Asphaltene Precipitation/Deposition Estimation and Inhibition through Nanotechnology: A Comprehensive Review
Abstract
1. Introduction
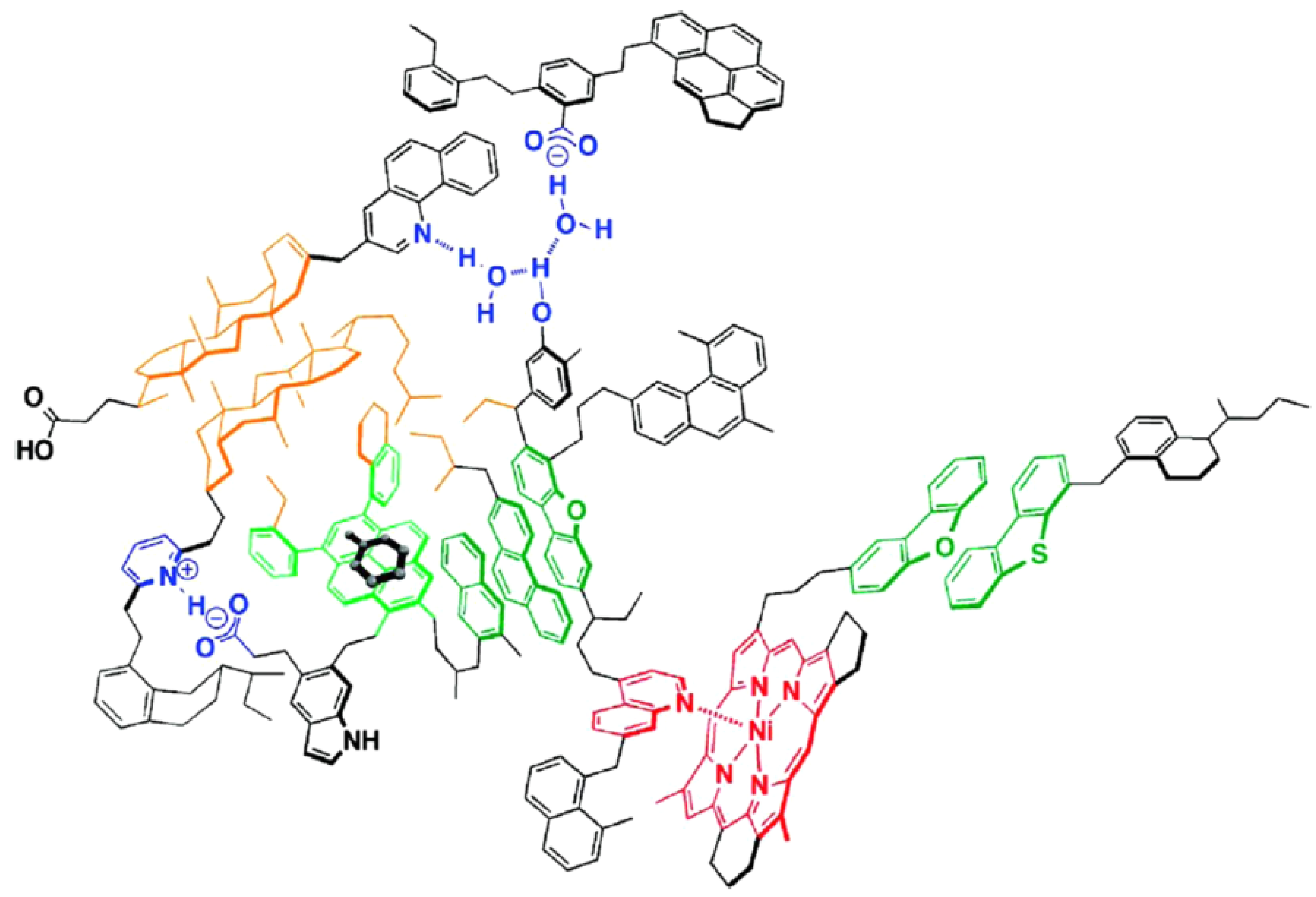
2. Asphaltenes
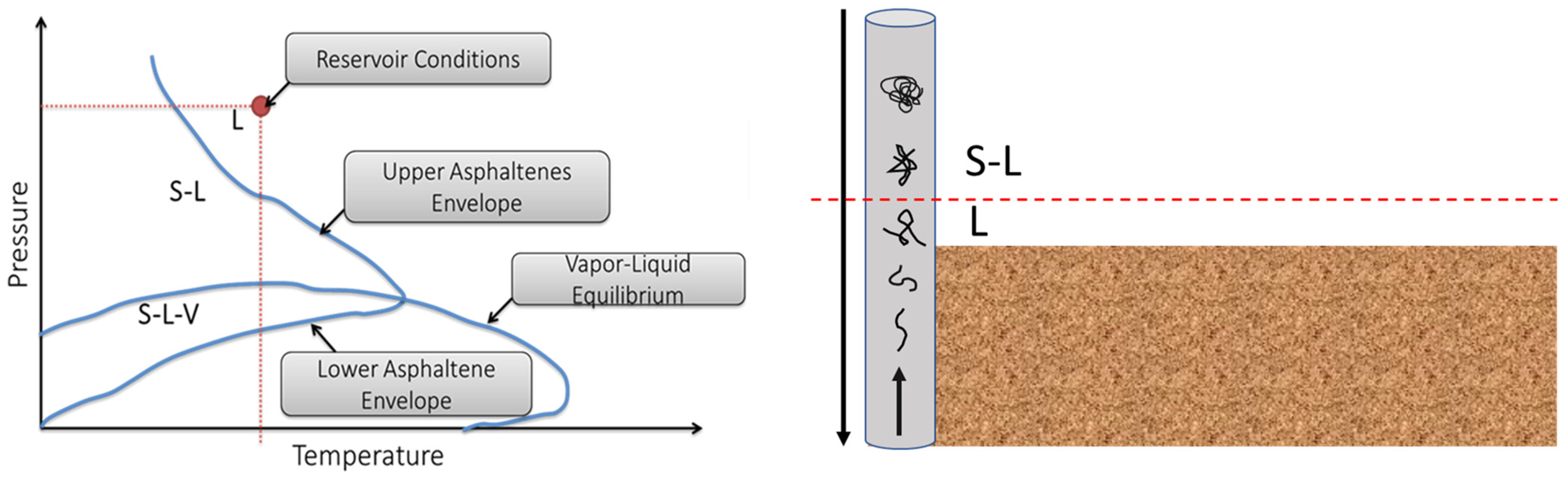
2.1. Asphaltene Precipitation
2.2. Thermodynamic Models of Asphaltene Precipitation
3. Nanotechnology for the Inhibition of Asphaltene Precipitation/Deposition
3.1. Synthesis of Nanoparticles for the Inhibition of the Precipitation/Deposition of Asphaltenes
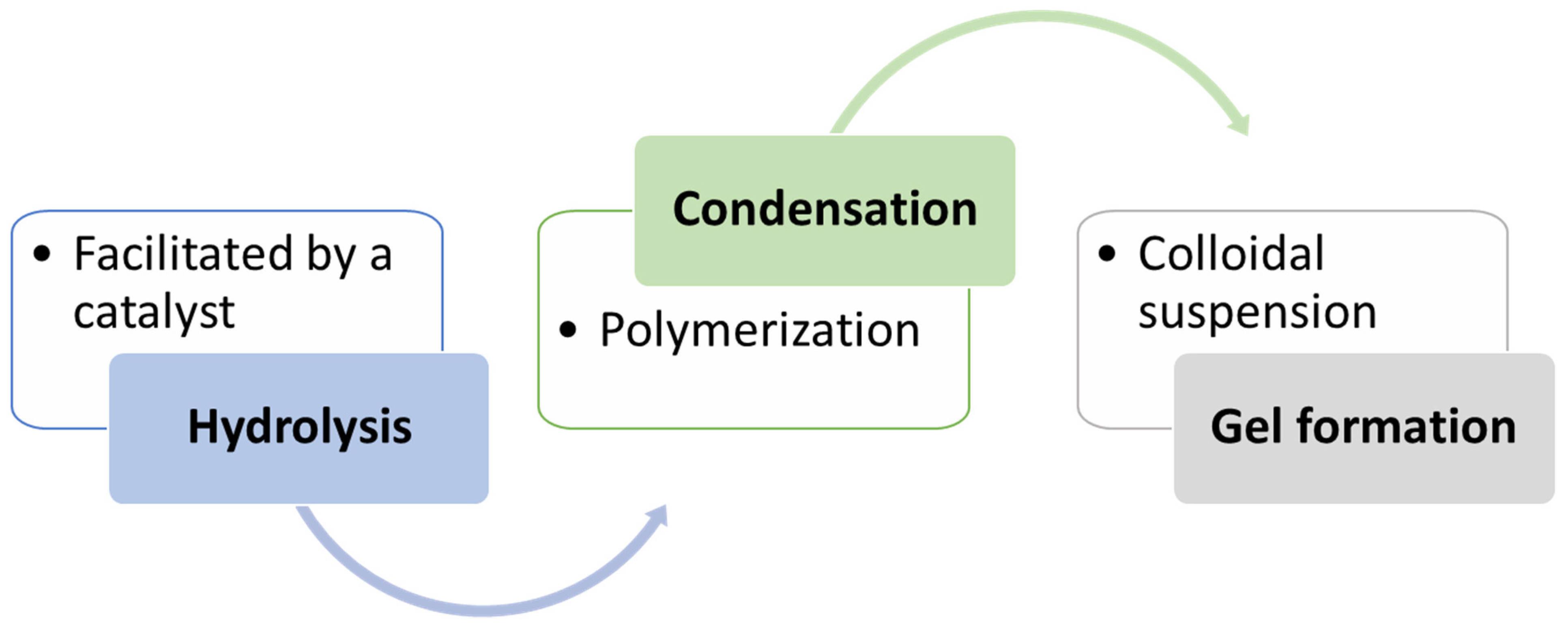
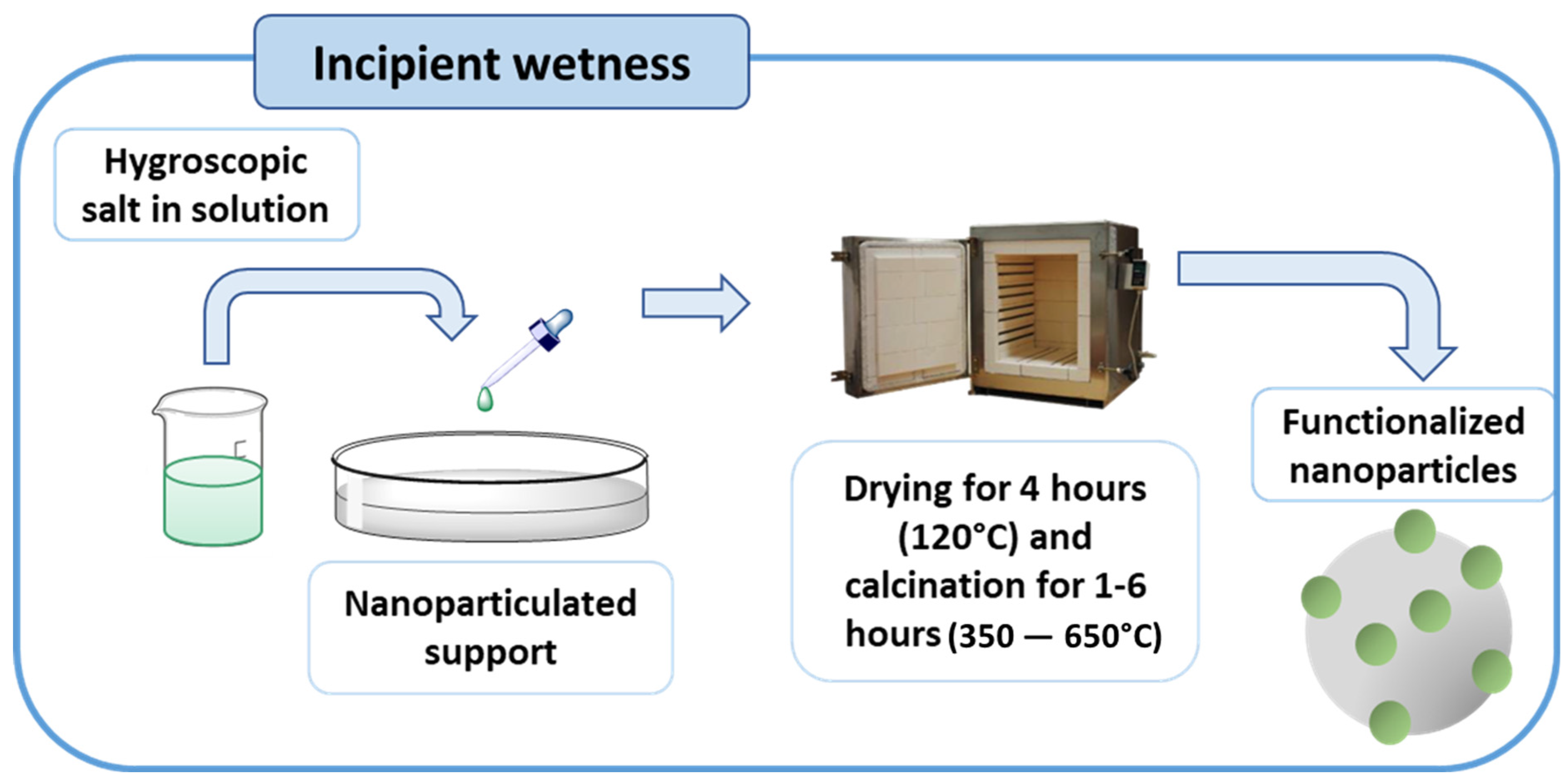
3.1.1. Sol–Gel
3.1.2. Coprecipitation
3.1.3. Nanoparticle Functionalization
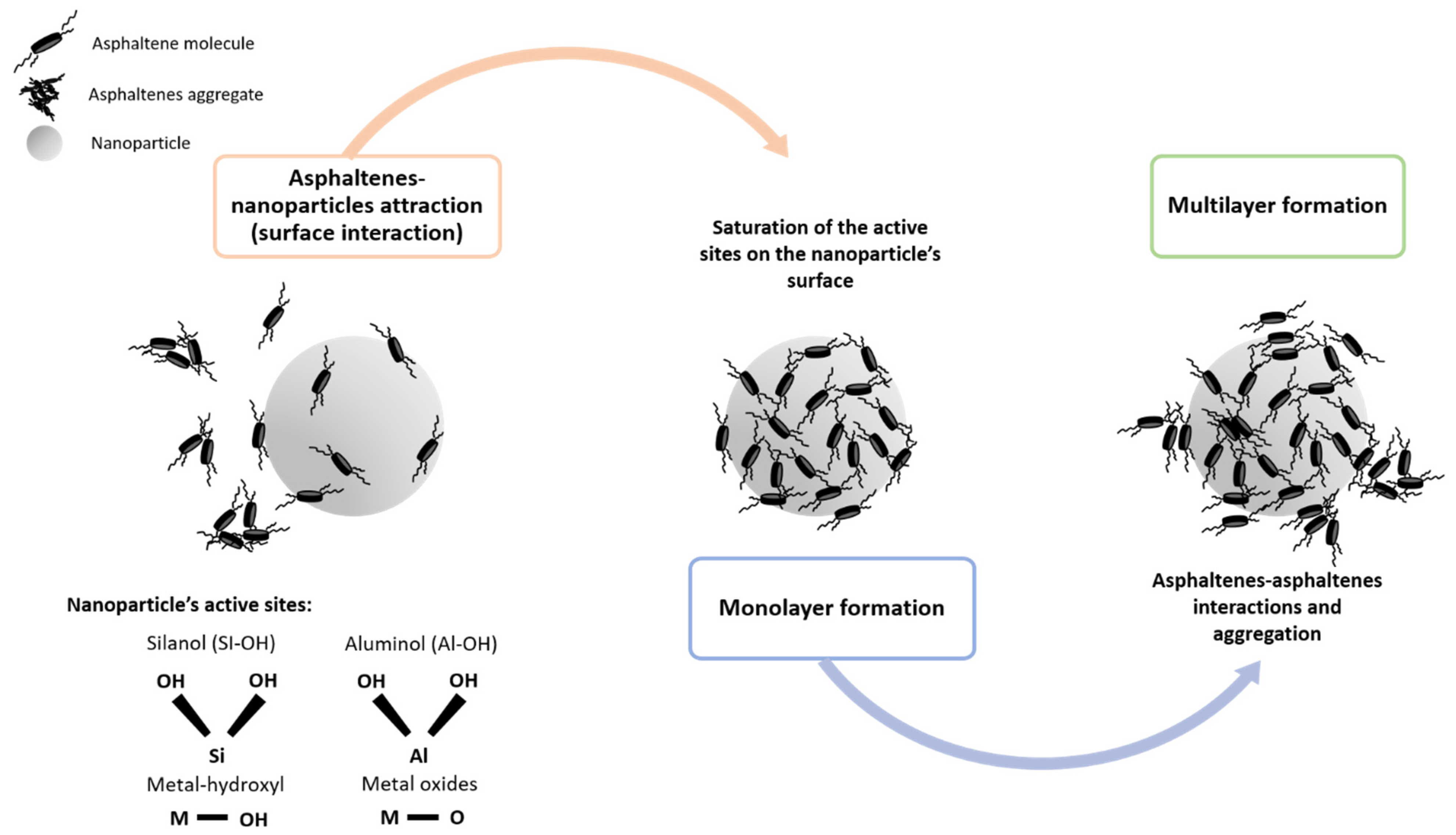
3.2. Phenomenological Approaches to Asphaltene–Nanoparticle Interactions
3.2.1. Behavior of the Crude Oil Fractions in Terms of Their Interaction with Nanoparticles
3.2.2. Adsorption Isotherms: Construction and Modeling
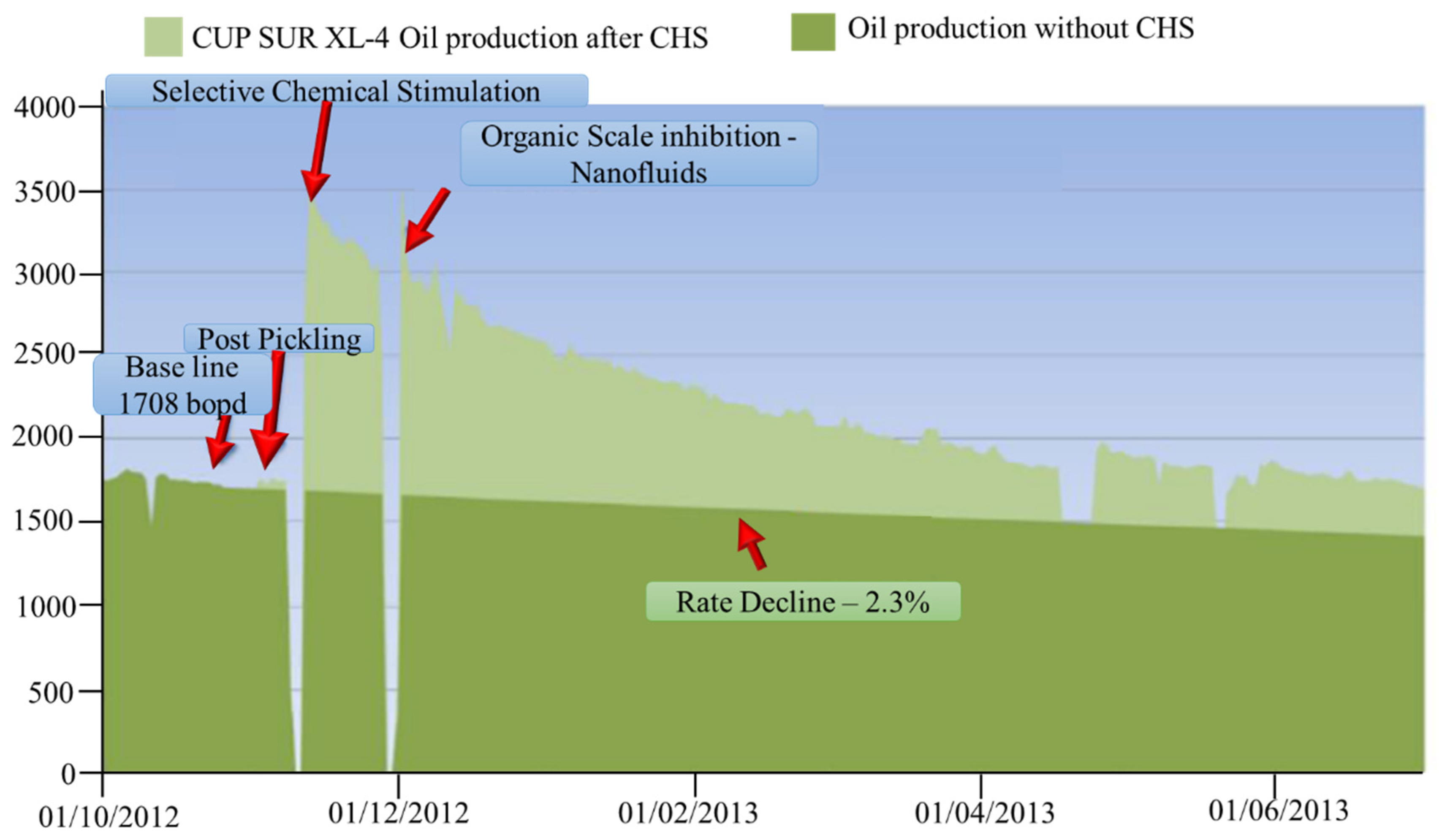
3.3. Inhibition of Asphaltene Precipitation/Deposition: Upscaling through Coreflooding Tests and Field Trial Application
4. Outlook and New Technologies
5. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- León, E.A.; Quintero, J.F.G.; Cervantes, A.A.B.; Rangel, E.R.S. Análisis de la simulación de precipitación de asfaltenos en el crudo del campo Colorado. Fuentes Reventón Energético 2012, 10, 6. [Google Scholar]
- Leon, E.A. Efecto de la Estructura Quimica y la Concentracion de Los Asfaltenos Sobre las Propiedades Reologicas y Termodinamicas de la Cristalizacion de Parafinas en Crudos del Campo Colorado; Universidad Industrial de Santander, Escuela De Ing. Quimica: Bucaramaga, Colombia, 2016. [Google Scholar]
- Asomaning, S. Test methods for determining asphaltene stability in crude oils. Pet. Sci. Technol. 2003, 21, 581–590. [Google Scholar] [CrossRef]
- Demirbas, A. Deposition and flocculation of asphaltenes from crude oils. Pet. Sci. Technol. 2016, 34, 6–11. [Google Scholar] [CrossRef]
- Leontaritis, K.; Amaefule, J.; Charles, R. A systematic approach for the prevention and treatment of formation damage caused by asphaltene deposition. SPE Prod. Facil. 1994, 9, 157–164. [Google Scholar] [CrossRef]
- Soulgani, B.S.; Tohidi, B.; Jamialahmadi, M.; Rashtchian, D. Modeling formation damage due to asphaltene deposition in the porous media. Energy Fuels 2011, 25, 753–761. [Google Scholar] [CrossRef]
- Khanifar, A.; Demiral, B.; Alian, S.S.; Drman, N. Study of asphaltene precipitation and deposition phenomenon. In Proceedings of the 2011 National Postgraduate Conference, Kuala Lumpur, Malaysia, 17–19 July 2011; pp. 1–6. [Google Scholar]
- Hassanzadeh, M.; Abdouss, M. Molecular Structure: The First and Most Significant Factor in the Precipitation of Asphaltenes. SPE J. 2023, 28, 894–907. [Google Scholar] [CrossRef]
- Mohammed, I.; AlShehri, D.; Mohamed, M.; Mohammed Kamal, S.; Olalekan Saheed, A.; Abdullah, S.; Patil, S. Exploration of Novel Sacrificial Fluids for Asphaltene Adsorption Remediation. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 19–21 February 2023. [Google Scholar]
- Chamkalani, A.; Mohammadi, A.H.; Eslamimanesh, A.; Gharagheizi, F.; Richon, D. Diagnosis of asphaltene stability in crude oil through “two parameters” SVM model. Chem. Eng. Sci. 2012, 81, 202–208. [Google Scholar] [CrossRef]
- Akbarzadeh, K.; Hammami, A.; Kharrat, A.; Zhang, D.; Allenson, S.; Creek, J.; Solbakken, T. Asphaltenes—Problematic but rich in potential. Oilfield Rev. 2007, 19, 22–43. [Google Scholar]
- Figuera, L.; Marin, M.; Lopez, G.L.R.; Marin, E.; Gammiero, A.; Granado, C. Characterization and Modelling of Asphaltene Precipitation and Deposition in a Compositional Reservoir. In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 20–22 September 2010. [Google Scholar]
- Ahmadi, M.A.; Shadizadeh, S.R. New approach for prediction of asphaltene precipitation due to natural depletion by using evolutionary algorithm concept. Fuel 2012, 102, 716–723. [Google Scholar] [CrossRef]
- Guerrero-Martin, C.A.; Montes-Páez, E.; de Oliveira, M.C.K.; Campos, J.; Lucas, E.F. Calculating Asphaltenes Precipitation Onset Pressure by Using Cardanol as Precipitation Inhibitor: A Strategy to Increment the Oil Well Production. In Proceedings of the SPE Trinidad and Tobago Section Energy Resources Conference, Port of Spain, Trinidad and Tobago, 25–26 June 2018. [Google Scholar]
