes. One such factor is the pelletization pressure applied during the manufacturing process. Pelletization pressure directly impacts the porosity and interface resistances of the electrolyte, thereby influencing the overall cell performance. Therefore, optimizing the pelletization pressure is crucial to achieving the desired electrochemical performance of the solid-state battery [13]. Another critical parameter that significantly affects the solid electrolyte’s conductivity and cell performance is temperature. Temperature influences the mobility of ions within the electrolyte, thereby impacting the overall ionic conductivity. The optimization of temperature conditions during the manufacturing process and battery operation is pivotal for achieving enhanced performance and stability. However, the existing literature on the effect of temperature and pressure on ceramic solid electrolytes remains limited. Most available studies primarily focus on the effects of solid electrolytes on dendritic growth in lithium-metal batteries and related issues. Consequently, there is a need for further investigation to comprehend the intricate relationship between temperature, pressure, and solid-electrolyte conductivity [14]. Moreover, understanding the interplay between these parameters is vital for the commercialization of solid-state batteries. For example, the optimal stack pressure of a solid electrolyte for use in an all-solid-state battery (ASSB) with a lithium-metal anode remains a topic of debate. Lower operating cell pressure can lead to reduced apparent ionic conductivity due to poor contact between the electrolyte and electrodes. A model proposed by Zhang et al. suggests a minimum operational pressure of 20 MPa [13]. However, experimental research conducted by Doux et al. found that a stack pressure of 5 MPa resulted in a cell capable of cycling for over 1000 h, while 25 MPa caused the cell to short-circuit after less than 50 h of cycling due to lithium dendrite growth [15]. These findings highlight the intricate balance required in selecting the appropriate stack pressure to achieve optimal performance and prevent detrimental effects.
The existing body of research on argyrodite-based solid electrolytes has predominantly focused on Li6PS5Cl, with different synthesis methods being employed in various laboratories and varying pelletizing and operating pressures being applied during processing and testing. In fact, many papers neglect to even mention what operating pressures they use when performing EIS testing. As a result, there is considerable inconsistency in the reported results among these studies, as can be seen in Table 1, which limits the comparability and generalizability of their findings [16]. Moreover, recent research has indicated that Li6PS5Cl may not be the most optimal formulation of argyrodite, and alternative formulations such as Li6PS5Cl0.5Br0.5 exhibit superior electrochemical properties [15]. To address these issues and to enhance the applicability of the findings, this study centers its attention on Li6PS5Cl0.5Br0.5, a readily available material on the market. This choice ensures that the material being investigated is synthesized using well-established methods and is easily accessible to researchers and companies alike. By focusing on this specific formulation, the study aims to investigate the effects of pelletization pressure, temperature, operating pressure, and temperature on the ionic conductivity of Li6PS5Cl0.5Br0.5. Understanding the influence of these parameters on the ionic conductivity of argyrodite electrolytes is not only crucial for fabricating high-performance solid-state cells but also facilitates the modeling of solid-state lithium batteries to assess electrolyte performance [16], determine battery operating voltage [17], analyze microstructure heterogeneity [18], evaluate battery energy efficiency [19], and design suitable cooling systems for the battery [20]. By gaining insights into the impact of pelletization pressure and temperature, as well as operating pressure and temperature, on the ionic conductivity of Li6PS5Cl0.5Br0.5, this study contributes valuable knowledge that can advance multiple aspects of solid-state battery research and development. The findings of this study will shed light on the optimal conditions for fabricating argyrodite-based solid electrolytes with enhanced ionic conductivity. This information can be utilized to improve the performance and stability of solid-state batteries, ultimately contributing to the development of next-generation energy storage systems. Moreover, the study’s outcomes will serve as a foundation for further investigations into the broader implications of these parameters on battery performance and design.

Table 1.
Comparison of argyrodite ionic conductivities, pelletizing pressures, and operating pressures among journal articles.
