Study on Slagging Characteristics of Co-Combustion of Meager Coal and Spent Cathode Carbon Block
Abstract
1. Introduction
2. Experiment
2.1. Preparation of Ash Sample
2.2. Determination of Ash Melting Point
2.3. Characterization of Ash
2.4. Analysis Method
2.5. Thermodynamic Calculation
3. Results and Discussion
3.1. Ash Composition Analysis
3.2. Analysis of Ash Melting Characteristics
3.2.1. Characteristic Index Analysis
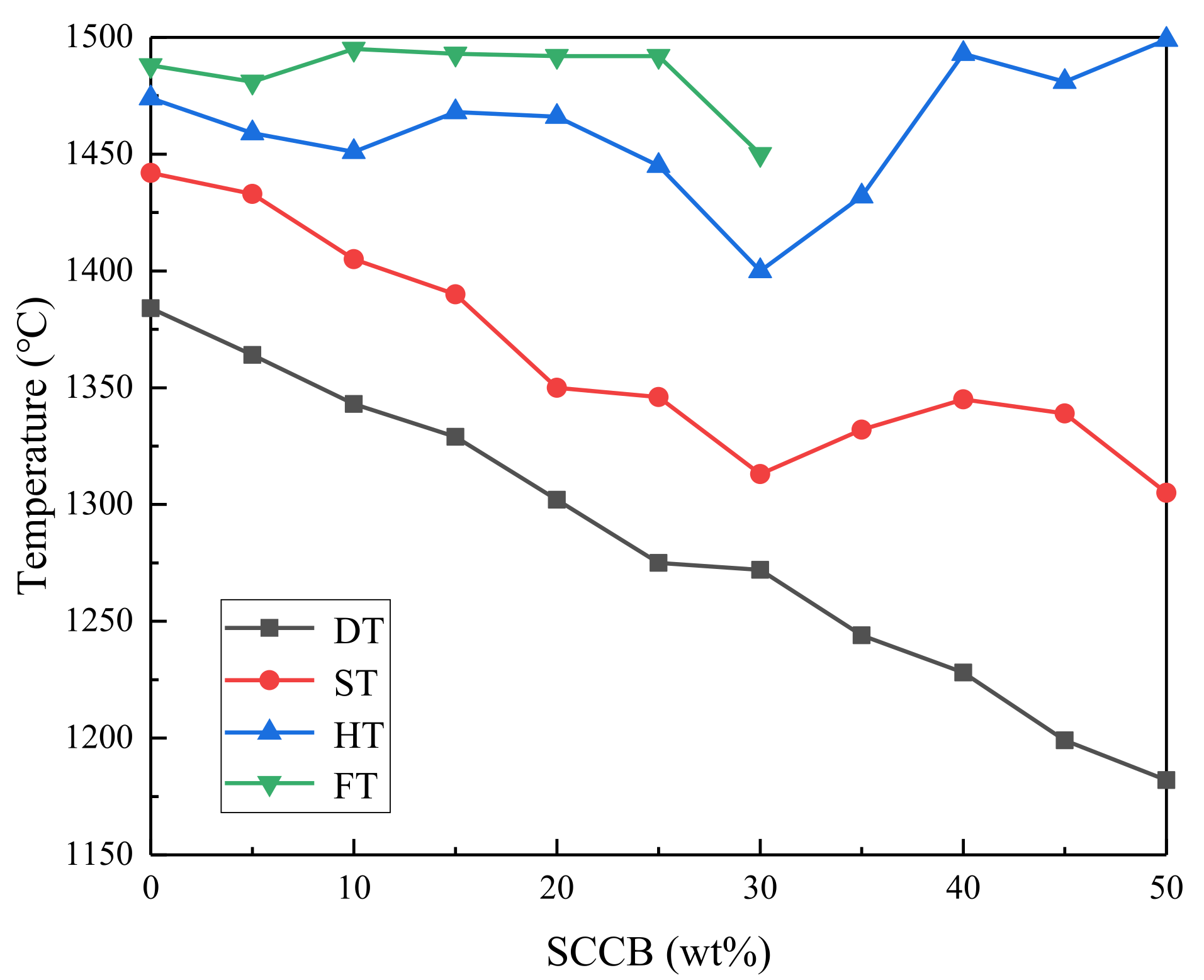
3.2.2. AFT Analysis
3.2.3. Correlation Analysis between DT and Characteristic Index
3.3. Relationship between Ash Melting Point and Ash Content
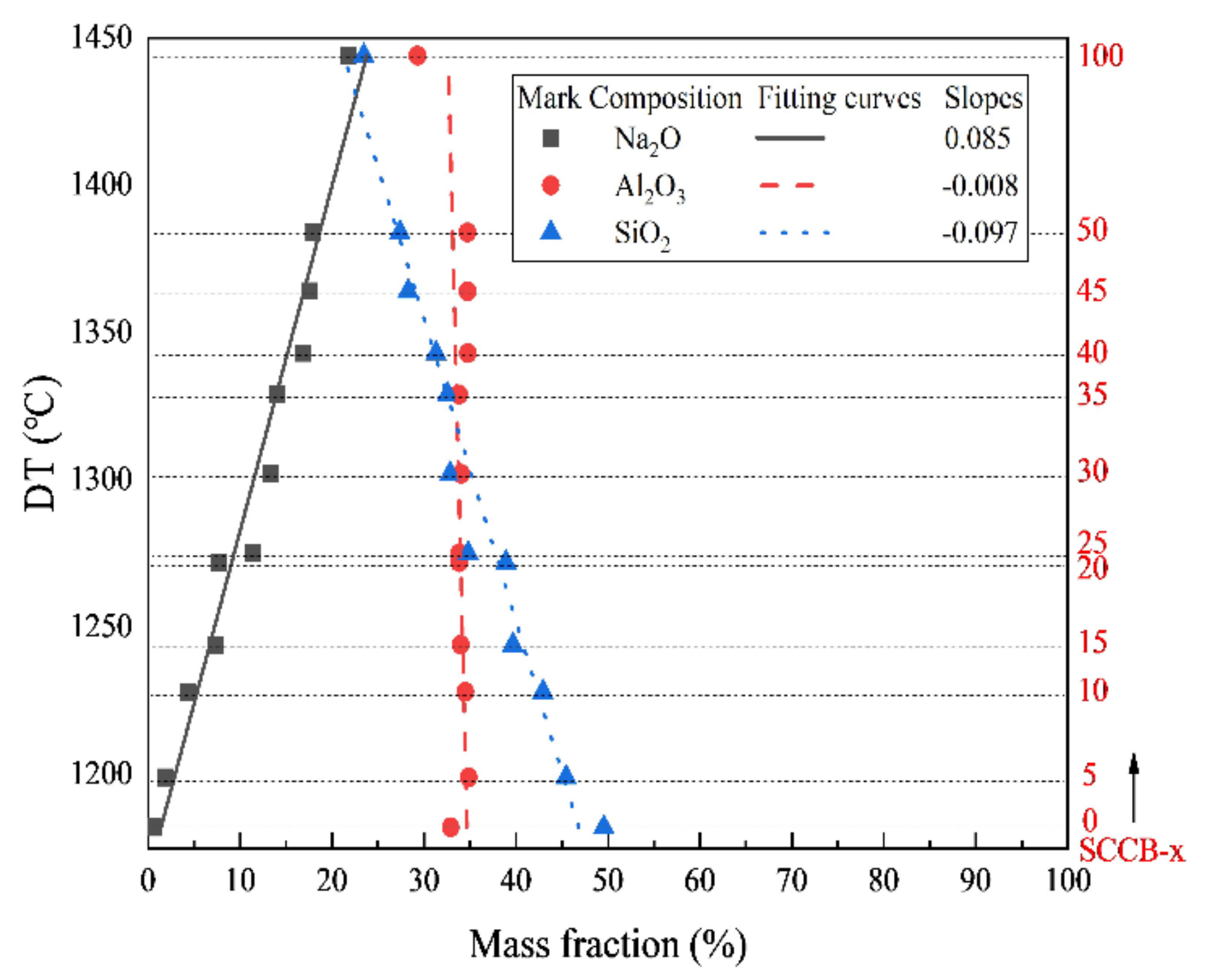
3.3.1. Single Oxide Evaluation
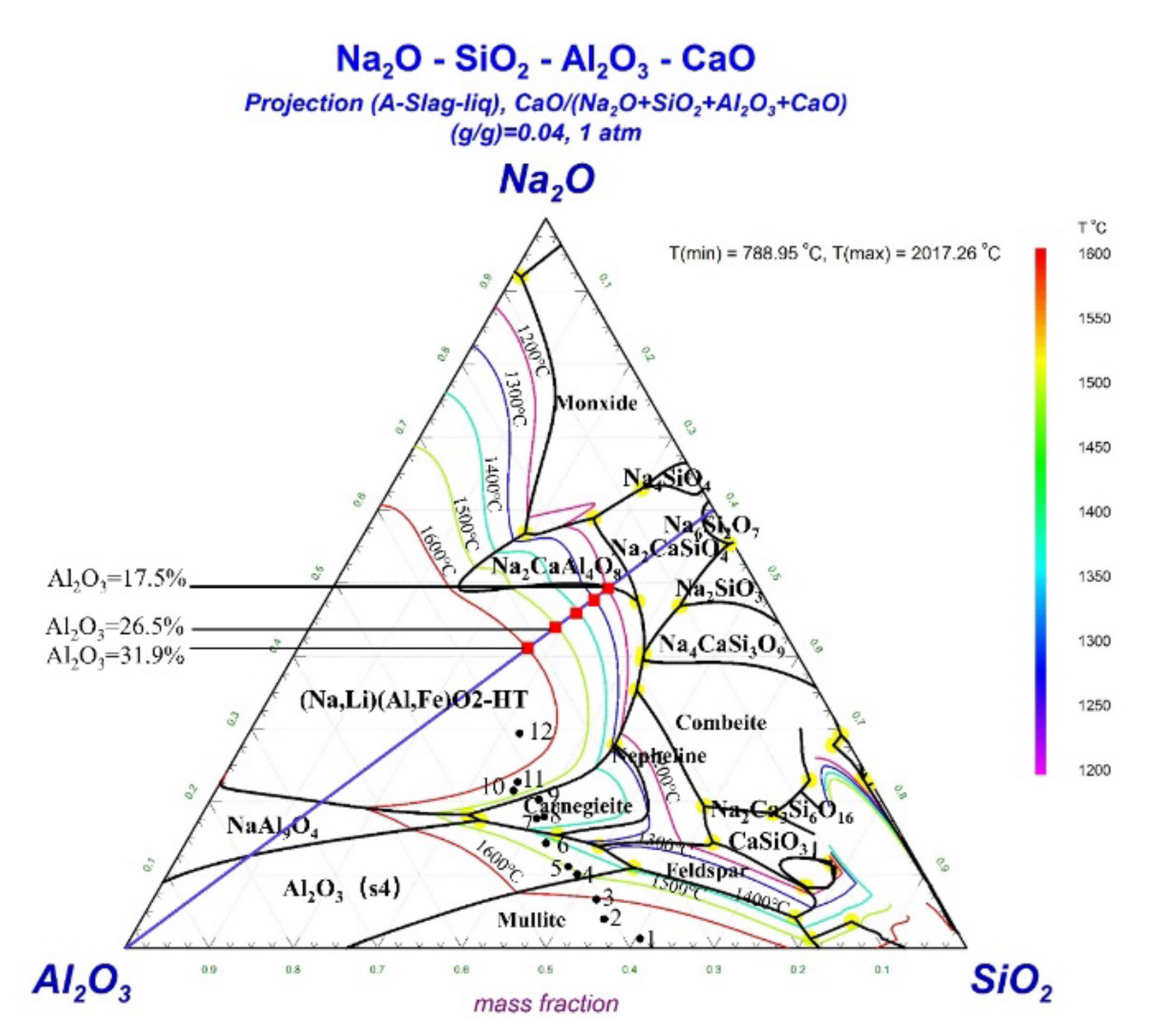
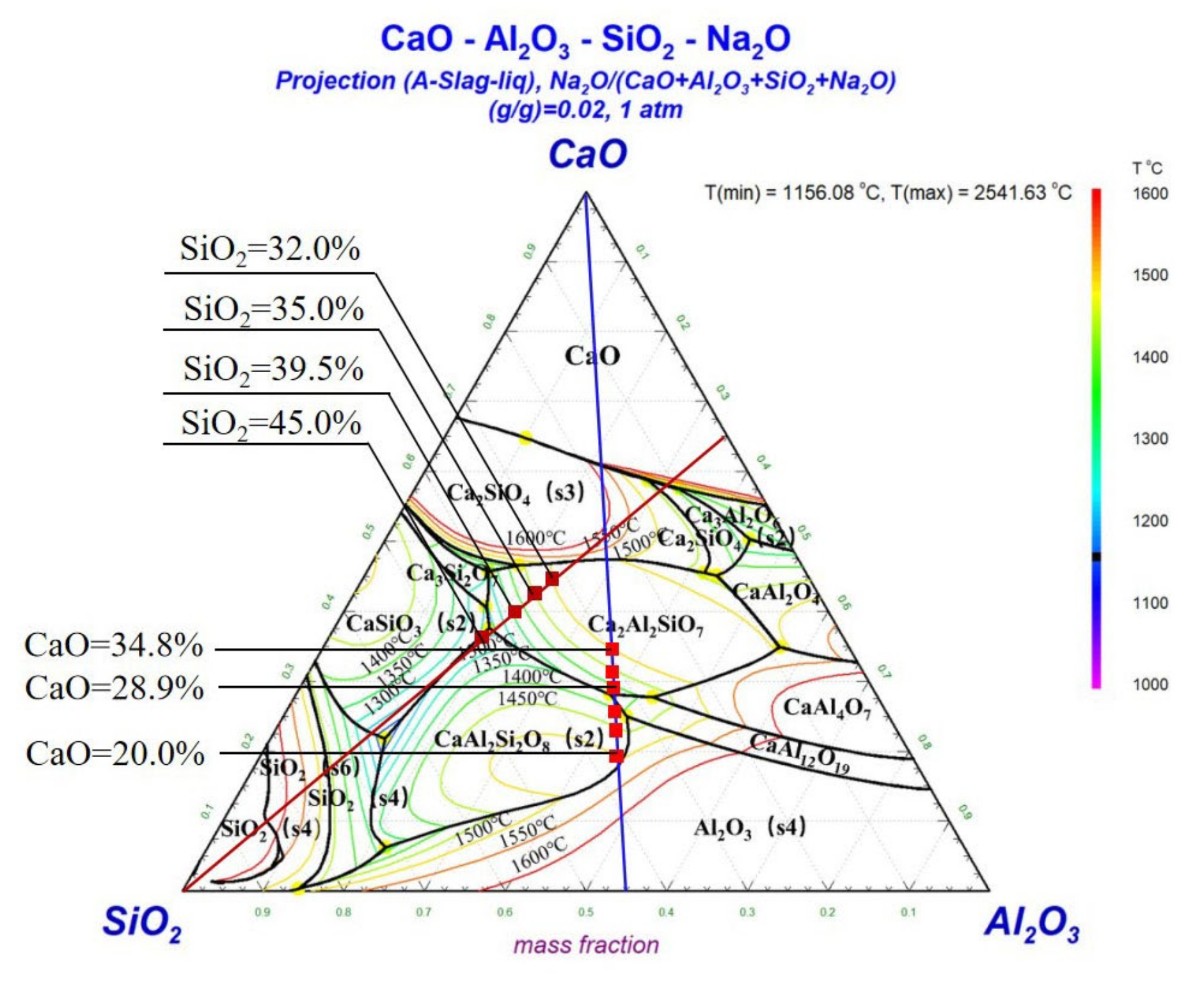
3.3.2. Phase Diagram Analysis
3.4. Mineral Composition during Ash Melting
3.5. Mineral Analysis of Ash
3.6. Analysis of Viscous Temperature Characteristics of Ash
3.7. Analysis of Ash Surface Morphology
3.8. Influence of Additive on the Co-Combustion Ash of SCCB-5
3.8.1. Effect of Additive on Mineral Composition
3.8.2. Effect of Additive on Viscosity Characteristics of Ash
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.L.; Liu, F.Q.; Xie, M.Z.; Liu, W.; Sohn, H.Y. Recycling and utilization of spent potlining by different high temperature treatments. J. Clean. Prod. 2021, 289, 125704. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, L.Y.; Yuan, J.; Yao, Z.; Tang, L.; Wang, Z.; Zhang, Z. Co-utilization of spent pot lining and coal gangue by hydrothermal acid-leaching method to prepare silicon carbide powder. J. Clean. Prod. 2018, 204, 848–860. [Google Scholar] [CrossRef]
- Silveira, B.I.; Dantas, A.E.; Blasquez, J.; Samtos, R.K.P. Characterization of inorganic fraction of spent potliners: Evaluation of the cyanides and fluorides content. J. Hazard. Mater. 2002, 89, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.J.; Andrade, L.F.; Trento, M.V.C.; Eleuterio, M.W.D.; Luber, J.; Davide, L.C.; Marcuss, S. Cytogenotoxic effects of spent pot liner (SPL) and its main components on human leukocytes and meristematic cells of Allium cepa. Water Air Soil Pollution. 2016, 227, 156. [Google Scholar] [CrossRef]
