Abstract
Recent research on the role of nanomaterials in gas hydrate science and a few review papers have highlighted the positive synergies between gas hydrates and metal–organic frameworks (MOFs) for gas separation and storage. Metal–organic frameworks consist of metal nodes and organic linkers connected by coordination bonds to form programmable modular structures that are symmetric and have tunable properties. Metal–organic frameworks, also known as microporous or nanoporous materials, provide a large pore volume and surface area suitable for capturing, separating and storing gases through physisorption mechanisms. However, water and water interactions within the nanopores, open metal sites, coordination bonds and surface make metal–organic framework usage in water-based technologies an exciting research topic. Water-based gas hydrate technology could be potential technology that can take advantage of MOF tunable properties, such as a large surface area and a high pore volume, to improve its efficiency and formation mechanism. For the authors of this review, the synergy of MOFs and gas hydrates resembles a Pandora’s box of unanswered questions and revelations. Therefore, this review examines the current state of the art, including present research on gas storage and separation using gas hydrates in the presence of a MOF. In addition, critical technical aspects, such as the water stability of MOFs, the nano confinement effect and water properties in the nanopores, are presented to stimulate critical thinking among scientists in hydrate research to fully exploit the synergies between MOFs and hydrates. This review ends with the authors’ opinion on potential research areas, unanswered questions and practical implications and prospects.
1. Introduction
Society faces two pressing global issues: first, ensuring an environmentally friendly energy supply for a growing population, and second, the threat of catastrophic climate change due to global warming caused by greenhouse gases, such as CO2. Therefore, technological development is focused both on developing cleaner fuels and reducing CO2 emissions through capture and storage (from industrial emission sources such as the cement, power and chemical industries) [1]. In controlling, capturing and storing CO2 emissions, the focus is on developing and promoting newer technologies and introducing new environmentally friendly fuels with lower carbon emissions, such as hydrogen as a fuel. Currently, nations and various industries rely on natural gas as an environmentally friendly conventional fuel to meet energy and consumption needs. However, efforts are being made to reduce cost differences between conventional and non-conventional (environmentally friendly) energy usage. Currently, the role of hydrogen as a fuel in various sectors is being discussed, and technologies are developing to produce, transport and store it economically to accelerate the transition [2,3,4]. Carbon capture and sequestration (CCS) technologies are therefore necessary to reduce emissions in the current industry and to enable the inclusion of newer fuels in the current energy mix [5,6,7]. For example, CCS is being used to convert brown hydrogen into blue hydrogen. The CCS technology platform is based on CO2 capture (chemical adsorption or physical adsorption) from a gas mixture containing CO2. The CO2 concentration in the gas mixture depends on the source, e.g., cement, chemical or power plants [8,9,10].
The right choice of CCS technology platform requires a complex balancing of economic, technical and environmental advantages and disadvantages [11]. Such a technology platform concerns developing novel materials, such as adsorbents and absorbents, and processing improvements to increase efficiency, reduce costs and simplify operations. The role of materials in introducing new technologies and improving existing ones was highlighted in an article in Nature, which reported that seven chemical separation processes (complex and energy-intensive) that have the potential to change the world require extensive work on technologies based on either adsorption (solid porous materials) or absorption (liquid solvents) [12]. In this context, novel adsorbents, including 3D materials, have received considerable attention due to their wide range of applications and tailored properties [13,14,15].
Over the past decade, intensive research has led to a better understanding of gas hydrates as a potential medium for the storage of certain gases of interest (CH4, CO2 and H2), for separation, as a phase change material [16] and as an energy storage material [17,18]. However, despite an improved understanding of hydrate formation, stability and dissociation, critical technical challenges remain, such as low formation kinetics, long induction times, and probabilistic hydrate nucleation under high-pressure and low-temperature requirements, limiting practical bulk-phase applications. In this context, metal–organic framework materials offer the possibility to overcome the above limitations, e.g., by improving the gas–liquid contact area to promote mass transfer and rapid hydrate growth.
Recent experimental studies have shown that porous metal–organic framework materials have opened a new field of research, namely the study of the hydrate formation process in a nano-confined space. Recent review articles on MOFs and hydrate synergy have highlighted the potential application of these novel porous materials to enhance gas hydrate formation. Some noteworthy review articles include the state of the art [19], the role of nano reactors [20], the confined space affecting gas hydrate formation [21] and CH4 hydrates in carbon, zeolites and metal–organic frameworks [22]. The nano-confined space in MOFs, together with the presence of water, leads to specific new effects that influence hydrate formation kinetics. The authors believe that a better understanding of the stability of MOFs, surface chemistry, including functionalization and material chemistry, and water properties in a confined nano space would be essential in advancing hydrate research, but recent review articles have not addressed these topics.
Therefore, in this review, the authors address issues such as the current state of the art, the stability of metal–organic frameworks in water, the properties of water in nanopores and the main experimental variables under high-pressure–low-temperature conditions (see Figure 1) that affect the synergy between hydrates and metal–organic frameworks. It is expected that new insights into this synergy will raise some new research questions, provide some new insights and further improve our understanding of the formation and dissociation of gas hydrates in nanoporous materials, especially metal–organic frameworks.
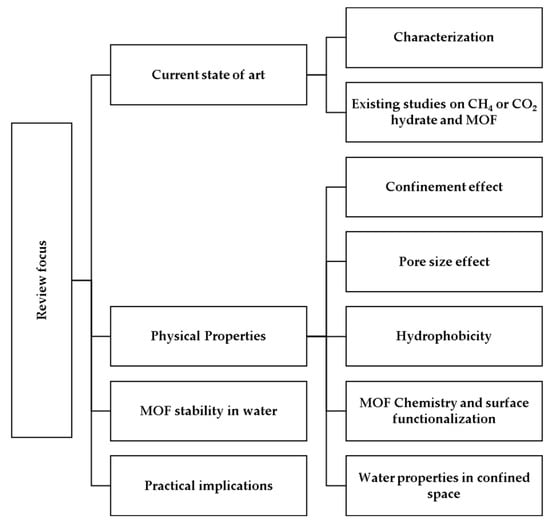
Figure 1.
Focus of the current review paper.
2. Gas Hydrates Introduction
Gas hydrates are water-based structures but have an exceptionally high gas density. Gas hydrates form when gas and water come together under hydrate-forming conditions (high pressure and low temperature). Under these operating conditions, water molecules form an ice-like host lattice with the help of hydrogen bonds and have a cavity for gas molecules, stabilized by van der Waals forces [23]. Gas structures are essentially three known cubic structures (sI, sII and sH) that differ from each other in terms of lattice parameters, the total number of cages (a mixture of small and large cages), cavity radius, the number of cavities and the number of water molecules. Many gases form gas hydrates. Gas molecules with small diameters (0.4 nm or less), such as H2, N2 and O2, form sII hydrates, whereas gases with larger diameters (0.4–0.6 nm), such as CH4 or CO2, form sI hydrates [24] (refer to Figure 2).
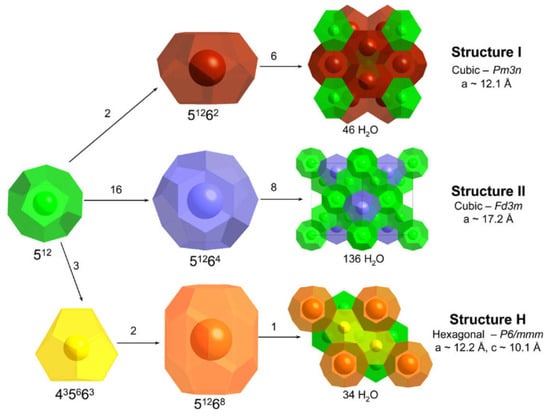
Figure 2.
Discovered clathrate hydrate structures: cages, water units and unit cage [25] (Reprinted from [25], Copyright (2009), with permission from Elsevier).
Hydrate-based technology is believed to be an economically competitive, environmentally friendly and non-explosive method and a technically simple, safer and less hazardous way to store and transport natural gas. It could fill the technology gap for end users if they expect simplified technology and safer gas delivery. Gas hydrates as a storage medium are also an attractive medium because of the high reusability of the material and low maintenance costs. Research on gas hydrate technology has focused on CH4 storage [26], CO2 storage, CO2 capture from flue and fuel gases [27] and the storage of H2 rich natural gas mixtures in hydrates [28]. Despite the above advantages, gas hydrates have not gained acceptance due to specific technical issues. Significant challenges include the probabilistic nature of hydrate formation and its slow growth rate, long induction time and mass transfer barrier, leading to low gas storage rates, so the commercial introduction of gas hydrates has been delayed [29]. The mass transfer barrier formed at the gas–liquid interface hinders gas diffusion into the water phase, and the exothermic nature of hydrate formation can retard the hydrate formation process if the released heat is not rapidly dissipated elsewhere. Among the various possibilities, hydrate formation in porous media (sands and clays) has been intensively studied because hydrates also occur naturally under high pressures and at low temperatures [30].
