Abstract
Nitrogen reduction reaction (NRR) and nitrate reduction reaction (NO3−RR) provide a potential sustainable route by which to produce ammonia, a next-generation energy carrier. Many studies have been conducted over the years, mainly emphasizing material design and strategies to improve catalytic performance. Despite significant achievements in material design and corresponding fundamental knowledge, the produced ammonia is still very limited, which makes it prone to bias. The presence of interferants (e.g., cations and sacrificial reagents), the pH of the solution, and improper analytical procedure can lead to the over or underestimation of ammonia quantification. Therefore, the selection of the appropriate ammonia quantification method, which meets the sample solution condition, along with the proper analytical procedures, is of great importance. In this review, the state-of-the-art ammonia quantification method is summarized, emphasizing the advantages, limitations, and practicality for NRR and NO3−RR studies. Fundamental knowledge of the quantification method is introduced. Perspective on the considerations for selecting the suitable quantification method and for performing the quantification process is also provided. Although non exhaustive, this focused review can be useful as a guide to design the experimental setup and procedure for more reliable ammonia quantification results.
1. Introduction
Ammonia (NH3) is an essential feedstock chemical for the fertilizer, pharmaceutical, and nitrogen-containing chemicals industries. Today, it is also considered a next-generation energy carrier due to its high energy density for hydrogen (17.6%) [1,2,3,4,5,6]. Up to now, ammonia production has heavily relied on the conventional Haber-Bosch process, which requires a high energy supply (temperature 400–500 °C and pressure 100–200 atm) [7,8,9], and is responsible for 1.2% of global CO2 emissions [10,11,12,13]. Nitrogen reduction reactions (NRR) and nitrate reduction reactions (NO3−RR) to ammonia have attracted increasing attention because they can be performed under ambient conditions and are potentially more sustainable [14,15,16]. NRR and NO3−RR can be performed in photocatalytic (PC), electrocatalytic (EC), or photoelectrocatalytic (PEC) systems [17,18,19,20,21,22,23,24]. In the last five years, publications relating to this field have significantly increased, indicating the noticeable potential of these processes (Figure 1).
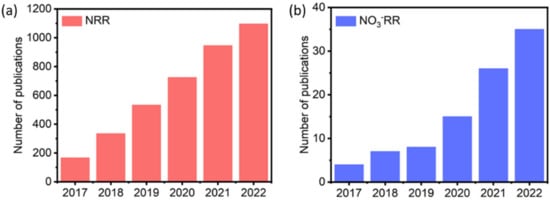
Figure 1.
Number of publications in (a) NRR and (b) NO3−RR studies. Data were obtained from the Web of Science based on an article title of “nitrogen reduction reaction” and “nitrate reduction reaction”, respectively.
The advancement of the photo(electro)catalytic NRR and NO3−RR has been reported by many researchers over the years [25,26,27,28]. Most of the works have emphasized material design and strategies to improve catalytic performances, i.e., ammonia production rate and selectivity. For example, Utomo et al. reported that copper loading on the surface of oxygen-deficient TiO2 could enhance its catalytic performance over EC-NRR due to a strong metal support interaction (SMSI) between the copper nanoparticles and TiO2 substrates [28]. The SMSI led to an enlarged electrochemically active surface area, increased electron density, and promoted electron transfer and nitrogen activation, which resulted in an enhanced NH3 yield of 13.6 µg mgcat−1 h−1 ( mg L−1 NH3-N) at −0.5 V vs. RHE compared to the bare TiO2. In PC-NRR, Liu et al. reported the deposition of the Bi4O5Br photocatalyst on the surface of hydrophobic zeolitic imidazolate framework-8 (ZIF-8), which created the triphasic contact between nitrogen gas, liquid (water-based) electrolyte, and the solid photocatalyst [29]. The hydrophobic nature of ZIF-8 retarded the diffusion of the liquid (water-based) electrolyte and facilitated the direct contact between the nitrogen gas with the Bi4O5Br photocatalyst. As a result, ammonia generation reached 327.388 µmol gcat−1 h−1 ( mg L−1 NH3-N), which was 3.6-fold higher compared to pristine Bi4O5Br. Recently, Cu-based materials have also been reported as promising catalysts in EC-NO3−RR. A Cu/Cu2O catalyst showed a high ammonia yield of up to 0.2449 mmol·cm−2 h−1 ( mg L−1 NH3-N) at −0.85 V vs. RHE. The high performance was attributed to the electron transfer from Cu2+ to Cu0, which facilitated the formation of NOH intermediate (*NOH) as one of the key intermediates and suppressed the hydrogen evolution reaction [30].
Regardless of the significant achievements in material design, and crucially fundamental knowledge that has been established, production rates and selectivity of the current catalytic system are still far from gaining industrial interest. For instance, the production rate of NRR mostly ranges from a few to 35 µg mgcat−1 h−1, which is approximately still at the micromolar level [25]. In comparison, the NO3−RR performs better, with production rates reaching the millimolar level [31]. The wide range of ammonia production leads to the need for compatible quantification methods. The low ammonia production rates make it prone to interference from impurities in the catalytic system, which deviates the measured ammonia from its true value [32]. Moreover, the ammonia quantification, which is performed without carefully considering the condition of the sample solution and the specification of the quantification method, may raise concerns about the reliability of the measurements.
Several ammonia quantification methods have been used in many applications. Spectrophotometric methods, better known as the indophenol blue and Nessler’s method, are two typical standard ammonia detection methods widely used in water quality control [33]. Today, these methods were also frequently used in NRR and NO3−RR. The ion chromatography (IC) and 1H nuclear magnetic resonance (1H NMR) methods have also been used as primary or supporting detection methods in NRR [25,31]. Other methods, such as ion selective electrodes (ISE) and fluorometric methods, are also known for ammonia detection, but application is still limited in terms of water quality control [14]. The selection of the most suitable ammonia quantification method depends on the catalytic system under investigation and the specification of each method. Some papers have reported the limitations of spectrophotometric and IC methods for NRR studies by subjecting the methods to some possible interferants, such as pH, cations, and sacrificial reagents [34,35]. Some reviews have also introduced several ammonia detection methods as an additional part of their discussion [14,25,31,36]. However, to the best of our knowledge, a focused review concentrating on ammonia quantification methods in NRR and NO3−RR is still under-discussed. Therefore, it is timely to insightfully discuss the ammonia quantification methods and their compatibility with the conditions of the sample solutions obtained from NRR or NO3−RR works.
In this focused review, we summarize the state-of-the-art ammonia quantification methods, emphasizing the advantages, limitations, and practicality for NRR and NO3−RR. We introduce fundamental knowledge for a better understanding of each method. We also provide our perspective on the considerations for selecting the suitable detection methods based on the sample solution’s condition and for conducting the measurement process. Therefore, a proper ammonia quantification procedure can be designed, which makes the results more convincing and reliable.
2. Ammonia in Solution
Ammonia is a polar molecule that can dissolve in water due to its high solubility (482 g L−1 at 24 °C). In an aqueous solution, ammonia can simultaneously exist in two forms of species, either in the form of unionized ammonia (NH3) or ammonium ion (NH4+). These two species are in equilibrium (Equation (1)) and the equilibrium can be shifted to a specific species by changing the temperature or pH value. As shown in Equations (2) and (3), the fraction of unionized NH3 increases with increasing temperature and pH.
where pKa is the dissociation constant of the NH4+ ion and T is the temperature (°C) [31]. At 25 °C, NH4+ ions are the predominant species at a pH lower than 9.25, while the unionized NH3 becomes a dominant species at a pH higher than 9.25 (Figure 2) [37,38,39]. Because of this property, the determination of ammonia is mostly performed in the aqueous solution. In this case, a certain amount of acid or alkaline solution can be added to the solution to shift the equilibrium toward unionized NH3 or NH4+ ions. In NRR and NO3−RR, either unionized NH3 or NH4+ can be used to represent the total ammonia production rate.
pKa = 0.09018 + 2729.92 (273.15 + T)−1
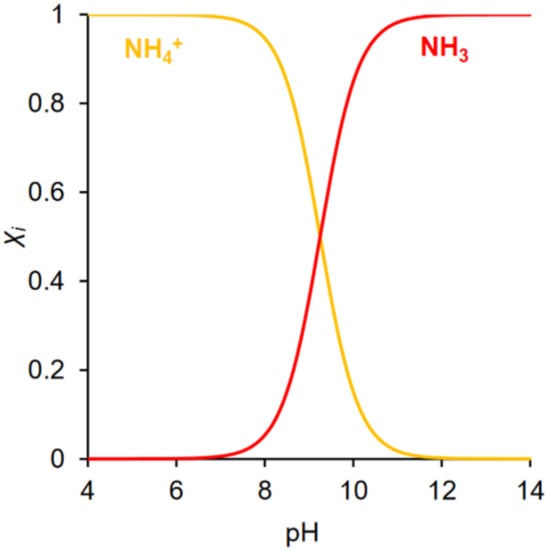
Figure 2.