- Shi, Z.N.; Li, W.; Hu, X.W.; Ren, B.J.; Gao, B.L.; Wang, Z.W. Recovery of carbon and cryolite from spent pot lining of aluminium reduction cells by chemical leaching. Trans. Nonferrous Met. Soc. China 2012, 22, 222–227. [Google Scholar] [CrossRef]
- Xie, M.Z.; Li, R.B.; Zhao, H.L.; Liu, W.; Lu, T.T.; Liu, F.Q. Detoxification of spent cathode carbon blocks from aluminum smelters by joint controlling temperature-vacuum process. J. Clean. Prod. 2020, 249, 119370. [Google Scholar] [CrossRef]
- Xiao, J.; Yuan, J.; Tian, Z.L.; Yang, K.; Yao, Z.; Yu, B.L.; Zhang, L.Y. Comparison of ultrasound-assisted and traditional caustic leaching of spent cathode carbon (SCC) from aluminum electrolysis. Ultrason. Sonochemistry 2018, 40, 21–29. [Google Scholar] [CrossRef]
- Li, N.; Xie, G.; Wang, Z.X.; Hou, Y.Q.; Li, R.X. Recycle of spent potlining with low carbon grade by floatation. In Chemical, Material and Metallurgical Engineering, 3rd ed.; Trans Tech Publications Ltd.: Bäch, Switzerland, 2014. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chen, X.P. Discussion on recycling of spent cathode carbon blocks from aluminum smelters. Light Met. 2020, 5, 31–35. [Google Scholar] [CrossRef]
- Renó, M.L.G.; Torres, F.M.; Silva, R.J.; Santos, J.J.C.S.; Melo, M.D.N.M. Exergy analyses in cement production applying waste fuel and mineralizer. Energy Convers. Manag. 2013, 75, 98–104. [Google Scholar] [CrossRef]
- Zhao, H.L.; Ma, B.Z.; Hong, S.; Huang, H.; Liu, F.Q.; Sohn, H.Y. Recovery of Copper and Cobalt from Converter Slags via Reduction–Sulfurization Smelting Using Spent Pot Lining as the Reductant. ACS Sustain. Chem. Eng. 2021, 9, 4234–4246. [Google Scholar] [CrossRef]
