Abstract
Anaerobic digestion is one of the most widely used treatment methods for animal manure. Chicken manure has high methane production potential and is thus a suitable substrate for biogas plants. However, high nitrogen content inhibits the metabolism of anaerobic microorganisms and thus hinders methane production from chicken manure. Enhancing the performance of anaerobic digestion for chicken manure is indeed a long-standing challenge. This review presents new insights into maintaining methanogens’ activities, the decomposition of acetate, and the dynamics of methanogenic pathways under high ammonia stress. This review also analyzed the possible strategies for alleviating ammonia inhibition effects, including supplementing trace elements, co-digestion with nitrogen-less materials, in-situ ammonia removal, and long adaptation of anaerobic consortia to ammonia stress. The insights obtained in this paper may provide helpful information for a better understanding of anaerobic digestion technology for chicken manure and other nitrogen-rich waste and wastewater.
1. Introduction
Anaerobic digestion technology, the primary treatment strategy for animal manure, can efficiently treat organic matter to generate clean energy. In China, the annual output of animal manure reaches 3800 million tons []. This potential bioenergy from anaerobic treatment represents an ideal alternative to fossil energy sources and can reduce greenhouse gas emissions. Chicken manure is a bioenergy source that is very special as compared to pig and cow manure. Firstly, the total solids (TS) content of chicken manure is greater than 20%. It can be treated by high solids anaerobic digestion technology. However, a large amount of water is required to dilute chicken manure to about TS 10% before anaerobic treatment in the actual project []. This increases the production of digestate and operating costs. In addition, chicken manure contains considerable protein, uric acid, and other nitrogen-containing organic matter, with the nitrogen content usually being higher than 4% []. Anaerobic digestion technology, however, recommends a suitable carbon/nitrogen (C/N) ratio of 20–30 [], while the C/N ratio of chicken manure is as low as 5–10 [].
High ammonia level (total ammonia nitrogen, TAN > 3 g/L) is the primary bottleneck and is one of the main factors affecting microbial community structure and the methanogenic pathway in the anaerobic digestion of chicken manure. Previous studies demonstrated that hydrogenotrophic methanogens (converting H2 and CO2 into methane) were more tolerant of ammonia nitrogen than acetoclastic methanogens (cracking acetate to produce methane), which were dominant in high ammonia conditions []. However, recent studies found that acetate generated methane through syntrophic acetate oxidation combined with the hydrogenotrophic methanogenesis pathway under high ammonia stress []. Syntrophic oxidation is the primary metabolism of acetate at high ammonia levels []. Therefore, comprehensive knowledge of the links among ammonia levels, microbial community structure, and methanogenic pathways is critical to improving biogas production performance through improved operating strategies.
Previous studies focused on the anaerobic treatment of high nitrogen content substrates and employed deliberate strategies to enhance the ammonia tolerance of anaerobic microorganisms or to alleviate ammonia inhibition, including adding trace elements, co-digestion, adaptation, dilution, etc. In particular, the addition of trace elements, such as iron, nickel, cobalt, selenium, etc., can significantly improve the activity of methanogens despite high ammonia levels []. Co-digestion with a substrate with high carbon content or dilution with water can reduce the substrate’s nitrogen content []. In addition, nitrogen can be removed by in-situ, side-stream, post, and pre-anaerobic digestion stripping to avoid ammonia inhibition and to realize the recovery of nitrogen resources []. These methods significantly impact the community structure of microorganisms.
This review discusses the dynamics of the methanogenic pathway and the key functional microbial community structure in the anaerobic digestion of chicken manure. It illustrates the impact of different intentional manipulation strategies to enhance the process performance in the methanogenic pathway, aiming to optimize methane production performance from a biological perspective.
2. Ammonia Inhibition
2.1. Anaerobic Decomposition of Protein and Uric Acid in Chicken Manure
During the anaerobic treatment of chicken manure, process instability may occur due to high levels of ammonia nitrogen produced as a byproduct of protein and uric acid degradation. About 40%–70% of organic nitrogen in chicken manure comes from uric acid and 30%–60% from protein []. Protein was converted into amino acids by hydrolytic bacteria and then further converted into organic acids and ammonia by the action of acidogenic bacteria (Table 1). The protein degradation efficiency in chicken manure is less than 50% []. Notably, the degradation of proteins is complex and more sensitive to ammonia inhibition in the hydrolysis stage. For example, the peptone degradation efficiency was 50% under TAN levels of 2.0 g/L and rapidly decreased to 30% when the TAN increased to 5.0 g/L. However, peptone degradation almost ceased at a TAN of 6.5 g/L, and high ammonia levels mainly inhibit the deamination of peptone []. Therefore, due to the low degradability of proteins, uric acid degradation is one of the leading causes of high ammonia levels in the anaerobic digestion of chicken manure.

Table 1.
Anaerobic degradation of protein and uric acid.
The degradation of uric acid is complicated. Uric acid can be anaerobically degraded by Clostridium sp., Enterobacter sp., Streptococcus sp., Bacteroides sp., and so on (Table 1) []. These microorganisms were ubiquitous in anaerobic digestion systems, especially Clostridium and Bacteroides sp. []. Clostridium purinolyticum, for example, degrades uric acid into acetate, formate, glycine, and CO2 (Equation (2)) []. In addition, the uric acid degradation by Streptococcus sp. requires the presence of formate, which acts as a reducing agent (Equation (3)) []. The degradation of uric acid by Bacteroides sp. does not require the participation of formate (Equation (4)) []. Therefore, the anaerobic degradation of uric acid involves a variety of microbial metabolic reactions. The degradation of uric acid should be given adequate attention during anaerobic digestion. Current research lacks knowledge of nitrogen balance for the anaerobic treatment of chicken manure.
2.2. Ammonia Inhibition Threshold for Anaerobic Consortia
Anaerobic digestion is performed by various microorganisms, with the degradation of substrate divided into hydrolysis, acidogenesis, acetogenesis, and methanogenesis (Figure 1) []. These steps need to be performed in balance in order to obtain stable operation. Ammonia nitrogen exists mainly as ammonium bicarbonate (NH4HCO3) under anaerobic alkaline conditions []. Ammonium bicarbonate can provide alkalinity, buffer the pH value of the digestate and enhance the buffering capacity of the system (Figure 1). In addition, minor TAN levels (0.05–0.2 g/L) benefit microorganisms []. High ammonia nitrogen levels (TAN > 3 g/L), however, can inhibit the activity of anaerobic consortia, especially acetoclastic methanogens. The anaerobic digestion process produces undesirably higher TAN concentrations, even more than 10 g/L for chicken manure []. TAN is composed of free ammonia nitrogen (FAN, NH3) and ammonium ions (NH4+). Temperature and pH modulate the balance between NH4+ and NH3, and the latter has been reported to be the leading cause of microbial inhibition [].
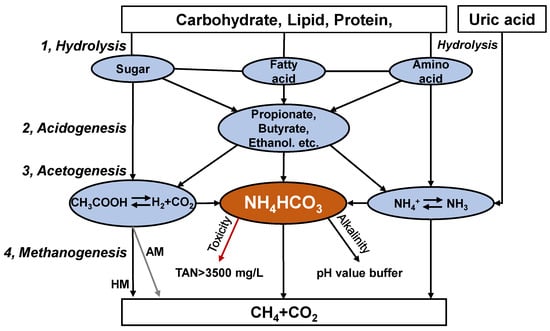
Figure 1.
Biological metabolism of organic matter in chicken manure during anaerobic digestion.
Hydrolysis and acidogenesis bacteria have higher ammonia tolerance than methanogens, which is the main reason for organic acid accumulation at high ammonia levels []. The IC50 (50% inhibition) of hydrolysis and acidogenesis efficiency was reached at TAN of 5.7 and 5.3 g/L in thermophilic conditions []. The hydrolysis efficiency of chicken manure was 75–77% (FAN of 1.1 g/L) under mesophilic conditions and only about 66% under thermophilic conditions (FAN of 2.2 g/L) with a similar TAN concentration (5.6–5.7 g/L) []. The IC50 of the hydrolysis and acidogenesis processes was 2.3 and 2.2 g/L of FAN []. This also illustrates that the hydrolysis and acidogenesis processes were less susceptible to ammonia inhibition.
The concentration of ammonia nitrogen can influence acetogenesis efficiency. The efficiency of acetogenesis increased by 52% when the concentration of TAN was decreased from 5.6 g/L to 3.8 g/L []. Because the acetogenic step is not considered the bottleneck, few studies have examined the effect of ammonia levels on acetogenic bacteria. However, it has been shown that an increased ammonia concentration (TAN 0.8 to 6.9 g/L) dramatically influenced the putative acetogenic population structure and caused two distinct changes in the most abundant microbial members []. In addition to converting organic matter to acetate, hydrogen can be converted to acetate through homoacetogens, the process being thermodynamically positive (ΔG0′ = −104.6 kJ/mol). Homoacetogens are suited to survival under low temperatures and acidic or alkaline conditions and are more competitive for hydrogen than hydrogenophilic methanogens []. The FAN concentration increased from 0.1 to 0.4 g/L, and the hydrogen consumption efficiency of the homoacetogenic process decreased from 68.5% to 4.5% []. It can be seen that homoacetogens have a lower ammonia inhibition threshold, being more sensitive to ammonia inhibition than hydrolysis, acidogenesis, and acetogenesis processes.
Ammonia levels have been found to inhibit methane production performance, with the ammonia threshold for inhibition ranging widely from 1.5 g/L to 7.0 g/L [,]. Inhibition phenomena were observed at 1.5–2.5 g/L of TAN during the anaerobic digestion of chicken manure [,]. The acetoclastic Methanosaeta sp. tolerated TAN concentrations of up to 3.0 g/L. In comparison, the facultative hydrogenotrophic Methanosarcina sp. could grow in environments containing as much as 7.0 g/L, whereas growth of hydrogenotrophic Methanobacterium sp. was observed up to 9.0 g/L []. Hydrogenophilic methanogens can tolerate higher ammonia levels and often were most dominant in high ammonia-level reactors.
