Purified Glycerine from Biodiesel Production as Biomass or Waste-Based Green Raw Material for the Production of Biochemicals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
- Separation of impurities by sedimentation.
- Reaction with an alkaline agent (strong base) at 80 °C for 1.5 h.
- Filtration of the reaction mixture under reduced pressure.
- Removal of water on a vacuum evaporator at 20 mmHg.
- Vacuum distillation at 1 mm Hg.
- Sorption on activated carbon.
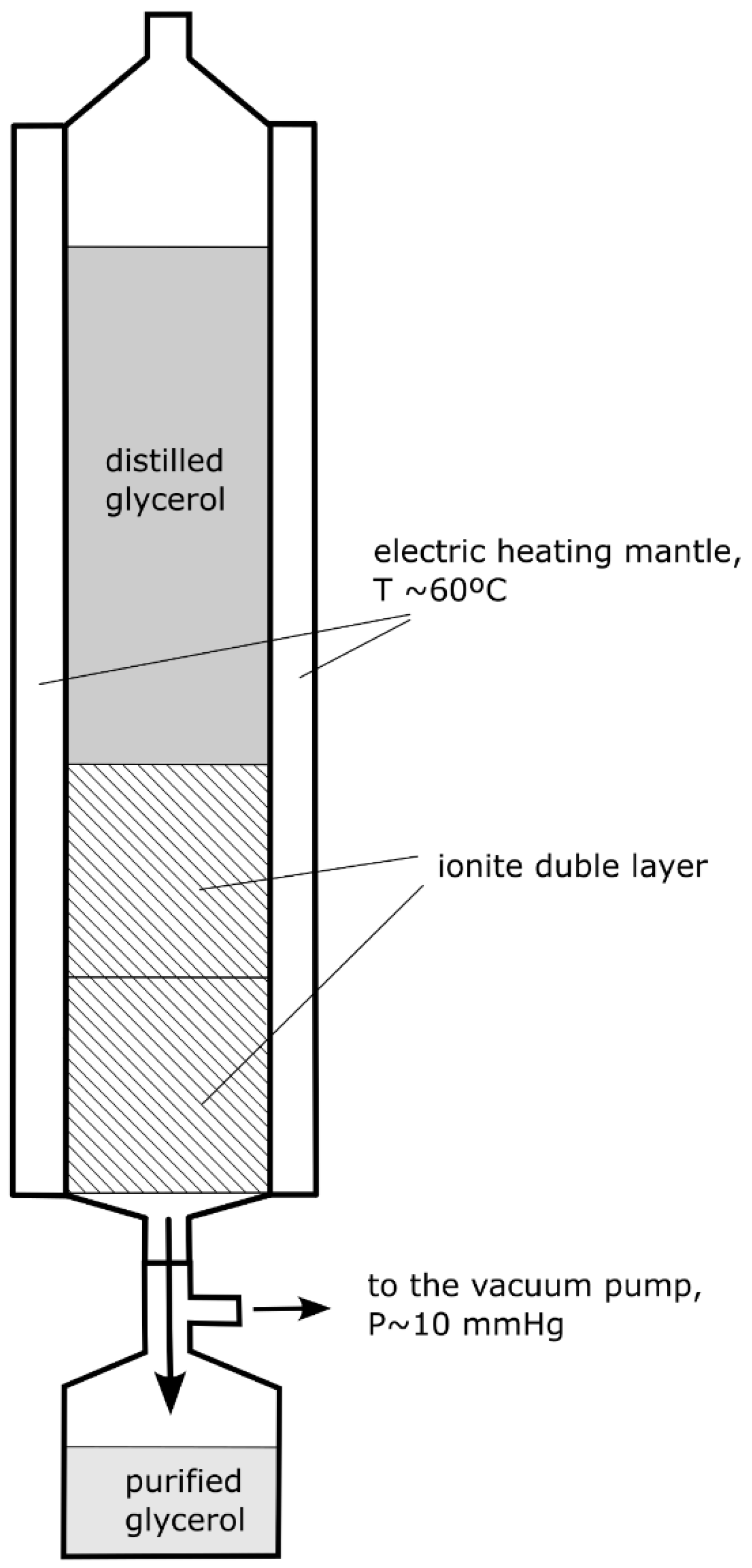
2.2. Glycerine Purification Tests with Ion Exchange Resins
- Temperature: 60 °C,
- The flow of glycerine through the ionite bed at a rate of approx. 2–5 cm3/min forced by a vacuum generated by a diaphragm pump,
- The column had a stabilisation time of approx. 20 min,
- The contact time between the glycerine and the ionite was approximately 2–5 min.