The molar fraction of NH4+ ions and unionized NH3 as a function of pH. Adapted from Ref. [39] Copyright 2022, MDPI.
3. Ammonia Quantification Methods
There are several methods and analytical instruments that can be used for ammonia quantification. These include, but are not limited to, spectrophotometric methods, IC, ISE, fluorometric, enzymatic, conductivity, and titrimetric methods. Among these methods, spectrophotometric methods are widely used for ammonia quantification in NRR and NO3−RR due to their high accuracy, low detection limits, reproducibility, and simplicity. In recent years, the IC method and 1H NMR have also been used. Combining with 15N2 isotopes as a feed gas during the NRR, the 1H NMR method can verify the origin of the produced ammonia. In the following discussion, ammonia quantification methods will be discussed in detail, focusing on the spectrophotometric methods, IC, 1H NMR, ISE, and fluorometric methods as the major quantification methods used in NRR. Meanwhile, additional methods will also be introduced to give a broader perspective on ammonia quantification.
3.1. Spectrophotometric Method
The spectrophotometric method is a facile and inexpensive analytical technique to measure the absorbance or transmittance of light. In ammonia-related fields, this method was initially used for ammonia quantification in water quality control [33]. Today, this method is also widely used for ammonia quantification in NRR and NO3−RR because it has high accuracy, low detection limits, and good repeatability [15,40].
The spectrophotometric method involves the absorbance of ultraviolet-visible (UV-vis) light by molecules. The UV light covers wavelengths from 100–380 nm, while the visible light ranges up to 800 nm. Most UV-vis spectrophotometers possess a working wavelength from 200–1100 nm. However, in many ammonia-related fields, including NRR and NO3−RR, the practical wavelength ranges from 200 to 800 nm, in which the specific absorption wavelengths used for quantification vary depending on the color of the compound in the solution. In this regard, compounds having a conjugated unsaturated covalent bond that can absorb UV-vis light at a particular wavelength are called chromophores, and they confer color of the substance. Water and alcohol are considered excellent media for UV-vis measurements since they are transparent and do not absorb UV-vis light. In comparison, when dealing with compounds that are not dissolved in water or alcohol, acetone and dimethylformamide (DMF) can be alternative solvents. However, both acetone and DMF absorb light below 320 and 275, respectively [41].
UV-vis spectroscopy is based on the electronic transition of molecules that absorb light [42]. The light absorption results in the excitation of electrons from the ground state or lower energy orbital (highest occupied molecular orbital, HOMO) to the excited state or higher energy orbital (lowest unoccupied molecular orbital, LUMO). The energy for the absorbed wavelength is equal to the energy gap between HOMO and LUMO. The relationship between the light absorption with the concentration of molecules is correlated by the Beer-Lambert law, as shown in Equation (4).
For a given wavelength, A is the light absorbance, which is proportional to the l, length of sample cell (cm), c, molar concentration of the solute, and ε, molar absorptivity, which is specific for every compound. During the UV-vis measurement, the light intensity of the sample solution, I, is measured with respect to the intensity of the reference, I0 [43]. The concentration of the sample solution is then determined after calibration using a series of standard solutions [41]. Notably, the l and ε are the same between the sample solution and the standard solutions used for calibration.
Technically, a UV-vis spectrophotometer directs light to a sample, and the transmitted light is detected by the detector on the opposite side (Figure 3a). The transmittance indicates the portion of light that is being absorbed by the sample at each wavelength. The absorbance values are then plotted (y-axis) as the function of wavelength (x-axis) in a UV-vis spectrum. The wavelength with the highest peak (λmax) is typically used for quantification [41,42]. Spectrophotometric ammonia determination can be divided into two specific methods, i.e., indophenol blue method and Nessler’s method, which will be discussed in detail in the following sections.
3.1.1. Indophenol Blue Method
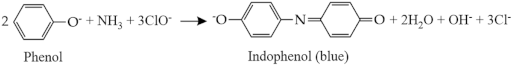
The indophenol blue method is based on the reaction of ammonia with hypochlorite and phenol in an alkaline condition, which produces blue-colored indophenol. This reaction is called the Berthelot reaction, and because it involves the use of phenol, the indophenol blue method is also known as the phenate method. The reaction in the indophenol blue method proceeds via several steps, including the reaction between ammonia and hypochlorite at pH 9.7–11.5 as the first step. This reaction produces monochloramine, which further reacts with the phenol, producing quinone chloramine. The formation of quinone chloramine is facilitated by the addition of sodium nitroprusside or sodium nitroferricyanide as a catalyst. Subsequently, the quinone chloramine further reacts with phenol to form indophenol, which dissociates in the alkaline solution resulting in a blue color. Therefore, the indophenol product can be quantitatively determined using a UV-vis spectrophotometer at a wavelength of 630–655 nm. The overall process of the reaction in the indophenol blue method is presented in Equation (5) [44].

Modification of the indophenol blue method was then developed, specifically by substituting the phenol using sodium salicylate or salicylic acid. This modified method is also known as the “salicylate method”. In addition to being less harmful than phenol, the use of salicylate compounds is to avoid the formation of a harmful substance, such as o-chlorophenol, during the process. However, because of the lower reactivity of the salicylate compared to phenol, the salicylate method requires a much higher concentration of salicylate to reach a similar sensitivity as phenol. The reaction involves the same reaction step between the ammonia and hypochlorite to form monochloramine, as in the original indophenol blue method. However, in the salicylate method, the monochloramine reacts with salicylate, producing 5-aminosalicylate. The 5-aminosalicylate is oxidized, and it further reacts with salicylate, forming a colored compound. The apparent color may be varied depending on the ammonia concentration in the sample. The color may change from yellow to green and then to blue, along with increasing ammonia concentration (Figure 3b,c). The overall reaction is shown in Equation (6).
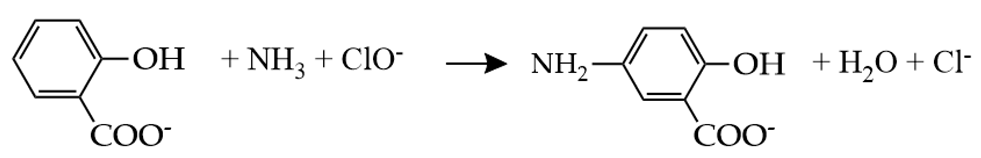

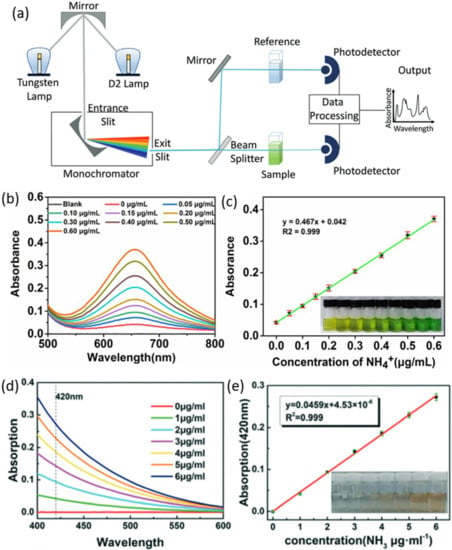
Figure 3.
(a) Schematic illustration of UV-vis spectrophotometer for double beam instrument. The tungsten lamp and D2 lamp emit visible and ultraviolet lights, respectively. The light is directed to the monochromator, which determines the wavelength of the sample. Adapted with permission from Ref. [41] Copyright 2018, Wiley-VCH. Ammonia determination using indophenol blue method, (b) UV-vis spectra of standard solutions with different concentrations, and (c) corresponding calibration curve. The absorbance values for the calibration curve were obtained from a wavelength of 655 nm. Inset of (c) is the photograph of the standard solutions after 2 h of reaction with indophenol reagent. Adapted with permission from Ref. [45] Copyright 2021 Royal Society of Chemistry. Ammonia determination using Nessler’s method. (d) UV-vis spectra of the standard solution with different concentrations, and (e) corresponding calibration curve. The absorbance values for the calibration curve were obtained from the wavelength of 420 nm. Inset of (e) is the photograph of the standard solution after 20 min of reaction with Nessler’s reagent. Adapted with permission from Ref. [46] Copyright 2020, Royal Society of Chemistry.
In both the indophenol blue method and the salicylate method, citrate buffer is often used to stabilize the pH and prevent the precipitation of hardness cations, such as magnesium or calcium ions, at a high pH [35,47]. The indophenol blue method and the salicylate method are able to measure ammonia at low concentrations (0–0.6 mg L−1 of NH3-N) with good accuracy and repeatability [14]. However, it takes a relatively long time for the samples to be ready for measurement (1–2 h). It is worth noting that most of the NRR and NO3−RR reports employed the salicylate method to quantify the produced ammonia, according to the reagent that they used (they used a salicylate compound instead of phenol). However, they referred to their method as the indophenol blue method instead of the salicylate method, possibly due to the origin of the salicylate method derived from the indophenol blue method and the same principle between them, or just for simplification purposes. Therefore, we will use the term indophenol blue method in the following discussion.