3. Methanogenic Activities under Ammonia Stress
3.1. Methanogenic Activities Consuming Acetate
Methanogens are essential microorganisms in anaerobic digestion, but low growth rates and sensitivity to environmental stress make methanogenesis the most critical stage of the process. Specific methanogenic activity (SMA) is a method that can measure methane production from both the cleavage of acetate and the reduction in CO2 by hydrogen. []. The SMA of acetate has been studied [], whereas the SMA for dissolved hydrogen has been reported infrequently []. Studies have shown that increasing the organic loading rate (OLR) by reducing the hydraulic retention time (HRT) decreased methanogenic activity []. Higher solids in the feedstock may increase ammonia concentration above inhibiting levels under an identical HRT, which results in a lower SMA (Table 2). A high SMA of 0.56 g-COD/(g-volatile suspended solids (VSS)·d) was reported at a low TAN level of 2.4 g/L []. The ammonia concentration was reduced from 6.8 to 5.8 g/L through in-situ ammonia stripping, and the SMA increased three-fold []. The SMA decreased by 39% and 64% under TAN concentrations of 4.9 and 5.8 g/L, respectively, compared to the control (TAN 1.2 g/L) []. In addition, the SMA determination also has a more significant relationship with acetate concentration. The acetate concentration was set to 2.0 and 10.0 g/L (different acetate concentrations used in methanogenic activity assays), and the SMA was 0.091 and 0.357 g-COD/(g-VSS·d), respectively []. This illustrates that the methanogenic activity positively correlates with the acetate concentration in a specific range (<10.0 g/L). The main factor inhibiting SMA is the ammonia level rather than the acetate concentration in the stable fermentation system. Reduced methanogen activity causes volatile fatty acid (VFA) accumulation, a decrease in pH, and, ultimately, the failure of the anaerobic operation.
The SMA was lower than that of the mesophilic condition due to a higher FAN concentration under the thermophilic condition with the same ammonia level []. The SMA was 0.15 g-COD/(g-VSS·d) at 37 °C, which was higher than that at 55 °C (0.10 g-COD/(g-VSS·d) at a low ammonia level (TAN of 2.4 g/L) []. The methanogenic activity was more sensitive to ammonia under thermophilic than mesophilic conditions []. It can be seen from Figure 2 that the effects of OLR and NH4+ on SMA were challenging to distinguish. TAN increases with the OLR. Undoubtedly, a higher OLR can also cause the accumulation of various organic acids. Although acetoclastic methanogens tolerated higher acetate concentrations, macromolecular fatty acids other than acetate were negative, especially propionate. Thus, high OLR and TAN jointly curtail the activity of acetoclastic methanogens. In addition, as can be seen from Figure 2b, higher FAN significantly reduced the activity of acetoclastic methanogens, even at a lower OLR (<3.0 g-VS (L·d)). At a higher OLR, the tolerance concentration of FAN by acetoclastic methanogens was low (<0.5 g/L). It is essential to control the OLR to less than 3.0 g-volatile solid (VS)/(L·d) or regulate the FAN concentration to less than 0.5 g/L. Therefore, a thermophilic condition is not advisable for the anaerobic treatment of chicken manure.
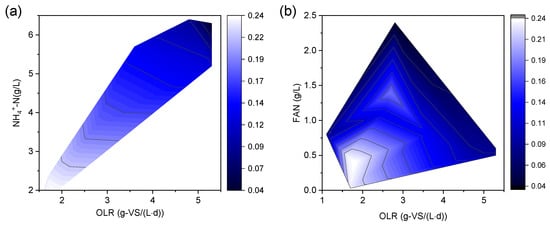
Figure 2.
Effect of NH4+ (a) and NH3 (b) levels on specific methanogenic activity. Note: This figure was drawn using OriginLab software based on the data in Table 2.

Table 2.
The methanogenic activity of acetate for the mesophilic and thermophilic digesters in treating chicken manure.
Table 2.
The methanogenic activity of acetate for the mesophilic and thermophilic digesters in treating chicken manure.
| HRT (d) | OLR (g-VS/(L·d)) | TAN (g/L) | FAN (g/L) | Acetate a (g/L) | SMA (g-COD/(g-VSS·d)) | References |
|---|---|---|---|---|---|---|
| 20 | 4.8 | 6.9 | 0.5 | 4.0 | 0.090 | [] |
| 20 | 5.3 | 6.9 | 0.6 | 4.0 | 0.040 | [] |
| 20 | 5.3 | 6.8 | 0.5 | 2.0 | 0.090 | [] |
| 20 | 3.6 | 6.5 | 0.8 | 4.0 | 0.13 | [] |
| 33 | 2.5 | 6.2 | 1.1 | 2.0 | 0.085 | [] |
| 20 | 2.8 | 6.0 | 2.4 | 2.0 | 0.035 | [] |
| 20 | 5.3 | 5.8 | 0.6 | 4.0 | 0.12 | [] |
| 20 | 2.5 | 5.6 | 1.1 | 2.0 | 0.17 | [] |
| 20 | 2.7 | 5.0 | 1.4 | 4.0 | 0.18 | [] |
| 50 | 1.1 | 3.9 | 0.8 | 2.0 | 0.082 | [] |
| 20 | 1.6 | 2.5 | 0.5 | 4.0 | 0.23 | [] |
| 20 | 1.7 | 2.4 | 0.03 | 4.0 | 0.23 | [] |
| 20 | 1.8 | 2.3 | 0.4 | 4.0 | 0.24 | [] |
Note: HRT: Hydraulic retention time; OLR: Organic loading rate; TAN: Total ammonia nitrogen; FAN: Free ammonia nitrogen; SMA: Specific methanogenic activity; VSS: Volatile suspended solids; COD: chemical oxygen demand; a: Acetate concentration for the SMA test.
3.2. Hydrogentrophic Methanogenic Activities
The hydrogen concentration in digesters correlates closely with the anaerobic digestion process stability. Low hydrogen benefits the conversion and degradation of organic acid []. The SMA can evaluate the rate of hydrogen consumption by hydrogenophilic methanogens. Although hydrogenophilic methanogens have a more robust tolerance to ammonia nitrogen, the effect of ammonia cannot be ignored. The TAN of the digester was reduced from 6.8 to 5.8 g/L, and the SMA of dissolved hydrogen was increased from 0.15 to 0.20 g-COD/(g-VSS·d) []. The TAN concentration was 1.0 and 7.0 g/L, respectively. The methane yields obtained at high ammonia levels (7.0 g/L) were 22% and 41% lower than those obtained at low ammonia levels (1.0 g/L) under mesophilic and thermophilic conditions []. There are still relatively few studies on the SMA of hydrogen. Nevertheless, it is undeniable that enhancing hydrogenotrophic activity and comprehensive knowledge is very important for improving methane production and predicting fermentation performance. Understanding the effects of ammonia nitrogen and the activity of hydrogenophilic methanogens requires further research.
4. Dynamics of Functional Microbes in the Anaerobic Digestion of Chicken Manure
4.1. Methanogenic Archaea Community
Acetoclastic methanogens, represented by the genera Methanosaeta and Methanosarcina sp., are essential in efficiently reducing acetate concentration and maintaining performance stability (Table 3). Research has shown that Methanosaeta sp. exhibits a high affinity for acetate but a relatively slow growth rate. In contrast, Methanosarcina sp. has a lower acetate affinity but a higher growth rate in comparison []. In addition, Methanosaeta sp. is a strict acetoclastic methanogen [], while Methanosarcina sp. can utilize acetate, H2/CO2, formate, and methanol []. The filamentous shape of Methanosaeta sp. expands its surface area, thus making it more sensitive to high VFAs and toxic substances such as ammonia. Methanosarcina sp. can be present in microbial clusters, protecting them from inhibitors and thus making them more resistant to adverse factors []. The characteristics of different methanogens determine their responsiveness to environmental changes.
It can be seen from Table 4 and Figure 3 that the facultative Methanosarcina sp. was frequently observed in the high-ammonia digesters. The TAN concentration was 6.8 g/L, and the acetoclastic Methanosarcina sp. relative abundance was 73%. In contrast, when the TAN concentration was 5.8 g/L with stripping, Methanosarcina sp. increased to 83% []. The concentration of TAN gradually increased from 8.0 g/L to 15.0 g/L, Methanosaeta sp. decreased from 58% to an undetectable level, and acetoclastic Methanosarcina sp. was also reduced from 58% to 6% []. Methanosaeta sp. contributes significantly in digesters with low ammonia levels, and it is essential for efficiently degrading acetate and maintaining low organic acid levels. In addition, different nitrogen sources were observed to affect the methanogens community. It was found that urea has a more substantial inhibitory effect on methanogens than NH4Cl []. The nitrogen source in chicken manure is protein and uric acid []. The inhibitory effect on acetoclastic methanogens is more prominent. It causes the accumulation of VFAs, thus forming a dual-inhibition environment of high ammonia and organic acids []. Therefore, the microbial community structure for chicken manure anaerobic treatment is more complicated than other substrates.
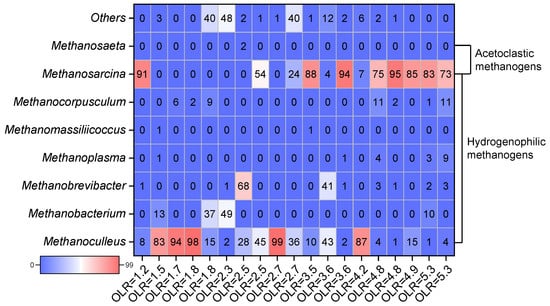
Figure 3.
Microbial community structure under different OLR levels of chicken manure digestion Note: OriginLab software made this figure based on the data in Table 4.
Hydrogenophilic methanogens, such as Methanoculleus sp. and Methanothermobacter sp., were more robust to ammonia and had a shorter doubling time than acetoclastic methanogens (Table 3). Hydrogenotrophic methanogens still performed acceptably when the TAN was as high as 8.0 g/L, as exemplified by the Methanobacterium sp. []. In an acetate-based reactor, increasing the TAN concentration from 0.2 g/L to 4.0 g/L resulted in a 34% decrease in methane yield. In contrast, the methane yield was reduced by only 13% in the H2/CO2-based reactor []. At high ammonia levels (TAN > 5.0 g/L), changes in the TAN concentration may not cause a significant reduction in methanogenesis due to the presence of ammonia-tolerant hydrogenotrophic methanogens. Methane production efficiency was inhibited when the TAN reached 4.8 g/L (FAN 0.7 g/L) and was almost completely inhibited at TAN 13.0 g/L []. The TAN concentration decreased from 5.7 g/L to 2.5 g/L, and the methanogenesis efficiency increased by only 4% (from 61.5% to 63.9%) []. In addition, Methanoculleus sp. and Methanobacterium sp. were dominant at a high ammonia level and low OLR (<3.0 g-VS/(L·d)), while Methanosarcina sp. was dominant, regardless of the ammonia level at high OLR (Figure 3). Therefore, hydrogenotrophic methanogens play an essential role under high-ammonia conditions for methane production.

Table 3.
The characteristics of the main methanogens observed in the anaerobic digestion of chicken manure.
Table 3.
