HYSCORE Spectroscopy to Resolve Electron–Nuclear Structure of Vanadyl Porphyrins in Asphaltenes from the Athabasca Oil Sands In Situ Conditions
Abstract
:1. Introduction
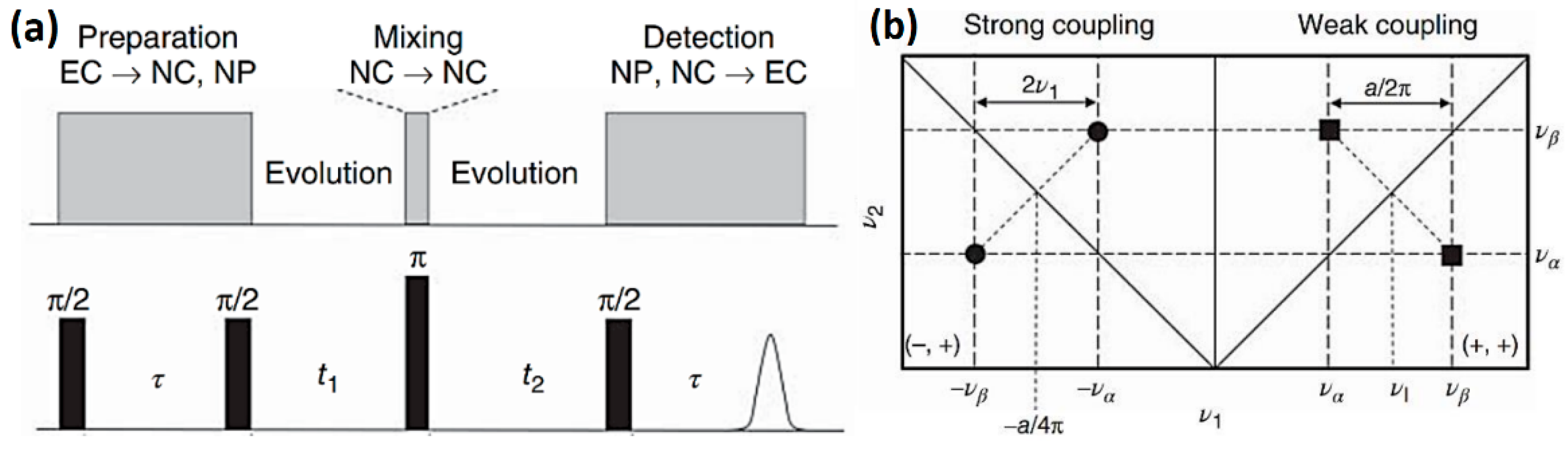
2. Materials and Methods
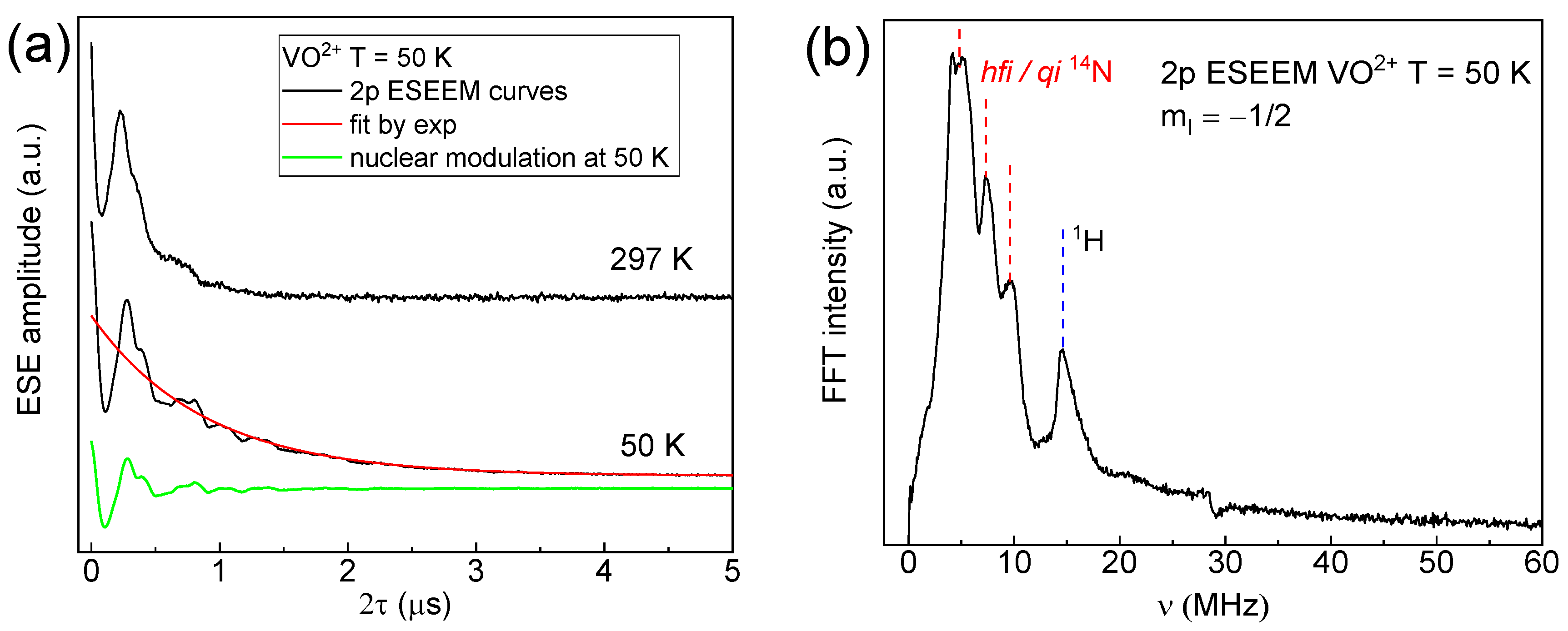
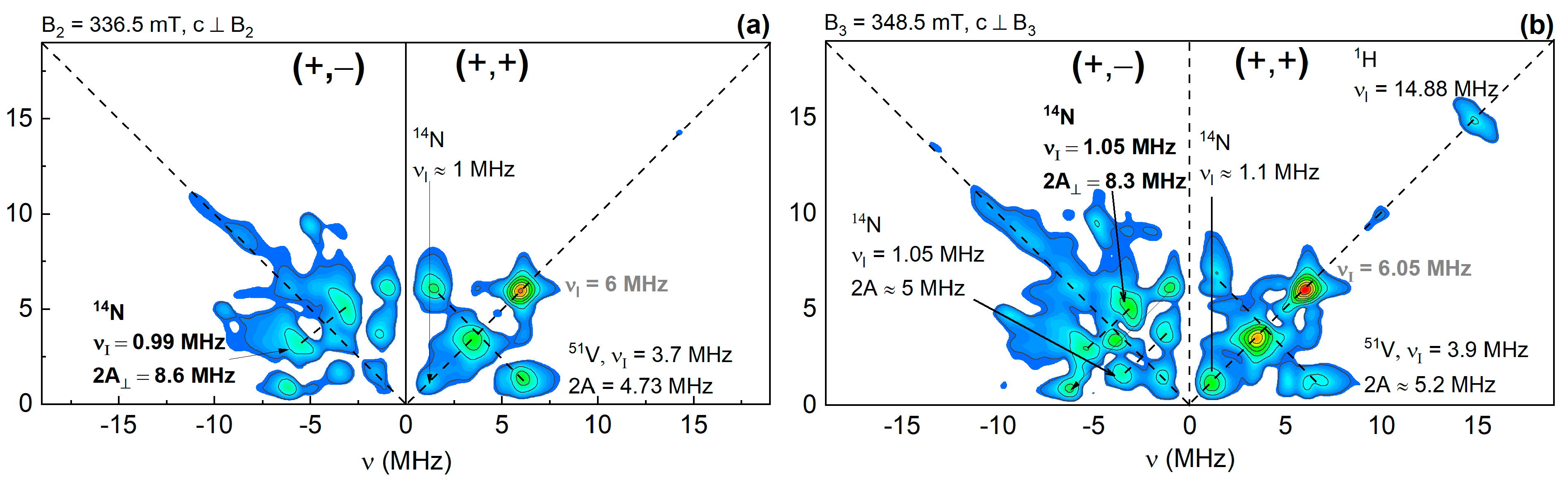
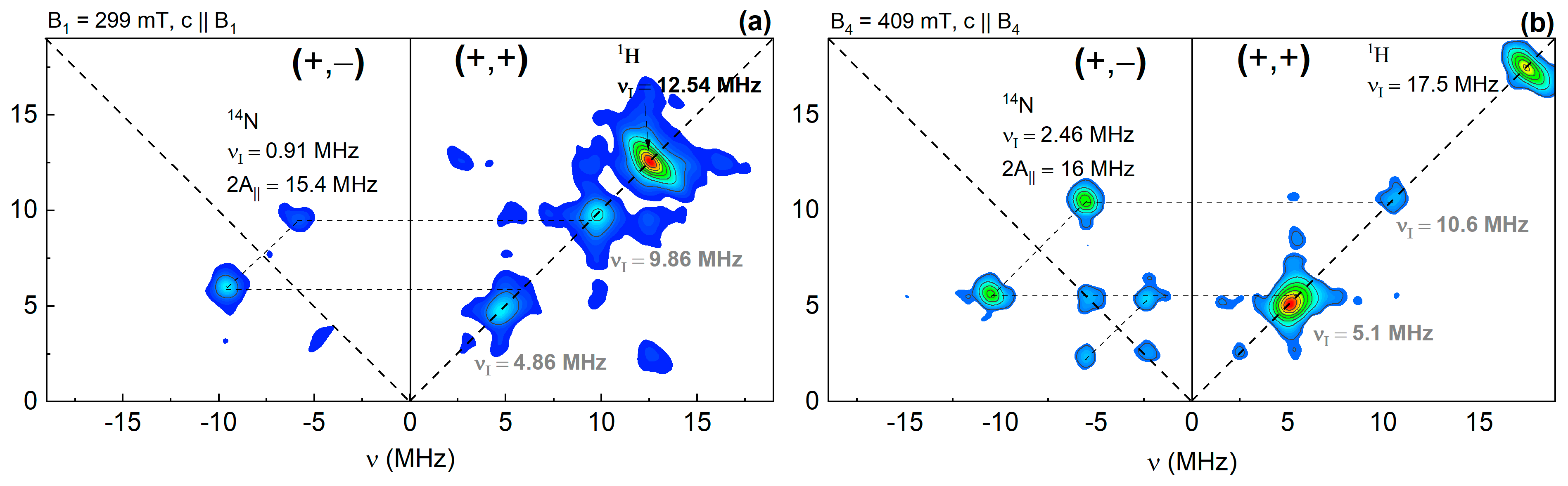
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yakubov, M.; Abilova, G.; Tazeeva, E.; Yakubova, S.; Tazeev, D.; Mironov, N.; Milordov, D. A Comparative Analysis of Vanadyl Porphyrins Isolated from Resins of Heavy Oils with High and Low Vanadium Content. Processes 2021, 9, 2235. [Google Scholar] [CrossRef]
- Gotico, P.; Halime, Z.; Aukauloo, A. Recent Advances in Metalloporphyrin-Based Catalyst Design towards Carbon Dioxide Reduction: From Bio-Inspired Second Coordination Sphere Modifications to Hierarchical Architectures. Dalton Trans. 2020, 49, 2381–2396. [Google Scholar] [CrossRef] [PubMed]
- Devatha, C.P.; Vishnu Vishal, A.; Purna Chandra Rao, J. Investigation of Physical and Chemical Characteristics on Soil Due to Crude Oil Contamination and Its Remediation. Appl. Water Sci. 2019, 9, 89. [Google Scholar] [CrossRef]
- Yakubov, M.R.; Milordov, D.V.; Yakubova, S.G.; Abilova, G.R.; Sinyashin, K.O.; Tazeeva, E.G.; Borisova, U.U.; Mironov, N.A.; Morozov, V.I. Vanadium and Paramagnetic Vanadyl Complexes Content in Asphaltenes of Heavy Oils of Various Productive Sediments. Pet. Sci. Technol. 2017, 35, 1468–1472. [Google Scholar] [CrossRef]
- Biktagirov, T.; Gafurov, M.; Mamin, G.; Gracheva, I.; Galukhin, A.; Orlinskii, S. In Situ Identification of Various Structural Features of Vanadyl Porphyrins in Crude Oil by High-Field (3.4 T) Electron–Nuclear Double Resonance Spectroscopy Combined with Density Functional Theory Calculations. Energy Fuels 2017, 31, 1243–1249. [Google Scholar] [CrossRef]