2. Materials and Methods
In this work, the sulfide electrolyte powder studied was the AmpceraTM (Tucson, AZ, USA) Argyrodite Li6PS5Cl0.5Br0.5 with a D50 of about 10 µm from batch number 20220923. The supplier claims an ionic conductivity of 3.5 mS/cm [12], but does not disclose its processing or testing conditions. For this study, 100 mg of powder was used for pelletizing in an internally designed test cell. The test cell was made of A2-die steel plungers, electrically insulative polymer discs, and a ceramic sleeve. Brass leads were drilled into the steel plungers for easy access to electrical connections. The diameter of the pressing surface was 10 mm, and the thickness of the pellet was measured by first measuring the distance between the two plungers with a caliper before adding the powder, and then, after adding the powder and pelletizing, calculating the difference between the two values. The values were verified by removing the pellet after testing and measuring the thickness using a micrometer. This test setup allows for an SS/SS symmetric cell to be assembled, processed, and tested all within the same tooling. The end of the stainless-steel plungers that contact the electrolyte were very precisely sanded and polished to create a flat mirror finish on the steel. This was accomplished by first machining the surface until it is perfectly flat, then sanding it with grit paper, starting with FEPA P280 grit paper and working up to P4000 grit paper, then finishing with a 1 µm polish to reach a mirrored finish. The surface of the stainless-steel plungers was checked between each test to ensure that the finish was mirrored, and if it was not, it was polished before conducting the next test to ensure that the surface of the testing and pelletizing fixture remained the same for each sample. To verify that the amount of force on the cell was constant over the time it was being held for the high-temperature pelletizing process, a load cell was integrated into the cell. The test cell was pressed using a TMAX-SYP-24T hydraulic press with a digital pressure gauge in a glovebox with an argon atmosphere. The pelletizing pressures used at room temperature were 180, 360, 540, 720, and 900 MPa. For each pelletizing pressure, the pellet was held under the desired pressure for one minute three times, with a 120° rotation of the cell between each pelletization. The operating pressure was set using the same hydraulic press. Operating pressures tested at room temperature were 10, 25, 50, 75, 100, 150, 250, and 350 MPa. Elevated temperature testing was also carried out at 50 °C and 75 °C using pelletizing pressures of 180, 540, and 900 MPa and operating pressures of 10, 50, 100, 150, and 250 MPa. The trials conducted at 180 MPa only used operating pressures up to 150 MPa. The experimental setup is shown in Figure 1. The test-cell assemblies were placed in an environmental chamber for one hour at the desired temperature to allow the cell to reach thermal equilibrium before the EIS tests were performed on the cells. Three repetitions were conducted for each measurement. Reduced temperature testing was also performed at −20 °C and 0 °C using pelletizing pressures of 180, 360, and 540 MPa and operating pressures of 50, 100, 150, and 250 MPa. Further studies were carried out by testing the cell under different pelletizing pressure and temperature conditions. Two temperatures were tested, starting at 25 °C and 100 °C, at each pelletizing pressure (180 MPa, 360 MPa, 540 MPa, 720 MPa, and 900 MPa). Each of the temperature and pressure combinations was held for 1 h, and then the cell was brought back down to 25 °C for electrochemical characterization at each operating pressure (10 MPa, 50 MPa, 100 MPa, 150 MPa, and 250 MPa). Next, 135 °C, 150 °C, and 180 °C were tested at 540 MPa pelletizing pressure using the same methods. The results of this study will help to understand the range of pelletization and the operating pressures and temperatures of all-solid-state lithium-ion batteries made with the Li6PS5Cl0.5Br0.5 crystalline electrolyte material.
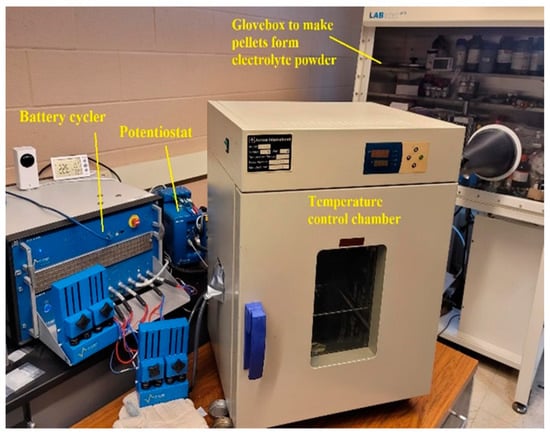
Figure 1.
Experimental setup.
The ionic conductivity for each of these combinations was tested by performing electrochemical impedance spectroscopy (EIS) with a Biologic SP-150 (France) potentiostat. EIS measurements were taken in potentiostatic mode with an excitation voltage of 20 mV in a frequency range of 1 MHz to 1 kHz. The total resistance, Rt, was determined from fitting the impedance data to a Nyquist plot, and the conductivity was calculated using Equation (1):
where k is the ionic conductivity of the electrolyte, δ is the thickness of the electrolyte pellet, Rt is the total resistance measured by the potentiostat, and A is the pellet’s surface area. The thickness was measured by measuring the thickness of the full cell assembly while empty and subtracting that value from the full cell assembly with pelletized electrolyte powder in it. This measurement was verified by carefully removing a few pellets after pelletization and measuring their thickness using a micrometer for comparison. Figure 2 shows the Nyquist plot for one of the low-temperature tests performed. The raw data as well as a fit made in Zview 3.5g software are shown on the plot. The fit shows a single semicircle that does not start at 0, signifying that the resistance used for the ionic conductivity tests is not the bulk resistance. Rather, it is most likely a combination of bulk resistance and grain boundary resistance [30,32,33]. Results based on the Zview fit indicate that there is a grain boundary resistance of 90 Ω and a bulk resistance of 105 Ω; this means that the total resistance is 195 Ω, which is the value that was used to calculate the ionic conductivity of the electrolyte. Only the testing performed at −20 °C shows the trend, because it is believed that grain boundary resistance increases as temperature decreases [30,32,33]. The grain boundary and bulk resistance will not be individually investigated in this study, but it is important to know that the ionic conductivity measurements are coming from a combination of the bulk and grain boundary resistance. Further study could be done to see how the pelletizing parameters affect the bulk and grain boundary resistance individually when much lower temperature testing is conducted in combination with a higher EIS frequency.
k = δ/(Rt A)
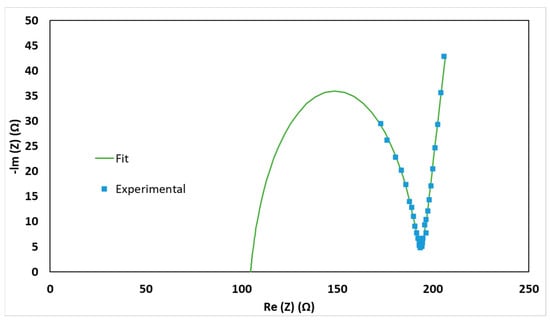
Figure 2.