On the other hand, various artificial solid media (porous and nonporous nanomaterials) have been tested for their effects on hydrate studies. These nanomaterials include zeolites, activated carbon and carbon nanotubes; MOFs traditionally have a larger specific surface area that enhances the mass transfer process. This review paper focuses only on metal–organic frameworks and the subclass of metal–organic frameworks.
3. Metal–Organic Framework
3.1. Introduction
Metal–organic frameworks (also known as porous coordination polymers (PCPs)) are crystalline and porous 3D materials containing metal ion clusters and multifunctional organic molecules [31] (refer to Figure 3). Organic linkers can be monovalent, divalent, trivalent or tetravalent ligands. Metal ion clusters and organic linkers are connected by robust chemical coordination bonds between organic linkers and metal-containing clusters [32]. In this coordination bond, metal ion clusters are electron donors, and the carboxyl groups of the linkers are electron acceptors. Metal ion clusters have different coordination domains, and by modifying the metal ion clusters or the organic linkers, various porous structures with different surface areas, desired pore sizes, shapes and densities can be designed and synthesized [33]. MOFs differ from microporous inorganic materials, such as zeolites, because they are more flexible in terms of designing the material architecture and functionalizing the pores, they have permanent porosity, they do not collapse in the absence of guest molecules and they retain their crystalline structures both in the solute state and at high temperatures.
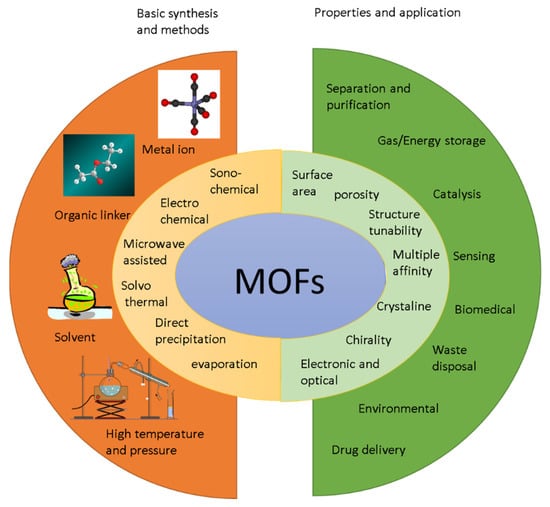
Figure 3.
MOF synthesis building blocks, synthesis methods, properties and various applications.
Pores of different sizes can be present in a framework, and the presence of pores is a more stringent criterion for classification as an MOF than the size of the pores [34]. Pores are classified into three types: (a) microporous (<2 nm), mesoporous (2–50 nm) and macroporous (>50 nm). Most MOFs have pores of microscopic sizes and are, therefore, referred to as microporous or nanoporous compounds. MOFs can also have different pore classes within the same structure due to defects during synthesis or as a characteristic property [35]. Previous studies have focused on gas storage and gas separation systems. The effects of humidity and water on the structural stability of MOFs and their working efficiency [36,37,38,39] have recently attracted attention due to the water-sensitive metal–organic linkages that could become unstable in the presence of water [40].
In the last 25 years, since the MOF terminology was coined in 1995, MOF chemistry and MOF research have made great strides. Currently, MOF chemistry and MOF research are in their fourth generation [41], providing a unique opportunity for gas hydrate researchers to explore new MOF chemistry that goes far beyond the second generation of MOFs with permanent porosity (refer to Figure 4). As MOF chemistry continues to develop, it offers immense opportunities for the gas hydrate community to look for suitable MOFs beyond the second generation that offer synergistic value for improving gas hydrate science.
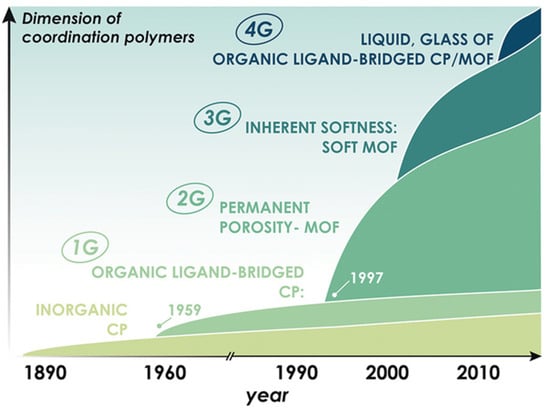
Figure 4.
MOF advancement divided into four levels (four generations). Gas science and technology have immensely benefitted from permanent porosity-based MOFs (2nd generation). Current research on MOF development are focused on third and fourth generation MOFs (soft crystals and flexible MOFs) and fourth generation MOFs (glassy MOFs derived from crystalline states) [41] (reprinted from [41] with permission from © 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany).
Current second generation MOFs are attractive 3D porous materials for gas storage and for the separation of some necessary gases such as CH4 or CO2 [42,43] due to their material-related properties, such as high specific area, high porosity, structural chemistry and tunable surface functionalization [44,45,46]. Primarily, MOFs are used for gas storage and gas separation under high or low-pressure conditions at elevated temperatures or ay room temperature. Under such conditions, MOFs are expected to have storage and separation functions through the physical adsorption and utilization of the pore surface. However, not all pore spaces are fully utilized [43]. However, the application of MOFs in the gas hydrate technology platform requires high pressure, low temperatures (less than 6 °C) and the presence of water. MOF materials are extensively studied with physisorption in the dry state, but in the presence of water, the mechanism for storing gas in the form of gas hydrates could be drastically different from what is initially expected. Furthermore, the gas hydrate phase diagram is also affected in nanoporous materials [47]. This introduces additional complexity and challenges that need to be further investigated.
3.2. MOF–Hydrate Potential Synergy
Recently, there has been discussion of potential synergies between nanomaterials and hydrates, which are complementary and offer potential synergies in gas storage and separation technologies [19,20,21]. The synergy between the two technologies stems from the fact that porous nanomaterials (metal–organic frameworks) offer a large surface area, pore volume and tunable chemistry. An increased surface area can increase the gas–liquid contact area, improve the conversion of water to hydrates and shorten the induction time, so this likely plays a role in improved hydrate formation. Another reason for looking for synergies is the idea of lower energy consumption. It is expected that a new process combining gas adsorption processes (based on MOFs) and crystallization energy (based on gas hydrates) can reduce overall energy consumption compared with the adsorption-only process (see the Figure 5).
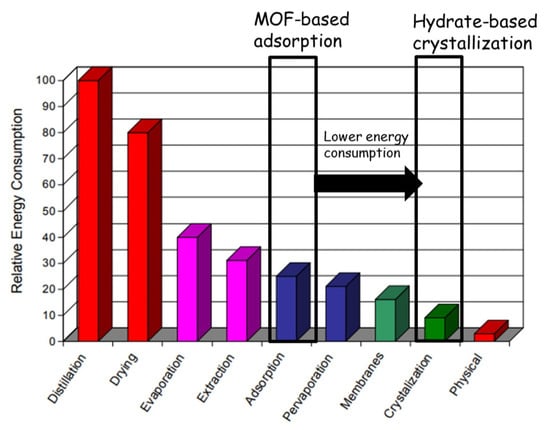
Figure 5.
Relative energy use by various separation technologies [48] (Figure used here is from the open access report available at the following link: https://www1.eere.energy.gov/manufacturing/industries_technologies/imf/pdfs/separationsreport.pdf) (Accessed on 21 December 2022).
Figure 5 suggests that, although MOF-based adsorption is an energy-intensive technology [48] for the separation process, combining it with gas-hydrate-based crystallization can reduce energy consumption, making it an attractive proposition. Gas hydrate is a technology used for water crystallization under high gas pressures and low temperatures, and it consumes less energy compared with adsorption. Some of the previously tested porous materials, such as carbon nanotubes, porous silica gel and zeolites, have been tested in studies to enhance gas hydrate formation, but they lack the nano-confinement effect due to a larger pore size at the meso and macro scales. MOF and gas hydrate technologies complement each other, as both prove effective in separation. For example, the right MOF material for the separation process is selected based on material thickness and affinity for a particular component of the mixture. In contrast, the separation process for gas hydrates is based on the different thermodynamic stability pressures of the hydrate-forming gases.
When considering hydrate–MOF synergies, it is also advisable to consider the right type of MOF under different pressure and temperature conditions and gas types (see Figure 6). For example, MOFs suitable for gas storage must have a larger pore volume, whereas MOFs suitable for separation processes must be sticky or have high selectivity (see Table 1). Similarly, certain gases are known to form gas hydrates at different thermodynamic hydrate stability pressures, which also contribute to the selective separation of a gas from a given gas mixture. Gases also differ in terms of their polarity and molecular size. Because gases in hydrate technologies have different thermodynamic stability pressures, the pressure requirements for gas storage differ from the pressure requirements for gas separation. For example, in the separation of CO2 impurities from a CH4/CO2 gas mixture (biogas mixture), the pressure should be lower than the CH4 hydrate stability pressure but higher than the CO2 hydrate stability pressure, and the selected MOF should have a higher selectivity toward CO2 than that toward CH4 gas in a certain pressure range and temperature.