- Thomanetz, E. Solid recovered fuels in the cement industry with special respect to hazardous waste. Waste Manag. Res. 2012, 30, 404–412. [Google Scholar] [CrossRef]
- Jiang, X.G.; Li, Y.H.; Yan, J. Hazardous waste incineration in a rotary kiln: A review. Waste Dispos. Sustain. Energy. 2019, 1, 3–37. [Google Scholar] [CrossRef]
- Zhang, S.R.; Jiang, X.G.; Lv, G.J.; Liu, B.X.; Jin, Y.Q.; Yan, J.H. SO2, NOx, HF, HCl and PCDD/Fs emissions during Co-combustion of bituminous coal and pickling sludge in a drop tube furnace. Fuel 2016, 186, 91–99. [Google Scholar] [CrossRef]
- Han, D.; Jiang, X.G.; Lv, G.J.; Yong, C.; Yan, J.H. Co-combustion of tannery sludge in a commercial circulating fluidized bed boiler. Waste Manag. 2015, 46, 227–233. [Google Scholar] [CrossRef]
- Zhang, J.; Teng, Z.C.; Han, K.H.; Li, Y.J.; Wang, M.M. Co-combustion characteristics and kinetics of meager coal and spent cathode carbon block by TG-MS analysis. Arab. J. Chem. 2021, 14, 103198. [Google Scholar] [CrossRef]
- Dai, H.L.; Shi, S.W.; Yang, L.; Guo, C.; Chen, X. Recent progress on the corrosion behavior of metallic materials in HF solution. Corros. Res. 2021, 39, 313–337. [Google Scholar] [CrossRef]
- Li, X.M.; Zhi, L.F.; Shi, W.J.; Kong, L.X.; Bai, J.; Yu, J.L.; Reinmoller, M.; Guhl, S.; Meyer, B.; Li, W. Effect of K2O/Na2O on fusion behavior of coal ash with high silicon and aluminum level. Fuel 2020, 265, 116964. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Kitano, K.; Takedab, S.; Tsurue, T. Influence of mineral and chemical composition of coal ashes on their fusibility. Fuel Process. Technol. 1995, 45, 27–51. [Google Scholar] [CrossRef]
- Oleschko, H.; Muller, M. Influence of coal composition and operating conditions on the release of alkali species during combustion of hard coal. EnergyFuels 2007, 21, 3240–3248. [Google Scholar] [CrossRef]