Nyquist plot for Ampcera Li6PS5Cl0.5Br0.5 at −20 °C, 540 MPa pelletizing and 250 MPa operating pressure. Results indicate that there is a grain boundary resistance of 90 Ω and a bulk resistance of 105 Ω based on the Zview fit, which means that the total resistance is 195 Ω. This is the value that was used to calculate the ionic conductivity of the electrolyte. This figure is for visual explanation only; the accuracy of the bulk and grain boundary measurements have not been studied or verified.
3. Results and Discussion
It is noted that for each parameter set, electrolyte ionic conductivity measurements were made on three separate pellets, and the average of the three values and the standard deviation are presented using data points with error bars. The error bars on some of the graphs are not visible because the standard deviation is very low compared with others. In addition, the data points are larger than the actual error.
3.1. Effect of Pelletizing Pressure on Electrolyte Pellet Thickness
First, the effect of pelletization pressure on the electrolyte thickness was investigated. A quantity of 100 mg electrolyte was added to a cell with a diameter of 10 mm and the electrolyte thickness was measured at different pelletizing pressures. We first applied the pressure, then measured the thickness after the pressure was released. The results of the thickness test are shown in Figure 3. As can be seen, by increasing the pelletization pressure, the thickness of the electrolyte decreases, which indicates an increase in the relative density of the electrolyte. However, this decrease is not significant for pelletization pressures of more than about 600 MPa, which indicates that the electrolyte thickness is mostly stabilized beyond that pressure. Using two trials, we established a correlation that gives the electrolyte thickness with the pelletization pressure. This correlation is stated in Equation (2).
where the pelletization pressure, pp, is in MPa and the electrolyte thickness, δ, is in micro-meters.
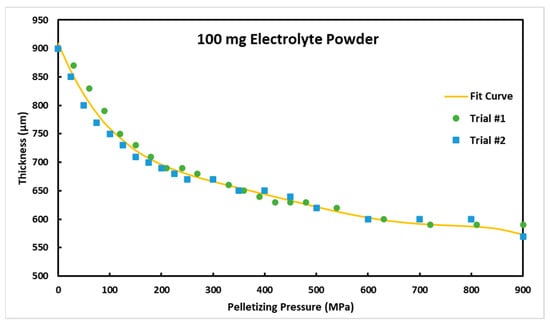
Figure 3.
Electrolyte thickness versus the pelletization pressure for 100 mg electrolyte powder and the die with a circular diameter of 10 mm.
The equation is not a completely accurate representation of how powder compaction of the argyrodite powder will respond for modeling purposes but gives a good estimate for the purpose of this paper. A study using a linear variable-differential transformer (LVDT) sensor with a constant strain rate would give a better representation of how the argyrodite powder compacts as pressure increases. Finding a repeatable and accurate way to measure the density of the pellets would also be beneficial for future studies but was not employed in this study because of the difficulty of removing the pellets in one complete piece and the air sensitivity of the samples leading to densimeter testing outside of the glovebox being unreliable or not possible. Furthermore, relative density calculations would show the same trend, as the area and mass of each measurement is identical, and the only value changing is thickness.
3.2. Effect of Pelletizing and Operating Pressure on Ionic Conductivity
Figure 4a shows the electrolyte ionic conductivity versus the cell operating pressure at several pelletization pressures and the lab temperature (~25°C). As seen, the ionic conductivity increases with the increase in the cell operating pressure. In the range of operating pressures of 10 MPa to 50 MPa, the rate of increase in the ionic conductivity is relatively fast, while this rate decreases when the operating pressure is more than 100 MPa. The low ionic conductivity at low operating pressures is due to poor contact between the current collector and pellet as well as poor particle-to-particle contact, as there is a relaxation of the particles after pelletization [31]. The increased operating pressure improves these contacts, but eventually hits a plateau point where that contact can no longer be improved, and further increasing the operating pressure could have negative effects such as defects forming in the crystal lattice, such as vacancies or dislocations. It is desirable to have a certain amount of these defects as it will improve the conductivity of the electrolyte, but too many or a poor distribution of them can have a negative impact on the conductivity [34]. For real-world application, an operating pressure above 5 MPa is unrealistic, and efforts need to be made to lower the operating pressure to values below 5 MPa while keeping the ionic conductivity at an acceptable level [35]. Unfortunately, it appears that there is not a significant difference in ionic conductivity at low pressures based on the pelletizing pressure alone. However, it is most likely that when scaled up to commercial cell levels that would use a much thinner electrolyte (~30 µm), the effect of operating pressure would be different, and further study would need to be conducted to find the optimal operating pressure based on electrolyte thickness, surface area, and electrode stack size.
