Comparative Analysis of a New Class of Symmetric and Asymmetric Supercapacitors Constructed on the Basis of ITO Collectors
Abstract
1. Introduction
1.1. Context of the Study and Related Works
1.2. Research Gap
1.3. Motivation and Contribution
- Construction of tested supercapacitors with a unique combination of active materials; the unique design results from proper selection of electrode materials and electrolyte active ingredients;
- Development of a method for selecting search ranges of the parameters of supercapacitor models and a method of normalization of the measured characteristics;
- Selection of models known in the literature that best approximate the characteristics of the supercapacitors studied, verification of the parameters of selected fractional-order models, and evaluation of the quality of their matching to the examined elements.
2. Materials and Methods
2.1. Materials Used for Symmetric and Asymmetric Supercapacitors
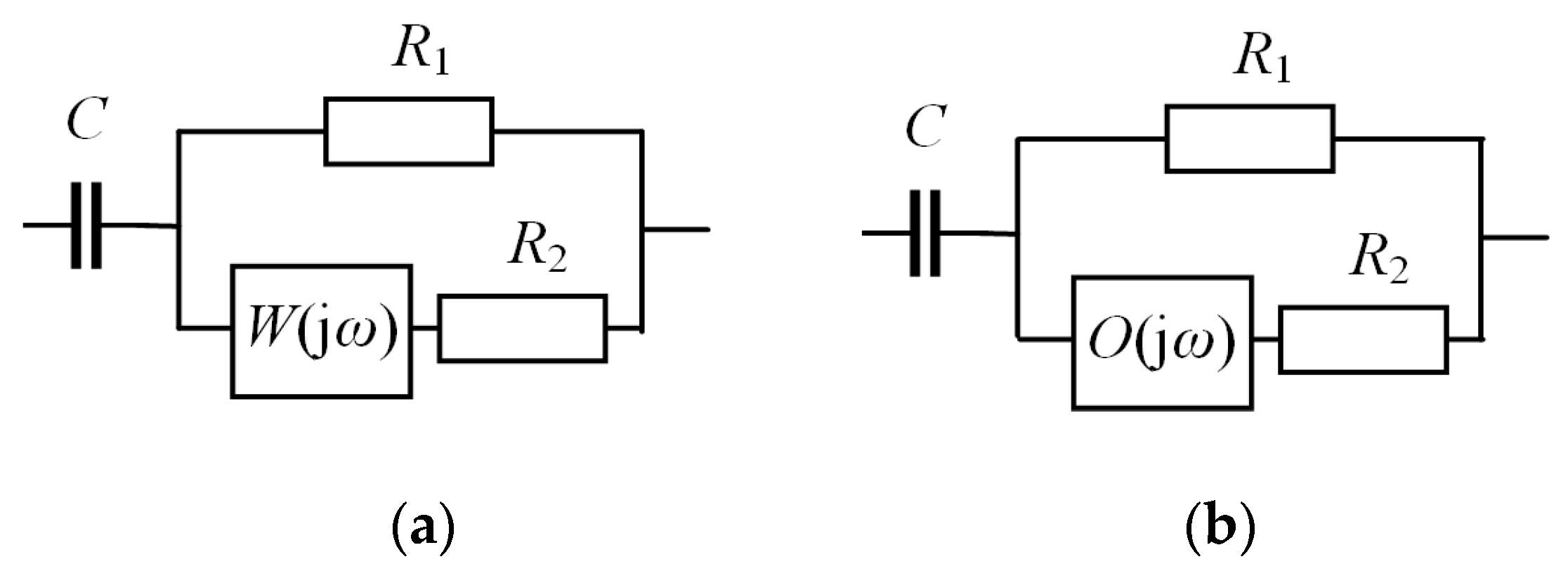
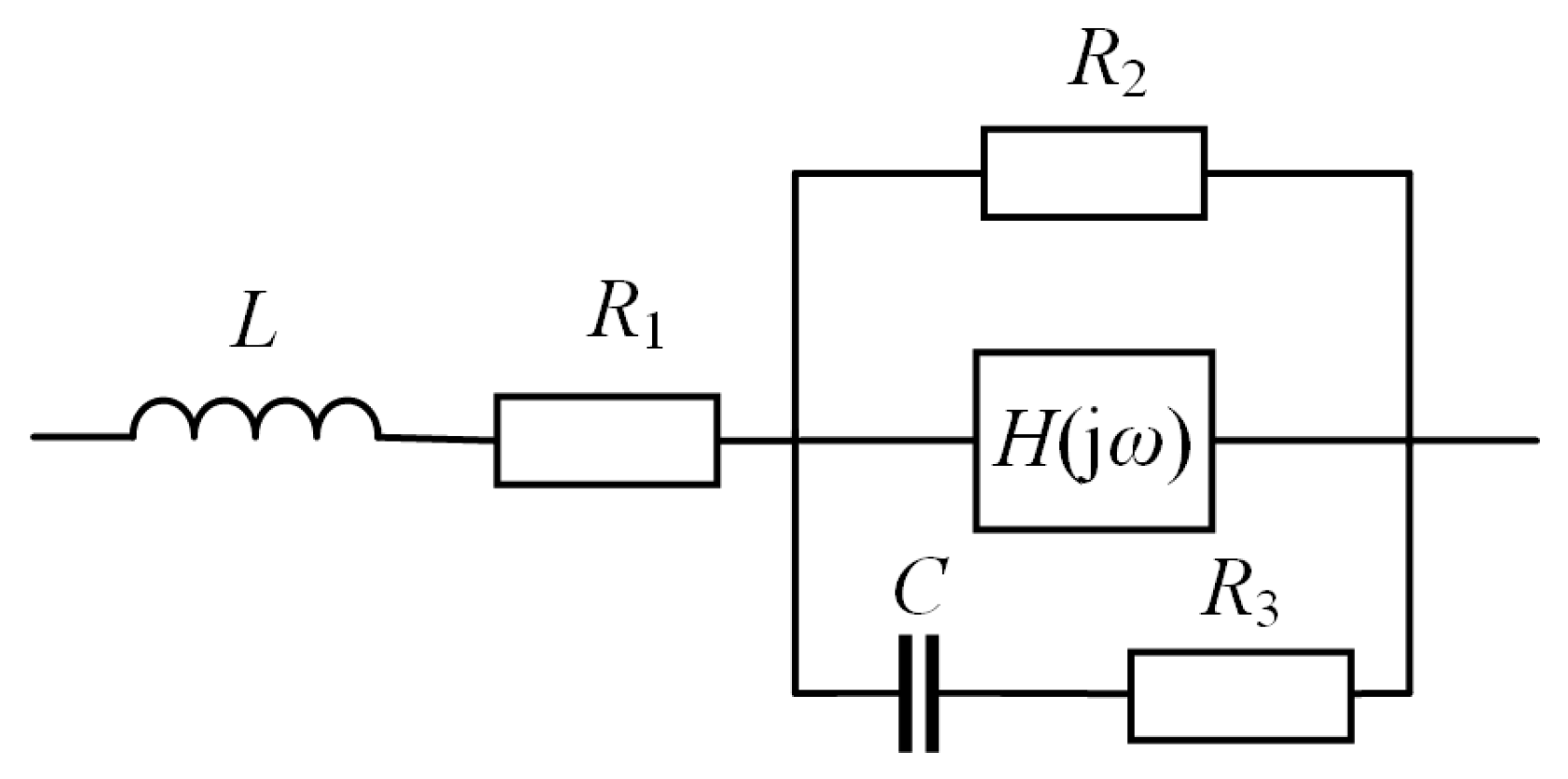
2.2. Fractional-Order Supercapacitor Models in Frequency Domain
- Warburg impedance:
- Warburg limited impedance:
- Havriliak–Negami function:where: Z0, B—parameters; C0, C∞—supercapacitor’s capacitance at low (f → 0) and high frequencies (f → ∞); τ—parameter related to operating temperature; μ, Φ—fractional-order parameters.
