Metal-Tolerant Bioinoculant Pseudomonas putida KNP9 Mediated Enhancement of Soybean Growth under Heavy Metal Stress Suitable for Biofuel Production at the Metal-Contaminated Site
Abstract
1. Introduction
2. Materials and Methods
2.1. Heavy Metal Tolerance Experiment
2.2. Heavy Metal Removal Studies
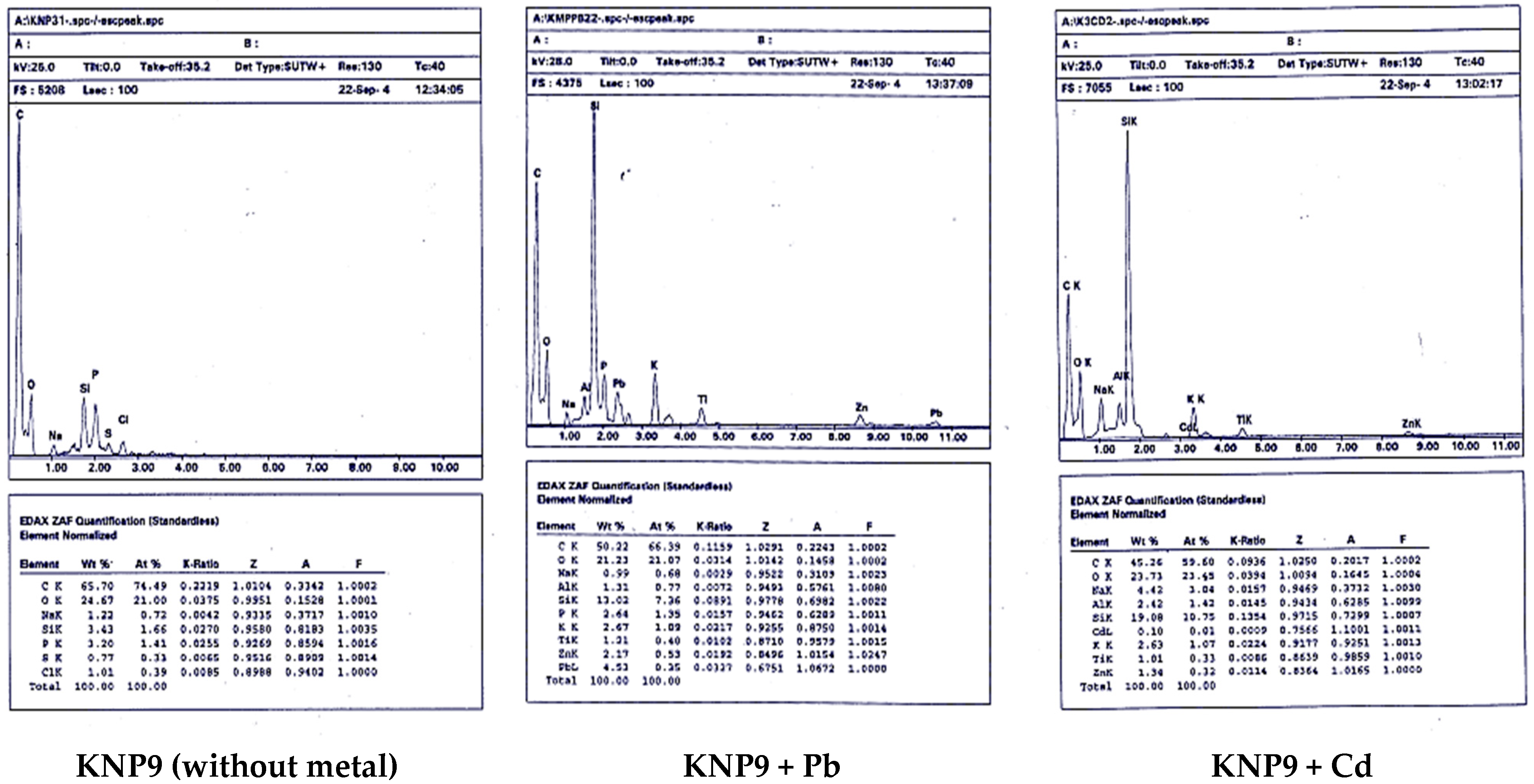
2.3. SEM-EDX Analysis
2.4. Pot Trial Experiment
2.5. Metal Quantification in Plant and Soil Samples
3. Results
3.1. Heavy Metal Tolerance Study
3.2. Heavy Metal Removal Studies
3.3. SEM-EDX Analysis
3.4. In Situ Pot Trial Experiment on Soybean
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. Heavy Met. 2018, 10, 115–132. [Google Scholar]
- Kumar, S.; Choudhary, A.K.; Suyal, D.C.; Makarana, G.; Goel, R. Leveraging arsenic resistant plant growth-promoting rhizobacteria for arsenic abatement in crops. J. Hazard. Mater. 2022, 425, 127965. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Ur Rehman, M.; Ahmad, B.; Ali, I.; Younas, M.; Aslam, M.S.; Rahman, A.U.; Taheri, E.; Fatehizadeh, A.; Rezakazemi, M. Assessment of heavy metals accumulation in agricultural soil, vegetables and associated health risks. PLoS ONE 2022, 17, e0267719. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, Y.-T.; Lai, M.-C.; Chiou, T.-Y.; Liao, C.-S. Continuous Biodiesel Production from Waste Soybean Oil Using a Nano-Fe3O4 Microwave Catalysis. Processes 2021, 9, 756. [Google Scholar] [CrossRef]
- Staton, M. Nutrient Management Recommendations for Profitable Soybean Production. Available online: https://www.canr.msu.edu/news/nutrient_management_recommendations_for_profitable_soybean_production (accessed on 12 April 2018).
- Kirillova, A.V.; Danilushkina, A.A.; Irisov, D.S.; Bruslik, N.L.; Fakhrullin, R.F.; Zakharov, Y.A.; Bukhmin, V.S.; Yarullina, D.R. Assessment of Resistance and Bioremediation Ability of Lactobacillus Strains to Lead and Cadmium. Int. J. Microbiol. 2017, 2017, 9869145. [Google Scholar] [CrossRef]
- Dabir, A.; Heidari, P.; Ghorbani, H.; Ebrahimi, A. Cadmium and lead removal by new bacterial isolates from coal and aluminum mines. Int. J. Environ. Sci. Technol. 2019, 16, 8297–8304. [Google Scholar] [CrossRef]
- Chellaiah, E.R. Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: A minireview. Appl. Water Sci. 2018, 8, 154. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef]
- Verma, S.; Kuila, A. Bioremediation of heavy metals by microbial process. Environ. Technol. Innov. 2019, 14, 100369. [Google Scholar] [CrossRef]
