Metal–Organic Framework (MOF)-Derived Catalyst for Oxygen Reduction Reaction (ORR) Applications in Fuel Cell Systems: A Review of Current Advancements and Perspectives
Abstract
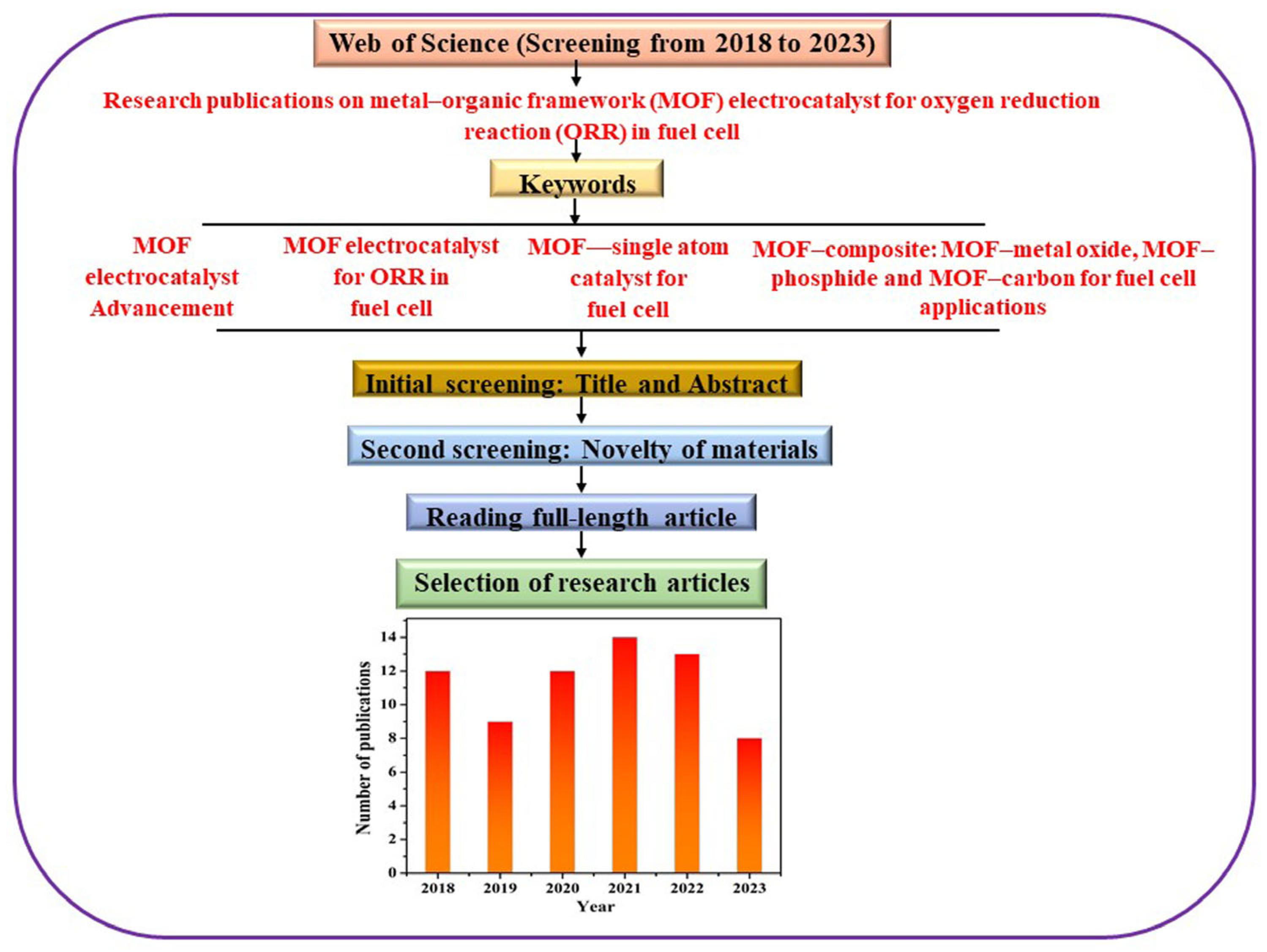
1. Introduction
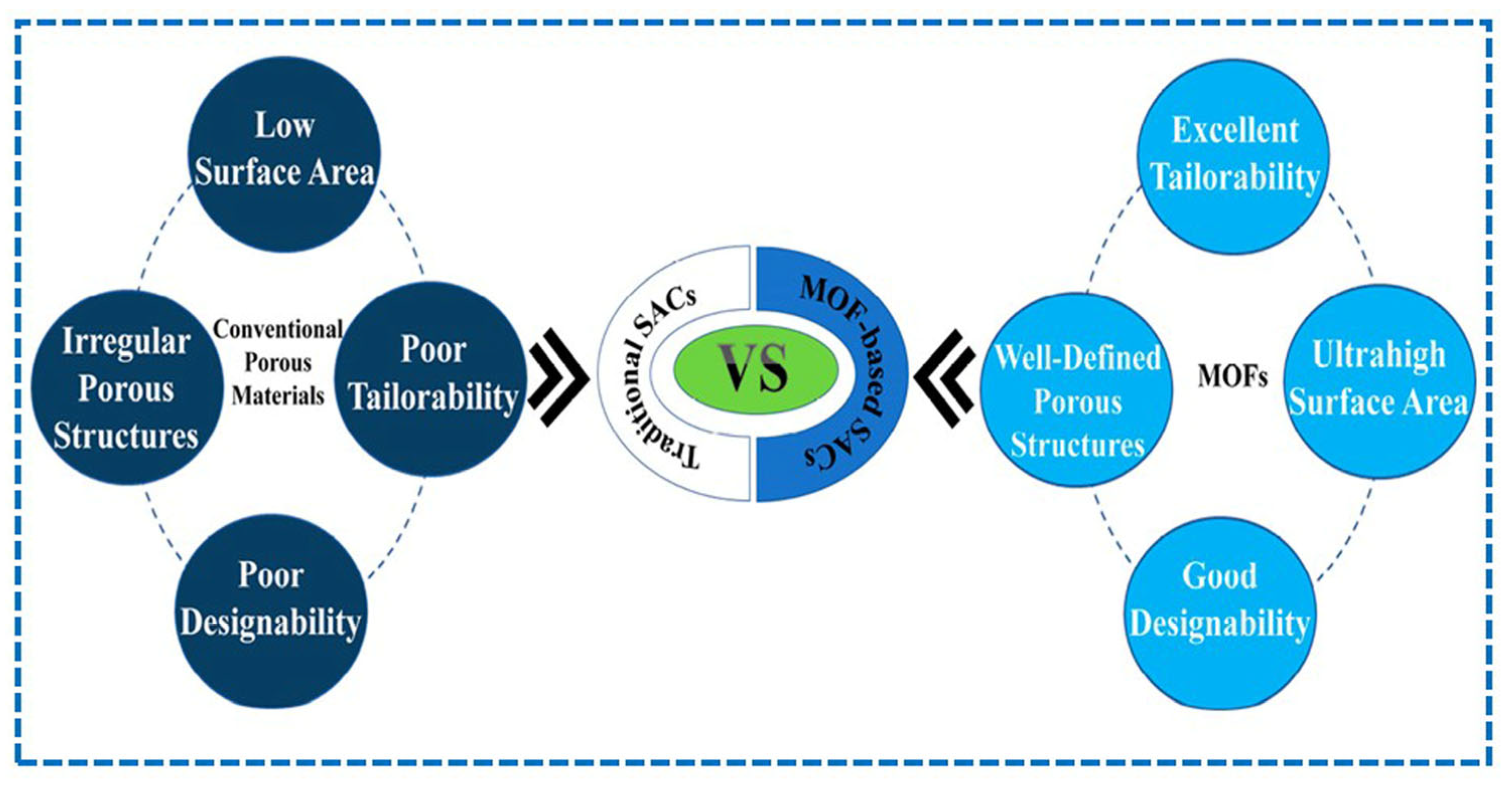
2. A MOF-Based Conceptual Highlight
2.1. Advancement of MOFs
2.2. MOF-Directed Tuning of Intrinsic and Extrinsic Frameworks
2.3. Influences of a High Graphitization Scale
2.4. Porous Structure Tuning
2.5. The Influence of Size and Shape
3. Oxygen Reduction Reaction (ORR) in MOFs
- Associative and dissociative appears on the surface of the catalysts:O2(g) + * = O2* (O* + O*) pH = 14
- Electron transfer from anode region to associative O2:O2* + H2O + e− = OOH* + OH− pH = 14
- Intermediate O=O dissociation:OOH* + e− = O* + OH− pH = 14
- Generation of OH− from catalysts surface to solution:O* + H2O + e− = OH* + OH− pH = 14
- Associative O2 appears on the surface of the catalysts.O2(g) + * = O2* (O* + O*) pH = 0
- Electron transfer from anode to associative O2.O2* + H+ + e− = OOH* pH = 0
- Intermediate dissociation.OOH* + H+ + e− = OH−* pH = 0
- Generation of the H2O byproduct from the ORR pathway.OH* + H+ +e− = H2O pH = 0
4. MOF Derivatives
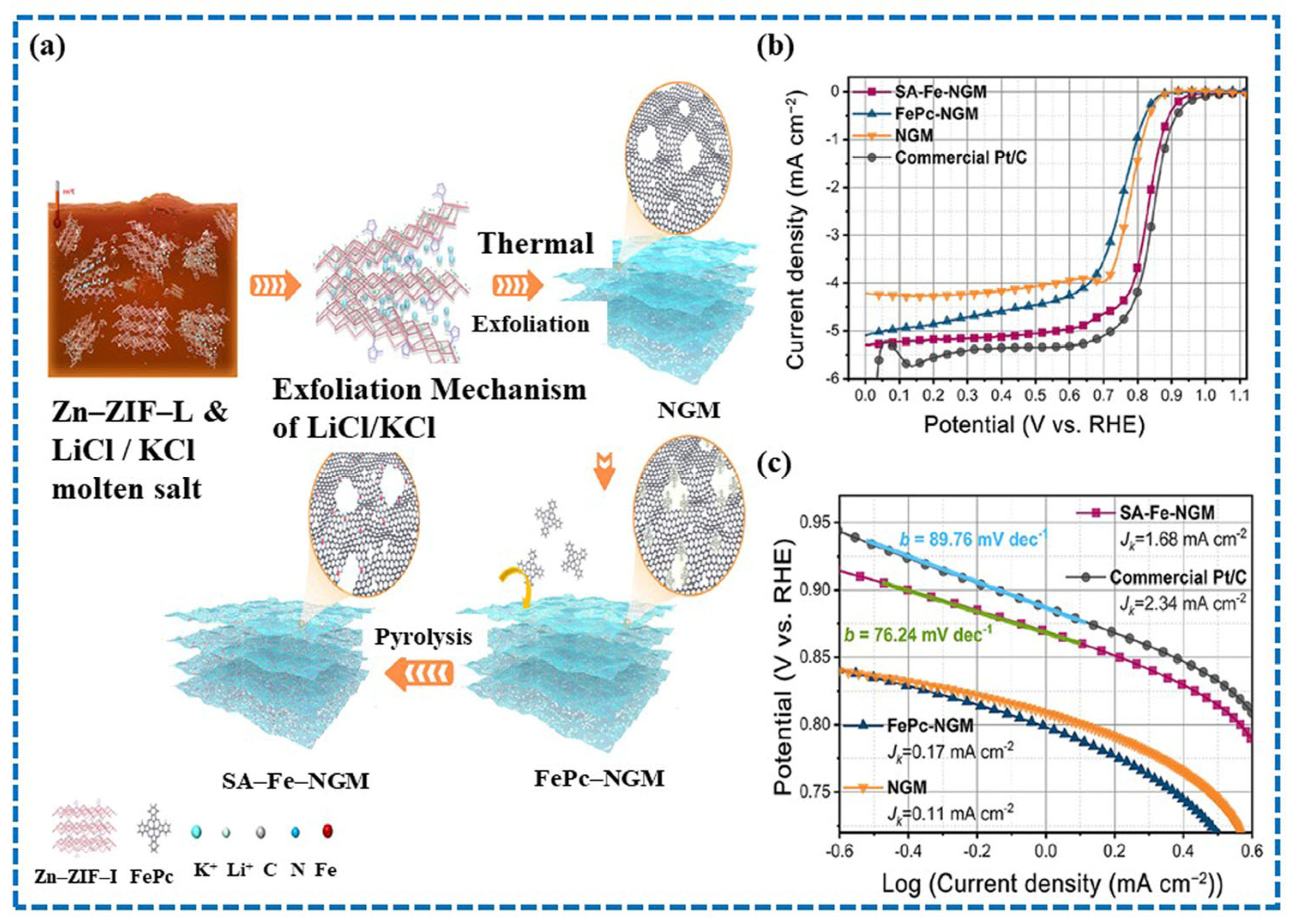
4.1. Single-Atom Catalyst (SAC) in MOFs for ORR
4.2. MOF–Carbon Composite
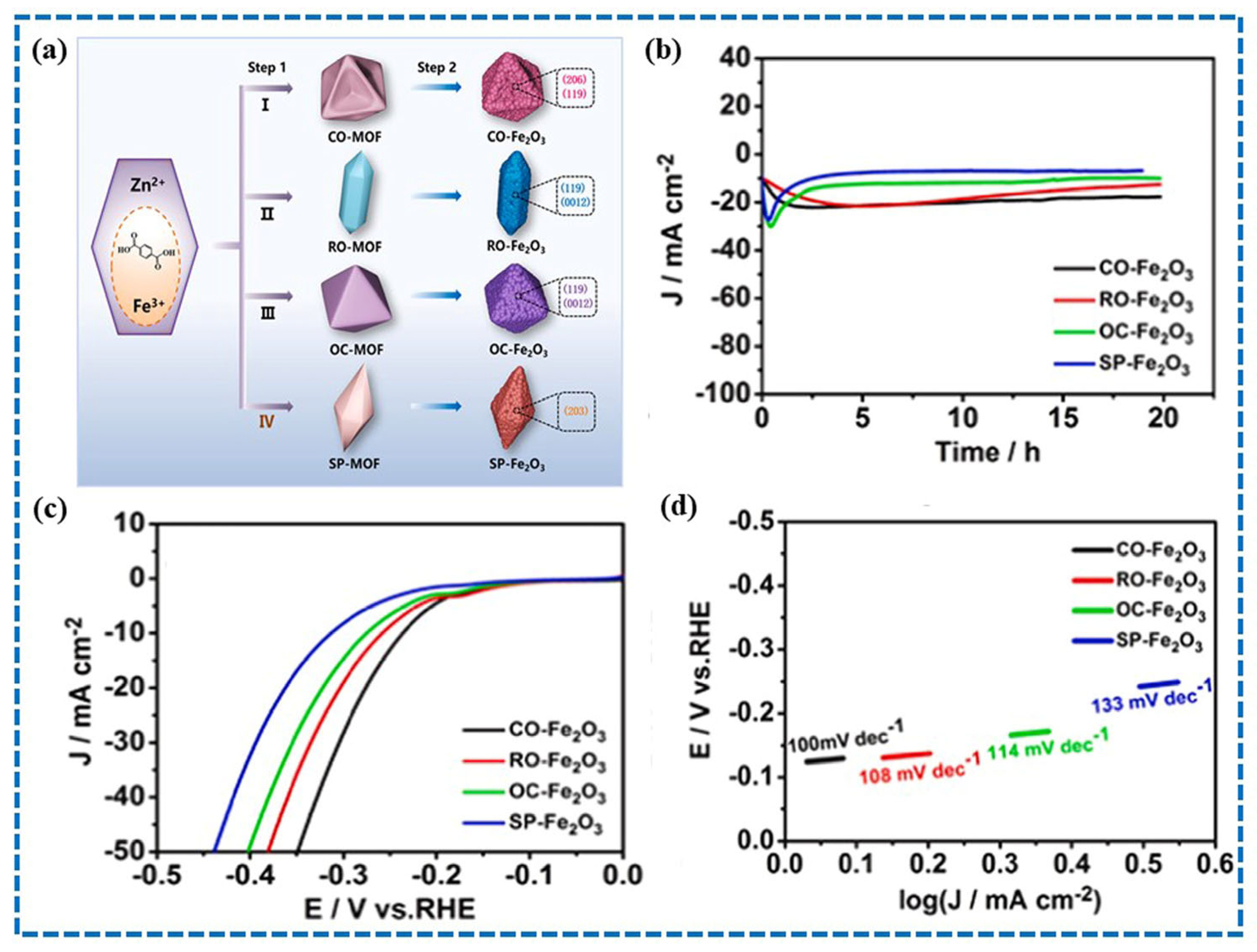
4.3. Metal Oxides Derived from MOFs
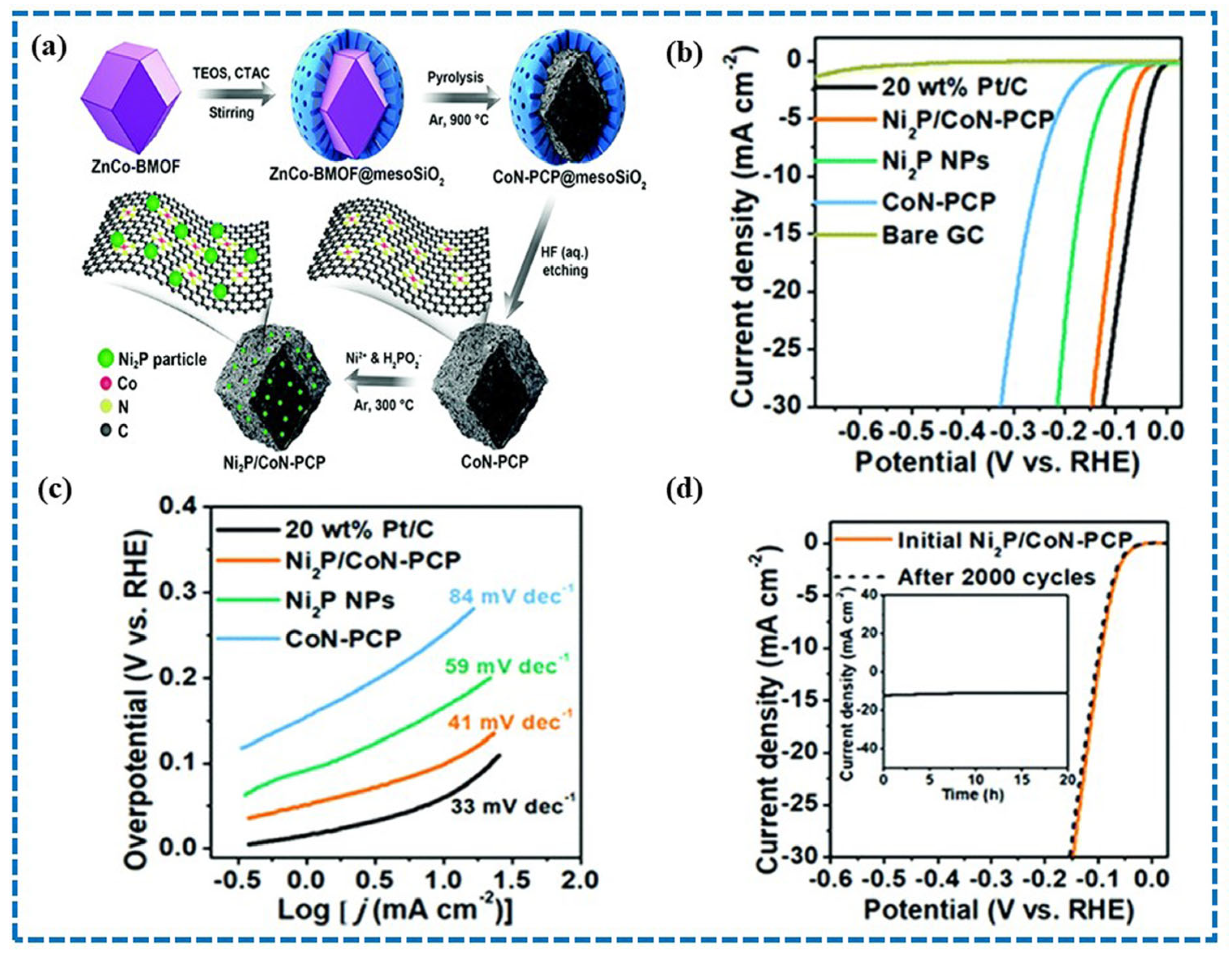
4.4. Metal Phosphide Derived from MOFs
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baz, K.; Cheng, J.; Xu, D.; Abbas, K.; Ali, I.; Ali, H.; Fang, C. Asymmetric impact of fossil fuel and renewable energy consumption on economic growth: A nonlinear technique. Energy 2021, 226, 120357. [Google Scholar] [CrossRef]
- Hunt, J.D.; Nascimento, A.; Nascimento, N.; Vieira, L.W.; Romero, O.J. Possible pathways for oil and gas companies in a sustainable future: From the perspective of a hydrogen economy. Renew. Sustain. Energy Rev. 2022, 160, 112291. [Google Scholar] [CrossRef]
- Kataria, A.; Khan, T.I. Necessity of Paradigm Shift from Non-renewable Sources to Renewable Sources for Energy Demand. In Urban Growth and Environmental Issues in India; Kateja, A., Jain, R., Eds.; Springer: Singapore, 2021; pp. 337–352. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Dhanabalan, K.; Roh, S.-H.; Kim, T.-H.; Park, K.-W.; Jung, S.; Kurkuri, M.D.; Jung, H.-Y. A comprehensive review on unitized regenerative fuel cells: Crucial challenges and developments. Int. J. Hydrog. Energy 2017, 42, 4415–4433. [Google Scholar] [CrossRef]
