An Exploratory Study of Direct Injection (DI) Diesel Engine Performance Using CNSL—Ethanol Biodiesel Blends with Hydrogen
Abstract
1. Introduction
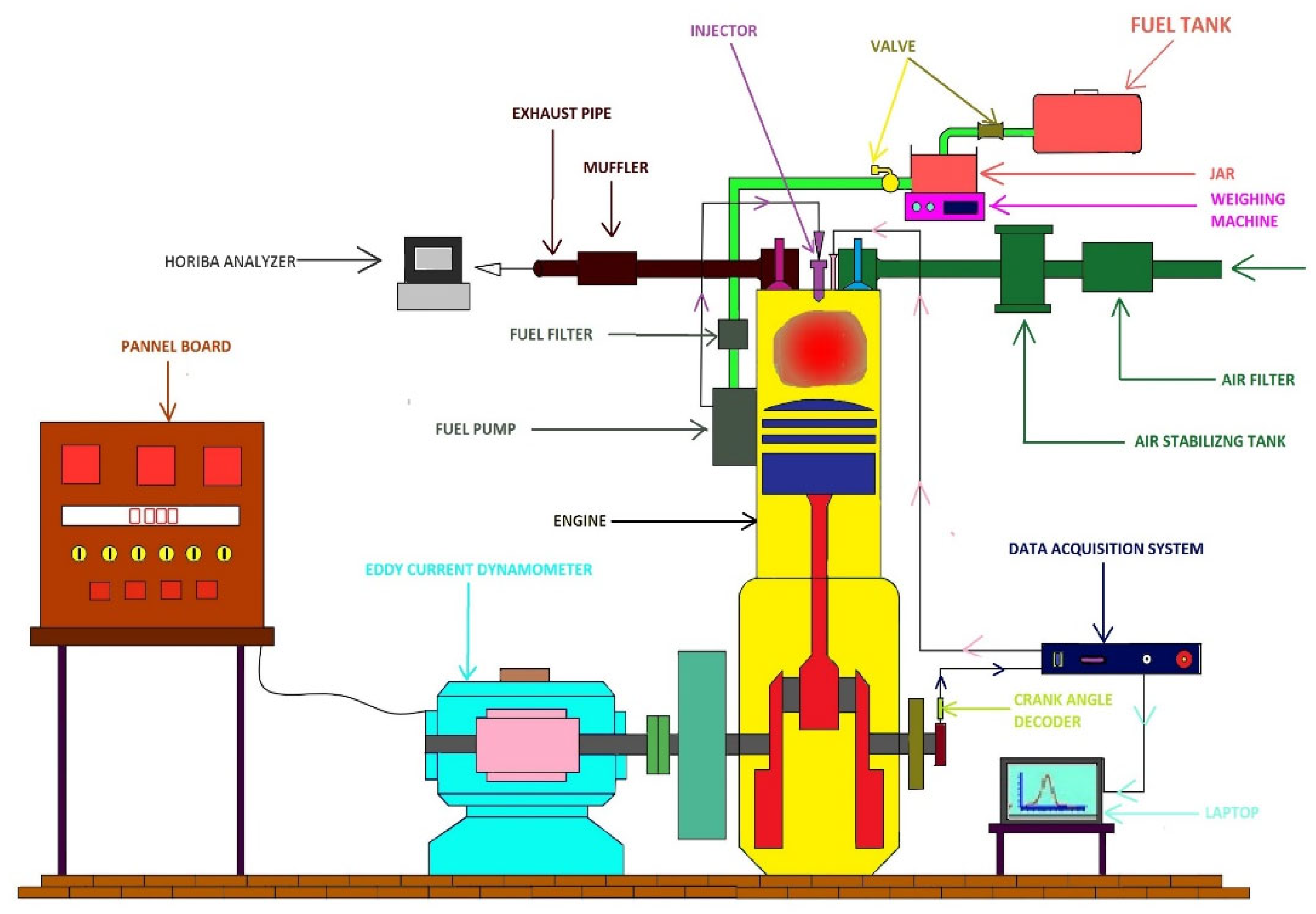
2. Materials and Methods
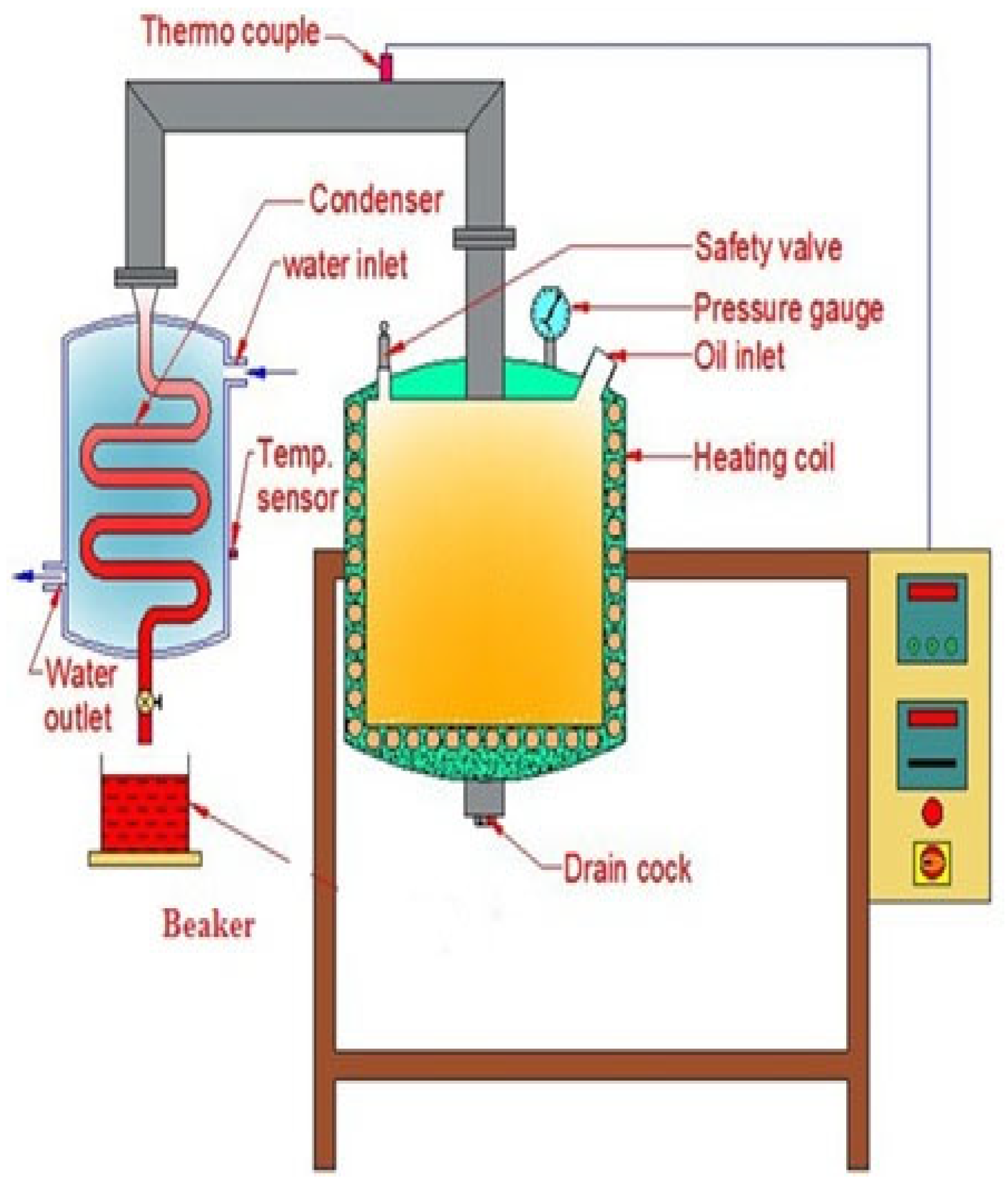
2.1. Liquid Biofuel from Thermal-Cracked Cashew Nut Shells
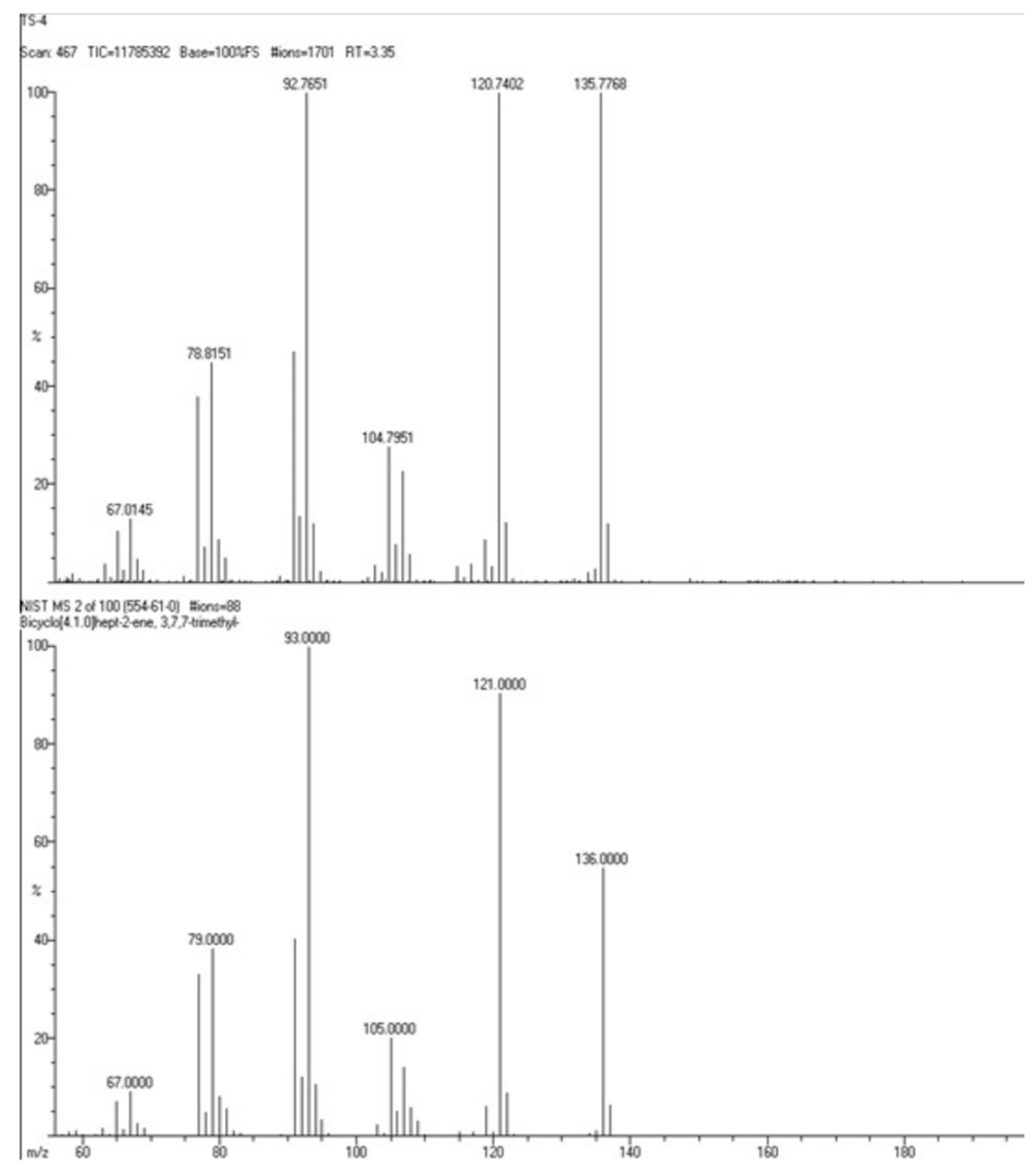
2.2. Gas Chromatography–Mass Spectrum (GCMS) Examination
2.3. Examination of the Updraft-Cracked CNSL
3. Results and Discussion
3.1. Experimental Characteristics of CNSL- Ethanol Biodiesel Blend with the Hydrogen Addition
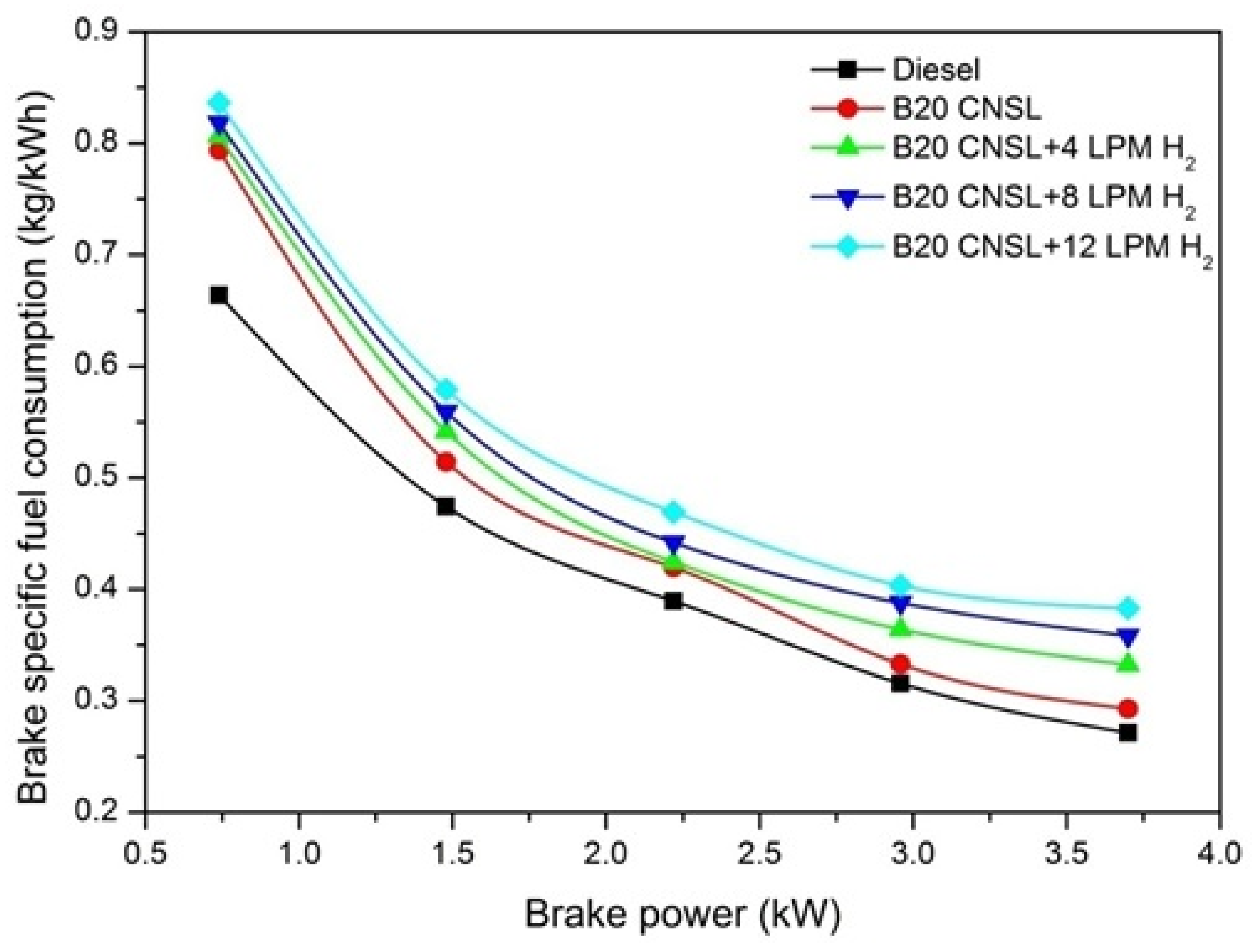
3.1.1. Brake-Specific Fuel Consumption
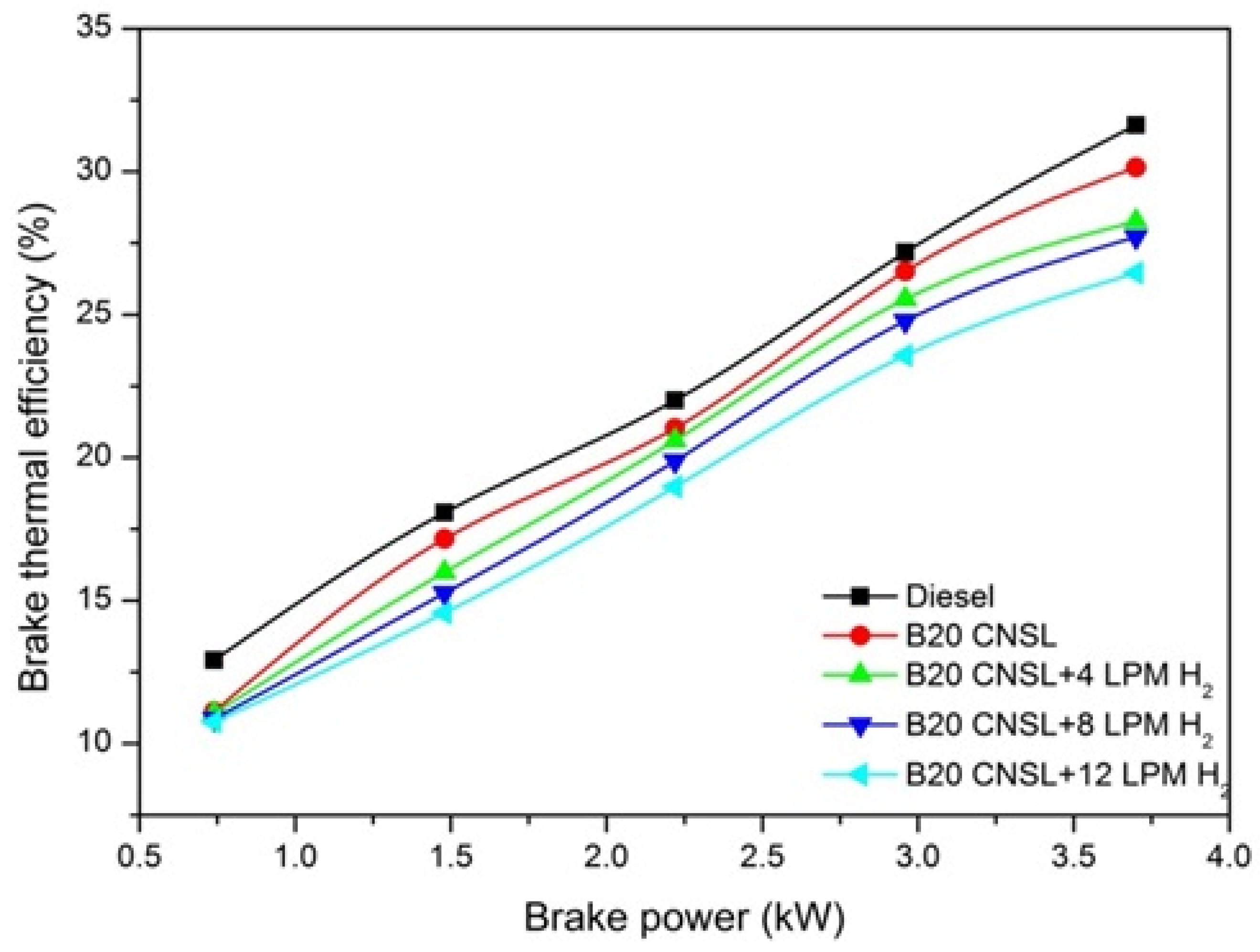
3.1.2. Brake Thermal Efficiency
3.1.3. Exhaust Gas Temperature
3.1.4. NOx Emissions
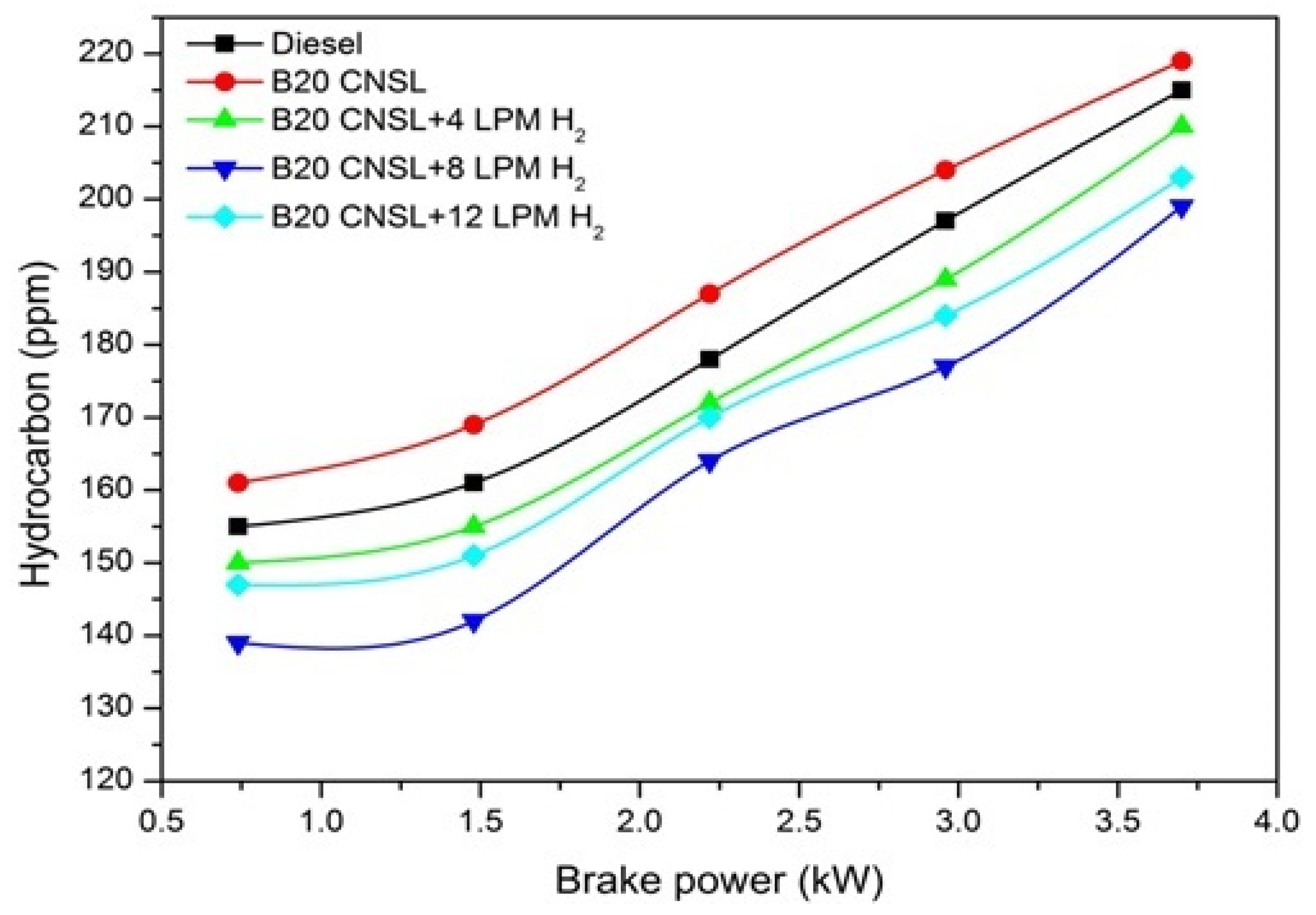
3.1.5. HC Emissions
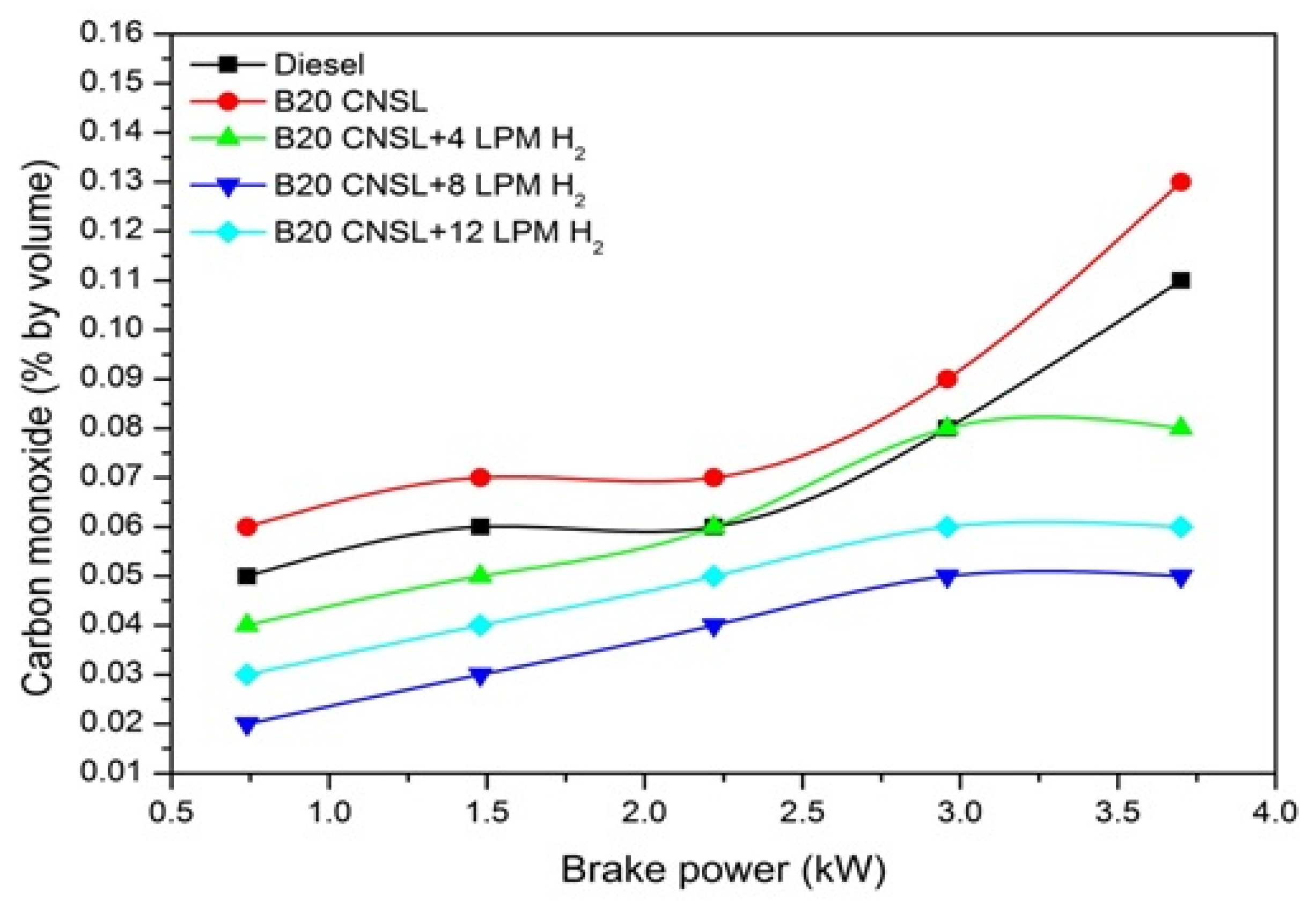
3.1.6. CO Emissions
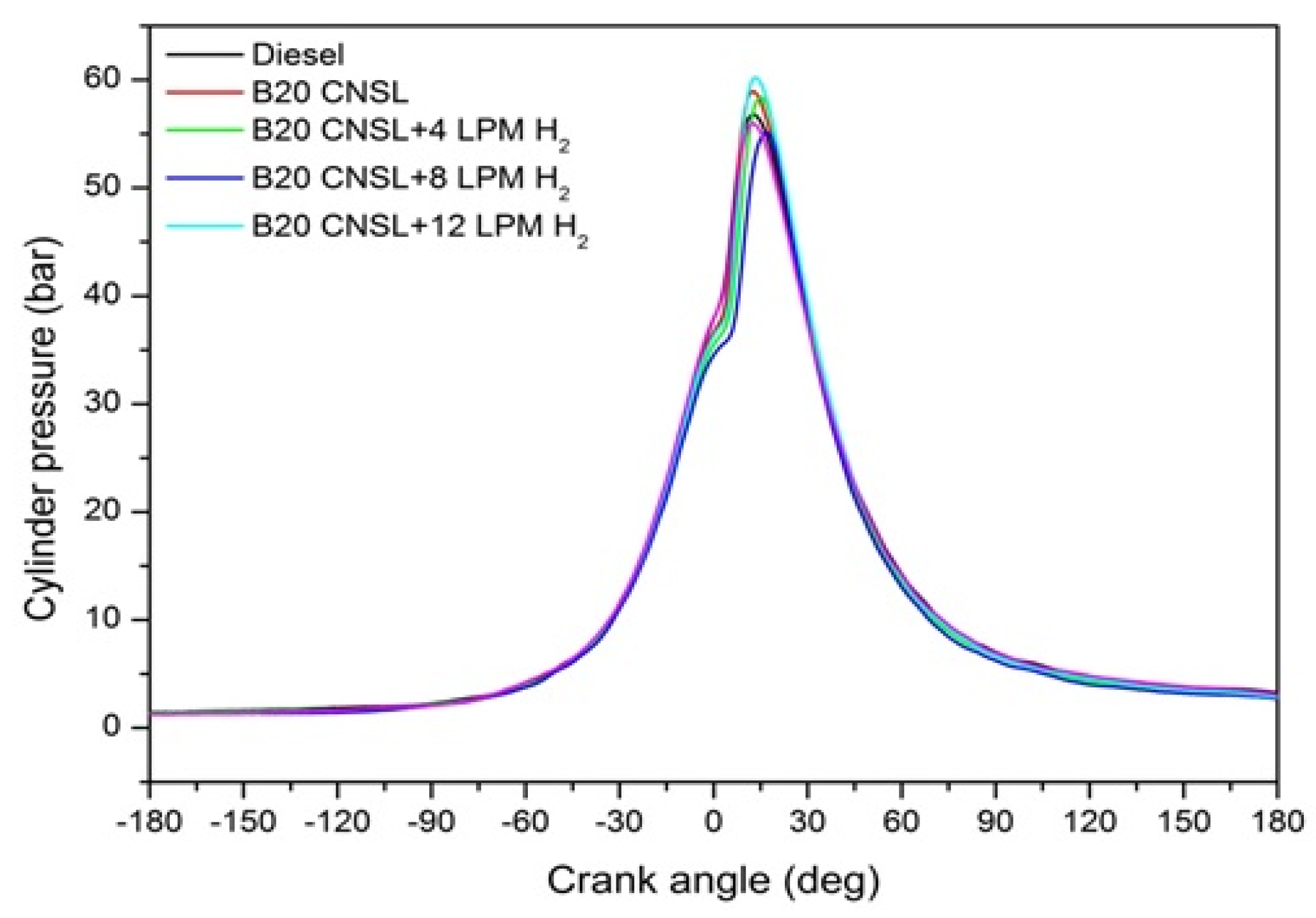
3.1.7. Variations in the Combustion In-Cylinder Pressure with the Crank Angle
3.1.8. Variations in the Heat-Release Rate with the Crank Angle
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ravi, R.; Pachamuthu, S. Design and development of innovative protracted-finned counter flow heat exchanger (PFCHE) for an engine WHR and its impact on exhaust emissions. Energies 2018, 11, 2717. [Google Scholar] [CrossRef]