3.1.2. Nessler’s Method
Nessler’s method refers to the reagent being used, i.e., Nessler’s reagent. This reagent consists of mercury (II) iodide or potassium iodide in an alkaline solution, either in sodium hydroxide or potassium hydroxide. Upon reaction with ammonia, the Nessler’s reagent forms a yellow-colored compound, which can be determined using a spectrophotometer at wavelengths of 410–425 nm. The color intensifies from yellow to yellow-orange/reddish-brown along with an increase in ammonia concentration (Figure 3d,e) [47]. The overall reaction is shown in Equation (7).
The detection of ammonia using Nessler’s method can be interfered with by various metal cations, especially the hardness metal cations. The metal cations may precipitate as hydroxides in the high alkaline solution, which interfere with the spectrophotometric measurements. The typical approach to remove the turbidity in the sample solution is by adding zinc sulfate and sodium hydroxide, followed by filtration. However, this process is relatively tedious and time-consuming. Alternatively, a Rochelle salt (potassium sodium tartrate) can be put in to prevent turbidity.
Nessler’s method can detect ammonia concentrations at a comparable detection limit to the indophenol blue method (0.025–5.0 mg L−1 of NH3-N), but with a relatively faster process [14]. Due to the sensitivity of this method to the reaction time, the measurement of the colored sample using a spectrophotometer is recommended to be performed around 10–30 min after mixing the sample solution with Nessler’s reagent for an accurate result. However, one should note that Nessler’s method employs a mercury-containing chemical, which is toxic and harmful. Therefore, the reagent and the related samples should be properly handled, stored, and disposed of [35,47].
3.2. IC Method
Ion chromatography is a separation method that works based on the interaction between polar molecules or ions with the ion exchanger (stationary phase) and an eluent (mobile phase) [48]. In NRR and NO3−RR, the cationic exchangers, which possess a negatively charged functional group, are used because the ammonia is detected in the form of NH4+ ions. The cationic exchangers are embedded in the column. A low-capacity ion-exchange column allows the use of the dilute ionic solution as an eluent. This eluent has an adequately low conductivity background. Therefore, the conductivity of the ions can be directly measured. This technique is known as the non-suppressed IC. Meanwhile, if the eluent is diluted so it converts to a neutral form after the separation, then the technique is called suppressed IC. Both techniques demonstrate high sensitivity up to mg L−1 level with the ability to detect multiple ions within minutes [49].
The primary equilibrium in the IC is the ion-exchange displacement of an eluent ion (E) that is initially attached to the stationary phase (denoted by the subscript r) by an analyte (A) in the mobile phase (denoted by subscript m). The equilibrium for cation exchange of a single charged ion is shown in Equation (8).
The charge of the cation is balanced by anions (of the same charge as cations) in the mobile phase. However, this anion does not involve the cation exchange process. The common eluent for cation exchange is H+, coming from sulfuric acid, citric acid, nitric acid, or tartaric acid. Specifically, for suppressed IC, sulfuric acid or methanesulfonic acid (MSA) is usually used. In IC, the selectivity is mostly altered by modifying the nature of the stationary phase or by just using the suitable stationary phase. In this case, the selectivity in IC can be described as the tendency of the exchange process between the ion in the analyte of interest, and the eluent ion in the stationary phase. Cation exchange selectivity is commonly altered using different types of functional groups, such as sulfonate, carboxylate, and phosphate [49].
A typical IC instrument includes a pump, an injector, a column, a suppressor, and a recorder or data system (Figure 4a). Ion exchange separation mainly occurs in the column packed with an ion exchanger, which is commercially available. When the samples containing certain cations are injected into the chromatography instruments, the cations flow with the eluent through a column. The speed of the cations flowing through the column differs depending on their interaction with the ion exchanger, and they are then separated. The conductivity detector is then usually used to detect the separated cation, which results in a series of peaks with different retention times. Each cation is identified based on its retention time, and the area of the peak represents the concentration of the cation (Figure 4b). In this case, the peak area is proportional to the concentration of the cation. By comparing the obtained peak area with the standard calibration curve, the concentration of the cation can be quantified (Figure 4c,d).
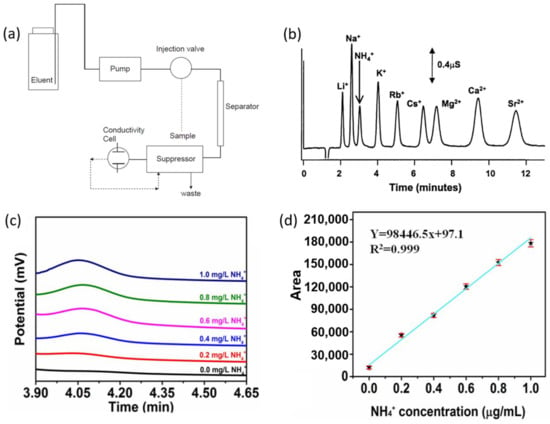
Figure 4.
(a) Schematic diagram of standard IC instrument. Adapted from Ref. [50] Copyright 2017, Elsevier. (b) High-speed separation of nine common cations at 27 °C. Experiment was performed using Dionex CS-12A cation exchange column (carboxylate-functionalized cation-exchange column), eluent: 17 mM MSA at 0.5 mL/min, 20 µL injection, 50 µM analyte concentration. Adapted with permission from Ref. [51] Copyright 2003, Elsevier. (c) Chromatogram of NH4+ standard solutions with various concentrations and (d) corresponding calibration curve. Adapted with permission from Ref. [45] Copyright 2021, Royal Society of Chemistry 2021.
Compared to the spectrophotometric method, the IC method has some advantages. First, the IC method can measure NH3-N concentrations ranging from 0.02 to 40 mg L−1 [14,25]. Moreover, in a single operation process, it can simultaneously detect multiple cations with high sensitivity and repeatability. Second, it has high selectivity because it can quantify inorganic and organic cations by adjusting the parameter and operational procedure. Third, many types of cations can be detected and quantified in one running process [35]. Unfortunately, the peaks originating from the cations with closed retention times may overlap with each other, which interferes with the detection. For example, the peaks of NH4+ ions may overlap with the peaks of Na+, K+, and Li+, which limits its application for NH4+ quantification in electrolytes containing those ions [14,47]. Therefore, other approaches are needed to improve the resolution of the peaks; for example, by using a column with a higher cation-exchange capacity or applying a column-switching technique to improve the separation of NH4+ and Na+ peaks [31].
3.3. 1H NMR Method
Many nuclear isotopes, including 1H, 14N, and 15N, are magnetically active with non-zero spin and the NMR instruments measure the radio frequency (RF) associated with those nuclei. Similar to other spectroscopy methods, the NMR analyzes the structure or chemical composition of molecules based on their absorption of electromagnetic radiation. In this case, the NMR specifically works at the radio-wave frequency, which affects a transition in the nuclear spin level.
Many atomic nuclei have a property called spin, by which we can illustrate as if they are spinning. The energy of spin states is not equal in an applied magnetic field. Because nuclei are charged particles, they have their own magnetic moments (µ), which are generated by their charge and spin. In an applied magnetic field, there are two magnetic moment alignments of nuclei. The alignment can be in the same direction (+½ spin state) or in the opposite direction (−½ spin state) relative to the magnetic field direction. The +½ spin state has a lower energy level [43]. The resonance occurs when the nuclei with the same alignment as the applied magnetic field absorb energy, which changes their orientation (Figure 5a). The absorbed energy must be equal to the difference between the two states, as shown in Equation (9).
where is the energy absorbed by the nuclei, is the energy of −½ spin state (opposite direction against the magnetic field direction), is the energy of +½ spin state (same direction as the magnetic field direction), is the Planck constant, and is the frequency of the electromagnetic radiation [43].
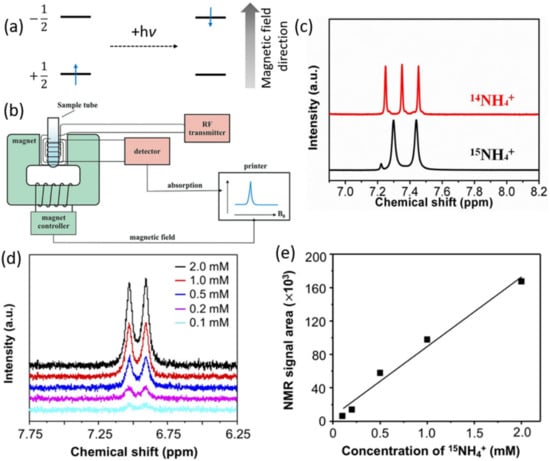
Figure 5.
