Particle Agglomeration of Biomass and Plastic Waste during Their Thermochemical Fixed-Bed Conversion
Abstract
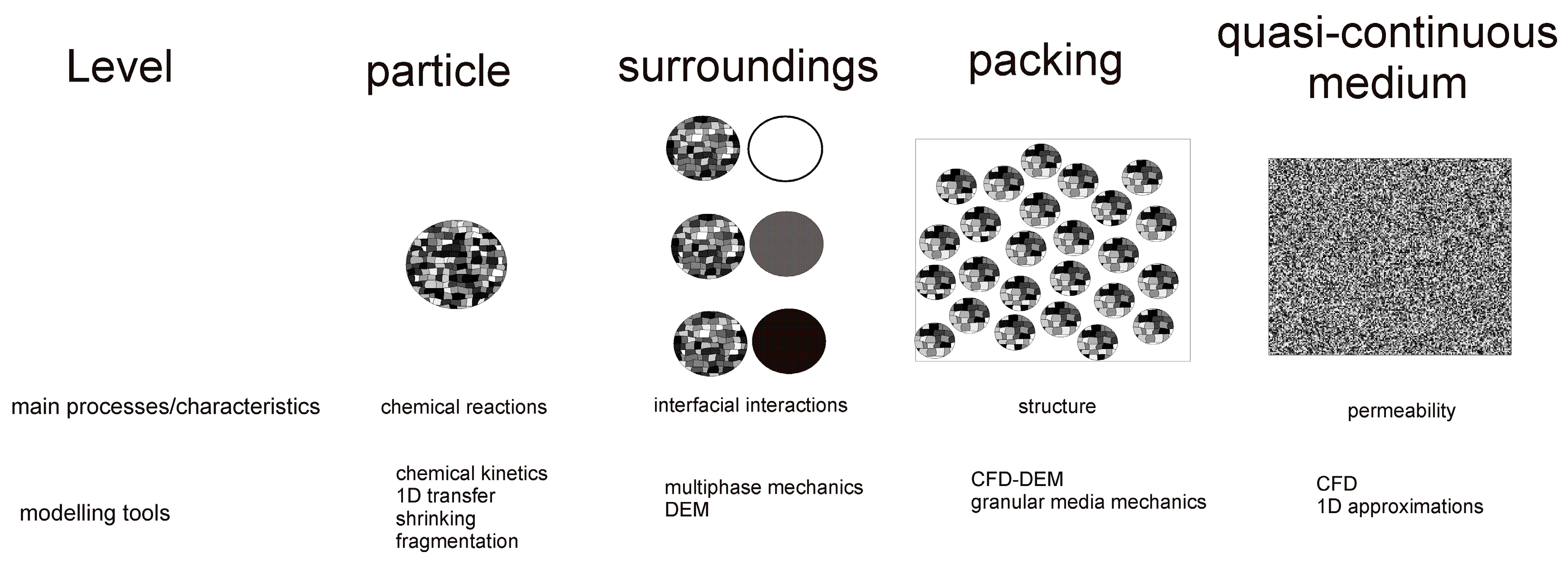
1. Introduction
2. Features of Phase Transitions of the Mineral and Organic Parts of Fuels
2.1. Ash Melting
2.2. Organic Matter Melting and Decomposition
3. Agglomeration in Thermochemical Conversion Reactors
3.1. Fluidised Beds
3.2. Fixed Beds
3.2.1. Basic Schemes of Fixed Bed Conversion and Control Possibilities
3.2.2. Experimental Results
| Ref. | Conversion Process | Reactor Type and Dimensions | Gaseous Reagent | Fuel Composition | Phenomena Observed |
|---|---|---|---|---|---|
| [104] | Combustion | Grate combustor (0.5 MWth) | Air | Wood. agricultural waste | Ash sintering |
| [105] | Gasification | Shaft reactor (length 400 mm, internal diameter 66 mm) | Air, steam | Peat | Ash-sand sintering |
| [108] | Gasification | Updraft gasifier (bed height 1.5 m, internal diameter 0.28 m) | Air, oxygen, steam | Wood | Bridging at high fuel moisture |
| [98] | Gasification | Downdraft gasifier (bed height 0.8 m, internal diameter 0.92 m) | Air | Wood | Channelling |
| [109] | Gasification | Downdraft gasifier (bed height 0.4 m, internal diameter 0.35 m) | Air | Wood | Bridging |
| [110] | Gasification | Downdraft gasifier with rotating grate (bed height 0.26 m, internal diameter 0.22 m) | Air | Garden waste | Accumulated ash sintering |
| [111] | Gasification | Downdraft gasifier with rotating grate (bed height 0.26 m, internal diameter 0.22 m) | Air | Biomass waste, coal | Accumulated ash sintering |
| [112] | Gasification | Shaft reactor (bed height 0.3 m, internal diameter 45 mm) | Air | Charcoal, polyethylene | Oxidation front instability due to melting polymer flow |
| [113] | Gasification | Shaft reactor (bed height 0.3 m, internal diameter 45 mm) | Air | Charcoal, polyurethane | Oxidation front instability due to melting polymer flow |
| [118] | Gasification | Downdraft gasifier (reactor height 2.3 m, internal diameter 0.6 m) | Air | Municipal waste, straw | Agglomeration at MSW fraction of 60% |
| [119] | Gasification | Updraft gasifier (bed height 0.35 m, internal diameter 0.15 m) | Air | Sawdust, polyethylene | Agglomeration, deposition |
| [121] | Gasification | Downdraft gasifier (bed height 0.5 m, internal diameter 0.16–0.3 m) | Air | Wood, sewage sludge | Ash agglomeration |
| [127] | Gasification | Downdraft gasifier with rotating grate (bed height 0.26 m, internal diameter 0.22 m) | Air | Garden waste, polyethylene | Ash sintering |
| [128] | Gasification | Downdraft gasifier (bed height 0.4 m, internal diameter 0.35 m) | Air | Fiber-plastic waste | Ash agglomeration |
| [130] | Gasification | Downdraft gasifier GEK | Air | Paper industry waste | Agglomeration caused by plastic melting in the pyrolysis zone |
| [131] | Gasification | Downdraft gasifier (bed height 0.15 m, internal diameter 0.1 m) | Air | Refuse derived fuel | Ash agglomeration |
| [132] | Gasification | Updraft gasifier (bed height 1.1 m, internal diameter 0.15 m) | Oxygen | Waste | Difficult operation at bed height above 0.7 m |
| [139] | Combustion | Updraft gasifier (bed height 1.9 m, internal diameter 0.15 m) | Air | Wood, marine plastics | Flame extinction under high plastic content |
3.2.3. An Attempt to Classify the Plastic-Containing Fuel Conversion Conditions with Respect to Agglomeration
4. Mathematical Modelling
4.1. Numerical Models of Combustion and Gasification Processes
4.2. Filtration in Reactive Media
4.3. Interfacial Interactions
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, K.D.; Jain, S. Municipal solid waste generation, composition, and management: The global scenario. Soc. Responsib. J. 2020, 16, 917–948. [Google Scholar] [CrossRef]
- Cai, W.; Luo, Z.; Zhou, J.; Wang, Q. A review on the selection of raw materials and reactors for biomass fast pyrolysis in China. Fuel Process. Technol. 2021, 221, 106919. [Google Scholar] [CrossRef]
- Malkow, T. Novel and innovative pyrolysis and gasification technologies for energy efficient and environmentally friendly sound MSW disposal. Waste Manag. 2004, 24, 53–79. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef]
- Tugov, A.N.; Ryabov, G.A.; Shtegman, A.V.; Ryzhii, I.A.; Litun, D.S. All-Russia Thermal Engineering Institute experience in using difficult to burn fuels in the power industry. Therm. Eng. 2016, 63, 455–462. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martinez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Goh, Y.R.; Lim, C.N.; Zakaria, R.; Chan, K.H.; Reynolds, G.; Yang, Y.B.; Siddal, R.G.; Nasserzadeh, V.; Swithenbank, J. Mixing, modelling and measurements of incinerator bed combustion. Process Saf. Environ. Prot. 2000, 78, 21–32. [Google Scholar] [CrossRef]
- Ocfemia, K.C.S.; Bell, P.S. Thermal Decomposition Process for Reducing Agglomerate Formation. U.S. Patent 9,416,006, 16 August 2016. [Google Scholar]
- Gryaznov, N.S. Coking Theory Basics; Metallurgiya: Moscow, Russia, 1976. (In Russian) [Google Scholar]
- Chen, Y.; Lee, S.; Tahmasebi, A.; Bai, J.; Mahoney, M.; Yu, J. A review of the state-of-the-art research on carbon structure evolution during the coking process: From plastic layer chemistry to 3D carbon structure establishment. Fuel 2020, 271, 117657. [Google Scholar] [CrossRef]
- Kang, S.-W.; Sarofim, A.F.; Beer, J.M. Agglomerate formation during coal combustion: A mechanistic model. Combust. Flame 1991, 86, 258–268. [Google Scholar] [CrossRef]
- Nomura, S.; Thomas, K.M. Fundamental aspects of coal structural changes in the thermoplastic phase. Fuel 1998, 77, 829–836. [Google Scholar] [CrossRef]
- Riva, L.; Cardarelli, A.; Andersen, G.J.; Buo, T.V.; Barbanera, M.; Bartocci, P.; Fantozzi, F.; Nielsen, H.K. On the self-heating behavior of upgraded biochar pellets blended with pyrolysis oil: Effects of process parameters. Fuel 2020, 278, 118395. [Google Scholar] [CrossRef]
- Zaini, I.N.; Wen, Y.; Mousa, E.; Jonsson, P.G.; Yang, W. Primary fragmentation behavior of refuse derived fuel pellets during rapid pyrolysis. Fuel Process. Technol. 2021, 216, 106796. [Google Scholar] [CrossRef]
- Kolapkar, S.S.; Zinchik, S.; Burli, P.; Lin, Y.; Hartley, D.S.; Klinger, J.; Handler, R.; Bar-Ziv, E. Integrated torrefaction-extrusion system for solid fuel pellet production from mixed fiber-plastic wastes: Techno-economic analysis and life cycle assessment. Fuel Process. Technol. 2022, 226, 107094. [Google Scholar] [CrossRef]
- Ge, Z.; Cao, X.; Zha, Z.; Ma, Y.; Zeng, M.; Wu, Y.; Li, F.; Zhang, H. The sintering analysis of biomass waste ash based on the in-situ exploration and thermal chemical calculation in the gasification process. Combust. Flame 2022, 245, 112381. [Google Scholar] [CrossRef]
- Litun, D.S.; Ryabov, G.Y. Study of the Microstructure and Chemical Composition of Coke Particles Formed after Fragmentation During Rapid Pyrolysis of Wood. Russ. Int. J. Ind. Eng. 2018, 6, 28–32. [Google Scholar] [CrossRef]
- Kuba, M.; Skoglund, N.; Ohman, M.; Hofbauer, H. A review on bed material particle layer formation and its positive influence on the performance of thermo-chemical biomass conversion in fluidized beds. Fuel 2021, 291, 120214. [Google Scholar] [CrossRef]
- Sharma, T.; Ratner, A. Analysis and Characterization of Metallic Nodules on Biochar from Single-Stage Downdraft Gasification. Processes 2021, 9, 533. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, Z.; Li, J.; Zhang, B.; Zhou, H.; Xu, K. Experimental investigation of physicochemical and slagging characteristics of inorganic constituents in ash residues from gasification of different herbaceous biomass. Energy 2020, 198, 117367. [Google Scholar] [CrossRef]
- Bie, N.; Wang, J.; Lv, P.; Zhang, Y.; Bai, Y.; Song, X.; Su, W.; Yu, G. In-situ release characteristic of alkali metals during co-pyrolysis of coal and biomass in a visual fixed bed combined with laser-induced breakdown spectroscopy. Fuel 2023, 331, 125868. [Google Scholar] [CrossRef]