- Gafurov, M.R.; Gracheva, I.N.; Mamin, G.V.; Ganeeva, Y.M.; Yusupova, T.N.; Orlinskii, S.B. Study of Organic Self-Assembled Nanosystems by Means of High-Frequency ESR/ENDOR: The Case of Oil Asphaltenes. Russ. J. Gen. Chem. 2018, 88, 2374–2380. [Google Scholar] [CrossRef]
- Asaoka, S.; Nakata, S.; Shiroto, Y.; Takeuchi, C. Asphaltene Cracking in Catalytic Hydrotreating of Heavy Oils. 2. Study of Changes in Asphaltene Structure during Catalytic Hydroprocessing; ACS Publications: Washington, DC, USA, 1983. [Google Scholar] [CrossRef]
- Mironov, N.A.; Milordov, D.V.; Abilova, G.R.; Yakubova, S.G.; Yakubov, M.R. Methods for Studying Petroleum Porphyrins (Review). Pet. Chem. 2019, 59, 1077–1091. [Google Scholar] [CrossRef]
- Djimasbe, R.; Varfolomeev, M.A.; Al-muntaser, A.A.; Yuan, C.; Suwaid, M.A.; Feoktistov, D.A.; Rakhmatullin, I.Z.; Milovankin, A.A.; Murzakhanov, F.; Morozov, V.; et al. Deep Insights into Heavy Oil Upgrading Using Supercritical Water by a Comprehensive Analysis of GC, GC–MS, NMR, and SEM–EDX with the Aid of EPR as a Complementary Technical Analysis. ACS Omega 2021, 6, 135–147. [Google Scholar] [CrossRef]
- Trukhan, S.N.; Kazarian, S.G.; Martyanov, O.N. Electron Spin Resonance of Slowly Rotating Vanadyls–Effective Tool to Quantify the Sizes of Asphaltenes in Situ. Energy Fuels 2017, 31, 387–394. [Google Scholar] [CrossRef]
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Khan, M.A.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef]
- Trukhan, S.N.; Yakushkin, S.S.; Martyanov, O.N. Fine-Tuning Simulation of the ESR Spectrum─Sensitive Tool to Identify the Local Environment of Asphaltenes In Situ. J. Phys. Chem. C 2022, 126, 10729–10741. [Google Scholar] [CrossRef]
- Mannikko, D.; Stoll, S. Vanadyl Porphyrin Speciation Based on Submegahertz Ligand Proton Hyperfine Couplings. Energy Fuels 2019, 33, 4237–4243. [Google Scholar] [CrossRef]