(a) Absorption process in NMR. (b) Schematic representation of a typical NMR spectrometer. Adapted with permission from Ref. [52] Copyright 2019, Georg Thieme Verlag KG. (c) 1H NMR spectra of 14NH4+ and 15NH4+ produced from NRR reaction using 14N2 and 15N2 isotopes. Adapted with permission from Ref. [45] Copyright 2021, Royal Society of Chemistry. (d) 1H NMR spectra of 15NH4+ standard solutions with various concentrations and (e) corresponding calibration curve. Adapted with permission from Ref. [53] Copyright 2020, American Chemical Society.
In practice, energy difference in the energy levels (and therefore, the nuclei population) is a function of the applied magnetic field. The stronger the magnetic field, the higher the energy difference between the possible spin states. In an applied magnetic field, the nuclei precess in their axis with various alignments. The frequency at which a nucleus precess is proportional to the strength of the applied magnetic field. In this case, each isotope has its characteristic frequency. For example, in a 7.5 T of magnetic field, the 1H nucleus precesses at 300 MHz, while at 21.1 T, 1H precesses at 900 MHz [54].
The resonance occurs when external electromagnetic radiation matches with the nucleus precession. As also shown in Figure 5a, during this resonance process, the spin alignment of the nuclei changes from +½ spin state to −½ spin state, which increases the population of higher energy spin states. This process continues until the population of the high energy spin states is equal to the lower energy spin states. This condition is called saturation, which should be avoided as no net signal can be observed. At this stage, external electromagnetic radiation is turned off. Therefore, the resonance stops. With cessation of resonance, the system changes back to the initial condition, where the high energy spin state (−½) nuclei relax to the lower energy (+½) spin state. The relaxation process is accompanied by RF emission. Therefore, the detector can record the intensity of RF from each nucleus. This process is reiterated, which strengthens the signal from each nucleus and reduces the noise. The software then calculates peak properties, such as chemical shift, intensity, multiplicity, and J-coupling (Figure 5b) [43,54].
In NRR and NO3−RR, 1H NMR is mostly used, instead of 15N NMR, to detect 15NH3 because of the extremely low gyromagnetic ratio of 15N. In this case, 1H NMR can differentiate the 15NH4+ and 14NH4+ because 14NH4+ is a spin-1 nucleus and 15NH4+ is a spin-½ nucleus. Therefore, the coupling between 1H and 15N in 15NH4+ will generate a doublet signal with a spacing of 73 Hz. Meanwhile, the coupling between 1H and 14N in 14NH4+ results in a triplet signal with a spacing of 52 Hz (Figure 5c) [36,47,53].
Measurement using 1H NMR in NRR usually aims to confirm the origin of the produced ammonia. Before 1H NMR detection, the NRR experiments are first performed by using the 15N2 as an N source instead of 14N2. Therefore, the detection of 15NH3 in NMR spectra verifies that the produced ammonia is originated from N2 gas instead of other impurities. In addition to confirming the origin of ammonia qualitatively, more recently, 1H NMR was also used for 15NH3 quantification, as reported by Jang et al. For this purpose, measurements of a series of standard solutions were performed. The peak area of the 1H NMR spectra was then plotted as a function of concentration to obtain the calibration curve (Figure 5d,e). Prior to measurements, the sample solutions were acidified to reach a pH of 3 by adding HCl to convert 15NH3 to 15NH4+ [53].
The 1H NMR method offers high sensitivity, appreciable repeatability, and straightforward discrimination against the contaminant ammonia [34]. Furthermore, it does not need any advanced chemical manipulation, as required in the spectrophotometric method. However, it requires complex and expensive instrumentation.
3.4. ISE Method
There are typically two types of electrodes that can be used to measure the dissolved ammonia; i.e., an ammonium ion-selective electrode and an ammonia gas sensing electrode. As its name suggests, the ammonium ion-selective electrode (NH4+-ISE) measures NH4+ ions dissolved in the solution. It measures the potential difference between the reference electrode and the ion electrode (Figure 6a) [55]. This electrode is equipped with an NH4+ ion-specific membrane, typically a polyvinylchloride (PVC) membrane. Before performing ammonia detection using NH4+-ISE, the sample solution is acidified to convert NH3(aq) into NH4+ ions. When the electrode is placed into the sample solution, the potential in the ion electrode develops against a reference electrode (typically Ag/AgCl) [56]. The value of the potential is proportional to the concentration of the NH4+ ion in the solution, in accordance with the Nernst equation (Equation (10)). The concentration of NH4+ can be quantified using the calibration curve.
where E is the measured voltage (V), E0 is the reference constant (V), R is the universal gas constant (J mol−1 K−1), T is the temperature (K), z = charge of ion, F is the Faraday constant (C mol−1), and Q is the reaction quotient of the cell reaction [57].
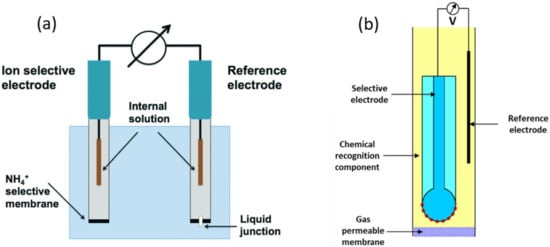
Figure 6.
Schematic diagram of (a) NH4+ ion selective electrode. Adapted with permission from Ref. [57] Copyright 2019, Royal Society of Chemistry. (b) A typical membrane-based electrochemical gas sensor. For the ammonia gas sensing electrode, the pH electrode is typically used as a selective electrode. Adapted from Ref. [58] Copyright 2017, Elsevier.
NH4+-ISE has a wide range of detection limits: 0.06–20,000 mg L−1 NH3-N (Radiometer Analytical ISE25NH4-9) [14]. This value varies for each commercial product. The major limitation of NH4+-ISE is disturbance from the cations, which have similar behavior to ion electrodes, such as Na+ and K+. Hence, the other cations can be detected as ammonium, which results in a false positive. The interference by the other cations can be quantified based on the cross-sensitivity ratio, as shown in Equation (11).
where is the NH4+ ions concentration (mg L−1), is the concentration of interference cation, and CSR is the cross-sensitivity ratio. The CSR value for K+ is 1:(15–30) against NH4+. This CSR value means that 20 mg L−1 of K+ ions will have the same potential difference as 1 mg L−1 NH4+. Meanwhile, Na+ has a CSR value of 1:(1000–1300) against NH4+. The interference of Na+ to NH4+ is estimated in the same way as K+ [57].
The ammonia gas-sensing electrode measures the dissolved ammonia in the form of unionized NH3. It has a wide detection range of 0.01–17,000 mg L−1 NH3-N (Orion 9512HPBNWP) and the detection range can be varied in different commercial products [14]. The electrode consists of a pH electrode, a hydrophobic gas-permeable membrane, and a reference electrode (Figure 6b) [58]. It typically uses ammonium chloride solution as an internal solution. The gas permeable membrane separates this internal solution to the sample solution. Prior to ammonia detection, a strong base is added to the sample solution to reach a pH value higher than 11. Therefore, the dissolved NH4+ ions in the sample solution are converted into dissolved NH3(aq), which then diffuse through the membrane to the internal solution until the NH3 partial pressure of both the sample solution and the internal solution reaches equilibrium. The diffusion of NH3(aq) from the sample solution to the internal solution changes the pH of the internal solution, and these pH changes can be detected using a pH electrode [14,31]. In this regard, the pH change is proportional to the concentration of the NH3(aq) in the sample solution [44]. However, because the gas-sensing electrode detects the ammonia in the form of dissolved ammonia gas (NH3(aq)), there is a possibility that the NH3(aq) can be released from the sample solution. Therefore, it is recommended to have a good sealing between the internal solution and sample solution, or to use a closed container with a small opening to tackle the NH3(aq) releasing problem. Moreover, the performance of the ammonia gas sensing electrode is highly affected by the concentration of dissolved ammonia and the dissolved ions. It slowly works in a low concentration of ammonia. However, turbidity or color stability, which can be problems in the spectrophotometer method, do not influence the performance of the gas-sensing electrode [31].
Both NH4+-ISE and the NH3 gas-sensing electrode provide a facile and rapid process for ammonia determination. They are also able to measure ammonia in a much wider range of concentrations. However, their detection accuracy, precision, and repeatability in low ammonia concentrations (i.e., <0.5 mg L−1 NH3-N) is lower than in higher concentrations. Furthermore, because of the deterioration of the membrane, the lifetime of the ISE is limited to between months to a year [14].
