Experimental Study on the Preparation of Hydrogen-Rich Gas by Gasifying of Traditional Chinese Medicine Residue in a DFB Based on Calcium Looping
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Model
2.2. Experimental Device
2.3. Raw Materials
3. Results and Discussion
3.1. Temperature and Pressure Characteristics of DFB
3.1.1. Temperature Characteristics
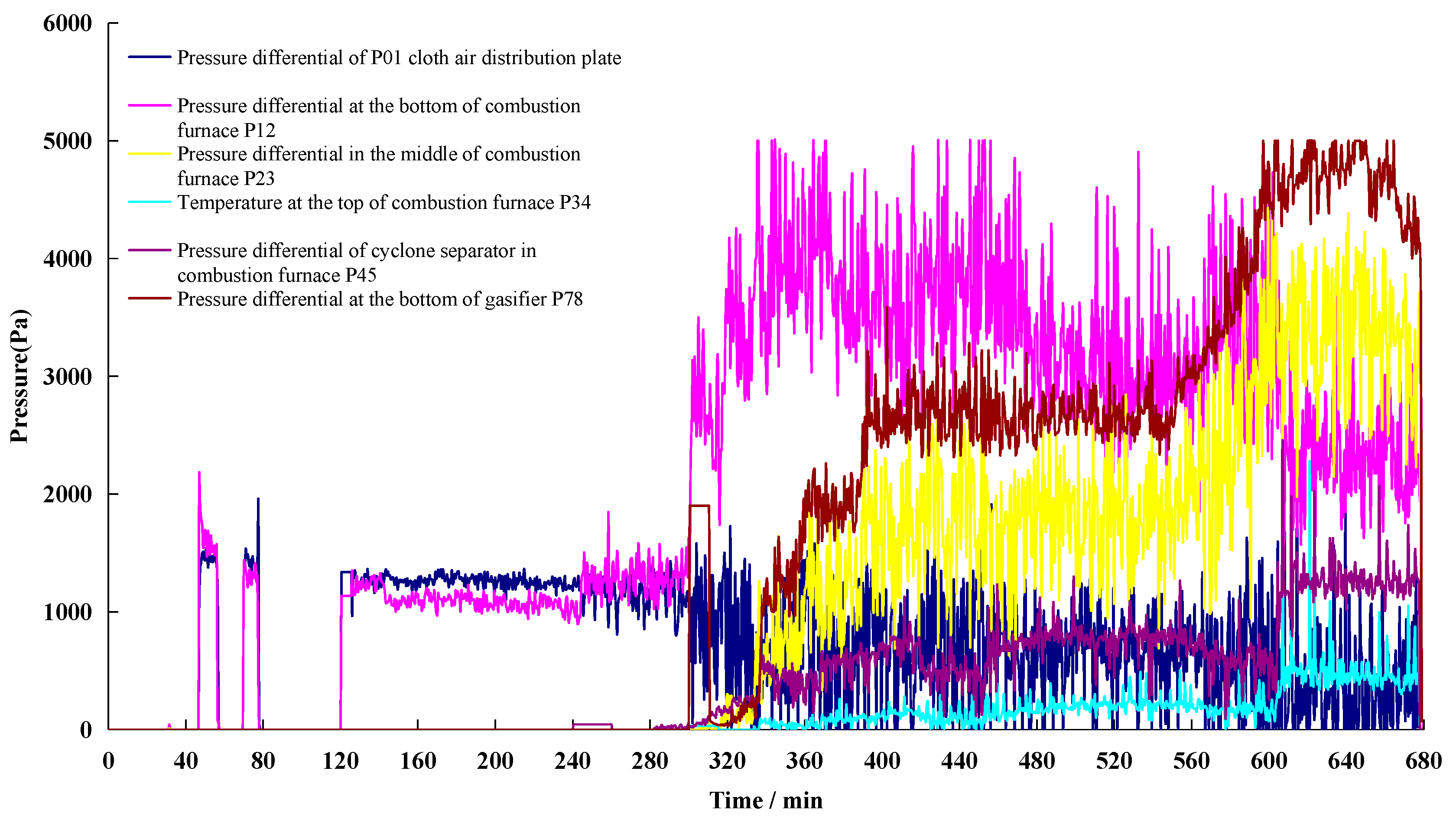
3.1.2. Pressure Variation Characteristics
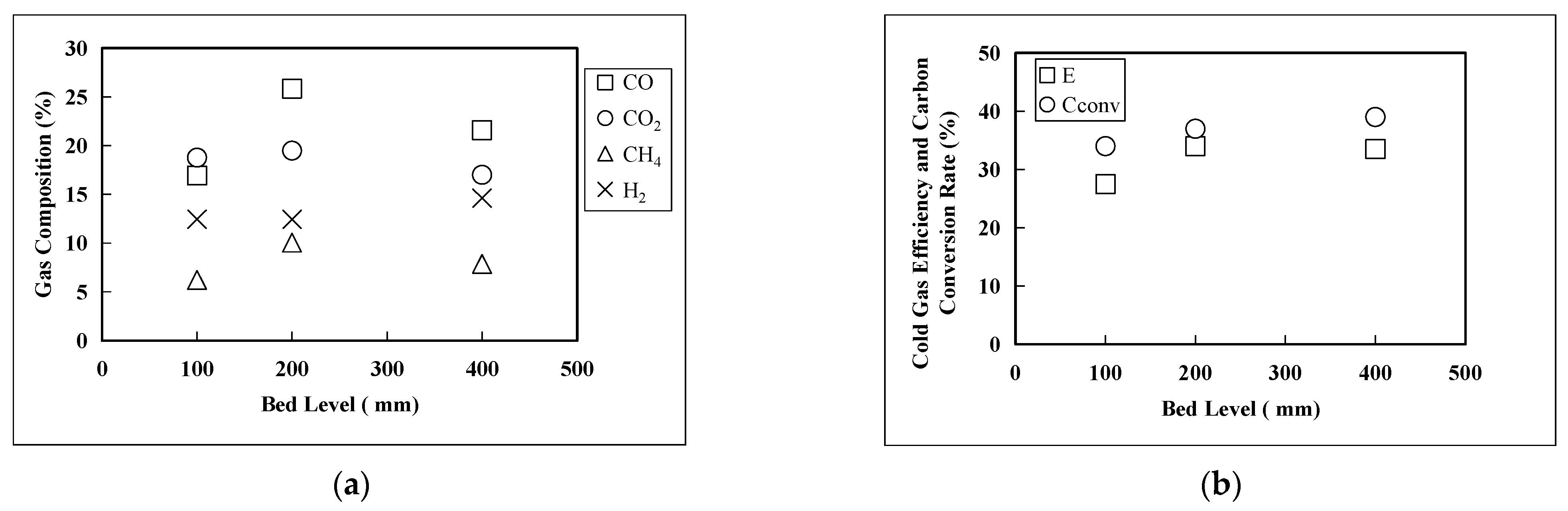
3.2. Gasification Performance of DFB
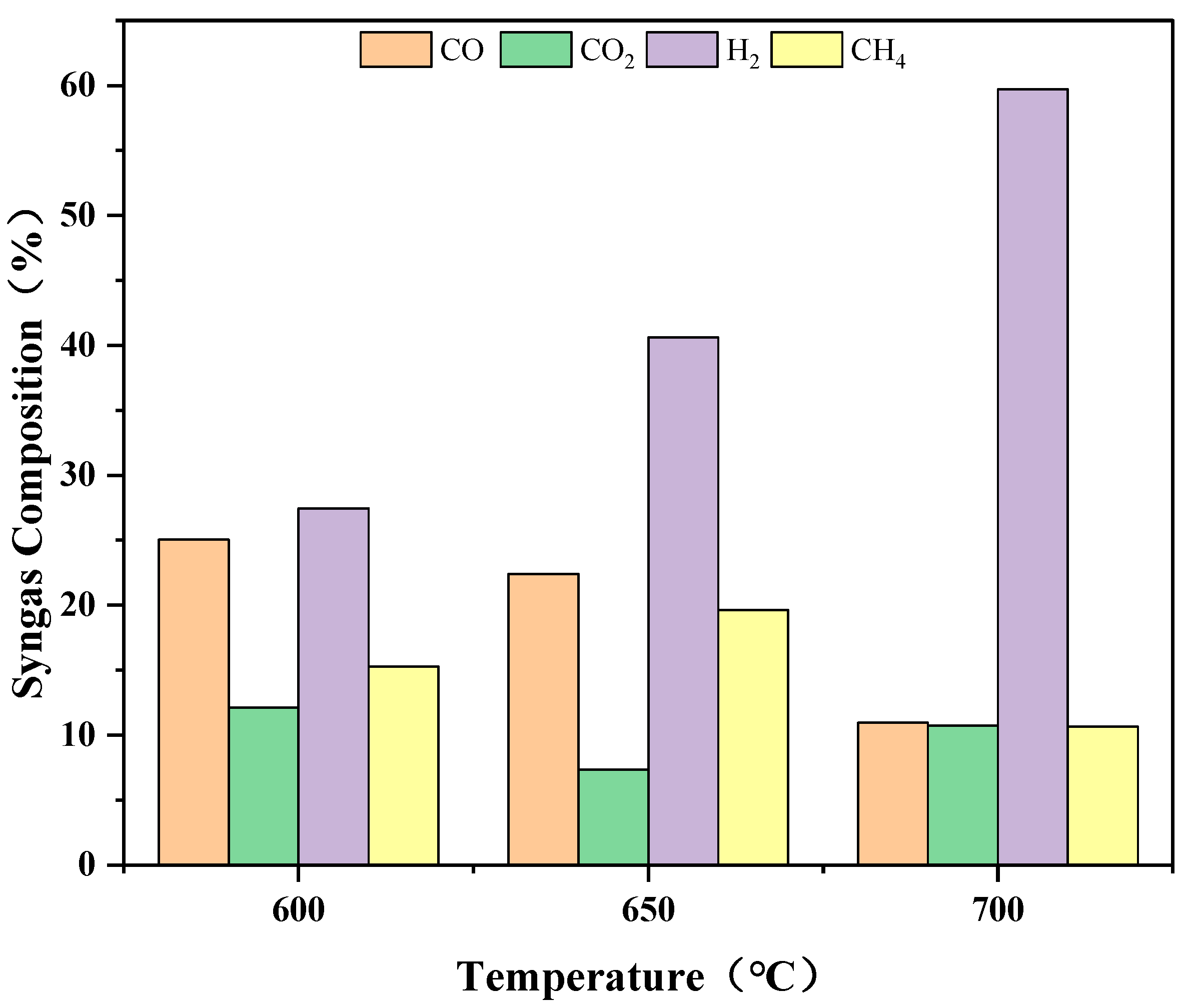
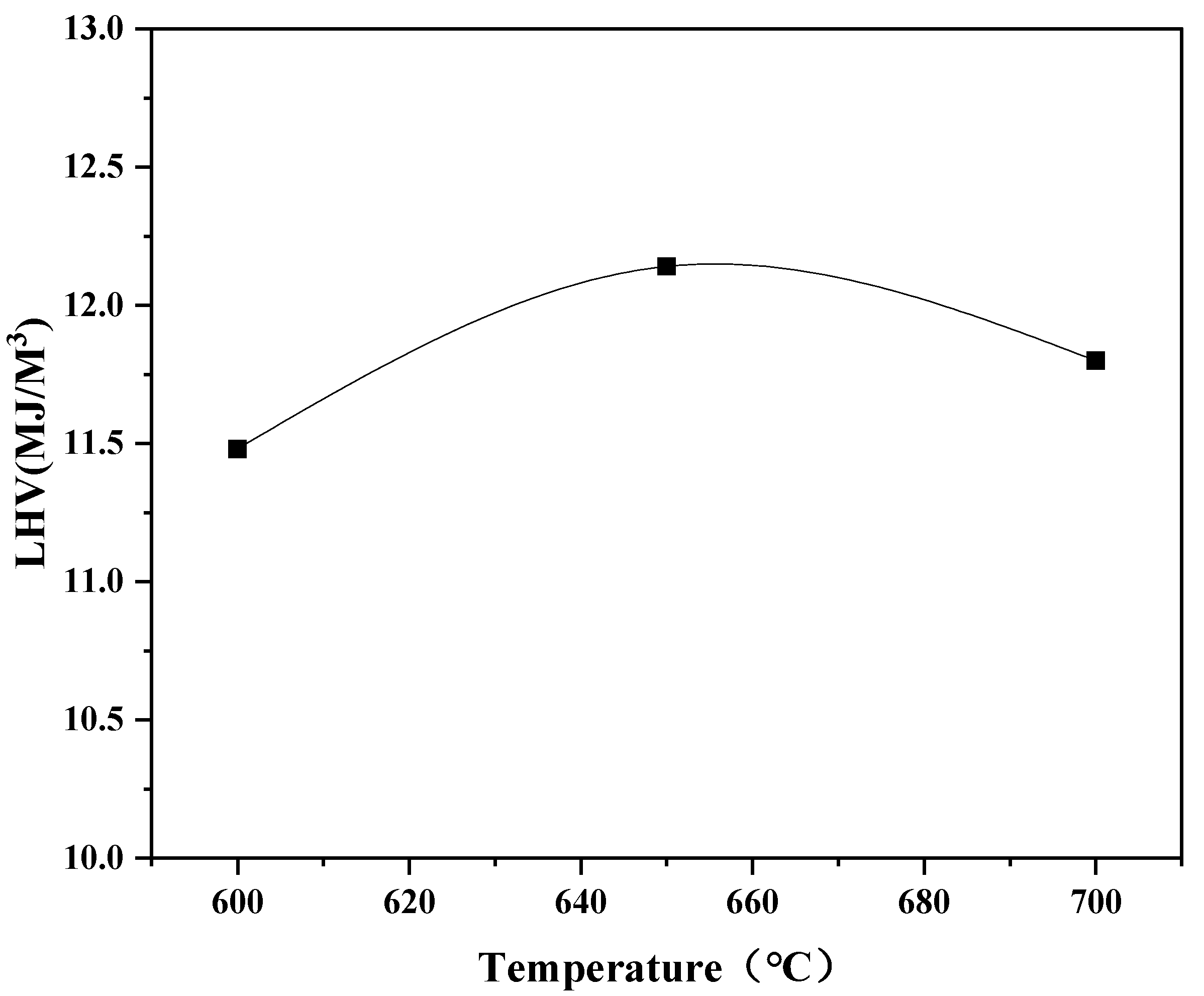
3.2.1. Influence of Temperature
3.2.2. The Effect of S/B
3.2.3. The Effect of Ca/C
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, G.; Lü, Q.; Qi, L. Experimental Research on Coal Gasification Characteristics in Single and Dual Circulating Fluidized Beds. Proc. CSEE 2009, 29, 24–29. [Google Scholar]
- Zhu, X.; Imtiaz, Q.; Donat, F.; Müller, C.R.; Li, F. Chemical looping beyond combustion—A perspective. Energy Environ. Sci. 2020, 13, 772–804. [Google Scholar] [CrossRef]
- Anders, L.; Leckner, B.; Mattisson, T. A fluidized-bed combustion process with inherent CO2 separation; application of chemical-looping combustion. Chem. Eng. Sci. 2001, 56, 3101–3113. [Google Scholar]