- Ravi, R.; Pachamuthu, S. Experimental investigation on innovatory waste heat recovery system impacts on DIESEL engine exhaust emissions. Energy Sources Part A Recov. Util. Environ. Eff. 2020, 1–24. [Google Scholar] [CrossRef]
- Altin, R.; Çetinkaya, S.; Yücesu, H.S. Potential of using vegetable oil fuels as fuel for diesel engines. Energy Convers. Manag. 2001, 42, 529–538. [Google Scholar] [CrossRef]
- Ravi, R.; Senthilkumar, P.; Padmanathan, K. Computational and experimental investigation on effective utilization of waste heat from diesel engine exhaust using a fin protracted heat exchanger. Energy 2020, 200, 117489. [Google Scholar] [CrossRef]
- Hansen, A.C.; Lyne, P.W.; Zhang, Q. Ethanol-Diesel Blends: A Step towards a Bio-Based Fuel for Diesel Engines; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2013; pp. 16–48. [Google Scholar] [CrossRef]
- Babajide, O.; Petrik, L.; Musyoka, N.; Amigun, B.; Ameer, F. Application of coal fly ash as a solid basic catalyst in producing biodiesel. In Proceedings of the 2020 AIChE Annual Meeting, Online Event, 16–18 November 2020. [Google Scholar]
- Shivaprasad, K.; Kumar, G.; Guruprasad, K. Performance, emission, and fuel induction system of hydrogen fuel operated spark ignition engine—A review. Int. J. Mod. Eng. Res 2012, 2, 565–571. [Google Scholar]