- Dhanabalan, K.; Sadhasivam, T.; Park, J.H.; Jung, H.Y.; Kim, B.S.; Roh, S.H. Advances in Metal−Organic Ligand Systems for Polymer Electrolyte Membranes: A Review. Fuel Cells 2017, 17, 278–287. [Google Scholar] [CrossRef]
- Etesami, M.; Mehdipour-Ataei, S.; Somwangthanaroj, A.; Kheawhom, S. Recent progress of electrocatalysts for hydrogen proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2022, 47, 41956–41973. [Google Scholar] [CrossRef]
- Wu, D.; Shen, X.; Pan, Y.; Yao, L.; Peng, Z. Platinum Alloy Catalysts for Oxygen Reduction Reaction: Advances, Challenges and Perspectives. ChemNanoMat 2020, 6, 32–41. [Google Scholar] [CrossRef]
- Abd Aziz, A.J.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A. Review of composite cathodes for intermediate-temperature solid oxide fuel cell applications. Ceram. Int. 2020, 46, 23314–23325. [Google Scholar] [CrossRef]
- Alipour Moghadam Esfahani, R.; Kong, F.; Black-Araujo, K.; Easton, L.J.; Ebralidze, I.I.; Easton, E.B. A doped metal oxide PGM-free electrocatalyst for the oxygen reduction reaction. Electrochim. Acta 2023, 438, 141564. [Google Scholar] [CrossRef]
- Osmieri, L. Transition metal–nitrogen–carbon (M–N–C) catalysts for oxygen reduction reaction. Insights on synthesis and performance in polymer electrolyte fuel cells. ChemEngineering 2019, 3, 16. [Google Scholar] [CrossRef]
- Wang, R.; Lou, M.; Zhang, J.; Sun, Z.; Li, Z.; Wen, P. Zif-8@zif-67-derived co embedded into nitrogen-doped carbon nanotube hollow porous carbon supported pt as an efficient electrocatalyst for methanol oxidation. Nanomaterials 2021, 11, 2491. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, R.; Yang, Z.; Chen, Y.; Zhou, L.; Yuan, Y. Rapeseed meal-based autochthonous N and S-doped non-metallic porous carbon electrode material for oxygen reduction reaction catalysis. Int. J. Hydrog. Energy 2021, 46, 508–517. [Google Scholar] [CrossRef]
- Chen, K.; Shi, L.; Zhang, Y.; Liu, Z. Scalable chemical-vapour-deposition growth of three-dimensional graphene materials towards energy-related applications. Chem. Soc. Rev. 2018, 47, 3018–3036. [Google Scholar] [CrossRef]