The characteristics of the main methanogens observed in the anaerobic digestion of chicken manure.
| Genus | Species | Source | Metabolic Substrate | Optimum Temperature (°C) | Optimum pH | Doubling Time (h) | References |
|---|---|---|---|---|---|---|---|
| Methanosaeta | M. concilii | ND | Acetate | 35–40 | 7.1–7.5 | 65.0 | [] |
| Methanosaeta | M. harundinacea | UASB reactor | Acetate | 34–37 | 7.2–7.6 | 28.0 | [] |
| Methanosaeta | M. pelagica | Marine Tidal | Acetate | 30 | 7.5 | 298.0 | [] |
| Methanosaeta | M. thermophila | ND | Acetate | 55–60 | 7.0 | ND | [] |
| Methanosaeta | M. sp | Thermophilic digester | Acetate | 55 | 6.7 | 36.0 | [] |
| Methanosarcina | M. barkeri | Pyruvate digester | H2/CO2, Formate, Acetate | 37 | 6.5 | 25.0 | [] |
| Methanosarcina | M. thevmophila | Sludge digester | H2/CO2, Formate, Acetate | 50 | 6.5–6.8 | 12.0 | [] |
| Methanosarcina | M. flavescens | Full-scale biogas plant | H2/CO2, Formate, Acetate | 45 | 7.0 | 20.4 | [] |
| Methanoculleus | M. receptaculi | Oil field | H2/CO2, Formate | 50–55 | 7.5–7.8 | 8.3 | [] |
| Methanoculleus | M. palmolei | Palm oil | H2/CO2, Formate | 40 | 6.9–7.5 | 13.5 | [] |
| Methanocalculus | M. taiwanensis | Estuary | H2/CO2, Formate | 37 | 6.7 | 8.2 | [] |
| Methanocalculus | M. halotolerans | Oil field | H2/CO2, Formate | 38 | 7.6 | 12.0 | [] |
| Methanocalculus | M. pumilus | Waste disposal site | H2/CO2, Formate | 35 | 6.5–7.5 | 12.0 | [] |
| Methanobacterium | M. congolense | Cassava peel digester | H2/CO2, Formate | 37–42 | 7.2 | 7.5 | [] |
| Methanobacterium | M. petrolearium | Crude oil storage tank | H2/CO2, Formate | 35 | 6.5 | 39.5 | [] |
| Methanobacterium | M. thermoflexum | Sludge digester | H2/CO2, Formate | 55 | 7.9–8.2 | 3.5 | [] |
| Methanobacterium | M. defluvii | Sludge digester | H2/CO2, Formate | 60 | 7.0 | 1.5 | [] |
| Methanobrevibacter | M. acididurans | Sour digester | H2/CO2 | 35 | 6.0 | 16.5 | [] |
| Methanococcus | M. maripaludis | Salt marsh sediment | H2/CO2, Formate | 38 | 6.8–7.2 | 2.0 | [] |
| Methanomassiliicoccus | M. luminyensis | Human feces | H2/CO2, Methanol | 37 | 7.6 | ND | [] |
Note: ND: No data.

Table 4.
Community structure of methanogens obtained in the anaerobic digestion of chicken manure.
Table 4.
Community structure of methanogens obtained in the anaerobic digestion of chicken manure.
| Order | OLR (g-VS/(L·d) | HRT (d) | TAN (g/L) | FAN (g/L) | TVFA (g/L) | Methanogen and Its Substrate | Abundance | Methane Yield (L-CH4/g-VS) | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.9 | 20 | 0.7 | 0.03 | 0.2 | Methanosarcina sp./ Acetate, H2/CO2 | 91% | 0.27 | [] |
| 2 | 1.5 | 60 | 7.5 | 1.5 | 0.5 | Methanoculleus sp./ Formate, H2/CO2 | 83% | 0.33 | [] |
| 3 | 1.7 | 20 | 2.4 | 0.7 | 0.4 | Methanoculleus sp./ Formate, H2/CO2 | 94% | 0.38 | [] |
| 4 | 1.8 | 20 | 2.5 | 0.4 | 0.2 | Methanoculleus sp./ Formate, H2/CO2 | 98% | 0.36 | [] |
| 5 | 1.8 | 40 | 5.6 | 0.6 | 0.6 | Methanobacterium sp./ Formate, H2/CO2 | 37% | 0.35 | [] |
| 6 | 2.3 | 40 | 7.2 | 1.8 | 6.4 | Methanobrevibacter sp./ Formate, H2/CO2 | 49% | 0.32 | [] |
| 7 | 2.5 | 40 | 6.2 | 1.1 | 2.1 | Methanobrevibacter sp./ Formate, H2/CO2 | 68% | 0.31 | [] |
| 8 | 2.5 | 30 | 2.3 | 0.3 | 2.0 | Methanosarcina sp./ Acetate, H2/CO2 | 54% | 0.25 | [] |
| 9 | 2.7 | 20 | 5.0 | 1.4 | 2.2 | Methanoculleus sp./ Formate, H2/CO2 | 99% | 0.34 | [] |
| 10 | 2.7 | 30 | 4.0 | 1.2 | 10.0 | Methanoculleus sp./ Formate, H2/CO2 | 36% | 0.34 | [] |
| 11 | 3.5 | 35 | 6.9 | 0.8 | 6.1 | Methanosarcina sp./ Acetate, H2/CO2 | 88% | 0.18 | [] |
| 12 | 3.6 | 20 | 6.5 | 0.8 | 4.5 | Methanoculleus sp./ Formate, H2/CO2 | 43% | 0.28 | [] |
| 13 | 3.6 | 20 | 6.6 | 0.9 | 6.7 | Methanosarcina sp./ Acetate, H2/CO2 | 94% | 0.29 | [] |
| 14 | 2.0 | 20 | 6.7 | 1.2 | 20.0 | Methanoculleus sp./ Formate, H2/CO2 | 87% | 0.05 | [] |
| 15 | 4.8 | 20 | 6.9 | 0.5 | 22.3 | Methanosarcina sp./ Acetate, H2/CO2 | 75% | 0.18 | [] |
| 16 | 4.8 | 20 | 6.8 | 0.6 | 15.1 | Methanosarcina sp./ Acetate, H2/CO2 | 95% | 0.24 | [] |
| 17 | 4.9 | 25 | 6.5 | 0.6 | 4.0 | Methanosarcina sp./ Acetate, H2/CO2 | 85% | 0.19 | [] |
| 18 | 5.3 | 20 | 5.8 | 0.6 | 15.2 | Methanosarcina sp./ Acetate, H2/CO2 | 83% | 0.25 | [] |
| 19 | 5.3 | 20 | 6.8 | 0.5 | 21.6 | Methanosarcina sp./ Acetate, H2/CO2 | 73% | 0.19 | [] |
| 20 | 5.3 | 20 | 6.9 | 0.6 | 13.6 | Methanosarcina sp./ Acetate, H2/CO2 | 73% | 0.19 | [] |
| 21 | 7.1 | 20 | 8.5 | 0.5 | 25.2 | Methanosarcina sp./ Acetate, H2/CO2 | 37% | 0.02 | [] |
Note: OLR: Organic loading rate; HRT: Hydraulic retention time; TAN: Total ammonium nitrogen; FAN: Free ammonia nitrogen; TVFA: Total volatile fatty acids; VS: Volatile solids.
4.2. Syntrophic Acetate Oxidation Bacteria in the Anaerobic Digestion of Chicken Manure
Pure cultures of isolated syntrophic acetate oxidation bacteria (SAOB) currently mainly include Schnuerera ultunense [], Thermacetogenium phaeum [], Pseudothermotoga/Thermotoga lettingae [], Syntrophaceticus schinkii [], and Tepidanaerobacter acetatoxydans []. Most SAOBs were detected and isolated from the high ammonia levels digesters (TAN, 6.0–7.0 g/L), as shown in Table 5. These bacteria can be detected in diverse mesophilic and thermophilic environments, ammonia concentrations, HRT, OLR, reactor types, etc. []. For example, S. schinkii was isolated from sludge from a mesophilic methane production digester operating at a high ammonia level (TAN 6.4 g/L). The addition of NH4Cl (0.6 M) in the modified basal medium can achieve higher cell densities of S. schinkii []. T. acetatoxydans also has a broader range of growth conditions and was observed at a TAN of 14.0 g/L [].
Table 6 shows the abundances of SAOB and total bacteria in 13 large-scale biogas plants. The average gene abundance of S. schinkii was 105–1010/mL, and C. ultunense, T. acetatoxydans, and T. phaeum were 0–108/mL under total bacteria of 1011–1013/mL []. However, a higher relative abundance of SAOB can also be found in extremely high ammonia nitrogen concentration digesters. The relative abundance of total syntrophic bacteria (Syntrophaceticus, Tepidanaerobacter, and Clostridium) was 15.9% at high ammonia levels (6.8 g/L), more than that of relatively low ammonia (5.8 g/L) digester (14.9%) []. In addition, previous studies have also found that the relative abundance of syntrophic bacteria increased significantly alongside hydrogenotrophic methanogens in extreme ammonia levels (7.0 g/L TAN) []. The ammonia inhibition threshold of most SAOBs is generally high, but no uniform conclusion can be made, as it depends on the configuration of the reactor, including temperature, retention time, etc.

Table 6.
Syntrophic acetate oxidation bacteria in the anaerobic digestion of chicken manure.

Table 5.
Characteristics and isolation of a pure culture of syntrophic acetate oxidation bacteria.
Table 5.
Characteristics and isolation of a pure culture of syntrophic acetate oxidation bacteria.
| Phylum | Class | Order | Family | Genus | Species | Isolation Source | Temperature Scale (°C) | pH Scale | NH4+-N Limit (g/L) | Doubling Time (h) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillota | Clostridia | Thermoanaero- bacterales | Thermoanaero-bacterales Family III. Incertae Sedis | Syntrophaceticus | S. schinkii | Up-flow anaerobic filter (NH4+-N 6.4 g/L) a | 25–40 | 6.0–8.0 | 8.4 | ND | [] |
| Bacillota | Clostridia | Thermosedimini- bacterales | Tepidanaero- bacteraceae | Tepidanaerobacter | T. acetatoxydans | Ammonium-rich, mesophilic systems (NH4+-N 6.4 g/L) | 25–55 | 4.0–9.5 | 9.8–14.0 | ND | [] |
| Bacillota | Clostridia | Eubacteriales | Clostridiaceae | Clostridium | C. ultunense | Swine manure digester (NH4+-N 7 g/L) | 15–50 (37) b | 5.0 –10.0 (7.0) | 11.2 | 48–120 | [] |
| Bacillota | Clostridia | Thermoanaero- bacterales | Thermoanaero- bacteraceae | Thermacetogenium | T. phaeum | Kraft-pulp wastewater | 40–65 (58) | 5.9–8.4 (6.8) | ND | 23 | [] |
| Thermotogota | Thermotogae | Thermotogales | Thermotogaceae | Thermotoga | T. lettingae | Sulfate-reducing bioreactor | 50–75 (65) | 6.0–8.5 (7.0) | ND | 4 | [] |
Note: ND: No data; a: Ammonia nitrogen concentration in the reactor; b: () = optimal value.