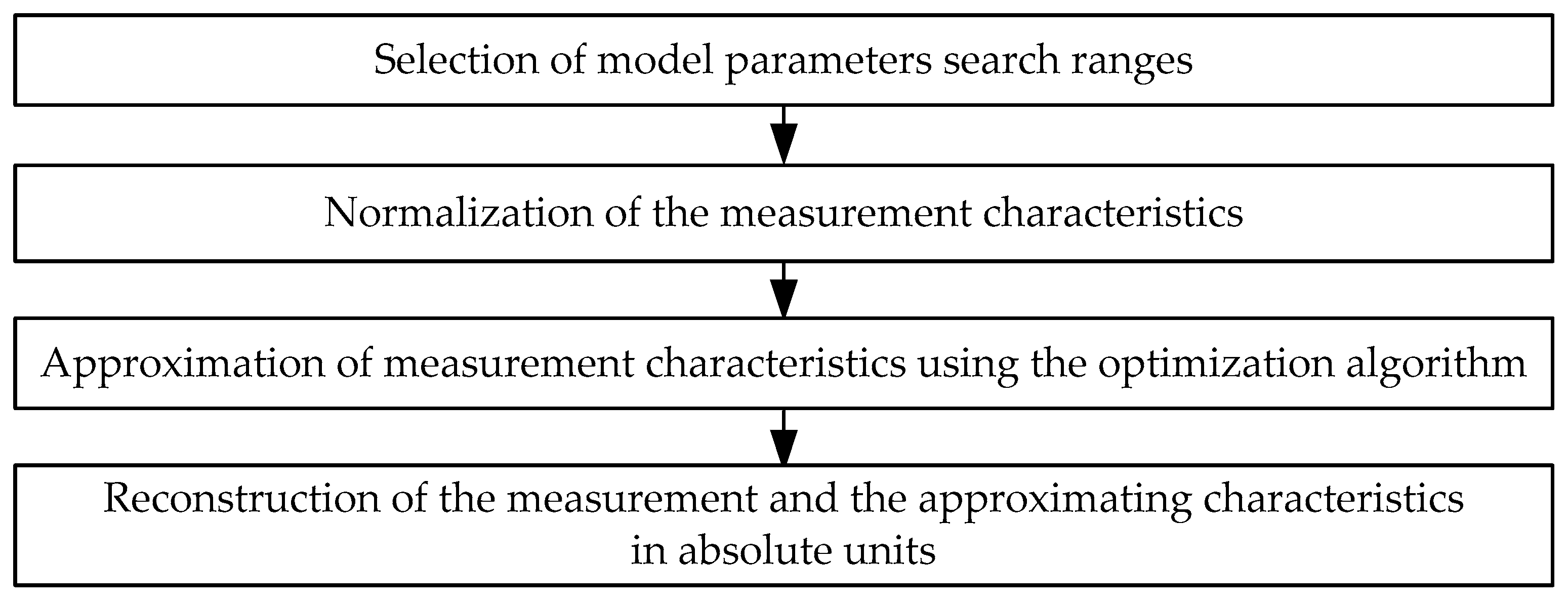
2.3. The Method of Estimation of the Model Parameters
3. Results
- sym pp—symmetric supercapacitor with collectors containing polypyrrole;
- Sym po—symmetric supercapacitor with collectors containing poly(3-n-octylpyrrole);
- asym p_w—asymmetric supercapacitor, a working electrode connected to a collector containing polypyrrole;
- asym o_w—asymmetric supercapacitor, a working electrode connected to a collector containing poly(3-n-octylpyrrole).
4. Discussion
- From the SEM analysis, we can know the local structure of polypyrrole and poly(3-n-octylpyrrole) surfaces. The polypyrrole surface is continuous and has three-dimensional structures that are evenly distributed over the surface. The surface of poly(3-n-octylpyrrole) has surface defects and uneven three-dimensional structures. For this reason, it can be assumed that the better electrochemical properties of polypyrrole result from its more ordered structure and the continuity of the polymer surface on the ITO plate;
- Among the polymers studied, polypyrrole turned out to be the best electrode material used as the electrode material. Supercapacitors containing this polymer covered ITO plates and an electrolyte containing lithium perchlorate (LiClO4) showed the highest specific capacitance of 49 F/g (Table 1). The tested asymmetric supercapacitors have two different electrode materials: polypyrrole and poly(3-n-octylpyrrole). The values of the parameters tested in asymmetric supercapacitors, such as specific capacitance, turned out to be higher than the parameters obtained during the study of supercapacitors based on poly(3-n-octylpyrrole), but lower than those of systems containing polypyrrole;
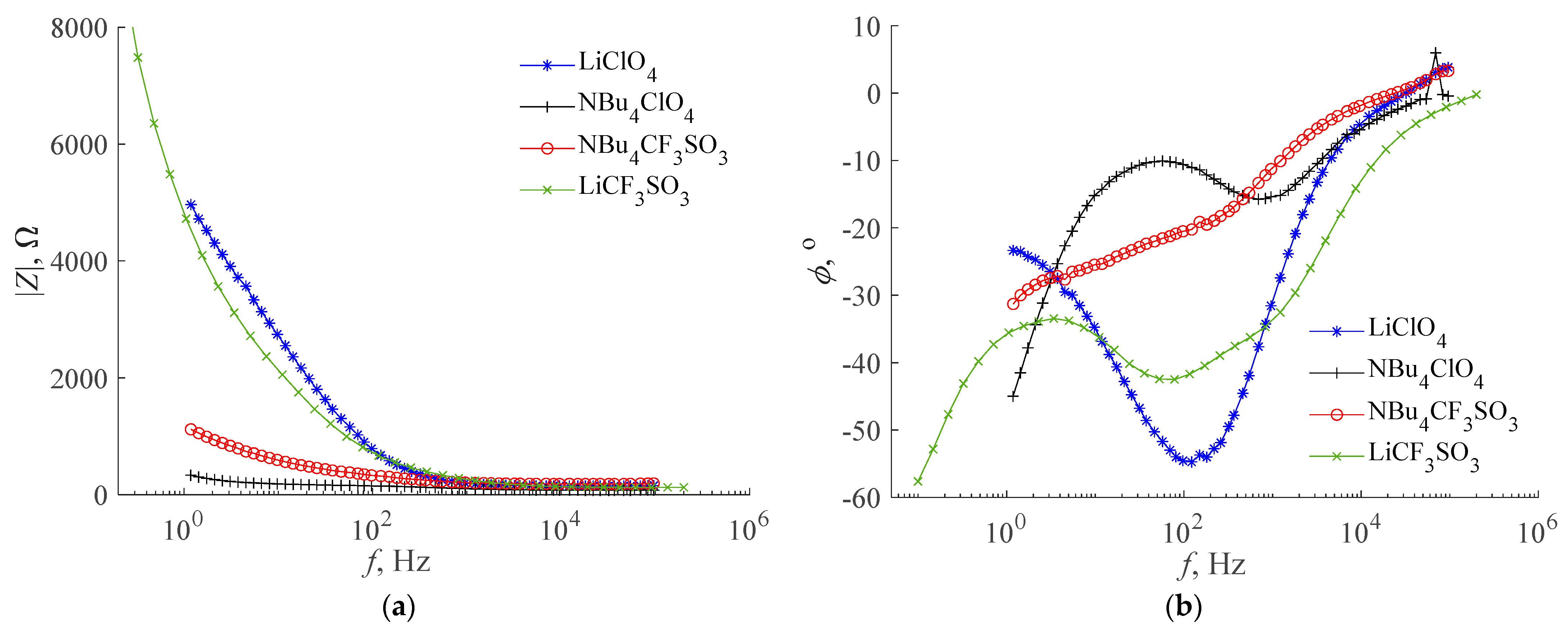
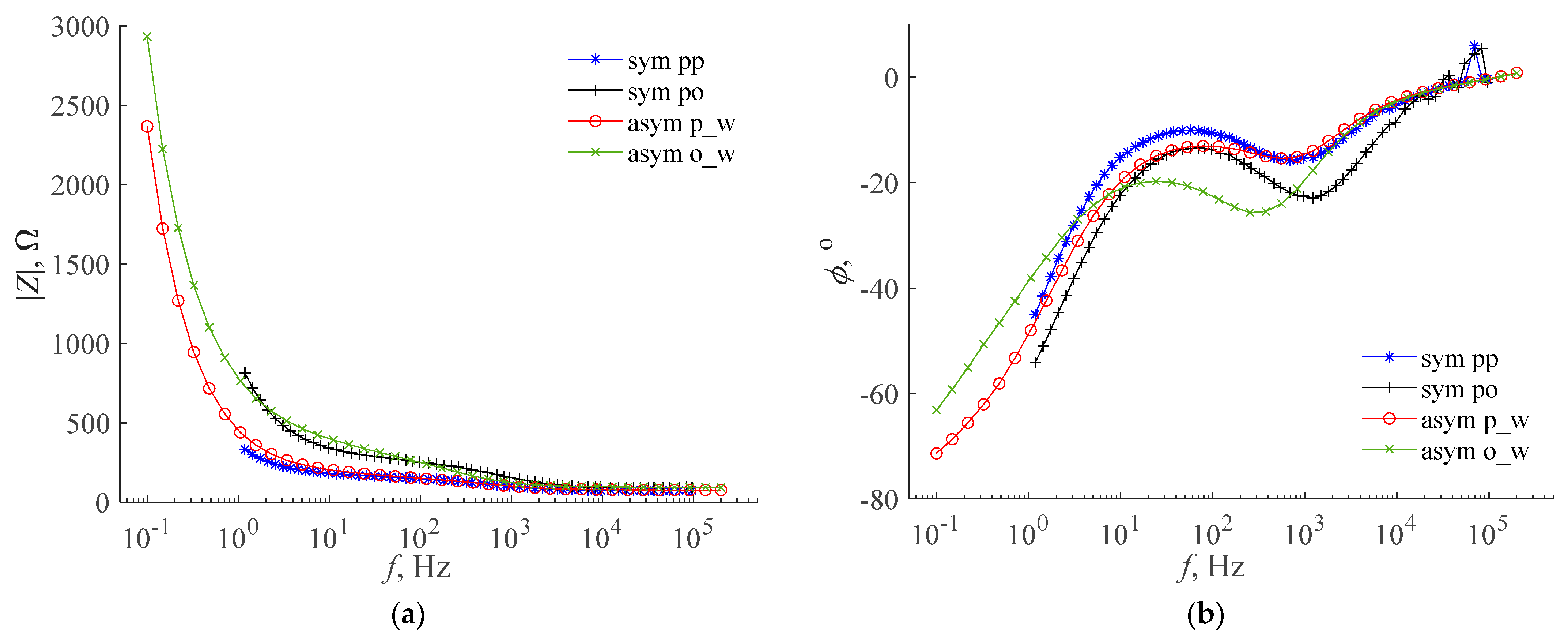
- The use of normalization of the measurement and approximation characteristics allows for the use of the error value ε to compare the approximation accuracy of the characteristics of different supercapacitors, although these characteristics have different ranges of values [32]. For example, Figure 10 shows that the accuracy of the approximation of the impedance argument of a symmetric supercapacitor with collectors containing polypyrrole, containing LiClO4 as the active electrolyte component, is satisfactory for the model based on poles and zeros of fractional-order impedance, which corresponds to a small error value ε in Table 2. On the other hand, the approximation accuracy of the impedance argument of this supercapacitor is not satisfactory for the model with the Warburg limited impedance, which corresponds to the large error value ε in Table 3;
- Among all optimization algorithms tested (including a hybrid algorithm [38,39,40,41], which is a combination of a genetic and a Newton gradient algorithm, used in the previous work of the author on evaluating the stability of the power system, including [42,43]), the best effectiveness and accuracy of the characteristic approximation were obtained for the Particle Swarm Optimization (PSO) algorithm [33,34,35,36,37,38]. The paper [38] presents a ranking of the effectiveness of various optimization algorithms used to analyze the optimal sizing of batteries in microgrids based