- Shivaprasad, K.V.; Rajesh, R.; Wogasso, W.A.; Nigatu, B.; Addisu, F. Usage of hydrogen as a fuel in spark ignition engine. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Moodbidri, India, 2–3 March 2018; p. 376. [Google Scholar] [CrossRef]
- Das, L.M. Fuel induction techniques for a hydrogen-operated engine. Int. J. Hydrogen Energy 1990, 15, 833–842. [Google Scholar] [CrossRef]
- Shivaprasad, K.V.; Chitragar, P.R.; Kumar, G.N. Effect of hydrogen addition on combustion and emissions performance of a high-speed spark ignited engine at idle condition. Therm. Sci. 2018, 22, 1405–1413. [Google Scholar] [CrossRef]
- Chitragar, P.R.; Shivaprasad, K.V.; Kumar, G.N. Experimental Analysis of Four-Cylinder 4-Stroke Gasoline Engine Using Hydrogen Fractions for Performance and Emission Parameters (No. 2017-26-0063); SAE Technical Paper; SAE: Warrendale, PA, USA, 2017. [Google Scholar]
- Bose, P.K.; Maji, D. An experimental investigation on engine performance and emissions of a single-cylinder diesel engine using hydrogen as inducted fuel and diesel as injected fuel with exhaust gas recirculation. Int. J. Hydrogen Energy 2009, 34, 4847–4854. [Google Scholar] [CrossRef]
- Kasiraman, G.; Nagalingam, B.; Balakrishnan, M. Performance, emission and combustion improvements in a direct injection diesel engine using cashew nut shell oil as fuel with camphor oil blending. Energy 2012, 47, 116–124. [Google Scholar] [CrossRef]