- McKenna, A.M.; Chacón-Patiño, M.L.; Salvato Vallverdu, G.; Bouyssiere, B.; Giusti, P.; Afonso, C.; Shi, Q.; Combariza, M.Y. Advances and Challenges in the Molecular Characterization of Petroporphyrins. Energy Fuels 2021, 35, 18056–18077. [Google Scholar] [CrossRef]
- Sarkar, T.K.; Saraswat, V.; Mitra, R.K.; Obot, I.B.; Yadav, M. Mitigation of Corrosion in Petroleum Oil Well/Tubing Steel Using Pyrimidines as Efficient Corrosion Inhibitor: Experimental and Theoretical Investigation. Mater. Today Commun. 2021, 26, 101862. [Google Scholar] [CrossRef]
- Jumina, J.; Kurniawan, Y.S.; Siswanta, D.; Purwono, B.; Zulkarnain, A.K.; Winarno, A.; Waluyo, J.; Ahmad, J.S.M. The Origin, Physicochemical Properties, and Removal Technology of Metallic Porphyrins from Crude Oils. Indones. J. Chem. 2021, 21, 1023–1038. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, C.; Shi, Q. Porphyrins in Heavy Petroleums: A Review. In Structure and Modeling of Complex Petroleum Mixtures; Xu, C., Shi, Q., Eds.; Structure and Bonding; Springer International Publishing: Cham, Switzerland, 2016; pp. 39–70. [Google Scholar] [CrossRef]
- Gafurov, M.R.; Ponomarev, A.A.; Mamin, G.V.; Rodionov, A.A.; Murzakhanov, F.F.; Arash, T.; Orlinskii, S.B. Application of Pulsed and High-Frequency Electron Paramagnetic Resonance Techniques to Study Petroleum Disperse Systems. Georesursy 2020, 22, 2–14. [Google Scholar] [CrossRef]
- Gafurov, M.; Mamin, G.; Gracheva, I.; Murzakhanov, F.; Ganeeva, Y.; Yusupova, T.; Orlinskii, S. High-Field (3.4 T) ENDOR Investigation of Asphaltenes in Native Oil and Vanadyl Complexes by Asphaltene Adsorption on Alumina Surface. Geofluids 2019, 2019, e3812875. [Google Scholar] [CrossRef]