on different features of the battery. The Ranker Search algorithm [38] ranks first, and the PSO algorithm ranks second. For our research, the Ranker Search algorithm is not a good solution, because it is looking for an optimal solution in a previously prepared data set. Creating such a data set would require the discretization of the value of the parameters sought. The PSO algorithm searches for the space of the objective function parameters in a continuous manner, without the need to create a discrete data set;
- For all supercapacitors and mathematical models tested, a satisfactory approximation accuracy of the impedance modulus characteristics was obtained. In each case, this accuracy was better than for the characteristics of the impedance argument. This regularity is also confirmed by the research conducted by the authors so far, among others described in [32];
- For most of the supercapacitors tested, the accuracy of the approximation of impedance characteristics was better for the model based on poles and zeros of the fractional-order impedance (3) than for the models with the Warburg impedance (4) and (5). This is especially noticeable with supercapacitors containing LiClO4 as the active electrolyte component. In the studies described in [32], a similar regularity was also observed;
- For all cases, a very high value of parameter B of the model with limited Warburg impedance was obtained. Therefore, in formula (5), the value of the coth function is always close to 1 and this model can be reduced to the model with the Warburg impedance (4), while the values of the other parameters will not change. In the study, the parameters of the model (4) were also estimated and the obtained values of its parameters were very similar to the values of the parameters of the model (5) [32];
- The parameter k in the model based on poles and zeros of the fractional-order impedance is related to the reciprocal of the supercapacitor capacity. Based on the results from Table 2, it can be seen that in most cases the highest capacitance values and the smallest RC resistance values were obtained for asymmetric supercapacitors. In each of the investigated cases of asymmetric supercapacitors, a higher capacity was obtained when the working electrode was connected to a polypyrrole-containing collector. The asymmetric supercapacitor, which contains LiClO4 as the active electrolyte component, had the highest capacitance and relatively small RC resistance;
- In the case of the model with limited Warburg impedance, on the basis of the results in Table 3, similar conclusions can be drawn. However, the analysis should not take into account results with large values of the ε error;
- In Table 4, a comparison has been made between the proposed approach of the optimization process and fractional-order model estimations presented in the paper, and the state-of–the-art solutions, mostly used in supercapacitors studies.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Subasinghage, K.; Gunawardane, K.; Padmawansa, N.; Kularatna, N.; Moradian, M. Modern Supercapacitors Technologies and Their Applicability in Mature Electrical Engineering Applications. Energies 2022, 15, 7752. [Google Scholar] [CrossRef]