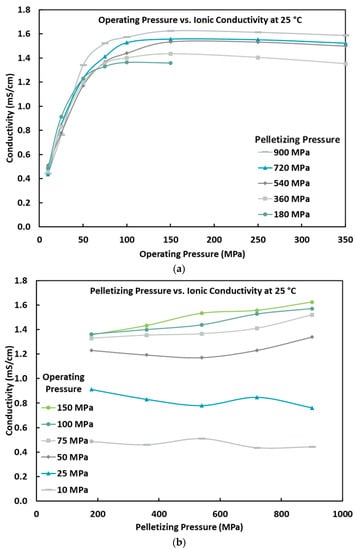
Figure 4.
(a) Ionic conductivity of the Li6PS5Cl0.5Br0.5 electrolyte versus the cell operating pressure at different pelletization pressures and at room temperature (25 °C). (b) Ionic conductivity of the Li6PS5Cl0.5Br0.5 electrolyte versus the cell pelletizing pressure at different operating pressures and at room temperature (25 °C).
The pelletization pressure was expected to play a significant role in the ionic conductivity of the solid electrolyte; however, as can be seen in Figure 4b, there is very little difference in ionic conductivity from changing the pelletizing pressures at operating pressures below 50 MPa. Previous studies have shown that by increasing the pelletizing pressure, a more densified pellet can be created, which allows for a reduction in voids and an increase in particle-to-particle interaction [31]. This explains why there is a slight increase in ionic conductivity as the pelletizing pressures increase at operating pressures of 50 MPa and above. The supplier of the electrolyte claims an ionic conductivity of 3.5 mS/cm, which is twice the value reached during this testing. This discrepancy could be due to batch-to-batch variation or the supplier’s unknown pelletizing process.
3.3. Effect of Operating Temperature on Ionic Conductivity
The effect of cell operating temperature is shown in Figure 5. As can be seen, the effect of temperature is significant. It seems its effect is greater than the operation and pelletization pressures. Every pelletizing pressure that was tested at high temperatures showed a significant increase in conductivity. If a cell is tested at a temperature of 75 °C and pressure of 250 MPa and the pelletization pressure is 540 MPa, the ionic conductivity reaches 12 mS/cm, which is comparable to or greater than many commercial liquid electrolytes.
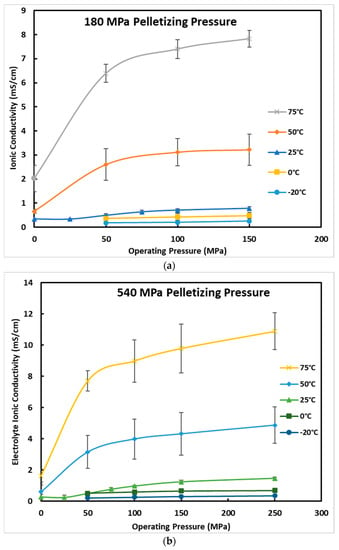
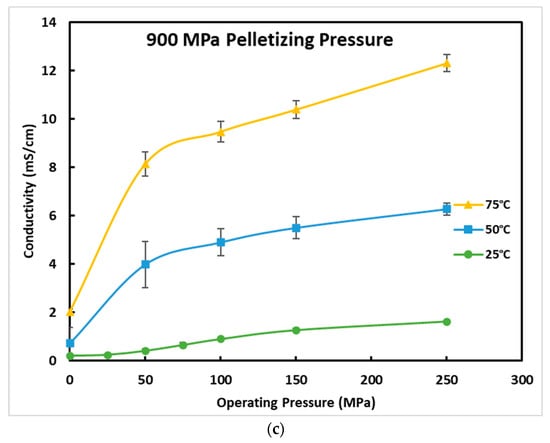
Figure 5.
Ionic conductivity of the Li6PS5Cl0.5Br0.5 electrolyte versus the cell operating pressure at different battery operating temperatures and pelletization pressures of (a) 180 MPa (b) 540 MPa and (c) 900 MPa. Error bars are presented to show one standard deviation range for each result.
If the operating temperature is not a design constraint for the battery and the battery application, it is possible to significantly decrease the pelletization pressure, which affects the battery manufacturing cost, and to decrease the operating pressure, which may decrease the battery-pack design complexity and cost.
Low operating temperatures likewise show a clear decrease in ionic conductivity, which is not a good sign for EV applications as most standards for EVs currently require operating temperatures as low as −40 °C. There is concern here not only for the performance of the battery, but also for the longevity of the battery due to lithium dendrite growth and other degradation mechanisms. Figure 6 shows an Arrhenius plot for the ionic conductivity of the electrolyte at the operating pressure of 250 MPa and in the temperature range of −20 to 75 °C. The Arrhenius plot for the ionic conductivity of the electrolyte at the operating pressure of 50 MPa, 100 MPa, and 150 MPa are also shown in Figure S1 in the supplementary document. The values in the Arrhenius plots are also listed in Table S1 in the supplementary document. The activation energy, Ea, was calculated from the slope of the linearized Arrhenius plot shown in Equation (3),
where k is the conductivity, A is the pre-exponential constant, kb is the Boltzmann constant, and T is the absolute temperature. The linear dependance of ln(k) vs. (1000/T) follows the Arrhenius law, which verifies the purity and thermal stability of the electrolyte powder used as well as the accuracy of the testing results produced in this paper. The activation energy at 250 MPa operating pressure and 540 pelletizing pressure was found to be 0.275 eV, which was very similar to the activation energy values of 2.85, 2.80, and 2.75 eV for 50, 100, and 150 MPa, respectively. There is a slight decrease in activation energy as the operating pressure increases.