- Van Dyk, J.; Waanders, F.B.; Benson, S.A.; Laumb, M.L.; Hack, K. Viscosity predictions of the slag composition of gasified coal, utilizing FactSage equilibrium modelling. Fuel 2009, 88, 67–74. [Google Scholar] [CrossRef]
- Su, S.; Pohl, J.H.; Holcombe, D.; Hart, J.A. Techniques to determine ignition, flame stability and burnout of blended coals in p.f. power station boilers. Prog. Energy Combust. Sci. 2001, 27, 75–98. [Google Scholar] [CrossRef]
- Su, S.; Pohl, J.; Holcombe, D.; Hart, J.A. Slagging propensities of blended coals. Fuel 2001, 80, 1351–1360. [Google Scholar] [CrossRef]
- Qiu, J.R.; Li, F.; Zheng, Y.; Zheng, C.G.; Zhou, H.C. The influences of mineral behaviour on blended coal ash fusion characteristics. Fuel 1999, 78, 963–969. [Google Scholar] [CrossRef]
- Wall, T.F.; Creelman, R.A.; Gupta, R.P.; Gupta, S.K.; Coin, C.; Lowe, A. Coal ash fusion temperatures-New characterization techniques, and implications for slagging and fouling. Prog. Energy Combust. Sci. 1998, 24, 345–353. [Google Scholar] [CrossRef]
- Bryers, R.W. Fireside slagging, fouling, and high-temperature corrosion of heat-transfer surface due to impurities in steam-raising fuels. Prog. Energy Combust. Science. 1996, 22, 29–120. [Google Scholar] [CrossRef]
- Degereji, M.U.; Ingham, D.B.; Ma, L.; Pourkashanian, M.; Williams, A. Prediction of ash slagging propensity in a pulverized coal combustion furnace. Fuel 2012, 101, 171–178. [Google Scholar] [CrossRef]
- Huang, L.Y.; Norman, J.; Pourkashanian, M.; Williams, A. Prediction of ash deposition on superheater tubes from pulverized coal combustion. Fuel 1996, 75, 271–279. [Google Scholar] [CrossRef]
- Adell, V.; Cheeseman, C.R.; Ferraris, M.; Salvo, M.; Smeacetto, F.; Boccaccini, A.R. Characterising the sintering behaviour of pulverised fuel ash using heating stage microscopy. Mater. Charact. 2007, 58, 980–988. [Google Scholar] [CrossRef]
- Wang, L.; Skjevrak, G.; Hustad, J.; Grønli, M. Sintering characteristics of sewage sludge ashes at elevated temperatures. Fuel Process. Technol. 2012, 96, 88–97. [Google Scholar] [CrossRef]