- Gilev, B.; Andreev, M.; Hinov, N.; Angelov, G. Modeling and Simulation of a Low-Cost Fast Charging Station Based on a Micro Gas Turbine and a Supercapacitor. Energies 2022, 15, 8020. [Google Scholar] [CrossRef]
- Khodaparastan, M.; Mohamed, A. Supercapacitors for Electric Rail Transit Systems. In Proceedings of the 6th International Conference on Renewable Energy Research and Applications (ICRERA), San Diego, CA, USA, 5–8 November 2017; pp. 896–901. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nawaz, A.; Sreekumar, P.; Natsheh, A.; Akre, V.; Van, T.L. Implementation and Validation for Multitasks of a Cost-Effective Scheme Based on ESS and Braking Resistors in PMSG Wind Turbine Systems. Energies 2022, 15, 8282. [Google Scholar] [CrossRef]
- Jamal, S.; Pasupuleti, J.; Rahmat, N.A.; Tan, N.M.L. Energy Management System for Grid-Connected Nanogrid during COVID-19. Energies 2022, 15, 7689. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Wang, Z.; Ni, S.; Yang, Y.; Tian, P. Adaptive state of energy evaluation for supercapacitor in emergency power system of more-electric aircraft. Energy 2023, 263, 125632. [Google Scholar] [CrossRef]
- Geng, Z.; Mannerhagen, F.; Thiringer, T. Characterization of Lithium Ion Supercapacitors; Institute of Electrical and Electronics Engineers: Piscataway, NJ, USA, 2020; ISBN 978-9-0758-1536-8. [Google Scholar] [CrossRef]
- Pullanchiyodan, A.; Manjakkal, L.; Dahiya, R. Metal Coated Fabric Based Asymmetric Supercapacitor for Wearable Applications. IEEE Sens. J. 2021, 21, 26208–26214. [Google Scholar] [CrossRef]
- Bogusz, W.; Krok, F. Solid Electrolytes, Electrical Properties and Methods of Their Measurement, Wyd; Naukowo-Techniczne: Warszawa, Poland, 1995. (In Polish) [Google Scholar]
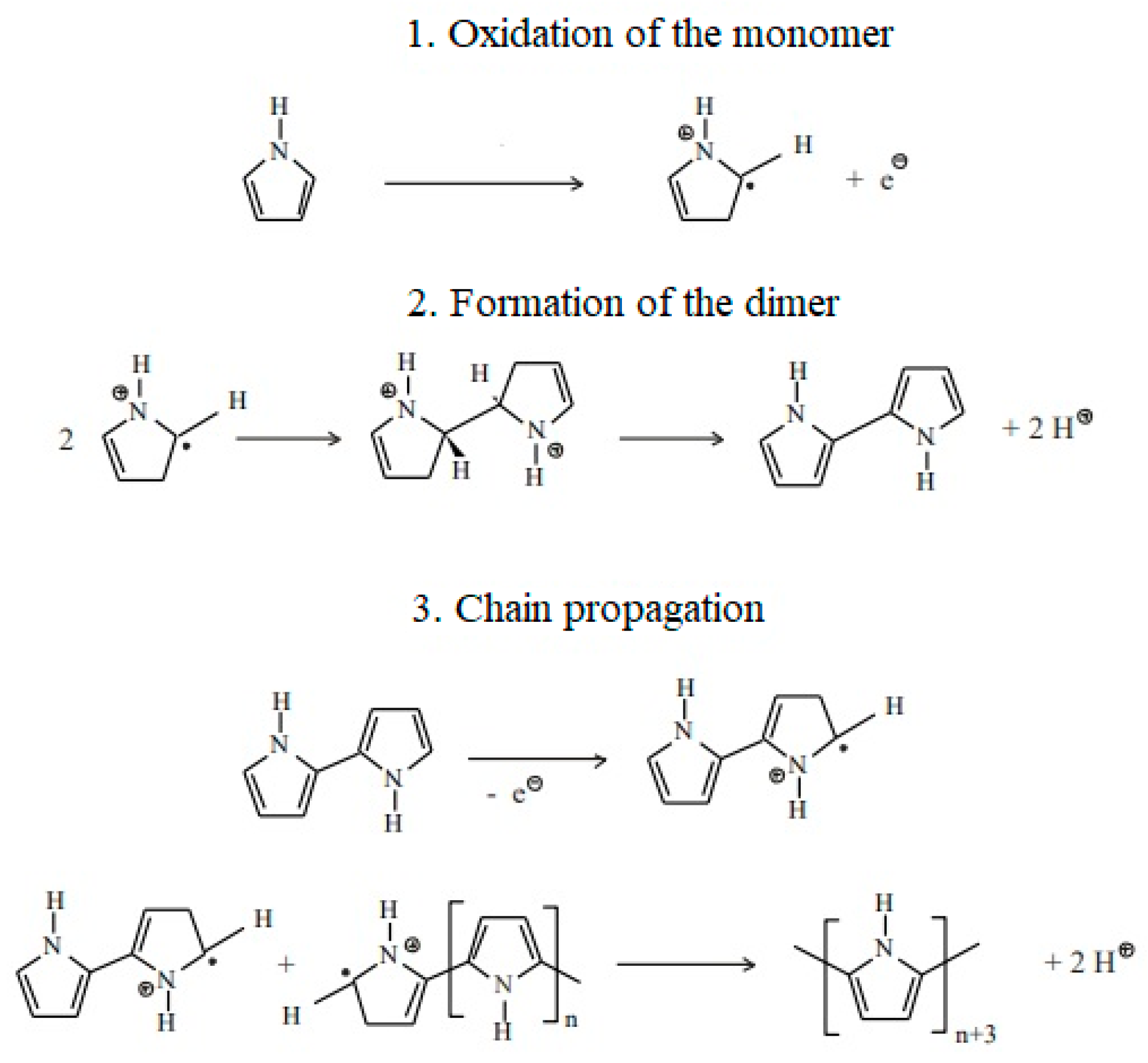
- Gocki, M. Research on the Application of Selected Conductive Polymers for Electricity Storage. Master’s Thesis, Department of Physicochemistry and Polymer Technology, Faculty of Chemistry, Silesian University of Technology, Gliwice, Poland, 2021. (In Polish). [Google Scholar]
- Song, Y.; Shang, M.; Li, J.; Su, Y. Continuous and controllable synthesis of MnO2/PPy composites with core-shell structures for supercapacitors. Chem. Eng. J. 2020, 405, 127059. [Google Scholar] [CrossRef]
- Tong, L.; Jiang, C.; Cai, K.; Wei, P. High-performance and freestanding PPy/Ti3C2Tx composite film for flexible all-solid-state supercapacitors. J. Power Sources 2020, 465, 228267. [Google Scholar] [CrossRef]
- Wen, J.; Xu, B.; Zhou, J.; Chen, Y. Novel high-performance asymmetric supercapacitors based on nickel-cobalt composite and PPy for flexible and wearable energy storage. J. Power Sources 2018, 402, 91–98. [Google Scholar] [CrossRef]
- Krajewska, A. Development of Electrochemical Sensors for the Determination of Acrylamide and Acrylic Acid in Food Products. Ph.D. Dissertation, Faculty of Chemistry, Gdańsk University of Technology, Gdańsk, Poland, 2009. (In Polish). [Google Scholar]
- Geiger, W.E.; Barričre, F. Organometallic Electrochemistry Based on Electrolytes Containing Weakly-Coordinating Fluoroarylborate Anions. Accounts Chem. Res. 2010, 43, 1030–1039. [Google Scholar] [CrossRef]