- Khan, J.; Liu, H.; Xiao, J.; Zhu, Y.; Hayat, A.; Ullah, H.; Ahmed, G.; Zhang, H.; Sun, Y.; Han, L. Synthesis of heteroatom incorporated porous carbon encapsulated Fe-doped Co9S8 as an efficient bifunctional electrocatalyst for overall water splitting. J. Phys. Chem. Solids 2023, 175, 111220. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent Progress in Metal-Organic Frameworks for Applications in Electrocatalytic and Photocatalytic Water Splitting. Adv. Sci. 2017, 4, 1600371. [Google Scholar] [CrossRef]
- Zhou, J.; Zeng, C.; Ou, H.; Yang, Q.; Xie, Q.; Zeb, A.; Lin, X.; Ali, Z.; Hu, L. Metal-organic framework-based materials for full cell systems: A review. J. Mater. Chem. C Mater. 2021, 9, 11030–11058. [Google Scholar] [CrossRef]
- Dang, S.; Zhu, Q.L.; Xu, Q. Nanomaterials derived from metal-organic frameworks. Nat. Rev. Mater. 2017, 3, 17075. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, J.; Lee, C.; Li, M.; Han, B.; Wu, T.; Pan, H.; Geng, D.; Yan, Q. Integration of Metal–Organic Frameworks and Metals: Synergy for Electrocatalysis. Small 2023, 2300916. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Yang, W.; Shen, W.; Xue, H.; Pang, H. MOF-Derived Metal Oxide Composites for Advanced Electrochemical Energy Storage. Small 2018, 14, 1704435. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, H.L. Metal-Organic-Framework-Based Single-Atom Catalysts for Energy Applications. Chem 2019, 5, 786–804. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Yuan, S.; Zhou, J.; Wang, B. Challenges and recent advances in MOF-polymer composite membranes for gas separation. Inorg. Chem. Front. 2016, 3, 896–909. [Google Scholar] [CrossRef]
- Li, K.; Yang, J.; Gu, J. Hierarchically Porous MOFs Synthesized by Soft-Template Strategies. Acc. Chem. Res. 2022, 55, 2235–2247. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, L.; Wang, S.M.; Han, Z. MOF-templated nitrogen-doped porous carbon materials as efficient electrocatalysts for oxygen reduction reactions. Inorg. Chem. Front. 2017, 4, 1231–1237. [Google Scholar] [CrossRef]
- Nath, K.; Bhunia, K.; Pradhan, D.; Biradha, K. MOF-templated cobalt nanoparticles embedded in nitrogen-doped porous carbon: A bifunctional electrocatalyst for overall water splitting. Nanoscale Adv. 2019, 1, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Pan, Y.; Pan, C.; Xu, L. Metal−organic framework as anode materials for lithium-ion batteries with high capacity and rate performance. ACS Appl. Energy Mater. 2020, 3, 10776–10786. [Google Scholar] [CrossRef]
- Hou, C.C.; Zou, L.; Xu, Q. A Hydrangea-Like Superstructure of Open Carbon Cages with Hierarchical Porosity and Highly Active Metal Sites. Adv. Mater. 2019, 31, 1904689. [Google Scholar] [CrossRef]