- Morris, J.D.; Daood, S.S.; Chilton, S.; Nimmo, W. Mechanisms and mitigation of agglomeration during fluidized bed combustion of biomass: A review. Fuel 2018, 230, 452–473. [Google Scholar] [CrossRef]
- Fang, X.; Jia, L. Experimental study on ash fusion characteristics of biomass. Bioresour. Technol. 2012, 104, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, T.; Xing, P.; Pourkashanian, M.; Darvell, L.I.; Jones, J.M.; Nimmo, W. Prediction of biomass ash fusion behaviour by the use of detailed characterisation methods coupled with thermodynamic analysis. Fuel 2015, 141, 275–284. [Google Scholar] [CrossRef]
- Ge, Z.; Cao, X.; Zha, Z.; Ma, Y.; Zeng, M.; Wu, K.; Chu, S.; Tao, Y.; Zhang, H. The mineral transformation and molten behaviors of biomass waste ashes in gasification-melting process. Fuel Process. Technol. 2022, 226, 107095. [Google Scholar] [CrossRef]
- Dunnu, G.; Maier, J.; Scheffknecht, G. Ash fusibility and compositional data of solid recovered fuels. Fuel 2010, 89, 1534–1540. [Google Scholar] [CrossRef]
- Namkung, H.; Lee, Y.-J.; Park, J.-H.; Song, G.-S.; Choi, J.W.; Choi, Y.-C.; Park, S.-J.; Kim, J.-G. Blending effect of sewage sludge and woody biomass into coal on combustion and ash agglomeration behavior. Fuel 2018, 225, 266–276. [Google Scholar] [CrossRef]
- Mlonka-Medrala, A.; Magdziarz, A.; Gajek, M.; Nowinska, K.; Nowak, W. Alkali metals association in biomass and their impact on ash melting behaviour. Fuel 2020, 261, 116421. [Google Scholar] [CrossRef]
- Reinmoller, M.; Schreiner, M.; Guhl, S.; Neuroth, M.; Meyer, B. Ash behavior of various fuels: The role of the intrinsic distribution of ash species. Fuel 2019, 253, 930–940. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Petrova, N.L. Thermal behaviour of biomass ashes in air and inert atmosphere with respect to their decarbonation. Fuel 2022, 314, 122766. [Google Scholar] [CrossRef]
- Ohman, M.; Boman, C.; Hedman, H.; Nordin, A.; Bostrom, D. Slagging tendencies of wood pellet ash during combustion in residential pellet burners. Biomass Bioenergy 2004, 27, 585–596. [Google Scholar] [CrossRef]
- Litun, D.S.; Ryabov, G.A.; Folomeev, O.M.; Shorina, E.A.; Smirnova, O.A. Fragmentation and agglomeration of biomass in fluidised bed pyrolysis and combustion. J. Phys. Conf. Ser. 2020, 1565, 012004. [Google Scholar] [CrossRef]
- Li, F.; Yu, B.; Wang, G.; Fan, H.; Wang, T.; Guo, M.; Fang, Y. Investigation on improve ash fusion temperature (AFT) of low-AFT coal by biomass addition. Fuel Process. Technol. 2019, 191, 11–19. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Li, Y.; Zhang, P.; Mao, R.; Ren, L.; Luo, J. Experimental Study of the Pore Structure during Coal and Biomass Ash Sintering Based on X-ray CT Technology. Energy Fuels 2021, 35, 2098–2109. [Google Scholar] [CrossRef]
- Yao, X.; Hu, Y.; Ge, J.; Ma, X.; Mao, J.; Sun, L.; Xu, K.; Xu, K. A comprehensive study on influence of operating parameters on agglomeration of ashes during biomass gasification in a laboratory-scale gasification system. Fuel 2020, 276, 118083. [Google Scholar] [CrossRef]
- Moradian, F.; Tchoffor, P.A.; Davidsson, K.O.; Pettersson, A.; Backman, R. Thermodynamic equilibrium prediction of bed agglomeration tendency in dual fluidized-bed gasification of forest residues. Fuel Process. Technol. 2016, 154, 82–90. [Google Scholar] [CrossRef]
- Akindele, O.D. Influence of Additives on Agglomeration Behavious/Formation in a Laboratory-Scale Fixed Bed Combustion of Biomass Fuels. Ph.D. Thesis, The University of Sheffield, Sheffield, UK, 2018. [Google Scholar]
- Sharypov, V.I.; Marin, N.; Beregovtsova, N.G.; Baryshnikov, S.V.; Kuznetsov, B.N.; Cebolla, V.L.; Weber, J.V. Co-pyrolysis of wood biomass and synthetic polymer mixtures. Part I: Influence of experimental conditions on the evolution of solids, liquids and gases. J. Anal. Appl. Pyrolysis 2002, 64, 15–28. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Huang, Q.; Cai, J. Thermogravimetric characteristics and kinetic of plastic and biomass blends co-pyrolysis. Fuel Process. Technol. 2006, 87, 963–969. [Google Scholar] [CrossRef]
- Ephraim, A.; Minh, D.P.; Lebonnois, D.; Pelegrina, C.; Sharrock, P.; Nzihou, A. Co-pyrolysis of wood and plastics: Influence of plastic type and content on product yield, gas composition and quality. Fuel 2018, 231, 110–117. [Google Scholar] [CrossRef]
- Ma, W.; Rajput, G.; Pan, M.; Lin, F.; Zhong, L.; Chen, G. Pyrolysis of typical MSW components by Py-GC/MS and TG-FTIR. Fuel 2019, 251, 693–708. [Google Scholar] [CrossRef]
- Burra, K.R.G.; Gupta, A.K. Nonlinear Synergistic Effects in Thermochemical Co-processing of Wastes for Sustainable Energy. In Innovations in Sustainable Energy and Cleaner Environment: Green Energy and Technology; Springer: Singapore, 2020; pp. 117–148. [Google Scholar] [CrossRef]
- Esso, S.B.E.; Xiong, Z.; Chaiwat, W.; Kamara, M.F.; Longfei, X.; Xu, J.; Ebako, J.; Jiang, L.; Su, S.; Hu, S.; et al. Review on synergistic effects during co-pyrolysis of biomass and plastic waste: Significance of operating conditions and interaction mechanism. Biomass Bioenergy 2022, 159, 106415. [Google Scholar] [CrossRef]
- Vo, T.A.; Tran, Q.K.; Ly, H.V.; Kwon, B.; Hwang, H.T.; Kim, J.; Kim, S.-S. Co-pyrolysis of lignocellulosic biomass and plastics: A comprehensive study on pyrolysis kinetics and characteristics. J. Anal. Appl. Pyrolysis 2022, 163, 105464. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Bouadili, A.; Nadifiyine, M.; Benchanaa, M.; Mokhlisse, A. Pyrolysis kinetics of olive residue/plastic mixtures by non-isothermal thermogravimetry. Fuel Process. Technol. 2009, 90, 722–728. [Google Scholar] [CrossRef]
- Dominguez, A.; Blanco, C.G.; Barriocanal, C.; Alvarez, R.; Diez, M.A. Gas chromatographic study of the volatile products from co-pyrolysis of coal and polyethylene wastes. J. Chromatogr. A 2001, 918, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Fujita, K.; Kameda, T.; Yoshioka, T. Interactions of beech wood–polyethylene mixtures during co-pyrolysis. J. Anal. Appl. Pyrolysis 2016, 122, 531–540. [Google Scholar] [CrossRef]
- Gu, J.; Fan, H.; Wang, Y.; Zhang, Y.; Yuan, H.; Chen, Y. Co-pyrolysis of xylan and high-density polyethylene: Product distribution and synergistic effects. Fuel 2020, 267, 116896. [Google Scholar] [CrossRef]
- Jeske, H.; Schirp, A.; Cornelius, F. Development of a thermogravimetric analysis (TGA) method for quantitative analysis of wood flour and polypropylene in wood plastic composites (WPC). Thermochim. Acta 2012, 543, 165–171. [Google Scholar] [CrossRef]
- Donskoy, I.G.; Kozlov, A.N.; Kozlova, M.A.; Penzik, M.V.; Shamanskiy, V.A. Thermochemical interaction of wood and polyethylene during co-oxidation in the conditions of thermogravimetric analysis. React. Kinet. Mech. Catal. 2020, 131, 845–857. [Google Scholar] [CrossRef]
- Shi, L.; Cheng, X.; Liu, Q.; Liu, Z. Reaction of volatiles from a coal and various organic compounds during co-pyrolysis in a TG-MS system. Part 1. Reaction of volatiles in the void space between particles. Fuel 2018, 213, 37–47. [Google Scholar] [CrossRef]
- Esso, S.B.E.; Yan, Q.; Xiong, Z.; Hu, X.; Xu, J.; Jiang, L.; Su, S.; Hu, S.; Wang, Y.; Xiang, J. Importance of char-volatiles interactions during co-pyrolysis of polypropylene and biomass components. J. Environ. Chem. Eng. 2022, 10, 108202. [Google Scholar] [CrossRef]
- Lin, X.; Kong, L.; Cai, H.; Zhang, Q.; Bi, D.; Yi, W. Effects of alkali and alkaline earth metals on the co-pyrolysis of cellulose and high density polyethylene using TGA and Py-GC/MS. Fuel Process. Technol. 2019, 191, 71–78. [Google Scholar] [CrossRef]
- Xinjie, L.; Singh, S.; Yang, H.; Wu, C.; Zhang, S. A thermogravimetric assessment of the tri-combustion process for coal, biomass and polyethylene. Fuel 2021, 287, 119355. [Google Scholar] [CrossRef]