- Siddig, A.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2021, 764, 142892. [Google Scholar]
- Alalwan, H.A.; Alminshid, A.H. CO2 capturing methods: Chemical looping combustion (CLC) as a promising technique. Sci. Total Environ. 2021, 788, 147850. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.M.; Alobaid, F.; Dieringer, P.; Epple, B. Biomass-based chemical looping gasification: Overview and recent developments. Appl. Sci. 2021, 11, 7069. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Wang, Y.; Huo, R.; Huang, Z.; Liu, M.; Wei, G.; Zhao, Z.; Li, H.; Fang, Y. Review of biomass chemical looping gasification in China. Energy Fuels 2020, 34, 7847–7862. [Google Scholar] [CrossRef]
- Goel, A.; Moghaddam, E.M.; Liu, W.; He, C.; Konttinen, J. Biomass chemical looping gasification for high-quality syngas: A critical review and technological outlooks. Energy Convers. Manag. 2022, 268, 116020. [Google Scholar] [CrossRef]
- Samprón, I.; de Diego, L.F.; García-Labiano, F.; Izquierdo, M.T.; Adánez, A.A.J. Biomass Chemical Looping Gasification of pine wood using a synthetic Fe2O3/Al2O3 oxygen carrier in a continuous unit. Bioresour. Technol. 2020, 316, 123908. [Google Scholar] [CrossRef]