- Tripathi, M.; Munot, H.P.; Shouche, Y.; Meyer, J.M.; Goel, R. Isolation and functional characterization of siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9. Curr. Microbiol. 2005, 50, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Burd, G.I.; Dixon, D.G.; Glick, B.R. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J. Microbiol. 2000, 46, 237–245. [Google Scholar] [CrossRef]
- Aiking, H.; Kok, K.; van Heerikhuizen, H.; van’t Riet, J. Adaptation to Cadmium by Klebsiella aerogenes Growing in Continuous Culture Proceeds Mainly via Formation of Cadmium Sulfide. Appl. Environ. Microbiol. 1982, 44, 938–944. [Google Scholar] [CrossRef]
- Mathivanan, K.; Chandirika, J.U.; Vinothkanna, A.; Yin, H.; Liu, X.; Meng, D. Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment—A review. Ecotoxicol. Environ. Saf. 2021, 226, 112863. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.A.; Hamdan, A.M.; El-Naggar, M.Y. Assessment of the heavy metal bioremediation efficiency of the novel marine lactic acid bacterium, Lactobacillus plantarum MF042018. Sci. Rep. 2020, 10, 314. [Google Scholar] [CrossRef]
- El-Naggar, N.E.; Hamouda, R.A.; Mousa, I.E.; Abdel-Hamid, M.S.; Rabei, N.H. Statistical optimization for cadmium removal using Ulva fasciata biomass: Characterization, immobilization and application for almost-complete cadmium removal from aqueous solutions. Sci. Rep. 2018, 8, 12456. [Google Scholar] [CrossRef]
- Chakravarty, R.; Banerjee, P.C. Morphological changes in an acidophilic bacterium induced by heavy metals. Extremophiles 2008, 12, 279–284. [Google Scholar] [CrossRef]
- Husna; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Qadir, M.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O.; Lee, I.J. Phytohormones producing rhizobacteria alleviate heavy metals stress in soybean through multilayered response. Microbiol. Res. 2023, 266, 127237. [Google Scholar] [CrossRef] [PubMed]
- Sahile, A.A.; Khan, M.A.; Hamayun, M.; Imran, M.; Kang, S.-M.; Lee, I.-J. Novel Bacillus cereus Strain, ALT1, Enhance Growth and Strengthens the Antioxidant System of Soybean under Cadmium Stress. Agronomy 2021, 11, 404. [Google Scholar] [CrossRef]
- Miransari, M. Soybean production and heavy metal stress. In Abiotic and Biotic Stresses in Soybean Production; Miransari, M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 197–216. [Google Scholar]

| Days | Pb in µM | Cd in µM | ||
|---|---|---|---|---|
| Pellets | Supernatant | Pellets | Supernatant | |