- Mansur, C.R.; Guimaraes, A.R.; Gonzalez, G.; Lucas, E.F. Determination of the onset of asphaltene precipitation by visible ultraviolet spectrometry and spectrofluorimetry. Anal. Lett. 2009, 42, 2648–2664. [Google Scholar] [CrossRef]
- Lima, A.F.; Mansur, C.R.; Lucas, E.F.; Gonza, G. Polycardanol or sulfonated polystyrene as flocculants for asphaltene dispersions. Energy Fuels 2010, 24, 2369–2375. [Google Scholar] [CrossRef]
- Garreto, M.; Gonzalez, G.; Ramos, A.; Lucas, E. Looking for a Model Solvent to Disperse Asphaltenes. Chem. Chem. Technol. 2010. Available online: http://science2016.lp.edu.ua/sites/default/files/Full_text_of_%20papers/full_text_341.pdf (accessed on 15 January 2023).
- Garreto, M.; Mansur, C.; Lucas, E. A model system to assess the phase behavior of asphaltenes in crude oil. Fuel 2013, 113, 318–322. [Google Scholar] [CrossRef]
- Ferreira, S.R.; Louzada, H.F.; Dip, R.M.M.; Gonza, G.; Lucas, E.F. Influence of the architecture of additives on the stabilization of asphaltene and water-in-oil emulsion separation. Energy Fuels 2015, 29, 7213–7220. [Google Scholar] [CrossRef]
- Ferreira, S.R.; Barreira, F.R.; Spinelli, L.S.; Leal, K.Z.; Seidl, P.; Lucas, E.F. Comparison between asphaltenes (sub) fractions extracted from two different asphaltic residues: Chemical characterization and phase behavior. Química Nova 2016, 39, 26–31. [Google Scholar] [CrossRef]
- Palermo, L.C.; Lucas, E.F. Asphaltene aggregation: Influence of composition of copolymers based on styrene-stearyl methacrylate and styrene-stearyl cinnamate containing sulfate groups. Energy Fuels 2016, 30, 3941–3946. [Google Scholar] [CrossRef]
- Figueira, J.N.; Simão, R.A.; Soares, B.G.; Lucas, E.F. The influence of chemicals on asphaltenes precipitation: A comparison between atomic force microscopy and near infrared techniques. Fuentes Reventón Energético 2017, 15, 7–17. [Google Scholar] [CrossRef]
- Mazzeo, C.P.; Stedille, F.A.; Mansur, C.R.; Ramos, A.N.C.; Lucas, E.F. Flocculation of asphaltenes by polymers: Influence of polymer solubility conditions. Energy Fuels 2018, 32, 1087–1095. [Google Scholar] [CrossRef]
- Barreira, F.R.; Reis, L.G.; Nunes, R.D.C.P.; Filipakis, S.D.; Lucas, E.F. Asphaltenes Precipitation Onset: Influence of the Addition of a Second Crude Oil or Its Asphaltenes Fractions (C3I and C5I). Energy Fuels 2018, 32, 10391–10397. [Google Scholar] [CrossRef]
- Nunes, R.C.P.; Valle, M.R.T.; Reis, W.R.D.; Aversa, T.M.; Filipakis, S.D.; Lucas, E.F. Model Molecules for Evaluating Asphaltene Precipitation Onset of Crude Oils. J. Braz. Chem. Soc. 2019, 30, 1241–1251. [Google Scholar] [CrossRef]
- Buckley, J.S. Asphaltene Deposition. Energy Fuels 2012, 26, 4086–4090. [Google Scholar] [CrossRef]
- Wang, J.; Buckley, J.S.; Creek, J.L. Asphaltene Deposition on Metallic Surfaces. J. Dispers. Sci. Technol. 2004, 25, 287–298. [Google Scholar] [CrossRef]
- Buckley, J.S. Microscopic Investigation of the Onset of Asphaltene Precipitation. Fuel Sci. Technol. Int. 1996, 14, 55–74. [Google Scholar] [CrossRef]
- Buckley, J.S.; Wang, J. Crude oil and asphaltene characterization for prediction of wetting alteration. J. Pet. Sci. Eng. 2002, 33, 195–202. [Google Scholar] [CrossRef]
- Kurup, A.S.; Wang, J.; Subramani, H.J.; Buckley, J.; Creek, J.L.; Chapman, W.G. Revisiting Asphaltene Deposition Tool (ADEPT): Field Application. Energy Fuels 2012, 26, 5702–5710. [Google Scholar] [CrossRef]
- Buckley, J.S. Predicting the Onset of Asphaltene Precipitation from Refractive Index Measurements. Energy Fuels 1999, 13, 328–332. [Google Scholar] [CrossRef]
- Wang, J.; Buckley, J.S. Asphaltene Stability in Crude Oil and Aromatic SolventsThe Influence of Oil Composition. Energy Fuels 2003, 17, 1445–1451. [Google Scholar] [CrossRef]
- Yang, M.-G.; Eser, S. Fractionation and Molecular Analysis of a Vacuum Residue Asphaltenes. Fuel Chem. Am. Chem. Soc. 2006, 44, 762–768. Available online: https://d1wqtxts1xzle7.cloudfront.net/75009198/FRACTIONATION_AND_MOLECULAR_ANALYSIS_OF_20211122-1750-1vsfv0h.pdf?1637589666=&response-content-disposition=inline%3B+filename%3DFractionation_and_molecular_analysis_of.pdf&Expires=1687059206&Signature=SpRRaAFsgO2g84AI5lnEWDseeE4Sy~2l4T3jHrk1vzknSL3wfvJJaHyo7~JoKoExzC~hGE422WPwUsLMBHiv3PZ~BscMP8yFG7~uSwaeZwWo-qjgabXLRMgbjwF6uLtAqGtvb4Z4WMomn-PL1qKHDnXBApsgg5b7Osump3f6~o4rWgltNeoPGaHG~LpIkmD6KtvoELOsGkN4uSCqdPLLYsKfsKUVFsn6gnC-TIdUf4-ZzsluaqRtAhLqry5vutl8BIJPpL7ZTc6AmTNH-APaqUw91IolRHvofflTVHyeI-ywff8gVnuX6qUsq-DTSdlX7dZa4cBPsgmm525pNqU7iQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 25 January 2023).
