Syngas Production from Protective Face Masks through Pyrolysis/Steam Gasification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermogravimetric Analysis
2.3. Fluidized Bed Reactor System
2.4. Gasification Process Conditions
2.5. Composition of Evolved Gases Analysis
2.6. Composition of Tars Analysis
2.7. Evaluation of Carbon Content in Products
3. Results and Discussion
3.1. Thermogravimetric Analysis
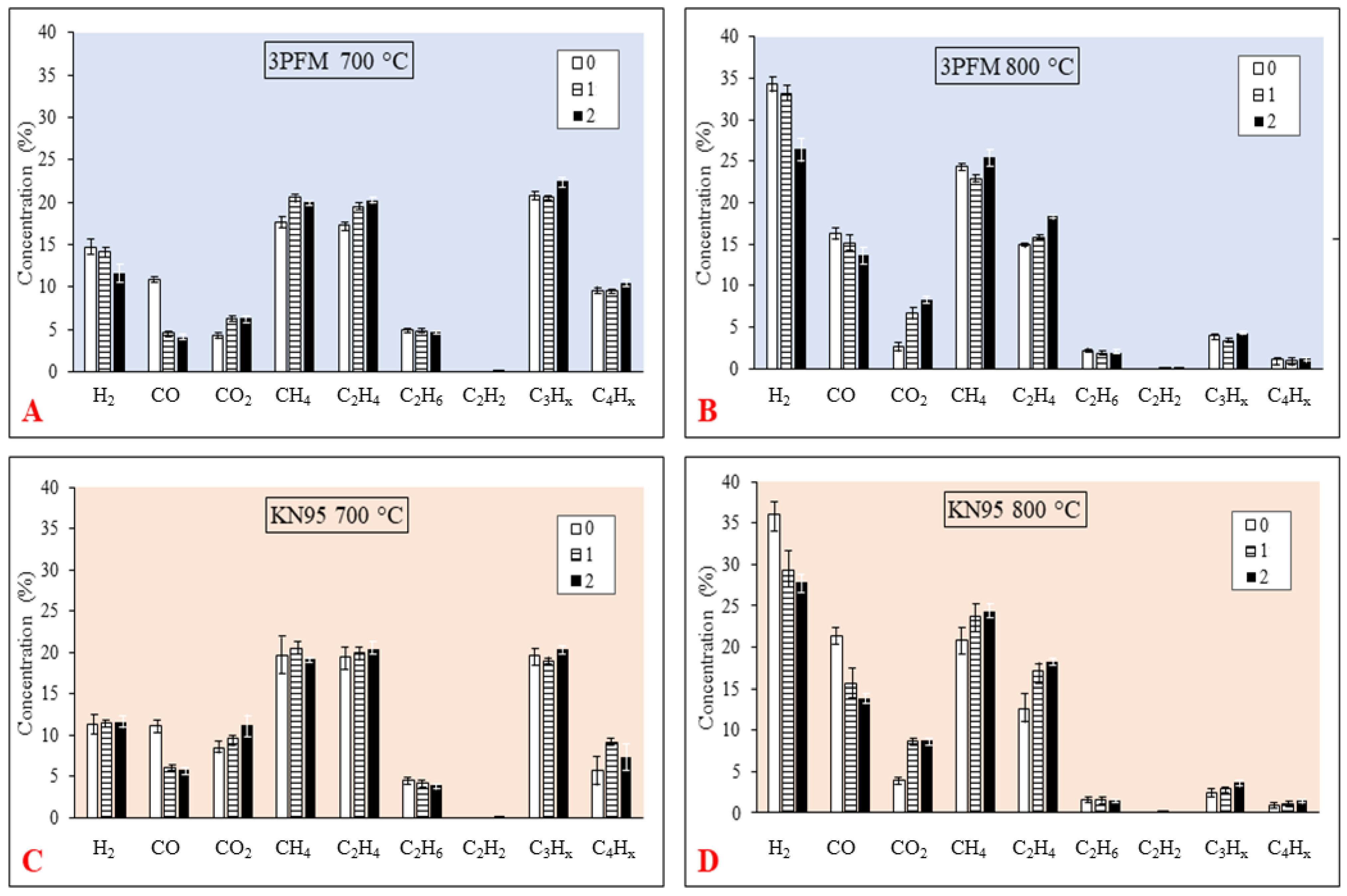
3.2. The Influence of Gasification Temperature on Gases Formation
3.3. The Influence of Steam to Carbon Ratio on Gases Formation
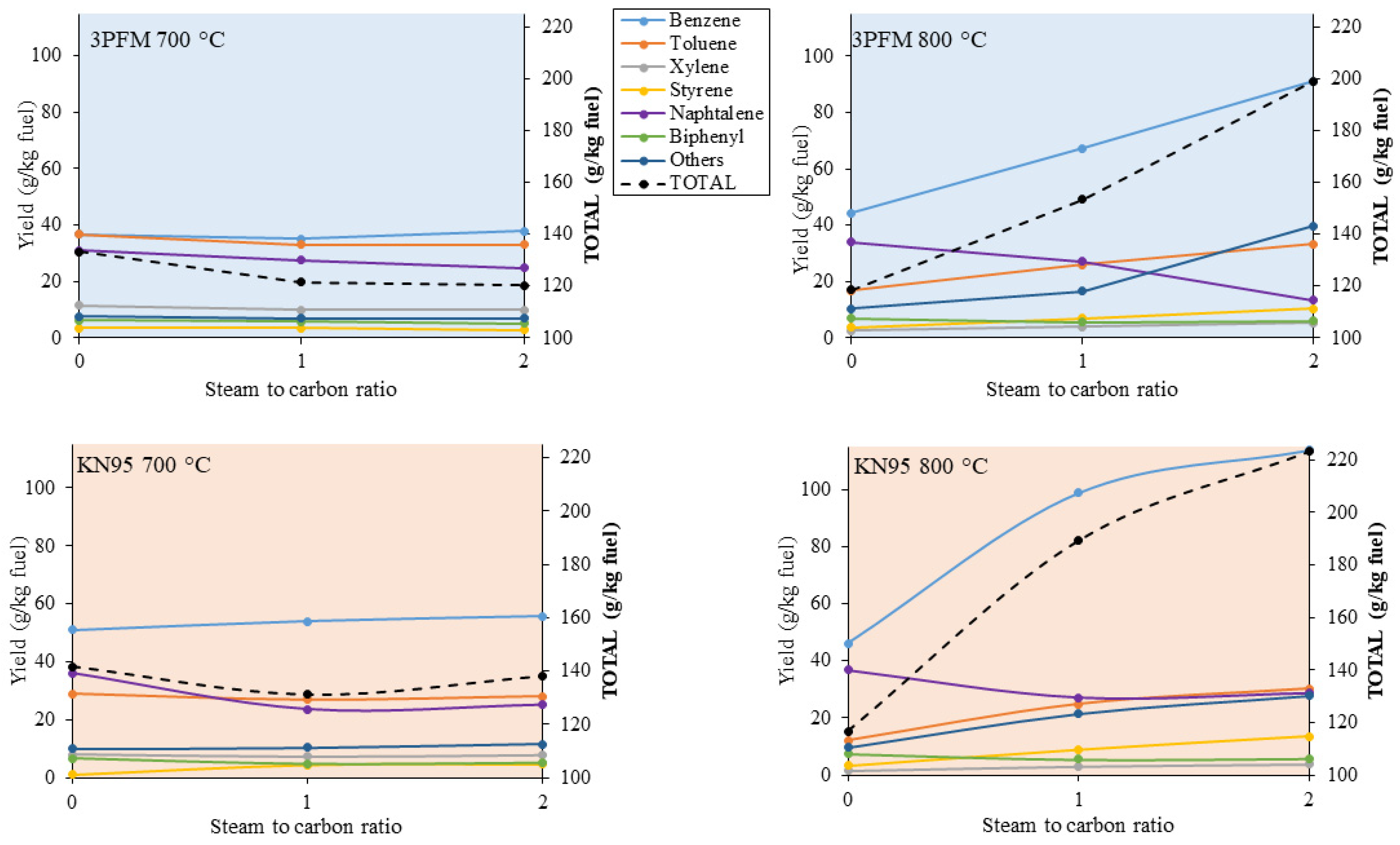
3.4. The Influence of Gasification Conditions on the Composition of Tars
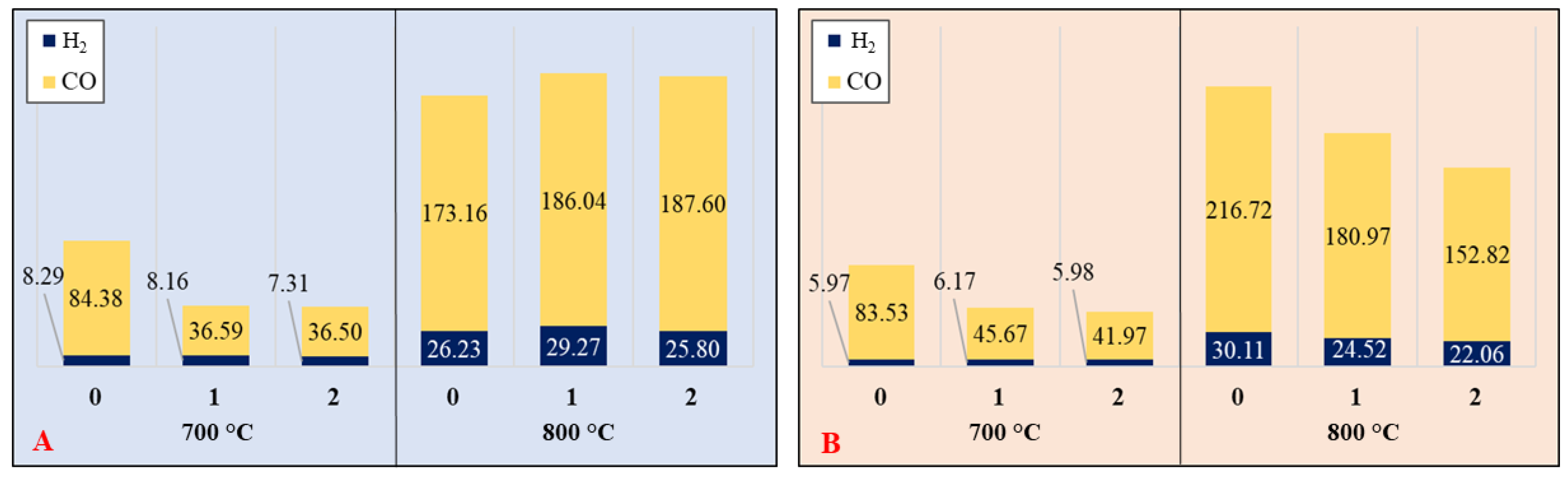
3.5. Product Yields—Conversion Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus Disease (COVID-19) Pandemic—Overview. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 6 December 2022).