3.5. Fluorometric Method
The fluorometric method offers high sensitivity for ammonia detection at nanomolar concentrations (1.0 × 10−4–2.8 × 10−3 mg L−1 NH3-N) [14]. This method is based on the fluorescence process of molecules. When molecules are subjected to light, the molecules absorb light (excitation), which changes their electronic state from the ground state (S0) to one of the vibrational levels of the excited electronic state (S1). The fluorescence process occurs when the electron relaxes from the singlet excited state (S1) to the singlet ground state (S0), which is accompanied by light emission (Figure 7a) [59]. The lifetime of fluorescence is very short (1 × 10−9 to 1 × 10−7 s). The energy (or wavelength) of the emitted light from the fluorescence process is determined by the energy difference between the ground state (S1) and the singlet excited state (S1), and is also influenced by the energy lost due to vibrational relaxation. This relationship is shown by Equation (12).
where is the energy of emitted fluorescence, is the absorbed energy, is the vibrational relaxation energy, and is the energy associated with the solvent cage of molecules to reorient itself in the state. Moreover, due to the energy loss, the wavelength of the emitted fluorescence shifts to a lower energy (longer wavelength) than the excitation wavelength (Stokes shift) [60].
Ammonia detection using the fluorometric method employs o-phthaldialdehyde (OPA) and sulfite as the reagents, which react with ammonia forming a highly fluorescent isoindole derivative (Equation (13)) [61,62,63,64]. The fluorescent product has maximum excitation and emission wavelengths at 362.5 and 423.0 nm, respectively (Figure 7b) [63,65]. The fluorescence detector is then used to detect the emitted fluorescence signal, which is proportional to the concentration of ammonia.
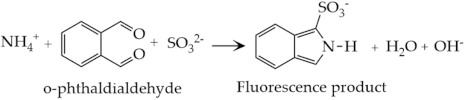

Practically, in a typical flow injection fluorescence system (Figure 7c), the sample is injected to the sample loops, where the carrier facilitates the sample to react with OPA and sulfite, producing fluorescence products in the reaction coil. The product is then carried to the detector [66]. Note that the presence of amines and amino acids in the solution interferes with the ammonia detection, since both compounds may also react with the OPA, producing fluorescence products [62]. To avoid such interference, a purge-and-trap strategy can be helpful. In this technique, the sample solution is added with sodium hydroxide to convert all NH4+ ions to NH3(aq). Subsequently, the solution is purged with argon gas to release the NH3(aq). The released NH3 is then trapped in a hydrochloric acid solution. The NH4+ concentration in the hydrochloric acid solution is then determined using the fluorometric method [66].
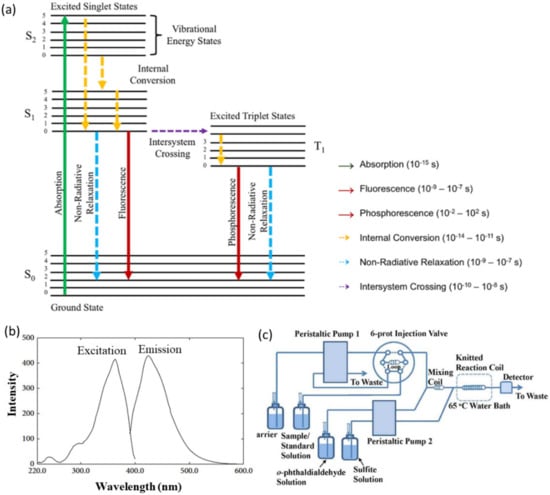
Figure 7.
(a) Jablonski diagram. S is the singlet states (paired spin), and T is the triplet states (unpaired spin). Solid lines represent radiative processes, while dashed lines represent nonradiative processes. Adapted with permission from Ref. [60] Copyright 2019, Wiley VCH. (b) Excitation and emission spectra from the product of ammonia-o-phthaldialdehyde-sulfite. Adapted with permission from Ref. [65] Copyright 2014, Hindawi. (c) Schematic diagram of the flow injection-fluorescence system. Adapted with permission from Ref. [66] Copyright 2016, Taylor & Francis Publisher.
In NRR or NO3−RR works, where the reaction does not involve the use or the formation of amine or amino acid-related compounds, the interference from amines and amino acids can be neglected. Because of its sensitivity, the fluorescence method can be considered for ammonia detection in NRR because the produced ammonia is still often very limited (at micromolar level). However, for NO3−RR, which typically produces a more significant amount of ammonia (at the millimolar level), the fluorometric method may not be suitable for ammonia detection because too much dilution is needed to fit the method’s linearity ranges, which may lead to errors.
3.6. Enzymatic, Conductivity, and Titrimetric Methods
In addition to the aforementioned five major ammonia quantification methods, there are some other methods that are also known, i.e., the enzymatic method, the conductivity method, and the titrimetric method [31]. However, these methods are rarely used at the present time, likely due to sensitivity issues and complex procedures. In this following discussion, the three methods are briefly introduced.
The first method is the enzymatic method, which uses glutamate dehydrogenase enzyme to catalyze the reaction of 2-oxoglutarate and reduced nicotinamide adenine dinucleotide (NADH) in the presence of ammonia. Specifically, the enzyme catalyzes the reductive amination of 2-oxoglutarate, where NADH is oxidized during the glutamate formation from the ammonia present (Equation (14)). Therefore, the concentration of ammonia is determined based on the decrease of NADH concentration, which can be spectrophotometrically detected by the change of the NADH absorbance peak at 340 nm. However, this method is highly vulnerable to pH and temperature [31,67,68,69,70].
The second is the conductivity method, which is based on changes in solution conductivity due to the formation of salt as a result of the ammonia reaction with the acid solution. In this process, the gas containing ammonia is flowed into the sulfuric acid solution. The ammonia will react with the sulfuric acid, producing salts that decrease the solution’s conductivity. The change in conductivity is then monitored using a conductivity meter. The decrease in conductivity is proportional to the amount of ammonia [71]. Despite its simplicity, this method is prone to interferences because conductivity is not only specifically influenced by ammonia. The presence of other ions also changes conductivity. Therefore, impurities originating from the samples, feed gas, instruments, or side products may easily deviate from ammonia detection [31].
The last method is the titrimetric method, which is usually used for the detection of ammonia with a concentration higher than 5 mg L−1. In this method, the pH of the sample solution is increased to 9.5 to convert NH4+ ion into NH3(aq). Then, the distillation process is performed on the sample solution to release all the NH3(aq), in which the distillate is accommodated in a container containing boric acid solution and an indicator. Titration using a standard sulfuric acid solution is then performed on the distillate solution to obtain ammonia concentration. This process requires the measurement of blank samples for control experiments [31,72]. However, the requirements for the distillation process using this method may make this method less practical.
3.7. Comparison of Ammonia Quantification Methods and Their Application in Nitrogen/Nitrate Reduction Reaction
A comparison of the five major ammonia detection methods is presented in Table 1 and is illustrated in Figure 8. In Table 1, we also provide some representative catalysts, together with their activity (ammonia yield, in mg L−1 NH3-N), to represent the current ammonia production levels in NRR and NO3−RR. Among the five ammonia detection methods, most NRR or NO3−RR studies employed spectrophotometric methods as the main ammonia detection method. The IC and 1H NMR methods have also been used. The isotope labeling experiment using 1H NMR has been mostly used to qualitatively verify the origin of the produced ammonia. The ISE and fluorescence methods have been mostly utilized in water quality control. Here, the fluorometric method showed the lowest detection limit, which could detect up to the nanomolar level. Fortunately, there have been many reagents and kits for ammonia quantification that are now commercially available. These commercial products can potentially simplify experimental procedures. Readers are directed to the excellent review paper that provided the list of those commercial products for further information [31].

Table 1.
Comparison of the ammonia quantification methods with some representative catalysts.
Figure 8.
Illustration of five major quantification methods that have been used or can potentially be used for ammonia quantification in nitrogen/nitrate reduction reactions.
4. Perspective for Selecting Method and Performing Ammonia Quantification
The reaction conditions that are usually employed either in EC, PC, or PEC NRR (and NO3−RR), such as concentrations of produced ammonia, pH, cations, and sacrificial reagents, can influence detection accuracy. The indophenol blue method, the Nessler’s method, and the IC method show high accuracy in ammonia concentrations below 0.41 mg L−1 NH3-N. However, above this concentration, the indophenol blue method shows a positive bias [35]. Therefore, dilution is necessary for the sample solution containing concentrated ammonia, where it will be measured using the indophenol blue method to achieve high accuracy.
Recently, Zhao et al. also reported that, at low ammonia concentrations (<0.16 mg L−1 NH3-N), the indophenol blue method exhibited high accuracy over a wide range of pH. Meanwhile, the Nessler’s method worked well in neutral pH. In acidic conditions, such as in H2SO4 solution or electrolyte, the IC method is a good option considering less interference compared to the spectrophotometric method [34]. However, in the presence of Na+ or K+, the NH4+ peaks may overlap with the Na+ or K+ peaks; therefore, the IC instrument needs to be optimized first before performing the ammonia detection to obtain a better resolution of each ion peak.