- Fuchs, A.; Mannhardt, P.; Hirschle, P.; Wang, H.; Zaytseva, I.; Ji, Z.; Yaghi, O.; Wuttke, S.; Ploetz, E. Single Crystals Heterogeneity Impacts the Intrinsic and Extrinsic Properties of Metal–Organic Frameworks. Adv. Mater. 2022, 34, 2104530. [Google Scholar] [CrossRef]
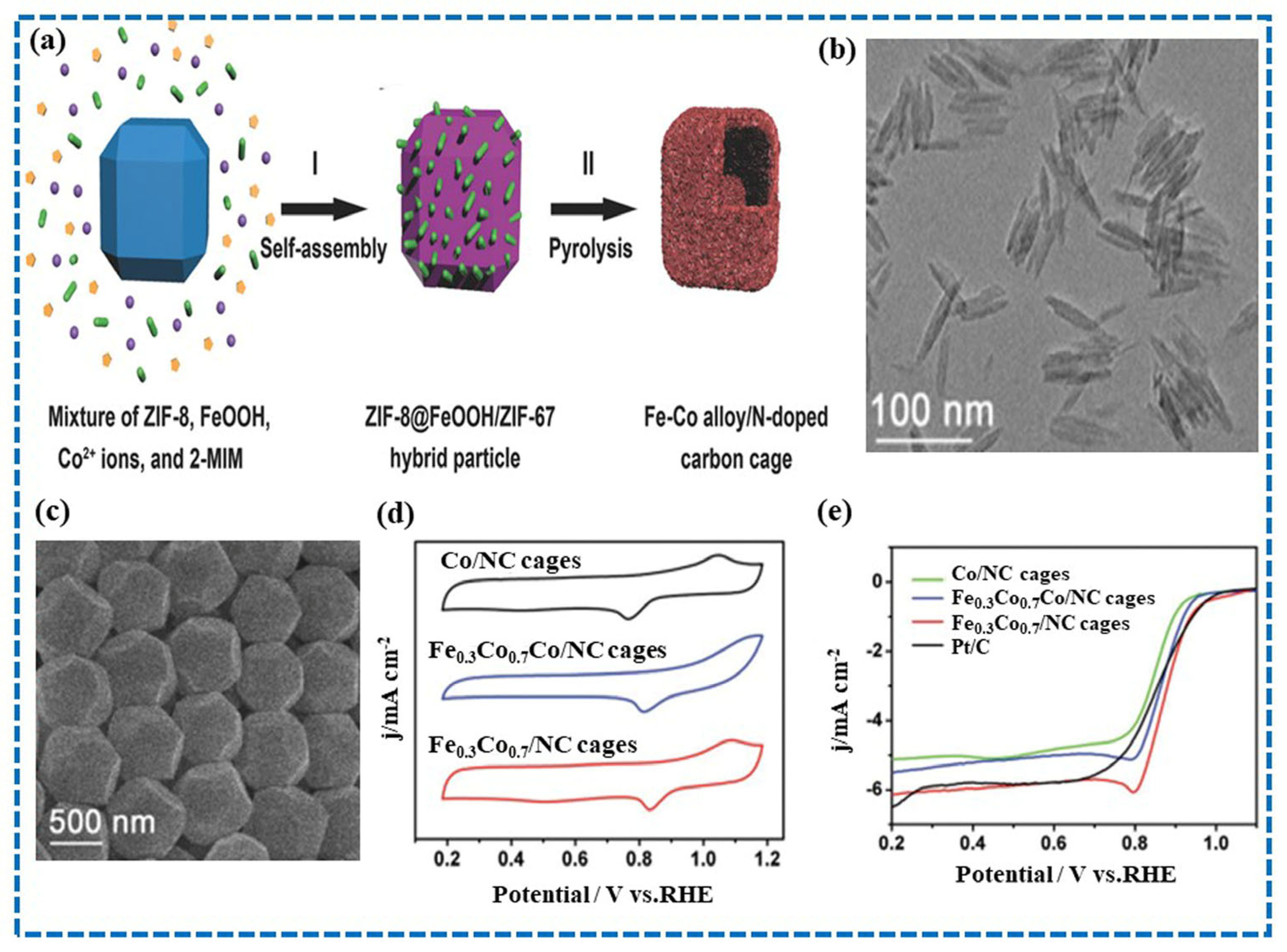
- Guan, B.Y.; Lu, Y.; Wang, Y.; Wu, M.; Lou, X.W.D. Porous Iron–Cobalt Alloy/Nitrogen-Doped Carbon Cages Synthesized via Pyrolysis of Complex Metal–Organic Framework Hybrids for Oxygen Reduction. Adv. Funct. Mater. 2018, 28, 1706738. [Google Scholar] [CrossRef]
- Huang, Q.; Guo, Y.; Wang, X.; Chai, L.; Ding, J.; Zhong, L.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. In-MOF-derived ultrathin heteroatom-doped carbon nanosheets for improving oxygen reduction. Nanoscale 2020, 12, 10019–10025. [Google Scholar] [CrossRef]
- Jing, Y.; Cheng, Y.; Wang, L.; Liu, Y.; Yu, B.; Yang, C. MOF-derived Co, Fe, and Ni co-doped N-enriched hollow carbon as efficient electrocatalyst for oxygen reduction reaction. Chem. Eng. J. 2020, 397, 125539. [Google Scholar] [CrossRef]
- Li, L.; Dai, P.; Gu, X.; Wang, Y.; Yan, L.; Zhao, X. High oxygen reduction activity on a metal-organic framework derived carbon combined with high degree of graphitization and pyridinic-N dopants. J. Mater. Chem. A Mater. 2017, 5, 789–795. [Google Scholar] [CrossRef]
- Jin, M.; Lu, S.-Y.; Zhong, X.; Liu, H.; Liu, H.; Gan, M.; Ma, L. Spindle-Like MOF Derived TiO2@NC-NCNTs Composite with Modulating Defect Site and Graphitization Nanoconfined Pt NPs as Superior Bifunctional Fuel Cell Electrocatalysts. ACS Sustain. Chem. Eng. 2020, 8, 1933–1942. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, A.; Huang, Q.; Li, Q.; Hu, Y.; Qian, J.; Huang, S. Hierarchical N-doped C.N.T.s grafted onto MOF-derived porous carbon nanomaterials for efficient oxygen reduction. J. Colloid Interface Sci. 2022, 606, 1833–1841. [Google Scholar] [CrossRef]
- Rong, J.; Gao, E.; Liu, N.; Chen, W.; Rong, X.; Zhang, Y.; Zheng, X.; Ao, H.; Xue, S.; Huang, B.; et al. Porphyrinic MOF-derived rich N-doped porous carbon with highly active CoN4C single-atom sites for enhanced oxygen reduction reaction and Zn-air batteries performance. Energy Storage Mater. 2023, 56, 165–173. [Google Scholar] [CrossRef]