- Murzakhanov, F.F.; Mamin, G.V.; Goldberg, M.A.; Knotko, A.V.; Gafurov, M.R.; Orlinskii, S.B. EPR of Radiation-Induced Nitrogen Centers in Hydroxyapatite: New Approaches to the Study of Electron-Nuclear Interactions. Russ. J. Coord. Chem. 2020, 46, 729–737. [Google Scholar] [CrossRef]
- Murzakhanov, F.F.; Mamin, G.V.; Orlinskii, S.B.; Gerstmann, U.; Schmidt, W.G.; Biktagirov, T.; Aharonovich, I.; Gottscholl, A.; Sperlich, A.; Dyakonov, V.; et al. Electron–Nuclear Coherent Coupling and Nuclear Spin Readout through Optically Polarized VB–Spin States in HBN. Nano Lett. 2022, 22, 2718–2724. [Google Scholar] [CrossRef]
- Roessler, M.M.; Salvadori, E. Principles and Applications of EPR Spectroscopy in the Chemical Sciences. Chem. Soc. Rev. 2018, 47, 2534–2553. [Google Scholar] [CrossRef]
- Gourier, D.; Delpoux, O.; Bonduelle, A.; Binet, L.; Ciofini, I.; Vezin, H. EPR, ENDOR, and HYSCORE Study of the Structure and the Stability of Vanadyl−Porphyrin Complexes Encapsulated in Silica: Potential Paramagnetic Biomarkers for the Origin of Life. J. Phys. Chem. B 2010, 114, 3714–3725. [Google Scholar] [CrossRef] [PubMed]
- Ben Tayeb, K.; Delpoux, O.; Barbier, J.; Marques, J.; Verstraete, J.; Vezin, H. Applications of Pulsed Electron Paramagnetic Resonance Spectroscopy to the Identification of Vanadyl Complexes in Asphaltene Molecules. Part 1: Influence of the Origin of the Feed. Energy Fuels 2015, 29, 4608–4615. [Google Scholar] [CrossRef]
- Strausz, O.P.; Lown, E.M. The Chemistry of Alberta Oil Sands, Bitumens, and Heavy Oils; Alberta Energy Research Institute (AERI): Calgary, AB, Canada, 2003. [Google Scholar]
- Elofson, R.M.; Schulz, K.F.; Hitchon, B. Geochemical Significance of Chemical Composition and ESR Properties of Asphaltenes in Crude Oils from Alberta, Canada. Geochim. Cosmochim. Acta 1977, 41, 567–580. [Google Scholar] [CrossRef]