- Hu, Q.; Shen, Y.; Chew, J.W.; Ge, T.; Wang, C.-H. Chemical looping gasification of biomass with Fe2O3/CaO as the oxygen carrier for hydrogen-enriched syngas production. Chem. Eng. J. 2020, 379, 122346. [Google Scholar] [CrossRef]
- Abanades, J.C.; Murillo, E.; Fernandez, J.R.; Grasa, G.; Martínez, I. New CO2 capture process for hydrogen production combining Ca and Cu chemical loops. Environ. Sci. Technol. 2010, 44, 6901–6904. [Google Scholar] [CrossRef] [PubMed]
- Di Giuliano, A.; Gallucci, K. Sorption enhanced steam methane reforming based on nickel and calcium looping: A review. Chem. Eng. Process.-Process Intensif. 2018, 130, 240–252. [Google Scholar] [CrossRef]
- Weimer, T.; Berger, R.; Hawthorne, C.; Abanades, J.C. Lime enhanced gasification of solid fuels: Examination of a process for simultaneous hydrogen production and CO2 capture. Fuel 2008, 87, 1678–1686. [Google Scholar] [CrossRef]
- Connell, D.P.; Lewandowski, D.A.; Ramkumar, S.; Phalak, N.; Statnick, R.M.; Fan, L.-S. Process simulation and economic analysis of the Calcium Looping Process (CLP) for hydrogen and electricity production from coal and natural gas. Fuel 2013, 105, 383–396. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Suzuki, Y.; Hatano, H.; Harada, M. Developing an innovative method, HyPr-RING, to produce hydrogen from hydrocarbons. Energy Convers. Manag. 2002, 43, 1283–1290. [Google Scholar] [CrossRef]