- Kumar, R.S.; Loganathan, M.; Gunasekaran, E.J. Performance, emission and combustion characteristics of CI engine fuelled with diesel and hydrogen. Front. Energy 2015, 9, 486–494. [Google Scholar] [CrossRef]
- Loganathan, M.; Thanigaivelan, V.; Madhavan, V.M.; Anbarasu, A.; Velmurugan, A. The synergetic effect between hydrogen addition and EGR on cashew nut shell liquid biofuel-diesel operated engine. Fuel 2020, 266, 117004. [Google Scholar] [CrossRef]
- Bedar, P.; Chitragar, P.R.; Shivaprasad, K.V.; Kumar, G.N. Performance and emission analysis of single-cylinder CI engine using Simarouba glauca biodiesel. In Fluid Mechanics and Fluid Power–Contemporary Research; Springer: New Delhi, India, 2017; pp. 1519–1527. [Google Scholar] [CrossRef]
- Thanigaivelan, V.; Loganathan, M. Effect of ethanol addition on performance and emission of CNSL biodiesel-Hydrogen operated di dual fuel engine. Int. J. Mech. Eng. Technol. 2019, 10, 1209–1220. [Google Scholar]
- Douadi, O.; Ravi, R.; Faqir, M.; Essadiqi, E. A conceptual framework for waste heat recovery from compression ignition engines: Technologies, working fluids & heat exchangers. Energy Convers. Manag. 2022, 16, 100309. [Google Scholar] [CrossRef]
- Thanigaivelan, V.; Balaji, G.; Loganathan, M.; Saravanan, C.G. Recital and emanation individuality of cashew nut shell with methanol blends. IOP Conf. Ser. Mater. Sci. Eng. 2018, 402, 012108. [Google Scholar] [CrossRef]