2.3. Analytical Methods
3. Results and Discussion
3.1. Test of Ion Exchange Resin with Crude Glycerol
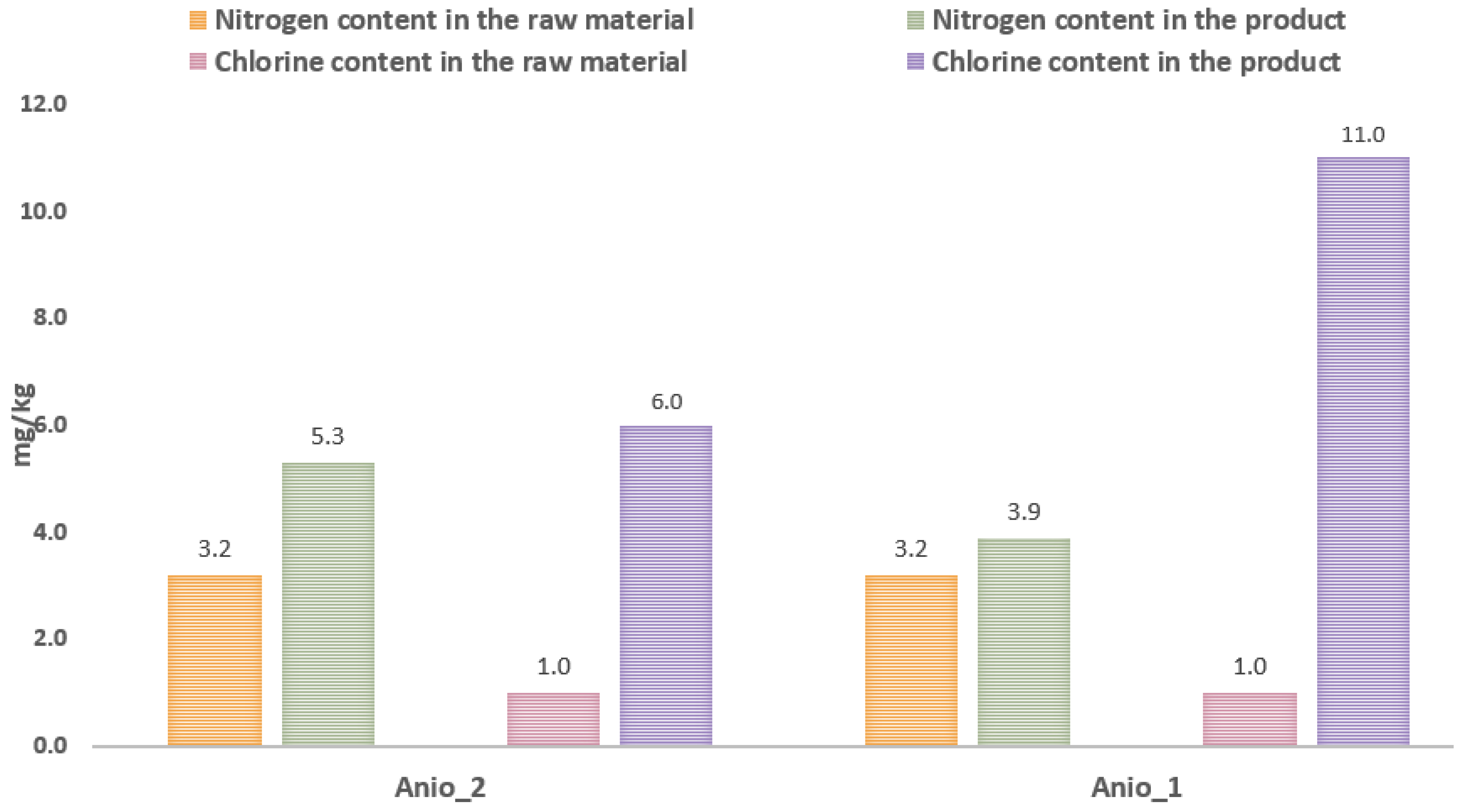
3.2. Test of Anion Exchange Resin with Distilled Glycerol
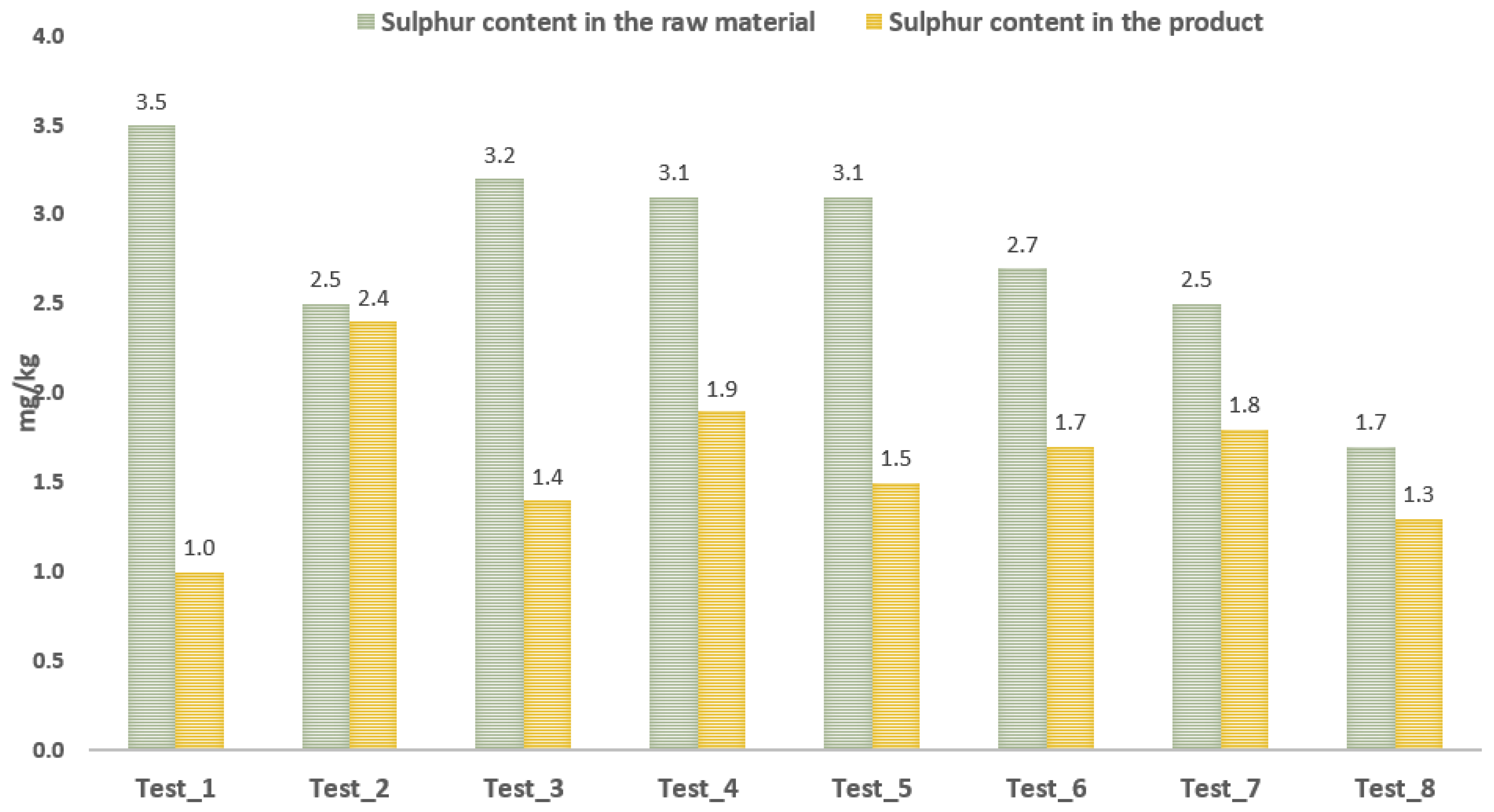
3.3. Test of Cation Exchange Resin with Distilled Glycerol
4. Conclusions
- Glycerines as a by-product of biodiesel production, obtained from vegetable raw materials, do not contain significant amounts of impurities in the form of sulphur, chlorine, and nitrogen compounds.