- McGhee, B.; Norton, F.; Snape, C.E.; Hall, P.J. The copyrolysis of poly(vinylchloride) with cellulose derived materials as a model for municipal waste derived chars. Fuel 1995, 74, 28–31. [Google Scholar] [CrossRef]
- Xiong, S.; Zhuo, J.; Zhou, H.; Pang, R.; Yao, Q. Study on the co-pyrolysis of high density polyethylene and potato blends using thermogravimetric analyzer and tubular furnace. J. Anal. Appl. Pyrolysis 2015, 112, 66–73. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Study of the thermal behavior, kinetics, and product characterization of biomass and low-density polyethylene co-pyrolysis by thermogravimetric analysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2018, 133, 185–197. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Wang, J.; Xue, Q. Low-temperature treatment of polyethylene plastics and semi-coke mixture and CO2 gasification of finely ground products. Fuel 2021, 285, 119215. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Van Geem, K.M.; Fathi, M.; Bazgir, H.; Ghadiri, M. The pyrolysis of oak with polyethylene, polypropylene and polystyrene using fixed bed and stirred reactors and TGA instrument. Energy 2021, 232, 121085. [Google Scholar] [CrossRef]
- Bouafif, H.; Koubaa, A.; Perre, P.; Cloutier, A.; Riedl, B. Wood particle/high-density polyethylene composites: Thermal sensitivity and nucleating ability of wood particles. Appl. Polym. Sci. 2009, 113, 593–600. [Google Scholar] [CrossRef]
- Rago, Y.P.; Collard, F.-X.; Gorgens, J.F.; Surroop, D.; Mohee, R. Torrefaction of biomass and plastic from municipal solid waste streams and their blends: Evaluation of interactive effects. Fuel 2020, 277, 118089. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Q.; Arnold, L.; Yang, W.; Blasiak, W. A study of the pyrolysis behaviors of pelletized recovered municipal solid waste fuels. Appl. Energy 2013, 107, 173–182. [Google Scholar] [CrossRef]
- Donskoy, I.; Svishchev, D. Experimental Study of Model Refuse-Derived Fuel Pellets Swelling during Heating and Combustion. Processes 2023, 11, 995. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Karlsson, M.; Hupa, M.; Frankenhaeuser, M. Combustion and Gasification Properties of Plastics Particles. J. Air Waste Manag. Assoc. 1997, 47, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.N.; Jensen, P.A.; Hjuler, K.; Nielsen, M.; Dam-Johansen, K. Agglomeration and Deposition Behavior of Solid Recovered Fuel. Energy Fuels 2016, 30, 7858–7866. [Google Scholar] [CrossRef]
- Nakhaei, M.; Wu, H.; Grevain, D.; Jensen, L.S.; Glarborg, P.; Clausen, S.; Dam-Johansen, K. Experiments and modeling of single plastic particle conversion in suspension. Fuel Process. Technol. 2018, 178, 213–225. [Google Scholar] [CrossRef]
- Bartels, M.; Lin, W.; Nijenhuis, J.; Kapteijn, F.; van Ommen, J.R. Agglomeration in fluidized beds at high temperatures: Mechanisms, detection and prevention. Progress Energy Combust. Sci. 2008, 34, 633–666. [Google Scholar] [CrossRef]
- Scala, F. Particle agglomeration during fluidized bed combustion: Mechanisms, early detection and possible countermeasures. Fuel Process. Technol. 2018, 171, 31–38. [Google Scholar] [CrossRef]
- Nascimento, F.R.M.; Gonzalez, A.M.; Lora, E.E.S.; Ratner, A.; Palacio, J.C.E.; Reinaldo, R. Bench-scale bubbling fluidized bed systems around the world—Bed agglomeration and collapse: A comprehensive review. Int. J. Hydrog. Energy 2021, 46, 18740–18766. [Google Scholar] [CrossRef]
- Yu, C.; Tang, Z.; Zeng, L.; Chen, C.; Gong, B. Experimental determination of agglomeration tendency in fluidized bed combustion of biomass by measuring slip resistance. Fuel 2014, 128, 14–20. [Google Scholar] [CrossRef]
- Werther, J.; Saenger, M.; Hartge, E.-U.; Ogada, T.; Siagi, Z. Combustion of agricultural residues. Progress Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Royo, J.; Canalis, P.; Quintana, D. Chemical study of bottom ash sintering in combustion of pelletized residual agricultural biomass. Fuel 2022, 310B, 122145. [Google Scholar] [CrossRef]
- Anicic, B.; Lin, W.; Dam-Johansen, K.; Wu, H. Agglomeration mechanism in biomass fluidized bed combustion—Reaction between potassium carbonate and silica sand. Fuel Process. Technol. 2018, 173, 182–190. [Google Scholar] [CrossRef]
- Bandara, J.C.; Jaiswal, R.; Nielsen, H.K.; Moldestad, B.M.E.; Eikeland, M.S. Air gasification of wood chips, wood pellets and grass pellets in a bubbling fluidized bed reactor. Energy 2021, 233, 121149. [Google Scholar] [CrossRef]
- Fryda, L.E.; Panopoulos, K.D.; Kakaras, E. Agglomeration in fluidised bed gasification of biomass. Powder Technol. 2008, 181, 307–320. [Google Scholar] [CrossRef]
- Boujjat, H.; Rodat, S.; Abanades, S. Solar-hybrid Thermochemical Gasification of Wood Particles and Solid Recovered Fuel in a Continuously-Fed Prototype Reactor. Energies 2020, 13, 5217. [Google Scholar] [CrossRef]
- Kikuchi, K.; Suzuki, A.; Mochizuki, T.; Endo, S.; Imai, E.; Tanji, Y. Ash-agglomerating gasification of coal in a spouted bed reactor. Fuel 1985, 64, 368–372. [Google Scholar] [CrossRef]
- Atakul, H.; Hilmioglu, B.; Ekinci, E. The relationship between the tendency of lignites to agglomerate and their fusion characteristics in a fluidized bed combustor. Fuel Process. Technol. 2005, 86, 1369–1383. [Google Scholar] [CrossRef]
- Gupta, S.; De, S. An experimental investigation of high-ash coal gasification in a pilot-scale bubbling fluidized bed reactor. Energy 2022, 244B, 122868. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, M.; Wu, H. Bed agglomeration during fast pyrolysis of bio-oil derived fuels in a fluidized-bed reactor. Fuel 2022, 328, 125359. [Google Scholar] [CrossRef]
- Urciuolo, M.; Solimene, R.; Ammendola, P.; Krusch, S.; Scherer, V.; Salatino, P.; Chirone, R.; Senneca, O. On the agglomeration tendency of carbonaceous fuels in fluidized beds. Fuel 2020, 277, 118187. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Arena, U. Bed defluidisation during the fluidised bed pyrolysis of plastic waste mixtures. Polym. Degrad. Stab. 2004, 85, 1051–1058. [Google Scholar] [CrossRef]
- Robinson, T.; Bronson, B.; Gogolek, P.; Mehrani, P. Comparison of the air-blown bubbling fluidized bed gasification of wood and wood-PET pellets. Fuel 2016, 178, 263–271. [Google Scholar] [CrossRef]
- Cho, M.-H.; Mun, T.-Y.; Kim, J.-S. Production of low-tar producer gas from air gasification of mixed plastic waste in a two-stage gasifier using olivine combined with activated carbon. Energy 2013, 58, 688–694. [Google Scholar] [CrossRef]
- Lin, C.-L.; Weng, W.-C. Effects of different operating parameters on the syngas composition in a two-stage gasification process. Renew. Energy 2017, 109, 135–143. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Song, J.; Pan, Y.; Sima, J.; Zhu, C.; Gao, H.; Guo, L.; Zhang, J.; Huang, Q. Fuel gas production through waste polyethylene gasification using bauxite residue as the oxygen carrier. Fuel 2022, 321, 123878. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Choi, Y.-K.; Kim, J.-S. Three-stage air gasification of waste polyethylene: In-situ regeneration of active carbon used as a tar removal additive. Energy 2019, 166, 335–342. [Google Scholar] [CrossRef]
- Park, K.-B.; Jeong, Y.-S.; Guzelciftci, B.; Kim, J.-S. Characteristics of a new type continuous two-stage pyrolysis of waste polyethylene. Energy 2019, 166, 343–351. [Google Scholar] [CrossRef]
- Ennis, B.J.; Tardos, G.; Pfeffer, R. A microlevel-based charasterization of granulation phenomena. Powder Technol. 1991, 65, 257–272. [Google Scholar] [CrossRef]
- Khadilkar, A.B.; Rozelle, P.L.; Pisupati, S.V. A study on initiation of ash agglomeration in fluidized bed gasification systems. Fuel 2015, 152, 48–57. [Google Scholar] [CrossRef]
- Ryabov, G.A.; Litun, D.S. Agglomeration during Fluidized Bed Combustion and Gasification of Fuels. Therm. Eng. 2019, 66, 635–651. [Google Scholar] [CrossRef]