- Hartmann, D.; Lopes, H.E.; Teixeira, C.L.S.; de Oliveira, M.C.K.; Gonzalez, G.; Lucas, E.F.; Spinelli, L.S. Alkanes Induced Asphaltene Precipitation Studies at High Pressure and Temperature in the Presence of Argon. Energy Fuels 2016, 30, 3693–3706. [Google Scholar] [CrossRef]
- Gray, M.R. Consistency of Asphaltene Chemical Structures with Pyrolysis and Coking Behavior. Energy Fuels 2003, 17, 1566–1569. [Google Scholar] [CrossRef]
- Nellensteyn, F. The constitution of asphalt. J. Inst. Pet. Technol. 1924, 10, 311–323. [Google Scholar]
- Pfeiffer, J.P.; Saal, R.N.J. Asphaltic Bitumen as Colloid System. J. Phys. Chem. 1940, 44, 139–149. [Google Scholar] [CrossRef]
- Dickie, J.P.; Yen, T.F. Macrostructures of the asphaltic fractions by various instrumental methods. Anal. Chem. 1967, 39, 1847–1852. [Google Scholar] [CrossRef]
- Wiehe, I.A. The pendant-core building block model of petroleum residua. Energy Fuels 1994, 8, 536–544. [Google Scholar] [CrossRef]
- Mullins, O.C. The modified Yen model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Garreto, M.D.S.E. Influência do Parâmetro de Solubilidade Sobre a Estabilidade de Asfaltenos no Petróleo. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2011. Available online: http://objdig.ufrj.br/64/teses/756142.pdf (accessed on 7 January 2023).
- Mack, C. Colloid chemistry of asphalts. J. Phys. Chem. 2002, 36, 2901–2914. [Google Scholar] [CrossRef]
- Leyva, C.; Ancheyta, J.; Centeno, G. Effect of alumina and silica–alumina supported NiMo catalysts on the properties of asphaltenes during hydroprocessing of heavy petroleum. Fuel 2014, 138, 111–117. [Google Scholar] [CrossRef]
- Moura, L.G.M.D.; Rosa, P.D.T.V.E. Avaliação de asfaltenos precipitados em diferentes condições de composição, temperatura e pressão. Química Nova 2018, 41, 157–162. [Google Scholar] [CrossRef]
- Centeno, G.; Trejo, F.; Ancheyta, J.; Carlos, A. Precipitación de asfaltenos del crudo Maya en un sistema a presión. Rev. Soc. Química México 2004, 48, 179–188. [Google Scholar]
- ASTM D893–14(2018); Standard Test Method for Insolubles in Used Lubricating Oils. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D2006; Standard Test Method for Characteristic Groups in Rubber Extender and Processing Oils by the Precipitation Method. ASTM International: West Conshohocken, PA, USA, 1970.
- Bulmer, J.; Starr, J. Syncrude Analytical Methods for Oil Sand and Bitumen Processing; AOSTRA: Edmonton, AB, Canada, 1979. [Google Scholar]
- ASTM D2007-19; Standard Test Method for Characteristic Groups in Rubber Extender and Processing Oils and Other Petroleum-Derived Oils by the Clay-Gel Absorption Chromatographic Method. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM 6560; Standard Test Method for Determination of Asphaltenes (Heptane Insolubles) in Crude Petroleum and Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D3279-19; Standard Test Method for n-Heptane Insolubles. ASTM International: West Conshohocken, PA, USA, 2019.
- Escobedo, J.; Mansoori, G.A. Viscometric determination of the onset of asphaltene flocculation: A novel method. SPE Prod. Facil. 1995, 10, 115–118. [Google Scholar] [CrossRef]
- Leontaritis, K.J. The asphaltene and wax deposition envelopes. Fuel Sci. Technol. Int. 1996, 14, 13–39. [Google Scholar] [CrossRef]
- Moreno, L.F.C.; Leon, E.A.; Garcia, R.P.; Nino, J.C.L. Evaluation of the Effect of the Asphaltene Accumulation in Porous Media at Dynamic Conditions for a Colombian Crude Oil. In Proceedings of the SPE Heavy and Extra Heavy Oil Conference, Medellín, Colombia, 24–26 September 2014. [Google Scholar]
- Mullins, O.C. Review of the molecular structure and aggregation of asphaltenes and petroleomics. SPE J. 2008, 13, 48–57. [Google Scholar] [CrossRef]
- Fuentes, J.F.; Montes, D.; Lucas, E.F.; Montes-Páez, E.G.; Szklo, A.; Guerrero-Martin, C.A. Nanotechnology applied to the inhibition and remediation of formation damage by fines migration and deposition: A comprehensive review. J. Pet. Sci. Eng. 2022, 216, 110767. [Google Scholar] [CrossRef]
- Ibañez-Gómez, L.F.; Albarracín-Quintero, S.; Céspedes-Zuluaga, S.; Montes-Páez, E.; Ando Junior, O.H.; Carmo, J.P.; Siqueira, A.A.; Guerrero-Martin, C.A. Process optimization of the flaring gas for field applications. Energies 2022, 15, 7655. [Google Scholar] [CrossRef]
- Bello-Angulo, D.; Mantilla-Duarte, C.; Montes-Paez, E.; Guerrero-Martin, C. Box–Jenkins Methodology Application to Improve Crude Oil Production Forecasting: Case Study in a Colombian Field. Arab. J. Sci. Eng. 2022, 47, 11269–11278. [Google Scholar] [CrossRef]
- Hirschberg, A.; Dejong, L.N.; Schipper, B.; Meijer, J. Influence of temperature and pressure on asphaltene flocculation. Soc. Pet. Eng. J. 1984, 24, 283–293. [Google Scholar] [CrossRef]
- Hammami, A.; Phelps, C.H.; Monger-McClure, T.; Little, T. Asphaltene precipitation from live oils: An experimental investigation of onset conditions and reversibility. Energy Fuels 2000, 14, 14–18. [Google Scholar] [CrossRef]
- Rassamdana, H.; Dabir, B.; Nematy, M.; Farhani, M.; Sahimi, M. Asphalt flocculation and deposition: I. The onset of precipitation. AIChE J. 1996, 42, 10–22. [Google Scholar] [CrossRef]
- Zendehboudi, S.; Shafiei, A.; Bahadori, A.; James, L.A.; Elkamel, A.; Lohi, A. Asphaltene precipitation and deposition in oil reservoirs–Technical aspects, experimental and hybrid neural network predictive tools. Chem. Eng. Res. Des. 2014, 92, 857–875. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Morozov, E.V.; Subramani, V.; Martyanov, O.N.; Kazarian, S.G. Chemical visualization of asphaltenes aggregation processes studied in situ with ATR-FTIR spectroscopic imaging and NMR imaging. J. Phys. Chem. C 2015, 119, 2646–2660. [Google Scholar] [CrossRef]
- Burke, N.E.; Hobbs, R.E.; Kashou, S.F. Measurement and Modeling of Asphaltene Precipitation (includes associated paper 23831). J. Pet. Technol. 1990, 42, 1440–1446. [Google Scholar] [CrossRef]
- Novosad, Z.; Costain, T. Experimental and modeling studies of asphaltene equilibria for a reservoir under CO2 injection. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 23–26 September 1990. [Google Scholar]
- Kokal, S.L.; Najman, J.; Sayegh, S.G.; George, A.E. Measurement and correlation of asphaltene precipitation from heavy oils by gas injection. J. Can. Pet. Technol. 1992, 31. [Google Scholar] [CrossRef]
- Leontaritis, K. Asphaltene deposition: A comprehensive description of problem manifestations and modeling approaches. In Proceedings of the SPE Production Operations Symposium, Oklahoma City, OK, USA, 13–14 March 1989. [Google Scholar]
- Gupta, A.K. A Model for Asphaltene Flocculation Using an Equation of State. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 1986. [Google Scholar]
- Thomas, F.; Bennion, D.; Bennion, D.; Hunter, B. Experimental and theoretical studies of solids precipitation from reservoir fluid. J. Can. Pet. Technol. 1992, 31. [Google Scholar] [CrossRef]
- Nghiem, L.; Hassam, M.; Nutakki, R.; George, A. Efficient modelling of asphaltene precipitation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1993. [Google Scholar]
- Chung, F.; Sarathi, P.; Jones, R. Modeling of Asphaltene and Wax Precipitation; National Inst. for Petroleum and Energy Research: Bartlesville, OK, USA, 1991. [Google Scholar]
- Peng, D.-Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Nghiem, L.X.; Coombe, D.A. Modelling asphaltene precipitation during primary depletion. SPE J. 1997, 2, 170–176. [Google Scholar] [CrossRef]
- Nghiem, L.X.; Coombe, D.A.; Ali, S. Compositional simulation of asphaltene deposition and plugging. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 27–30 September 1998. [Google Scholar]
- Fussell, L.T. A Technique for Calculating Multiphase Equilibria (includes associated papers 8734 and 8746). Soc. Pet. Eng. J. 1979, 19, 203–210. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N. On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Victorov, A.I.; Firoozabadi, A. Thermodynamic micellizatin model of asphaltene precipitation from petroleum fluids. AIChE J. 1996, 42, 1753–1764. [Google Scholar] [CrossRef]
- Chapman, W.G.; Sauer, S.G.; Ting, D.; Ghosh, A. Phase behavior applications of SAFT based equations of state—From associating fluids to polydisperse, polar copolymers. Fluid Phase Equilibria 2004, 217, 137–143. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Hasdbjerg, C. PC-SAFT equation of state applied to petroleum reservoir fluids. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007. [Google Scholar]
- Yen, T.; Chilingarian, G. Asphaltenes and Asphalts, Develop-Ments in Petroleum Science; Elsevier Science BV: Los Angeles, CA, USA, 1994. [Google Scholar]
- Mullins, O.C. The asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. [Google Scholar] [CrossRef]
- Mullins, O.C.; Andrews, B.; Pomerantz, A.; Dong, C.; Zuo, J.Y.; Pfeiffer, T.; Larter, S. Impact of Asphaltene nanoscience on understanding oilfield reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Metal oxide nanoparticles for asphaltene adsorption and oxidation. Energy Fuels 2011, 25, 1017–1023. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Carbognani, L.; Lopez-Linares, F.; Pereira-Almao, P. Iron oxide nanoparticles for rapid adsorption and enhanced catalytic oxidation of thermally cracked asphaltenes. Fuel 2012, 95, 257–262. [Google Scholar] [CrossRef]
- Xie, K.; Karan, K. Kinetics and thermodynamics of asphaltene adsorption on metal surfaces: A preliminary study. Energy Fuels 2005, 19, 1252–1260. [Google Scholar] [CrossRef]
- Gray, M.R.; Tykwinski, R.R.; Stryker, J.M.; Tan, X. Supramolecular assembly model for aggregation of petroleum asphaltenes. Energy Fuels 2011, 25, 3125–3134. [Google Scholar] [CrossRef]
- Castillo, J.; Vargas, V. Metal porphyrin occlusion: Adsorption during asphaltene aggregation. Pet. Sci. Technol. 2016, 34, 873–879. [Google Scholar] [CrossRef]
- Rogel, E. Asphaltene aggregation: A molecular thermodynamic approach. Langmuir 2002, 18, 1928–1937. [Google Scholar] [CrossRef]
- Koots, J.A.; Speight, J.G. Relation of petroleum resins to asphaltenes. Fuel 1975, 54, 179–184. [Google Scholar] [CrossRef]
- León, O.; Contreras, E.; Rogel, E.; Dambakli, G.; Acevedo, S.; Carbognani, L.; Espidel, J. Adsorption of native resins on asphaltene particles: A correlation between adsorption and activity. Langmuir 2002, 18, 5106–5112. [Google Scholar] [CrossRef]
- Pereira, J.C.; López, I.; Salas, R.; Silva, F.; Fernández, C.; Urbina, C.; López, J.C. Resins: The molecules responsible for the stability/instability phenomena of asphaltenes. Energy Fuels 2007, 21, 1317–1321. [Google Scholar] [CrossRef]
- Carnahan, N.F.; Salager, J.-L.; Antón, R.; Dávila, A. Properties of resins extracted from Boscan crude oil and their effect on the stability of asphaltenes in Boscan and Hamaca crude oils. Energy Fuels 1999, 13, 309–314. [Google Scholar] [CrossRef]
- Porte, G.; Zhou, H.; Lazzeri, V. Reversible description of asphaltene colloidal association and precipitation. Langmuir 2003, 19, 40–47. [Google Scholar] [CrossRef]
- León, O.; Contreras, E.; Rogel, E.; Dambakli, G.; Espidel, J.; Acevedo, S. The influence of the adsorption of amphiphiles and resins in controlling asphaltene flocculation. Energy Fuels 2001, 15, 1028–1032. [Google Scholar] [CrossRef]
- Ghloum, E.F.; Al-Qahtani, M.; Al-Rashid, A. Effect of inhibitors on asphaltene precipitation for Marrat Kuwaiti reservoirs. J. Pet. Sci. Eng. 2010, 70, 99–106. [Google Scholar] [CrossRef]
- Yen, A.; Yin, Y.R.; Asomaning, S. Evaluating asphaltene inhibitors: Laboratory tests and field studies. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001. [Google Scholar]
- Junior, L.C.R.; Ferreira, M.S.; da Silva Ramos, A.C. Inhibition of asphaltene precipitation in Brazilian crude oils using new oil soluble amphiphiles. J. Pet. Sci. Eng. 2006, 51, 26–36. [Google Scholar] [CrossRef]
- Franco, C.A.; Zabala, R.; Cortés, F.B. Nanotechnology applied to the enhancement of oil and gas productivity and recovery of Colombian fields. J. Pet. Sci. Eng. 2017, 157, 39–55. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Ruiz, M.A.; Pereira-Almao, P.; Corte, F.B. Nanoparticles for inhibition of asphaltenes damage: Adsorption study and displacement test on porous media. Energy Fuels 2013, 27, 2899–2907. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Mirzaei, S.; Asghari, M.; Ivakpour, J. Aluminum oxide nanoparticles for highly efficient asphaltene separation from crude oil using ceramic membrane technology. Oil Gas Sci. Technol. Rev. D’ifp Energ. Nouv. 2017, 72, 34. [Google Scholar] [CrossRef]
- Parsaei, R.; Kazemzadeh, Y.; Riazi, M. Study of Asphaltene Precipitation during CO2 Injection into Oil Reservoirs in the Presence of Iron Oxide Nanoparticles by Interfacial Tension and Bond Number Measurements. ACS Omega 2020, 5, 7877–7884. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Aminshahidy, B. Effects of hydrophobic CaO and SiO2 nanoparticles on Asphaltene Precipitation Envelope (APE): An experimental and modeling approach. Oil Gas Sci. Technol. Rev. D’ifp Energ. Nouv. 2018, 73, 56. [Google Scholar] [CrossRef]