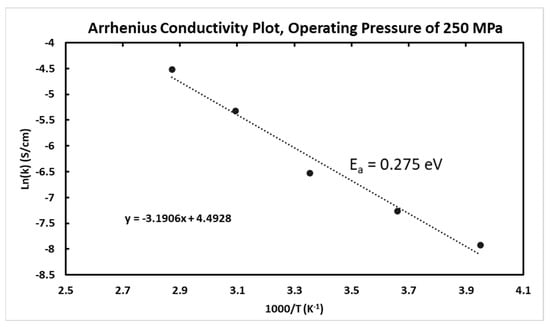
Figure 6.
Arrhenius ionic conductivity plot of AmpceraTM Li6PS5Cl0.5Br0.5 at 250 MPa from −20 to 75 °C. The activation energy was found to be 0.275 eV.
3.4. Effect of Pelletizing Temperature on Ionic Conductivity
The effect of cell pelletizing temperatures and operating pressure is shown in Figure 7. The effect of pelletizing temperature does not seem to have a linear relationship with ionic conductivity. At low operating pressures there is an increase in ionic conductivity from 25 °C up to 100 °C, but the ionic conductivity decreases at temperatures above 100 °C pelletizing temperature. As operating pressure increases, the trend changes and it appears the highest ionic conductivity occurs at 100 °C pelletizing temperature with operating pressures above 100 MPa.
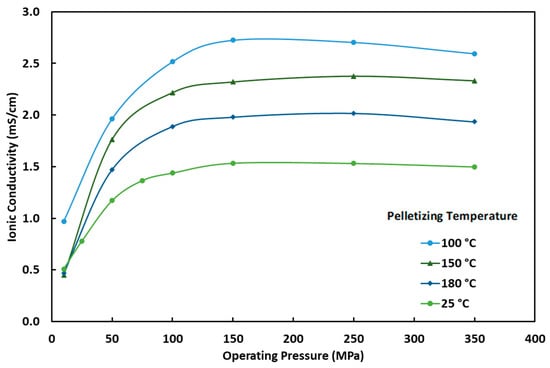
Figure 7.
Ionic conductivity of the Li6PS5Cl0.5Br0.5 electrolyte versus the cell operating pressure at different battery pelletizing temperatures and operating pressures at a constant pelletizing pressure of 540 MPa.
Testing was further conducted at 100 °C pelletizing temperature to see the effect of pelletizing pressure at an elevated temperature. Figure 8 shows the resulting ionic conductivity at pelletizing pressures from 180 MPa to 900 MPa and operating pressures from 10 MPa to 350 MPa at 100 °C pelletizing pressure compared to the best conductivity results from room temperature pelletizing, which is at a 900 MPa pelletizing pressure. The 180 MPa pelletizing pressure at 100 °C ionic conductivity values are significantly higher than the 900 MPa and 25 °C pelletizing results, which makes it very clear that pelletizing temperature has a larger impact on the ionic conductivity compared to pelletizing pressure. Also, the ionic conductivity results at differing pelletizing pressures are significantly different when pelletized at 100 °C, which is different than was observed at the room temperature pelletizing. This is important because it means it is possible to increase the performance of a solid-state battery by increasing the pelletizing temperature even at lower pelletizing pressures, which would lead to lower manufacturing costs and simpler manufacturing methods.
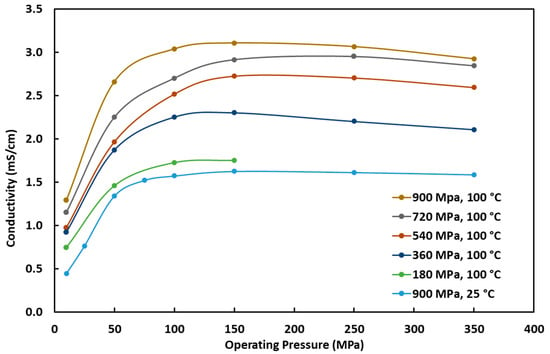
Figure 8.
Ionic conductivity of the Li6PS5Cl0.5Br0.5 electrolyte versus the cell operating pressure at different battery pelletizing pressures and 100 °C pelletizing temperature compared with the best room-temperature pelletizing temperature result.