- Glycerines obtained from mixed raw materials (vegetable oils, UCOs and animal fats) do not contain significant amounts of impurities in the form of chlorine compounds. Still, they are contaminated with sulphur and nitrogen compounds. Glycerine obtained from UCOs has high levels of impurities in the form of nitrogen compounds.
- The use of ion exchange resins at the preliminary purification stage (before distillation) is ineffective because the MONG-type impurities present in the glycerine are released as a separate phase which block the flow of glycerine through the ionite bed.
- Using anionites to purify distilled glycerine of waste origin is not recommended due to the introduction of impurities such as chlorine and nitrogen compounds during the process.
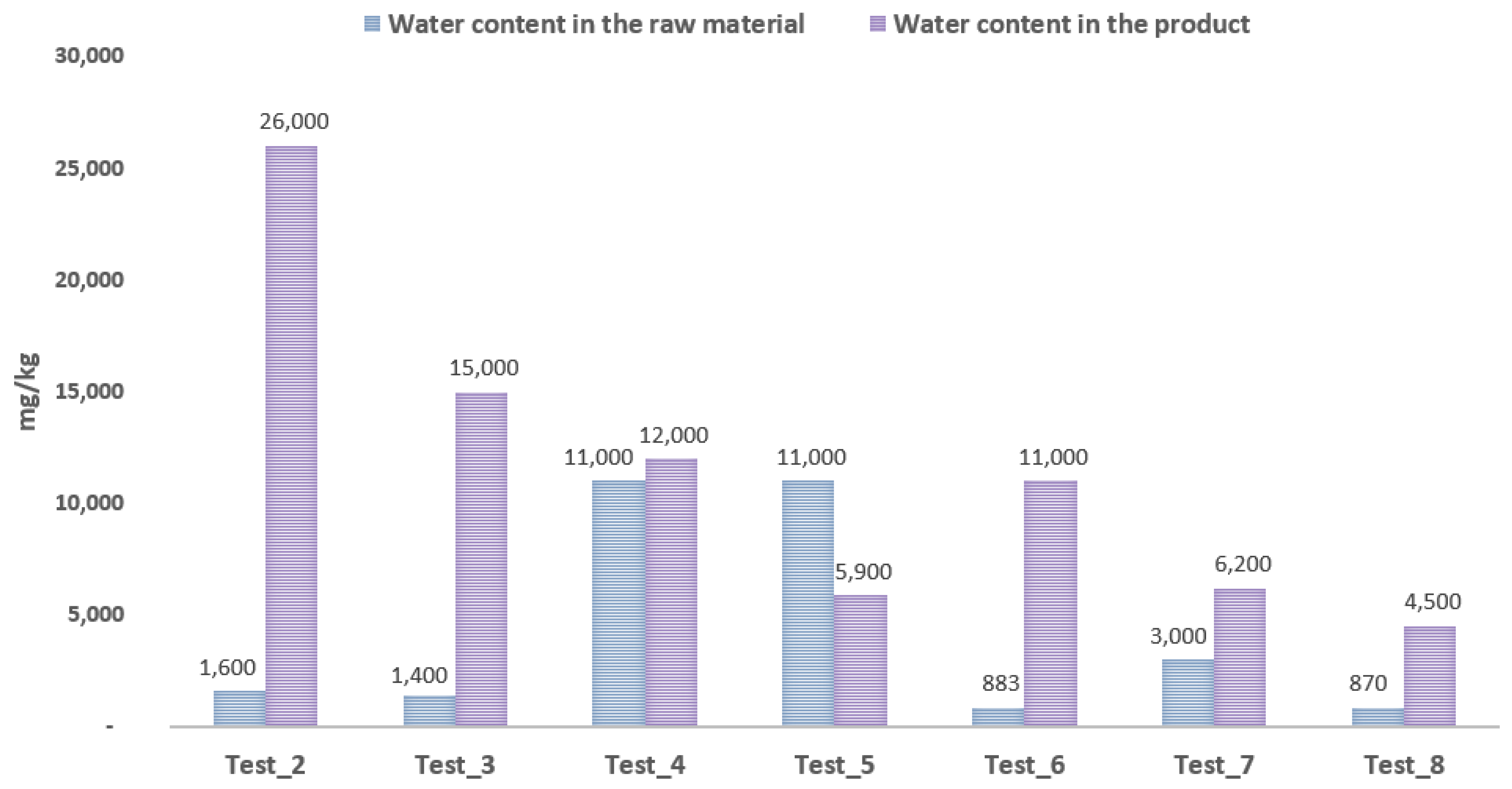
- The use of cationites in the purification process of distilled glycerine of waste origin is promising due to the reduction in the sulphur content and the significant elimination of impurities in the form of nitrogen compounds. The disadvantage is that the cationites cause an increase in the water content of the glycerine samples. Following the sorption on the cationite, it is advisable to carry out the standard steam stripping process. Research is required to verify the performance of this process in laboratory conditions.
- The use of cationites which are a highly porous material is not recommended for the purification process of glycerines, in contrast to cationites in the form of gel grains.
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fauzi, A.H.M.; Amin, N.A.S. An overview of ionic liquids as solvents in biodiesel synthesis. Renew. Sustain. Energy Rev. 2012, 16, 5770–5786. [Google Scholar] [CrossRef]
- Hudha, M.I.; Daryono, E.D.; Rastini, E.K. Potential crude glycerol from Transesterification WCO (Waste Cooking Oil) as an anti-fungisida Spray. ChemTech Res. 2017, 10, 55–61. [Google Scholar]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Hambali, E.; Fitria, R.; Sari, V.I. Biorefinery of Oil Producing Plants for Value-Added Products, Glycerol and Derivatives; Wiley: Hoboken, NJ, USA, 2022; p. 24. [Google Scholar] [CrossRef]
- Datta, I.; Ghosh, A.; Acharjee, A.; Rakshit, A.; Saha, B. Overview on biodiesel market, Vietnam. J. Chem. 2021, 59, 271–284. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2010; pp. 2765–2767. [Google Scholar]
- The United States Pharmacopeia, the National Formulary; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2009.