- Dean, C.C.; Blamey, J.; Florin, N.H.; Al-Jeboori, M.J.; Fennell, P.S. The calcium looping cycle for CO2 capture from power generation, cement manufacture and hydrogen production. Chem. Eng. Res. Des. 2011, 89, 836–855. [Google Scholar] [CrossRef]
- Bahzad, H.; Katayama, K.; Boot-Handford, M.E.; Dowell, N.M.; Shah, N.; Fennell, P.S. Iron-based chemical-looping technology for decarbonising iron and steel production. Int. J. Greenh. Gas Control 2019, 91, 102766. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Chu, Z.; Fang, Y. Thermodynamic analysis of integrated sorption-enhanced staged-gasification of biomass and in-situ CO2 utilization by methane reforming process based on calcium looping. Energy Convers. Manag. 2023, 278, 116710. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; He, Z.; Zhao, J.; Wang, D. Microtubular Fe/Mn-promoted CaO-Ca12Al14O33 bifunctional material for H2 production from sorption enhanced water gas shift. Appl. Catal. B Environ. 2022, 314, 121474. [Google Scholar] [CrossRef]
- Yan, X.; Li, Y.; Ma, X.; Bian, Z.; Zhao, J.; Wang, Z. CeO2-modified CaO/Ca12Al14O33 bi-functional material for CO2 capture and H2 production in sorption-enhanced steam gasification of biomass. Energy 2020, 192, 116664. [Google Scholar] [CrossRef]