- Lisowska-Oleksiak, A.; Nowak, A.P.; Wilamowska, M. Supercapacitors as Energy Storage Materials. Acta Energetica 2015, 7, 71–78. (In Polish) [Google Scholar]
- Filip, A.; Musat, V.; Tigau, N.; Polosan, S.; Pimentel, A.; Ferreira, S.; Gomes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. ZnO nanostructures grown on ITO coated glass substrate by hybrid microwave-assisted hydrothermal method. Optik 2020, 208, 164372. [Google Scholar] [CrossRef]
- Gualous, H.; Bouquain, D.; Berthon, A.; Kauffmann, J. Experimental study of supercapacitor serial resistance and capacitance variations with temperature. J. Power Sources 2003, 123, 86–93. [Google Scholar] [CrossRef]
- Helseth, L. Comparison of methods for finding the capacitance of a supercapacitor. J. Energy Storage 2021, 35, 102304. [Google Scholar] [CrossRef]
- Gocki, M.; Nowak, A.J. Analysis of the Characteristics of Electrically Conductive Polymers Using the Electrochemical Impedance Spectroscopy Method. In International Student Scientific Conference TalentDetector2022_Winter, Works of the Depart. of Eng. and Bio. Mat.; Silesian University of Technology: Gliwice, Poland, 2022; pp. 231–238. (In Polish) [Google Scholar]
- Buller, S.; Karden, E.; Kok, D.; De Doncker, R. Modeling the dynamic behavior of supercapacitors using impedance spectroscopy. IEEE Trans. Ind. Appl. 2002, 38, 1622–1626. [Google Scholar] [CrossRef]
- Freeborn, T.J.; Maundy, B.; Elwakil, A.S. Fractional-order models of supercapacitors, batteries and fuel cells: A survey. Mater. Renew. Sustain. Energy 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Omori, T.; Nakanishi, M.; Tashima, D. Modeling of Equivalent Circuit Analysis of Degraded Electric Double-Layer Capacitors. Materials 2021, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Quintana, J.J.; Ramos, A.; de la Nuez, I. Modeling electrochemical double layer capacitor, from classical to fractional impedance. In Proceedings of the 14th Mediterranean Electrotechnical Conference, Ajaccio, France, 5–7 May 2008; pp. 61–66. [Google Scholar] [CrossRef]
- Martin, R.; Quinatana, J.; Ramos, A.; Nuez, I.; Martín, A.R. Fractional equivalent impedance of electrochemical double layer capacitors combinations. J. Eur. Des Syst. Autom. 2008, 42, 923–928. [Google Scholar] [CrossRef]
- Martín, A.R.; Quintana, J.J.; Ramos, A.; De La Nuez, I. Modeling of Electrochemical Double Layer Capacitors by Means of Fractional Impedance. J. Comput. Nonlinear Dyn. 2008, 3, 61–66. [Google Scholar] [CrossRef]
- Hidalgo-Reyes, J.; Gómez-Aguilar, J.; Jiménez, R.E.; Alvarado-Martinez, V.; Lopez-Lopez, M. Determination of supercapacitor parameters based on fractional differential equations. Int. J. Circuit Theory Appl. 2019, 47, 1225–1253. [Google Scholar] [CrossRef]
- Czerwiński, W.; Kaczmarek, H.; Kędziera, D. Conductive and Photosensitive Polymer Materials; Nicolaus Copernicus University: Toruń, Poland, 2012. [Google Scholar]
- Kwon, H.; Han, D.J.; Lee, B.Y. All-solid-state flexible supercapacitor based on nanotube-reinforced polypyrrole hollowed structures. RSC Adv. 2020, 10, 41495–41502. [Google Scholar] [CrossRef] [PubMed]