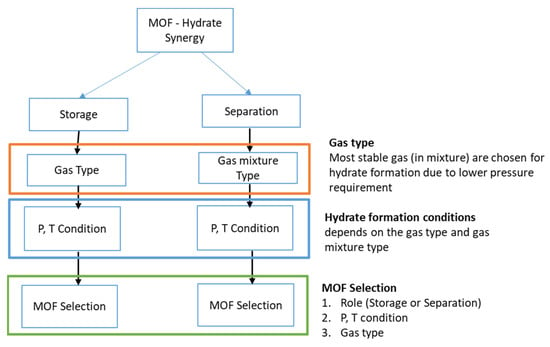
Figure 6.
Possible decision matrix concerning the selection of MOFs for MOF–hydrate synergy.

Table 1.
Differences in MOF design criteria as an adsorbent for CO2 capture and CH4 storage [43].
4. MOF–Hydrate Synergy: Current State of the Art
Hydrate–MOF synergies have been investigated mainly in CH4 gas storage and in CO2 gas storage and capture, and the initial results are promising. CH4 gas is a major component of natural gas, and higher natural gas consumption is promoted due to lower CO2 emissions and higher octane values compared with petroleum or coal consumption [49]. Currently, natural gas is transported either in a compressed form or by liquefaction under difficult conditions (high pressures and very low temperatures) because natural gases have a low density, low boiling point, high critical pressure and high diffusivity, and their transportation and storage consume excessive energy [50]. Therefore, hydrates provide an alternative medium for storage and transportation under moderate conditions and in safer environments [51,52]. When CH4 gas is stored in hydrates, the storage capacity varies between 160 and 180 v/v, and the CH4 gas molecules do not fill all hydrate cages. Therefore, different methods (stirring, promoters and porous media) are being tested to improve kinetics and storage capacity at the laboratory scale [53]. Research on the synergy between hydrates and MOFs is limited to CH4 and CO2 hydrates, and no such studies exist for the storage of hydrogen hydrates in MOFs. However, recent publications using activated carbon have confirmed that nanomaterials can positively accelerate hydrogen hydrates in confined spaces at lower pressures and can enable higher conversion of water to hydrates [54].
4.1. MOF Material Characterization
The characterization of the process of hydrate formation and dissociation and its structure in a confined space is challenging due to the limitations of visualization techniques (size of the confined space < 100 nm) and the possibility of a phase change in the host structure or the main water phase [55]. Visualization techniques, such as magnetic resonance imaging (MRI) and X-ray computed tomography (CT), are not always effective due to their lower resolutions (7.5 µm) and inability to distinguish hydrates from the ice phase. Therefore, alternative techniques, such as the high-pressure adsorption technique, could be sufficient for pre-wetted material [55]. Inelastic neutron scattering (INS) at low temperatures (about 4 K) and in the presence of deuterated water could also be used to identify hydrogen atoms in gas hydrates, either in bulk or in a confined nano space, such as metal–organic frameworks or the cavities of nanoporous carbons [56,57]. Synchrotron X-ray diffraction (SXRD) is another technique that offers additional advantages over single-crystal X-ray diffraction due to its high intensity and associated shorter sampling time. Some of the first methods for characterizing CH4 hydrates in confined spaces using SXRD show a decrease in unit cell parameters in microporous and mesoporous carbons [58]. Other SXRD results confirmed the thermal stability of CH4 and CO2 hydrates in confined spaces [59]. Raman and 13C NMR spectroscopy techniques are other potential techniques that could be used to characterize gas hydrates at the nanoscale. However, sufficient research is still needed in this direction because there are few studies on this topic [60,61]. Scanning electron microscopy (SEM) has been used to investigate crystal morphology and stability before and after the MOF and hydrate system went through a heating and cooling cycle [62]. Recently, differential scanning calorimetry has been used to study the conversion of water to hydrates in nanoporous materials, such as MOFs, zeolites, porous organic cages, etc. [62,63,64].
Although the above techniques are well known and influential for the characterization of hydrates in confined spaces, the sample size is in the range of mg or less. To confirm the phenomenon on an industrial scale, a larger sample size (in grams) is required for laboratory experiments. In these cases, additional challenges, such as heat and mass transfer, can pose a much greater problem than those with smaller sample sizes. Unfortunately, there are very few examples of combined hydrate–MOF studies that focus on large MOF sample sizes to confirm and compare results due to upscaling. In one recent study [65], the authors argued that a packed column system is the best choice to study MOF–hydrate synergies upon upscaling. Therefore, they tested the synergy of HKUST-1 and CH4 hydrate in a packed column configuration (60 cm3) using chenille fabric and found that HKUST-1, when spread over the fabric, effectively reduces induction time, showing reproducible results and improving recyclability.
4.2. MOF–Hydrate Synergy for CH4 Storage
Most studies on the application of MOFs to improve gas hydrate technology are related to the formation of CH4 hydrates (see Table 2). Used MOFs can be divided into hydrophobic and hydrophilic MOFs. Water adsorption isotherms are generally used to characterize MOFs as hydrophobic and hydrophilic [66]. Studied MOF–hydrate synergies include Al metals (MIL-53), Fe based (MIL-100), Cr (MIL-101) and Cu metals (HKUST-1), and Cr-based MOF-1 and Y-shaped MOF-5 are used. MIL-53 and HKUST-1 are widely studied MOFs for gas adsorption due to their higher hydrothermal stability [67,68]. The water adsorption isotherm suggests that MIL-101 and MIL-100 (Fe) are the most stable MOFs in water as hydrophilic mesoporous compounds and compared with HKUST-1, and zeolitic imidazolate frameworks (ZIF-8 and ZIF-67) are characterized as hydrophobic MOFs [66]. In one study, amine-functionalized UiO-66 was used to perform flow assurance studies of natural gas hydrates.
Another common difference among MOFs used so far is the difference in their thermal conductivity. MOFs are known to have low thermal conductivity and are considered thermal insulators (thermal conductivity < 2 Wm−1 K−1 at room temperature) [69,70,71,72], and their thermal conductivity further decreases in the presence of adsorbates, such as liquids (water, ethanol and methanol) and gases (CH4 or CO2) [69]. (For example, the following are the thermal conductivities of selected MOFs: HKUST-1 = 0.44–0.73 W/(m K) [69], ZIF-8 = 0.32 W/(m K) [71], CH4-Cr-soc-MOF-1 = 1.3 W/(m K) [70] and CH4-Y-shp-MOF-5 = 0.32 W/m K [72]). HKUST- 1 has a relatively higher thermal conductivity, which facilitates local heat dissipation during hydrate formation and creates a suitable environment for hydrate growth. Available research also indicates that, in the presence of hydrates and ice, the overall thermal conductivity of the system improves [73,74], and the difference in the conductivity of the porous material also affects the dissociation rate of the hydrates [75].
In the case of MIL-53, Kim et al. [76] found that CH4 hydrates can only form in meso- and macroporous spaces and in interparticle spaces, with no hydrates forming in micropores (the micropore size was 0.6 nm, and the CH4 hydrate unit cell length was 1.2 nm). In this study, the authors also pointed out weaker thermodynamic inhibition compared with other porous materials, such as silica gel or carbon. In addition, two different zones of hydrate formation were found. First, gas hydrates formed at higher pressures; in the second zone, hydrates formed at lower pressures in a confined space.

Table 2.
Available research on MOF–hydrate synergy for CH4 storage (n = maximum CH4 stored in H2O system in mmol/g, Rw = ratio of water/MOF (g/g), Cmax = Maximum water to hydrate conversion %, Td = dissociation temperature at Cmax, WC = water content %).
Table 2.