5. Methanogenic Pathways under Ammonia Stress
5.1. Acetate Metabolic Pathway
The production and consumption of acetate involve primarily anaerobic digestion steps, including acidogenesis, acetogenesis, syntrophic acetate oxidation, and methanogenesis. The methanogenesis pathway of acetate cracking is a thermodynamically positive reaction (Table 7) that can decompose organic acids. However, the reaction can be inhibited by high ammonia nitrogen levels, causing the accumulation of organic acids, mainly acetate. Under such conditions, acetate can instead be decomposed by ammonia-tolerant syntrophic acetate oxidation bacteria to CO2 and H2 or formate (Table 7) []. Under standard conditions, the syntrophic acetate oxidation (SAO) process is energetically unfavorable (ΔG0′ = +104.6 kJ/mol), but it is feasible if the H2 or the formate levels are kept low by hydrogenotrophic methanogens. Thus, close cooperation between the SAOB and the hydrogenotrophic methanogens (SAO-HM pathway) can make the acetate conversion exergonic and has the same stoichiometry (ΔG0′ = −31.0 kJ/mol) as with the acetoclastic methanogenesis (AM) pathway. It has been shown that AM pathway would be inhibited at high ammonia levels []. Therefore, adjusting the operating parameters to improve the activity of critical anaerobic consortia, such as SAOB and hydrogenotrophic methanogens, may be an effective strategy to improve process stability and biogas yield significantly.

Table 7.
The primary reactions during the anaerobic digestion process.
5.2. Ammonia Threshold for the Dynamic of the Methane Production Pathway
The isotopic labeling method has demonstrated that the AM pathway was indispensable under high ammonia conditions (Table 8). Using stable carbon isotopes (δ13C) to determine the AM pathway, it was 17% under a TAN of 6.8 g/L in the mesophilic condition. But the TAN was reduced to 5.8 g/L by ammonia stripping, and the AM pathway increased to 23% []. Acetoclastic methanogens were more competitive with acetate in mesophilic conditions. The concentration of TAN decreased from 5.3 g/L to 2.5 g/L, and the AM pathway increased from 17% to 60% []. In addition, the radiolabelling carbon isotope [2–14C] 14C quantifies the proportion of different methanogenic pathways at different TAN concentrations (from 0.2 to 11.1 g/L). The study found that the proportion of the AM pathway was 25–32% at high ammonia (4.5–11.1 g/L) levels, while it reached 73–91% at a low ammonia level (0.2–1.5 g/L) []. The changes in methanogenic pathways depended on the TAN concentration with thresholds of 1.0, 3.5, and 6.0 g/L []. Factors affecting the AM pathway mainly include OLR, HRT, TAN, and VFA concentrations, as illustrated in Figure 4b. The ammonia level affecting the AM pathway was divided into three regions: TAN < 2 g/L, 2–5 g/L, and >5 g/L (Figure 4a). The corresponding proportions of AM are higher than 70%, 30–70%, and below 30%, respectively.

Table 8.
Comparison of methanogenic pathways obtained in the anaerobic digestion of chicken manure.
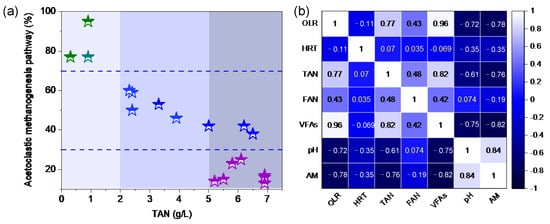
Figure 4.
The proportion of the AM pathway at different ammonia concentrations (a), Spearman rank correlation among OLR, TAN, FAN, VFA, HRT and AM (b). Note: This figure shows the Spearman correlation analysis of the data in Table 8 using OriginLab software.
6. Challenges and Perspectives in Maintaining the Process Stability
6.1. Optimizing Operating Parameters
An extended HRT can maintain the advantages of acetoclastic methanogens. SAOB has a longer doubling time (2–5 d) under mesophilic conditions []. The doubling time of SAOB is shortened, generally 31–72 h under thermophilic conditions. The growth rate of SAOB can exceed that of acetoclastic methanogens, which enhances the competitiveness of SAOB for acetate []. SAOB was detected in digesters with different HRT conditions (17–130 d), especially in digesters with high ammonia nitrogen levels []. As discussed above, the doubling time of acetoclastic methanogens is longer (10–65 h) [], and hydrogenotrophic methanogens have shorter doubling times (2–20 h) under optimal growth conditions []. Although the residence time (20–30 d) associated with anaerobic digestion technology can meet the doubling time of different anaerobic consortia, the time can increase significantly at high ammonia levels. Under TAN concentrations of 0.5–2.8 g/L, the doubling time of Methanoculleus bourgensis was 10–13 d. However, the doubling time increased to 23–50 d when TAN reached 4.8 g/L []. Therefore, an appropriate HRT should be selected to avoid microbial loss in high ammonia-level digesters.
Some studies gradually increase the TS concentration of the substrate and keep the constant HRT or gradually reduce the HRT and keep the constant TS concentration of the substrate to increase the OLR, which is to obtain the maximum processing capacity of the reactor and obtain the best operating parameters [,,]. In addition, changing the HRT and TS concentration of the substrate to obtain the constant OLR can be used to investigate the effect of operating conditions on methane production performance []. Therefore, the optimization of the experiment is diverse and complex. In addition, high ammonia nitrogen concentration and OLR are interrelated, and high ammonia levels must accompany higher OLR, so separating the effects of both on fermentation performance is difficult. One study showed that keeping the ammonia nitrogen concentration unchanged and increasing the OLR does not change the type and quantity of SAOB []. This indicates that the ammonia level, rather than the OLR, directly affects the structure of the SAOB flora. However, OLR is an important parameter affecting acetoclastic methanogens and may alter growth conditions for SAOB. In addition, the OLR was one of the critical factors affecting the microbial network related to anaerobic digestion, including the acetogenic community structure []. In anaerobic systems, there was a syntrophic and competitive relationship between different microorganisms, and OLR may also change the community structure and abundance of potential SAOB.
OLR typically ranges from 1.0 to 5.0 g-VS/(L·d) for continuously stirred tank reactors. The OLR was usually below 3.0 g-VS/(L·d) for chicken manure []. OLR and HRT strongly affected the methane yield in the anaerobic digestion of chicken manure, as shown in Table 9 and Figure 5a. Different OLRs and HRTs can be divided into four regions. When the OLR was lower than 3.0 g-VS/(L·d), the ideal fermentation performance was achieved, and HRT was lower than 40 d (Q1); when the HRT was higher than 40 d, stable operation of the system was ensured, and a higher methane yield was achieved (Q2). While the OLR was higher than 3.0 g-VS/(L·d), the methane yield was very unsatisfactory when the HRT was lower than 40 d (Q4), and when the HRT was over 40 days, stable methane production was achieved. Nevertheless, the long-term stable operation was not maintained (Q3). In addition, organic acids were decomposed at low OLR (<3.0 g-VS/(L·d)) even though there were higher ammonia levels, which resulted in higher FAN concentrations. However, this did not inhibit methane production. The degree of ammonia inhibition was closely related to OLR and alleviated or overcome under lower OLR conditions [,]. Thus, methane yield and FAN were positively correlated at lower OLR (Figure 5b); however, this was not generalizable.
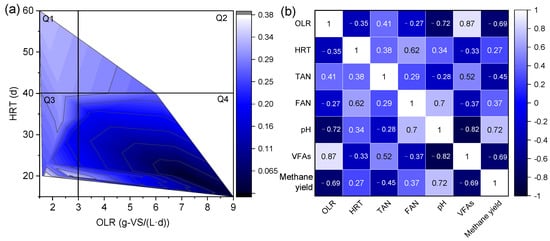
Figure 5.
Effects of HRT and OLR on methane yield (a), the links between operating parameters and methane yield (b) in the anaerobic digestion of chicken manure. Note: This figure shows the Spearman correlation analysis based on the data of Table 9 using OriginLab software.
Moreover, it needs to be noted that OLR directly affects the composition of the methanogen community structure. Methanosaeta sp. dominated at lower OLR levels and was gradually replaced by Methanosarcina sp. with higher OLR. For example, the OLR level of 0.9–1.9 g-VS/(L·d) was dominated by Methanosaeta sp., and when the OLR was 2.9–3.7 g-VS/(L·d), the role of Methanosarcina sp. was more prominent in the treatment of pharmaceutical wastewater []. Additionally, the SAO-HM pathway increased from 33% to 47% with increasing OLR (from 6.0 to 17.0 g-COD/(L·d)) in the anaerobic digestion of maize silage at low ammonia levels (TAN 0.5 g/L) []. This may be because the high VFA concentration inhibited the activity of the AM pathway, enhancing the dominance of hydrogenotrophic methanogens and the contribution to methane formation. Therefore, a low but acceptable OLR (about 3.0 g-VS/(L·d)) and an extended HRT (>40 d) are key operating parameters to ensure efficient methane production.

Table 9.
Methane production performance of chicken manure under different operating conditions.
Table 9.
Methane production performance of chicken manure under different operating conditions.
| TS (%) | OLR (g-VS/(L·d)) | HRT (d) | TAN (g/L) | FAN (g/L) | pH Value | TVFA (g/L) | Methane Yield (L/g-VS) | References |
|---|---|---|---|---|---|---|---|---|
| 19.0 | 9.0 | 15 | 6.0 | 0.03 | 6.55 | 43.0 | 0.03 | [] |
| 20.0 | 7.1 | 20 | 8.5 | 0.5 | 7.7 | 25.2 | 0.02 | [] |
| 15.0 | 6.0 | 40 | 6.9 | 0.9 | 8.0 | 9.3 | 0.27 | [] |
| 15.0 | 5.3 | 20 | 6.9 | 0.6 | 7.9 | 13.6 | 0.19 | [] |
| 15.0 | 5.3 | 20 | 6.8 | 0.5 | 7.8 | 21.6 | 0.19 | [] |
| 15.0 | 5.3 | 20 | 5.8 | 0.6 | 7.9 | 15.2 | 0.25 | [] |
| 15.0 | 4.8 | 20 | 6.9 | 0.5 | 7.8 | 22.3 | 0.18 | [] |
| 15.0 | 4.8 | 20 | 6.8 | 0.6 | 7.9 | 15.1 | 0.24 | [] |
| 10.0 | 3.6 | 20 | 6.5 | 0.8 | 8.0 | 6.7 | 0.28 | [] |
| 15.0 | 3.5 | 35 | 6.6 | 0.6 | 7.9 | 5.4 | 0.19 | [] |
| 11.0 | 2.8 | 30 | 8.0 | 1.6 | 8.3 | 5.0 | 0.18 | [] |
| 8.0 | 2.7 | 30 | 5.0 | 0.8 | 8.2 | 2.0 | 0.20 | [] |
| 7.5 | 2.7 | 20 | 5.0 | 1.4 | 8.5 | 2.2 | 0.34 | [] |
| 7.6 | 2.5 | 20 | 5.7 | 1.1 | 8.3 | 3.0 | 0.25 | [] |
| 9.0 | 2.3 | 33 | 6.2 | 1.1 | 8.2 | 2.1 | 0.30 | [] |
| 15.0 | 2.3 | 40 | 7.2 | 2.3 | 8.5 | 6.4 | 0.31 | [] |
| 5.0 | 1.8 | 20 | 2.3 | 0.4 | 8.2 | 0.4 | 0.36 | [] |
| 13.5 | 1.8 | 23 | 5.9 | 0.4 | 7.8 | 7.6 | 0.26 | [] |
| 5.0 | 1.7 | 20 | 2.4 | 0.6 | 8.4 | 0.3 | 0.38 | [] |
| 5.0 | 1.6 | 20 | 2.5 | 0.5 | 8.3 | 0.4 | 0.25 | [] |
| 15.0 | 1.5 | 60 | 7.5 | 1.8 | 8.3 | 0.5 | 0.33 | [] |
Note: OLR: Organic loading rate; HRT: Hydraulic retention time; TAN: Total ammonium nitrogen; FAN: Free ammonia nitrogen; TVFA: Total volatile fatty acids; VS: Volatile solids.