- Chen, Z.H.; Liu, J.; Chen, L.G.; Evrendilek, F.; Xie, W.M.; Wu, X.Y.; Hu, J.W.; Li, W.X. Emission-to-ash detoxification mechanisms of co-combustion of spent pot lining and pulverized coal. J. Hazard. Mater. 2021, 418, 126380. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, Y.; Wang, D.X.; Dong, C.Q.; Yang, Y.P.; Xiao, X.B.; Lu, Q.; Zhao, Y. Effect of sodium oxides in ash composition on ash fusibility. Energy Fuels 2016, 30, 1437–1444. [Google Scholar] [CrossRef]
- Dai, X.; Bai, J.; Huang, Q.; Liu, Z.; Bai, X.J.; Cao, R.G.; Wen, X.D.; Li, W.; Du, S.Y. Viscosity temperature properties from molecular dynamics simulation: The role of calcium oxide, sodium oxide and ferrous oxide. Fuel 2019, 237, 163–169. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Tan, H.Z.; Niu, Y.Q.; Wang, X.B. Experimental study on ash fusion characteristics and slagging potential using simulated biomass ashes. J. Energy Inst. 2019, 92, 1889–1896. [Google Scholar] [CrossRef]
- Ma, X.W.; Li, F.H.; Ma, M.J.; Fang, Y.T. Investigation on blended ash fusibility characteristics of biomass and coal with high silica-alumina. Energy Fuels 2017, 31, 7941–87951. [Google Scholar] [CrossRef]
- Shi, W.J.; Kong, L.X.; Bai, J.; Xu, J.; Li, W.C.; Bai, Z.Q.; Li, W. Effect of CaO/Fe2O3 on fusion behaviors of coal ash at high temperatures. Fuel Process. Technol. 2018, 181, 18–24. [Google Scholar] [CrossRef]
- Vargas, S.; Frandsen, F.; Dam-Johansen, K. Rheological properties of high-temperature melts of coal ashes and other silicates. Prog. Energy Combust. Sci. 2001, 27, 237–429. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, Y.M.; Bao, S.X.; Chen, T.J.; Liu, X. Effect of stone coal chemical composition on sintering behavior during roasting. Ind. Eng. Chem. Res. 2014, 53, 157–163. [Google Scholar] [CrossRef]