- Thanigaivelan, V.; Loganathan, M. Performance analysis of addition of hydrogen to compression ignition diesel engine with biodiesel blends. In Proceedings of the 2017 Third International Conference on Science Technology Engineering & Management, Chennai, India, 23–24 March 2018; pp. 1123–1128. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.F.; Zhong, X.; Hu, B.; Liu, H.; Yao, M. Experimental study on the combustion and emissions fueling biodiesel/n-butanol, biodiesel/ethanol and biodiesel/2,5-dimethylfuran on a diesel engine. Energy 2016, 115, 539–549. [Google Scholar] [CrossRef]
- Zheng, Z.; Xia, M.; Liu, H.; Wang, X.; Yao, M. Experimental study on combustion and emissions of dual fuel RCCI mode fueled with biodiesel/n-butanol, biodiesel/2,5-dimethylfuran and biodiesel/ethanol. Energy 2018, 148, 824–838. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Geng, Z.; Pang, X.; Wang, X.; Ji, J.; Wang, G.; Liu, H. Effects of various co-solvents on the solubility between blends of soybean oil with either methanol or ethanol. Fuel 2019, 244, 461–471. [Google Scholar] [CrossRef]
- Kannan, G.R.; Anand, R. Experimental investigation on diesel engine with dienestrol-water microemulsions. Energy 2011, 36, 1680–1687. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Elango, A.; Prathima, A. The effect of cerium oxide additive on the performance and emission characteristics of a CI engine operated with rice bran biodiesel and its blends. Int. J. Green Energy 2013, 13, 267–273. [Google Scholar] [CrossRef]
- Lubi, M.C.; Thachil, E.T. Cashew nut shell liquid (CNSL)—A versatile monomer for polymer synthesis. Des. Monomers Polym. 2000, 3, 123–153. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Larroche, C.; Kim, S.H.; Pandey, A. Valorization of cashew nut processing residues for industrial applications. Ind. Crops Prod. 2020, 152, 112550. [Google Scholar] [CrossRef]
- Sukjit, E.; Herreros, J.M.; Dearn, K.D.; García-Contreras, R.; Tsolakis, A. The effect of the addition of individual methyl esters on the combustion and emissions of ethanol and butanol-diesel blends. Energy 2012, 42, 364–374. [Google Scholar] [CrossRef]
- Lu, Q.; Xiong, W.M.; Li, W.Z.; Guo, Q.X.; Zhu, X.F. Catalytic pyrolysis of cellulose with sulfated metal oxides: A promising method for obtaining high yield of light furan compounds. Bioresour. Technol. 2009, 100, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N. Comparative analysis of biodiesel-ethanol-diesel and biodiesel-methanol-diesel blends in a diesel engine. Energy 2012, 40, 210–213. [Google Scholar] [CrossRef]