Overall, the results indicate that pelletization pressure has a limited influence on the electrolyte’s ionic conductivity, especially at lower operating pressures unless paired with in-creased pelletizing temperatures. This suggests that other factors, such as electrode stack size and surface area, may play a more significant role in determining conductivity at lower pressures. Further investigation is necessary to understand the interplay between these factors and optimize the solid-state battery’s performance. The effect of operating temperature on ionic conductivity was found to be substantial. Higher temperatures led to increased conductivity, while lower temperatures resulted in decreased conductivity. This poses a challenge for EV applications that require operation at very low temperatures, as it may affect battery performance and longevity. Additional research is needed to develop strategies for improving conductivity and mitigating the negative impact of low temperatures on solid-state batteries. The findings also highlight the importance of pelletizing temperature in influencing ionic conductivity. Increasing the pelletizing temperature, even at lower pelletizing pressures, was found to enhance the electrolyte’s performance. This opens possibilities for improving battery performance while reducing manufacturing costs and simplifying manufacturing methods. Overall, these results provide valuable in-sights into the factors affecting the performance of Li6PS5Cl0.5Br0.5 electrolyte in solid-state batteries and establishes a baseline for performance of an easy to access commercial electrolyte. The understanding gained from this study can guide further optimization efforts and contribute to the development of safe, high-performance solid-state batteries for various applications. The authors also refer the readers to references [36,37,38,39,40] for further information on argyrodite electrolytes and solid-state lithium batteries.
4. Conclusions
In this study the effect of four parameters, cell operating temperature, cell operating pressure, electrolyte pelletization pressure and electrolyte pelletizing temperature, on the ionic conductivity of Li6PS5Cl0.5Br0.5 argyrodite electrolyte was investigated. It was shown that all four parameters played a significant role in determination of the ionic conductivity of this electrolyte material. Of course, it seems that the effect of operating temperature is more significant than operating pressure on ionic conductivity, and pelletizing temperature’s effect is greater than pelletizing pressure’s effect on ionic conductivity. The increase in pelletizing temperature and pressure increases the density of the pellet, which should lead to lower grain boundary resistances and reduce the porosity of the pellet. Increased operating pressure reduces the activation energy barrier for ions to flow through the solid electrolyte and improves particle to particle interaction as well as particle to current collector interaction. If a desired electrolyte conductivity is needed for a cell, the battery engineer can alter these parameters to reach this desired value. For example, if the high operating pressure of a cell is a constraint for a battery application, the three possibilities to achieve the desired conductivity are the cell operating temperature, the electrolyte pelletization pressure and the electrolyte pelletizing temperature during the battery manufacturing. The results provided in this study can be used as an initial guide for engineers to find a balance between these four parameters and choose the temperature and pressures based on the constraint that they may have for manufacturing the battery and/or the battery usage in a particular application. Also, it creates a baseline for the possible maximum pelletizing temperature before undesirable degradation of the electrolyte may occur. The low temperature performance of the argyrodite electrolyte is very poor, with ionic conductivity values below 1 mS/cm. This performance is not satisfactory for EV applications because vehicles need to be able to operate at low temperatures. It is assumed the operating conditions of solid-state batteries will be the limiting condition for designing future EVs, so further studies should be done to improve the ionic conductivity of the electrolyte while minimizing operating pressure and not relying on a high operating temperature operation. The next parameters to investigate, therefore, would be pelletizing time as well as pelletizing method as uniaxial pelletizing is not the only method when looking at scaling up the processing of solid electrolytes. Also, the reason for ionic conductivity decreasing at pelletizing temperatures above 135 °C needs to be further investigated to understand what causes this response. Lastly, different chemistries may have different trends or limitations, but this study establishes an initial baseline for processing and operating conditions of a commercially available Li6PS5Cl0.5Br0.5 argyrodite electrolyte.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en16135100/s1, Figure S1: Arrhenius ionic conductivity plot of AmpceraTM Li6PS5Cl0.5Br0.5 at 50, 100, and 150 MPa from the temperature of −20 to 75 °C.; Table S1: Arrhenius ionic conductivity values of AmpceraTM Li6PS5Cl0.5Br0.5 as plotted in Figure S1.
Author Contributions
Conceptualization, J.D. and S.F.; methodology, J.D.; software, J.D. and C.-Y.Y.; validation, J.D., C.-Y.Y., R.G. and S.F.; data analysis, J.D., S.F. and J.C.; investigation, J.D. and J.C.; resources, J.D., J.C. and R.F.; data curation, J.D. and J.C.; writing—original draft preparation, J.D. and S.F.; writing—review and editing, J.D. and S.F.; visualization, J.D.; supervision, J.D., S.F. and R.F.; project administration, R.F. and S.F. All authors have read and agreed to the published version of the manuscript.
Funding
The University of Akron received external research funding from the Schaeffler Group to contribute and conduct this study.