6.2. Microbial Adaptation Enhances the AM Pathway
Acetoclastic methanogens have a stronger affinity for acetate than hydrogenotrophic methanogens and can significantly reduce the acetate level. For example, facultative hydrogenotrophic Methanosarcina sp. has higher growth rates but requires acetate concentrations above 1.0 mM, whereas acetoclastic Methanosaeta sp. predominates at lower acetate levels (<1.0 mM) []. As shown in Figure 3, Methanosarcina sp. played a significant role in methane production under a high ammonia and organic acid environment. The existence of a higher abundance of Methanosarcina sp. was the key to ensuring efficient methane production for the anaerobic digestion of nitrogen-rich substrates. For example, the tolerance concentration of Methanosarcina sp. to ammonia nitrogen and organic acid can reach 7.0 and 15.0 g/L, respectively []. However, the high concentration of organic acid residues indicated poor fermentation performance. In contrast, Methanosaeta sp. was able to reduce acetate to a lower level, but the tolerance to ammonia nitrogen was significantly lower than that of Methanosarcina sp. For example, Methanosaeta thermophila has an ammonia inhibition threshold of 3.0 g/L and was completely inhibited at 4.5 g/L []. These acetoclastic methanogens should play complementary roles at high ammonia levels. On the one hand, the activity and quantity of Methanosaeta sp. can be enhanced by adaptation or adding trace elements, maintaining its dominance at relatively low ammonia levels (<5.0 g/L). However, Methanosaeta sp. lost its competitive advantage over acetate at higher ammonia levels (>5.0 g/L), and enhancing the acetate metabolic activity of Methanosarcina sp. was the key strategy. Furthermore, and interestingly, maintaining a specific organic acid concentration seems to be an effective way to alleviate ammonia inhibition at higher ammonia levels, which avoids the irreversible toxicity of higher FAN to methanogens. Enhancing the activity of acetoclastic methanogens is the key to efficient methane production in nitrogen-rich manure and this will require more in-depth research on the metabolic mechanism.
The adaptation of the microbial community is often achieved by gradually increasing the ammonia levels. It can take several retention times before the process becomes stable and adequate adaptation of the microorganisms may take two months or more []. In an undisturbed anaerobic digestion system, an ammonia nitrogen concentration below 3.0 g/L does not cause inhibition []. However, the adaptation of anaerobic sludge did not cause poisoning, even though the TAN concentration was as high as 5.0–6.0 g/L []. It was essential to carefully monitor the VFA levels, methane content, and pH during this adaptation period. If the VFA levels increase, it is crucial to decrease the OLR and increase the HRT for a period to allow the microbial community to grow and maintain itself in the digesters []. Adaptation allows ammonia-tolerant microorganisms to grow and perform the respective degradation steps. Ammonia adaptation includes the development of an acetate-degrading pathway involving SAOB that cooperates with hydrogenophilic methanogens []. Under high ammonia levels, the AM pathway is inhibited, and a longer time is required to meet the growth of acetoclastic methanogens and SAOB.
Adaptation improves the ammonia resistance of various microorganisms, which is beneficial to the stable operation of the digestion system. The relative abundance of acetoclastic Methanosaeta sp. reached 5% through adaptation for more than 10 years in a chicken manure biogas plant at a TAN of 6.2 g/L []. Interestingly, Methanosaeta sp. was less abundant and contributed 42% of the methane production. The tolerance to ammonia inhibition of acetoclastic Methanosaeta can significantly improve after long-term adaptation. To our knowledge, the anaerobic consortia, including acetoclastic methanogens and hydrogenotrophic methanogens, have a certain tolerance to ammonia inhibition. The methane yield reached 0.34 L/g-VS when the FAN concentration reached 1.42 g/L through an 87 d adaptation []. It can also be seen that the anaerobic digestion of chicken manure can still stably operate under the FAN concentration of 1.0~2.0 g/L [,]. The FAN gradually increased from 0.8 to 3.2 g/L (54 d), and the methanogenesis process was inhibited entirely []. The adaptation of methanogenesis to ammonia is well above those considered inhibitory levels []. Based on the physiological characteristics of anaerobic consortia, gradual adaptation is a common and effective way to improve tolerance to ammonia inhibition. The critical role of acetoclastic methanogens under high ammonia conditions cannot be ignored and needs re-evaluation and rethought.
6.3. Co-Digestion
Reducing the nitrogen content of the substrate by co-digestion and modifying the C/N ratio of the substrate to a suitable range to affect digestion may be the primary strategy to alleviate ammonia inhibition during the anaerobic digestion of nitrogen-rich substrates, and it is easy to implement. Nitrogen-rich substrates such as animal manure, especially chicken manure, are co-digested with carbon-rich substances such as straw and cattle manure. This increases biogas production and is essential for the economics of biogas plants. For example, co-digestion of pig manure with corn stover, rice straw, or energy crops significantly improves microbial diversity, fermentation stability, and methane production performance [,]. Chicken manure and pig manure were used for co-digestion with rice straw, and the C/N ratio reached the maximum methanogenic potential at 25 and showed significant ammonia inhibition at 15 under mesophilic conditions []. In addition, food waste, cattle manure, and corn stover are highly complementary in raw material characteristics, especially C/N []. Co-digestion of 75% food waste and 25% corn straw resulted in a methane yield of 500 mL/g-VS, significantly higher than the anaerobic treatment of food waste or corn straw as a single substrate []. Carbon-rich substrates were properly added to make the C/N ratio of the substrate around 20:1 to obtain better methane production performance in the anaerobic digestion of chicken manure. In addition, the methane production performance of the mixed substrate was better than that of single chicken manure (Table 10). The co-digestion of animal manure and carbon-rich materials significantly improved the treatment of animal manure and the stability of the fermentation process and methane production. Moreover, co-digestion technology has some advantages in reducing ammonia inhibition, such as no need for additional equipment; it can simultaneously realize the stable treatment of various wastes and is conducive to using digestate as fertilizer.
It should be noted that if biogas plants can easily obtain the co-digestion substrate, the co-digestion strategy can increase biogas production and obtain higher profits for the biogas plant. However, for most biogas plants, the co-digestion of carbon-rich substrates often needs to be purchased and transported over long distances, which can inevitably increase the operating costs of the biogas plant. In addition, seasonal production of carbon-rich substrates such as corn, wheat, and rice straw cannot guarantee sufficient co-digestive substrates. Moreover, materials such as straw need to be crushed, and pretreatments such as hydrolysis and the acidogenesis process destroy stubborn components such as lignocellulose, which increases operating costs and processing steps. In addition, the improper storage of carbon-rich substrates causes rot, mildew, odor, and energy loss. All of the above are issues that need to be carefully considered for co-digestion technology. It is necessary to ensure that the economic benefits of the biogas plant are higher than the additional investment costs. This is the critical factor in ensuring the long-term operation of biogas plants.

Table 10.
Co-digestion of chicken manure with carbon-rich substrates.
Table 10.
Co-digestion of chicken manure with carbon-rich substrates.
| Substrate and Mixture Ratio | C/N Ratio | Methane Yield (mL/g-VS) | Findings | Reference |
|---|---|---|---|---|
| Chicken manure: Dairy manure = 50:50 | 27.0 | 234 |
| [] |
| Chicken manure: Chlorella 1067 = 8:2 | 8.9 | 239 |
| [] |
| Chicken manure: Cardboard waste = 35:65 a | 18.7 | 319 |
| [] |
| Chicken manure: Chlorella sp. = 8:2 a | 10.7 | 169 |
| [] |
| Chicken manure: Corn stover = 2:1 a | 20.5 | 450 |
| [] |
| Chicken manure: Corn stover = 1:3 a | 50.0 | 298 |
| [] |
| Chicken manure: Corn stover = 1:1 b | 30.8 | 255 |
| [] |
Note: ND: Data not involved; a: based on the volatile solids; b: based on total solids.
6.4. Supplementation of Trace Elements
Adding trace elements is also critical to maintaining stable operation during the anaerobic digestion of chicken manure. It can improve the tolerance of anaerobic consortia to ammonia, especially for Methanosaeta sp. with weak ammonia tolerance []. Adding Fe2+ and Ni2+ enhanced the acetoclastic methanogen and hydrogenotrophic methanogen activity in the anaerobic digestion of high-solids chicken manure. It increased the contribution of the AM pathway, which was mainly converted by Methanosarcina sp. []. In addition, formate dehydrogenase catalyzes the anaerobic oxidation of formate, which is highly dependent on the trace elements selenium, molybdenum, and tungsten []. Moreover, iron, cobalt, and nickel are essential cofactors for coenzyme M methyltransferase and carbon monoxide dehydrogenase, which play important roles in the hydrogenotrophic methanogenesis process []. Therefore, in theory, adding these trace elements can enhance the metabolic activity of hydrogenotrophic methanogens, promoting hydrogen consumption and keeping it at a low level, thus making the SAO process more thermodynamically favorable.
The addition of trace elements to an anaerobic digestion process dominated by the SAO process has been shown to significantly increase methane production and promote the degradation of organic acids []. However, adding trace elements did not significantly affect the relative abundance of SAOB. Regardless of whether trace elements were added, the relative abundance of SAOB was between 0.02–0.04% in chicken manure digestion []. Quantitative analysis showed no significant changes in the abundance of known SAOB in digesters supplemented with trace element additives in the anaerobic digestion of the organic fraction of municipal solid waste supplemented with egg albumin powder []. The dynamic of the methanogenesis pathway affected by trace elements seems to mainly change the community structure of methanogens in the digester []. Therefore, adding trace elements to reactors with high ammonia levels mainly affects the structure of the microbial community, especially acetoclastic methanogens. Currently, the mechanism of promotion of the methanogen community by trace elements is still unclear, primarily due to the complex types and dosages of the trace elements, with further research thus being necessary.