- Niizuma, S.; Iwaizumi, M.; Strausz, O.P. ENDOR [electron nuclear double resonance] study of free radicals in Athabasca asphaltene. AOSTRA J. Res. Alta. Oil Sands Technol. Auth. Can. 1991, 7, 217–223. [Google Scholar]
- Elkahky, S.; Lagat, C.; Sarmadivaleh, M.; Barifcani, A. A Comparative Study of Density Estimation of Asphaltene Structures Using Group Contribution Methods and Molecular Dynamic Simulations for an Australian Oil Field. J. Pet. Explor. Prod. Technol. 2019, 9, 2699–2708. [Google Scholar] [CrossRef]
- Gray, M.R.; Chacón-Patiño, M.L.; Rodgers, R.P. Structure–Reactivity Relationships for Petroleum Asphaltenes. Energy Fuels 2022, 36, 4370–4380. [Google Scholar] [CrossRef]
- Ganeeva, Y.M.; Barskaya, E.E.; Okhotnikova, E.S.; Yusupova, T.N. Features of the Composition of Compounds Trapped in Asphaltenes of Oils and Bitumens of the Bavly Oil Field. Energy Fuels 2021, 35, 2493–2505. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Nguyen, D.L.T.; Xia, C.; Nguyen, T.B.; Shokouhimehr, M.; Sana, S.S.; Grace, A.N.; Aghbashlo, M.; Tabatabaei, M.; Sonne, C.; et al. Recent Advances in Asphaltene Transformation in Heavy Oil Hydroprocessing: Progress, Challenges, and Future Perspectives. Fuel Process. Technol. 2021, 213, 106681. [Google Scholar] [CrossRef]
- Prisner, T.; Rohrer, M.; MacMillan, F. Pulsed EPR spectroscopy: Biological applications. Annu. Rev. Phys. Chem. 2001, 52, 279–313. [Google Scholar] [CrossRef] [Green Version]
- Lund, A.; Shiotani, M.; Shimada, S. Principles and Applications of ESR Spectroscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Stoll, S.; Schweiger, A. EasySpin, a Comprehensive Software Package for Spectral Simulation and Analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Gracheva, I.N.; Gafurov, M.R.; Mamin, G.V.; Biktagirov, T.B.; Rodionov, A.A.; Galukhin, A.V.; Orlinskii, S.B. ENDOR Study of Nitrogen Hyperfine and Quadrupole Tensors in Vanadyl Porphyrins of Heavy Crude Oil. Magn. Reson. Solids Electron. J. 2016, 18, 16102. [Google Scholar]