- Chen, X.; Wang, N.; Shen, K.; Xie, Y.; Tan, Y.; Li, Y. MOF-Derived Isolated Fe Atoms Implanted in N-Doped 3D Hierarchical Carbon as an Efficient ORR Electrocatalyst in Both Alkaline and Acidic Media. ACS Appl. Mater. Interfaces 2019, 11, 25976–25985. [Google Scholar] [CrossRef]
- Lu, X.; Wu, M.; Lu, Z.; Hu, J.; Xie, J.; Hao, A.; Cao, Y. Construction of multiple active sites by solution-free self-generating dual-template strategy: Boosting the ORR performance of NiFe/N-doped 3D porous carbon nanosheets. J. Alloys Compd. 2023, 942, 169095. [Google Scholar] [CrossRef]
- Li, J.; Xia, W.; Tang, J.; Tan, H.; Wang, J.; Kaneti, Y.V.; Bando, Y.; Wang, T.; He, J.; Yamauchi, Y. MOF nanoleaves as new sacrificial templates for the fabrication of nanoporous Co-N:X/C electrocatalysts for oxygen reduction. Nanoscale Horiz. 2019, 4, 1006–1013. [Google Scholar] [CrossRef]
- Siburian, R.; Kondo, T.; Nakamura, J. Size Control to a Sub-Nanometer Scale in Platinum Catalysts on Graphene. J. Phys. Chem. C 2013, 117, 3635–3645. [Google Scholar] [CrossRef]
- Xili, D.; Zhou, Q.; Zhang, L. Well-defined Co-N-C catalyst based on ZIF-67 in mixed solvents with low amount of ligands for efficient oxygen reduction reaction. J. Alloys Compd. 2022, 911, 165072. [Google Scholar] [CrossRef]
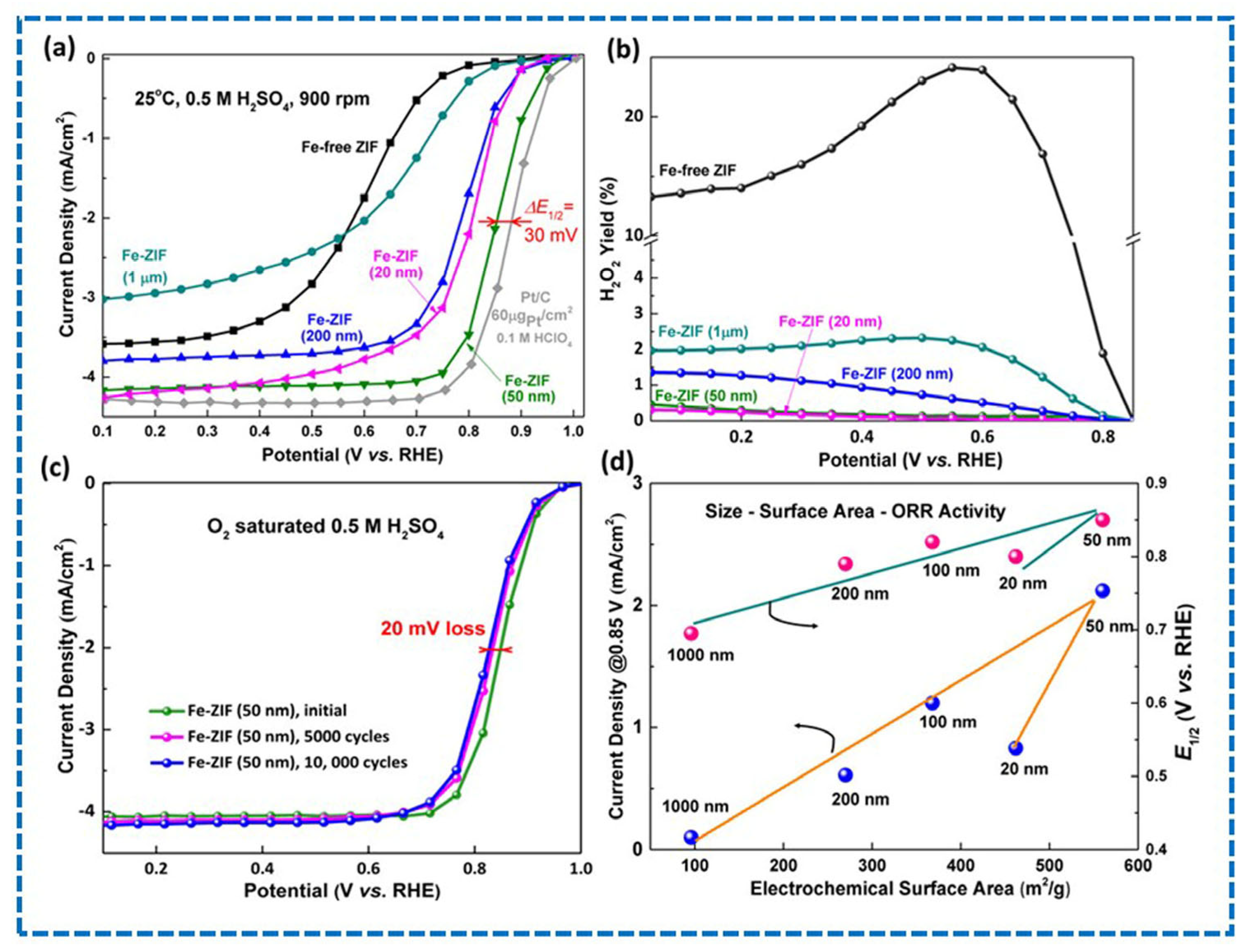
- Zhang, H.; Hwang, S.; Wang, M.; Feng, Z.; Karakalos, S.; Luo, L.; Qiao, Z.; Xie, X.; Wang, C.; Su, D.; et al. Single Atomic Iron Catalysts for Oxygen Reduction in Acidic Media: Particle Size Control and Thermal Activation. J. Am. Chem. Soc. 2017, 139, 14143–14149. [Google Scholar] [CrossRef]
- Zhong, L.; Li, S. Unconventional Oxygen Reduction Reaction Mechanism and Scaling Relation on Single-Atom Catalysts. ACS Catal. 2020, 10, 4313–4318. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, D.; Jiang, S.; Xia, H.; Yang, Y.; Yan, W.; Hu, J.; Zhang, J. Rational confinement engineering of MOF-derived carbon-based electrocatalysts toward CO2 reduction and O2 reduction reactions. InfoMat 2022, 4, e12257. [Google Scholar] [CrossRef]
- Kim, M.; Yi, J.; Park, S.H.; Park, S.S. Heterogenization of Molecular Electrocatalytic Active Sites through Reticular Chemistry. Adv. Mater. 2023, 35, 2203791. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, P.; Bai, Y.; Zhang, Z.; Li, X.; Xiong, W.; Zhang, J.; Yuan, A.; Zheng, F. High synergetic transition metal phosphides@ nitrogen doped porous carbon nanosheets hybrids derived from silk fibroin and phytic acid self-assembly for ultra-high performance lithium storage. J. Alloys Compd. 2022, 920, 165832. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Sun, L.; Wang, L. Metal/metal oxide nanostructures derived from metal-organic frameworks. RSC Adv. 2015, 5, 7267–7279. [Google Scholar] [CrossRef]