- Stanger, L.; Schirrer, A.; Benedikt, F.; Bartik, A.; Jankovic, S.; Müller, S.; Kozek, M. Dynamic modeling of DFB steam gasification for control design. Energy 2023, 265, 126378. [Google Scholar] [CrossRef]
- Mauerhofer, A.M.; Fuchs, J.; Müller, S.; Benedikt, F.; Schmid, J.C.; Hofbauer, H. CO2 gasification in a DFB reactor system: Impact on the product gas composition. Fuel 2019, 253, 1605–1616. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Luo, K.; Li, D.; Fan, J. Study of biomass gasification in an industrial-scale dual circulating fluidized bed (DCFB) using the Eulerian-Lagrangian method. Particuology 2023, 83, 156–168. [Google Scholar] [CrossRef]
- Fuchs, J.; Schmid, J.C.; Müller, S.; Hofbauer, H. DFB gasification of biomass with selective carbon dioxide removal and limestone as bed material: A review. Renew. Sustain. Energy Rev. 2019, 107, 212–231. [Google Scholar] [CrossRef]
- Yaghoubi, E.; Xiong, Q.; Doranehgard, M.H.; Yeganeh, M.M.; Shahriari, G.; Bidabadi, M. The effect of different operational parameters on hydrogen rich syngas production from biomass gasification in a DFB gasifier. Chem. Eng. Process.-Process Intensif. 2018, 126, 210–221. [Google Scholar] [CrossRef]