- Yao, X.Y. Relationship of coal ash fusibility to chemical composition. J. Fuel Chem. Technol. 1965, 6, 151–161. [Google Scholar]
- Wang, Y.; Wang, D.X.; Dong, C.Q.; Yang, Y.P. The behavior and reactions of sodium containing minerals in ash melting process. J. Energy Inst. 2017, 90, 167–173. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Jensen, P.A.; Jensen, A.D. A kinetic study of gaseous potassium capture by coal minerals in a high temperature fixed-bed reactor. Fuel 2008, 87, 3304–3312. [Google Scholar] [CrossRef]
- Clery, D.S.; Mason, P.E.; Rayner, C.M.; Jones, J.M. The effects of an additive on the release of potassium in biomass combustion. Fuel 2018, 214, 647–655. [Google Scholar] [CrossRef]
- Mu, L.; Zhao, C.; Zhao, L.; Chen, B.W.; Xu, Z.L.; Yang, Z.Q.; Shang, Y.; Yin, H.C. Mineralogical composition evolution and thermogravimetric characteristics of sewage sludge ash at different ashing temperatures. Energy Fuels 2018, 32, 12617–12629. [Google Scholar] [CrossRef]
- Liu, S.Q.; Qi, C.; Jiang, Z.; Zhang, Y.J.; Niu, M.F.; Li, Y.Y.; Dai, S.F.; Finkelman, R.B. Mineralogy and geochemistry of ash and slag from coal gasification in China: A review. Int. Geol. Rev. 2018, 60, 717–735. [Google Scholar] [CrossRef]
- Xie, L.C.; Xu, L.; Ma, X.X. Influence of the direct coal liquefaction residue on the fusion characteristics of Jincheng coal with a high ash fusion temperature. Energy Fuels 2020, 34, 1355–1364. [Google Scholar] [CrossRef]
- Li, F.H.; Li, M.; Fan, H.L.; Fan, Y.T. Understanding Ash Fusion and Viscosity Variation from Coal Blending Based on Mineral Interaction. Energy Fuels 2018, 32, 142–151. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Fan, J.; Li, P.; Wang, B.H.; Zhao, H. Mineral transmutation of high alkali Zhundong coal in ash melting process. J. Zhejiang Univ. 2015, 49, 1559–1564. [Google Scholar] [CrossRef]
- Labbafan, A.; Ghassemi, H. Numerical modeling of an E-Gas entrained flow gasifier to characterize a high-ash coal gasification. Energy Convers. Manag. 2016, 112, 337–349. [Google Scholar] [CrossRef]