- Yang, J.; Li, P.; Wang, L.; Guo, X.; Guo, J.; Liu, S. In-situ synthesis of Ni-MOF@CNT on graphene/Ni foam substrate as a novel self-supporting hybrid structure for all-solid-state supercapacitors with a high energy density. J. Electroanal. Chem. 2019, 848, 113301. [Google Scholar] [CrossRef]
- Huang, H.; Shen, K.; Chen, F.; Li, Y. Metal-organic frameworks as a good platform for the fabrication of single-atom catalysts. ACS Catal. 2020, 10, 6579–6586. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, P.; Zhang, Y.; Liu, B.; Gao, P.; Guo, S. 3D star-like atypical hybrid MOF derived single-atom catalyst boosts oxygen reduction catalysis. J. Energy Chem. 2021, 55, 355–360. [Google Scholar] [CrossRef]
- Ding, S.; Barr, J.A.; Shi, Q.; Zeng, Y.; Tieu, P.; Lyu, Z.; Fang, L.; Li, T.; Pan, X.; Beckman, S.P.; et al. Engineering Atomic Single Metal–FeN4Cl Sites with Enhanced Oxygen-Reduction Activity for High-Performance Proton Exchange Membrane Fuel Cells. ACS Nano 2022, 16, 15165–15174. [Google Scholar] [CrossRef]
- Xie, X.; Shang, L.; Xiong, X.; Shi, R.; Zhang, T. Fe Single-Atom Catalysts on MOF-5 Derived Carbon for Efficient Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. Adv. Energy Mater. 2022, 12, 2102688. [Google Scholar] [CrossRef]
- Li, J.; Xia, W.; Tang, J.; Gao, Y.; Jiang, C.; Jia, Y.; Chen, T.; Hou, Z.; Qi, R.; Jiang, D.; et al. Metal-Organic Framework-Derived Graphene Mesh: A Robust Scaffold for Highly Exposed Fe-N4Active Sites toward an Excellent Oxygen Reduction Catalyst in Acid Media. J. Am. Chem. Soc. 2022, 144, 9280–9291. [Google Scholar] [CrossRef]
- Wang, K.; Chu, Y.; Zhang, X.; Zhao, R.; Tan, X. Facile Method to Synthesize a High-Activity S-Doped Fe/S.N.C. Single-Atom Catalyst by Metal–Organic Frameworks for Oxygen Reduction Reaction in Acidic Medium. Energy Fuels 2021, 35, 20243–20249. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrog. Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
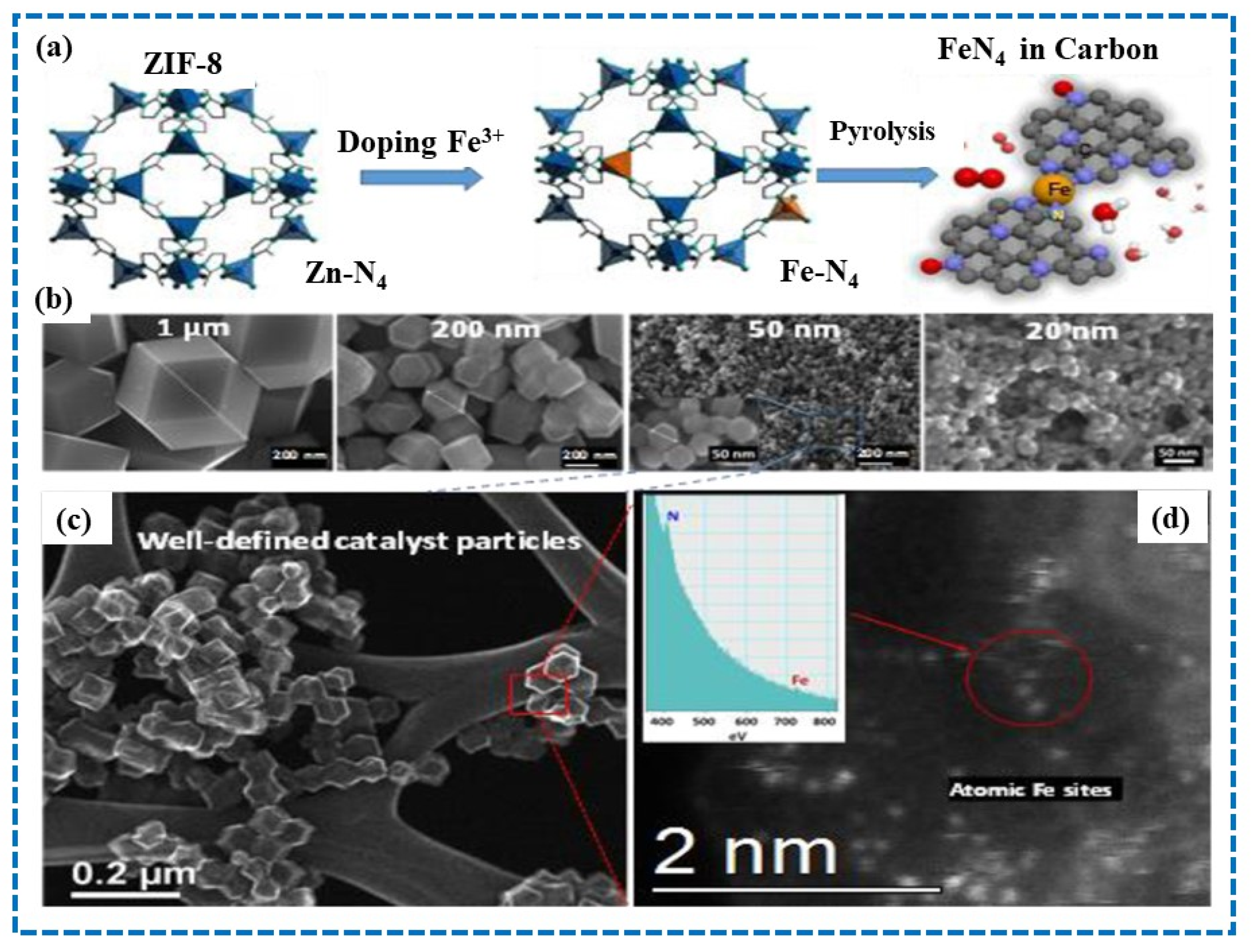