- Sun, H.; Bao, G.; Yang, S.; Hu, J.; Wang, H. Numerical study of the biomass gasification process in an industrial-scale DFB gasifier with 8MWth input. Renew. Energy 2023, 211, 681–696. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, W.; Chen, S.; Duan, L.; Xiang, W. Investigations on biomass gasification of compact-fast DFB calcium looping. J. Clean. Prod. 2023, 405, 137065. [Google Scholar] [CrossRef]
- Hanchate, N.; Ramani, S.; Mathpati, C.S.; Dalvi, V.H. Biomass gasification using DFB gasification systems: A review. J. Clean. Prod. 2021, 280, 123148. [Google Scholar] [CrossRef]
- Karl, J.; Pröll, T. Steam gasification of biomass in DFB gasifiers: A review. Renew. Sustain. Energy Rev. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Bishnu, A.; Dutta, A.; Basu, P. An investigation into steam gasification of biomass for hydrogen enriched gas production in presence of CaO. Int. J. Hydrog. Energy 2010, 35, 1582–1589. [Google Scholar]
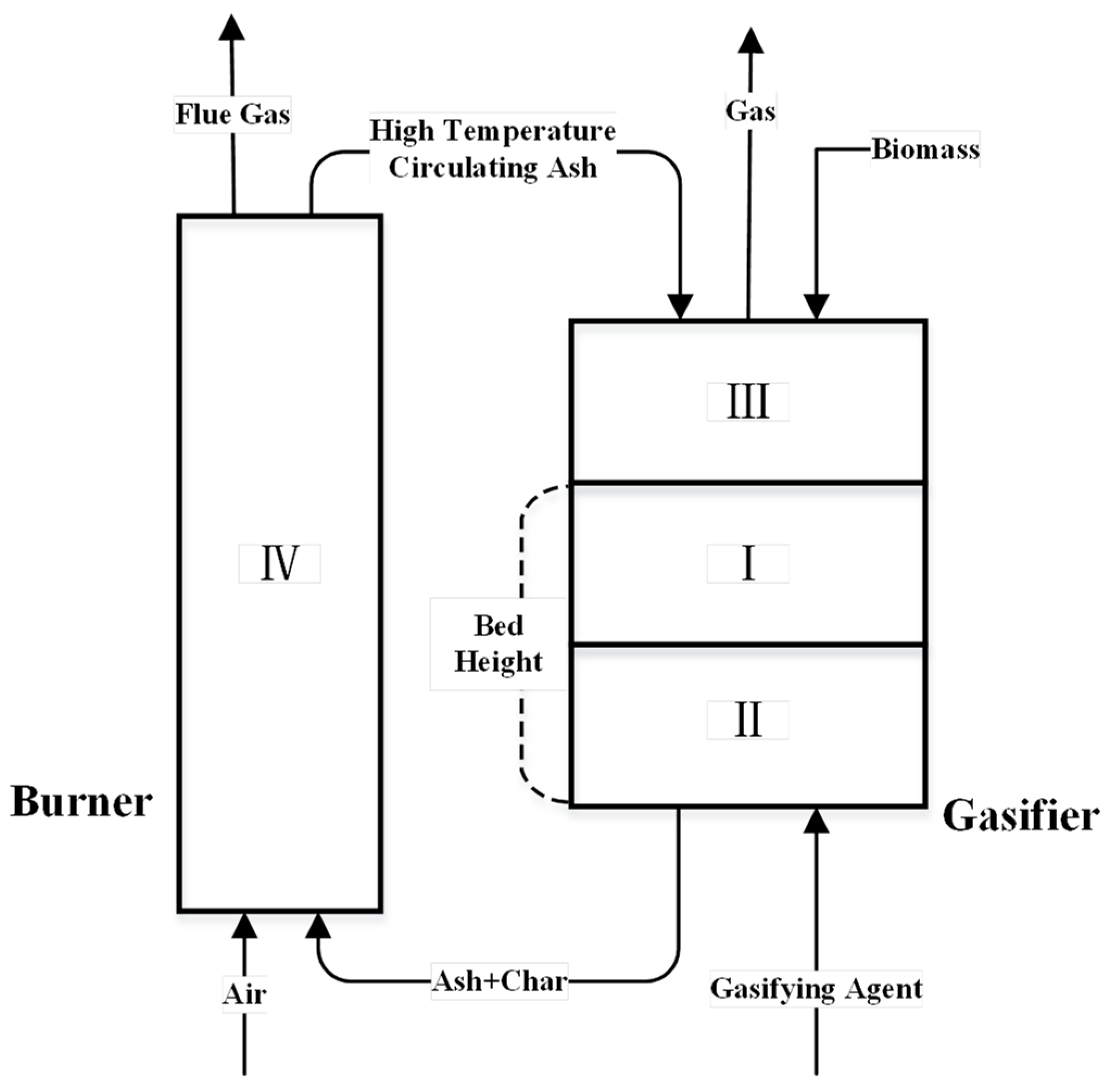
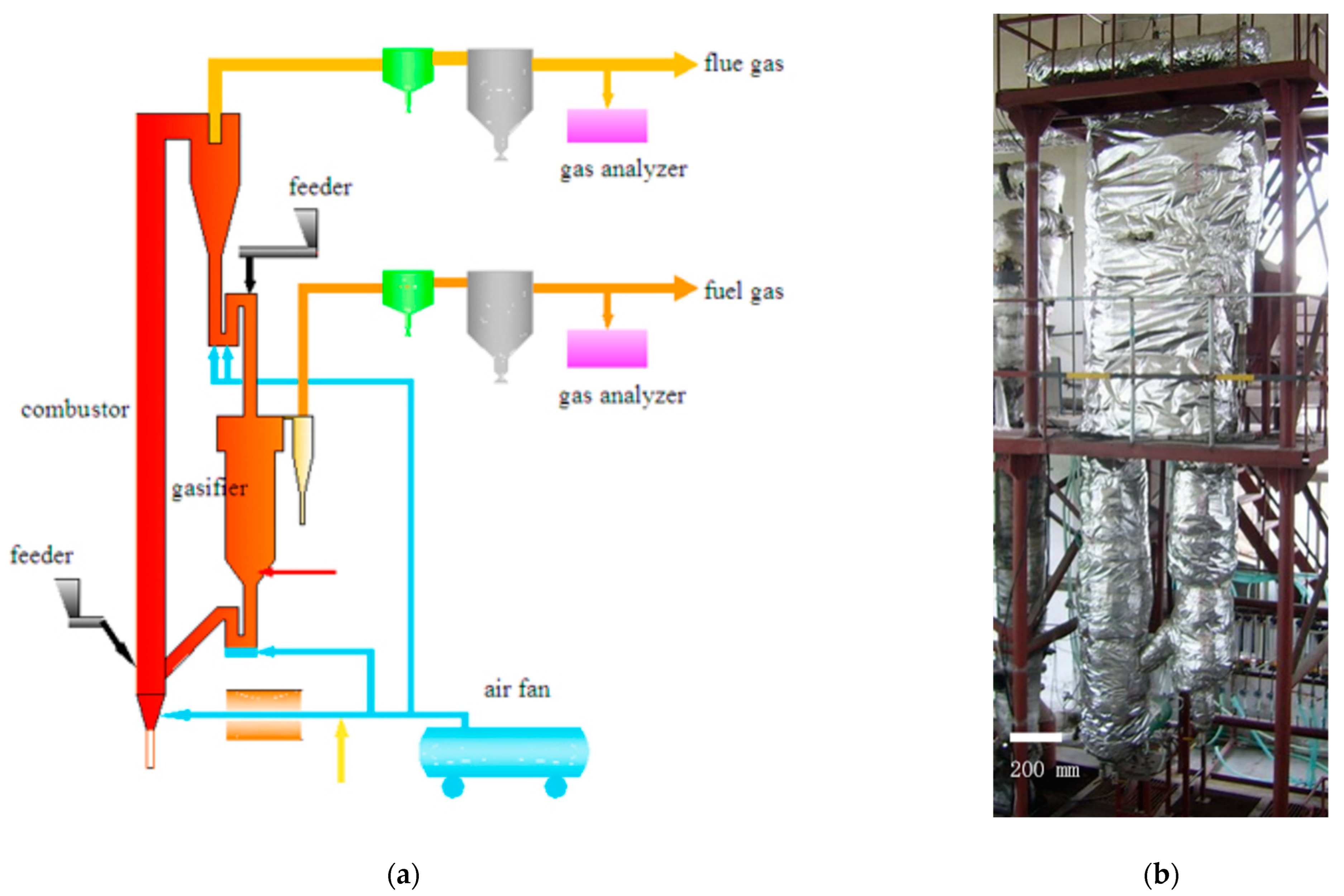

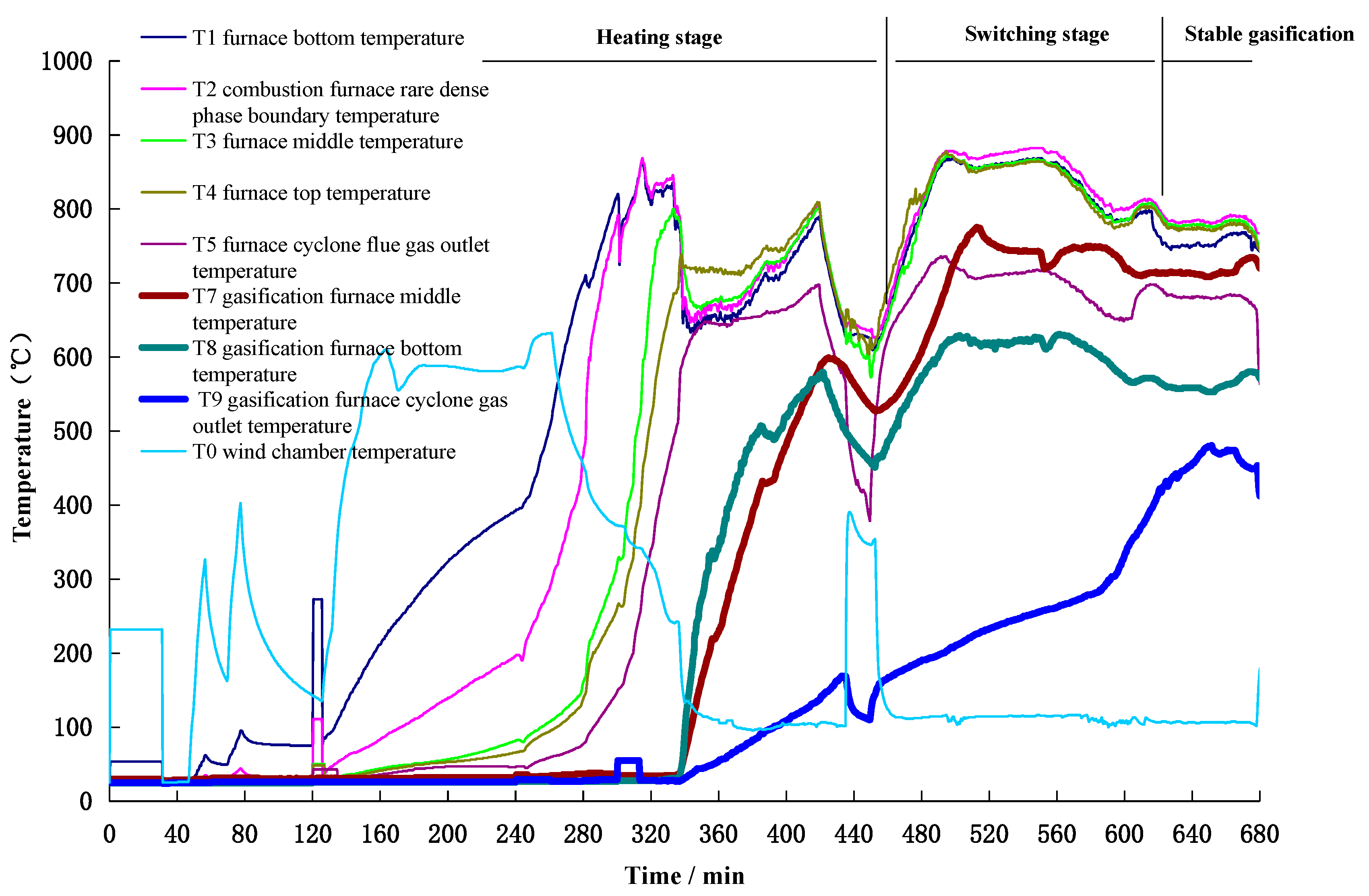
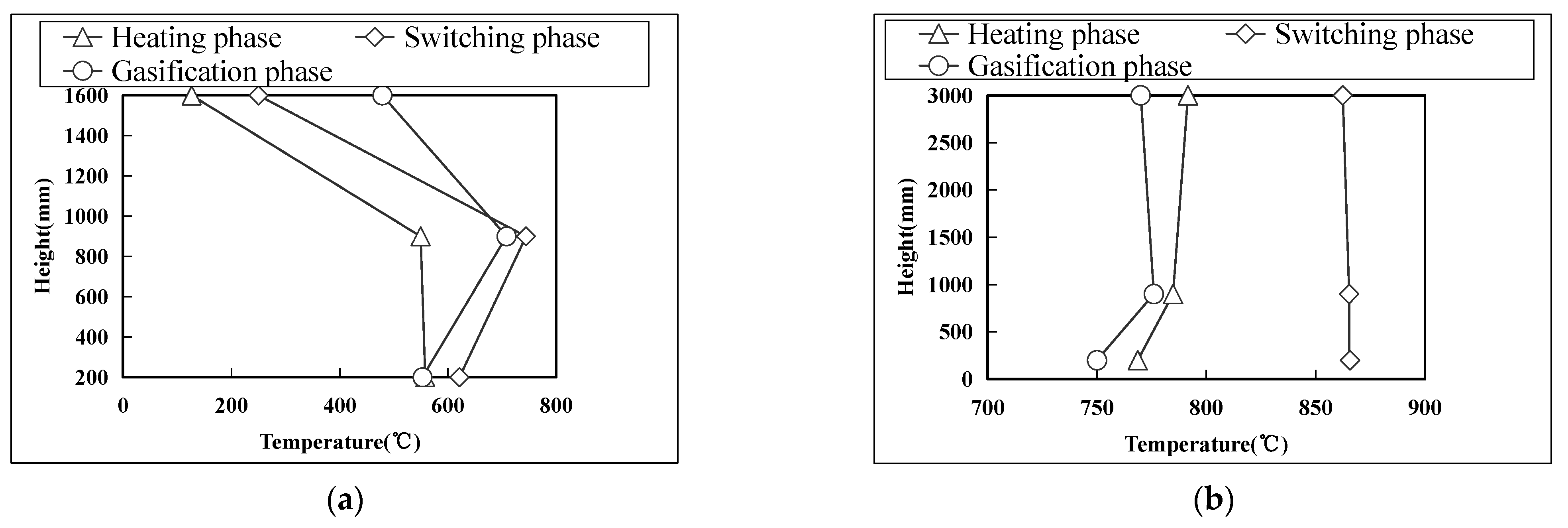
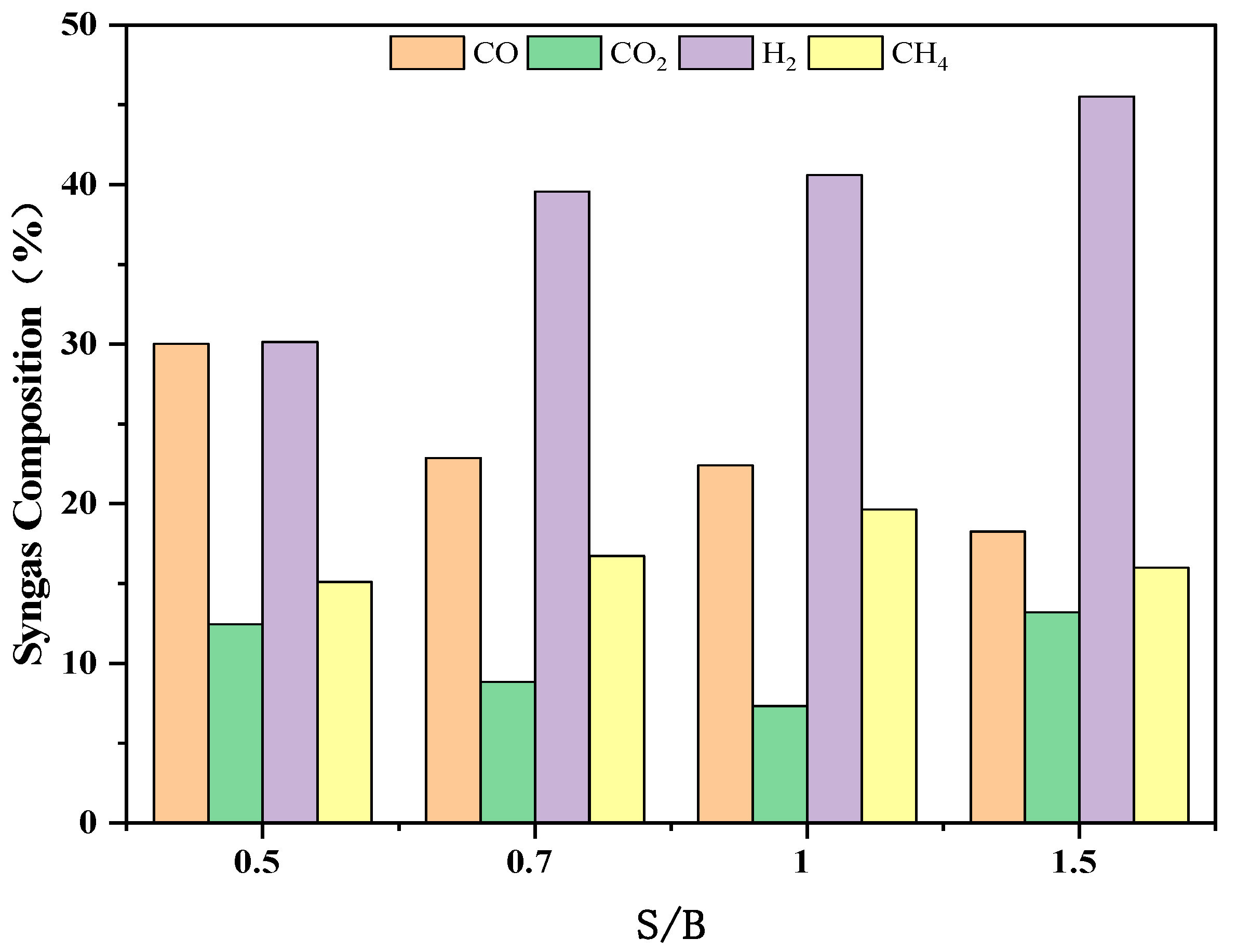
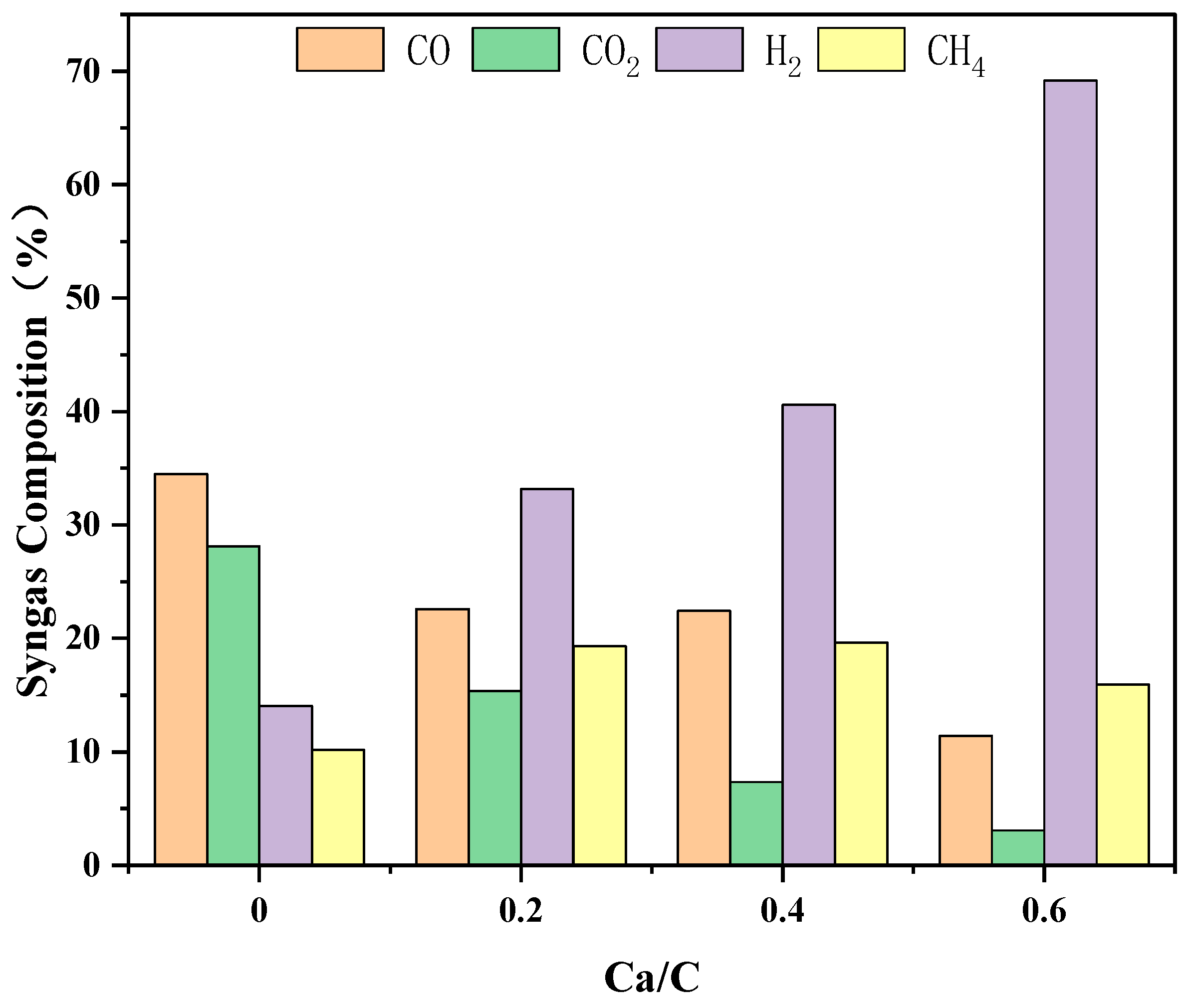
| Title | Symbol | Position (Air Distribution Plate Distance/mm) |
|---|---|---|
| Air chamber temperature | T0 | −40 |
| Dense phase zone temperature | T1 | 200 |