- Debnath, B.K.; Saha, U.K.; Sahoo, N. Effect of hydrogen-diesel quantity variation on brake thermal efficiency of a dual dual-fuelled engine. J. Power Technol. 2012, 92, 55–67. [Google Scholar]
- Thanigaivelan, V.; Loganathan, M. Performance and emission characteristics of dual fuel DI diesel engine using cashew nut shell biodiesel hydrogen as fuel. Int. J. Mech. Eng. Technol. 2019, 10, 847–857. [Google Scholar]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Kakaras, E.C.; Giakoumis, E.G. Effects of ethanol-diesel fuel blends on the performance and exhaust emissions of heavy-duty DI diesel engine. Energy Convers. Manag. 2008, 49, 3155–3162. [Google Scholar] [CrossRef]
- Rathinam, S.; Balan, K.N.; Subbiah, G.; Sajin, J.B.; Devarajan, Y. Emission study of a diesel engine fueled with higher alcohol-biodiesel blended fuels. Int. J. Green Energy 2019, 16, 667–673. [Google Scholar] [CrossRef]
- Shivaprasad, K.V.; Chitragar, P.R.; Nayak, V.; Kumar, G.N. Influence of spark timing on the performance and emission characteristics of gasoline–hydrogen-blended high-speed spark-ignition engine. Int. J. Ambient. Energy 2017, 38, 605–612. [Google Scholar] [CrossRef]






| Properties | Measurement Standards | Diesel | TC-CNSL (Cardanol) | TC-CNSL |
|---|---|---|---|---|
| Density 25 °C (g/cc) | ASTM D1298 | 0.8/0.84 | 0.9326 | 0.821 |
| Flash point (°C) | ASTM D93 | 80 | 198 | <28 |
| Calculated cetane index | ASTM D976 | 52 | 28 | 45 |