- Ren, H.J.; Zhang, Y.Q.; Fang, Y.T. Co-gasification behavior of meat and bone meal char and coal char. Fuel Process. Technol. 2011, 92, 298–307. [Google Scholar] [CrossRef]
- Ji, H.S.; Li, Z.Q.; Zhou, Y.; Qi, Y.; He, Z.X. The research on viscosity-temperature characteristics of the mixed slag of biomass and bituminous coal. J. Fuel Chem. Technol. 2021, 49, 11–19. [Google Scholar] [CrossRef]
- Zhang, C.X.; Li, Y.J.; He, Z.R.; Zhao, J.L.; Wang, D. Microtubular Fe/Mn-promoted CaO-Ca12Al14O33 bifunctional material for H2 production from sorption enhanced water gas shift. Appl. Catal. B Environ. 2022, 314, 121474. [Google Scholar] [CrossRef]



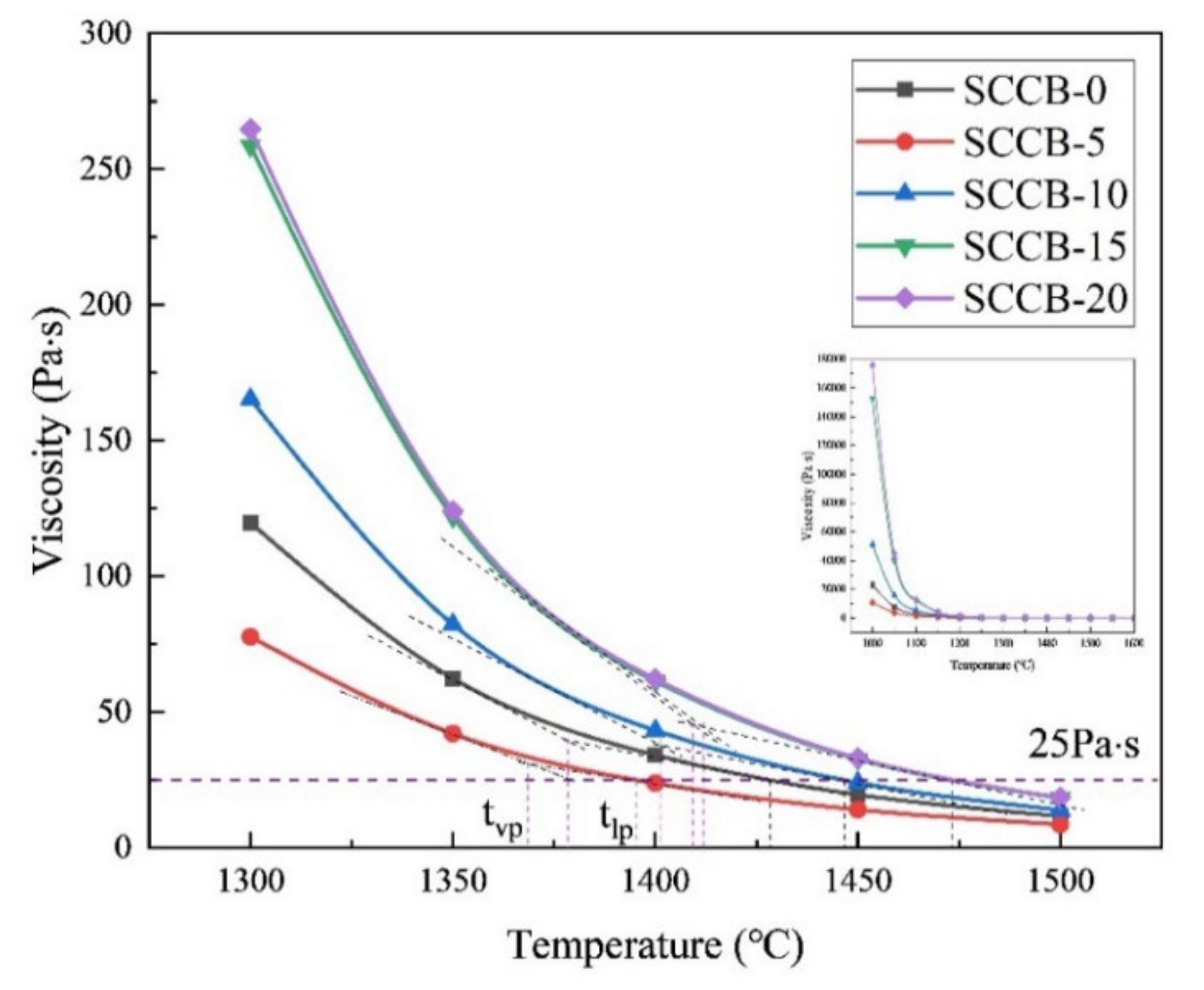
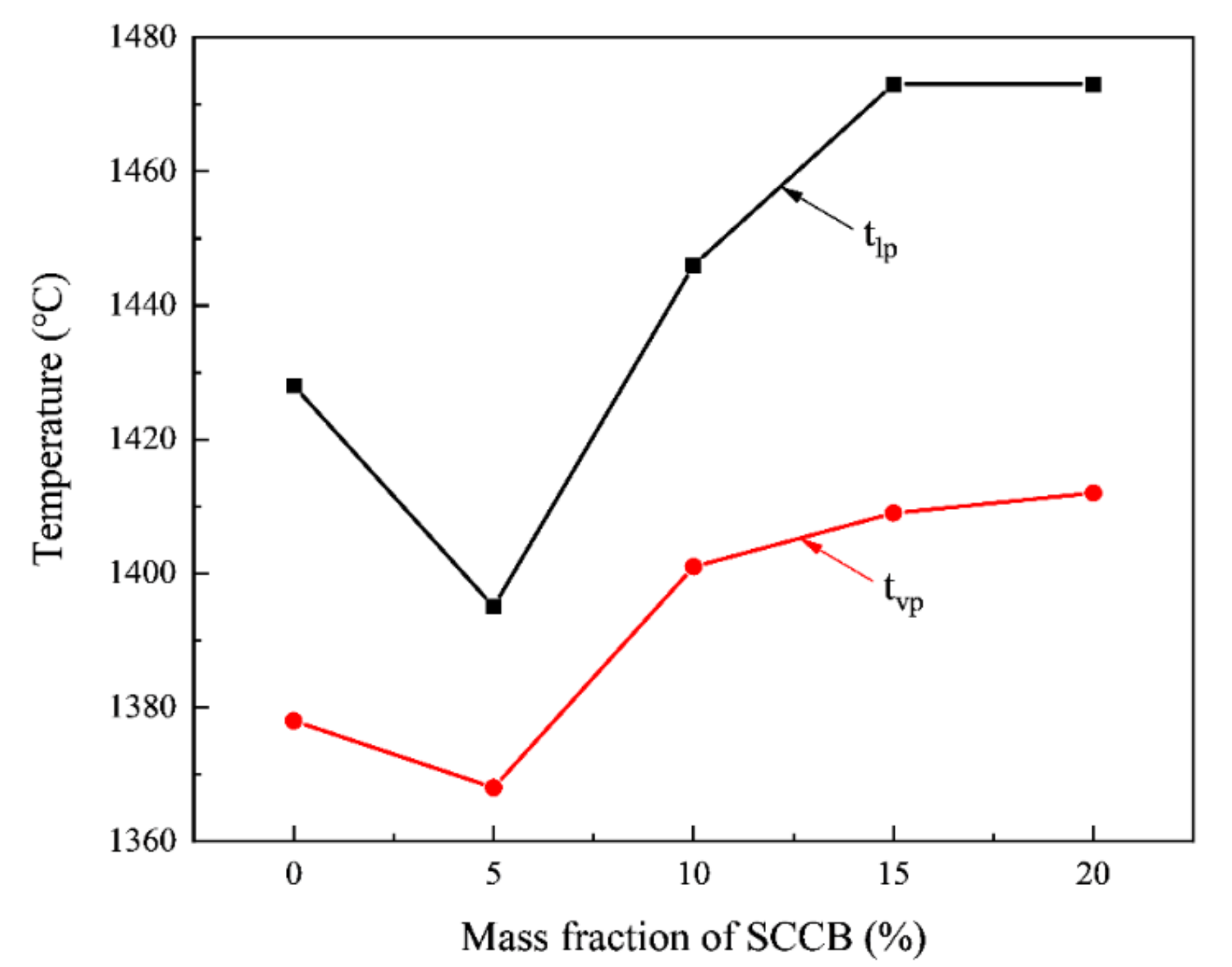
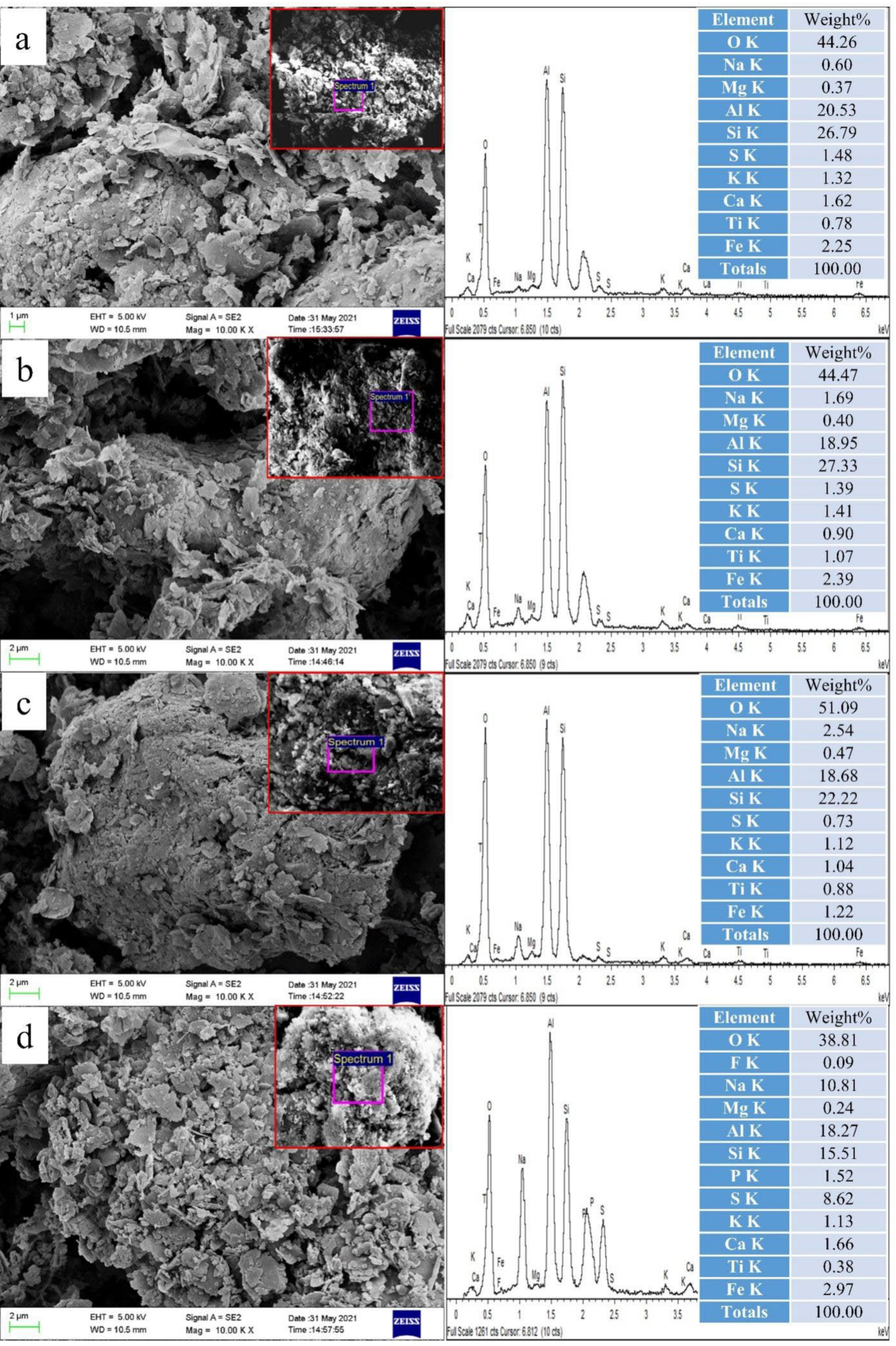

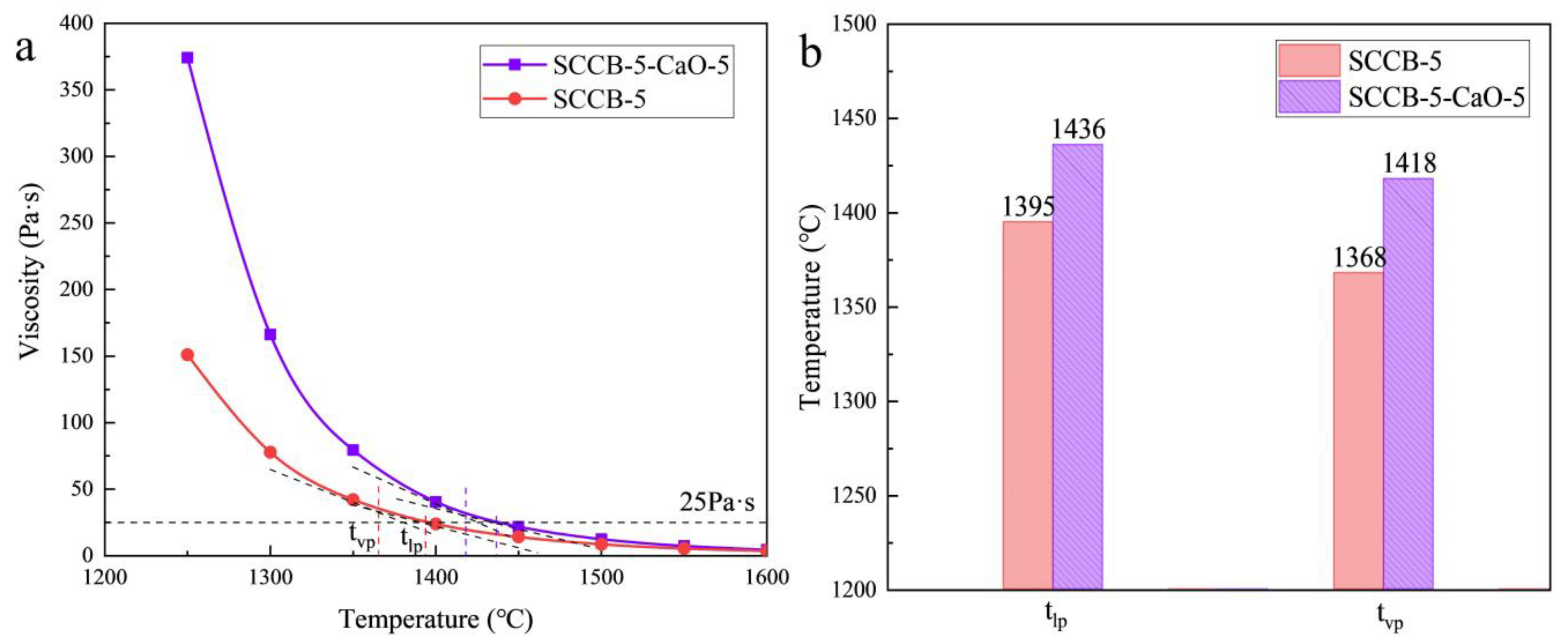
| Index | Index Expression | Slagging Potential | |||
|---|---|---|---|---|---|
| Low | Medium | High | Severe | ||
| <0.206 | 0.206–0.4 | >0.4 | --- | ||
| >78.8 | 66.1–78.8 | <66.1 | --- | ||
| <0.6 | --- | 0.6-40 | >40 | ||
| >1343 °C | 1232–1343 °C | 1149–1232 °C | <1149 °C | ||
| Sample | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 | Fe2O3 | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCCB-0 | 0.62 | 0.83 | 32.91 | 49.56 | 0.19 | 1.03 | 0.98 | 3.17 | 1.03 | 6.28 | 3.40 |
| SCCB-5 | 1.86 | 0.39 | 34.87 | 45.45 | 0.94 | 3.94 | 1.24 | 2.62 | 1.45 | 5.76 | 1.48 |
| SCCB-10 | 4.31 | 0.38 | 34.39 | 42.94 | 0.88 | 5.12 | 1.16 | 3.52 | 1.33 | 5.43 | 0.54 |
| SCCB-15 | 7.31 | 0.38 | 33.99 | 39.67 | 0.37 | 6.71 | 1.09 | 3.66 | 1.24 | 5.18 | 0.40 |
| SCCB-20 | 7.70 | 0.44 | 33.83 | 38.92 | 0.93 | 5.78 | 1.47 | 3.84 | 1.29 | 5.27 | 0.53 |
| SCCB-25 | 11.43 | 0.41 | 33.83 | 34.82 | 0.62 | 6.92 | 1.18 | 4.07 | 1.12 | 5.04 | 0.56 |
| SCCB-30 | 13.36 | 0.36 | 34.04 | 32.83 | 0.55 | 7.16 | 0.93 | 4.23 | 1.11 | 4.88 | 0.55 |