- Ryabov, G.A.; Folomeev, O.M.; Smirnova, O.A.; Litun, D.S. A Study into the Influence of Different Factors on the Behavior of Alkaline Element Concentrations that Cause Bed Agglomeration. Therm. Eng. 2021, 68, 72–81. [Google Scholar] [CrossRef]
- Dixon, A.G.; Walls, G.; Stanness, H.; Nijemeisland, M.; Stitt, E.H. Experimental validation of high Reynolds number CFD simulations of heat transfer in a pilot-scale fixed bed tube. Chem. Eng. J. 2012, 200, 344–356. [Google Scholar] [CrossRef]
- Glazov, S.V.; Kislov, V.M.; Salgansky, E.A.; Rabinovich, O.S.; Malinouski, A.I.; Salganskaya, M.V.; Pilipenko, E.N.; Kolesnikova, Y.Y. Effect of local rearrangements in the particle bed on the stability of filtration combustion of solid fuel. Int. J. Heat Mass Transf. 2017, 108B, 1602–1609. [Google Scholar] [CrossRef]
- Podlesniy, D.N.; Zaichenko, A.Y.; Salgansky, E.A.; Salganskaya, M.V. Regularities of filtration combustion of bidisperse fuel mixtures in an inclined rotary reactor. Int. J. Heat Mass Transf. 2018, 127C, 183–187. [Google Scholar] [CrossRef]
- Allesina, G.; Pedrazzi, S.; Tartarini, P. Modeling and investigation of the chanelling phenomenon in downdraft stratified gasifiers. Bioresour. Technol. 2013, 146, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, S. Airflow Channeling Through Fixed Wheat Straw Bed in an Updraft Gasifier Before the Initiation of Gasification. Energy Sources 2003, 25, 1183–1191. [Google Scholar] [CrossRef]
- Varunkumar, S.; Rajan, N.K.S.; Mukunda, H.S. Single Particle and Packed Bed Combustion in Modern Gasifier Stoves—Density Effects. Combust. Sci. Technol. 2011, 183, 1147–1163. [Google Scholar] [CrossRef]
- Jaganathan, V.M.; Ambatipudi, M.K.; Varunkumar, S. The Phenomenon of Flame Jump in Counter–current Flame Propagation in Biomass Packed Beds—Experiments and Theory. Combust. Sci. Technol. 2022, 194, 1199–1212. [Google Scholar] [CrossRef]
- Salgansky, E.A.; Zaichenko, A.Y.; Podlesniy, D.N.; Salganskaya, M.V.; Toledo, M. Coal dust gasification in the filtration combustion mode with syngas production. Int. J. Hydrog. Energy 2017, 42, 11017–11022. [Google Scholar] [CrossRef]
- Tanui, J.K.; Kioni, P.N.; Mirre, T.; Nowitzki, M.; Todorova, D.A. Application of CFD-DEM method in modeling of wood combustion in a fixed bed. Bulg. Chem. Commun. 2020, 52, 396–403. [Google Scholar]
- Fernandez, M.J.; Mediavilla, I.; Barro, R.; Borjabad, E.; Ramos, R.; Carrasco, J.E. Sintering reduction of herbaceous biomass when blended with woody biomass: Predictive and combustion tests. Fuel 2019, 239, 1115–1124. [Google Scholar] [CrossRef]
- Tsvetkov, M.V.; Zyukin, I.V.; Freiman, V.M.; Salganskaya, M.V.; Tsvetkova, Y.Y. Possible Ways to Prevent Ash Slagging in Peat Gasification in the Filtration Combustion Mode. Russ. J. Appl. Chem. 2017, 90, 1706–1711. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, S.; Shaddix, C.R.; Hui, S. Kinetic modeling of the formation and growth of inorganic nano-particles during pulverized coal char combustion in O2/N2 and O2/CO2 atmospheres. Combust. Flame 2016, 173, 195–207. [Google Scholar] [CrossRef]
- Li, J.; Bai, X.; Dong, Z.; Chen, Y.; Yang, H.; Wang, X.; Chen, H. Influence of additives on lignin agglomeration and pyrolysis behavior. Fuel 2020, 263, 116629. [Google Scholar] [CrossRef]
- Backer, E.G.; Mudge, L.K.; Mitchell, D.H. Oxygen/steam gasification of wood in a fixed-bed gasifier. Ind. Eng. Chem. Process Des. Dev. 1984, 23, 725–728. [Google Scholar] [CrossRef]
- Madadian, E.; Lefsrud, M.; Lee, C.P.; Roy, Y. Gasification of Pelletized Woody Biomass Using a Downdraft Reactor and Impact of Material Bridging. J. Energy Eng. 2016, 142, 04016001. [Google Scholar] [CrossRef]
- Siddiqui, H.; Thengane, S.K.; Sharma, S.; Mahajani, S.M. Revamping downdraft gasifier to minimize clinker formation for high-ash garden waste as feedstock. Bioresour. Technol. 2018, 266, 220–231. [Google Scholar] [CrossRef]
- Thengane, S.K.; Gupta, A.; Mahajani, S.M. Co-gasification of high ash biomass and high ash coal in downdraft gasifier. Bioresour. Technol. 2019, 273, 159–168. [Google Scholar] [CrossRef]
- Salganskaya, M.V.; Glazov, S.V.; Salganskii, E.A.; Zholudev, A.F. Filtration combustion of charcoal-polyethylene systems. Russ. J. Phys. Chem. B 2010, 4, 928–933. [Google Scholar] [CrossRef]
- Salganskaya, M.V.; Glazov, S.V.; Salganskii, E.A.; Zholudev, A.F.; Stesik, L.N. Filtration combustion of systems with polymer materials. Khimicheskaya Fiz. 2013, 32, 57–61. (In Russian) [Google Scholar]
- Pan, R.; Debenest, G. Numerical investigation of a novel smoldering-driven reactor for plastic waste pyrolysis. Energy Convers. Manag. 2022, 257, 115439. [Google Scholar] [CrossRef]
- Pan, R.; Debenest, G.; Zanoni, M.A.B. Numerical study of plastic waste pyrolysis driven by char smoldering. Process Saf. Environ. Prot. 2022, 165, 46–56. [Google Scholar] [CrossRef]
- Gonzalez, H.; Caro, S.; Toledo, M.; Olguin, H. Syngas production from polyethylene and biogas in porous media combustion. Int. J. Hydrog. Energy 2018, 43, 4294–4304. [Google Scholar] [CrossRef]
- Orihuela, M.P.; Espinoza, L.; Ripoll, N.; Chacartegui, R.; Toledo, M. Natural gas-supported gasification of polyethylene and wood mixtures in a porous medium reactor. Energy Convers. Manag. 2021, 233, 113901. [Google Scholar] [CrossRef]
- Bhoi, P.R.; Huhnke, R.L.; Kumar, A.; Indrawan, N.; Thapa, S. Co-gasification of Municipal Solid Waste and Biomass in a Commercial Scale Downdraft Gasifier. Energy 2018, 163, 513–518. [Google Scholar] [CrossRef]
- Donskoy, I.; Kozlov, A.; Svishchev, D.; Penzik, M. Experimental study on fixed-bed combustion and agglomeration of sawdust–polyethylene mixtures. Energy Sources A 2022, in press. [Google Scholar] [CrossRef]
- Garcia-Bacaioca, P.; Mastral, J.F.; Ceamanos, J.; Berrueco, C.; Serrano, S. Gasification of biomass/high density polyethylene mixtures in a downdraft gasifier. Bioresour. Technol. 2008, 99, 5485–5491. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.; Cheng, Y.; Maneerung, T.; Yao, Z.; Tong, Y.W.; Wang, C.-H.; Dai, Y. Co-gasification of woody biomass and sewage sludge in a fixed-bed downdraft gasifier. AIChE J. 2015, 61, 2508–2521. [Google Scholar] [CrossRef]
- Vonk, G.; Piriou, B.; Dos Santos, P.F.; Wolbert, D.; Vaitilingom, G. Comparative analysis of wood and solid recovered fuels gasification in a downdraft fixed bed reactor. Waste Manag. 2019, 85, 106–120. [Google Scholar] [CrossRef]
- Basha, M.H.; Sulaiman, S.A.; Uemura, Y. Co-gasification of palm kernel shell and polystyrene plastic: Effect of different operating conditions. J. Energy Inst. 2020, 93, 1045–1052. [Google Scholar] [CrossRef]
- Sherratt, J. The Effect of Thermoplastics Melt Flow Behaviour on the Dynamics of Fire Growth. Ph.D. Thesis, University of Edinburgh, Edinburgh, Scotland, 2001. [Google Scholar]
- Joseph, P.; Tretsiakova-McNally, S. Melt-Flow Behaviours of Thermoplastic Materials under Fire Conditions: Recent Experimental Studies and Some Theoretical Approaches. Materials 2015, 8, 8793–8803. [Google Scholar] [CrossRef]
- Matsuoka, T.; Takagi, A.; Katagiri, Y.; Nakamura, Y. Three-dimensional image reconstruction of a thermoplastic rod burning with and without melting. Combust. Flame 2022, 242, 112199. [Google Scholar] [CrossRef]
- Fazil, A.; Kumar, S.; Mahajani, S.M. Downdraft co-gasification of high ash biomass and plastics. Energy 2022, 243, 123055. [Google Scholar] [CrossRef]
- Madadian, E.; Crowe, C.; Lefsrud, M. Evaluation of composite fiber-plastics biomass clinkering under the gasification conditions. J. Clean. Prod. 2017, 164, 137–145. [Google Scholar] [CrossRef]
- Wang, J.; Kong, L.; Bai, J.; Xue, K.; Zhu, X.; Luo, Y.; Zhao, X.; Li, H.; Guo, Z.; Bai, Z.; et al. Characterization of slag from anthracite gasification in moving bed slagging gasifier. Fuel 2021, 292, 120390. [Google Scholar] [CrossRef]