In the PC-NRR (and NO3−RR), which typically employ sacrificial reagents such as methanol, the indophenol blue and Nessler’s methods showed deviations (Figure 9), possibly due to complex formations with ammonia [35]. Therefore, one can consider using the IC method for ammonia detection when working with sacrificial reagents. The 15NH3 isotope labeling or 14NH3 measurement using 1H NMR shows excellent stability and reproducibility in various electrolytes. However, it requires sophisticated and expensive instruments. Moreover, quantification of low-concentration ammonia dissolved in concentrated electrolytes is still challenging. As an alternative, the fluorometric method can be employed in low ammonia concentrations, such as in EC-, PC-, and PEC-NRR. Meanwhile, the ISE method can be a practical method for ammonia detection in a system that produces a large amount of ammonia, such as in PC- and EC-NO3−RR. Note that the spectrophotometric method and the fluorometric method involve a reaction between the ammonia and the reagents, where the reaction between the reagent and the ammonia is concentration dependent. Underestimation of ammonia concentrations may occur due to incomplete reactions. Therefore, a specified concentration of reagents and a strict time of analysis are crucial to ensure the completeness of the reaction.
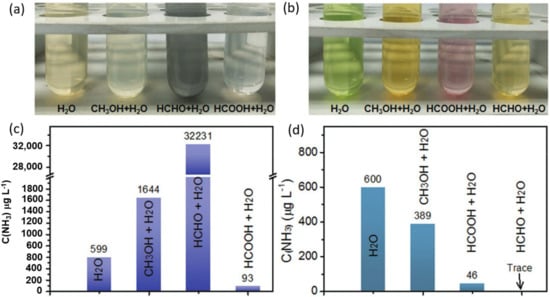
Figure 9.
Photograph of the various ammonia solutions in the presence of 40% volume of methanol as the sacrificial reagent and its derived oxidation products determined by (a,c) Nessler’s method and (b,d) indophenol blue method. The ammonia concentration was 600 µg L−1 in all experiments. Adapted with permission from Ref. [35] Wiley-VCH 2021.
Regardless of the methods and instruments being used, it is recommended to prepare the calibration curve from the standard solutions which closely mimic the real condition of the sample solution; e.g., use the same solvent to prepare the standard solution as in the real reaction, add an equivalent amount of sacrificial reagent, or adjust the pH. Therefore, possible interference from the solution background can be minimized. The quantification of the blank solution (or solution taken before the reaction is started) is also highly recommended to correct the measurement results of the sample solution after the reaction. Finally, it is also highly recommended to verify the results of one method with other quantitative methods (perform two separate ammonia quantification with different methods from the same sample solution) to make the ammonia quantification more reliable.
5. Conclusions
An overview of the current methods for ammonia quantification, either those that have been used or can potentially be used for present NRR and NO3−RR studies and in the future, was provided. In general, the selection of ammonia quantification methods should consider the condition of the sample solution and the predicted ammonia concentration. The sensitivity or detection limits of the selected quantification method should meet the range of ammonia concentrations. Moreover, possible interferants in the solution, such as cations, sacrificial reagents, and pH, must also be considered. Although the list of quantification methods described here is not exhaustive, this focused review is expected to provide a guide by which to choose the most suitable ammonia quantification method in NRR and NO3−RR studies and properly perform measurements. Therefore, the obtained ammonia values will be more convincing and reliable.
Author Contributions
Conceptualization, Y.H.N., writing—original draft preparation, W.P.U.; writing—review and editing, W.P.U., H.W. and Y.H.N., supervision, H.W. and Y.H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hong Kong Research Grant Council (RGC) General Research Fund (GRF) CityU11305419, CityU11306920, CityU11308721 and the General Program of Science and Technology Innovation Committee of Shenzhen Municipality JCYJ20190808181805621.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.; Xia, M.; Wang, H.; Huang, K.; Qian, C.; Maravelias, C.T.; Ozin, G.A. Greening Ammonia toward the Solar Ammonia Refinery. Joule 2018, 2, 1055–1074. [Google Scholar] [CrossRef]
- Comer, B.M.; Fuentes, P.; Dimkpa, C.O.; Liu, Y.-H.; Fernandez, C.A.; Arora, P.; Realff, M.; Singh, U.; Hatzell, M.C.; Medford, A.J. Prospects and Challenges for Solar Fertilizers. Joule 2019, 3, 1578–1605. [Google Scholar] [CrossRef]
- Hollevoet, L.; Jardali, F.; Gorbanev, Y.; Creel, J.; Bogaerts, A.; Martens, J.A. Towards Green Ammonia Synthesis through Plasma-Driven Nitrogen Oxidation and Catalytic Reduction. Angew. Chem. Int. Ed. 2020, 59, 23825–23829. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kang, D.W.; Kang, M.; Lee, J.-H.; Choe, J.H.; Chae, Y.S.; Choi, D.S.; Yun, H.; Hong, C.S. High Ammonia Uptake of a Metal–Organic Framework Adsorbent in a Wide Pressure Range. Angew. Chem. Int. Ed. 2020, 59, 22531–22536. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Bahadoran, A.; Liu, Q.; Ramakrishna, S.; Sadeghi, B.; De Castro, M.M.; Cavaliere, P.D. Hydrogen Production as a Clean Energy Carrier through Heterojunction Semiconductors for Environmental Remediation. Energies 2022, 15, 3222. [Google Scholar] [CrossRef]
- Appl, M. Ammonia, 2. Production Processes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-30673-2. [Google Scholar]
- Martín, A.J.; Shinagawa, T.; Pérez-Ramírez, J. Electrocatalytic Reduction of Nitrogen: From Haber-Bosch to Ammonia Artificial Leaf. Chem 2019, 5, 263–283. [Google Scholar] [CrossRef]
- Mallouppas, G.; Ioannou, C.; Yfantis, E.A. A Review of the Latest Trends in the Use of Green Ammonia as an Energy Carrier in Maritime Industry. Energies 2022, 15, 1453. [Google Scholar] [CrossRef]
- Ham, C.J.M.v.d.; Koper, M.T.M.; Hetterscheid, D.G.H. Challenges in Reduction of Dinitrogen by Proton and Electron Transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef]
- Elishav, O.; Mosevitzky Lis, B.; Miller, E.M.; Arent, D.J.; Valera-Medina, A.; Grinberg Dana, A.; Shter, G.E.; Grader, G.S. Progress and Prospective of Nitrogen-Based Alternative Fuels. Chem. Rev. 2020, 120, 5352–5436. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and Future Role of Haber–Bosch Ammonia in a Carbon-Free Energy Landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Xu, X.; Liu, E.; Zhu, N.; Liu, F.; Qian, F. Review of the Current Status of Ammonia-Blended Hydrogen Fuel Engine Development. Energies 2022, 15, 1023. [Google Scholar] [CrossRef]
- Cui, X.; Tang, C.; Zhang, Q. A Review of Electrocatalytic Reduction of Dinitrogen to Ammonia under Ambient Conditions. Adv. Energy Mater. 2018, 8, 1800369. [Google Scholar] [CrossRef]
- Tang, C.; Qiao, S.-Z. How to Explore Ambient Electrocatalytic Nitrogen Reduction Reliably and Insightfully. Chem. Soc. Rev. 2019, 48, 3166–3180. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Chen, R.; Yan, C.; Zhao, P.; Hu, Y.; Zhang, W.; Yang, S.; Jin, Z. Review on Photocatalytic and Electrocatalytic Artificial Nitrogen Fixation for Ammonia Synthesis at Mild Conditions: Advances, Challenges and Perspectives. Nano Res. 2019, 12, 1229–1249. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, C.; Yu, Y.; Lu, S.; Zhang, B. Recent Advances in Non-Noble Metal Electrocatalysts for Nitrate Reduction. Chem. Eng. J. 2021, 403, 126269. [Google Scholar] [CrossRef]