| Boiling point (°C) | ASTM D1160 | 180–340 | 225 | 180–380 |
| Kinetic viscosity at 30 °C (cP) | ASTM D445 | 2.0 to 4.5 | 17.2 | 4.43 |
| Calorific value (kJ/kg) | ASTM D240 | 44,000 | 39,600 | 41,780 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadivelu, T.; Ramanujam, L.; Ravi, R.; Vijayalakshmi, S.K.; Ezhilchandran, M. An Exploratory Study of Direct Injection (DI) Diesel Engine Performance Using CNSL—Ethanol Biodiesel Blends with Hydrogen. Energies 2023, 16, 415. https://doi.org/10.3390/en16010415
Vadivelu T, Ramanujam L, Ravi R, Vijayalakshmi SK, Ezhilchandran M. An Exploratory Study of Direct Injection (DI) Diesel Engine Performance Using CNSL—Ethanol Biodiesel Blends with Hydrogen. Energies. 2023; 16(1):415. https://doi.org/10.3390/en16010415
Chicago/Turabian StyleVadivelu, Thanigaivelan, Lavanya Ramanujam, Rajesh Ravi, Shivaprasad K. Vijayalakshmi, and Manoranjitham Ezhilchandran. 2023. "An Exploratory Study of Direct Injection (DI) Diesel Engine Performance Using CNSL—Ethanol Biodiesel Blends with Hydrogen" Energies 16, no. 1: 415. https://doi.org/10.3390/en16010415
APA StyleVadivelu, T., Ramanujam, L., Ravi, R., Vijayalakshmi, S. K., & Ezhilchandran, M. (2023). An Exploratory Study of Direct Injection (DI) Diesel Engine Performance Using CNSL—Ethanol Biodiesel Blends with Hydrogen. Energies, 16(1), 415. https://doi.org/10.3390/en16010415