Available research on MOF–hydrate synergy for CH4 storage (n = maximum CH4 stored in H2O system in mmol/g, Rw = ratio of water/MOF (g/g), Cmax = Maximum water to hydrate conversion %, Td = dissociation temperature at Cmax, WC = water content %).
| MOF Properties | |||
|---|---|---|---|
| Gas Name + MOF Reference Paper/Wettability | Pore Diameter (nm) and Specific Surface Area in m2/g | Pore Volume (cm3/g) | P (Max), T Condition/Key Observation |
| CH4-MIL-53 [76] (Hydrophilic) | 0.6, >10 nm (1500) | 0.18 (micro), 0.49 (Meso) | p = 94 bar T = 276 − 285 K Phase line for bulk and confined hydrates WC = 30% |
| CH4-HKUST-1 [62] (Hydrophilic) | 1.0 (1000) | p = 80 bar, T = −10 °C to 30 °C n = 8.1 mmol/gram at Rw = 1.02, Cmax = 87.2%, Td (Cmax) = 10.7 °C | |
| CH4-MIL-53 (Al) [77] (Hydrophilic) CH4-HKUST–1 (Hydrophilic) CH4-ZIF-8 (Hydrophobic) | 0.85 0.39, 0.9 1.16 | 0.59 0.79 0.65 | p = 120 bar, T = 1 °C WC = 0–35% MIL-53, n = 0.77 (wet) HKUST-1, n = 1.26 (wet) For ZIF-8, n = 8 (wet) |
| CH4-MIL-100 (Fe) [57] (Hydrophilic) CH4-ZIF-8 (Hydrophobic) | 2.4–2.9 (1476) 1.2 (1565) | 0.87 0.72 | T = 2 °C p = 100 bar Rw = 0–0.56 (MIL-100 (Fe)) nCH4 (max wt%) = 8% (Rw = 0). MIL-100 (Fe) Rw = 0–0.6 (ZIF-8) nCH4 (max wt%) = 16% (Rw = 0.6) |
| CH4-Cr-soc-MOF-1 [78] (Hydrophilic) CH4-Y-shp-MOF-5 (Hydrophilic) | 1.5, 1.7 (4500) 1.2 (1550) | 0.63 | T = 1.85 °C (275 K) Rw = 0–4 (MOF-1) nCH4 (max wt%) = 24% (Rw = 3.4) p = 70–80 bar Rw = 0–0.75 (MOF-5) nCH4 (max wt%) = 12% (Rw = 0) p = 60–70 bar |
| CH4-ZIF-8 [79] (Hydrophobic) | (1273) | 0.61 | T = −4 °C and 1 °C p = 110 bars Wc = 0–35.1% nCH4 = 9.304 mmol/g at T = 269.15 K and p = 28.5 bar (WC = 35.1%) |
| CH4-ZIF-8 (Zinc-based) [63] (Hydrophobic) CH4-ZIF-67 (Cobalt-based) (Hydrophobic) | 1.2 (1052) 1.2 (1585) | 0.6 | p = 80 bar, T = −15 °C to 30 °C Rw = 0–1.01, Cmax = 80% (ZIF-8) n = 7.9 mmol/g at Rw = 1.01 Rw = 0–0.82, Cmax = 83.6% (ZIF-67) n = 8.1 mmol/g at Rw = 0.82 |
| CH4-MIL-101 [80] (Simulation study) | 2.9–3.4 (nm) | p = 500 bar, T = 275 K Rw = 0, 0.68, 9.46 | |
| CH4-ZIF-8 [81] Hydrophobic (Simulation study) | 1.16 (nm) | p = 100 bar, T = 275 K Wc = 30% | |
| CH4-UIO-66-NH2 [82] Hydrophobic (Flow assurance study) | n/a | n/a | T = 272–285 K p = 100 bar |
Zeolitic imidazolate frameworks (ZIF) are a subclass of organometallic frameworks that contain metal ions (inorganic) and imidazolate linkers (organic) and form a framework of tetrahedral structural units, similar to zeolites [83]. The main difference between ZIF-8 and ZIF-67 is the different metal ions (Zn in ZIF-8 and Co in ZIF-67). ZIF-8 has been extensively tested for CH4 storage in hydrates due to its high methane absorption capacity (∼8 mmol/g at 80 bar) [77,84] and its hydrophobic properties, which increase the stability of ZIF in the presence of water [85] and under high pressures (up to 800 bar) [84]. ZIF is mainly a microporous MOF (pore size < 1.2 nm), whereas other common MOFs also have a sufficient amount of meso- and macropores. Available research results indicate that ZIF-8 has higher gas uptake than other available MOFs, such as HKUST-1 and MIL-53, in both the dry and wet states, which is due to its large surface area and hydrophobic nature [77].
Although ZIF’s framework exhibits low water adsorption (0.2 mmol/g–0.5 mmol/g under various relative pressures) [86,87], it has been reported that ZIFs have a flexible structure, and the pore windows expand under high pressures (3.4 Å) to allow the diffusion and transport of CH4 gas molecules (3.8 Å). This is referred to as the “gate opening phenomenon” [88] and shows that the adsorption of CH4 molecules under high-pressure conditions increases from 1.8 mmol/g (at 10 bar) to 8.5 mmol/g at 100 bar [77], confirming why ZIF exhibits higher gas uptake under both dry and wet conditions. In another study [63], the performance of microporous ZIF-8 and ZIF-67 was compared in CH4 hydrate formation, and no significant difference was found due to different metal sites (Zn and Co). Both ZIFs showed increased gas uptake under hydrate formation conditions, and hydrate formation occurred in the interstitial space between MOF crystals, which was attributed to the hydrophobic nature of the pores, enhanced gas diffusion through the crystals due to pore expansions and high gas adsorption in the inner pore walls (see Figure 7).
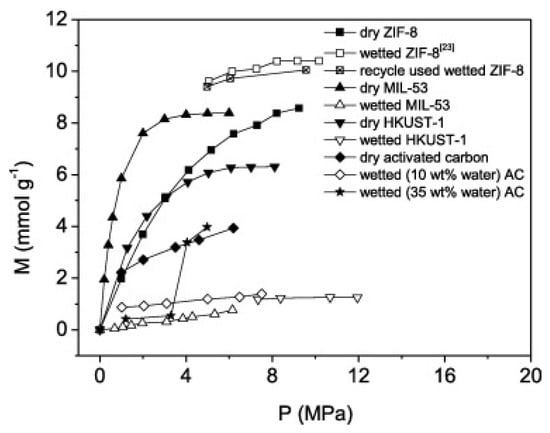
Figure 7.
Methane adsorption in dry ZIF-8, 30 wt% wetted ZIF-8, once-recycled 30 wt% wetted ZIF-8, dry and 25 wt% wetted MIL-53, dry and 30 wt% wetted HKUST-1 and nanoporous activated carbon (AC) with water contents of 0, 10 and 35 wt% at 274.15 K (Reprinted from [77], Copyright (2018), with permission from Elsevier).
4.3. MOF–Hydrate Synergy for CO2 Storage and/or Separation
Although CH4 and CO2 hydrates both form sI hydrates, the ability of CO2 hydrate to stabilize cages is better than that of CH4 hydrate because the molecular size of CO2 (5.12 Å) is larger than that of CH4 (4.36 Å), which also leads to differences in their thermodynamic stability [89,90]. Of the common gases that form hydrates, such as N2, CO2, CH4 and O2, CO2 forms the most stable hydrate. Therefore, it is more thermodynamically stable and has faster formation kinetics. This difference is used to capture and separate CO2 from gas mixtures containing CO2, e.g., CO2/N2 mixtures [91] or CO2/H2 mixtures [92]. Although the thermodynamic driving force to separate CO2 gas from the gas mixture is strong, the method suffers from low separation efficiency and is limited by operating conditions. Most work on the synergy between hydrates and MOF is limited to CH4 hydrates, and minimal research is available for pure CO2 gas (see the Table 3) and CO2 gas separation and storage from a mixture.

Table 3.
Available research on MOF–hydrate Synergy for CO2 storage (n/a = not available).
The kinetics of the CO2 hydrate formation of water-wetted HKUST-1 were studied using experiments and computer simulations [36]. The authors justified the selection of HKUST-1 based on its well-established pore structure, relative stability in water-rich environments and the extensive experimental and modeled data available as a gas adsorbent [93]. The HKUST-1 used in this study had hydrophobic (micro) and hydrophilic (meso/macro) pores. The authors used partially saturated HKUST-1 crystals (36 wt% water) to study the kinetics of CO2 hydrate formation. CO2 hydrate formation was better than that of H2 and N2 hydrates because the CO2 molecule has a negative chemical potential (due to the higher quadruple moment of CO2), so both physisorption and gas hydrate formation can proceed better compared with H2 and N2 gases, making it a better candidate for gas separation [36].
Furthermore, it was also found that CO2 molecules tend to settle in small hydrophobic pores at low pressures and in large pores at higher pressures. The authors also suggested that CO2 storage occurs in the pore space partially surrounded by water molecules because the chemical potential of CO2 in water-saturated HKUST-1 is negative enough to replace water because of it having a higher quadrupole moment than that of H2 or N2. Therefore, the authors argued that the different gas storage capacities of HKUST-1 can be used for CO2 gas separation from gas mixtures, such as CO2 + N2 and CO2 + H2 (see Figure 8).
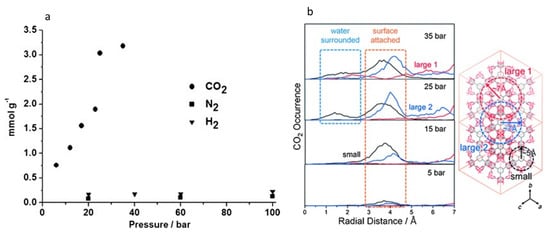
Figure 8.
HKUST-1-based CO2 gas stored under hydrate formation conditions: (a) Gas storage (mmol/g) of water-saturated HKUST-1 at different pressures; (b) Spatial distributions of CO2 along the radial direction from the center of three different pore types (one small pore and two large pores) (Reprinted from [36], copyright (2015) with permission from WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany).