- Mofijur, M.; Fattah, I.M.R.; Alam, M.A.; Islam, A.B.M.S.; Ong, H.C.; Rahman, S.M.A.; Najafi, G.; Ahmed, S.F.; Uddin, A.; Mahlia, T.M.I. Impact of COVID-19 on the Social, Economic, Environmental and Energy Domains: Lessons Learnt from a Global Pandemic. Sustain. Prod. Consum. 2021, 26, 343–359. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus Disease (COVID-19): How Is It Transmitted? Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-how-is-it-transmitted#:~:text=The%20virus%20can%20spread%20from,%2C%20speak%2C%20sing%20or%20breathe (accessed on 7 December 2022).
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic Waste Release Caused by COVID-19 and Its Fate in the Global Ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2111530118. [Google Scholar] [CrossRef]
- WHO. Tonnes of COVID-19 Health Care Waste Expose Urgent Need to Improve Waste Management Systems. Available online: https://www.who.int/news/item/01-02-2022-tonnes-of-covid-19-health-care-waste-expose-urgent-need-to-improve-waste-management-systems#:~:text=Tens%20of%20thousands%20of%20tonnes,to%20a%20new%20WHO%20report (accessed on 9 December 2022).
- McCarthy, R.; Gino, B.; d’Entremont, P.; Barari, A.; Renouf, T.S. The Importance of Personal Protective Equipment Design and Donning and Doffing Technique in Mitigating Infectious Disease Spread: A Technical Report. Cureus 2020, 12, e12084. [Google Scholar] [CrossRef]
- Jiangtao, S.; Zheng, W. Coronavirus: China Struggling to Deal with Mountain of Medical Waste Created by Epidemic. Available online: https://www.scmp.com/news/china/society/article/3065049/coronavirus-china-struggling-deal-mountain-medical-waste-created (accessed on 20 December 2022).