- Fokum, E.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. Recent technological and strategical developments in the biomanufacturing of 1,3-propanediol from glycerol. Int. J. Environ. Sci. Technol. 2021, 18, 2467–2490. [Google Scholar] [CrossRef]
- Konur, O. Propanediol Production from Glycerol, Biodiesel Fuels—Science Technology Health and Environment; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9780367456238. [Google Scholar]
- Dolejš, I.; Líšková, M.; Krasňan, V.; Markošová, K.; Rosenberg, M.; Lorenzini, F.; Marr, A.C.; Rebroš, M. Production of 1,3-Propanediol from Pure and Crude Glycerol Using Immobilized Clostridium butyricum. Catalysts 2019, 9, 317. [Google Scholar] [CrossRef]
- Berdechowski, K. Analysis of biohydrogen production methods in terms of GHG emission value. Nafta-Gaz 2019, 4, 230–235. [Google Scholar] [CrossRef]
- Zhou, C.H.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Chilakamarry, H.R.; Mimi Sakinah, A.M.; Zularisam, A.W.; Pandey, A.; Vo, D.V.N. Technological perspectives for utilisation of waste glycerol for the production of biofuels: A review. Environ. Technol. Innov. 2021, 24, 101902. [Google Scholar] [CrossRef]
- Zakaria, Z.Y.; Linnekoski, J.; Amin, N.A.S. Catalyst screening for conversion of glycerol to light olefins. J. Chem. Eng. 2012, 207, 803–813. [Google Scholar] [CrossRef]
- Bhargava, A.; Shelke, S.; Dilkash, M.; Chaubal-Durve, N.; Patil, P.; Nadar, S.; Marghade, D.; Tiwari, M. A comprehensive review on catalytic etherification of glycerol to value-added products. Rev. Chem. Eng. 2022. [Google Scholar] [CrossRef]
- Clauser, N.M.; González, G.; Mendieta, C.M.; Kruyeniski, J.; Area, M.C.; Vallejos, M.E. Biomass Waste as Sustainable Raw Material for Energy and Fuels. Sustainability 2021, 13, 794. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Zhang, L.; Chang, Y.; Hao, Y. Converting waste cooking oil to biodiesel in China: Environmental impacts and economic feasibility. Renew. Sustain. Energy Rev. 2021, 140, 110661. [Google Scholar] [CrossRef]
- Velenturf, A.P.M.; Purnell, P. Principles for a sustainable circular economy. Sustain. Prod. Consum. 2021, 27, 1437–1457. [Google Scholar] [CrossRef]
- Rogowska, D.; Berdechowski, K. Ocena wpływu sposobu alokacji emisji w procesie produkcji biopaliwa na wartość emisji gazów cieplarnianych. Nafta-Gaz 2013, 4, 226–234. [Google Scholar]
- Mai-Moulin, T.; Hoefnagels, R.; Grundmann, P.; Junginger, M. Effective sustainability criteria for bioenergy: Towards the implementation of the european renewable directive II. Renew. Sustain. Energy Rev. 2021, 138, 110645. [Google Scholar] [CrossRef]
- Tan, H.W.; Aziz, A.A.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Ziels, N.W. Recovery and purification of glicerol. J. Am. Oil Chem. Soc. 1956, 33, 556–565. [Google Scholar] [CrossRef]
- Khok, Y.T.; Ooi, C.H.; Matsumoto, A.; Yeoh, F.Y. Reactivation of spent activated carbon for glycerine purification. Adsorption 2020, 26, 1015–1025. [Google Scholar] [CrossRef]
- Chol, G.; Dhabhai, R.; Dalai, A.K.; Reaney, M. Purification of crude glycerol derived from biodiesel production process: Experimental studies and techno-economic analyses. Fuel Process. Technol. 2018, 178, 78–87. [Google Scholar] [CrossRef]
- Maquirriain, M.A.; Neyertz, C.A.; Querini, C.A.; Pisarello, M.L. Crude glycerine purification by solvent extraction. Braz. J. Chem. Eng. 2022, 39, 235–249. [Google Scholar] [CrossRef]