Data Availability Statement
No additional data is available to report.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Hao, F.; Han, F.; Liang, Y.; Wang, C.; Yao, Y. Architectural design and fabrication approaches for solid-state batteries. MRS Bull. 2018, 43, 775–781. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Xu, Y.; Zhang, G.; Li, S.; Li, C.; Zhang, X.; Sun, X.; Ge, X.; Ma, Y. Strategies to Boost Ionic Conductivity and Interface Compatibility of Inorganic–Organic Solid Composite Electrolytes. Energy Storage Mater. 2021, 36, 291–308. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, R.; Yang, L.; Zheng, J.; Zhang, K.; Mo, S.; Lin, Z.; Pan, F. Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Mater. 2018, 10, 139–159. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, L.; Sun, G.; Yu, X.; Li, H.; Chen, L. A stabilized PEO-based solid electrolyte via a facile interfacial engineering method for a high voltage solid-state lithium metal battery. Chem. Commun. 2020, 56, 5633–5636. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, K.; Day, C.S.; Gross, M.D.; Lachgar, A.; Holzwarth, N.A.W. A new crystalline LiPON electrolyte: Synthesis, properties, and electronic structure. Solid State Ion. 2013, 233, 95–101. [Google Scholar] [CrossRef]
- Zhou, L.; Park, K.-H.; Sun, X.; Lalère, F.; Adermann, T.; Hartmann, P.; Nazar, L.F. Solvent-Engineered Design of Argyrodite Li6PS5X (X = Cl, Br, I) Solid Electrolytes with High Ionic Conductivity. ACS Energy Lett. 2019, 4, 265–270. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Han, F.; Yao, X.; Wang, C. Lithium/Sulfide All-Solid-State Batteries using Sulfide Electrolytes. Adv. Mater. 2021, 33, 2000751. [Google Scholar] [CrossRef]
- Wang, H.; Yu, C.; Ganapathy, S.; van Eck, E.R.H.; van Eijck, L.; Wagemaker, M. A lithium argyrodite Li6PS5Cl0.5Br0.5 electrolyte with improved bulk and interfacial conductivity. J. Power Sources 2019, 412, 29–36. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.J.; Harrison, K.L.; Roberts, S.A.; Harris, S.J. Pressure-Driven Interface Evolution in Solid-State Lithium Metal Batteries. Cell Rep. Phys. Sci. 2020, 1, 100012. [Google Scholar] [CrossRef]
- Doux, J.-M.; Nguyen, H.; Tan, D.H.S.; Banerjee, A.; Wang, X.; Wu, E.A.; Jo, C.; Yang, H.; Meng, Y.S. Stack Pressure Considerations for Room-Temperature All-Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2020, 10, 1903253. [Google Scholar] [CrossRef]
- Doux, J.-M.; Yang, Y.; Tan, D.H.; Nguyen, H.; Wu, E.A.; Wang, X.; Banerjee, A.; Meng, S.Y. Pressure effects on sulfide electrolytes for all solid-state batteries. J. Mater. Chem. A 2020, 8, 5049–5055. [Google Scholar] [CrossRef]
- Zarrin, H.; Farhad, S.; Hamdullahpur, F.; Chabot, V.; Yu, A.; Fowler, M.; Chen, Z. Effects of Diffusive Charge Transfer and Salt Concentration Gradient in Electrolyte on Li-ion Battery Energy and Power Densities. Electrochimica Acta 2014, 125, 117–123. [Google Scholar] [CrossRef]
- Mastali, M.; Samadani, E.; Farhad, S.; Fraser, R.; Fowler, M. Three-dimensional Multi-Particle Electrochemical Model of LiFePO4 Cells based on a Resistor Network Methodology. Electrochimica Acta 2016, 190, 574–587. [Google Scholar] [CrossRef]
- Kashkooli, A.G.; Amirfazli, A.; Farhad, S.; Lee, D.U.; Felicelli, S.; Park, H.W.; Feng, K.; De Andrade, V.; Chen, Z. Representative volume element model of lithium-ion battery electrodes based on X-ray nano-tomography. J. Appl. Electrochem. 2017, 47, 281–293. [Google Scholar] [CrossRef]
- Farhad, S.; Nazari, A. Introducing the energy efficiency map of lithium-ion batteries. Int. J. Energy Res. 2019, 43, 931–944. [Google Scholar] [CrossRef]
- Mohammed, A.H.; Esmaeeli, R.; Aliniagerdroudbari, H.; Alhadri, M.; Hashemi, S.R.; Nadkarni, G.; Farhad, S. Dual-purpose cooling plate for thermal management of prismatic lithium-ion batteries during normal operation and thermal runaway. Appl. Therm. Eng. 2019, 160, 114106. [Google Scholar] [CrossRef]
- Yu, C.; Ganapathy, S.; Hageman, J.; van Eijck, L.; van Eck, E.R.H.; Zhang, L.; Schwietert, T.; Basak, S.; Kelder, E.M.; Wagemaker, M. Facile Synthesis toward the Optimal Structure-Conductivity Characteristics of the Argyrodite Li6PS5Cl Solid-State Electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 33296–33306. [Google Scholar] [CrossRef]
- Boulineau, S.; Courty, M.; Tarascon, J.-M.; Viallet, V. Mechanochemical synthesis of Li-argyrodite Li6PS5X (X=Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ion. 2012, 221, 1–5. [Google Scholar] [CrossRef]