- Oliveira, G.; Clarindo, J.; Santo, K.; Souza, F., Jr. Chemical modification of cobalt ferrite nanoparticles with possible application as asphaltene flocculant agent. Mater. Res. 2013, 16, 668–671. [Google Scholar] [CrossRef]
- Corte, F.B.; Mejía, J.M.; Ruiz, M.A.; Benjumea, P.; Riffel, D.B. Sorption of asphaltenes onto nanoparticles of nickel oxide supported on nanoparticulated silica gel. Energy Fuels 2012, 26, 1725–1730. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Eshraghi, S.E.; Kazemi, K.; Sourani, S.; Mehrabi, M.; Ahmadi, Y. Behavior of asphaltene adsorption onto the metal oxide nanoparticle surface and its effect on heavy oil recovery. Ind. Eng. Chem. Res. 2015, 54, 233–239. [Google Scholar] [CrossRef]
- Shojaati, F.; Riazi, M.; Mousavi, S.H.; Derikvand, Z. Experimental investigation of the inhibitory behavior of metal oxides nanoparticles on asphaltene precipitation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 99–110. [Google Scholar] [CrossRef]
- Mohammadi, M.; Akbari, M.; Fakhroueian, Z.; Bahramian, A.; Azin, R.; Arya, S. Inhibition of asphaltene precipitation by TiO2, SiO2, and ZrO2 nanofluids. Energy Fuels 2011, 25, 3150–3156. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Comparative oxidation of adsorbed asphaltenes onto transition metal oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 145–149. [Google Scholar] [CrossRef]
- Tarboush, B.J.A.; Husein, M.M. Adsorption of asphaltenes from heavy oil onto in situ prepared NiO nanoparticles. J. Colloid Interface Sci. 2012, 378, 64–69. [Google Scholar] [CrossRef]
- Shayan, N.N.; Mirzayi, B. Adsorption and removal of asphaltene using synthesized maghemite and hematite nanoparticles. Energy Fuels 2015, 29, 1397–1406. [Google Scholar] [CrossRef]
- Zabala Romero, R.D.; Acuna, H.M.; Cortes, F.; Patino, J.E.; Cespedes Chavarro, C.; Mora, E.; Botero, O.F.; Guarin, L. Application and evaluation of a nanofluid containing nanoparticles for asphaltenes inhibition in well CPSXL4. In Proceedings of the OTC Brasil, Rio de Janeiro, Brazil, 29–31 October 2013. [Google Scholar]
- Al-Jabari, M.; Nassar, N.; Husein, M. Separation of asphaltenes from heavy oil model-solutions by adsorption on colloidal magnetite nanoparticles. In Proceeding of the International Congress of Chemistry Environment (3rd ICCE), Kuwait City, Kuwait, 18–20 November 2007. [Google Scholar]
- Hosseinpour, N.; Khodadadi, A.A.; Bahramian, A.; Mortazavi, Y. Asphaltene adsorption onto acidic/basic metal oxide nanoparticles toward in situ upgrading of reservoir oils by nanotechnology. Langmuir 2013, 29, 14135–14146. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Sun, Q.; Lan, W.; Guo, X. Effect of nanoparticles on asphaltene aggregation in a microsized pore. Ind. Eng. Chem. Res. 2018, 57, 9009–9017. [Google Scholar] [CrossRef]
- Azizkhani, A.; Gandomkar, A. A novel method for application of nanoparticles as direct asphaltene inhibitors during miscible CO2 injection. J. Pet. Sci. Eng. 2020, 185, 106661. [Google Scholar] [CrossRef]
- Varamesh, A.; Hosseinpour, N. Prediction of asphaltene precipitation in reservoir model oils in the presence of Fe3O4 and NiO nanoparticles by cubic plus association equation of state. Ind. Eng. Chem. Res. 2019, 58, 4293–4302. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Fan, W.; Zhang, X.; Lv, Q. Nanoparticles for inhibition of asphaltenes deposition during CO2 flooding. Ind. Eng. Chem. Res. 2016, 55, 6723–6733. [Google Scholar] [CrossRef]
- Ezeonyeka, N.L.; Hemmati-Sarapardeh, A.; Husein, M.M. Asphaltenes adsorption onto metal oxide nanoparticles: A critical evaluation of measurement techniques. Energy Fuels 2018, 32, 2213–2223. [Google Scholar] [CrossRef]
- Nassar, N.N.; Betancur, S.; Acevedo, S.C.; Franco, C.A.; Corte, F.B. Development of a population balance model to describe the influence of shear and nanoparticles on the aggregation and fragmentation of asphaltene aggregates. Ind. Eng. Chem. Res. 2015, 54, 8201–8211. [Google Scholar] [CrossRef]
- Tarboush, B.J.A.; Husein, M.M. Dispersed Fe2O3 nanoparticles preparation in heavy oil and their uptake of asphaltenes. Fuel Process. Technol. 2015, 133, 120–127. [Google Scholar] [CrossRef]
- Hashemi, S.I.; Fazelabdolabadi, B.; Moradi, S.; Rashidi, A.M.; Shahrabadi, A.; Bagherzadeh, H. On the application of NiO nanoparticles to mitigate in situ asphaltene deposition in carbonate porous matrix. Appl. Nanosci. 2016, 6, 71–81. [Google Scholar] [CrossRef]
- Betancur, S.; Carmona, J.C.; Nassar, N.N.; Franco, C.A.; Corte, F.B. Role of particle size and surface acidity of silica gel nanoparticles in inhibition of formation damage by asphaltene in oil reservoirs. Ind. Eng. Chem. Res. 2016, 55, 6122–6132. [Google Scholar] [CrossRef]
- Amin, F.; Nazar, A.R.S. Assessing the asphaltene adsorption on metal oxide nanoparticles. Iran. J. Oil Gas Sci. Technol. 2016, 5, 62–72. [Google Scholar]
- Hosseini-Dastgerdi, Z.; Maleki, A.; Elyassi, E.; Rashidi, A. Silica/polyacrylamide nanocomposite for inhibition of asphaltene precipitation from unstable crude oils. Pet. Sci. Technol. 2022, 1–19. [Google Scholar] [CrossRef]
- Lopez, D.; Giraldo, L.J.; Lucas, E.F.; Riazi, M.; Franco, C.A.; Cortes, F.B. Cardanol/SiO2 nanocomposites for inhibition of formation damage by asphaltene precipitation/deposition in light crude oil reservoirs. Part I: Novel nanocomposite design based on SiO2–cardanol interactions. Energy Fuels 2020, 34, 7048–7057. [Google Scholar] [CrossRef]
- Bagherpour, S.; Riazi, M.; Riazi, M.; Cortés, F.B.; Mousavi, S.H. Investigating the performance of carboxylate-alumoxane nanoparticles as a novel chemically functionalized inhibitor on asphaltene precipitation. ACS Omega 2020, 5, 16149–16164. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi Alemi, F.; Mousavi Dehghani, S.A.; Rashidi, A.; Hosseinpour, N.; Mohammadi, S. Potential application of Fe2O3 and functionalized SiO2 nanoparticles for inhibiting asphaltene precipitation in live oil at reservoir conditions. Energy Fuels 2021, 35, 5908–5924. [Google Scholar] [CrossRef]
- Marana, A.L.O.; Martin, C.A.G.; Montes-Páez, E.; Junior, O.H.A. Modelling and simulation of a thermoelectric waste heat recovery system–TWRHS. Dyna 2021, 88217, 265–272. [Google Scholar] [CrossRef]
- Gandomkar, A.; Nasriani, H.R. The role of direct asphaltene inhibitors on asphaltene stabilization during gas injection. Fuel 2020, 282, 118827. [Google Scholar] [CrossRef]
- Guerrero-Martin, C.A.; Guerrero-Martin, L.E.; Szklo, A. Mitigation options to control greenhouse gas emissions in a colombian oil field. In Proceedings of the SPE International Conference and Exhibition on Health, Safety, Environment, and Sustainability, Virtual, 27–31 July 2020. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Parak, W.J.; Kanaras, A.G. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Shen, B.; Sun, S. Chemical synthesis of magnetic nanoparticles for permanent magnet applications. Chem.–Eur. J. 2020, 26, 6757–6766. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.D.; Shahriar, S.M.; Ahammad, A.S.; Shim, Y.Y.; Reaney, M.; Cho, J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Mesbah, M.; Igwegbe, C.A.; Ezeliora, C.D.; Osagie, C.; Khan, N.A.; Salari, M.; Dehghani, M.H. Sono electro-chemical synthesis of LaFeO3 nanoparticles for the removal of fluoride: Optimization and modeling using RSM, ANN and GA tools. J. Environ. Chem. Eng. 2021, 9, 105320. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Uddin, M.T.; Islam, M.A.; Awual, M.R.; Rahman, M.M. One-step wet-chemical synthesis of ternary ZnO/CuO/Co3O4 nanoparticles for sensitive and selective melamine sensor development. New J. Chem. 2019, 43, 4849–4858. [Google Scholar] [CrossRef]
- Mahhouti, Z.; El Moussaoui, H.; Mahfoud, T.; Hamedoun, M.; El Marssi, M.; Lahmar, A.; El Kenz, A.; Benyoussef, A. Chemical synthesis and magnetic properties of monodisperse cobalt ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 14913–14922. [Google Scholar] [CrossRef]
- Benhammada, A.; Trache, D.; Kesraoui, M.; Tarchoun, A.F.; Chelouche, S.; Mezroua, A. Synthesis and characterization of α-Fe2O3 nanoparticles from different precursors and their catalytic effect on the thermal decomposition of nitrocellulose. Thermochim. Acta 2020, 686, 178570. [Google Scholar] [CrossRef]
- Varanda, L.C.; Souza, C.G.; Moraes, D.A.; Neves, H.R.; Souza Junior, J.B.; Silva, M.F.; Silva, T.L.; Beck Junior, W. Size and shape-controlled nanomaterials based on modified polyol and thermal decomposition approaches. A brief review. An. Acad. Bras. Ciências 2019, 91, e20181180. [Google Scholar] [CrossRef]
- Abdelghany, M.M.; Ahmed, I.S.; Dessouki, H.A.; Abdelrahman, E.A. Facile synthesis of CuO and Ag nanoparticles by thermal decomposition of novel Schiff base complexes. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4281–4299. [Google Scholar] [CrossRef]
- Abakumov, M.; Nizamov, T.; Yanchen, L.; Shchetinin, I.; Savchenko, A.; Zhukov, D.; Majouga, A. Versatile seed-mediated method of CoxFe3-xO4 nanoparticles synthesis in glycol media via thermal decomposition. Mater. Lett. 2020, 276, 128210. [Google Scholar] [CrossRef]
- Shahjuee, T.; Masoudpanah, S.M.; Mirkazemi, S.M. Thermal decomposition synthesis of MgFe 2 O 4 nanoparticles for magnetic hyperthermia. J. Supercond. Nov. Magn. 2019, 32, 1347–1352. [Google Scholar] [CrossRef]
- Ramalingam, V.; Harshavardhan, M.; Kumar, S.D. Wet chemical mediated hematite α-Fe2O3 nanoparticles synthesis: Preparation, characterization and anticancer activity against human metastatic ovarian cancer. J. Alloy. Compd. 2020, 834, 155118. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Yousuf, W.E.; Mohammed, A.A.; Mohammed, R.S.; Darwish, D.B.; Abdeen, E.E. Comparative study between zinc oxide nanoparticles synthesis by biogenic and wet chemical methods in vivo and in vitro against Staphylococcus aureus. Microb. Pathog. 2020, 147, 104384. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Vocciante, M.; Salerno, M.; Caratto, V.; Fabiano, B. Bi Nanoparticles Synthesis by a Bottom-up Wet Chemical Process. CET J. Chem. Eng. Trans. 2019, 73. [Google Scholar] [CrossRef]
- Garcia, P.R.; Prymak, O.; Grasmik, V.; Pappert, K.; Wlysses, W.; Otubo, L.; Epple, M.; Oliveira, C.L. An in situ SAXS investigation of the formation of silver nanoparticles and bimetallic silver–gold nanoparticles in controlled wet-chemical reduction synthesis. Nanoscale Adv. 2020, 2, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Martin, C.A.; Fernández-Ramírez, J.S.; Arturo-Calvache, J.E.; Milquez-Sanabria, H.A.; da Silva Fernandes, F.A.; Costa Gomes, V.J.; Lucas, E.F. Exergy Load Distribution Analysis Applied to the Dehydration of Ethanol by Extractive Distillation. Energies 2023, 16, 3502. [Google Scholar] [CrossRef]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion synthesis of superparamagnetic nanoparticles for bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef]
- Asab, G.; Zereffa, E.A.; Abdo Seghne, T. Synthesis of silica-coated Fe3O4 nanoparticles by microemulsion method: Characterization and evaluation of antimicrobial activity. Int. J. Biomater. 2020, 9, 2020. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Saberi, A.H.; McClements, D.J.; Lin, M. Microemulsions as nanoreactors for synthesis of biopolymer nanoparticles. Trends Food Sci. Technol. 2019, 86, 118–130. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.A.; Moreno-Trejo, M.B.; Meléndez-Zaragoza, M.J.; Collins-Martínez, V.; López-Ortiz, A.; Martínez-Guerra, E.; Sánchez-Domínguez, M. Spinel-type ferrite nanoparticles: Synthesis by the oil-in-water microemulsion reaction method and photocatalytic water-splitting evaluation. Int. J. Hydrogen Energy 2019, 44, 12421–12429. [Google Scholar] [CrossRef]
- Bibi, I.; Maqbool, H.; Iqbal, S.; Majid, F.; Kamal, S.; Alwadai, N.; Iqbal, M. La1-xGdxCr1-yNiyO3 perovskite nanoparticles synthesis by micro-emulsion route: Dielectric, magnetic and photocatalytic properties evaluation. Ceram. Int. 2021, 47, 5822–5831. [Google Scholar] [CrossRef]
- Ren, D.; Xu, J.; Chen, N.; Ye, Z.; Li, X.; Chen, Q.; Ma, S. Controlled synthesis of mesoporous silica nanoparticles with tunable architectures via oil-water microemulsion assembly process. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125773. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: A review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Díaz de Greñu, B.; de Los Reyes, R.; Costero, A.M.; Amorós, P.; Ros-Lis, J.V. Recent progress of microwave-assisted synthesis of silica materials. Nanomaterials 2020, 10, 1092. [Google Scholar] [CrossRef]
- Kheradmandfard, M.; Minouei, H.; Tsvetkov, N.; Vayghan, A.K.; Kashani-Bozorg, S.F.; Kim, G.; Hong, S.; Kim, D.E. Ultrafast green microwave-assisted synthesis of high-entropy oxide nanoparticles for Li-ion battery applications. Mater. Chem. Phys. 2021, 262, 124265. [Google Scholar] [CrossRef]
- Ayinde, W.B.; Gitari, W.M.; Samie, A. Optimization of microwave-assisted synthesis of silver nanoparticle by Citrus paradisi peel and its application against pathogenic water strain. Green Chem. Lett. Rev. 2019, 12, 225–234. [Google Scholar] [CrossRef]
- Nano-Por-un-Dia. Métodos de Nanofabricación. ed. Fundación Argentina de Nanotecnologia 2016. Available online: https://www.nanoporundia.org/estudiantes/ (accessed on 14 January 2023).