- Semary, M.S.; Fouda, M.E.; Hassan, H.; Radwan, A.G. Realization of fractional-order capacitor based on passive symmetric network. J. Adv. Res. 2019, 18, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska-Ciszek, A.; Walczak, J. Frequency Method for Determining the Equivalent Parameters of Fractional-Order Elements LβCα. In Proceedings of the Conference on Non-Integer Order Calculus and Its Applications RRNR 2018, Białystok, Poland, 20–21 September 2018; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 250–267. [Google Scholar] [CrossRef]
- Gocki, M.; Jakubowska-Ciszek, A.; Pruski, P. Measurement Estimation of the Fractional-Order Models Parameters of a New Class of Symmetrical Polymer Supercapacitors. Przegląd Elektrotechniczny 2022, 11, 224–228. (In Polish) [Google Scholar] [CrossRef]
- Poli, R.; Kennedy, J.; Blackwell, T. Particle Swarm Optimization. An Overview. Swarm Intell. 2007, 1, 33–57. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Rosa, A.C.; Fachada, N.; Laredo, J.L.J.; Merelo, J.J. Particle Swarm and Population Structure. In Proceedings of the Genetic and Evolutionary Computation, Conference Companion CECCO, Kyoto, Japan, 15–19 July 2018; pp. 85–86. [Google Scholar] [CrossRef]
- Cleghorn, C.W.; Engelbrecht, A.P. Fitness-Distance-Ratio Particle Swarm Optimization: Stability Analysis. In Proceedings of the Genetic and Evolutionary Computation, Conference Companion CECCO, Kyoto, Japan, 15–19 July 2018; pp. 12–18. [Google Scholar] [CrossRef]
- Fan, Y.; An, H.; Zhao, R. Double Traction Strategy Particle Swarm Optimization Algorithm. In Proceedings of the International Conference on Computers, Information Processing and Advanced Education CIPAE, Ottawa, ON, Canada, 16–18 October 2020; pp. 206–210. [Google Scholar] [CrossRef]
- Li, W.; Fan, Y.; Jiang, Q.; Xu, Q. Velocity-Driven Particle Swarm Optimization. In Proceedings of the 8th International Conference on Computing and Pattern Recognition ICCPR, Beijing, China, 23–25 October 2019; pp. 9–16. [Google Scholar] [CrossRef]
- Khan, H.; Nizami, I.F.; Qaisar, S.M.; Waqar, A.; Krichen, M.; Almaktoom, A.T. Analyzing Optimal Battery Sizing in Microgrids Based on the Feature Selection and Machine Learning Approaches. Energies 2022, 15, 7865. [Google Scholar] [CrossRef]
- Khanh, D.V.; Vasant, P.; Elamvazuthi, I.; Dieu, V.N. Optimization Of Thermo-Electric Coolers Using Hybrid Genetic Algorithm And Simulated Annealing. Arch. Control Sci. 2014, 24, 155–176. [Google Scholar] [CrossRef]
- Paszek, S.; Nocoń, A. Optimisation and Polyoptimisation of Power System Stabilizer Parameters; LAMBERT Academic Publishing: Saarbrücken, Germany, 2014. [Google Scholar]
- Wörner, L.; Kulig, S.; Willing, M.; Winzer, P. Genetic Algorithm Embedded into a Quality-Oriented Workflow of Methods for the Development of a Linear Drive used in Intralogistic Systems. Arch. Electr. Eng. 2014, 63, 647–665. [Google Scholar] [CrossRef]
- Pruski, P.; Paszek, S. Location of generating units most affecting the angular stability of the power system based on the analysis of instantaneous power waveforms. Arch. Control Sci. 2020, 30, 273–293. [Google Scholar] [CrossRef]
- Pruski, P.; Paszek, S. Calculations of power system electromechanical eigenvalues based on analysis of instantaneous power waveforms at different disturbances. Appl. Math. Comput. 2018, 319, 104–114. [Google Scholar] [CrossRef]