- Deng, Y.; Chi, B.; Li, J.; Wang, G.; Zheng, L.; Shi, X.; Cui, Z.; Du, L.; Liao, S.; Zang, K.; et al. Atomic Fe-Doped MOF-Derived Carbon Polyhedrons with High Active-Center Density and Ultra-High Performance toward PEM Fuel Cells. Adv. Energy Mater. 2019, 9, 1802856. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, X.; Wang, H.; Bao, L.; Li, C.; Cong, Y.; Zhao, Q. MOF-derived carbon nanotubes as an highly active electrocatalyst for oxygen reduction reaction in alkaline and acidic media. Int. J. Electrochem. Sci. 2023, 18, 100131. [Google Scholar] [CrossRef]
- Ali Khan, I.; Qian, Y.; Badshah, A.; Arif Nadeem, M.; Zhao, D. Highly Porous Carbon Derived from MOF-5 as a Support of ORR Electrocatalysts for Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 17268–17275. [Google Scholar] [CrossRef]
- Gamal, S.; Kospa, D.A.; Gebreil, A.; El-Hakam, S.A.; Ahmed, A.I.; Ibrahim, A.A. NiCo2O4 spinel supported N-dopped porous Hollow carbon derived MOF functionalized SiO2 for efficient ORR electrocatalysis. Int. J. Hydrog. Energy 2023, 48, 18890–18905. [Google Scholar] [CrossRef]
- Kuang, X.; Luo, Y.; Kuang, R.; Wang, Z.; Sun, X.; Zhang, Y.; Wei, Q. Metal organic framework nanofibers derived Co3O4-doped carbon-nitrogen nanosheet arrays for high efficiency electrocatalytic oxygen evolution. Carbon 2018, 137, 433–441. [Google Scholar] [CrossRef]
- Niu, Y.; Yuan, Y.; Zhang, Q.; Chang, F.; Yang, L.; Chen, Z.; Bai, Z. Morphology-controlled synthesis of metal-organic frameworks derived lattice plane-altered iron oxide for efficient trifunctional electrocatalysts. Nano Energy 2021, 82, 105699. [Google Scholar] [CrossRef]
- Xu, G.; Xu, G.-C.; Ban, J.-J.; Zhang, L.; Lin, H.; Qi, C.-L.; Sun, Z.-P.; Jia, D.-Z. Cobalt and cobalt oxides N-codoped porous carbon derived from metal-organic framework as bifunctional catalyst for oxygen reduction and oxygen evolution reactions. J. Colloid Interface Sci. 2018, 521, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tuo, Y.; Lu, Q.; Lu, H.; Zhang, S.; Zhou, Y.; Zhang, J.; Liu, Z.; Kang, Z.; Feng, X.; et al. Hierarchical trimetallic Co-Ni-Fe oxides derived from core-shell structured metal-organic frameworks for highly efficient oxygen evolution reaction. Appl. Catal. B 2021, 287, 119953. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, S.; Xu, L.; Wang, D.; Li, Y. An efficient multifunctional hybrid electrocatalyst: Ni2P nanoparticles on MOF-derived Co,N-doped porous carbon polyhedrons for oxygen reduction and water splitting. Chem. Commun. 2018, 54, 12101–12104. [Google Scholar] [CrossRef]