Although the effectiveness of trace elements has been confirmed in the anaerobic digestion process of different substrates, including animal manure [], biomass straw [], food waste [], etc., there are significant differences in the added types and concentrations of trace elements in the different feedstocks. It is necessary to develop different kinds of trace elements for different substrates and determine how various trace elements promote performance. In addition to higher levels of protein, the nitrogen-rich substrate is accompanied by higher levels of sulfur. Sulfur combines with trace elements to form metal-sulfide precipitates, reducing metal element bioavailability during anaerobic digestion []. This process involves metallic iron only, including zero-valent iron, ferrous iron, iron oxides (magnetite and hematite), etc. []. The addition of iron reduces the hydrogen sulfide level in the biogas and reduces the biotoxicity to anaerobic microorganisms. Studies have shown that iron oxide can enhance the syntrophic oxidation of the acetate process and methane production []. It shows that adding iron to the high ammonia digesters is an effective strategy to improve fermentation performance. Further research is needed to understand the role of trace elements fully.
6.5. Ammonia Removal from Digesters
Based on the high solid characteristic of chicken manure, it is very suitable for high-solids anaerobic digestion technology. However, ammonia inhibition is an inevitable bottleneck. Anaerobic digestion of high-solids chicken manure under low-ammonia conditions by ammonia removal is feasible. For example, anaerobic digestion of high-solids chicken manure with a feed TS concentration of 15% can be achieved by ammonia stripping. The methane yield can be increased by 34% compared with the control reactor without ammonia stripping []. In addition, by using activated carbon, zeolite, and other ammonia absorption materials to reduce the ammonia nitrogen concentration, the solids concentration of the chicken manure feedstock can be increased to 22% []. However, the method of absorbing ammonia nitrogen faces the disadvantage of ammonia saturation. It requires regular replacement of absorption materials and subsequent treatment, which may not be expedient in engineering applications. These studies, however, illustrate a vital direction for further research: achieving high solids/dry anaerobic digestion of chicken manure at relatively low ammonia levels.
Decreasing the ammonia levels will undoubtedly increase methane production, but it is also accompanied by changes in methanogenesis pathways and microbial community structures, enhancing system stability. Therefore, it is necessary to adopt appropriate ammonia removal methods (ammonia stripping and absorption, ion exchange, etc.) to alleviate ammonia inhibition and achieve stable processing capacity. The most important issue is that reducing the ammonia level is conducive to transforming the methanogenesis pathway from the reduction in carbon oxidation to the AM pathway, enhancing the degradation of organic acids, and providing positive feedback to fermentation performance. For example, the ammonia nitrogen concentration of the reactor for the anaerobic digestion of chicken manure was reduced from 6.8 to 5.8 g/L by ammonia stripping, the proportion of the AM pathway increased from 17% to 23%, and the concentration of organic acids in the system also decreased from 21.6 to 15.2 g/L []. In addition, by using membrane absorption (TAN decreased from 6.6 to 2.9 g/L) in a chicken manure anaerobic digestion reactor, Methanosaeta sp. (17%) was detected in the membrane reactor, organic acid concentration also decreased significantly (from 12.3 to 6.3 g/L) []. This fully demonstrates that reducing the ammonia level is conducive to the growth of acetoclastic methanogens, promoting the deep degradation of organic acids. In addition, ammonia stripping or ammonia absorption can control the degree of ammonia removal by regulating the frequency of stripping, the concentration of the acid absorbent, and the membrane area, which are conducive to controlling the cost of ammonia removal within a reasonable range. Additionally, there are various configurations of ammonia stripping in anaerobic digestion, including pretreatment, in-situ, side-stream, and post-treatment []. In-situ ammonia removal would find more applications, both from the economic and the technical point of view.
7. Conclusions
The properties of chicken manure determine the complexity of its microbial community structure, activities, and methanogenic pathway compared to other animal manures during anaerobic treatment. The ammonia level is the main factor affecting methane production performance. Selecting more suitable process parameters can alleviate the ammonia inhibition impacts to a certain extent, such as longer residence time or lower organic loading rate. In addition, adaptation, adding trace elements, co-digestion, and ammonia removal can significantly improve the anaerobic digestion performance of chicken manure. Therefore, anaerobic treatment with the feeding of high-solid chicken manure is possible through deliberate operational strategies. Increased knowledge of syntrophic acetate oxidation, hydrogenotrophic and acetoclastic methanogenesis is helpful for maintaining process stability. This requires an in-depth analysis of methane formation from the perspective of microbiology and an analysis of changes in microbial community structure at the fundamental level.
Author Contributions
Y.S.: Investigation, Writing an original draft. W.Q.: Writing review & editing, Supervision, Project administration, Funding acquisition, Conceptualization. J.Z.: Writing original draft. R.D.: Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Beijing, China (No. 6222029) and the Key Research and Development Program of Hainan Province of China (ZDYF2021SHFZ065).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kang, Y.; Yang, Q.; Bartocci, P.; Wei, H.; Liu, S.S.; Wu, Z.; Zhou, H.; Yang, H.; Fantozzi, F.; Chen, H. Bioenergy in China: Evaluation of domestic biomass resources and the associated greenhouse gas mitigation potentials. Renew. Sustain. Energy Rev. 2020, 127, 109842. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef]
- Ileleji, K.E.; Martin, C.; Jones, D. Chapter 17: Basics of Energy Production through Anaerobic Digestion of Livestock Manure. In Bioenergy; Academic Press: Cambridge, MA, USA, 2015; pp. 287–295. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of temperature and carbon-nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: Focusing on ammonia inhibition. PLoS ONE 2014, 9, e97265. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, Y.; Zhadan, S.; Bochmann, G.; Salyuk, A.; Nykyforov, V. Dry Anaerobic Digestion of Chicken Manure: A Review. Appl. Sci. 2020, 10, 7825. [Google Scholar] [CrossRef]
- Westerholm, M.; Moestedt, J.; Schnurer, A. Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef]
- Feng, X.M.; Karlsson, A.; Svensson, B.H.; Bertilsson, S. Impact of trace element addition on biogas production from food industrial waste—Linking process to microbial communities. FEMS Microbiol. Ecol. 2010, 74, 226–240. [Google Scholar] [CrossRef]
- Fitamo, T.; Treu, L.; Boldrin, A.; Sartori, C.; Angelidaki, I.; Scheutz, C. Microbial population dynamics in urban organic waste anaerobic co-digestion with mixed sludge during a change in feedstock composition and different hydraulic retention times. Water Res. 2017, 118, 261–271. [Google Scholar] [CrossRef]
- Palakodeti, A.; Azman, S.; Rossi, B.; Dewil, R.; Appels, L. A critical review of ammonia recovery from anaerobic digestate of organic wastes via stripping. Renew. Sustain. Energy Rev. 2021, 143, 110903. [Google Scholar] [CrossRef]
- Fuchs, W.; Wang, X.; Gabauer, W.; Ortner, M.; Li, Z. Tackling ammonia inhibition for efficient biogas production from chicken manure: Status and technical trends in Europe and China. Renew. Sustain. Energy Rev. 2018, 97, 186–199. [Google Scholar] [CrossRef]
- Niu, Q.; Takemura, Y.; Kubota, K.; Li, Y.Y. Comparing mesophilic and thermophilic anaerobic digestion of chicken manure: Microbial community dynamics and process resilience. Waste Manag. 2015, 43, 114–122. [Google Scholar] [CrossRef]
- Gallert, C.; Bauer, S.; Winter, J. Effect of ammonia on the anaerobic degradation of protein by a mesophilic and thermophilic biowaste population. Appl. Microbiol. Biotechnol. 1998, 50, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Thong-On, A.; Suzuki, K.; Noda, S.; Inoue, J.; Kajiwara, S.; Ohkuma, M. Isolation and characterization of anaerobic bacteria for symbiotic recycling of uric acid nitrogen in the gut of various termites. Microbes Environ. 2012, 27, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Schnurer, A.; Schink, B.; Svensson, B.H. Clostridium ultunense sp. nov., a Mesophilic Bacterium Oxidizing Acetate in Syntrophic Association with a Hydrogenotrophic Methanogenic Bacterium. Int. J. Syst. Bacteriol. 1996, 46, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Dfirre, P.; Andreesen, J.R. Anaerobic Degradation of Uric Acid via Pyrimidine Derivatives. Arch. Microbiol. 1982, 131, 255–260. [Google Scholar] [CrossRef]
- Potrikus, C.J.; Breznak, J.A. Anaerobic Degradation of Uric Acid by Gut Bacteria of termetest. Appl. Environ. Microbiol. 1980, 40, 125–132. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Hao, T.; Xiao, Y.; Varjani, S. Transiting from the inhibited steady-state to the steady-state through the ammonium bicarbonate mediation in the anaerobic digestion of low-C/N-ratio food wastes. Bioresour. Technol. 2022, 351, 127046. [Google Scholar] [CrossRef]
- Orhan, Y.; Burak, D. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Niu, Q.; Hojo, T.; Qiao, W.; Qiang, H.; Li, Y.-Y. Characterization of methanogenesis, acidogenesis and hydrolysis in thermophilic methane fermentation of chicken manure. Chem. Eng. J. 2014, 244, 587–596. [Google Scholar] [CrossRef]
- Bi, S.; Qiao, W.; Xiong, L.; Ricci, M.; Adani, F.; Dong, R. Effects of organic loading rate on anaerobic digestion of chicken manure under mesophilic and thermophilic conditions. Renew. Energy 2019, 139, 242–250. [Google Scholar] [CrossRef]
- Yin, D.; Qiao, W.; Negri, C.; Adani, F.; Fan, R.; Dong, R.-J. Enhancing hyper-thermophilic hydrolysis pretreatment of chicken manure for biogas production by in-situ gas phase ammonia stripping. Bioresour. Technol. 2019, 287, 121470. [Google Scholar] [CrossRef]