- Pershaanaa, M.; Bashir, S.; Ramesh, S.; Ramesh, K. Every bite of Supercap: A brief review on construction and enhancement of supercapacitor. J. Energy Storage 2022, 50, 104599. [Google Scholar] [CrossRef]
- Gómez, F.; Rosales, J.; Guía, M. RLC electrical circuit of non-integer order. Open Phys. 2013, 11, 1361–1365. [Google Scholar] [CrossRef]
- Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef]
- Freeborn, T.J.; Maundy, B.; Elwakil, A.S. Measurement of Supercapacitor Fractional-Order Model Parameters From Voltage-Excited Step Response. IEEE J. Emerg. Sel. Top. Circuits Syst. 2013, 3, 367–376. [Google Scholar] [CrossRef]
- Vicentini, R.; Da Silva, L.M.; Cecilio Junior, E.P.; Alves, T.A.; Nunes, W.G.; Zanin, H. How to Measure and Calculate Equivalent Series Resistance of Electric Double-Layer Capacitors. Molecules 2019, 24, 1452. [Google Scholar] [CrossRef] [PubMed]
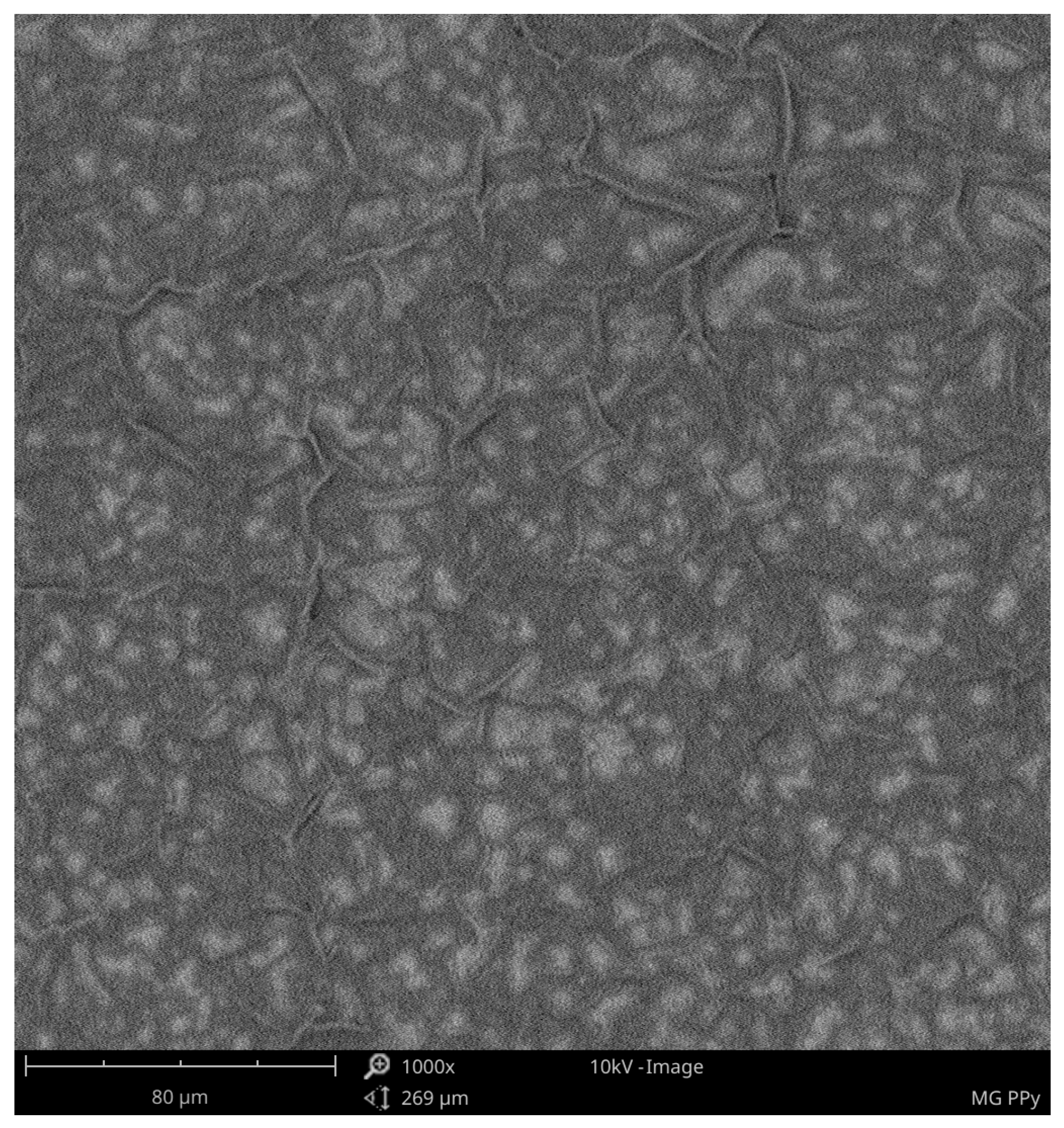
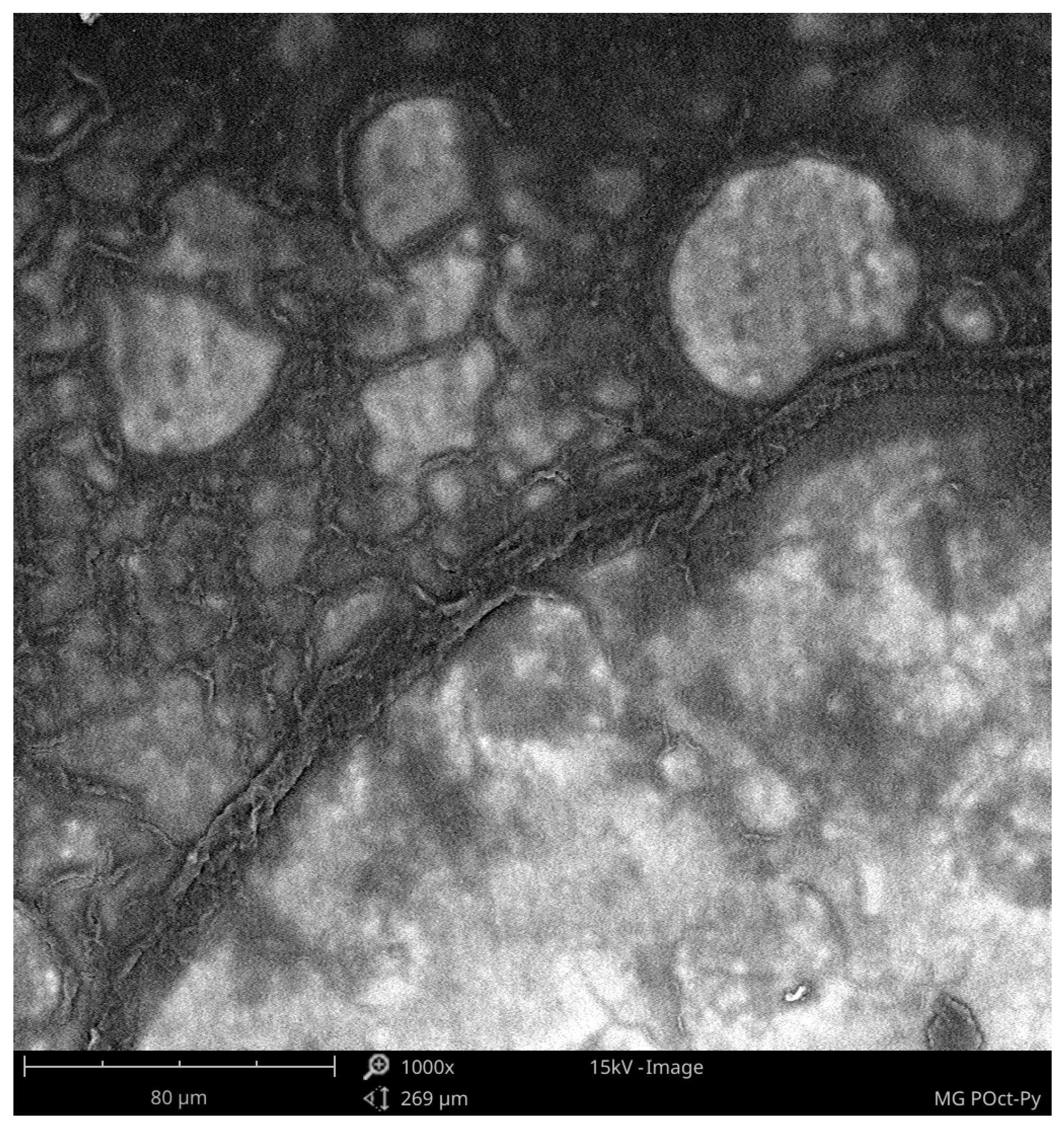


| Type of supercapacitors | Cp, F/g |
| lithium perchlorate (LiClO4) | |
| sym pp | 49.04 |
| sym po | 10.23 |
| asym p_w | 23.80 |
| asym o_w | 23.65 |
| tetrabutylammonium perchlorate (NBu4ClO4) | |
| sym pp | 39.56 |
| sym po | 8.05 |
| asym p_w | 22.95 |
| asym o_w | 19.60 |
| tetrabutylammonium triflate (NBu4CF3SO3) | |
| sym pp | 34.31 |
| sym po | 3.75 |
| asym p_w | 16.91 |
| asym o_w | 12.75 |
| lithium trifluoromethanesulfonate (LiCF3SO3) | |
| sym pp | 37.37 |
| sym po | 5.04 |
| asym p_w | 15.24 |
| asym o_w | 18.38 |
| k | ω0 | α | β | RC | error ε | |
| lithium perchlorate (LiClO4) | ||||||
| sym pp | 8424.3 | 185.56 | −0.56367 | 0.27405 | 146.85 | 0.058637 |
| sym po | 3828.4 | 259.3 | −0.45725 | 0.33503 | 76.65 | 0.16693 |
| asym p_w | 1352.5 | 1347.4 | −0.59429 | 0.29479 | 72.014 | 0.19888 |
| asym o_w | 1616.4 | 477.76 | −0.56389 | 0.2352 | 62.64 | 0.061291 |
| tetrabutylammonium perchlorate (NBu4ClO4) | ||||||
| sym pp | 1465.8 | 8.0341 | 0.70812 | 0.92687 | 56.177 | 0.40087 |
| sym po | 4031.3 | 10.966 | 0.62814 | 0.8955 | 70.833 | 0.61297 |
| asym p_w | 1609.4 | 9.8724 | 0.52107 | 0.80999 | 66.543 | 0.043923 |
| asym o_w | 2040.1 | 23691 | −1.7018 | 0.4795 | 97.362 | 0.61096 |
| tetrabutylammonium triflate (NBu4CF3SO3) | ||||||
| sym pp | 2167.6 | 2440.5 | −0.47945 | 0.39377 | 184.62 | 0.062268 |
| sym po | 3426.5 | 639.38 | −0.45559 | 0.28419 | 110.46 | 0.15369 |
| asym p_w | 2120.2 | 8898.6 | −0.7408 | 0.36878 | 117.53 | 0.35233 |
| asym o_w | 3299.4 | 349.14 | −0.54399 | 0.25386 | 142.63 | 0.065157 |
| lithium trifluoromethanesulfonate (LiCF3SO3) | ||||||
| sym pp | 11115 | 1237.4 | −0.20086 | 0.45873 | 103.74 | 0.23864 |
| sym po | 2494.6 | 1370.9 | −0.60748 | 0.34568 | 111.98 | 0.56962 |
| asym p_w | 1707.3 | 15.035 | 0.54098 | 0.81436 | 67.561 | 0.012915 |
| asym o_w | 1928.1 | 10.482 | 0.53368 | 0.81183 | 56.655 | 0.02344 |
| C | R1 | R2 | Z0 | B | error ε | |
| lithium perchlorate (LiClO4) | ||||||
| sym pp | 0.068338 | 13763 | 158.65 | 21198 | 70168 | 2.3005 |
| sym po | 0.11296 | 6441.9 | 73.134 | 7715.2 | 157040 | 1.7026 |
| asym p_w | 5.1356 × 10−4 | 892.3 | 69.498 | 6289.3 | 23706 | 1.2981 |
| asym o_w | 0.0013897 | 1552.3 | 58.296 | 7293.1 | 67880 | 1.6432 |
| tetrabutylammonium perchlorate (NBu4ClO4) | ||||||
| sym pp | 5.8999 × 10−4 | 216.53 | 100.34 | 8137.8 | 46950 | 0.16901 |