- Yang, S.; Cheng, Y.; Liu, T.; Huang, S.; Yin, L.; Pu, Y.; Liang, G. Impact of Waste of COVID-19 Protective Equipment on the Environment, Animals and Human Health: A Review. Environ. Chem. Lett. 2022, 20, 2951–2970. [Google Scholar] [CrossRef]
- Spennemann, D.H.R. COVID-19 Face Masks as a Long-Term Source of Microplastics in Recycled Urban Green Waste. Sustainability 2021, 14, 207. [Google Scholar] [CrossRef]
- Akkajit, P.; Romin, H.; Assawadithalerd, M.; Al-Khatib, I.A. Assessment of Knowledge, Attitude, and Practice in Respect of Medical Waste Management among Healthcare Workers in Clinics. J. Environ. Public Health 2020, 2020, 8745472. [Google Scholar] [CrossRef]
- Jung, S.; Lee, S.; Dou, X.; Kwon, E.E. Valorization of Disposable COVID-19 Mask through the Thermo-Chemical Process. Chem. Eng. J. 2021, 405, 126658. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic Pollutants from Plastic Waste—A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Kurniawan, W.; Aziz, M. Technological Review on Thermochemical Conversion of COVID-19-Related Medical Wastes. Resour. Conserv. Recycl. 2021, 167, 105429. [Google Scholar] [CrossRef]
- Safdari, M.-S.; Amini, E.; Weise, D.R.; Fletcher, T.H. Heating Rate and Temperature Effects on Pyrolysis Products from Live Wildland Fuels. Fuel 2019, 242, 295–304. [Google Scholar] [CrossRef]
- Greco, G.; Videgain, M.; Di Stasi, C.; Pires, E.; Manyà, J.J. Importance of Pyrolysis Temperature and Pressure in the Concentration of Polycyclic Aromatic Hydrocarbons in Wood Waste-Derived Biochars. J. Anal. Appl. Pyrolysis 2021, 159, 105337. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic Pyrolysis of Plastic Waste: A Review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Anekwe, I.M.S.; Khotseng, L.; Isa, Y.M. The Place of Biofuel in Sustainable Living; Prospects and Challenges. In Comprehensive Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 226–258. [Google Scholar]
- Farooq, A.; Lee, J.; Song, H.; Ko, C.H.; Lee, I.-H.; Kim, Y.-M.; Rhee, G.H.; Pyo, S.; Park, Y.-K. Valorization of Hazardous COVID-19 Mask Waste While Minimizing Hazardous Byproducts Using Catalytic Gasification. J. Hazard. Mater. 2022, 423, 127222. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.Y.; Lee, T.R.; Tokmurzin, D.; Park, S.J.; Ra, H.W.; Yoon, S.J.; Mun, T.-Y.; Yoon, S.M.; Moon, J.H.; Lee, J.G.; et al. Hydrogen-Rich Gas Production from Disposable COVID-19 Mask by Steam Gasification. Fuel 2023, 331, 125720. [Google Scholar] [CrossRef] [PubMed]
- Oyeleke, O.O.; Ohunakin, O.S.; Adelekan, D.S. Catalytic Pyrolysis in Waste to Energy Recovery Applications: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1107, 012226. [Google Scholar] [CrossRef]