There are very few studies on the efficiency of the capture/sequestration of CO2 from CO2/N2 or CO2/H2 mixtures using the synergy of gas hydrates and MOFs. It has been argued and experimentally demonstrated that microporosity and mesoporosity play a more important role in enhancing hydrate formation than the pure bulk phase. Therefore, further studies should be carried out on the MOFs with microporosity and mesoporosity to investigate CO2 capture and storage under gas hydrate formation conditions [47].
5. MOF–Hydrate Synergy: Physical Properties
5.1. Confinement–Compression Effect in Nanopores
Nanopores experience both a confinement effect and a supercompression effect. The result of these two effects can control hydrate nucleation, growth kinetics and the conversion of water to hydrates, as well as formation pressure (see Figure 9).
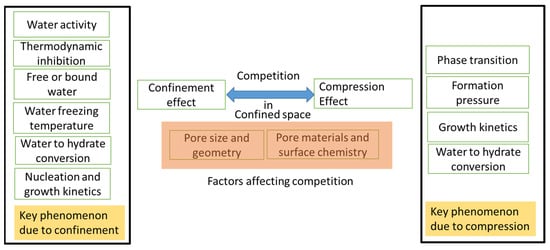
Figure 9.
Proposed competition governing hydrate formation and growth kinetics in confined nano space.
The existence of the compression effect in nanopores has been confirmed in several studies. Fujimori et al. [94] observed the formation of a monoatomic sulfur chain with exceptional conductivity properties, which could be due to the presence of ultra-high pressures (above 90 GPa) with one-dimensional carbon nanotubes under ambient pressures. Another phase change within nanotubes was successfully achieved when Urita et al. achieved the phase transition from B1 to B2 in KI crystals, a transition process that generally occurs above 1.9 GPa under bulk conditions [95]. Therefore, the high compression effect in nanopores can be used for phase changes or for the growth of other crystalline structures, such as ice or gas hydrates [22]. Studies on the pronounced compression effect during hydrate formation have not been adequately explored and therefore require further investigation.
The confinement effect occurs in pores whose sizes correspond to the lateral dimension of a few molecular diameters and affects the physicochemical properties of the liquid phase. In nanopores, the interactions (repulsive and attractive interactions) between the liquid–pore interface and the gas–liquid interface develop adsorption potential and control the properties of molecules or atoms. Due to the confinement effect, the liquid phase in the pores can undergo a phase transition compared with the liquid phase under bulk conditions [96]. Furthermore, the interface between the molecule and the surface is generally controlled by the surface curvature, so a curved surface has a van der Waals energy that is eight times larger than that of a flat surface [97]. Therefore, entrapped molecules exhibit various properties such as super mobility, strong physisorption and a high heat of adsorption [98].
To investigate the confinement effect, CH4 and C3H8 hydrates were formed in silica gel pores (radius 7 nm) [99]. It was found that the experimental formation pressure in the pores was higher than that in the bulk phase. Due to the confinement effect, the water activity decreases, the required hydrate formation pressure increases and the freezing temperature of water decreases [100]. In the case of extremely low water activity and an extreme decrease in the freezing temperature of water, the water in these nanopores can reach a supercooled state. The increase in thermodynamic stability pressure in nanopores compared with the main phase is also known as thermodynamic inhibition. Some key factors that may contribute to the enhancement of thermodynamic inhibition are the metastable state of the fluid, surface heterogeneities and pore interconnection effects [101]. The inhibition effect in a confined space could depend on the surface properties of the pores and the pore size and geometry [76]. As the pores become smaller, the water activity in the pore space becomes weaker, and the inhibition effect becomes stronger due to geometric constraints in the nanopores [102,103,104].
In another study conducted on confined water in the cavities of activated carbon under atmospheric pressure, the trapped water did not freeze in the micropores and did not undergo a phase transition from liquid to solid. However, water did freeze in large micropores and mesopores, albeit at a much lower freezing point. Moreover, at high pressures and in a CH4 gas environment, freezing (CH4 hydrate formation) was observed in confined micropores at higher temperatures than those in previous cases [105].
Thus, the authors hypothesize that there is competition between the compression and confinement effects (see Figure 9) and that the dominant mechanism controls gas hydrate nucleation and growth kinetics. It is also possible that different dominant mechanisms exist in different pore spaces of the same material. If the confining effect dominates, one would expect lower water to hydrate conversion, a high-required formation pressure and a long nucleation time. On the other hand, there are also experimental studies that confirm the presence of a dominant compression effect. For example, a recent study on hydrogen hydrates in nanopores showed a 30% lower formation pressure than that in the bulk phase and almost 100% conversion of water to hydrates using activated carbon [54].
5.2. Pore Size Effect
One of the main conclusions from the available studies and reviews is that nanomaterial surface chemistry and pore size control and influence hydrate formation in confined spaces [106]. Pore size controls water intrusions and extrusions, water properties in confined spaces, the hydrate formation mechanism and hydrate phase behavior [47,107]. Micropores with a diameter of <1.2 nm do not provide a sufficient internal volume for hydrate cages to assemble during hydrate nucleation [108] (see Figure 10), although some studies have discussed the possibility of defective nano hydrates [109]. Due to the lack of advanced characterization techniques, it is difficult to quantify hydrates and the formation mechanism in micropores [22]. It would not be wrong to conclude that microporosity is not exploited for hydrate formation. However, micropores can be exploited for gas adsorption due to their high gas solubility [110].
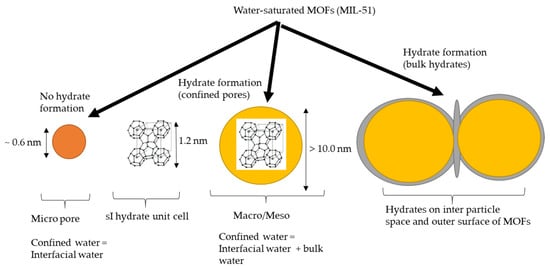
Figure 10.
Hydrate formation mechanism in the presence of MOF. Role of surface area and pore size.
On the other hand, mesoporosity facilitates water intrusion by capillary condensation [111] and generates the gas–liquid interface in addition to the liquid–solid interface [112]. It has also been found that hydrates form first in macroporous pores and then in mesoporous pores at higher pressures [110]. The difference in pore size also controls hydrate stability and dissociation temperatures. Hydrates that form in larger pores have a higher dissociation temperature [113]. As pore size decreases, the ability to form hydrates within the pores decreases, and hydrates are more likely to form in the interparticle space and on the outer surface.
Experimental results show that hydrate crystals do not form in wetted micropores, and hydrate dissociation is similar to that in the bulk phase. In wetted mesopore nanomaterials (diameter > 6.2 nm), the hydrate forms in the pores at higher pressure and lower temperature conditions, whereas in wetted macropore nanomaterials (diameter > 100 nm), there is no effect of pore space on the hydrate stability line [77].
Pore diameter also affects the amount of interfacial water trapped in the pore space. For example, interfacial water at a distance of 0.5 nm from the surface was found to have different structural properties compared with that of the bulk phase (e.g., loss of hydrogen bonding and no freezing on cooling) [114]. Therefore, it is difficult to form hydrates in microporous materials with a pore diameter of <1 nm, because the interfacial water has different structural properties.
Currently, the formation of hydrates in nanomaterials appears to be most promising if the mesopores can be optimized. Therefore, nanomaterials with dominant mesopores have shown higher conversion of water to hydrates compared with other microporous compounds. For example, up to 95% conversion of water to hydrates has been observed in activated carbon with well-developed mesoporosity [115,116]. In another study, the CH4 adsorption capacity in a water-saturated activated model porous carbon increased from 18% (pore size = 0.8 nm) to 102% and to 170% (pore size 10 nm–25 nm) when the pores become mesopores [58]. This experimental evidence confirms the positive role of mesoporous carbon and suggests that metal–organic frameworks with well-defined mesoporosity can be beneficial, whereas microporosity can support methane adsorption at the inner pore surface.
5.3. Hydrophobicity
MOFs are generally hydrophilic (>10,000 MOFs), and hydrophobic MOFs and MOF-derived composites are a small subclass of MOFs (<100 MOFs) [117]. The hydrophobicity of MOFs can be divided into surface hydrophobicity and internal pore hydrophobicity. The degree of hydrophobicity controls the competition between water adsorption and gas molecule adsorption. In the early days of MOF-based studies, hydrophobicity was largely overlooked. Today, however, hydrophobic MOFs are of great interest because hydrophobic MOFs are believed to improve mechanical and chemical stability, water stability, working efficiency, durability and reusability in various applications.