- Montes, D.; Cortés, F.B.; Franco, C.A. Reduction of heavy oil viscosity through ultrasound cavitation assisted by NiO nanocrystals-functionalized SiO2 nanoparticles. Dyna 2018, 85, 153–160. [Google Scholar] [CrossRef]
- Montes, D.; Henao, J.; Taborda, E.A.; Gallego, J.; Corte, F.B.; Franco, C.A. Effect of Textural Properties and Surface Chemical Nature of Silica Nanoparticles from Different Silicon Sources on the Viscosity Reduction of Heavy Crude Oil. ACS Omega 2020, 5, 5085–5097. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Jung, K.Y.; Park, S.B. Enhanced photoactivity of silica-embedded titania particles prepared by sol–gel process for the decomposition of trichloroethylene. Appl. Catal. B Environ. 2000, 25, 249–256. [Google Scholar] [CrossRef]
- Montes, D.; Orozco, W.; Taborda, E.A.; Franco, C.A.; Cortés, F.B. Development of nanofluids for perdurability in viscosity reduction of extra-heavy oils. Energies 2019, 12, 1068. [Google Scholar] [CrossRef]
- Montes, D.; Taborda, E.A.; Minale, M.; Cortés, F.B.; Franco-Ariza, C.A. Effect of the NiO/SiO2 Nanoparticles-Assisted Ultrasound Cavitation Process on the Rheological Properties of Heavy Crude Oil: Steady State Rheometry and Oscillatory Tests. Energy Fuels 2019, 33, 9671–9680. [Google Scholar] [CrossRef]
- Taborda, E.A.; Alvarado, V.; Cortés, F.B. Effect of SiO2-based nanofluids in the reduction of naphtha consumption for heavy and extra-heavy oils transport: Economic impacts on the Colombian market. Energy Convers. Manag. 2017, 148, 30–42. [Google Scholar] [CrossRef]
- Cortés, F.B.; Montoya, T.; Acevedo, S.; Nassar, N.N.; Franco, C.A. Adsorption-desorption of n-c7 asphaltenes over micro- and nanoparticles of silica and its impact on wettability alteration. CTF Cienc. Tecnol. Y Futuro 2016, 6, 89–106. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.-T.; Lin, V.S.-Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol–gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal-Fontal, J.E.; Cortés, F.B.; Franco, C.A. Viscosity reduction of extra heavy crude oil by magnetite nanoparticle-based ferrofluids. Adsorpt. Sci. Technol. 2017, 36, 23–45. [Google Scholar] [CrossRef]
- Betancur, S.; Franco, C.A.; Cortés, F.B. Magnetite-silica nanoparticles with a core-shell structure for inhibiting the formation damage caused by the precipitation/deposition of asphaltene. J. Magnetohydrodyn. Plasma Res. 2016, 21, 289–322. [Google Scholar]
- Franco, C.; Patiño, E.; Benjumea, P.; Ruiz, M.A.; Cortés, F.B. Kinetic and thermodynamic equilibrium of asphaltenes sorption onto nanoparticles of nickel oxide supported on nanoparticulated alumina. Fuel 2013, 105, 408–414. [Google Scholar] [CrossRef]
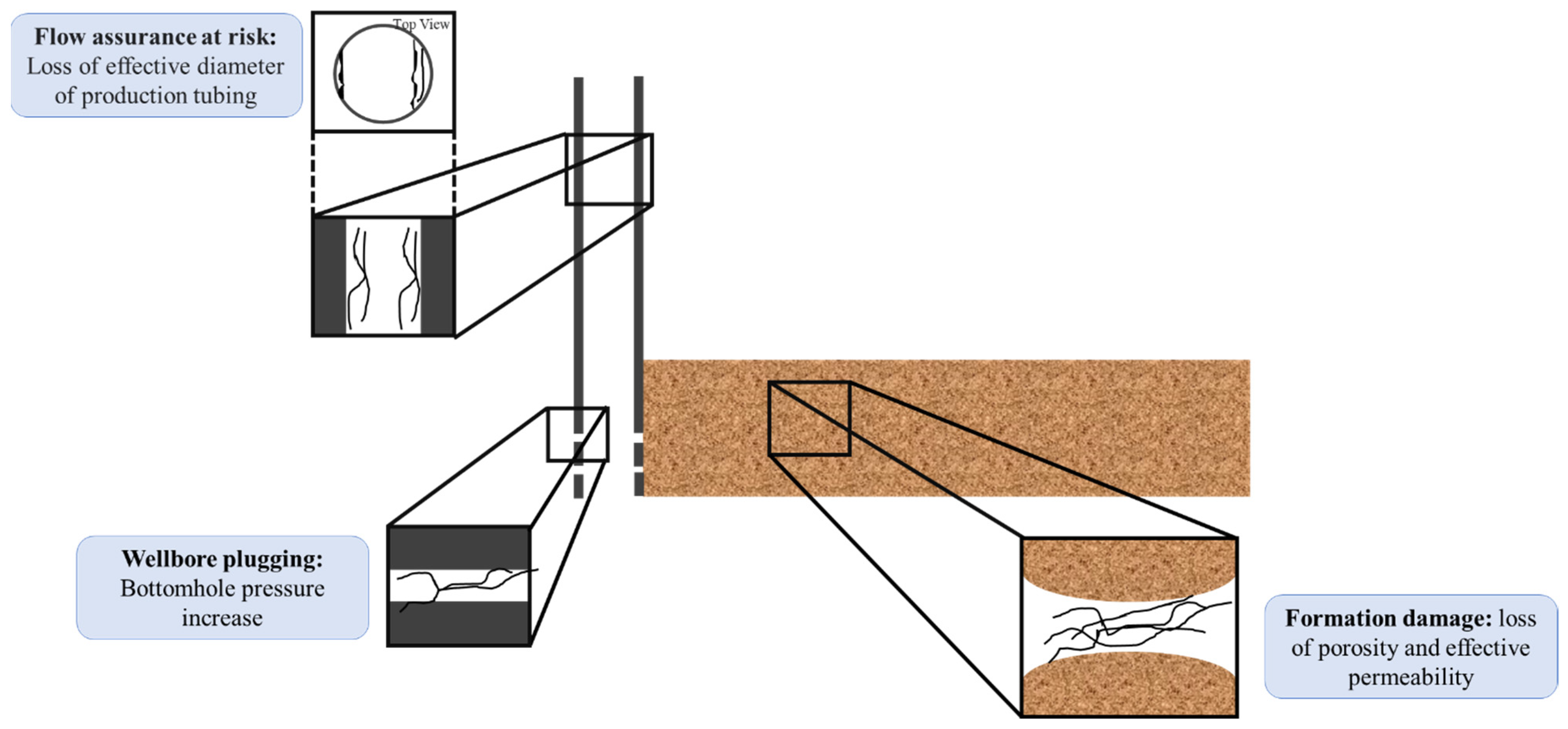
- Bennion, D.B. An overview of formation damage mechanisms causing a reduction in the productivity and injectivity of oil and gas producing formations. J. Can. Pet. Technol. 2002, 41, PETSOC-02-11-DAS. [Google Scholar] [CrossRef]
- Acevedo, S.; Cordero, T.J.M.; Carrier, H.; Bouyssiere, B.; Lobinski, R. Trapping of paraffin and other compounds by asphaltenes detected by laser desorption ionization–time of flight mass spectrometry (LDI–TOF MS): Role of A1 and A2 asphaltene fractions in this trapping. Energy Fuels 2009, 23, 842–848. [Google Scholar] [CrossRef]
- Acevedo, S.; Castro, A.; Vásquez, E.; Marcano, F.; Ranaudo, M.A.A. Investigation of physical chemistry properties of asphaltenes using solubility parameters of asphaltenes and their fractions A1 and A2. Energy Fuels 2010, 24, 5921–5933. [Google Scholar] [CrossRef]
- Acevedo, S.C.; Castillo, J.; Vargas, V.; Castro, A.; Delgado, O.; Corte, F.B.; Bouyssiere, B. Suppression of phase separation as a hypothesis to account for nuclei or nanoaggregate formation by asphaltenes in Toluene. Energy Fuels 2018, 32, 6669–6677. [Google Scholar] [CrossRef]
- Acevedo, S.; Guzman, K.; Ocanto, O. Determination of the number average molecular mass of asphaltenes (M n) using their soluble A2 fraction and the vapor pressure osmometry (VPO) technique. Energy Fuels 2010, 24, 1809–1812. [Google Scholar] [CrossRef]
- Torkaman, M.; Bahrami, M.; Dehghani, M.R. Influence of temperature on aggregation and stability of asphaltenes. II. Orthokinetic aggregation. Energy Fuels 2018, 32, 6144–6154. [Google Scholar] [CrossRef]
- Rahmani, N.H.; Masliyah, J.H.; Dabros, T. Characterization of asphaltenes aggregation and fragmentation in a shear field. AIChE J. 2003, 49, 1645–1655. [Google Scholar] [CrossRef]
- Soler, C.A.C.; Malagueta, D.C.; Martin, C.A.G. Feasibility of implementation of solar thermal energy in steam-assisted gravity drainage (SAGD) in extra-heavy oil field in Colombia. Geoenergy Sci. Eng. 2023, 222, 211463. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene adsorption, a literature review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Montes, D. Upscaling Process for Nanofluids Usage for Heavy Oil Mobility Enhancement: Experimental Design and Field Trial Application. In Proceedings of the SPE Annual Technical Conference and Exhibition, Virtual, 27–29 October 2020. [Google Scholar]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Effect of the particle size on asphaltene adsorption and catalytic oxidation onto alumina particles. Energy Fuels 2011, 25, 3961–3965. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Application of nanotechnology for heavy oil upgrading: Catalytic steam gasification/cracking of asphaltenes. Energy Fuels 2011, 25, 1566–1570. [Google Scholar] [CrossRef]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Pereira-Almao, P.; Corte, F.B. Adsorption and subsequent oxidation of colombian asphaltenes onto nickel and/or palladium oxide supported on fumed silica nanoparticles. Energy Fuels 2013, 27, 7336–7347. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Arias-Madrid, D.; Cortés, F.B.; Franco, C.A. Optimization of the load of transition metal oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 nanoparticles in catalytic steam decomposition of n-C7 asphaltenes at low temperatures. Nanomaterials 2019, 9, 401. [Google Scholar] [CrossRef]
- Acevedo, S.; Ranaudo, M.A.; Escobar, G.; Gutiérrez, L.; Ortega, P. Adsorption of asphaltenes and resins on organic and inorganic substrates and their correlation with precipitation problems in production well tubing. Fuel 1995, 74, 595–598. [Google Scholar] [CrossRef]
- Guzma, J.D.; Betancur, S.; Carrasco-Marín, F.; Franco, C.A.; Nassar, N.N.; Corte, F.B. Importance of the adsorption method used for obtaining the nanoparticle dosage for asphaltene-related treatments. Energy Fuels 2016, 30, 2052–2059. [Google Scholar] [CrossRef]
- Franco, C.A.; Lozano, M.M.; Acevedo, S.; Nassar, N.N.; Corte, F.B. Effects of resin I on asphaltene adsorption onto nanoparticles: A novel method for obtaining asphaltenes/resin isotherms. Energy Fuels 2015, 30, 264–272. [Google Scholar] [CrossRef]
- Medina, O.E.; Olmos, C.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Nanotechnology Applied to Thermal Enhanced Oil Recovery Processes: A Review. Energies 2019, 12, 4671. [Google Scholar] [CrossRef]
- Nassar, N.N. Asphaltene adsorption onto alumina nanoparticles: Kinetics and thermodynamic studies. Energy Fuels 2010, 24, 4116–4122. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Luna, G.; Pereira-Almao, P. Kinetics of the catalytic thermo-oxidation of asphaltenes at isothermal conditions on different metal oxide nanoparticle surfaces. Catal. Today 2013, 207, 127–132. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Montoya, T.; Coral, D.; Franco, C.A.; Nassar, N.N.; Corte, F.B. A novel solid–liquid equilibrium model for describing the adsorption of associating asphaltene molecules onto solid surfaces based on the “Chemical Theory”. Energy Fuels 2014, 28, 4963–4975. [Google Scholar] [CrossRef]
- British Petroleum Company. Bp Statistical Review of World Energy, 71st ed.; BP p.l.c.: London, UK, 2022. [Google Scholar]
- Lyu, L.; Lingjia, F. A Study on EC Translation of BP Statistical Review of World Energy 2022 from the Perspective of Schema Theory. J. Linguist. Commun. Stud. 2023, 2, 10–14. [Google Scholar] [CrossRef]
- Moser, T. MNCs and sustainable business practice: The case of the Colombian and Peruvian petroleum industries. World Dev. 2001, 29, 291–309. [Google Scholar] [CrossRef]
- Ochoa, M.A.M.; Viloria, A.; Nieto, G.Y.C.; Ramírez, M.C. The Management of the Oil Sector as a Factor of Socioeconomic Control in Two Neighboring Countries: Colombia and Venezuela. Int. J. Control. Theory Appl. 2016, 9. Available online: https://d1wqtxts1xzle7.cloudfront.net/63976275/The_Management_of_the_Oil_Sector_as_a_Factor_of_Socioeconomic_RRR20200720-26542-w7ckjl-libre.pdf?1595293786=&response-content-disposition=inline%3B+filename%3DThe_Management_of_the_Oil_Sector_as_a_Fa.pdf&Expires=1687130744&Signature=HReW~iyXEeKVE~sRS3aw2navxwNM6mHCWcoILH3ou~SdulJuOd5m0F6-AIwLhN23u0qRifekXDVX6x1zIPmT9jbcEA2SPZ-WfET06iJH3HbjJggAik7jUiaXsrdQSL9J5Xwu12Ug6iOjm~i~zAZt~4fE61U23rZO~t1eSL3rFNfHAMBvhzIK3QiCzo0~I4jD1hFjb5TcNaMlM~54DDE5R2QjujuEcXsptIkHUB41oCZH~f~7ExmZzSTq3CavTKYsqDLLcGuVTI-GawtA53NW8dJMOxouDsal2VQ9ieNyi5klg8CKS2SNRLXdB4OdDfZ2tZEFg6xUpTHH5O-jmZstUg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 23 January 2023).
- Dhuldhoya, K.; Dusterhoft, R. Investigation of the Economic Impact of Parent Well Protection Treatments in Unconventional Plays. In Proceedings of the SPE Europec featured at 79th EAGE Conference and Exhibition, Paris, France, 12–15 June 2017. [Google Scholar]
- Rodriguez, J.; Joonghyeok, H.; Kee, H.K. The impact of hydraulic fracturing on groundwater quality in the Permian Basin, west Texas, USA. Water 2020, 12, 796. [Google Scholar] [CrossRef]
- Kuuskraa, V.; Stevens, S.H.; Moodhe, K.D. Technically Recoverable Shale Oil and Shale Gas Resources: An Assessment of 137 Shale Formations in 41 Countries Outside the United States; US Energy Information Administration, US Department of Energy: Washington, DC, USA, 2013. Available online: https://www.eia.gov/analysis/studies/worldshalegas/pdf/overview.pdf (accessed on 16 April 2023).