- Ouadi, M.; Brammer, J.G.; Kay, M.; Hornung, A. Fixed bed downdraft gasification of paper industry wastes. Appl. Energy 2013, 103, 692–699. [Google Scholar] [CrossRef]
- Tripathi, P.; Rao, L. Single particle and packed bed combustion characteristics of high ash and high plastic content refuse derived fuel. Fuel 2022, 308, 121983. [Google Scholar] [CrossRef]
- Na, J.I.; Park, S.J.; Kim, Y.K.; Lee, J.G.; Kim, J.H. Characteristics of oxygen-blown gasification for combustible waste in a fixed-bed gasifier. Appl. Energy 2003, 75, 275–285. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, X.; Bu, C.; Ma, J. Controlled air oxidation of plastic and biomass in a packed bed reactor. Chem. Eng. Technol. 2014, 37, 43–48. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Nipattummakul, N.; Gupta, A.K. Characteristics of syngas from co-gasification of polyethylene and woodchips. Appl. Energy 2011, 88, 165–174. [Google Scholar] [CrossRef]
- Pinto, F.; Franco, C.; Andre, R.N.; Miranda, M.; Gulyurtlu, I.; Cabrita, I. Co-gasification study of biomass mixed with plastic wastes. Fuel 2002, 81, 291–297. [Google Scholar] [CrossRef]
- Sahu, P.; Vairakannu, P. CO2 based synergistic reaction effects with energy and exergy (2E) analysis of high density polyethylene with high ash bituminous coal for syngas production. Fuel 2022, 311, 122500. [Google Scholar] [CrossRef]
- Weiland, F.; Lundin, L.; Celebi, M.; van der Vlist, K.; Moradian, F. Aspects of chemical recycling of complex plastic waste via the gasification route. Waste Manag. 2021, 126, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Kungkajit, C.; Prateepchaikul, G.; Kaosol, T. Influence of Plastic Waste for Refuse-Derived Fuel on Downdraft Gasification. Energy Proc. 2015, 79, 528–535. [Google Scholar] [CrossRef]
- Park, J.; Yu, S.; Kim, H.; Jo, H.; Min, K.; Lee, J.; Heo, J.; Ryu, C. Co-combustion of refuse plastic fuel from marine plastics with wood pellets in a fixed-bed: Identification of minimum cofiring ratio and ideal air flow rate. Fuel 2023, 344, 1278092. [Google Scholar] [CrossRef]
- Torero, J.L.; Fernandez-Pello, A.C. Forward smolder of polyurethane foam in a forced air flow. Combust. Flame 1996, 106, 89–109. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Li, S.; Tang, X.; Hao, J. Gasification of waste rigid polyurethane foam: Optimizing operational conditions. J. Mater. Cycles Waste Manag. 2015, 17, 560–565. [Google Scholar] [CrossRef]
- Dernbecher, A.; Dieguez-Alonso, A.; Ortwein, A.; Tabet, F. Review on modelling approaches based on computational fluid dynamics for biomass combustion systems. Focus on fixed bed and moving grate systems. Biomass Convers. Biorefinery 2019, 9, 129–182. [Google Scholar] [CrossRef]
- Gu, T.; Ma, W.; Berning, T.; Guo, Z.; Andersson, R.; Yin, C. Advanced simulation of a 750 t/d municipal solid waste grate boiler to better accommodate feedstock changes due to waste classification. Energy 2022, 254B, 124338. [Google Scholar] [CrossRef]
- Ma, W.; Ma, C.; Liu, X.; Gu, T.; Thengane, S.K.; Bourtsalas, A.; Chen, G. NOx formation in fixed-bed biomass combustion: Chemistry and modeling. Fuel 2021, 290, 119694. [Google Scholar] [CrossRef]
- Narobe, M.; Golob, J.; Klinar, D.; Francetic, V.; Likozar, B. Co-gasification of biomass and plastics: Pyrolysis kinetics studies, experiments on 100 kW dual fluidized bed pilot plant and development of thermodynamic equilibrium model and balances. Bioresour. Technol. 2014, 162, 21–29. [Google Scholar] [CrossRef]
- Couto, N.D.; Silva, V.B.; Rouboa, A. Thermodynamic evaluation of Portuguese municipal solid waste gasification. J. Clean. Prod. 2016, 139, 622–635. [Google Scholar] [CrossRef]
- Xu, P.; Hu, M.; Shao, Z.; Cheng, Y. Thermodynamic analysis of steam gasification of municipal solid waste. Energy Sources A 2018, 40, 623–629. [Google Scholar] [CrossRef]
- Kaydouh, M.-N.; El Hassan, N. Thermodynamic simulation of the co-gasification of biomass and plastic waste for hydrogen-rich syngas production. Res. Eng. 2022, 16, 100771. [Google Scholar] [CrossRef]
- Singh, M.; Salaudeen, S.A.; Gilroyed, B.H.; Dutta, A. Simulation of biomass-plastic co-gasification in a fluidized bed reactor using Aspen plus. Fuel 2022, 319, 123708. [Google Scholar] [CrossRef]
- van Kasteren, J.M.N. Co-gasification of wood and polyethylene with the aim of CO and H2 production. J Mater Cycles. Waste Manag. 2006, 8, 95–98. [Google Scholar] [CrossRef]
- Ma, W.; Chu, C.; Wang, P.; Guo, Z.; Liu, B.; Chen, G. Characterization of tar evolution during DC thermal plasma steam gasification from biomass and plastic mixtures: Parametric optimization via response surface methodology. Energy Convers. Manag. 2020, 225, 113407. [Google Scholar] [CrossRef]
- Hasanzadeh, R.; Mojaver, P.; Azdast, T.; Chitsaz, A.; Park, C.B. Low-emission and energetically efficient co-gasification of coal by incorporating plastic waste: A modeling study. Chemosphere 2022, 299, 134408. [Google Scholar] [CrossRef]
- Sabogal, O.S. Pyrolysis and Gasification of a Solid Recovered Fuel (SRF) and Its Model Materials. Ph.D. Thesis, Universite de Toulouse, Toulouse, France, 2022. [Google Scholar]
- Marques, T.E.; Santiago, Y.C.; Reno, M.L.G.; Maya, D.M.Y.; Sphaier, L.A.; Shi, Y.; Ratner, A. Environmental and Energetic Evaluation of Refuse-Derived Fuel Gasification for Electricity Generation. Processes 2021, 9, 2255. [Google Scholar] [CrossRef]
- Netzer, C.; Li, T.; Lovas, T. Surrogate Reaction Mechanism for Waste Incineration and Pollutant Formation. Energy Fuels 2021, 35, 7030–7049. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Progress Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Hoang, Q.N.; Vanierschot, M.; Blondeau, J.; Croymans, T.; Pittoors, R.; Van Caneghem, J. Review of numerical studies on thermal treatment of municipal solid waste in packed bed combustion. Fuel Commun. 2021, 7, 100013. [Google Scholar] [CrossRef]
- Levine, S.E.; Broadbelt, L.J. Detailed mechanistic modeling of high-density polyethylene pyrolysis: Low molecular weight product evolution. Polym. Degrad. Stab. 2009, 94, 810–822. [Google Scholar] [CrossRef]
- Ranzi, E.; Faravelli, T.; Manenti, F. Pyrolysis, Gasification, and Combustion of Solid Fuels. Adv. Chem. Eng. 2016, 49, 1–94. [Google Scholar] [CrossRef]
- Horton, S.R.; Zhang, Y.; Mohr, R.; Petrocelli, F.; Klein, M.T. Implementation of a Molecular-Level Kinetic Model for Plasma-Arc Municipal Solid Waste Gasification. Energy Fuels 2016, 30, 7904–7915. [Google Scholar] [CrossRef]
- Zhou, X.; Broadbelt, L.J.; Vinu, R. Mechanistic Understanding of Thermochemical Conversion of Polymers and Lignocellulosic Biomass. Adv. Chem. Eng. 2016, 49, 95–198. [Google Scholar] [CrossRef]
- Ohmukai, Y.; Hasegawa, I.; Mae, K. Pyrolysis of the mixture of biomass and plastics in countercurrent flow reactor Part I: Experimental analysis and modeling of kinetics. Fuel 2008, 87, 3105–3111. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Gebreegziabher, T.; Hui, C.W. Co-pyrolysis of biomass and plastics waste: A modelling approach. Chem. Eng. Trans. 2013, 35, 883–888. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, W. Effect of heat transfer model on the prediction of refuse-derived fuel pyrolysis process. Fuel 2015, 142, 46–57. [Google Scholar] [CrossRef]
- Sieradzka, M.; Rajca, P.; Zajemska, M.; Mlonka-Medrala, A.; Magdziarz, A. Prediction of gaseous products from refuse derived fuel pyrolysis using chemical modelling software—Ansys Chemkin-Pro. J. Clean. Prod. 2020, 248, 119277. [Google Scholar] [CrossRef]
- Hong, D.; Li, P.; Si, T.; Guo, X. ReaxFF simulations of the synergistic effect mechanisms during co-pyrolysis of coal and polyethylene/polystyrene. Energy 2021, 218, 119553. [Google Scholar] [CrossRef]