A recent study (see Figure 11) showed that, for MIL-100 (Fe) hydrophilic MOFs, the presence of moisture/water inhibits the hydrate formation process in the intrinsic porosity. The figure shows that the adsorption capacity, at a high pressure in the presence of water, was much lower than that of a dry sample because the water adsorbed and blocked the pores [57]. Using hydrophobic MOFs such as ZIF-8, it was found that wet and dry samples had similar adsorption isotherms up to a pressure of 30–40 bar (CH4 hydrate stability pressure); after that, the adsorption profile of the wet MOF increased dramatically, indicating the onset of gas hydrate formation. Hydrate formation occurred in the interparticle space, whereas the non-wettable microporosity only participated in the adsorption process by pure physisorption [57].
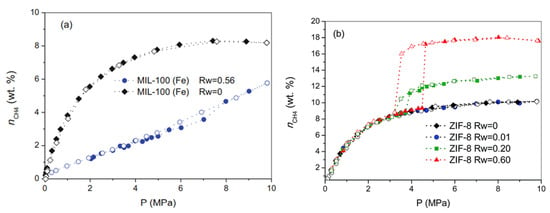
Figure 11.
High-pressure CH4 adsorption/desorption isotherms (275 K and up to 100 bar): (a) Hydrophilic framework of MIL-100 (Fe) (dry and wet); (b) Hydrophobic framework of ZIF-8 (dry and wet). Wet conditions correspond to different water contents (Rw is the amount of water in g/g) [57] (Reprinted from [57] due to open access permission).
The above study suggests that hydrophobic MOFs are better suited for gas hydrate formation because they can reduce competition between water and CO2. It is also suggested that the hydrophobic surface does not disturb the ordering of water molecules required to form hydrate cages around the guest molecule. In contrast, the hydrophilic surface disturbs the tetrahedral arrangement of the water structure and reduces gas density at the interface between the hydrophilic surface and the water [106]. Experimental work has also shown that a hydrophilic surface inhibits the nucleation of gas hydrates [114,118] because the interfacial water near the hydrophilic surface experiences a significant loss of tetrahedral water caused by H-bonds between the phases [114,118]. It was also discovered that dissolved gas molecules tend to collect closer to the hydrophobic surface–water interface in the form of surface nanobubbles [119] due to hydrophobic attraction [120,121]. A detailed discussion of surface nanobubbles and their role in hydrate formation can be found in another review article [122].
In hydrophobic MOFs, CO2 adsorption can occur in physisorption and hydrate formation. In hydrophobic MOFs, hydrates form only in the interparticle space, so the total surface area and micropores are not used (physisorption only). The above results contradict the idea that water spreads much more on hydrophilic surfaces than it does on hydrophobic ones. Therefore, the gas–liquid interface is higher, but the low water activity of hydrate formation overrides the ability to spread water on hydrophilic surfaces. It is also pointed out that hydrophilic MOFs with high water content can saturate the nanopores with water, limiting gas diffusivity through the MOFs. Therefore, further research should be conducted to optimize the water content of hydrophilic but water-stable MOFs to take advantage of high water dispersion on the surface and the nano-confinement properties of water. Efforts should focus on developing water-stable MOFs with superior water dispersibility, high surface water activity and a high degree of nanoscale confined water.
In a recent study, the effect of the weight ratio of water/HKUST-1 was investigated to study the conversion of water to CH4 hydrates using high-pressure µ-DSC [62]. When the ratio was increased from 0.39 to 1.08, the conversion of water to hydrates increased from 20.5% to 87.2%. The authors took advantage of the hydrophilic nature of HKUST-1 and its high specific surface area to synthesize hydrates on the surface of an MOF. Therefore, the hydrates formed here were dominated by bulk hydrates in the interstices between MOF particles and on the surface. The used HKUST-1 exhibited two types of nanopores with diameters of 1.4 nm and ~1 nm, but no hydrate formation was confirmed in the pores. After hydrate dissociation, the authors observed expansion of the pore space and the partial decomposition of HKUST-1 in SEM images. The MOF sample size used in this study was in the order of mg, and the sample preparation method was different from that used in previous studies. It should be considered that HKUST-1 has a hydrophilic surface, but the nanopores are hydrophobic and have a hydrophobic inner pore surface and can act as hydrophilic MOFs.
Another study investigated hydrophobic and hydrophilic mesoporous carbon materials for confined methane hydrate formation [112]. The results show that hydrophobicity controls water mobility at the pore walls, which controls water conversion to hydrates. It was also found that confined water in hydrophobic mesopores is converted to hydrates, and liquid water is uniformly distributed in hydrophilic mesopores but is not converted to hydrates.
Nanopores are inherently hydrophobic due to their small size, allowing water to enter and exit under high pressure. It is unclear whether water intrusion/extrusion can occur under hydrate formation conditions (high pressure and low temperature) and whether it can be sustained during multiple cycles of formation and dissociation [123]. A preliminary hydrophobic MOF ZIF-8 with a pore size of 0.34 nm allows water molecule intrusion (0.29 nm) between 19.9 MPa and 22 MPa [124,125].
Recycling and the water stability of MOFs control the lifetime and frequency of the replacement of MOF materials to maintain profitable commercial operations [126]. Although MOFs have good thermal conductivity, many MOFs tend to decompose in humid environments because the bonds between metal and oxygen are unstable and decompose by hydrolysis [127]. Zeolites imidazolate frameworks (ZIFs) are a subclass of MOFs known for their water, acid, chemical and thermal stability [127]. Recent research on zeolite-based hydrate formation shows that zeolites form gas hydrates in their lattice structures [79]. It was found that the growth of hydrates occurs in the interstitial space between the pores because water cannot penetrate into the hydrophobic inner pores. The study showed a 45% improvement in CH4 gas storage capacity in hydrates.
5.4. MOF Chemistry and Surface Functionalization
The surface chemistry of nanopore materials is expected to control growth kinetics. It has been proposed and experimentally proven that, when water is present in nanomaterials, thin water films develop at the water–solid interface, and hydrate nucleation starts at the solid–liquid interface rather than at the liquid–gas interface. The growth rate of the hydrate film is controlled by the mass-transfer-based driving force caused by the difference in methane saturation in the liquid phase at the gas–liquid interface and the liquid–solid interface [128,129,130]. It is argued that surface chemistry plays an important role because hydrate formation starts at the liquid–solid interface. For example, an experimental study [131] showed that activated carbon has improved growth kinetics and a shorter induction time (30 min) compared with nano-silica (84 min). This difference could be due to the interconnectivity of the pore space, which can facilitate the formation of crystal nuclei and can support hydrate growth during the transient hydrate formation and dissociation process [55].
There are few studies on the formation and dissociation of hydrates in the presence of MOFs, and there are no initial studies on the effects of MOF chemistry on hydrate formation. Since carbon-based nanomaterials (activated carbon, porous model carbon and multi-walled carbon nanotubes) have shown promising results for hydrate growth kinetics, the authors believe that carbon-based MOFs could be tested for hydrate formation and dissociation studies.
The kinetics of gas hydrate formation in a confined space depend on water–gas and water–solid surface interactions. The difference in induction time is the main difference between MOF materials and other microporous materials (carbon, silica or zeolite-based) in hydrate formation and dissociation. The difference in induction time is due to the different pore sizes and surface chemistry of the different materials [55]. Because MOFs are a rapidly developing field of research and new MOFs are discovered regularly, it is of great interest to thoroughly investigate the effects of MOF materials on formation and dissociation kinetics. The results of such a study could open the door to developing new MOF materials with a suitable surface chemistry, which is expected to optimize hydrate storage concerning specific gases, such as CH4, CO2 or H2.
In addition to controlling nucleation and growth kinetics by altering material chemistry, another means of control is using kinetic promoters in confined spaces. Kinetic promoters are a class of chemicals that, when added to water, affect kinetics without affecting thermodynamic stability. Common kinetic promoters include various surfactants [132] and amino acids. Their ability to promote kinetics has been tested in a porous sandy medium [133,134,135]. Few studies focus on the combined role of nanomaterials and kinetic promoters [136,137], which should be further explored. Another way to improve water activity and the interaction of water molecules at the water–solid interface is to change the surface chemistry via surface functionalization. When oxygen-containing surface groups are introduced into the pore surfaces of carbon nanomaterials, it was found that the formation of CH4 hydrates at lower pressure conditions (about 30–40 bar) was enhanced by promoting the formation of water clusters, leading to hydrate formation at lower pressures [138]. More detailed studies are needed, especially in the case of metal–organic frameworks, because surface functionalization is expected to enhance both physisorption and gas hydrate formation (Refer to Figure 12).
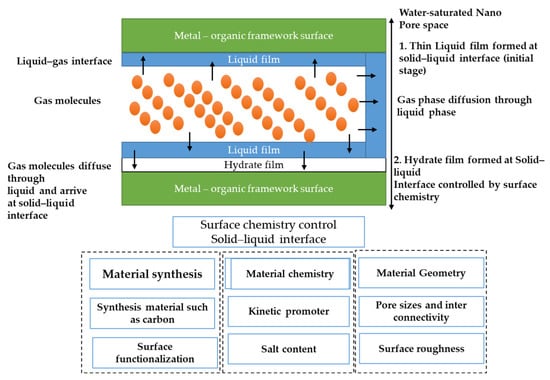
Figure 12.