- Borówka, G.; Krasodomski, W.; Semerjak, G.; Lubowicz, J.; Krzak, M. Technologie oczyszczania gliceryny technicznej. Przemysł Chemiczny. 2021, 100, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Ardi, M.S.; Aroua, M.K.; Awanis, H.N. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS removal by ion exchange resins: A review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef]
- Konstantinović, S.S.; Danilović, B.R.; Ćirić, J.T.; Ilić, S.B.; Savić, D.S.; Veljković, V.B. Valorization of crude glycerol from biodiesel production. Chem. Ind. Chem. Eng. Q. 2016, 22, 461–489. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Jahim, J.M.; Ismail, M.; Nasir, N.F.; Ba-Abbad, M.M.; Yarmo, M.A. Purification of crude glycerol from industrial waste: Experimental and simulation studies. J. Eng. Sci. Technol. 2016, 11, 1056–1072. [Google Scholar]
- Goh, B.H.H.; Chong, C.T.; Ge, Y.; Ong, H.C.; Ng, J.H.; Tian, B.; Józsa, V. Progress in utilisation of waste cooking oil for sustainable biodiesel and biojet fuel production. Energy Convers. Manag. 2020, 223, 113296. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudin, S.K. The potential of glycerol as a value-added commodity. J. Chem. Eng. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Aroua, M.K.; Cognet, P. Editorial: From Glycerol to Value-Added Products. Front. Chem. 2020, 8, 69. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Heterogeneous catalysis of the glycerol hydrogenolysis. Catal. Sci. Technol. 2011, 1, 179–190. [Google Scholar] [CrossRef]
- Miyuranga Viraj, K.A.; Udara Arachchige, S.P.R.; Randika Jayasinghe, A.; Samarakoon, G. Purification of Residual Glycerol from Biodiesel Production as a Value-Added Raw Material for Glycerolysis of Free Fatty Acids in Waste Cooking Oil. Energies 2022, 15, 8856. [Google Scholar] [CrossRef]
- PN-EN ISO 12185:2002; Ropa Naftowa i Przetwory Naftowe-Oznaczanie Gęstości-Metodą Oscylacyjną z U-Rurką. The Polish Committee for Standardization: Warszawa, Poland, 2002.
- PN-EN ISO 12937:2005; Przetwory Naftowe-Oznaczanie Wody-Miareczkowanie Kulometryczne Metodą Karla Fischera. The Polish Committee for Standardization: Warszawa, Poland, 2005.
- PN-EN ISO 20846:2012; Przetwory Naftowe-Oznaczanie Zawartości Siarki w Paliwach Do Pojazdów Samochodowych-Metoda Fluorescencji w Nadfiolecie. The Polish Committee for Standardization: Warszawa, Poland, 2012.
- ASTM D4629-17; Standard Test Method for Trace Nitrogen in Liquid Hydrocarbons by Syringe/Inlet Oxidative Combustion and Chemiluminescence Detection. ASTM International: Conshohocken, PA, USA, 2017.
- PN-EN 14077:2008; Ciekłe Przetwory Naftowe-Oznaczanie Zawartości Chlorowców Związanych Organicznie-Metoda Mikrokulometrii Oksydacyjnej. The Polish Committee for Standardization: Warszawa, Poland, 2008.
- Carmona, M.; Lech, A.; de Lucas, A.; Perez, A.; Rodriguez, J.F. Purification of glycerol/water solutions from biodiesel synthesis by ion exchange: Sodium and chloride removal. Part II. J. Chem. Technol. Biotechnol. 2009, 84, 1130–1135. [Google Scholar] [CrossRef]

| Number of Sample | Origin of Sample |
|---|---|
| Veg_1 | Vegetable oils |
| Veg_2 | |
| Veg_3 | |
| Mix_4 | Mixed: min. 90% vegetable oils, max. 10% UCO and II category animal fats |
| Mix_5 | |
| Mix_6 | |
| Mix_7 | |
| Mix_8 | |
| Mix_9 | |
| UCO_10 | UCO |
| Density at 15 °C | Glycerol Content | Sulphur Content | Nitrogen Content | Chlorine Content | Water Content | ||