- Wei, Z.; Li, Y.; Zhang, C.; Yang, L.; Chu, L. Revealing the mechanism on steam co-gasification of cellulose and polyethylene: A combined ReaxFF and DFT study. Fuel 2023, 334, 126784. [Google Scholar] [CrossRef]
- Janajreh, I.; Adeyemi, I.; Elagroudy, S. Gasification feasibility of polyethylene, polypropylene, polystyrene waste and their mixture: Experimental studies and modeling. Sustain. Energy Technol. Assess. 2020, 39, 100684. [Google Scholar] [CrossRef]
- Bieniek, A.; Jerzak, W.; Magdziarz, A. Numerical investigation of biomass fast pyrolysis in a free fall reactor. Arch. Thermodyn. 2021, 42, 173–196. [Google Scholar] [CrossRef]
- Tobo, Y.; Lotfi, A.; Virla, L.D.; Mahinpey, N. Fast pyrolysis multiphase CFD-kinetics model in a drop tube reactor. Fuel 2023, 340, 127524. [Google Scholar] [CrossRef]
- Popova, E.; Chernov, A.; Maryandyshev, P.; Brillard, A.; Kehrli, D.; Trouve, G.; Lyubov, V.; Brilhac, J.-F. Thermal degradation of wood biofuels, coals and hydrolysis lignin from the Russian Federation: Experiments and modeling. Bioresour. Technol. 2016, 218, 1046–1054. [Google Scholar] [CrossRef]
- Senneca, O.; Cerciello, F. Kinetics of combustion of lignocellulosic biomass: Recent research and critical issues. Fuel 2023, 347, 128310. [Google Scholar] [CrossRef]
- Donskoi, I.G. Process simulation of the co-gasification of wood and polymeric materials in a fixed bed. Solid Fuel Chem. 2018, 52, 121–127. [Google Scholar] [CrossRef]
- Donskoy, I.G. Mathematical modeling of coal and sewage sludge co-conversion using downdraft gasifier. Bull. Tomsk. Polytech. Univ. 2019, 330, 7–18. [Google Scholar] [CrossRef]
- Du, S.; Yuan, S.; Zhou, Q. Numerical investigation of co-gasification of coal and PET in a fluidized bed reactor. Renew. Energy 2021, 172, 424–439. [Google Scholar] [CrossRef]
- Couto, N.D.; Silva, V.B.; Monteiro, E.; Rouboa, A. Assessment of municipal solid wastes gasification in a semi-industrial gasifier using syngas quality indices. Energy 2015, 93, 864–873. [Google Scholar] [CrossRef]
- Cardoso, J.; Silva, V.; Eusebio, D. Process optimization and robustness analysis of municipal solid waste gasification using air-carbon dioxide mixtures as gasifying agent. Int. J. Energy Res. 2019, 43, 4715–4728. [Google Scholar] [CrossRef]
- Rahdar, M.H.; Lee, B.; Nasiri, F. Uncertainty Quantification of Biomass Composition Variability Effect on Moving-Grate Bed Combustion: An Experiment-Based Approach. Energy Fuels 2020, 34, 9697–9708. [Google Scholar] [CrossRef]
- Zachl, A.; Buchmayr, M.; Gruber, J.; Anca-Couce, A.; Scharler, R.; Hochenauer, C. Evaluation and extension of the load and fuel flexibility limits of a stratified downdraft gasifier. Energy 2022, 239D, 122279. [Google Scholar] [CrossRef]
- Glazov, S.V.; Salganskii, E.A.; Kislov, V.M.; Salganskaya, M.V.; Zholudev, A.F. Change in the structure of the filtration combustion wave due to a fuel composition change. Combust. Explos. Shock Waves 2010, 46, 286–292. [Google Scholar] [CrossRef]
- Rubinstein, J.; Torquato, S. Flow in random porous media: Mathematical formulation, variational principles, and rigorous bounds. J. Fluid Mech. 1989, 206, 25–46. [Google Scholar] [CrossRef]
- Buyevich, Y.A. Heat and mass transfer in disperse media—I. Averaged field equations. Int. J. Heat Mass Transfer 1992, 35, 2445–2452. [Google Scholar] [CrossRef]
- Li, Y.; Park, C.-W. Permeability of Packed Beds Filled with Polydisperse Spherical Particles. Ind. Eng. Chem. Res. 1998, 37, 2005–2011. [Google Scholar] [CrossRef]
- Schulz, R.; Ray, N.; Zech, S.; Rupp, A.; Krabner, P. Beyond Kozeny–Carman: Predicting the Permeability in Porous Media. Transp. Porous Media 2019, 130, 487–512. [Google Scholar] [CrossRef]
- de Lemos, M.J.S. Thermal Non-Equilibrium in Heterogeneous Media; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Cancelliere, A.; Chang, C.; Foti, E.; Rothman, D.H.; Succi, S. The permeability of a random medium: Comparison of simulation with theory. Phys. Fluids A 1990, 2, 2085. [Google Scholar] [CrossRef]
- Yang, Y.B.; Goodfellow, J.; Goh, Y.R.; Nasserzadeh, V.; Swithenbank, J. Investigation of Channel Formation Due to Random Packing in a Burning Waste Bed. Process Saf. Environ. Prot. 2001, 79, 267–277. [Google Scholar] [CrossRef]
- Donskoy, I.G. Mathematical modelling of the agglomeration in a reactive porous medium with variable permeability. Comp. Technol. 2020, 25, 22–35. (In Russian) [Google Scholar] [CrossRef]
- Yakovlev, I.; Maznoy, A.; Zambalov, S. Pore-scale study of complex flame stabilization phenomena in thin-layered radial porous burner. Combust. Flame 2021, 231, 111468. [Google Scholar] [CrossRef]
- Chorin, A.J. The instability of fronts in a porous medium. Commun. Math. Phys. 1983, 91, 103–116. [Google Scholar] [CrossRef]
- Aldushin, A.P.; Ivleva, T.P. Simulation of the hydrodynamic instability of a filtration combustion wave in a porous medium. Combust. Explos. Shock Waves 2015, 51, 107–115. [Google Scholar] [CrossRef]
- Lutsenko, N.A. Numerical model of two-dimensional heterogeneous combustion in porous media under natural convection or forced filtration. Combust. Theor. Model. 2018, 22, 359–377. [Google Scholar] [CrossRef]
- Zanoni, M.A.B.; Wang, J.; Gerhard, J.I. Understanding pressure changes in smouldering thermal porous media reactors. Chem. Eng. J. 2021, 412, 128642. [Google Scholar] [CrossRef]
- Nakamura, M.; Zhang, H.; Millrath, K.; Themelis, N.J. Modeling of Waste-to-Energy Combustion with Continuous Variation of the Solid Waste Fuel. In Proceedings of the 2003 ASME International Mechanical Engineering Congress and Exposition (IMECE’03), Washington DC, USA, 15–21 November 2003; pp. 69–78. [Google Scholar] [CrossRef]
- Bakarji, J.; Tartakovsky, D.M. Stochastic Pore Collapse Models in Granular Materials. arXiv 2020, arXiv:2002.12479. [Google Scholar] [CrossRef]
- Alalwan, N.; Arenas, A.; Estrada, E. Topological melting in networks of granular materials. J. Math. Chem. 2019, 57, 875–894. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Wang, Y.; Qin, C.; Li, B.; Luo, Y.; Feng, J. Investigating the Interaction Effects between Reservoir Deformation and Hydrate Dissociation in Hydrate-Bearing Sediment by Depressurization Method. Energies 2021, 14, 548. [Google Scholar] [CrossRef]
- Pen’kovskii, V.I. Two modeling problems for the motion of an aggressive liquid in a porous medium. J. Appl. Mech. Tech. Phys. 1968, 9, 781–785. [Google Scholar] [CrossRef]
- Daccord, G.; Lietard, O.; Lenormand, R. Chemical dissolution of a porous medium by a reactive fluid—II. Convection vs reaction, behavior diagram. Chem. Eng. Sci. 1993, 48, 179–186. [Google Scholar] [CrossRef]
- Bringedal, C.; Berre, I.; Pop, I.S.; Radu, F.A. Upscaling of Non-isothermal Reactive Porous Media Flow with Changing Porosity. Transp. Porous Media 2016, 114, 371–393. [Google Scholar] [CrossRef]
- Dayal, R.; Gambaryan-Roisman, T. Heat transfer in granular medium for application to selective laser melting: A numerical study. Int. J. Therm. Sci. 2017, 113, 38–50. [Google Scholar] [CrossRef]
- Xin, L.; Boutaous, M.; Xin, S.; Siginer, D.A. Multiphysical modeling of the heating phase in the polymer powder bed fusion process. Addit. Manufact. 2017, 18, 121–135. [Google Scholar] [CrossRef]
- Sasaki, A.; Aiba, S.; Fukusako, S. Numerical study on freezing heat transfer in water-saturated porous media. Numer. Heat Transf. A 1990, 18, 17–32. [Google Scholar] [CrossRef]
- Sajjadi, M.; Azaiez, J. Heat and mass transfer in melting porous media: Stable miscible displacements. Int. J. Heat Mass Transfer 2015, 88, 926–944. [Google Scholar] [CrossRef]
- Sibin, A.N.; Papin, A.A. Heat and mass transfer in melting snow. J. Appl. Mech. Tech. Phys. 2021, 62, 96–104. [Google Scholar] [CrossRef]
- Tanoue, K.; Nagao, M.; Yoshida, A.; Nishimura, T. Heat transfer and phase change in a polystyrene packed bed during melting. Int. J. Heat Mass Transfer 2014, 79, 324–331. [Google Scholar] [CrossRef]