- Westerholm, M.; Bettina, M.; Arthurson, V.; Anna, S. Changes in the Acetogenic Population in a Mesophilic Anaerobic Digester in Response to Increasing Ammonia Concentration. Microbes Environ. 2011, 26, 347–353. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, L.; Li, C.; Angelidaki, I.; Zhu, G. Deep insights into the network of acetate metabolism in anaerobic digestion: Focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef]
- Wang, D.; Duan, Y.; Yang, Q.; Liu, Y.; Ni, B.J.; Wang, Q.; Zeng, G.; Li, X.; Yuan, Z. Free ammonia enhances dark fermentative hydrogen production from waste activated sludge. Water Res. 2018, 133, 272–281. [Google Scholar] [CrossRef]
- Hendriksen, H.V.; Ahring, B.K. Effects of ammonia on growth and morphology of thermophilic hydrogen-oxidizing methanogenic bacteria. FEMS Microbiol. Ecol. 1991, 85, 241–245. [Google Scholar] [CrossRef]
- Ripoll, E.; López, I.; Borzacconi, L. Hydrogenotrophic activity: A tool to evaluate the kinetics of methanogens. J. Environ. Manag. 2020, 270, 110937. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.; Méndez, R.; Lema, J.M. Methanogenic and non-methanogenic activity tests. Theoretical basis and experimental set up. Water Res. 1993, 27, 1361–1376. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Qiao, W.; Xiong, L.; Mahdy, A.; Yin, D.; Song, Y.; Dong, R. Metabolic performance of anaerobic digestion of chicken manure under wet, high solid, and dry conditions. Bioresour. Technol. 2020, 296, 122342. [Google Scholar] [CrossRef]
- Jawed, M.; Tare, V. Methanogenic activity and performance of UASB, DSFF and USFF reactors. Water Sci. Technol. 1996, 34, 483–487. [Google Scholar] [CrossRef]
- Hao, L.P.; Lu, F.; He, P.J.; Li, L.; Shao, L.M. Predominant Contribution of Syntrophic Acetate Oxidation to Thermophilic Methane Formation at High Acetate Concentrations. Environ. Sci. Technol. 2011, 45, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Qiao, W.; Xiong, L.; Mandy, A.; Wandera, S.M.; Yin, D.; Dong, R. Improved high solid anaerobic digestion of chicken manure by moderate in situ ammonia stripping and its relation to metabolic pathway. Renew. Energy 2020, 146, 2380–2389. [Google Scholar] [CrossRef]
- Sung, S.; Tao, L. Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 2003, 53, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.M.; Westerholm, M.; Qiao, W.; Bi, S.J.; Wandera, S.M.; Fan, R.; Jiang, M.M.; Dong, R.J. An explanation of the methanogenic pathway for methane production in anaerobic digestion of nitrogen-rich materials under mesophilic and thermophilic conditions. Bioresour. Technol. 2018, 264, 42–50. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Qiao, W.; Mandy, A.; Xiong, L.; Yin, D.; Fan, R.; Dach, J.; Dong, R. Enhanced methanogenic performance and metabolic pathway of high solid anaerobic digestion of chicken manure by Fe 2+ and Ni 2+ supplementation. Waste Manag. 2019, 94, 10–17. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Hu, W.; Mahdy, A.; Dong, T.; Sun, Y.; Qiao, W.; Dong, R. The metabolic performance and microbial communities of anaerobic digestion of chicken manure under stressed ammonia condition: A case study of a 10-year successful biogas plant. Renew. Energy 2021, 167, 644–651. [Google Scholar] [CrossRef]
- Wandera, S.M.; Qiao, W.; Algapani, D.E.; Bi, S.; Yin, D.; Qi, X.; Liu, Y.; Dach, J.; Dong, R. Searching for possibilities to improve the performance of full scale agricultural biogas plants. Renew. Energy 2018, 116, 720–727. [Google Scholar] [CrossRef]
- Satoh, H.; Bandara, W.M.K.R.T.W.; Sasakawa, M.; Nakahara, Y.; Takahashi, M.; Okabe, S. Enhancement of organic matter degradation and methane gas production of anaerobic granular sludge by degasification of dissolved hydrogen gas. Bioresour. Technol. 2017, 244, 768–775. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Angelidaki, I. Ammonia inhibition on hydrogen enriched anaerobic digestion of manure under mesophilic and thermophilic conditions. Water Res. 2016, 105, 314–319. [Google Scholar] [CrossRef]
- Conklin, A.; Stensel, H.D.; Ferguson, J. Growth Kinetics and Competition Between Methanosarcina and Methanosaeta in Mesophilic Anaerobic Digestion. Water Environ. Res. 2006, 78, 486–496. [Google Scholar] [CrossRef]
- Kamagata, Y.; Mikami, E. Isolation and Characterization of a Novel Thermophilic Methanosaeta Strain. FEMS Microbiol. Lett. 1991, 179, 393–400. [Google Scholar] [CrossRef]
- Kern, T.; Fischer, M.A.; Deppenmeier, U.; Schmitz, R.A.; Rother, M. Methanosarcina flavescens sp. nov., a methanogenic archaeon isolated from a full-scale anaerobic digester. Int. J. Syst. Evol. Microbiol. 2016, 66, 1533–1538. [Google Scholar] [CrossRef]
- Abid, M.; Wu, J.; Seyedsalehi, M.; Hu, Y.-Y.; Tian, G. Novel insights of impacts of solid content on high solid anaerobic digestion of cow manure: Kinetics and microbial community dynamics. Bioresour. Technol. 2021, 333, 125205. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Fotidis, I.A.; Kissas, K.; Angelidaki, I. Effect of different ammonia sources on aceticlastic and hydrogenotrophic methanogens. Bioresour. Technol. 2018, 250, 390–397. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B.K. Thermophilic anaerobic digestion of livestock waste: The effect of ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Z.; Shi, G.; Arhin, S.G.; Papadakis, V.G.; Goula, M.A.; Zhou, L.; Zhang, Y.; Liu, G.; Wang, W. Methane production from acetate, formate and H2/CO2 under high ammonia level: Modified ADM1 simulation and microbial characterization. Sci. Total Environ. 2021, 783, 147581. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Qiao, W.; Qiang, H.; Hojo, T.; Li, Y.Y. Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: Stability, inhibition and recovery. Bioresour. Technol. 2013, 137, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.B.; Sprott, G.D. Methanosaeta concilii gen. nov. sp. nov. (“Methanothrix concilii”) and Methanosaeta thermoacetophila nom. rev., comb. nov. Int. J. Syst. Bacteriol. 1990, 40, 79–82. [Google Scholar] [CrossRef]
- Kai, M.; Liu, X.; Dong, X. Methanosaeta harundinacea sp. nov., a novel acetate-scavenging methanogen isolated from a UASB reactor. Int. J. Syst. Evol. Microbiol. 2006, 56, 127–131. [Google Scholar] [CrossRef]
- Mori, K.; Iino, T.; Suzuki, K.I.; Yamaguchi, K.; Kamagata, Y. Aceticlastic and NaCl-Requiring Methanogen “Methanosaeta pelagica” sp. nov., Isolated from Marine Tidal Flat Sediment. Appl. Environ. Microbiol. 2012, 78, 3416–3423. [Google Scholar] [CrossRef]
- Kamagata, Y.; Kawasaki, H.; Oyaizu, H.; Nakamura, K.; Mikami, E.; Endo, G.; Koga, Y.; Yamasato, K. Characterization of Three Thermophilic Strains of Methanothrix (Methanosaeta) thermophila sp. nov. and Rejection of Methanothrix (Methanosaeta) thermoacetophila. Int. J. Syst. Bacteriol. 1992, 42, 463–468. [Google Scholar] [CrossRef]
- Bock, A.K.; Prieger-Kraft, A.; Schönheit, P. Pyruvate—A novel substrate for growth and methane formation in Methanosarcina barkeri. Arch. Microbiol. 1994, 161, 33–46. [Google Scholar] [CrossRef]
- Zinder, S.H.; Mah, R.A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H2-CO2 for Methanogenesis. Appl. Environ. Microbiol. 1979, 38, 996. [Google Scholar] [CrossRef]
- Cheng, L.; Qiu, T.L.; Li, X.; Wang, W.D.; Deng, Y.; Yin, X.B.; Zhang, H. Isolation and characterization of Methanoculleus receptaculi sp. nov. from Shengli oil field, China. FEMS Microbiol. Lett. 2008, 285, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zellner, G.; Messner, P.; Winter, J.; Stackebrandt, E. Methanoculleus palmolei sp. nov., an irregularly coccoid methanogen from an anaerobic digester treating wastewater of a palm oil plant in North-Sumatra, Indonesia. Int. J. Syst. Bacteriol. 1998, 48, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-C.; Chen, S.-C.; Shu, C.-M.; Chiou, M.-S.; Wang, C.-C.; Chuang, M.-J.; Hong, T.-Y.; Liu, C.-C.; Lai, L.-J.; Hua, J.J. Methanocalculus taiwanensis sp. nov., isolated from an estuarine environment. Int. J. Syst. Evol. Microbiol. 2002, 52, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, B.; Fardeau, M.-L.; Cayol, J.-L.; Magot, M.; Patel, B.K.C.; Prensier, G.; Garcia, J.-L. Methanocalculus halotolerans gen. nov., sp. nov., isolated from an oil-producing well. Int. J. Syst. Bacteriol. 1998, 48, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Yamamoto, H.; Kamagata, Y.; Hatsu, M.; Takamizawa, K. Methanocalculus pumilus sp. nov., a heavy-metal-tolerant methanogen isolated from a waste-disposal site. Int. J. Syst. Evol. Microbiol. 2000, 50, 1723–1729. [Google Scholar] [CrossRef]
- Cuzin, N.; Ouattara, A.S.; Labat, M.; Garcia, J.L. Methanobacterium congolense sp. nov., from a methanogenic fermentation of cassava peel. Int. J. Syst. Evol. Microbiol. 2001, 51, 489–493. [Google Scholar] [CrossRef]
- Mori, K.; Harayama, S. Methanobacterium petrolearium sp. nov. and Methanobacterium ferruginis sp. nov., mesophilic methanogens isolated from salty environments. Int. J. Syst. Evol. Microbiol. 2011, 61, 138–143. [Google Scholar] [CrossRef]
- Kotelnikova, S.V.; Obraztsova, A.Y.; Gongadze, G.M.; Laurinavichius, K.S. Methanobacterium thermoflexum sp. nov. and Methanobacterium defluvii sp. nov., Thermophilic Rod-Shaped Methanogens Isolated from Anaerobic Digestor Sludge. Syst. Appl. Microbiol. 1993, 16, 427–435. [Google Scholar] [CrossRef]
- Savant, D.V.; Shouche, Y.S.; Prakash, S.; Ranade, D.R. Methanobrevibacter acididurans sp. nov., a novel methanogen from a sour anaerobic digester. Int. J. Syst. Evol. Microbiol. 2002, 52, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.J.; Paynter, M.J.B.; Gupta, R. Characterization of Methanococcus maripaludis sp. nov., a new methanogen isolated from salt marsh sediment. Arch. Microbiol. 1983, 135, 91–97. [Google Scholar] [CrossRef]
- Dridi, B.; Fardeau, M.-L.; Ollivier, B.; Raoult, D.; Drancourt, M. Methanomassiliicoccus luminyensis gen. nov., sp nov., a methanogenic archaeon isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2012, 62, 1902–1907. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Y.; Li, Y.; Chi, Y.; Yang, M. Rapid establishment of thermophilic anaerobic microbial community during the one-step startup of thermophilic anaerobic digestion from a mesophilic digester. Water Res. 2015, 69, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hu, W.; Qiao, W.; Westerholm, M.; Wandera, S.M.; Dong, R. Upgrading the performance of high solids feeding anaerobic digestion of chicken manure under extremely high ammonia level. Renew. Energy 2022, 194, 13–20. [Google Scholar] [CrossRef]