| SCCB-35 | 14.04 | 0.80 | 33.81 | 32.56 | 0.23 | 8.19 | 0.90 | 3.79 | 0.96 | 4.39 | 0.33 |
| SCCB-40 | 16.87 | 0.27 | 34.77 | 31.30 | 0.67 | 4.57 | 1.71 | 4.02 | 0.95 | 4.46 | 0.41 |
| SCCB-45 | 17.59 | 0.87 | 34.74 | 28.28 | 0.23 | 7.90 | 1.17 | 3.99 | 0.84 | 4.07 | 0.32 |
| SCCB-50 | 17.95 | 0.87 | 34.72 | 27.38 | 0.11 | 8.77 | 0.89 | 4.10 | 0.80 | 4.07 | 0.34 |
| SCCB-100 | 32.80 | 0.83 | 32.31 | 23.44 | 0.06 | 0.15 | 0.70 | 4.09 | 0.47 | 4.39 | 0.76 |
| Sample | ||||
|---|---|---|---|---|
| SCCB-0 | 0.142 | 82.82 | 0.088 | 1402 |
| SCCB-5 | 0.145 | 83.83 | 0.270 | 1383 |
| SCCB-10 | 0.188 | 82.15 | 0.810 | 1364.6 |
| SCCB-15 | 0.235 | 81.14 | 1.718 | 1356.8 |
| SCCB-20 | 0.253 | 80.30 | 1.948 | 1334.8 |
| SCCB-25 | 0.317 | 78.53 | 3.623 | 1309 |
| SCCB-30 | 0.350 | 77.61 | 4.676 | 1297.6 |
| SCCB-35 | 0.355 | 78.38 | 4.984 | 1281.6 |
| SCCB-40 | 0.408 | 78.15 | 6.883 | 1281 |
| SCCB-45 | 0.434 | 76.00 | 7.634 | 1255.4 |
| SCCB-50 | 0.443 | 75.18 | 7.952 | 1245.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, Z.; Li, X.; Wang, B.; Teng, Z.; Han, K. Study on Slagging Characteristics of Co-Combustion of Meager Coal and Spent Cathode Carbon Block. Energies 2023, 16, 736. https://doi.org/10.3390/en16020736
Zhang J, Liu Z, Li X, Wang B, Teng Z, Han K. Study on Slagging Characteristics of Co-Combustion of Meager Coal and Spent Cathode Carbon Block. Energies. 2023; 16(2):736. https://doi.org/10.3390/en16020736
Chicago/Turabian StyleZhang, Jigang, Zijun Liu, Xian Li, Bin Wang, Zhaocai Teng, and Kuihua Han. 2023. "Study on Slagging Characteristics of Co-Combustion of Meager Coal and Spent Cathode Carbon Block" Energies 16, no. 2: 736. https://doi.org/10.3390/en16020736
APA StyleZhang, J., Liu, Z., Li, X., Wang, B., Teng, Z., & Han, K. (2023). Study on Slagging Characteristics of Co-Combustion of Meager Coal and Spent Cathode Carbon Block. Energies, 16(2), 736. https://doi.org/10.3390/en16020736