- Snegirev, A.Y.; Kuznetsov, E.A.; Korobeinichev, O.P.; Shmakov, A.G.; Trubachev, S.A. Ignition and burning of the composite sample impacted by the Bunsen burner flame: A fully coupled simulation. Fire Saf. J. 2022, 127, 103507. [Google Scholar] [CrossRef]
- Fard, M.G.H.; Hostikka, S. Combustion characteristics of non-charring polymer cylinders—Experimental and numerical study. Combust. Flame 2023, 249, 112587. [Google Scholar] [CrossRef]
- Ramos, M.V.; Kasai, E.; Kano, J.; Nakamura, T. Numerical Simulation Model of the Iron Ore Sintering Process Directly Describing the Agglomeration Phenomenon of Granules in the Packed Bed. ISIJ Int. 2000, 40, 448–454. [Google Scholar] [CrossRef]
- Peters, B.; Banisadi, M.; Banisadi, M. The extended discrete element method (XDEM): An advanced approach to model blast furnace. In Iron Ores and Iron Oxide Materials; InTech: London, UK, 2018; p. 125. [Google Scholar] [CrossRef]
- Turnbull, B.; Swift, M.; Hill, R. Ice as a granular material. In Proceedings of the 24th Interntaional Conference of Theoretical and Applied Mechanics, Montreal, QC, Canada, 21–26 August 2016. [Google Scholar]
- Tsypkin, G.G. Formation of the impermeable layer in the process of methane hydrate dissociation in porous media. Fluid Dyn. 2017, 52, 657–665. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, Y.; Si, F.; Wang, P.; Zhou, J. Co-simulating fouling, erosion of gas-particle flow and morphologies predictions around circular tube via parallel CFD–DEM modeling. Fuel 2021, 294, 120464. [Google Scholar] [CrossRef]
- Chen, L.; Koh, C.A.; Sun, B. Insight into the plugging mechanism in water-continuous hydrate slurries. Fuel 2022, 316, 123360. [Google Scholar] [CrossRef]
- Glushkov, D.; Paushkina, K.; Vershinina, K.; Vysokomornaya, O. Slagging Characteristics of a Steam Boiler Furnace with Flare Combustion of Solid Fuel When Switching to Composite Slurry Fuel. Appl. Sci. 2023, 13, 434. [Google Scholar] [CrossRef]
- Davies, S.R.; Selim, M.S.; Sloan, E.D.; Bollavaram, P.; Peters, D.J. Hydrate plug dissociation. AIChE J. 2006, 52, 4016–4027. [Google Scholar] [CrossRef]
- Duan, X.; Shi, B.; Wang, J.; Song, S.; Liu, H.; Li, X.; Chen, Y.; Liao, Q.; Gong, J.; Chen, S.; et al. Simulation of the hydrate blockage process in a water-dominated system via the CFD-DEM method. J. Nat. Gas Sci. Eng. 2021, 96, 104241. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Perlmutter, D.D. A random pore model for fluid-solid reactions: I. Isothermal, kinetic control. AIChE J. 1980, 26, 379–386. [Google Scholar] [CrossRef]
- Sahimi, M.; Galavas, G.R.; Tsotsis, T.T. Statistical and continuum models of fluid-solid reactions in porous media. Chem. Eng. Sci. 1990, 45, 1443–1502. [Google Scholar] [CrossRef]
- Butakov, A.A.; Zanin, A.M. Effects of viscosity variation on an exothermic reaction in a flow system. Combust. Explos. Shock Waves 1978, 14, 628–631. [Google Scholar] [CrossRef]
- Dik, I.G. Critical conditions for thermal explosion of a viscous fluid flowing in a channel of finite length. Combust. Explos. Shock Waves 1976, 12, 70–77. [Google Scholar] [CrossRef]
- Vilyunov, V.N.; Dik, I.G. Thermal explosion in a viscous-liquid flow in a channel of finite length. Combust. Explos. Shock Waves 1978, 14, 370–372. [Google Scholar] [CrossRef]
- Tavakkol, S. Numerical Simulation of Wet Biomass Carbonization in Tubular Reactors. Ph.D. Thesis, Engler-Bunte-Institut, Karlsruher Institute of Technology, Karlsruhe, Germany, 2021. [Google Scholar]
- Zhang, M.; Zhang, Y.; Ma, D.; Li, A.; Fu, W.; Ji, G.; Dong, J. Numerical investigation on the heat transfer of plastic waste pyrolysis in a rotary furnace. Chem. Eng. J. 2022, 445, 136686. [Google Scholar] [CrossRef]
- Nakamura, M.; Themelis, N.J. Modeling of Solid Waste Flow and Mixing on the Traveling Grate of a Waste-to-Energy Combustion Chamber. In Proceedings of the 12th North American Waste to Energy Conference (NAWTEC 12), Savannah, GA, USA, 17–19 May 2004; Volume 3736, pp. 273–282. [Google Scholar]
- Yang, Y.B.; Lim, C.N.; Goodfellow, J.; Sharifi, V.N.; Swithenbank, J. A diffusion model for particle mixing in a packed bed of burning solids. Fuel 2005, 84, 213–225. [Google Scholar] [CrossRef]
- Scherer, V.; Monningmann, M.; Berner, M.O.; Sudbrock, F. Coupled DEM–CFD simulation of drying wood chips in a rotary drum—Baffle design and model reduction. Fuel 2016, 184, 896–904. [Google Scholar] [CrossRef]
- Wissing, F.; Wirtz, S.; Scherer, V. Simulating municipal solid waste incineration with a DEM/CFD method—Influences of waste properties, grate and furnace design. Fuel 2017, 206, 638–656. [Google Scholar] [CrossRef]
- Johansson, R.; Thunman, H.; Leckner, B. Influence of intraparticle gradients in modeling of fixed bed combustion. Combust. Flame 2007, 149, 49–62. [Google Scholar] [CrossRef]
- Haberle, I.; Skreiberg, O.; Lazar, J.; Haugen, N.E.L. Numerical models for thermochemical degradation of thermally thick woody biomass, and their application in domestic wood heating appliances and grate furnaces. Progress Energy Combust. Sci. 2017, 63, 204–252. [Google Scholar] [CrossRef]
- Hermansson, S.; Thunman, H. CFD modelling of bed shrinkage and chanelling in fixed bed combustion. Combust. Flame 2011, 158, 988–999. [Google Scholar] [CrossRef]
- Mahmoudi, A.H.; Hoffmann, F.; Peters, B. Detailed numerical modeling of pyrolysis in a heterogeneous packed bed using XDEM. J. Anal. Appl. Pyrolysis 2014, 106, 9–20. [Google Scholar] [CrossRef]
- Perera, K.U.C.; Narayana, M. Modelling of particle size effect on Equivalence Ratio requirement for wood combustion in fixed beds. Biomass Convers. Bioref. 2019, 9, 183–199. [Google Scholar] [CrossRef]
- Gomez, M.A.; Porteiro, J.; Patino, D.; Miguez, J.L. CFD modelling of thermal conversion and packed bed compaction in biomass combusiton. Fuel 2014, 117, 716–732. [Google Scholar] [CrossRef]
- Gomez, M.A.; Porteiro, J.; Patino, D.; Miguez, J.L. Eulerian CFD modelling for biomass combustion. Transient simulation of an under feed pellet boiler. Energy Convers. Manag. 2015, 101, 666–680. [Google Scholar] [CrossRef]
- Karim, M.R.; Naser, J. Numerical study of the ignition front propagation of different pelletised biomass in a packed bed furnace. Appl. Therm. Eng. 2018, 128, 772–784. [Google Scholar] [CrossRef]
- Miljkovic, B.; Pesenjanski, I.; Vicevic, M. Mathematical modelling of straw combustion in a moving bed combustor: A two dimensional approach. Fuel 2013, 104, 351–364. [Google Scholar] [CrossRef]
- Barroso, G.; Roth, S.; Nussbaumer, T. Investigation of biomass conversion on a moving grate by pyrolysis gas analysis and fuel bed modelling. Energy 2019, 174, 897–910. [Google Scholar] [CrossRef]
- Yang, Y.B.; Swithenbank, J. Mathematical modelling of particle mixing effect on the combustion of municipal solid wastes in a packed-bed furnace. Waste Manag. 2008, 28, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.; Shin, D.; Choi, S. Effect of fuel layer mixing in waste bed combustion. Adv. Environ. Res. 2001, 5, 259–267. [Google Scholar] [CrossRef]
- Duffy, N.T.M.; Eaton, J.A. Investigation of 3D flow and heat transfer in solid-fuel grate combustion: Measures to reduce high-temperature degradation. Combust. Flame 2016, 167, 422–443. [Google Scholar] [CrossRef]
- Yang, Y.B.; Nasserzadeh, V.; Goodfellow, J.; Swithenbank, J. Simulation of Channel Growth in a Burning Bed of Solids. Chem. Eng. Res. Des. 2003, 81, 221–232. [Google Scholar] [CrossRef]
- Duffy, N.T.M.; Eaton, J.A. Investigation of factors affecting channelling in fixed-bed solid fuel combustion using CFD. Combust. Flame 2013, 160, 2204–2220. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, B.; Cao, Z.; Chen, B.; Xue, K.; Zhou, H. Mathematical modeling for molten ash slagging moving bed coal gasifier considering the impact of particle behavior characteristics. Chem. Eng. Sci. 2022, 263, 118130. [Google Scholar] [CrossRef]