- Mahdy, A.; Bi, S.; Song, Y.; Qiao, W.; Dong, R. Overcome inhibition of anaerobic digestion of chicken manure under ammonia-stressed condition by lowering the organic loading rate. Bioresour. Technol. Rep. 2020, 9, 100359. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Ibragimov, E.M.; Vankov, P.Y.; Miluykov, V.A.; Ziganshin, A.M. Comparison of anaerobic digestion strategies of nitrogen-rich substrates: Performance of anaerobic reactors and microbial community diversity. Waste Manag. 2017, 59, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Lauterböck, B.; Nikolausz, M.; Lv, Z.; Baumgartner, M.; Liebhard, G. Improvement of anaerobic digestion performance by continuous nitrogen removal with a membrane contactor treating a substrate rich in ammonia and sulfide. Bioresour. Technol. 2014, 158, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Kamagata, Y.; Hanada, S.; Shoun, H. Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 2000, 50, 1601–1609. [Google Scholar] [CrossRef]
- Balk, M.; Weijma, J.; Stams, A.J.M. Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int. J. Syst. Evol. Microbiol. 2002, 52, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Roos, S.; Ürer, A.S. Syntrophaceticus schinkii gen. nov., sp. nov., an anaerobic, syntrophic acetate-oxidizing bacterium isolated from a mesophilic anaerobic filter. FEMS Microbiol. Lett. 2010, 309, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Roos, S.; Schnürer, A. Tepidanaerobacter acetatoxydans sp. nov., an anaerobic, syntrophic acetate-oxidizing bacterium isolated from two ammonium-enriched mesophilic methanogenic processes. Syst. Appl. Microbiol. 2011, 34, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Song, L.; Liu, X.; Dong, X. Tepidimicrobium xylanilyticum sp. nov., an anaerobic xylanolytic bacterium, and emended description of the genus Tepidimicrobium. Int. J. Syst. Evol. Microbiol. 2009, 59, 2698–2701. [Google Scholar] [CrossRef]
- Westerholm, M.; Leven, L.; Schnurer, A. Bioaugmentation of syntrophic acetate-oxidizing culture in biogas reactors exposed to increasing levels of ammonia. Appl. Environ. Microbiol. 2012, 78, 7619–7625. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef]
- Yan, Y.; Du, Z.; Zhang, L.; Feng, L.; Sun, D.; Dang, Y.; Holmes, D.E.; Smith, J.A. Identification of parameters needed for optimal anaerobic co-digestion of chicken manure and corn stover. RSC Adv. 2019, 9, 29609–29618. [Google Scholar] [CrossRef]
- Schnürer, A.; Nordberg, A. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature. Water Sci. Technol. 2008, 57, 735–740. [Google Scholar] [CrossRef]
- Jiang, Y.; Banks, C.; Zhang, Y.; Heaven, S.; Longhurst, P. Quantifying the percentage of methane formation via acetoclastic and syntrophic acetate oxidation pathways in anaerobic digesters. Waste Manag. 2018, 71, 749–756. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, J.; Guivernau, M.; Fernandez, B.; Vila, J.; Vinas, M.; Riau, V.; Prenafeta-Boldu, F.X. Functional biodiversity and plasticity of methanogenic biomass from a full-scale mesophilic anaerobic digester treating nitrogen-rich agricultural wastes. Sci. Total Environ. 2019, 649, 760–769. [Google Scholar] [CrossRef]
- Sun, L.; Muller, B.; Westerholm, M.; Schnurer, A. Syntrophic acetate oxidation in industrial CSTR biogas digesters. J. Biotechnol. 2014, 171, 39–44. [Google Scholar] [CrossRef]
- Westerholm, M.; Dolfing, J.; Schnurer, A. Growth Characteristics and Thermodynamics of Syntrophic Acetate Oxidizers. Environ. Sci. Technol. 2019, 53, 5512–5520. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Schmidt, T.; Lv, Z.; Liebetrau, J.; Richnow, H.H.; Kleinsteuber, S.; Nikolausz, M. Reduction of the hydraulic retention time at constant high organic loading rate to reach the microbial limits of anaerobic digestion in various reactor systems. Bioresour. Technol. 2016, 217, 62–71. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.H.; Zheng, Y.; Liu, J.B.; Xiong, W.P.; Zhang, Y.R.; Lu, Y.; Xue, W.J.; Fan, C.Z. Organic loading rate and hydraulic retention time shape distinct ecological networks of anaerobic digestion related microbiome. Bioresour. Technol. 2018, 262, 184–193. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.; Gao, J.; Xie, Y.; Li, L.; Qin, S.; Wang, Q.; Mao, D.; Luo, Y. Simultaneous removal of antibiotics and antibiotic resistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies. Water Res. 2019, 159, 511–520. [Google Scholar] [CrossRef]
- Gehring, T.; Klang, J.; Niedermayr, A.; Berzio, S.; Immenhauser, A.; Klocke, M.; Wichern, M.; Lubken, M. Determination of methanogenic pathways through carbon isotope (delta13C) analysis for the two-stage anaerobic digestion of high-solids substrates. Environ. Sci. Technol. 2015, 49, 4705–4714. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Yu, Q.; Ma, R. Removal of nitrogen from chicken manure anaerobic digestion for enhanced biomethanization. Fuel 2018, 232, 395–404. [Google Scholar] [CrossRef]
- Nie, H.; Jacobi, H.F.; Strach, K.; Xu, C.; Zhou, H.; Liebetrau, J. Mono-fermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Bayrakdar, A.; Molaey, R.; Sürmeli, R.Ö.; Sahinkaya, E.; Çalli, B. Biogas production from chicken manure: Co-digestion with spent poppy straw. Int. Biodeterior. Biodegrad. 2017, 119, 205–210. [Google Scholar] [CrossRef]
- Vrieze, J.D.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef]
- Kato, S.; Sasaki, K.; Watanabe, K.; Yumoto, I.; Kamagata, Y. Physiological and transcriptomic analyses of the thermophilic, aceticlastic methanogen Methanosaeta thermophila responding to ammonia stress. Microbes Environ. 2014, 29, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Müller, B.; Isaksson, S.; Schnürer, A. Trace element and temperature effects on microbial communities and links to biogas digester performance at high ammonia levels. Biotechnol. Biofuels 2015, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Xu, X.; Yang, F.; Li, C.; Zhou, M. Performance enhancement by rumen cultures in anaerobic co-digestion of corn straw with pig manure. Biomass Bioenergy 2018, 115, 120–129. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, W.; Luo, W.; Fang, H.; Lv, H.; Liu, R.; Niu, Q. Anaerobic co-digestion of chicken manure and cardboard waste: Focusing on methane production, microbial community analysis and energy evaluation. Bioresour. Technol. 2021, 321, 124429. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Yu, L.; Zou, D.; Yuan, H. Mesophilic anaerobic co-digestion of cattle manure and corn stover with biological and chemical pretreatment. Bioresour. Technol. 2015, 198, 431–436. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, T.; Xing, W.; Li, R.; Yang, T.; Yao, N.; Lv, D. Links between carbon/nitrogen ratio, synergy and microbial characteristics of long-term semi-continuous anaerobic co-digestion of food waste, cattle manure and corn straw. Bioresour. Technol. 2022, 343, 126094. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon-nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Li, R.; Duan, N.; Zhang, Y.; Liu, Z.; Li, B.; Zhang, D.; Lu, H.; Dong, T. Co-digestion of chicken manure and microalgae Chlorella 1067 grown in the recycled digestate: Nutrients reuse and biogas enhancement. Waste Manag. 2017, 70, 247–254. [Google Scholar] [CrossRef]
- Li, R.; Duan, N.; Zhang, Y.; Liu, Z.; Li, B.; Zhang, D.; Dong, T. Anaerobic co-digestion of chicken manure and microalgae Chlorella sp.: Methane potential, microbial diversity and synergistic impact evaluation. Waste Manag. 2017, 68, 120–127. [Google Scholar] [CrossRef]
- Qiong, Y.; Chen, S.; Ronghou, L.; Dominic, Y.; Zhu, X.; Bai, R.; Liu, M.; Sun, M. Anaerobic co-digestion of corn stover and chicken manure using continuous stirred tank reactor: The effect of biochar addition and urea pretreatment. Bioresour. Technol. 2020, 319, 124197. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Chen, C.; Liu, G.; He, Y.; Liu, X. Biogas production from co-digestion of corn stover and chicken manure under anaerobic wet, hemi-solid, and solid state conditions. Bioresour. Technol. 2013, 149, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Z.; Ran, W.; Yuan, H.; Li, X. Performance and microbial community dynamics in anaerobic co-digestion of chicken manure and corn stover with different modification methods and trace element supplementation strategy. Bioresour. Technol. 2021, 325, 124713. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Lee, C. A review of the effects of iron compounds on methanogenesis in anaerobic environments. Renew. Sustain. Energy Rev. 2019, 113, 109282. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Anaerobic digestion of chicken manure: Influence of trace element supplementation. Eng. Life Sci. 2019, 19, 143–150. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Calli, B. Long-term inuence of trace element deciency on anaerobic mono-digestion of chicken manure. J. Environ. Manag. 2018, 223, 743–748. [Google Scholar] [CrossRef]
- Cai, Y.; Hua, B.; Gao, L.; Hu, Y.; Yuan, X.; Cui, Z.; Zhu, W.; Wang, X. Effects of adding trace elements on rice straw anaerobic mono-digestion: Focus on changes in microbial communities using high-throughput sequencing. Bioresour. Technol. 2017, 239, 454–463. [Google Scholar] [CrossRef]
- Aquino, S.F.; Stuckey, D.C. Bioavailability and Toxicity of Metal Nutrients during Anaerobic Digestion. J. Environ. Eng. 2007, 133, 28–35. [Google Scholar] [CrossRef]
- Cai, Y.; Janke, L.; Meng, X.; Zheng, Z.; Zhao, X.; Pröter, J.; Schäfer, F. The absolute concentration and bioavailability of trace elements: Two vital parameters affecting anaerobic digestion performance of chicken manure leachate. Bioresour. Technol. 2022, 350, 126909. [Google Scholar] [CrossRef]
- Zhou, M.; Li, C.; Ni, F.; Chen, A.; Li, M.; Shen, G.; Deng, Y.; Deng, L. Packed activated carbon particles triggered a more robust syntrophic pathway for acetate oxidation-hydrogenotrophic methanogenesis at extremely high ammonia concentrations. Renew. Energy 2022, 191, 305–317. [Google Scholar] [CrossRef]
- Yellezuome, D.; Zhu, X.; Wang, Z.; Liu, R. Mitigation of ammonia inhibition in anaerobic digestion of nitrogen-rich substrates for biogas production by ammonia stripping: A review. Renew. Sustain. Energy Rev. 2022, 157, 112043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).