- Moreno-Enriquez, A.; Vargas-Silva, D.; Gambús-Ordaz, M.; Calderón-Carrillo, Z.; Robles-Albarracín, E. Assessment of the original gas volume in situ in unconventional gas-shale reservoirs through multiple models worldwide and its analogy to a Colombian formation. Boletín Geol. 2022, 44, 109–123. [Google Scholar] [CrossRef]
- Forero, C.A.; Forero, E.J.; Guerrero-Martin, L.; Szklo, A.; Rochedo, P.R.; Guerrero-Martin, C. Technical and economic assessment of the development of a Colombian Tight Oil reservoir: A simulation case study of Valle Medio del Magdalena basin. Dyna 2021, 88, 35–43. [Google Scholar] [CrossRef]
- Isaac, O.T.; Pu, H.; Oni, B.A.; Samson, F.A. Surfactants employed in conventional and unconventional reservoirs for enhanced oil recovery—A review. Energy Rep. 2022, 8, 2806–2830. [Google Scholar] [CrossRef]
- Gupta, I.; Rai, C.; Devegowda, D.; Sondergeld, C.H. Fracture hits in unconventional reservoirs: A critical review. SPE J. 2021, 26, 412–434. [Google Scholar] [CrossRef]
- Aslannezhad, M.; Kalantariasl, A.; You, Z.; Iglauer, S.; Keshavarz, A. Micro-proppant placement in hydraulic and natural fracture stimulation in unconventional reservoirs: A review. Energy Rep. 2021, 7, 8997–9022. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S. Insights to fracture stimulation design in unconventional reservoirs based on machine learning modeling. J. Pet. Sci. Eng. 2019, 174, 682–695. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, K.; Jin, G.; Moridis, G.; Kerr, E.; Scofield, R.; Johnson, A. Fracture-hit detection using LF-DAS signals measured during multifracture propagation in unconventional reservoirs. SPE Reserv. Eval. Eng. 2021, 24, 523–535. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Idris, A.K.; Chauhan, P.S. Nanoparticles applications for hydraulic fracturing of unconventional reservoirs: A comprehensive review of recent advances and prospects. J. Pet. Sci. Eng. 2019, 178, 41–73. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Malayeri, M.; Riazi, M.; Parsaei, R. Impact of Fe3O4 nanoparticles on asphaltene precipitation during CO2 injection. J. Nat. Gas Sci. Eng. 2015, 22, 227–234. [Google Scholar] [CrossRef]
- Zanganeh, P.; Ayatollahi, S.; Alamdari, A.; Zolghadr, A.; Dashti, H.; Kord, S. Asphaltene deposition during CO2 injection and pressure depletion: A visual study. Energy Fuels 2012, 26, 1412–1419. [Google Scholar] [CrossRef]
- Zabala, R.; Mora, E.; Botero, O.; Cespedes, C.; Guarin, L.; Franco, C.; Ospina, N. Nano-technology for asphaltenes inhibition in Cupiagua South Wells. In Proceedings of the IPTC 2014: International Petroleum Technology Conference, Doha, Qatar, 19–22 January 2014. [Google Scholar]
- Cano, N.A.; Céspedes-Zuluaga, S.; Guerrero-Martin, C.; Gallego, D. Exergy and emergy: Complementary tools for assessing the environmental sustainability use of biosolids generated in wastewater-treatment plant for energy-production. Química Nova 2022, 45, 4–15. [Google Scholar] [CrossRef]
- Alemi, F.M.; Mohammadi, S.; Dehghani, S.A.M.; Rashidi, A.; Hosseinpour, N.; Seif, A. Experimental and DFT studies on the effect of carbon nanoparticles on asphaltene precipitation and aggregation phenomena. Chem. Eng. J. 2021, 422, 130030. [Google Scholar] [CrossRef]
- Enayat, S.; Safa, M.A.; Tavakkoli, M.; Valdes, H.; Rashed, A.M.; Ghloum, E.F.; Santhanagopalan, S.; Vargas, F.M. Novel nanoparticle-based formulation to mitigate asphaltene deposition. Energy Fuels 2021, 35, 12974–12981. [Google Scholar] [CrossRef]
- Ngouangna, E.N.; Jaafar, M.Z.; Norddin, M.M.; Agi, A.; Oseh, J.O.; Mamah, S. Surface modification of nanoparticles to improve oil recovery Mechanisms: A critical review of the methods, influencing Parameters, advances and prospects. J. Mol. Liq. 2022, 360, 119502. [Google Scholar] [CrossRef]
- Sanjuan Navarro, L. Nanomaterials: Development of New Analysis Strategies. Ph.D. Thesis, Chemical Doctorate Program. Universidad de Valencia, Valencia, Spain, 2022. Available online: https://roderic.uv.es/bitstream/handle/10550/83930/TESIS%20LORENZO%20SANJUAN%20NAVARRO-SIGNED%20%281%29.pdf?sequence=1&isAllowed=y (accessed on 22 December 2022).
- Khidhir, D.; Sidiq, H. Efficacy of Green Oxide Nanofluids as Potential Dispersants for Asphaltene in Iraqi Crudes, Experimental, Tunning and Statistical Analysis. Energies 2022, 15, 6833. [Google Scholar] [CrossRef]
- Negi, H.; Singh, R.K. Chitosan-based amphiphilic compound synthesis and its use as an asphaltene dispersant and viscosity modifier. Waste Biomass Valorization 2023, 14, 781–794. [Google Scholar] [CrossRef]
- Sircar, A.; Rayavarapu, K.; Bist, N.; Yadav, K.; Singh, S. Applications of nanoparticles in enhanced oil recovery. Pet. Res. 2022, 7, 77–90. [Google Scholar] [CrossRef]
- Küçük, F.S.; Küçük, K.; Temizel, C. Importance and emergence of advanced materials in energy industry. In Sustainable Materials for Oil and Gas Applications (1–25); Gulf Professional Publishing: Cambridge, MA, USA; Kidlington, UK, 2021. [Google Scholar]
- Sandeep, R.; Jain, S.; Agrawal, A. Application of nanoparticles-based technologies in the oil and gas industry. In Nanotechnology for Energy and Environmental Engineering; Green Energy and Technology; Springer: Cham, Switzerland, 2020; pp. 257–277. [Google Scholar] [CrossRef]
- Unal, D.N.; Sadak, S.; Uslu, B. A Review on Electrochemical and Optical Sensing Platform Based on Ionic Liquids for Different Molecules Determination. Crit. Rev. Anal. Chem. 2023, 53, 798–824. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Experimental and field applications of nanotechnology for enhanced oil recovery purposes: A review. Fuel 2022, 324, 124669. [Google Scholar] [CrossRef]
- Mehra, D.; Rawat, P.; Kashyap, S.; Singh, D.; Pandey, G. Role of nanoparticles and their applications in enhanced oil recovery. In Proceedings of the AIP Conference Proceedings (2521, No. 1), Dehradun, India, 6–7 August 2021; AIP Publishing: Dehradun, India, 2023. [Google Scholar]
- Schuler, B.; Zhang, Y.; Liu, F.; Pomerantz, A.E.; Andrews, A.B.; Gross, L.; Banerjee, S.; Mullins, O.C. Overview of asphaltene nanostructures and thermodynamic applications. Energy Fuels 2020, 34, 15082–15105. [Google Scholar] [CrossRef]
- Hosseini-Dastgerdi, Z.; Meshkat, S.S. An experimental and modeling study of asphaltene adsorption by carbon nanotubes from model oil solution. J. Pet. Sci. Eng. 2019, 174, 1053–1061. [Google Scholar] [CrossRef]
- Satdive, A.; Tayde, S.; Toksha, B.; Kundu, D.; Naik, J.; Hazra, C.; Joshi, S.; Chatterjee, A. Superhydrophobic Hybrid Nanocomposites: Mapping the Current Research Trends and Recent Advances. Chem. Eng. Sci. 2023, 278, 118941. [Google Scholar]
- Sfameni, S.; Rando, G.; Marchetta, A.; Scolaro, C.; Cappello, S.; Urzì, C.; Visco, S.; Plutino, M.R. Development of eco-friendly hydrophobic and fouling-release coatings for blue-growth environmental applications: Synthesis, mechanical characterization and biological activity. Gels 2022, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Abdrabou, M.K.; Han, X.; Zheng, Y.; Zeng, Y.; Rohani, S. The effect of cationic surfactants on bitumen’s viscosity and asphaltene nanostructure under thermal partial upgrading. Energy Sci. Eng. 2022, 10, 2540–2560. [Google Scholar] [CrossRef]
- Lahjiri, F.; Mouillet, V.; Dony, A.; Ziyani, L.; Gazeau, S.; Delfosse, F.; Dieudonne-George, P.; Henn, F. Relationships between Chemical Composition, Asphaltene Nanostructures, and Thermochemical Properties of Bitumen before and after Accelerated Oxidative Aging. Energy Fuels 2023, 37, 8444–8455. [Google Scholar] [CrossRef]
- Tazikeh, S.; Amin, J.S.; Zendehboudi, S.; Shafiei, A. Effects of asphaltene structure and polythiophene-coated magnetite nanoparticles on surface topography and wettability alteration of silica surface. J. Mol. Liq. 2022, 349, 118470. [Google Scholar] [CrossRef]
- Tang, J.; Wang, H. Coarse grained modeling of nanostructure and asphaltene aggregation in asphalt binder using dissipative particle dynamics. Constr. Build. Mater. 2022, 314, 125605. [Google Scholar] [CrossRef]
- de S Araujo, M.V.; Sacramento, A.; Reis, A.N.; Alves, V.F.; da S Oliveira, M.A.; Martin, L.E.G.; Galindo, L.S.C.; Silva, R.S.; Martin, C.A.G. Análisis comparativo de la inyección cíclica de vapor utilizando el modelo de Borberg y Lantz en tres campos en Pará. Fuentes Reventón Energético 2022, 2, 55–63. [Google Scholar] [CrossRef]
- Meshkat, S.S.; Hosseini-Dastgerdi, Z.; Pakniya, F. Asphaltene removal from model oil solution by N doped graphene in a fixed bed column. J. Dispers. Sci. Technol. 2023, 1–8. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Gong, X.; Cui, P.; Wei, H. Stiffening and Toughening of Asphalt Mastic Induced by Bitumen–Mineral Selective Molecular Adsorption and Nanostructural Reconstruction. Sustainability 2023, 15, 4398. [Google Scholar] [CrossRef]
- Guerrero-Martin, C.A.; Montes-Paez, E.G.; Lucas, E.F. Teaching students to estimate industrial behavior from bench scale experiments: Polymer as asphaltenes deposition inhibitor. J. Mater. Educ. 2019, 41, 189–199. [Google Scholar]
- Razman Shah, N.B.B.; Sazali, R.A.B.; Sorbie, K.S.; Khalil, M.; Azizi, A. Nanomaterials for scaling prevention in alkaline–surfactant–polymer flooding: A review. Appl. Nanosci. 2022, 13, 3945–3974. [Google Scholar] [CrossRef]
- Long, Z.; Tang, X.; Ding, Y.; Miljković, M.; Khanal, A.; Ma, W.; You, L.; Xu, F. Influence of sea salt on the interfacial adhesion of bitumen–aggregate systems by molecular dynamics simulation. Constr. Build. Mater. 2022, 336, 127471. [Google Scholar] [CrossRef]




| Authors | Relevance |
|---|---|
| Nellensteyn [36] (1924) | Conceptual outline of the colloidal behavior of asphaltenes. Definition of the bases of the main asphaltene separation method by insolubility in heptane and pentane. |
| Pfeiffer and Saal [37] (1940) | Definition of the micellar structure of asphaltenes in oil. |
| Dickie and Yen [38] (1967) | Justification for the different values of molecular masses of asphaltenes obtained by different techniques, assigning the highest values to the existence of micelles. |
| Wiehe [39] (1994) | Introduction of the idea of compositional continuity of oil and its fractions. |
| Mack [42] (2002) | Relationship between viscosity and concentration of asphaltenes. |
| Mullins [40] (2010) | Improvement of the Yen model (1961). Effectiveness of critical concentration concepts for asphaltene aggregation. |
| Method | Precipitating Agent | Conditions | Rate Solvent/Sample (mL/g) | Methodology |
|---|---|---|---|---|
| ASTM D893 [46] | n-C5 commercial | 65 ± 5 °C. Filter solids with 150 mL n-C5 at room temperature | 10 | Centrifuge at 600–700 rpm for 20 min. To decant until only 3 of solution in the tube. Centrifuge again under the same conditions. Dry at ±105 °C for 30 min. |
| ASTM D2006 [47] | n-C5 commercial | No heating required | 50 | Leave to stand for 15 h, filter, and wash three times with 10 mL of n-C5 in each wash. |
| Bulmer et al. [48] | n-C5 analytical grade and commercial benzene | Heat to dissolve if necessary | 40 mL n-C5 and 1 mL benzene | Dissolve in benzene and heat if necessary. Add n-C5 and shake for 5 min. Leave to stand for 2 h. Filter under vacuum. Wash the balloon where the test was performed. Dry at 105 °C. |
| ASTM D2007 [49] | n-C5 commercial | Requires heating | 10 | Add n-C5 and shake well. Heat for a few seconds until dissolved. Leave to stand for 30 min. Wash with 10–20 mL of n-C5. |
| ASTM 6560 [50] | n-C7 and toluene | Requires reflow | 100 | Add n-C7 and reflux for 1 h. Cool for 1.5 to 2.5 h under light. Filter on Whatman No. 42 paper. Rinse the filter paper with hot n-C7 for 1 h. Keep under reflux with 30–60 mL of toluene in a water bath. Dry at 100–110 °C for 30 min. |
| ASTM D3279 [51] | n-C7 with purity > 99% | Requires reflow | 1000 | Add n-C7 and reflux for 15–20 min. Cool for 1 h. Filter under vacuum. Wash three times with 10 mL of n-C7 in each wash. Dry at 107 °C for 15 min. |
| Pressure | Translucence (%) |
|---|---|
| 580,151 | 110 |
| 290,075 | 123 |
| 145,038 | 143 |
| 72,519 | 161 |
| 36,259 | 190 |
| Authors | Year | Nanoparticle | Summary | Methodology | Experimental Ensamble | Source/Synthesis Method | Results |
|---|---|---|---|---|---|---|---|
| Rezakazemi et al. [101] | 2018 | γ-Al2O3 | Separation of the asphaltene using a ceramic membrane. | Dynamic light scattering (DLS) | Membrane cells | Commercial γ-Al2O3 (size, purity, and the specific surface area are 30 nm, 99.99%, and 90–160 m2/g) | Raman spectroscopy results revealed a significant rise in the estimated asphaltene molecular sheet diameter from 5.4818 to 13.7866 A. |
| The impact of adding alumina nanoparticles on expanding asphaltene’s molecular size. | According to DLS data, the addition of nano-alumina increased the molecular size of asphaltenes from 512.75 nm to 2949.55 nm. | ||||||
| Parsaei et al. [102] | 2020 | Iron oxide | The effect of nanoparticles on asphaltene precipitation was studied by measuring surface tension in the presence of CO2 at different temperatures and pressures. | IFT measurement by using pendant drop method in capillary tubes | Capillary tube | Commercial nanoparticles obtained from US Research Nanomaterials Inc. Houston, TX, USA | The addition of iron oxide nanoparticles to the oil solution reduces the interfacial tension at higher pressures with a steeper slope, showing that nanoparticles can decrease asphaltene precipitation. |
| The presence of nanoparticles reduced the amount of asphaltene that precipitated at 50 °C by 16.34% and at 70 °C by 19.65% depending on the temperature. | |||||||
| Ahmadi, Aminshahidy [103] | 2018 | CaO and SiO2 | The impact of CaO and SiO2 nanoparticle concentration on asphaltene precipitation in the presence of CO2 at different temperatures. | PVT cells to perform natural depletion | PVT cells | SiO2 was bought from Houston Brand company. For the CaO, 10 g CaCO3 was mixed with 5 mL acid solutions of succinic acid, tartaric acid, and citric acid (0.5 g acids dissolved in 5 mL water) and left to rest for 24 h. This mixture was then dried at 100 °C for 2 h. The samples were heated separately for 2 h at 900 °C. | Temperature increased from 90 °C to 100 °C during pressure reduction from 2500 Psi to 1500 Psi. |
| CaO decreased asphaltene precipitation from (0.32 wt%, 0.62 wt%) to (0.096 wt%, 0.214 wt%); SiO2 decreased asphaltene from (0.56 wt%, 1.10 wt.%) to (0.27 wt.%, 0.52 wt.%). | |||||||
| Oliveira et al. [104] | 2013 | Cobalt ferrite | The use of modified cobalt ferrite nanoparticles as a flocculant agent for asphaltenes. | Nanoparticles are annealed in an effort to modify their structural phase. | UV-Vis spectrophotometer Varian | Through the homogeneous precipitation method, cobalt ferrite nanoparticles were created using deionized water, a 2.0 mol/L solution of FeCl3, and a 1.0 mol/L solution of CoCl2. | The system’s asphaltene precipitation is unaffected by the presence of modified nanoparticles, indicating that the particles can help the asphaltene aggregate. |