Proposed overview of factors affecting surface chemistry during hydrate formation in pore spaces.
5.5. Water Properties in Confined Spaces
Water properties in confined spaces depend on pressure, water chemistry, the degree of water saturation, pore size, surface chemistry and surface properties. The liquid phase in nano-confined spaces is subject to the over-solubility phenomenon. Researchers have found that the solubility of nonpolar gases, such as CH4 and CO2, in supercooled water increases by almost 100 fold in a nano-confined space [56,139,140]. Research has confirmed that water in nanopores enters a supercooled state such that the melting temperature can be less than 50 K, depending on the pore size [141]. In this supercooled state, water exists in two states (low-density liquid and high-density liquid) [142] and can be investigated for the known thermal anomalies of water. Based on the authors’ interpretation and available research, the high gas uptake of water confined in nanopores of MOFs under hydrate formation conditions can be divided into four mechanisms [139,143,144] (see Figure 13).
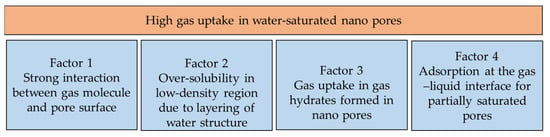
Figure 13.
Proposed contribution towards gas over-solubility in water confined in nano pore space.
It is not known what the relative contributions of the above factors are. The factors depend on the water saturation in the pore space and the surface chemistry of the pore space. The solubility of gases in a solvent confined in nanopores lies between Henry’s law and Langmuir’s curve [144]. The over-solubility of gases in a nano space can be caused by two main mechanisms [145], mainly adsorption or entrapment. Which mechanism ultimately dominates depends on the predominant interaction between the gas–solid interface and liquid–solid interactions. In cases where liquid–solid interactions predominate, gas over-solubility is driven by confinement. In this case, over-solubility can be further increased by decreasing the water saturation relative to full saturation, forming a gas–liquid interface. It has been argued that over-solubility does not depend on the specific surface area but can be optimized by controlling the pore size, because an optimal pore size allows layering of the liquid without strong curvature effects within the nano-confinement [145]. Thus, it is possible to take advantage of microporosity due to over-solubility because microporosity in a metal–organic framework is not involved in forming hydrate crystals. Therefore, metal–organic frameworks with optimal microporosity and mesoporosity distributions, optimal pore sizes and partial water saturation levels can optimize the overall gas uptake due to hydrate formation and over-solubility. Thus, it should be further investigated.
A thermodynamic study of CO2 hydrates in MIL-53 [76] confirmed the nano-confinement effect on water present in nanopores (see Figure 14). The hydrophilic MIL-53 exhibited micropores and meso/macropores due to a limitation in the laboratory synthesis method. When the pores were saturated with water and the hydrate phase diagram was plotted, it showed that two different hydrates were present (trapped and bulk hydrates), with the number of trapped hydrates in the meso/macropores being much higher than that of bulk hydrates. Due to CO2 over-solubility in micropores, a difference was observed in the formation and dissociation phase diagram. Based on the difference between the pore size and the hydrate unit size, it was assumed that hydrates cannot form in micropores and that CO2 is dissolved in water-saturated micropores via both physisorption and a confining effect. As a result, CO2 was dissolved in water-filled micropores, and CO2 hydrates formed in meso/macropores due to a weaker inhibition effect. In this study, complete dissociation did not result in complete gas recovery, so the gas remained highly soluble in the water-saturated nanopores even after complete dissociation. This confirmed the over-saturation of nonpolar CO2 gas in a confined space that is thermodynamically and kinetically stable [76]. Other studies have not reported such effects due to differences in hydrophilicity that allow water intrusion into nanopores, the degree of water saturation, compatible microporosity or differences in gas molecules. Although this phenomenon has been less observed in studies based on gas hydrates, available research indicates unusual water properties in nano-confinement, affecting gas solubility in water confined in nanopores.
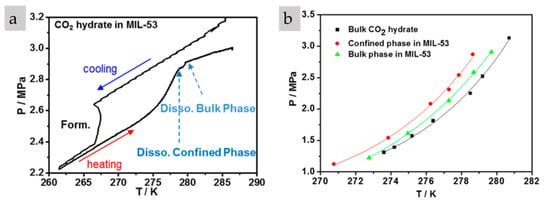
Figure 14.
CO2 hydrate phase diagram in the presence of water-saturated MIL-53 [76]: (a) Cooling and heating cycle; (b) Thermodynamic stability curve (Reproduced from [76], copyright (2015), with written permission from the American Chemical Society).
6. MOF–Hydrate Synergy: Metal–Organic Framework Stability in Water
MOFs are known for their good thermal stability, but when they come into contact with moist gases, many MOFs decay. However, ZIF MOFs have shown considerable thermal and chemical stability [127,146]. The water stability of MOF is of particular importance because large amounts of water vapor are present in various industries (natural gas streams, air pollution, air separation, etc.) where MOFs are to be used. Water vapor is also present in industrial flue gases (up to 10%), an important area for implementing hydrate-based gas separation, capture and storage. Because hydrate technology is water-based, the interaction between MOFs and water would be critical in MOF selection, controlling MOF efficiency and the cost of the overall process. The degree of hydrophobicity controls the water stability of MOFs. However, even hydrophobic MOFs can become water-unstable when the water loading is very high and exceeds the critical limit, but this phenomenon is not yet fully understood [40]. Hydrophilic MOFs can exhibit hydrophobic nanopores even under high compression if the pores are not large enough. Therefore, water intrusion needs to be further investigated in the context of hydrate formation in nanopores (especially mesopores and macropores) under high pressure. In addition to the chemical and thermal stability of ZIF-based MOFs, surface functionalization, e.g., with amine (-NH2), has also had a positive effect on the stability of MOFs and has improved CO2 uptake due to the interactions between amine and CO2 [126].
The stability of MOFs in a highly aqueous environment should also be considered when selecting MOFs for hydrate technology. MOFs should be structurally stable in the presence of water and after several formation/dissociation cycles. Currently, most available MOFs are hydrophilic, so their ability to survive in a water-rich environment under high pressures and low temperatures needs further investigation. Hydrophilic MOFs control the amount of water available for gas hydrate formation and facilitate water intrusion into the nanopores, and they usually have a nano-confinement effect. Metal nodes on MOFs tend to be hydrolysed in water, so further studies on hydrolytically stable MOFs need to be conducted. However, it is known that the metal ion charge controls hydrolysis reactions. Therefore, not all hydrophilic MOFs are water-unstable. For example, MIL-53 (Al-based) is more hydrophilic than MIL-100 and MIL-101 (Fe-based) but is also more water-stable than MIL-100,101 (due to the higher charge of Al in MIL-53). Therefore, it is necessary to identify hydrophilic MOFs with higher metal ion charges at the node and their relative water stability and to compare their performance at high water saturation. Another practical implication is to identify MOFs with optimal pore size distributions that can utilize micropores for gas absorption and meso/macropores for hydrate formation at lower thermodynamic pressures and enhanced hydrate growth.
The effects of water and high temperatures on the chemical and thermal stability of MOFs have been extensively studied, and many MOFs tend to undergo chemical decomposition due to either high temperatures or high water content. However, the chemical and thermodynamic stability of MOFs under hydrate formation conditions (high pressure, low temperature and presence of water) has not yet been studied. Recent experimental studies have shown that MOFs are relatively stable at lower temperatures for extended periods. For example, images from SEM showed that HKUST-1 is stable and does not chemically decompose after undergoing several cycles of formation and dissociation (in high-pressure differential scanning calorimetry(µ-DSC)) [62]. The chemical decomposition of HKUST-1 starts at the crystal structure [147], and its degradation can be controlled and reversed with an ethanol treatment, which improved the longevity of HKUST-1 crystals [148,149].
7. Practical Implications
Because MOFs are potential materials for improving gas hydrate technology, a successful pilot-scale demonstration requires scaling up the technology, which presents several challenges. First, there are very few success stories for commercializing MOF-based adsorption processes. For example, CALF-20, a water-stable MOF, was recently commercialized for CO2 capture and sequestration [150]. The combination of MOFs and hydrates can introduce additional complexity and further requirements (see Figure 15). Technology based on MOF–hydrate synergy should have a low cost and low energy consumption compared with competing technologies. Hydrates should have fast formation and dissociation kinetics in the presence of MOFs. MOFs should also exhibit higher gas selectivity, adsorption/storage capacity and hydrates. MOFs/hydrates should also be able to undergo multiple formations and dissociation cycles, with ideal MOFs being chemically, mechanically and thermally stable.
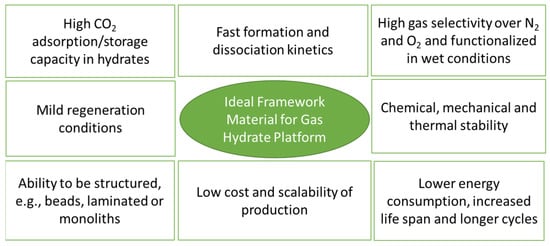
Figure 15.