- Yubuchi, S.; Teragawa, S.; Aso, K.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Preparation of high lithium-ion conducting Li6PS5Cl solid electrolyte from ethanol solution for all-solid-state lithium batteries. J. Power Sources 2015, 293, 941–945. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.-H.; Shen, Y.; Li, L.; Nan, C.-W. High-Conductivity Argyrodite Li6PS5Cl Solid Electrolytes Prepared via Optimized Sintering Processes for All-Solid-State Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 42279–42285. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide Solid Electrolyte with Favorable Mechanical Property for All-Solid-State Lithium Battery. Sci. Rep. 2013, 3, 2261. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.; Ma, Y.; Cao, Y.; Li, Q.; Wang, L.; Cheng, X.; Zuo, P.; Du, C.; Gao, Y.; Yin., G. Fabrication and Electrochemical Properties of Li4Ti5O12@Li6PS5Cl for All-solid-state Lithium Batteries using Simple Mechanical Method. Int. J. Electrochem. Sci. 2017, 12, 7795–7806. [Google Scholar] [CrossRef]
- Choi, S.; Ann, J.; Do, J.; Lim, S.; Park, C.; Shin, D. Application of Rod-Like Li6PS5Cl Directly Synthesized by a Liquid Phase Process to Sheet-Type Electrodes for All-Solid-State Lithium Batteries. J. Electrochem. Soc. 2018, 166, A5193. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, H.; Zheng, C.; Xia, Y.; Liang, C.; Huang, H.; Gan, Y.; Tao, X.; Zhang, W. All-solid-state batteries with slurry coated LiNi0.8Co0.1Mn0.1O2 composite cathode and Li6PS5Cl electrolyte: Effect of binder content. J. Power Sources 2018, 391, 73–79. [Google Scholar] [CrossRef]
- Ohno, S.; Bernges, T.; Buchheim, J.; Duchardt, M.; Hatz, A.-K.; Kraft, M.A.; Kwak, H.; Santhosha, A.L.; Liu, Z.; Minafra, N.; et al. How Certain Are the Reported Ionic Conductivities of Thiophosphate-Based Solid Electrolytes? An Interlaboratory Study. ACS Energy Lett. 2020, 5, 910–915. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Liu, Y.; Yu, C.; Yan, X.; Xu, B.; Wang, L. Synthesis and characterization of argyrodite solid electrolytes for all-solid-state Li-ion batteries. J. Alloys Compd. 2018, 747, 227–235. [Google Scholar] [CrossRef]
- Cronau, M.; Szabo, M.; König, C.; Wassermann, T.B.; Roling, B. How to Measure a Reliable Ionic Conductivity? The Stack Pressure Dilemma of Microcrystalline Sulfide-Based Solid Electrolytes. ACS Energy Lett. 2021, 6, 3072–3077. [Google Scholar] [CrossRef]
- Zhang, W.; Weber, D.A.; Weigand, H.; Arlt, T.; Manke, I.; Schröder, D.; Koerver, R.; Leichtweiss, T.; Hartmann, P.; Zeier, W.G.; et al. Interfacial Processes and Influence of Composite Cathode Microstructure Controlling the Performance of All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 17835–17845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Minafra, N.; Zeier, W.G.; Nazar, L.F. Innovative Approaches to Li-Argyrodite Solid Electrolytes for All-Solid-State Lithium Batteries. Acc. Chem. Res. 2021, 54, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Baktash, A.; Demir, B.; Yuan, Q.; Searles, D.J. Effect of defects and defect distribution on Li-diffusion and elastic properties of anti-perovskite Li3OCl solid electrolyte. Energy Storage Mater. 2021, 41, 614–622. [Google Scholar] [CrossRef]
- Zaman, W.; Hatzell, K.B. Processing and manufacturing of next generation lithium-based all solid-state batteries. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101003. [Google Scholar] [CrossRef]
- Dixit, M.; Beamer, C.; Amin, R.; Shipley, J.; Eklund, R.; Muralidharan, N.; Lindqvist, L.; Fritz, A.; Essehli, R.; Balasubramanian, M.; et al. The Role of Isostatic Pressing in Large-Scale Production of Solid-State Batteries. ACS Energy Lett. 2022, 7, 3936–3946. [Google Scholar] [CrossRef]
- Hassan, E.; Amiriyan, M.; Frisone, D.; Dunham, J.; Farahati, R.; Farhad, S. Effects of Coating on the Electrochemical Performance of a Nickel-Rich Cathode Active Material. Energies 2022, 15, 4886. [Google Scholar] [CrossRef]
- Ayoola, O.M.; Buldum, A.; Farhad, S.; Ojo, S.A. A Review on the Molecular Modeling of Argyrodite Electrolytes for All-Solid-State Lithium Batteries. Energies 2022, 15, 7288. [Google Scholar] [CrossRef]
- Frisone, D.; Amiriyan, M.; Hassan, E.; Dunham, J.; Farahati, R.; Farhad, S. Effect of LiNbO3 Coating on Capacity and Cycling of Nickel-Rich NMC Cathode Active Material. In Proceedings of the ASME 2021 International Mechanical Engineering Congress and Exposition (IMECE), Virtual, 1–5 November 2021. [Google Scholar] [CrossRef]
- Dunham, J.; Frisone, D.; Amiriyan, M.; Hassan, E.; Feng Hu, J.; Farahati, R.; Farhad, S. Effect of Pressure and Tempera-ture on the Performance of Argyrodite Li6PS5Cl0.5Br0.5 Electrolyte for All-Solid-State Lithium Battery. In Proceedings of the ASME 2021 International Mechanical Engineering Congress and Exposition (IMECE), Virtual, 1–5 November 2021. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).