| Cortés et al. [105] | 2012 | SiNi | Analyze the effect of temperature and NiO content on the asphaltene uptake by a hybrid nanomaterial composed of nickel oxide nanoparticles supported on a nanoparticulated matrix of silica gel. | UV-Vis technique to determine the asphaltene adsorption on the nanoparticles. | Nanoparticulated matrix of silica gel | Silica nanoparticles were synthesized by the sol–gel method following an acid route. The gel was prepared from TEOS (tetraethoxysilane), ethanol, water, and HNO3. | Asphaltene adsorption increased with increasing nickel oxide concentration in the hybrid nanomaterials at constant temperature. Regardless of asphaltene concentration, the hybrid nanomaterials’ ability to absorb asphaltene decreased as the temperature rose. |
| The synthesized nanosilica was impregnated with aqueous solutions of nickel nitrate Ni(NO3)2 in different concentrations (5 and 15 wt%) for 3 h and then dried at 120 °C for 6 h and cured for 6 h at 450 °C. | |||||||
| Kazemzadeh et al. [106] | 2014 | Fe3O4 | Examining the effect of Fe3O4 nanoparticles on asphaltene precipitation | Bond number measurement and IFT measurement using VIT technique. | The high-pressure chamber was made out of a capillary tube at the top. | Commercial nanoparticles. No provider reported. | The intensity of the asphaltene precipitation would be reduced as the the mass fraction of Fe3O4 nanoparticles increased. |
| Shojaati et al. [107] | 2017 | γ-Al2O3 Fe3O4 NiO | The impact of Fe3O4, NiO, and -Al2O3 metal oxide nanoparticles on synthetic oil was explored in this study in order to reduce the danger of asphaltene deposition and postpone the commencement of asphaltene precipitation. | An indirect technique as opposed to other onset measurement techniques. | Test tubes | Nanoparticles obtained from U.S. Research Nanomaterials, Inc., Houston, TX, USA. | Metal oxide nanoparticles showed a great effect on inhibition of asphaltene precipitation and can be applied as an inhibitor. |
| The instability of asphaltenes and the amount of asphaltene deposits were reduced in the presence of nanoparticles, in the following order of effectiveness: γ-Al2O3 > NiO > Fe3O4. | |||||||
| Mohammadi et al. [108] | 2011 | TiO2 ZrO2 SiO2 | Study the effect of metal oxide nanoparticles in organic-based nanofluids for stabilizing asphaltene particles in oil. | Oil titration method, making use of the polarized light microscopy technique, to check their potential in stabilizing or destabilizing asphaltene nanoaggregates. | The titration procedure is performed by gradual and step-by-step addition of n-heptane. Then, precipitation of asphaltene was investigated using a polarized light microscope. | TiO2 Nanoparticles: two different solutions were prepared. | Rutile (TiO2) fine nanoparticles can effectively enhance the asphaltene stability in acidic conditions and act inversely in basic conditions. It was found that the required amount of n-heptane for destabilizing the colloidal asphaltene is considerably higher in the presence of TiO2 nanofluids at pH below 4. FTIR spectroscopy shows the changes in n-heptane-insoluble asphaltenes when acidic nanoliquid TiO2 is used as an inhibitor. According to the results of FTIR spectroscopy, TiO2 nanoparticles can increase the stability of asphaltene nanoaggregates by forming hydrogen bonds in an acidic medium. At this time, the other materials used in this experiment, as well as the TiO2 nanoparticles, are not able to form a hydrogen bond under alkaline conditions; hence, they are not able to prevent the precipitation of asphaltenes. |
| Solution prepared by mixing 10 mL of tetraisopropyl orthotitanate with 25 mL of ethanol and 2 mL of ethylenediamine as template under vigorous stirring. | |||||||
| 3 mL HCl, 20 mL distilled water, and 10 mL ethanol. Then, it was slowly injected into solution 1 under 40–50 °C and stirred for about 4 h. | |||||||
| Zirconium oxychloride (ZrOCl2.8H2O) was used as the Zr source. The stock solution was prepared by mixing the metal salt solution with a solution of 1.5 g urea and 9 mL LNH3 (25 wt%) at a temperature of 60–80 °C and a pH between 9 and 10. As a surfactant, 2 g ethoxylated nonylphenol (20 mol) was added to form a nanoemulsion. | |||||||
| 20 mL of TEOS was dissolved in a mixture of isopropyl alcohol and ethanol and stirred at 50° C. for about 1 h. To this solution were then added 5 mL of ethylene diamine and 3 g of citric acid. The resulting solution was hydrolyzed to 65% by weight HNO3 solution for 2 h with vigorous stirring and then refluxed for 24 h. | |||||||
| Nassar et al. [109] | 2011 | NiO Co3O4 Fe3O4 | Asphaltenes have been investigated for their oxidation onto different types of nanoparticles, namely NiO, Co3O4, and Fe3O4. | The asphaltenes containing nanoparticles were separated by centrifugation. The supernatant was decanted and precipitated. Then, the samples were subjected to thermal analysis for estimating the adsorbed amount of asphaltenes and oxidation. | Batch adsorption experiments | Commercial nanoparticles purchased from Sigma Aldrich. | All tested nanoparticles showed high adsorption affinity and catalytic activity for the adsorption and oxidation of asphaltenes in the following order: NiO > Co3O4 > Fe3O4. The oxidation temperature of asphaltenes decreased by 140, 136, and 100 °C compared to non-catalytic oxidation in the presence of NiO, Co3O4, and Fe3O4 nanoparticles, respectively. |
| Tarboush et al. [110] | 2012 | NiO | Shows that NiO nanoparticles prepared in situ within heavy oil display much higher affinity toward asphaltene adsorption. | Oil characterization, before and after asphaltene adsorption, was conducted using density and viscosity measurements. | Viscosity measurements were determined using a cone–plate Brookfield viscometer model. | Nickel(II) nitrate hexahydrate (99.9985%, Puratronic) was used as the precursor salt. Commercially available nickel oxide (NiO) nanoparticles (dp < 50 nm, 99.8%) were used for comparison. | An asphaltene absorption of 2.8 g asphaltenes/g nanoparticles was reported. Commercial NiO nanoparticles in the same size range exposed to the same experimental conditions adsorbed only 15% of the above value. |
| Shayan and Mirzayi [111] | 2015 | γ-Fe2O3 α-Fe2O3 | Synthesized maghemite (γ-Fe2O3) and hematite (α-Fe2O3) nanoparticles were used for the adsorption and removal of asphaltenes from the prepared solution. | UV-vis spectrophotometer to determine the maximum peak of adsorption for asphaltene. | Batch adsorption experiments | FeCl3 (ferric chloride), FeCl2 4H2O (ferric chloride tetrahydrate), HCl (hydrochloric acid, 37%), ammonium hydroxide (NH4OH, 25% ammonia), methylene blue. | This work showed that the synthesized MNPs and HNPs can be considered as nanoadsorbents of asphaltenes, although MNPs are more efficient. |
| Zabala et al. [112] | 2013 | γ-Al2O3 | Describes the evolution of a fluid containing nanomaterial with high adsorption capacity for asphaltene inhibition. | Upscaling and field trial application | Real in-field conditions | Commercial silica nanoparticles obtained from Petroraza S.A. | Asphaltene content measured in the produced oil increased after the well treatment with the nanofluid containing alumina nanoparticles. |
| Al-Jabari et al. [113] | 2007 | Fe3O4 | Combination of nanoparticle adsorption and magnetic separation for the removal of asphaltenes from heavy oil by adsorption on colloidal magnetite. | Combination of nanoparticle adsorption and magnetic separation | Magnet and UV-Vis spectroscopy | Obtained from Nanostructured & Amorphous Materials, Inc., (130 Benton St, Garland, TX 75042,TX, USA) | Ultra-dispersed magnetite nanoparticles offer several advantages over conventional ones; for example, they provide a large surface of contact, reduce the distance traveled between the adsorbed species and the surface of the solid particles, and are excellent for phase separation with the aid of a magnetic medium. |
| Hosseinpour et al. [114] | 2013 | NiO CaCO3 Fe2O3 WO3 MgO ZrO2 | Three different categories of metal oxide nanoparticles with acidic, amphoteric, and basic surfaces were synthesized, and their textural, structural, and acid–base properties were characterized. Asphaltenes are extracted from the dead heavy oil sample, and their structure, elemental composition, and acid–base number are determined. The nanoparticles are then used to adsorb asphaltenes from asphaltene–toluene solutions. | Centrifugation followed by UV–vis spectroscopy of the supernatant liquid | The nanoparticles were mixed in tightly sealed vials | Precipitation method employed for obtaining the different nanostructures | The adsorption capacity of asphalt nanoparticles is between 1.23 and 3.67 mg/m2 and decreases in the order NiO > Fe2O3 > WO3 > MgO > CaCO3 > ZrO2, which is accompanied by the synergistic effects of acidity and surface charge. |
| Li et al. [115] | 2018 | NiO SiO2 Fe3O4 | Investigated effect of nanoparticles on the inhibition of asphaltene particle aggregation in a water-wet micro-sized pore. | Experimental methodology that directly observed asphaltene aggregation at the pore scale. | Water-wet microsized pore | Commercial nanoparticles. SiO2 nanoparticles (20 nm, ≥99.9%), NiO nanoparticles (40 nm, ≥99.9%), and Fe3O4 nanoparticles (20 nm, ≥99.5%) | The nanoparticles can act as inhibitors of asphaltenes, preventing the aggregation of asphaltenes and increasing the stability of asphaltenes in the microcapillaries. Asphaltene particles can easily aggregate with each other in the absence of nanoparticles. On the other hand, the presence of nanoparticles can prevent asphaltene particles from flocculating. This could be mainly due to the high surface area to volume ratio, good adsorption capacity, and high degree of suspension of the nanoparticles. |
| Azizkhani et al. [116] | 2019 | Fe3O4 γ-Al2O3 | Focused on the asphaltene precipitation by liquid-free asphaltene inhibitors at reservoir conditions. | The vanishing interfacial tension technique was implemented to evaluate the effect of the nanoparticles on minimum miscibility pressure. | PVT cells | Commercial nanoparticles. No provider reported. | Direct inhibitors of asphaltenes (liquid inhibitors) can be considered excellent candidates for field-scale mixed gas injection. Injection of CO2/nanoparticles reduced the precipitation of asphaltenes compared to injection of pure CO2 under reservoir conditions. Mixtures containing Fe3O4 can perform better than Al2O3 solutions as direct inhibitors of asphaltenes. |
| Varamesh et al. [117] | 2019 | Fe3O4 NiO | Development of a reliable and simple CPA EoS-based approach to model asphaltene precipitation in the presence of Fe3O4 and NiO nanoparticles. | Asphaltene onset in the presence and absence of nanoparticles was measured using dynamic light scattering. Cubic plus association equation of state (CPA EoS) was employed to predict the asphaltene precipitation in the presence and absence of the nanoparticles. | FTIR spectrophotometer | NiO and Fe3O4 nanoparticles were synthesized via precipitation from aqueous solutions. | CPA EoS can be used to develop chemical inhibitors of asphaltene precipitation by metal oxide nanoparticles. |
| Lu et al. [118] | 2016 | γ-Al2O3 | Investigated the adsorption of asphaltenes onto Al2O3 through 2 methods: (a) by adding a certain mass of nanoparticles in a fixed volume solution with different initial concentrations of asphaltenes | Coreflooding tests | Core | Commercial nanoparticles purchased from Aladdin Reagents Co. Ltd. (Shanghai, China). | The higher the mass fraction of Al2O3, the lower the precipitation intensity of asphaltenes. Al2O3 nanofluid injection can reduce the amount of oil and reduces permeability because nanoparticles can inhibit asphaltene deposition on the sand surface in a porous medium. |
| (b) by exposing a certain amount of asphaltenes in a fixed volume of solution with the addition of different amounts of nanoparticles | |||||||
| Ezeonyeka et. al. [119] | 2018 | Fe2O3 Fe3O4 γ-Al2O3 | Investigation of the adsorption of n-heptane-precipitated asphaltenes, C7 asphaltenes, from toluene model solutions onto three metal oxide NPs, Fe2O3, Fe3O4, and Al2O. | UV–vis spectroscopy at three different wavelengths was compared with thermogravimetric analysis (TGA) results | Sapphire measuring prism | Commercial Fe2O3 (dp <50 nm), Fe3O4 (20–30 nm), and Al2O3 (<50 nm particle size) were used as adsorbents. | Al2O3 showed the highest adsorption capacity with 385 ± 5 mg/g, followed by Fe3O4 and Fe2O3. Referring to mg/m2, however, Fe2O3 showed the highest adsorption capacity. TGA analysis showed that NPs promoted the oxidation of adsorbed asphaltenes in the reverse order of their adsorption capacity, qmax (mg/g) (Al2O3 > Fe2O3 ≈ Fe3O4). |
| Nassar et al. [120] | 2015 | SiO2 γ-Al2O3 Fe3O4 | Commercial nanoparticles of silica, γ-alumina, and magnetite were used as adsorbents to probe the chemical nature of the nanoparticles for asphaltene growth inhibition and to validate the model. | Experimental data on the kinetics of asphaltene aggregation were obtained using dynamic light scattering (DLS) measurements | UV-Vis spectrophotometer through asphaltene model solution in toluene | Commercial nanoparticles purchased from Sigma Aldrich | Under different conditions tested, all nanoparticles reduce the hydrodynamic diameter of large aggregates in solution to different degrees due to adsorption. The influence of the chemical nature of the nanoparticles, the origin of the asphaltenes, the heptol solution, and the temperature was successfully evaluated with DLS measurements. |
| Tarboush et al. [121] | 2014 | Fe2O3 | Presentation of the sol–gel/emulsion method for the in situ production of Fe2O3 nanoparticles in heavy oil from their aqueous precursor and comparison of their asphaltene adsorption with commercial Fe2O3 nanoparticles. | In situ prepared nanoparticles were recovered by centrifuging the crude oil for 10 min. The recovered samples were analyzed through TGA experiments. | In situ in heavy oil phase starting from their precursor aqueous iron (III) nitrate solution using a sol–gel/emulsion approach. | Iron (III) nitrate nonahydrate (used as the precursor salt), commercial iron (III) oxide (Fe2O3) nanoparticles (dp = 20–30 nm, 98%, used for comparison), toluene (99.8%), n-heptane (99%), and/or dichloromethane (DCM) (anhydrous, ≥99.8%, used to wash the nanoparticles recovered from the oil phase for microscopy). | The nanoparticles prepared in situ showed a much higher absorption, 2.6 ± 0.12 g/g, and were much more selective than the asphaltenes. Increasing the concentration of in situ generated particles showed a downward trend in absorption compared to the equilibrium concentration of asphaltenes. |