| sym po | 2.0493 × 10−4 | 419.49 | 105.75 | 11843 | 118380 | 0.32735 |
| asym p_w | 6.6418 × 10−4 | 424.6 | 104.02 | 2688.6 | 425780 | 0.14532 |
| asym o_w | 5.7118 × 10−4 | 973.77 | 99.314 | 5219.9 | 477820 | 0.17531 |
| tetrabutylammonium triflate (NBu4CF3SO3) | ||||||
| sym pp | 4.4654 × 10−4 | 1494.3 | 196.09 | 5923.3 | 99632 | 0.21563 |
| sym po | 2.5466 × 10−4 | 2527.3 | 104.72 | 13704 | 163990 | 1.1482 |
| asym p_w | 2.1319 × 10−4 | 909.13 | 126.47 | 9495.3 | 68987 | 0.39841 |
| asym o_w | 0.0017873 | 3603.9 | 131.39 | 12135 | 149330 | 1.5139 |
| lithium trifluoromethanesulfonate (LiCF3SO3) | ||||||
| sym pp | 1.5192 × 10−4 | 9991.4 | 116.73 | 18016 | 2054400 | 0.14238 |
| sym po | 1.9457 × 10−4 | 1282.7 | 112.81 | 9213.3 | 131880 | 1.0919 |
| asym p_w | 6.8501 × 10−4 | 839.92 | 115.35 | 1092.4 | 199870 | 0.098391 |
| asym o_w | 5.502 × 10−4 | 448.69 | 92.663 | 3060.1 | 467300 | 0.15339 |
| Traditional Approach | Authors’ Approach |
|---|---|
| The mechanism of storing electrical energy is based on an electrical double Helmholtz layer, which is formed at the interface between the electrolyte and the electrode [1,7,44]. | The mechanism of storing electrical energy is faradaic and based on a double Helmholtz layer and the reduction and oxidation reactions of conductive polymers [10,14,15,16,44]. |
| The parameters of supercapacitors (such as capacitance C and internal series resistance ESR) are determined based on charge and discharge waveforms by a constant current [18,19,45,46,47]. | The parameters of supercapacitors have been estimated on the basis of the frequency characteristics obtained using the electrochemical impedance spectroscopy (EIS) method [19,21,22]. The parameters are of fractional order [24,25,26,27]. |
| The series internal resistance ESR is usually determined by the voltage drop across a pre-charged supercapacitor over several tens of minutes [46,47,48]. | The series internal resistance ESR has been defined as the impedance value for high-frequency values when the capacitive part of the impedance tends to zero, within the use of the Particle Swarm Optimization for the determination of the model parameters [20,32,48]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gocki, M.; Jakubowska-Ciszek, A.; Pruski, P. Comparative Analysis of a New Class of Symmetric and Asymmetric Supercapacitors Constructed on the Basis of ITO Collectors. Energies 2023, 16, 306. https://doi.org/10.3390/en16010306
Gocki M, Jakubowska-Ciszek A, Pruski P. Comparative Analysis of a New Class of Symmetric and Asymmetric Supercapacitors Constructed on the Basis of ITO Collectors. Energies. 2023; 16(1):306. https://doi.org/10.3390/en16010306
Chicago/Turabian StyleGocki, Michał, Agnieszka Jakubowska-Ciszek, and Piotr Pruski. 2023. "Comparative Analysis of a New Class of Symmetric and Asymmetric Supercapacitors Constructed on the Basis of ITO Collectors" Energies 16, no. 1: 306. https://doi.org/10.3390/en16010306
APA StyleGocki, M., Jakubowska-Ciszek, A., & Pruski, P. (2023). Comparative Analysis of a New Class of Symmetric and Asymmetric Supercapacitors Constructed on the Basis of ITO Collectors. Energies, 16(1), 306. https://doi.org/10.3390/en16010306