| Temperature at the boundary of the lean and dense phase zone in the combustion furnace | T2 | 700 |
| Middle part temperature | T3 | 1500 |
| Middle part temperature | T4 | 2950 |
| Pressure of chamber | P0 | −40 |
| Bottom pressure of combustion furnace | P1 | 200 |
| Middle pressure of combustion furnace | P2 | 1500 |
| Top pressure of combustion furnace | P3 | 2950 |
| inlet | 400 |
| Title | Symbol | Position (Air Distribution Plate Distance/mm) |
|---|---|---|
| Bottom temperature | T8 | 255 |
| Middle temperature | T7 | 800 |
| Top temperature | T9 | 1600 |
| Bottom temperature | P8 | 255 |
| Middle pressure | P7 | 800 |
| Top pressure | P6 | 1600 |
| Bottom inlet | 100 | |
| Top inlet | 1650 |
| Project | Value | Method | Instrument | |
|---|---|---|---|---|
| Moisture | 5.45 | Industrial methods of coal analysis | scale (GB1302) drying oven (102A) | |
| Ash | 10.14 | |||
| Volatiles | 67.34 | scale (GB1302) | ||
| Fixed Carbon | 17.07 | muffle (RJM-1.8-10) | ||
| Lower Heating Value (LHV) (kJ/kg) | Heat Value | 15,300 | Method for determination of calorific value of coal | Calorimeter (AC-350 USA) |
| Ultimate Analysis (wt%) | C | 42.31 | Methods for determination of carbon and hydrogen in coal | TQ-3 type C and H element analyzer |
| H | 6.01 | |||