| 1st day | 615 ± 2.51 | 185 ± 4.04 | 106 ± 1.80 | 44 ± 0.50 |
| 3rd day | 753 ± 9.64 | 47 ± 3.60 | 111.5 ± 3.01 | 38.9 ± 0.58 |
| 4th day | 792 ± 4.00 | 7.21 ± 0.79 | 103 ± 1.52 | 47.3 ± 0.90 |
| 6th day | 647 ± 10.96 | 153 ± 1.00 | 32.14 ± 1.43 | 117.4 ± 0.30 |
| Treatments | Length (µ) | Breadth (µ) |
|---|---|---|
| Without metal (control) | 1.086 ± 0.01 | 0.37 ± 0.03 |
| In the presence of Pb | 2.26 ± 0.02 | 0.43 ± 0.04 |
| In the presence of Cd | 1.086 ± 0.01 | 0.607 ± 0.02 |
| Shoot Length b (cm) | Root Length b (cm) | Fresh Weight a (g) | Dry Weight a (g) | Chlorophyll c (mg g−1) | ||
|---|---|---|---|---|---|---|
| Without Metal | Mean (control) | 26 | 9.3 | 1.01 | 0.3 | 4.79 |
| Mean (treated) | 33.5 (28.8) | 12 (29.0) | 1.34 (32.6) | 0.32 (6.66) | 5.15 (7.5) | |
| With Lead | Mean (control) | 21.0 | 8.4 | 0.67 | 0.17 | 3.8 |
| Mean (treated) | 36.0 (70.6) | 10.8 (28.57) | 1.01 (50.74) | 0.30 (76.47) | 4.9 (28.94) | |
| Critical difference at 5% | 6.02 | 4.29 | 0.29 | 0.29 | 1.05 | |
| With Cadmium | Mean (control) | 24.60 | 6.4 | 0.85 | 0.22 | 2.27 |
| Mean (treated) | 35.70 (45.12) | 10.90 (70.3) | 1.24 (45.8) | 0.32 (45.0) | 4.5 (98.25) | |
| Critical difference at 5% | 4.91 | 2.16 | 0.23 | 0.24 | 1.34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, M.; Kumar, S.; Makarana, G.; Goel, R. Metal-Tolerant Bioinoculant Pseudomonas putida KNP9 Mediated Enhancement of Soybean Growth under Heavy Metal Stress Suitable for Biofuel Production at the Metal-Contaminated Site. Energies 2023, 16, 4508. https://doi.org/10.3390/en16114508
Tripathi M, Kumar S, Makarana G, Goel R. Metal-Tolerant Bioinoculant Pseudomonas putida KNP9 Mediated Enhancement of Soybean Growth under Heavy Metal Stress Suitable for Biofuel Production at the Metal-Contaminated Site. Energies. 2023; 16(11):4508. https://doi.org/10.3390/en16114508
Chicago/Turabian StyleTripathi, Manishi, Saurabh Kumar, Govind Makarana, and Reeta Goel. 2023. "Metal-Tolerant Bioinoculant Pseudomonas putida KNP9 Mediated Enhancement of Soybean Growth under Heavy Metal Stress Suitable for Biofuel Production at the Metal-Contaminated Site" Energies 16, no. 11: 4508. https://doi.org/10.3390/en16114508
APA StyleTripathi, M., Kumar, S., Makarana, G., & Goel, R. (2023). Metal-Tolerant Bioinoculant Pseudomonas putida KNP9 Mediated Enhancement of Soybean Growth under Heavy Metal Stress Suitable for Biofuel Production at the Metal-Contaminated Site. Energies, 16(11), 4508. https://doi.org/10.3390/en16114508