- Donskoy, I. Influence of heating conditions on formation and development of agglomerates in a reactive porous medium. Heat Transf. Res. 2022, 53, 25–36. [Google Scholar] [CrossRef]
- Donskoy, I.G. Mathematical modelling of the agglomeration in a reactive porous medium with variable permeability. J. Phys. Conf. Ser. 2020, 1565, 012101. [Google Scholar] [CrossRef]
- Donskoy, I.G.; Svishchev, D.A.; Kozlov, A.N.; Penzik, M.V. Thermal decomposition of polyethylene agglomerates in a porous medium. J. Phys. Conf. Ser. 2020, 1677, 012037. [Google Scholar] [CrossRef]
- Donskoy, I.; Kozlov, A.; Penzik, M.; Svishchev, D. Study of the formation and decomposition processes of agglomerates during fixed bed combustion of polymeric materials. E3S Web Conf. 2020, 209, 03010. [Google Scholar] [CrossRef]
- Yarovoi, T.A.; Zolotko, A.N.; Poletaev, N.I.; Vovchuk, Y.I. Ignition, combustion and extinction of high-ash coal particles. Part 1: Problem statement. In Aerodisperse Systems Physics; Astroprint: Odessa, Ukraine, 1998; Volume 37, pp. 82–85. (In Russian) [Google Scholar]
- Haugen, N.E.L.; Loong, B.K.Y.; Mitchell, R.E. Numerical approaches for thermochemical conversion of char. Progress Energy Combust. Sci. 2022, 91, 100993. [Google Scholar] [CrossRef]
- Pan, W.; Chen, D.; Hu, S.; Yu, D.; Yin, L.; Hu, Y. Direct melting of municipal solid wastes pyrolysis char and its promotion by the char combustion. Fuel 2022, 327, 125091. [Google Scholar] [CrossRef]
- Shi, W.; Bai, J.; Kong, L.; Li, H.; Bai, Z.; Vassilev, S.V.; Li, W. An overview of the coal ash transition process from solid to slag. Fuel 2021, 287, 119537. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, X.; Su, Y.; Zhang, T.; Xu, M. An improved model of fine particulate matter formation coupling the mechanism of mineral coalescence and char fragmentation during pulverized coal combustion. Proc. Combust. Inst. 2022, in press. [Google Scholar] [CrossRef]
- Liu, S.; Niu, Y.; Shang, Y.; Zhu, G.; Hui, S. A percolation model of fly ash formation during the combustion of non-uniform porous char. Combust. Flame 2023, 251, 112720. [Google Scholar] [CrossRef]
- Qu, S.; You, C. Direct numerical simulation (DNS) of alkali metals released during char combustion. Fuel 2019, 255, 115763. [Google Scholar] [CrossRef]
- Kleinhans, U.; Wieland, C.; Babat, S.; Spliethoff, H. Large Eddy Simulation of a particle-laden flow around a cylinder: Importance of thermal boundary layer effects for slagging and fouling. Fuel 2019, 241, 585–606. [Google Scholar] [CrossRef]
- Mu, L.; Wang, S.; Zhai, Z.; Shang, Y.; Zhao, C.; Zhao, L.; Yin, H. Unsteady CFD simulation on ash particle deposition and removal characteristics in tube banks: Focusing on particle diameter, flow velocity, and temperature. J. Energy Inst. 2020, 93, 1481–1494. [Google Scholar] [CrossRef]
- Zheng, Z.; Yang, W.; Yu, P.; Cai, Y.; Zhou, H.; Boon, S.K.; Subbaiah, P. Simulating growth of ash deposit in boiler heat exchanger tube based on CFD dynamic mesh technique. Fuel 2020, 259, 116083. [Google Scholar] [CrossRef]
- Fakourian, S.; McAllister, Z.; Fry, A.; Wang, Y.; Li, X.; Wendt, J.O.L.; Dai, J. Modeling ash deposit growth rates for a wide range of solid fuels in a 100 kW combustor. Fuel Process. Technol. 2021, 217, 106777. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Wei, J.; Guo, Q.; Gong, Y.; Yu, G. Numerical simulation of radiant syngas cooler with different connection to entrained-flow gasifier. Appl. Therm. Eng. 2022, 201A, 117804. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, H.; Wu, H. CFD modelling of biomass ash deposition under multiple operation conditions using a 2D mass-conserving dynamic mesh approach. Fuel 2022, 316, 123250. [Google Scholar] [CrossRef]
- Feng, D.; Li, H.; Zhu, M.; Han, L.; Zhou, Y. Insight into the interaction mechanism between liquid action and cone structure in liquid-containing gas-solid spouted fluidized bed reactors. Powder Technol. 2022, 408, 117693. [Google Scholar] [CrossRef]
- Khadilkar, A.; Rozelle, P.L.; Pisupati, S.V. Models of agglomerate growth in fluidized bed reactors: Critical review, status and applications. Powder Technol. 2014, 264, 216–228. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Y.; Lv, G.; Dong, L.; Zhao, Y.; Duan, C.; Zhou, E.; Hua, W. Agglomeration behavior of the dense medium in a gas–solid separation fluidized bed. Fuel 2023, 339, 127383. [Google Scholar] [CrossRef]
- Moseley, J.L.; O’Brien, T.J. A model for agglomeration in a fluidized bed. Chem. Eng. Sci. 1993, 48, 3043–3050. [Google Scholar] [CrossRef]
- Lin, W.; Dam-Johansen, K.; Frandsen, F. Agglomeration in bio-fuel fired fluidized bed combustors. Chem. Eng. J. 2003, 96, 171–185. [Google Scholar] [CrossRef]
- Zohdi, T.I. A computational framework for agglomeration in thermochemically reacting granular flows. Proc. R. Soc. Lond. A 2004, 460, 3421–3445. [Google Scholar] [CrossRef]
- Li, S.; Shang, L.; Teng, H.; Lu, Q. A model for agglomeration in bio-fuel fired fluidized bed. J. Therm. Sci. 2010, 19, 451–458. [Google Scholar] [CrossRef]
- Buck, B.; Lunewski, J.; Tang, Y.; Deen, N.G.; Kuipers, J.A.M.; Heinrich, S. Numerical investigation of collision dynamics of wet particles via force balance. Chem. Eng. Res. Des. 2018, 132, 1143–1159. [Google Scholar] [CrossRef]
- Gibson, L.; Soundarrajan, N.; Spenik, J.; Ma, J.; Shadle, L.; Pisupati, S.V. Application of Particle Population Model to Determine the Contribution to Slag, Flyash, and Syngas in Entrained Flow Gasification from Particle Size Distribution. Energy Fuels 2013, 27, 7681–7695. [Google Scholar] [CrossRef]
- Gu, C.; Zhao, H.; Xu, B.; Yang, J.; Zhang, J.; Du, M.; Liu, Y.; Tikhankin, D.; Yuan, Z. CFD-DEM simulation of distribution and agglomeration characteristics of bendable chain-like biomass particles in a fluidized bed reactor. Fuel 2023, 340, 127570. [Google Scholar] [CrossRef]
- Shampine, R.W.; Cohen, R.D.; Bayazitoglu, Y.; Anderson, C.F. Effect of agglomeration on pulverized-coal combustion. Combust. Flame 1995, 101, 185–191. [Google Scholar] [CrossRef]
- Netzer, C.; Li, T.; Seidel, L.; Mauss, F.; Lovas, T. Stochastic Reactor-Based Fuel Bed Model for Grate Furnaces. Energy Fuels 2020, 34, 16599–16612. [Google Scholar] [CrossRef]
- Geguzin, Y.E. Physics of Suntering; Nauka: Moscow, Russia, 1984. (In Russian) [Google Scholar]
- Bobkov, V.I.; Borisov, V.V.; Dli, M.I.; Meshalkin, V.P. Thermally Activated Chemical Technology Processes of Agglomeration of Phosphorites. Theor. Found Chem. Eng. 2018, 52, 35–41. [Google Scholar] [CrossRef]
- Troiano, M.; Solimene, R.; Montagnaro, F.; Salatino, P. Char/ash deposition and near-wall segregation in slagging entrained-flow gasification of solid fuels: From experiments to closure equations. Fuel 2020, 264, 116864. [Google Scholar] [CrossRef]
- Madanikashani, S.; Vandewalle, L.A.; De Meester, S.; De Wilde, J.; Van Geem, K.M. Multi-Scale Modeling of Plastic Waste Gasification: Opportunities and Challenges. Materials 2022, 15, 4215. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.F.; Harrison, D. (Eds.) Fluidization; Academic Press: London, UK, 1971. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donskoy, I. Particle Agglomeration of Biomass and Plastic Waste during Their Thermochemical Fixed-Bed Conversion. Energies 2023, 16, 4589. https://doi.org/10.3390/en16124589
Donskoy I. Particle Agglomeration of Biomass and Plastic Waste during Their Thermochemical Fixed-Bed Conversion. Energies. 2023; 16(12):4589. https://doi.org/10.3390/en16124589
Chicago/Turabian StyleDonskoy, Igor. 2023. "Particle Agglomeration of Biomass and Plastic Waste during Their Thermochemical Fixed-Bed Conversion" Energies 16, no. 12: 4589. https://doi.org/10.3390/en16124589
APA StyleDonskoy, I. (2023). Particle Agglomeration of Biomass and Plastic Waste during Their Thermochemical Fixed-Bed Conversion. Energies, 16(12), 4589. https://doi.org/10.3390/en16124589