- Lu, X.; Song, H.; Cai, J.; Lu, S. Recent Development of Electrochemical Nitrate Reduction to Ammonia: A Mini Review. Electrochem. Commun. 2021, 129, 107094. [Google Scholar] [CrossRef]
- Guo, C.; Ran, J.; Vasileff, A.; Qiao, S.-Z. Rational Design of Electrocatalysts and Photo(Electro)Catalysts for Nitrogen Reduction to Ammonia (NH3) under Ambient Conditions. Energy Environ. Sci. 2018, 11, 45–56. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, H.; Qu, K.; Wang, F.; Guan, P.; Xu, D.; Fan, W.; Shi, W. In-Situ Approach to Fabricate BiOI Photocathode with Oxygen Vacancies: Understanding the N2 Reduced Behavior in Photoelectrochemical System. Chem. Eng. J. 2019, 362, 349–356. [Google Scholar] [CrossRef]
- Cao, N.; Zheng, G. Aqueous Electrocatalytic N2 Reduction under Ambient Conditions. Nano Res. 2018, 11, 2992–3008. [Google Scholar] [CrossRef]
- Chen, X.; Li, N.; Kong, Z.; Ong, W.-J.; Zhao, X. Photocatalytic Fixation of Nitrogen to Ammonia: State-of-the-Art Advancements and Future Prospects. Mater. Horiz. 2018, 5, 9–27. [Google Scholar] [CrossRef]
- Wu, H.; Irani, R.; Zhang, K.; Jing, L.; Dai, H.; Chung, H.Y.; Abdi, F.F.; Ng, Y.H. Unveiling Carrier Dynamics in Periodic Porous BiVO4 Photocatalyst for Enhanced Solar Water Splitting. ACS Energy Lett. 2021, 6, 3400–3407. [Google Scholar] [CrossRef]
- Wu, H.; Tan, H.L.; Toe, C.Y.; Scott, J.; Wang, L.; Amal, R.; Ng, Y.H. Photocatalytic and Photoelectrochemical Systems: Similarities and Differences. Adv. Mater. 2020, 32, 1904717. [Google Scholar] [CrossRef] [PubMed]
- Utomo, W.P.; Leung, M.K.H.; Yin, Z.; Wu, H.; Ng, Y.H. Advancement of Bismuth-Based Materials for Electrocatalytic and Photo(Electro)Catalytic Ammonia Synthesis. Adv. Funct. Mater. 2022, 32, 2106713. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhang, C.; Liu, Y.; Xu, M.; Xue, Y.; Liu, L.; Leung, M.K.H. Bimetallic Mo–Co Nanoparticles Anchored on Nitrogen-Doped Carbon for Enhanced Electrochemical Nitrogen Fixation. J. Mater. Chem. A 2020, 8, 9091–9098. [Google Scholar] [CrossRef]
- Daiyan, R.; Chen, R.; Kumar, P.; Bedford, N.M.; Qu, J.; Cairney, J.M.; Lu, X.; Amal, R. Tunable Syngas Production through CO2 Electroreduction on Cobalt–Carbon Composite Electrocatalyst. ACS Appl. Mater. Interfaces 2020, 12, 9307–9315. [Google Scholar] [CrossRef]
- Utomo, W.P.; Wu, H.; Ng, Y.H. Modulating the Active Sites of Oxygen-Deficient TiO2 by Copper Loading for Enhanced Electrocatalytic Nitrogen Reduction to Ammonia. Small 2022, 18, 2200996. [Google Scholar] [CrossRef]
- Chen, P.; Shen, J.; Wang, T.; Dai, M.; Si, C.; Xie, J.; Li, M.; Cong, X.; Sun, X. Zeolitic Imidazolate Framework-67 Based Separator for Enhanced High Thermal Stability of Lithium Ion Battery. J. Power Sources 2018, 400, 325–332. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the Activity Origin of a Copper-Based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef]
- Qing, G.; Ghazfar, R.; Jackowski, S.T.; Habibzadeh, F.; Ashtiani, M.M.; Chen, C.-P.; Smith, M.R.; Hamann, T.W. Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia. Chem. Rev. 2020, 120, 5437–5516. [Google Scholar] [CrossRef]
- Choi, J.; Suryanto, B.H.R.; Wang, D.; Du, H.-L.; Hodgetts, R.Y.; Ferrero Vallana, F.M.; MacFarlane, D.R.; Simonov, A.N. Identification and Elimination of False Positives in Electrochemical Nitrogen Reduction Studies. Nat. Commun. 2020, 11, 5546. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on Water Quality Detection by UV-Vis Spectroscopy. Appl. Sci. 2020, 10, 6874. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, F.; Miao, Y.; Zhou, C.; Xu, N.; Shi, R.; Wu, L.-Z.; Tang, J.; Zhang, T. Revealing Ammonia Quantification Minefield in Photo/Electrocatalysis. Angew. Chem. Int. Ed. 2021, 60, 21728–21731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, R.; Bian, X.; Zhou, C.; Zhao, Y.; Zhang, S.; Wu, F.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; et al. Ammonia Detection Methods in Photocatalytic and Electrocatalytic Experiments: How to Improve the Reliability of NH3 Production Rates? Adv. Sci. 2019, 6, 1802109. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.; Kim, S.M.; Lee, Y.; Seok, J.; Jung, J.; Lee, J.S.; Jang, Y.J. Rational Design of Photocatalysts for Ammonia Production from Water and Nitrogen Gas. Nano Converg. 2021, 8, 22. [Google Scholar] [CrossRef]
- Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Langenfeld, N.J.; Kusuma, P.; Wallentine, T.; Criddle, C.S.; Seefeldt, L.C.; Bugbee, B. Optimizing Nitrogen Fixation and Recycling for Food Production in Regenerative Life Support Systems. Front. Astron. Space Sci. 2021, 8, 105. [Google Scholar] [CrossRef]
- Skolotneva, E.; Tsygurina, K.; Mareev, S.; Melnikova, E.; Pismenskaya, N.; Nikonenko, V. High Diffusion Permeability of Anion-Exchange Membranes for Ammonium Chloride: Experiment and Modeling. Int. J. Mol. Sci. 2022, 23, 5782. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Du, X.; Zeng, Y.; Chu, J.; Gong, C.; Huang, J.; Fan, C.; Wang, X.; Xiong, J. Atomic Structure Modification for Electrochemical Nitrogen Reduction to Ammonia. Adv. Energy Mater. 2020, 10, 1903172. [Google Scholar] [CrossRef]
- Rocha, F.S.; Gomes, A.J.; Lunardi, C.N.; Kaliaguine, S.; Patience, G.S. Experimental Methods in Chemical Engineering: Ultraviolet Visible Spectroscopy—UV-Vis. Can. J. Chem. Eng. 2018, 96, 2512–2517. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Essentials of Pharmaceutical Analysis; Springer Nature Singapore: Singapore, 2020; ISBN 9789811515460. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 5th ed.; Cengage Learning: Stamford, CT, USA, 2013. [Google Scholar]
- Zhou, L.; Boyd, C.E. Comparison of Nessler, Phenate, Salicylate and Ion Selective Electrode Procedures for Determination of Total Ammonia Nitrogen in Aquaculture. Aquaculture 2016, 450, 187–193. [Google Scholar] [CrossRef]
- Cong, M.; Chen, X.; Xia, K.; Ding, X.; Zhang, L.; Jin, Y.; Gao, Y.; Zhang, L. Selective Nitrogen Reduction to Ammonia on Iron Porphyrin-Based Single-Site Metal–Organic Frameworks. J. Mater. Chem. A 2021, 9, 4673–4678. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, K.; Cong, L.; Yu, Z.; Qu, L.; Huang, W. Facile Synthesis of a Ru-Dispersed N-Doped Carbon Framework Catalyst for Electrochemical Nitrogen Reduction. Catal. Sci. Technol. 2020, 10, 1336–1342. [Google Scholar] [CrossRef]
- Khalil, I.E.; Xue, C.; Liu, W.; Li, X.; Shen, Y.; Li, S.; Zhang, W.; Huo, F. The Role of Defects in Metal–Organic Frameworks for Nitrogen Reduction Reaction: When Defects Switch to Features. Adv. Funct. Mater. 2021, 31, 2010052. [Google Scholar] [CrossRef]
- Mikeš, O. Chapter 4.5. Ion Exchange Chromatography. In New Comprehensive Biochemistry; Deyl, Z., Ed.; Separation Methods; Elsevier: Amsterdam, The Netherlands, 1984; Volume 8, pp. 205–270. [Google Scholar]
- Lucy, C.A.; Hatsis, P. Chapter 4 Ion Chromatography. In Journal of Chromatography Library, 6th ed.; Heftmann, E., Ed.; chromatography; Elsevier: Amsterdam, The Netherlands, 2004; Volume 69, pp. 171–211. [Google Scholar]
- Srinivasan, K. Chapter Nine-Ion Chromatography Instrumentation for Water Analysis. In Chemistry and Water; Ahuja, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 329–351. ISBN 978-0-12-809330-6. [Google Scholar]
- Chong, J.; Hatsis, P.; Lucy, C.A. High-Speed Ion Chromatographic Separation of Cations at Elevated Temperature. J. Chromatogr. A 2003, 997, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.; Siddiqui, T.; Ali, S.; Farooq, I.; Zafar, M.S.; Khurshid, Z. Nuclear Magnetic Resonance Spectroscopy for Medical and Dental Applications: A Comprehensive Review. Eur. J. Dent. 2019, 13, 124–128. [Google Scholar] [CrossRef]