- Li, L.; Xie, W.; Chen, J.; Yang, J. ZIF-67 derived P/Ni/Co/NC nanoparticles as highly efficient electrocatalyst for oxygen reduction reaction (ORR). J. Solid State Chem. 2018, 264, 1–5. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Dou, S.; Deng, L.; Huo, J.; Wang, S. ZIF-67-derived Co-NC@CoP-NC nanopolyhedra as an efficient bifunctional oxygen electrocatalyst. J. Mater. Chem. A Mater. 2016, 4, 15836–15840. [Google Scholar] [CrossRef]
- Tang, B.; Wang, S.; Long, J. Novel in-situ P-doped metal-organic frameworks derived cobalt and heteroatoms co-doped carbon matrix as high-efficient electrocatalysts. Int. J. Hydrog. Energy 2020, 45, 32972–32983. [Google Scholar] [CrossRef]


| Sample | Hetero Atom | Metal Atom | Pyrolysis Temperature | ORR Applications | Ref. |
|---|---|---|---|---|---|
| Fe0.3Co0.7/NC cages | N | Fe, Co, and Zn | 800 °C | 0.98 V on set Potential | [29] |
| InFeCo–CNS900 | N and S | Fe/Co | 900 °C | 59.5 mV dec−1 | [30] |
| CoFeNi/N.C. | N | CoFeNi | 800 °C | 0.812 V | [31] |
| TiO2–NC−NCNTs | N | Ti/Co | 900 °C | 1.01 V | [33] |
| Pyridinic-N doped porous carbon (PNPC) | pyridinic-N | Al | 1000 °C | 0.890 | [32] |
| ZnHKUST-1 | N | Fe | 1000 °C | 0.997 V | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhanabalan, K.; Perumalsamy, M.; Sriram, G.; Murugan, N.; Shalu; Sadhasivam, T.; Oh, T.H. Metal–Organic Framework (MOF)-Derived Catalyst for Oxygen Reduction Reaction (ORR) Applications in Fuel Cell Systems: A Review of Current Advancements and Perspectives. Energies 2023, 16, 4950. https://doi.org/10.3390/en16134950
Dhanabalan K, Perumalsamy M, Sriram G, Murugan N, Shalu, Sadhasivam T, Oh TH. Metal–Organic Framework (MOF)-Derived Catalyst for Oxygen Reduction Reaction (ORR) Applications in Fuel Cell Systems: A Review of Current Advancements and Perspectives. Energies. 2023; 16(13):4950. https://doi.org/10.3390/en16134950
Chicago/Turabian StyleDhanabalan, Karmegam, Muthukumar Perumalsamy, Ganesan Sriram, Nagaraj Murugan, Shalu, Thangarasu Sadhasivam, and Tae Hwan Oh. 2023. "Metal–Organic Framework (MOF)-Derived Catalyst for Oxygen Reduction Reaction (ORR) Applications in Fuel Cell Systems: A Review of Current Advancements and Perspectives" Energies 16, no. 13: 4950. https://doi.org/10.3390/en16134950
APA StyleDhanabalan, K., Perumalsamy, M., Sriram, G., Murugan, N., Shalu, Sadhasivam, T., & Oh, T. H. (2023). Metal–Organic Framework (MOF)-Derived Catalyst for Oxygen Reduction Reaction (ORR) Applications in Fuel Cell Systems: A Review of Current Advancements and Perspectives. Energies, 16(13), 4950. https://doi.org/10.3390/en16134950