- Yung, M.M.; Starace, A.K.; Griffin, M.B.; Wells, J.D.; Patalano, R.E.; Smith, K.R.; Schaidle, J.A. Restoring ZSM-5 Performance for Catalytic Fast Pyrolysis of Biomass: Effect of Regeneration Temperature. Catal. Today 2019, 323, 76–85. [Google Scholar] [CrossRef]
- Li, S.; Cañete Vela, I.; Järvinen, M.; Seemann, M. Polyethylene Terephthalate (PET) Recycling via Steam Gasification—The Effect of Operating Conditions on Gas and Tar Composition. Waste Manag. 2021, 130, 117–126. [Google Scholar] [CrossRef]
- Mandviwala, C.; Berdugo Vilches, T.; Seemann, M.; Faust, R.; Thunman, H. Thermochemical Conversion of Polyethylene in a Fluidized Bed: Impact of Transition Metal-Induced Oxygen Transport on Product Distribution. J. Anal. Appl. Pyrolysis 2022, 163, 105476. [Google Scholar] [CrossRef]
- Israelsson, M.; Seemann, M.; Thunman, H. Assessment of the Solid-Phase Adsorption Method for Sampling Biomass-Derived Tar in Industrial Environments. Energy Fuels 2013, 27, 7569–7578. [Google Scholar] [CrossRef]
- Park, S.-J.; Seo, M.-K. Element and Processing. In Interface Science and Technology; Elsevier: Oxford, UK, 2011; pp. 431–499. [Google Scholar]
- Nawaz, A.; Kumar, P. Thermal Degradation of Hazardous 3-Layered COVID-19 Face Mask through Pyrolysis: Kinetic, Thermodynamic, Prediction Modelling Using ANN and Volatile Product Characterization. J. Taiwan Inst. Chem. Eng. 2022, 139, 104538. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Tzoganakis, C.; Mekonnen, T.H. Degradation Behavior of Polypropylene during Reprocessing and Its Biocomposites: Thermal and Oxidative Degradation Kinetics. Polymers 2020, 12, 1627. [Google Scholar] [CrossRef] [PubMed]
- Frączak, D. Chemical Recycling of Polyolefins (PE, PP): Modern Technologies and Products. In Waste Material Recycling in the Circular Economy—Challenges and Developments; IntechOpen: London, UK, 2022. [Google Scholar]
- Damayanti, D.; Saputri, D.R.; Marpaung, D.S.S.; Yusupandi, F.; Sanjaya, A.; Simbolon, Y.M.; Asmarani, W.; Ulfa, M.; Wu, H.S. Current Prospects for Plastic Waste Treatment. Polymers 2022, 14, 3133. [Google Scholar] [CrossRef] [PubMed]
- Wilk, V.; Hofbauer, H. Conversion of Mixed Plastic Wastes in a Dual Fluidized Bed Steam Gasifier. Fuel 2013, 107, 787–799. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Effects of Gasification Temperature and Catalyst Ratio on Hydrogen Production from Catalytic Steam Pyrolysis-Gasification of Polypropylene. Energy Fuels 2008, 22, 4125–4132. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Hydrogen Production by Steam Gasification of Polypropylene with Various Nickel Catalysts. Appl. Catal. B 2009, 87, 152–161. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Syngas from Steam Gasification of Polyethylene in a Conical Spouted Bed Reactor. Fuel 2013, 109, 461–469. [Google Scholar] [CrossRef]