- Mamin, G.V.; Gafurov, M.R.; Yusupov, R.V.; Gracheva, I.N.; Ganeeva, Y.M.; Yusupova, T.N.; Orlinskii, S.B. Toward the Asphaltene Structure by Electron Paramagnetic Resonance Relaxation Studies at High Fields (3.4 T). Energy Fuels 2016, 30, 6942–6946. [Google Scholar] [CrossRef] [Green Version]

| S, Mass % | Asphaltenes, Mass % | V, ppm | Ni, ppm |
|---|---|---|---|
| 4.0 | 18 | 250 | 100 |
| g⊥ | g|| | A⊥ | A|| | |
|---|---|---|---|---|
| VO2+ | 1.9868 | 1.965 | 158 MHz | 472 MHz |
| FR | giso=2.0038 | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadovnikova, M.A.; Murzakhanov, F.F.; Mamin, G.V.; Gafurov, M.R. HYSCORE Spectroscopy to Resolve Electron–Nuclear Structure of Vanadyl Porphyrins in Asphaltenes from the Athabasca Oil Sands In Situ Conditions. Energies 2022, 15, 6204. https://doi.org/10.3390/en15176204
Sadovnikova MA, Murzakhanov FF, Mamin GV, Gafurov MR. HYSCORE Spectroscopy to Resolve Electron–Nuclear Structure of Vanadyl Porphyrins in Asphaltenes from the Athabasca Oil Sands In Situ Conditions. Energies. 2022; 15(17):6204. https://doi.org/10.3390/en15176204
Chicago/Turabian StyleSadovnikova, Margarita A., Fadis F. Murzakhanov, Georgy V. Mamin, and Marat R. Gafurov. 2022. "HYSCORE Spectroscopy to Resolve Electron–Nuclear Structure of Vanadyl Porphyrins in Asphaltenes from the Athabasca Oil Sands In Situ Conditions" Energies 15, no. 17: 6204. https://doi.org/10.3390/en15176204
APA StyleSadovnikova, M. A., Murzakhanov, F. F., Mamin, G. V., & Gafurov, M. R. (2022). HYSCORE Spectroscopy to Resolve Electron–Nuclear Structure of Vanadyl Porphyrins in Asphaltenes from the Athabasca Oil Sands In Situ Conditions. Energies, 15(17), 6204. https://doi.org/10.3390/en15176204