| Hashemi et al. [122] | 2016 | NiO | Possible influence of nickel oxide (NiO) nanoparticles on the destabilization of asphaltene deposits in porous media in the presence of carbon dioxide. | Three experiments were designed to analyze the precipitation process of asphaltenes in the oil stream in porous media and the impact of the presence of nanoparticles in this process. | Carbonate porous matrix | The material used for the synthesis of nickel oxide nanoparticles was nickel acetate (C4H6NiO4). First, an appropriate amount of nickel acetate was dissolved in water, and then the solvent, citric acid, was added to the mixture in a stoichiometric ratio to form a homogeneous gel. The droplets of the prepared solution were dispersed in the carrier gas and transported to the reaction medium. | The accumulation of asphaltenes in the heart was reduced from 0.1033 (g) in EXP-2 to 0.0128 (g) in EXP-3 in essentially identical experimental situations. |
| The first experiment consisted of injecting live oil into the heart to analyze the effect of injection pressure and velocity, which also includes the mechanism of elimination of organic matter in the natural degradation process. | |||||||
| In the second experiment, the asphaltene precipitation inside the core was studied by injecting CO2 into the core. | |||||||
| In a third experiment, nickel oxide nanoparticles were dispersed in CO2 to study the effect of the presence of nanoparticles on asphaltene precipitation. | |||||||
| Betancur et al. [123] | 2016 | SiO2 | Studied the role of the particle size and surface acidity of silica nanoparticles on their interaction and adsorption of asphaltenes | Constructed adsorption isotherms through UV–visible spectrophotometry, as well as estimated the change in the asphaltene aggregation through dynamic light scattering (DLS). | UV–visible spectrophotometer, nanosizer, and core for dynamic tests | Implemented the sol–gel method for the synthesis of silica nanoparticles of different sizes from a tetraethyl orthosilicate (TEOS) precursor. The surface acidity of the nanoparticles was also modified. | It was observed that as the nanoparticle size increased, the adsorption was reduced due to a lesser availability of active sites in the adsorbent surface. Moreover, the acidity had a direct relation to the asphaltene adsorption and disaggregation. In addition, coreflooding tests were carried out with a nanofluid including the best nanoparticles, and the recovery factor had an increment of 11%. |
| Amin and Nazar [124] | 2016 | SiO2 γ-Al2O3 TiO2 | The influence of effective factors such as nanoparticle types, asphaltene types, nanoparticle-to-solution ratio of the asphaltene model, and temperature on the adsorption size of asphaltenes on metal oxide nanoparticles was evaluated. | The Taguchi design of experiments (DOE) approach, the toluene–asphaltene solution model, and a UV–visible spectrophotometer. | UV–visible spectrophotometer | Commercial nanoparticles purchased from TECNAN. | The nanoparticle type and nanoparticle structure of asphaltenes with an impact of 48.5% and 3.11%, respectively, have the highest and lowest proportions of the amount of adsorbed asphaltenes at selected concentrations. Alumina nanoparticles have the highest adsorption, and silica nanoparticles have the lowest adsorption. The temperature has no statistical significance. Asphaltenes with high aromaticity tend to adsorb more onto nanoparticles. |
| Hosseini-Dastgerdi et al. [125] | 2022 | SiO2 Polyacrylamide (PAM) | The study assesses how silica–polyacrylamide nanocomposite might be used for the first time to prevent asphaltene precipitation. | Techniques for polarized microscopy, dynamic light scattering, asphaltene dispersion testing, and viscometry | FTIR polarizing microscope (Olympus), FESEM technique | Using the vapor acid process with sulfuric acid over 1300 °C for 48 h, SiO2 nanoparticles were functionalized. The functionalized nanoparticle solution was then combined in a 1:1 mass ratio with polyacrylamide. By adding a certain quantity of synthesized nanocomposites to distillate water to reach a concentration of 1000 ppm by mass, the silica–PAM nanofluid was created. | The aggregate (asphaltene) size decreases as the dosage of the nanocomposite increases. It is anticipated that the heterogeneities of the nanocomposite surface will produce a number of sites for the adsorption of asphaltene, enhancing adsorption affinity and reducing asphaltene self-association. For the crude oil, the greatest dispersion effectiveness of the nanocomposite was 69% and 79% at doses of 1% and 2.5% nanofluid volume. |
| López et al. [126] | 2020 | SiO2 cardanol | Assess how cardanol/SiO2 nanocomposites behave in preventing asphaltene damage using a coreflooding test under reservoir circumstances. | Adsorption curves/desorption isotherms of cardanol onto SiO2 nanoparticles were constructed. Likewise, the relationship between the total surface acidity and the H and K of the SLE model was presented. | Fourier transform infrared spectroscopy (FTIR), dynamic light scattering (DLS) | To eliminate any dampness, SiO2 nanoparticles (SNs) were first dried at 120 °C for 4 h. The beginning wetness technique was used to secure the CDN to the SN surface. The mass of the cardanol sample per gram of SiO2 nanoparticles was then changed to produce three SiO2/cardanol nanocomposites (CSNs). | The developed nanocomposites demonstrate significant asphaltene precipitation/deposition inhibition capacity. Additionally, using nanocomposites improves oil recovery by more than 50% when compared to the scenario with asphaltene damage. |
| Bagherpour et al. [127] | 2023 | Carboxylate-alumoxane nanoparticles functionalized BMA and PBMA | In this study, the application of two types of carboxylate-alumoxane nanoparticles (functionalized boehmite by methoxyacetic acid (BMA) and functionalized pseudo-boehmite by methoxyacetic acid (PBMA)) for asphaltene adsorption and precipitation was investigated. | BMA and PBMA nanoparticle DLS analysis. Pore size distribution and nitrogen adsorption–desorption isotherm for PBMA were presented. | Ultraviolet–visible (UV–Vis) spectroscopy | Boehmite and pseudo-boehmite were functionalized using the acidic technique to create carboxylate-alumoxane nanoparticles, and are now known as BMA and PBMA, respectively. Aluminum oxide-hydroxide with varying concentrations of H2O molecules and variable crystal sizes makes up boehmite. Boehmite is frequently used as the main starting material when creating alumina phases. | In comparison to the onset point of the reference synthetic oil, the use of BMA and PBMA delays the commencement of precipitation by 17 and 26%, respectively. The adsorption of asphaltene on the surface of these functionalized nanoparticles is the most important factor for asphaltene removal in the presence of carboxylate-alumoxane nanoparticles. The carboxylate-alumoxane nanoparticles containing asphaltene are eliminated from the system upon centrifugation. |
| Mahmoudi Alemi et al. [128] | 2021 | Fe2O3 and functionalized SiO2 nanoparticles F-SiO2 | In a light live oil with a high danger of asphaltene deposition, this study investigates their impacts on asphaltene precipitation and aggregation. High pressure, high temperature (HPHT) reservoir conditions were used for the studies. | TGA mass loss curves of pure asphaltenes and asphaltenes adsorbed onto Fe2O3 and F-SiO2 nanoparticles | OPS technology with a LEUTERT one-phase sampler to have a representative oil sample; HPHT filtration experiments | A straightforward chemical precipitation technique was used to create pure iron oxide Fe2O3 nanostructures. In this technique, a quantity of iron(III) chloride hexahydrate (FeCl3, 6H2O) is used. | The results demonstrate that adding 150 ppm of F-SiO2 nanoparticles to live oil before depressurization at 274.9 °F delays the onset of asphaltene by over 600 psi; in contrast, adding the same amount of Fe2O3 nanoparticles before depressurization makes the oil more stable and prevents the precipitation of asphaltenes. |
| Simin Tazikeh [129] | 2022 | Fe3O4 | Investigate changes in the surface properties of silica during the precipitation of asphaltenes with magnetite (Fe3O4) nanoparticles. | Images captured by an AFM technique of an A2 asphaltene precipitation on a silica substrate. Changing wettability using the Young–Laplace and modified Wenzel models | Fourier transform infrared spectroscopy (FTIR); atomic force microscopy (AFM) | Polythiophene-coated Fe3O4 nanoparticles (Fe3O4-PTNP) are synthesized in two steps. First, Fe3O4 nanoparticles are synthesized by coprecipitation. Then, they are coated with polydopene using a chemical polymerization technique. | The results show that heteroatoms (e.g., O, N, and S) and aromatic rings as functional groups can affect the process of asphaltene agglomeration and adsorption onto a silica surface. Atomic force microscopy (AFM) is used to obtain adequate topography information. |
| Gandomkar and Nasrian [130] | 2020 | Metal oxide nanoparticles (GO, TiO2, SiO2, and MgO) | As direct asphaltene inhibitors (DAIs) on asphaltene stability over the period of miscible CO2 injection, metal oxide nanoparticles (GO, TiO2, SiO2, and MgO) have been addressed in this study in the liquid-free mode. | Four metal oxide nanoparticles (GO, TiO2, SiO2, MgO) were used as direct inhibitors to stabilize asphaltenes during CO2 injection into reservoir oil. The nanoparticles have acidic (SiO2 and TiO2) and basic (MgO and GO) characteristics. | IFT measurements of chemical properties. Bulk sample and dynamic asphaltene test | Commercial nanoparticles. | The CO2/GO mixture reduces asphaltene aggregation/deposition and improves oil recovery by 6–25% compared to CO2 injection alone. Direct asphaltene inhibitors reduce interfacial tension (IFT) and allow miscible gas injection at reservoir pressure and temperature. Metal oxide nanoparticles increase the solubility of asphaltene particles, keeping them in solution. |
| Azizkhani and Gandomkar [131] | 2020 | Fe3O4 Al2O3 | This study centered on the inhibition of liquid-free asphaltene precipitation under reservoir conditions. During CO2 injection, the Fe3O4 and Al2O3 nanoparticles were utilized as direct asphaltene inhibitors (DAIs). | The interfacial tension technique was used to evaluate the effect of DAIs on the minimum miscibility pressure during CO2/nanoparticle injection. Asphaltene precipitation in volatile and intermediate oils was studied by varying the concentration of DAI from 500 to 3000 ppm. | IFT (advanced drop shape analysis) PVT Cell | All the nanoparticles are commercially available, so Fe3O4 and Al2O3 were used as received. These nanoparticles were used in different concentrations such as 500, 1000, 2000, and 3000 ppm. | The addition of Fe3O4 and Al2O3 to CO2 reduces MMP in reservoirs. Mixtures with Fe3O4 are better asphaltene inhibitors than Al2O3 solutions. Solubility is more important than aggregation during CO2 nanoparticle injection. DAI concentrations above 2000 ppm are not favorable. |
| Method | Summary | Advantages | Problems |
|---|---|---|---|
| Chemical synthesis | Nanoparticle synthesis involves controlled chemical reactions using precursors and reducing agents. Common techniques include chemical reduction, chemical precipitation, coprecipitation, and microemulsion. | Chemical synthesis of nanoparticles offers precise control of size and shape, has diverse applications, and is easy to implement in the laboratory. | Chemical synthesis of nanoparticles can require toxic or expensive reagents, be a slow process, and be difficult to scale up for large-scale production. |
| Thermal decomposition method | Thermal decomposition generates nanoparticles by decomposing precursors at high temperatures in a controlled atmosphere to obtain metallic, semiconducting, or ceramic particles. | Allows the synthesis of nanoparticles at high temperatures with high purity, especially in the case of metallic and ceramic particles. | Requires special equipment, but there may be problems with stability, aggregation, and generation of unwanted by-products. |
| Wet synthesis | Uses an aqueous solution to generate nanoparticles. Methods such as sol–gel synthesis, hydrolysis, and precipitation in aqueous media are employed. This technique allows precise control of the size and shape of the nanoparticles. | Excellent purity and homogeneity, exact control of nanoparticle size and shape, and suitability for high-volume manufacturing. | It could need additional stages and agents, be sensitive to environmental factors, and demand rigorous supervision. |
| Microemulsion method | Stable colloidal systems of water, oil, and surfactant are used, achieving high uniformity in particle size and shape. | Guarantees high uniformity in size and shape, greater colloidal stability, and the production of very small particles. | The synthesis of nanoparticles with microemulsions is complex, requires specific temperature and pH conditions, and may involve additional purification and separation steps. |
| Microwave-assisted synthesis | Can be accelerated using microwave radiation, which allows rapid energy transfer to activate the chemical reaction efficiently. | It accelerates nanoparticle synthesis by providing rapid and uniform heating, reducing reaction time and increasing efficiency. It also allows precise control of reaction conditions. | Requires specialized microwave equipment and adjustments to reaction parameters. Production scale may be limited due to equipment constraints. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Martin, C.A.; Montes-Pinzon, D.; Meneses Motta da Silva, M.; Montes-Paez, E.; Guerrero-Martin, L.E.; Salinas-Silva, R.; Camacho-Galindo, S.; Fernandes Lucas, E.; Szklo, A. Asphaltene Precipitation/Deposition Estimation and Inhibition through Nanotechnology: A Comprehensive Review. Energies 2023, 16, 4859. https://doi.org/10.3390/en16134859
Guerrero-Martin CA, Montes-Pinzon D, Meneses Motta da Silva M, Montes-Paez E, Guerrero-Martin LE, Salinas-Silva R, Camacho-Galindo S, Fernandes Lucas E, Szklo A. Asphaltene Precipitation/Deposition Estimation and Inhibition through Nanotechnology: A Comprehensive Review. Energies. 2023; 16(13):4859. https://doi.org/10.3390/en16134859
Chicago/Turabian StyleGuerrero-Martin, Camilo Andrés, Daniel Montes-Pinzon, Mariana Meneses Motta da Silva, Erik Montes-Paez, Laura Estefanía Guerrero-Martin, Raúl Salinas-Silva, Stefanny Camacho-Galindo, Elizabete Fernandes Lucas, and Alexandre Szklo. 2023. "Asphaltene Precipitation/Deposition Estimation and Inhibition through Nanotechnology: A Comprehensive Review" Energies 16, no. 13: 4859. https://doi.org/10.3390/en16134859
APA StyleGuerrero-Martin, C. A., Montes-Pinzon, D., Meneses Motta da Silva, M., Montes-Paez, E., Guerrero-Martin, L. E., Salinas-Silva, R., Camacho-Galindo, S., Fernandes Lucas, E., & Szklo, A. (2023). Asphaltene Precipitation/Deposition Estimation and Inhibition through Nanotechnology: A Comprehensive Review. Energies, 16(13), 4859. https://doi.org/10.3390/en16134859