|---|---|---|---|---|---|---|---|
| Origin of the Sample | Sample No | kg/m3 | %(m/m) | mg/kg | mg/kg | mg/kg | mg/kg |
| Vegetable oils | Veg_1 | 1264.0 | 99.9 | <1.0 | <1.0 | <1.0 | 820 |
| Veg_2 | 1263.8 | 99.9 | <1.0 | <1.0 | <1.0 | 800 | |
| Veg_3 | 1263.7 | 99.8 | <1.0 | <1.0 | <1.0 | 2600 | |
| Mixed | Mix_4 | 1263.5 | 99.8 | 3.5 | 3.2 | <1.0 | 76 |
| Mix_5 | 1263.2 | 99.9 | 2.5 | 5.8 | <1.0 | 1600 | |
| Mix_6 | 1263.4 | 99.7 | 3.2 | 3.6 | <1.0 | 1400 | |
| Mix_7 | 1261.2 | 98.9 | 3.1 | 5.3 | <1.0 | 11,000 | |
| Mix_8 | 1263.7 | 99.9 | 2.7 | 5.5 | <1.0 | 883 | |
| Mix_9 | 1263.6 | 99.8 | 2.5 | 4.8 | <1.0 | 3000 | |
| UCO | UCO_10 | 1263.4 | 99.7 | 1.7 | 17.0 | <1.0 | 870 |
| Ion Exchange Resin | Designation | Particle Density [kg/m3] | Water Content [%m/m] | Total Exchange Capacity [eq/L] |
|---|---|---|---|---|
| Strong acid cation exchanger, gel form, H+ form | Kat_1 | 1190 | 48–58 | ≥1.80 |
| Strong acid cation exchanger, gel form, H+ form | Kat_2 | 1130 | 62–72 | ≥1.10 |
| Strong acid cation exchanger, highly porous type, H+ form | Kat_3 | 1190 | 45–55 | ≥1.50 |
| Strong base anion exchanger, gel form, Cl- form | Anio_1 | 1100 | 45–51 | ≥1.25 |
| Strong base anion exchanger, gel form, Cl- form | Anio_2 | 1070 | 49–59 | ≥1.20 |
| No. | Parameter | Unit | Description of the Method | Procedure/Standard |
|---|---|---|---|---|
| 1. | Density at 15 °C | kg/m3 | the oscillating method with U-tube | PN-EN ISO 12185:2002 [36] |
| 2. | Water content | mg/kg | coulometric titration by the Karl Fischer method | PN-EN ISO 12937:2005 [37] |
| 3. | Glycerol content | %m/m | gas chromatography with GC-FID flame ionization detection | GC method 1 |
| 4. | Sulphur content | mg/kg | ultraviolet fluorescence method | PN-EN ISO 20846:2012 [38] |
| 5. | Nitrogen content | mg/kg | oxidative combustion with chemiluminescence detection | ASTM D 4629-17 [39] |
| 6. | Chlorine content | mg/kg | oxidative microcoulometric method | PN-EN 14077:2008 [40] |
| Test No. | Cation Exchanger | Glycerine Sample |
|---|---|---|
| Test_1 | Kat_1 | Mix_4 |
| Test_2 | Kat_1 | Mix_5 |
| Test_3 | Kat_1 | Mix_6 |
| Test_4 | Kat_1 | Mix_7 |
| Test_5 | Kat_2 | Mix_7 |
| Test_6 | Kat_1 | Mix_8 |
| Test_7 | Kat_2 | Mix_9 |
| Test_8 | Kat_1 | UCO_10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borówka, G.; Semerjak, G.; Krasodomski, W.; Lubowicz, J. Purified Glycerine from Biodiesel Production as Biomass or Waste-Based Green Raw Material for the Production of Biochemicals. Energies 2023, 16, 4889. https://doi.org/10.3390/en16134889
Borówka G, Semerjak G, Krasodomski W, Lubowicz J. Purified Glycerine from Biodiesel Production as Biomass or Waste-Based Green Raw Material for the Production of Biochemicals. Energies. 2023; 16(13):4889. https://doi.org/10.3390/en16134889
Chicago/Turabian StyleBorówka, Grzegorz, Grzegorz Semerjak, Wojciech Krasodomski, and Jan Lubowicz. 2023. "Purified Glycerine from Biodiesel Production as Biomass or Waste-Based Green Raw Material for the Production of Biochemicals" Energies 16, no. 13: 4889. https://doi.org/10.3390/en16134889
APA StyleBorówka, G., Semerjak, G., Krasodomski, W., & Lubowicz, J. (2023). Purified Glycerine from Biodiesel Production as Biomass or Waste-Based Green Raw Material for the Production of Biochemicals. Energies, 16(13), 4889. https://doi.org/10.3390/en16134889