- Jang, Y.J.; Lindberg, A.E.; Lumley, M.A.; Choi, K.-S. Photoelectrochemical Nitrogen Reduction to Ammonia on Cupric and Cuprous Oxide Photocathodes. ACS Energy Lett. 2020, 5, 1834–1839. [Google Scholar] [CrossRef]
- Rigamonti, M.G.; Gatti, F.G.; Patience, G.S. Experimental Methods in Chemical Engineering: Nuclear Magnetic Resonance. Can. J. Chem. Eng. 2019, 97, 628–635. [Google Scholar] [CrossRef]
- Lindner, E.; Pendley, B.D. A Tutorial on the Application of Ion-Selective Electrode Potentiometry: An Analytical Method with Unique Qualities, Unexplored Opportunities and Potential Pitfalls; Tutorial. Anal. Chim. Acta 2013, 762, 1–13. [Google Scholar] [CrossRef]
- Berg, J. An Ion-Selective Electrode for Detection of Ammonium in Wastewater Treatment Plants; KTH Royal Institute of Technology: Stockholm, Sweden, 2021; p. 48. [Google Scholar]
- Cecconi, F.; Reifsnyder, S.; Ito, Y.; Jimenez, M.; Sobhani, R.; Rosso, D. ISE-Ammonium Sensors in WRRFs: Field Assessment of Their Influencing Factors. Environ. Sci. Water Res. Technol. 2019, 5, 737–746. [Google Scholar] [CrossRef]
- Li, T.; Wu, Y.; Huang, J.; Zhang, S. Gas Sensors Based on Membrane Diffusion for Environmental Monitoring. Sens. Actuators B Chem. 2017, 243, 566–578. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Introduction to Fluorescence. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 1–26. ISBN 978-0-387-46312-4. [Google Scholar]
- Gomes, A.J.; Lunardi, C.N.; Rocha, F.S.; Patience, G.S. Experimental Methods in Chemical Engineering: Fluorescence Emission Spectroscopy. Can. J. Chem. Eng. 2019, 97, 2168–2175. [Google Scholar] [CrossRef]
- Sarı, T.; Dede, S.; Yusufoğlu, B.; Karakuş, E. Determination of L-Phenylalanine in Human Plasma Samples with New Fluorometric Method. Appl. Biochem. Biotechnol. 2022, 194, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Roth, M. Fluorescence Reaction for Amino Acids. Anal. Chem. 1971, 43, 880–882. [Google Scholar] [CrossRef]
- Genfa, Z.; Dasgupta, P.K. Fluorometric Measurement of Aqueous Ammonium Ion in a Flow Injection System. Anal. Chem. 1989, 61, 408–412. [Google Scholar] [CrossRef]
- Felix, E.P.; Cardoso, A.A. A Method for Determination of Ammonia in Air Using Oxalic Acid-Impregnated Cellulose Filters and Fluorimetric Detection. J. Braz. Chem. Soc. 2012, 23, 142–147. [Google Scholar] [CrossRef]
- Hu, H.; Liang, Y.; Li, S.; Guo, Q.; Wu, C. A Modified o-Phthalaldehyde Fluorometric Analytical Method for Ultratrace Ammonium in Natural Waters Using EDTA-NaOH as Buffer. J. Anal. Methods Chem. 2014, 2014, e728068. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, D.; Lin, H.; Zhou, T. Determination of Ammonium in Seawater by Purge-and-Trap and Flow Injection with Fluorescence Detection. Anal. Lett. 2016, 49, 665–675. [Google Scholar] [CrossRef]
- Kun, E.; Kearney, E.B. Ammonia. In Methods of Enzymatic Analysis, 2nd ed.; Bergmeyer, H.U., Ed.; Academic Press: Cambridge, MA, USA, 1974; pp. 1802–1806. ISBN 978-0-12-091304-6. [Google Scholar]
- Guilbault, G.G. Use of Enzymes in Analytical Chemistry. Anal. Chem. 1966, 38, 527–536. [Google Scholar] [CrossRef]
- Hao, Y.-C.; Guo, Y.; Chen, L.-W.; Shu, M.; Wang, X.-Y.; Bu, T.-A.; Gao, W.-Y.; Zhang, N.; Su, X.; Feng, X.; et al. Promoting Nitrogen Electroreduction to Ammonia with Bismuth Nanocrystals and Potassium Cations in Water. Nat. Catal. 2019, 2, 448–456. [Google Scholar] [CrossRef]
- Ishihara, A.; Kurahasi, K.; Uehara, H. Enzymatic Determination of Ammonia in Blood Plasma. Clin. Chim. Acta 1972, 41, 255–261. [Google Scholar] [CrossRef]
- Wang, P.; Chang, F.; Gao, W.; Guo, J.; Wu, G.; He, T.; Chen, P. Breaking Scaling Relations to Achieve Low-Temperature Ammonia Synthesis through LiH-Mediated Nitrogen Transfer and Hydrogenation. Nat. Chem. 2017, 9, 64–70. [Google Scholar] [CrossRef]
- Bridgewater, L.L.; Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation, Eds.; American Public Health Association: Washington, DC, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- Li, L.; Tang, C.; Xia, B.; Jin, H.; Zheng, Y.; Qiao, S.-Z. Two-Dimensional Mosaic Bismuth Nanosheets for Highly Selective Ambient Electrocatalytic Nitrogen Reduction. ACS Catal. 2019, 9, 2902–2908. [Google Scholar] [CrossRef]
- Xing, Z.; Kong, W.; Wu, T.; Xie, H.; Wang, T.; Luo, Y.; Shi, X.; Asiri, A.M.; Zhang, Y.; Sun, X. Hollow Bi2MoO6 Sphere Effectively Catalyzes the Ambient Electroreduction of N2 to NH3. ACS Sustain. Chem. Eng. 2019, 7, 12692–12696. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Zu, X.; Zhang, X.; Wang, Y.; Wang, Y.; Fan, C. Photocatalytic Conversion of Nitrogen to Ammonia with Water on Triphase Interfaces of Hydrophilic-Hydrophobic Composite Bi4O5Br2/ZIF-8. Chem. Eng. J. 2019, 371, 796–803. [Google Scholar] [CrossRef]
- Daiyan, R.; Tran-Phu, T.; Kumar, P.; Iputera, K.; Tong, Z.; Leverett, J.; Ali Khan, M.H.; Esmailpour, A.A.; Jalili, A.; Lim, M.; et al. Nitrate Reduction to Ammonium: From CuO Defect Engineering to Waste NO x -to-NH 3 Economic Feasibility. Energy Environ. Sci. 2021, 14, 3588–3598. [Google Scholar] [CrossRef]
- Petsi, P.N.; Sarasidis, V.C.; Plakas, K.V.; Karabelas, A.J. Reduction of Nitrates in a Photocatalytic Membrane Reactor in the Presence of Organic Acids. J. Environ. Manag. 2021, 298, 113526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zheng, F.; Huang, B.; Ji, Y.; Shao, Q.; Li, Y.; Xiao, X.; Huang, X. Exploring Bi2Te3 Nanoplates as Versatile Catalysts for Electrochemical Reduction of Small Molecules. Adv. Mater. 2020, 32, 1906477. [Google Scholar] [CrossRef]
- Vesali-Kermani, E.; Habibi-Yangjeh, A.; Diarmand-Khalilabad, H.; Ghosh, S. Nitrogen Photofixation Ability of G-C3N4 Nanosheets/Bi2MoO6 Heterojunction Photocatalyst under Visible-Light Illumination. J. Colloid Interface Sci. 2020, 563, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Qiu, P.; Li, L.; Chen, H.; Jiang, F.; Wang, X. Bismuth Subcarbonate with Designer Defects for Broad-Spectrum Photocatalytic Nitrogen Fixation. ACS Appl. Mater. Interfaces 2018, 10, 25321–25328. [Google Scholar] [CrossRef]
- Fei, T.; Yu, L.; Liu, Z.; Song, Y.; Xu, F.; Mo, Z.; Liu, C.; Deng, J.; Ji, H.; Cheng, M.; et al. Graphene Quantum Dots Modified Flower like Bi2WO6 for Enhanced Photocatalytic Nitrogen Fixation. J. Colloid Interface Sci. 2019, 557, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, J.; Du, F.; Yang, L.; Huang, S.; Gao, J.; Li, C.; Guo, C. A Core–Shell Copper Oxides-Cobalt Oxides Heterostructure Nanowire Arrays for Nitrate Reduction to Ammonia with High Yield Rate. Green Energy Environ. 2022; in press. [Google Scholar] [CrossRef]
- Gao, X.; Shang, Y.; Liu, L.; Gao, K. Ag Plasmon Resonance Promoted 2D AgBr-δ-Bi2O3 Nanosheets with Enhanced Photocatalytic Ability. J. Alloys Compd. 2019, 803, 565–575. [Google Scholar] [CrossRef]
- Xu, F.; Wu, F.; Zhu, K.; Fang, Z.; Jia, D.; Wang, Y.; Jia, G.; Low, J.; Ye, W.; Sun, Z.; et al. Boron Doping and High Curvature in Bi Nanorolls for Promoting Photoelectrochemical Nitrogen Fixation. Appl. Catal. B 2021, 284, 119689. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, M.; Bao, D.; Meng, F.; Zhang, Q.; Zhou, Y.; Liu, K.; Zhang, Y.; Wang, J.; Chen, Z.; et al. Generating Defect-Rich Bismuth for Enhancing the Rate of Nitrogen Electroreduction to Ammonia. Angew. Chem. Int. Ed. 2019, 58, 9464–9469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).