| S | 0.25 | Method for determination of total sulfur in coal | CLS-1 Coulomb sulfur meter | |
| N | 3.23 | Method for elemental analysis of coal | Chemical titrator | |
| O | 32.61 | Method for determination of oxygen in coal | Flash Smart | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Yang, L.; Fan, X.; Li, X. Experimental Study on the Preparation of Hydrogen-Rich Gas by Gasifying of Traditional Chinese Medicine Residue in a DFB Based on Calcium Looping. Energies 2023, 16, 4434. https://doi.org/10.3390/en16114434
Zhou X, Yang L, Fan X, Li X. Experimental Study on the Preparation of Hydrogen-Rich Gas by Gasifying of Traditional Chinese Medicine Residue in a DFB Based on Calcium Looping. Energies. 2023; 16(11):4434. https://doi.org/10.3390/en16114434
Chicago/Turabian StyleZhou, Xiaoquan, Liguo Yang, Xiaoxu Fan, and Xuanyou Li. 2023. "Experimental Study on the Preparation of Hydrogen-Rich Gas by Gasifying of Traditional Chinese Medicine Residue in a DFB Based on Calcium Looping" Energies 16, no. 11: 4434. https://doi.org/10.3390/en16114434
APA StyleZhou, X., Yang, L., Fan, X., & Li, X. (2023). Experimental Study on the Preparation of Hydrogen-Rich Gas by Gasifying of Traditional Chinese Medicine Residue in a DFB Based on Calcium Looping. Energies, 16(11), 4434. https://doi.org/10.3390/en16114434