- Lazzarotto, I.P.; Ferreira, S.D.; Junges, J.; Bassanesi, G.R.; Manera, C.; Perondi, D.; Godinho, M. The Role of CaO in the Steam Gasification of Plastic Wastes Recovered from the Municipal Solid Waste in a Fluidized Bed Reactor. Process Saf. Environ. Prot. 2020, 140, 60–67. [Google Scholar] [CrossRef]
- Zhou, C.; Stuermer, T.; Gunarathne, R.; Yang, W.; Blasiak, W. Effect of Calcium Oxide on High-Temperature Steam Gasification of Municipal Solid Waste. Fuel 2014, 122, 36–46. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Park, K.-B.; Kim, J.-S. Hydrogen Production from Steam Gasification of Polyethylene Using a Two-Stage Gasifier and Active Carbon. Appl. Energy 2020, 262, 114495. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, G.; You, Y.; Xiao, B.; Liu, S.; He, P.; Guo, D.; Guo, X.; Zhang, G. Hydrogen-Rich Gas Production by Steam Gasification of Municipal Solid Waste (MSW) Using NiO Supported on Modified Dolomite. Int. J. Hydrogen Energy 2012, 37, 6503–6510. [Google Scholar] [CrossRef]
- Han, S.W.; Tokmurzin, D.; Lee, J.J.; Park, S.J.; Ra, H.W.; Yoon, S.J.; Mun, T.-Y.; Yoon, S.M.; Moon, J.H.; Lee, J.G.; et al. Gasification Characteristics of Waste Plastics (SRF) in a Bubbling Fluidized Bed: Use of Activated Carbon and Olivine for Tar Removal and the Effect of Steam/Carbon Ratio. Fuel 2022, 314, 123102. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Zhao, Z.; Felix, L.G.; Slimane, R.B.; Choi, C.W.; Ozkan, U.S. Olivine Catalysts for Methane- and Tar-Steam Reforming. Appl. Catal. B 2008, 81, 14–26. [Google Scholar] [CrossRef]
- Park, K.-B.; Jeong, Y.-S.; Kim, J.-S. Activator-Assisted Pyrolysis of Polypropylene. Appl. Energy 2019, 253, 113558. [Google Scholar] [CrossRef]
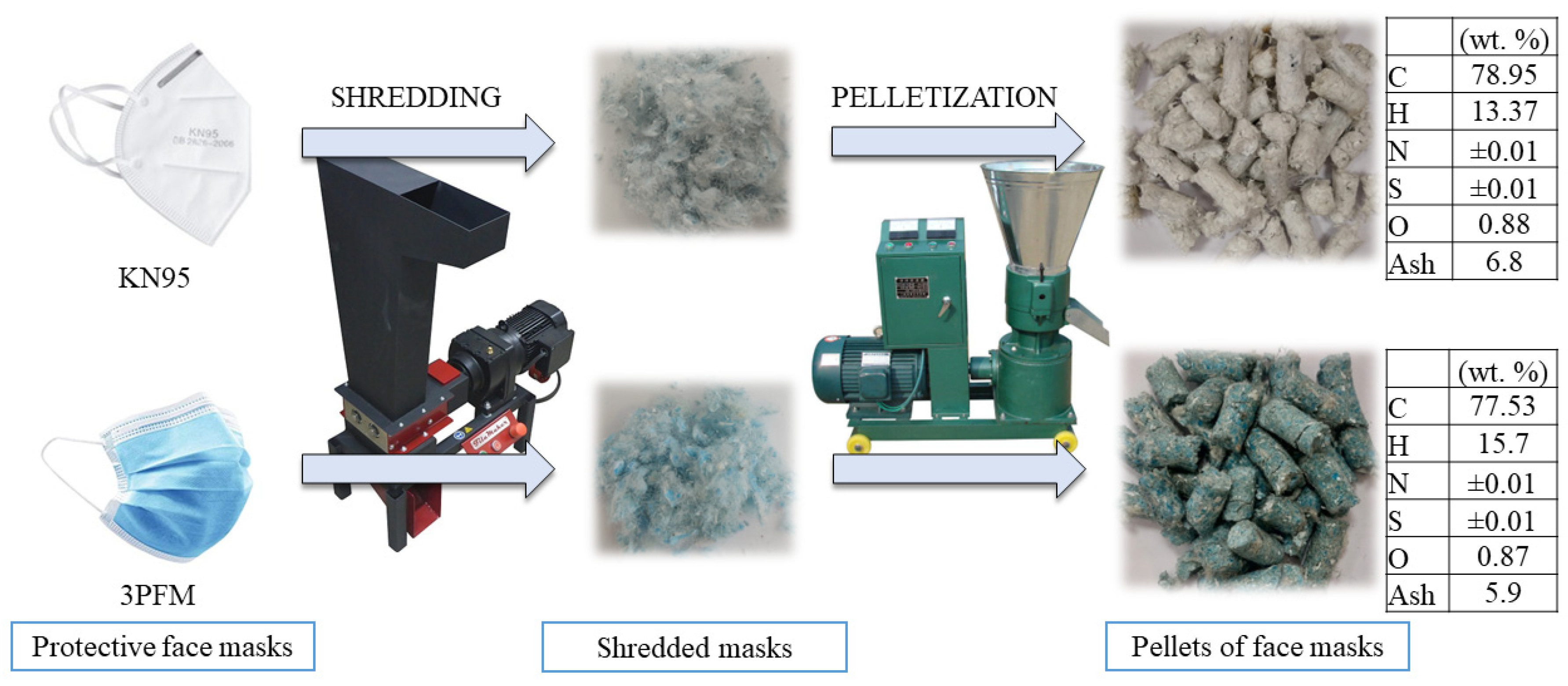



| SCR | Steam (g/min) | N2 (L/min) | He (L/min) | Air (L/min) |
|---|---|---|---|---|
| 0 | 0 | 5 | 0.05 | 0 |
| 1 | 1.63 | 3 | 0.05 | 0 |
| 2 | 3.26 | 2 | 0.05 | 0 |
| Combustion | 0 | 0 | 0.05 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiminaitė, I.; González-Arias, J.; Striūgas, N.; Eimontas, J.; Seemann, M. Syngas Production from Protective Face Masks through Pyrolysis/Steam Gasification. Energies 2023, 16, 5417. https://doi.org/10.3390/en16145417
Kiminaitė I, González-Arias J, Striūgas N, Eimontas J, Seemann M. Syngas Production from Protective Face Masks through Pyrolysis/Steam Gasification. Energies. 2023; 16(14):5417. https://doi.org/10.3390/en16145417
Chicago/Turabian StyleKiminaitė, Ieva, Judith González-Arias, Nerijus Striūgas, Justas Eimontas, and Martin Seemann. 2023. "Syngas Production from Protective Face Masks through Pyrolysis/Steam Gasification" Energies 16, no. 14: 5417. https://doi.org/10.3390/en16145417
APA StyleKiminaitė, I., González-Arias, J., Striūgas, N., Eimontas, J., & Seemann, M. (2023). Syngas Production from Protective Face Masks through Pyrolysis/Steam Gasification. Energies, 16(14), 5417. https://doi.org/10.3390/en16145417