Key factors responsible for high gas uptake in nano-confined water in MOFs.
Although MOFs are attractive materials for testing MOF–hydrate synergy, there are some shortcomings when comparing their properties with other available nanomaterials (see Figure 16) [151]. For example, most MOFs have spherical or cylindrical pore morphologies, which affect adsorption and storage performance. For example, studies have suggested that slit shape pore geometry in carbon nanomaterials has better gas adsorption than that of spherical or cylindrical geometry [152]. However, recent research on the role of pore geometry on hydrate formation suggests no significant effect on formation kinetics but an effect on hydrate stability [153]. In addition, MOFs contain metal nodes that affect the interaction between adsorbates and adsorbents (van der Waals interactions for non-polar molecules), which influence the regeneration process. Furthermore, MOFs have a lower thermal conductivity than those of carbon-based nanomaterials [154], which control the formation and dissociation steps in the combined MOF–hydrate process. Compared with carbon materials, MOFs also have lower mechanical strength and are, therefore, not good candidates for pellet and monolith fabrication, so there are fewer opportunities to reduce the volume occupied by MOFs in the combined process. Recently, MOFs have been proposed as a coating material for cheap packaging materials (first to improve the gas–water dispersion and to control the use of the expensive MOF) in large reactor designs (60 cm3) [65].
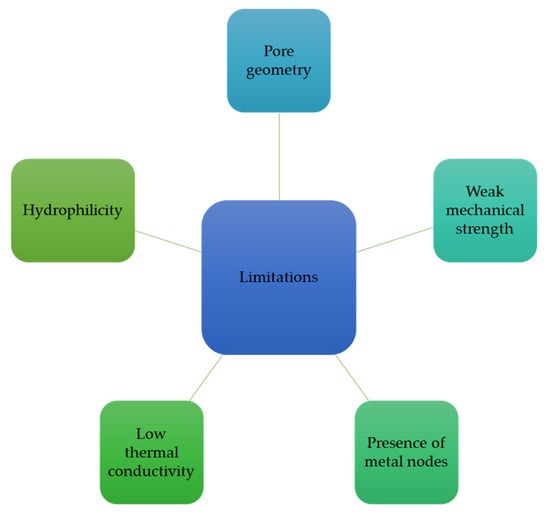
Figure 16.
Key limitations concerning metal–organic frameworks and their role in MOF–hydrate synergy.
The current state of art concerning MOF–hydrate synergy is summarized in the below figure (Figure 17).
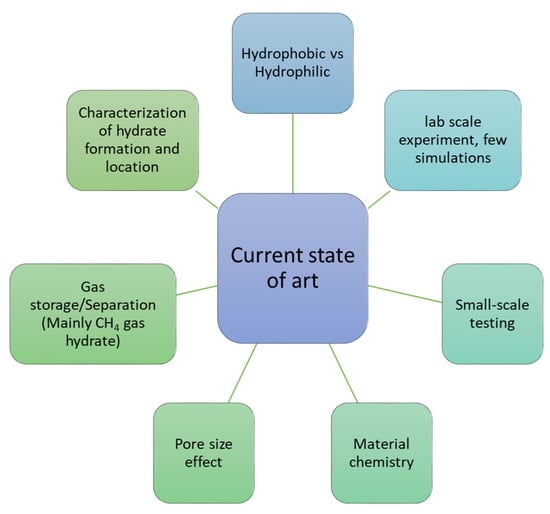
Figure 17.
Current state of art based on available research on MOF–hydrate synergy.
8. Conclusions and the Way Forward
This review discusses the current state of the art, highlights some scientific findings and their relevance and identifies unanswered questions that require further investigation. The current state of art and future prospects according to authors’ point of view are summarized in Figure 18.
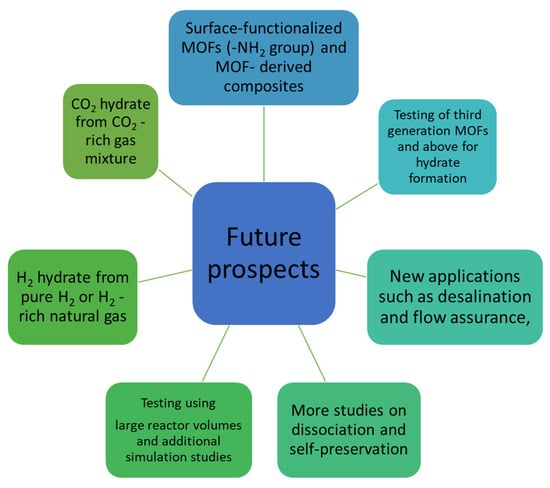
Figure 18.
Authors’ opinion concerning future research prospects studying MOF–hydrate synergy.
Some of the main conclusions and the way forward are highlighted below.
- The authors believe that the initial results are promising and that research should be further expanded, focusing on gases such as H2 [54] and H2-rich natural gas [3,155] and on CO2-enriched gas mixtures, such as CO2/N2, CO2/H2 and CO2/CH4. There is sufficient comprehensible literature on these gas mixtures in the field of gas hydrates that can serve as a starting point.
- Another research area where the application of MOF–hydrate synergies should be investigated is desalination. It is common practice to explore various porous media to enhance hydrate formation, and much of the emerging research is limited to silica-based porous materials. For example, hydrate-based desalination using CO2 hydrate formation may be one of the most promising areas. Current research is focused on using liquid or gaseous co-promoters (propane or cyclopentane) and porous silica systems to improve the separation of hydrates formed and water release [156,157]. Although MOFs have been studied for desalination by membranes [158,159], the role of MOFs in desalination through gas hydrates has not been explored.
- The synergy between MOFs and gas hydrates also raises many research questions. For example, the behavior of water in nano space under high pressures and low temperatures, the effects of pore sizes and degrees of water saturation in nanopores, the competition between high compression and the inhibition effect and its correlation with pore size, water intrusion and hydrophilic pores, and the effects of surface chemistry on hydrate nucleation and growth kinetics are some topics that need further investigation.
- A prerequisite for technological maturity is the cyclic nature of hydrate formation and dissociation and long-term stability during storage and transport. However, most of the available MOF–hydrate synergy studies have focused on gas hydrate formation studies, and there are very few studies on dissociation, total gas recovery and the self-preservation of gas hydrates in the presence of MOFs. Therefore, the authors recommend further investigation in this research direction.
- Preliminary research shows that hydrophobic MOFs are promising candidates with high water–hydrate conversion. Research also shows that the key factors affecting the nucleation and growth kinetics of gas hydrates in the presence of MOFs are (1) pore size distribution, (2) surface chemistry within the pores and (3) growth conditions. Available research indicates that the selection of MOFs depends on the gas type and MOF material, and the end application controls the chemistry and design of MOFs, as different MOFs need to be tailored and functionalized depending on the end application. The authors believe that MOFs with high thermal conductivity, lower production costs and better scaling-up feasibility should be considered.
- Laboratory-scale studies are limited to the microscale (volume < 1 cm3 and sample size < 1 mg). Various phenomena and synergies between hydrates and MOFs must be studied in large reactors to scale up the system. Hydrates in large volumes and metal–organic frameworks can use their self-preservation properties to remain stable during transport and storage at sub-freezing temperatures and at atmospheric pressure, compared with MOFs in the dry state under high-pressure conditions. Low MOF contents dispersed in various packaging materials should be further investigated under high-volume conditions.
- Previous studies have been limited to the use of pure water and saltwater in the study of MOF–hydrate synergies, ignoring the role of the kinetic promoter for further improvements. Therefore, further studies using environmentally friendly kinetic promoters (e.g., amino acids) to study hydrate nucleation and growth kinetics in MOFs would be interesting.
- Few studies [80,81] have used simulations to investigate the synergy between hydrates and MOFs. This leaves room for the broader application of simulation techniques to understand the mechanism and selection of the appropriate material from the MOF library.
Author Contributions
Conceptualization; Writing-original draft preparation, and review and editing; Resources; Funding acquisition: J.S.P.; Writing-review and editing; Resources, Supervision: N.v.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an international postdoctoral fellowship from the Independent Research Fund Grant Denmark (DFF), grant number 2031-00015B.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon Capture and Storage Update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Wang, P.; Li, K.; Yang, J.; Zhu, J.; Zhao, Y.; Teng, Y. Experimental and Theoretical Study on Dissociation Thermodynamics and Kinetics of Hydrogen-Propane Hydrate. Chem. Eng. J. 2021, 426, 131279. [Google Scholar] [CrossRef]
- Pandey, J.S.; Hansen, J.L.; von Solms, N. Hydrogen-Rich Natural Gas Hydrates Formation Kinetics in the Presence of Promoters. Chem. Eng. J. 2022, 432, 134295. [Google Scholar] [CrossRef]
- Gupta, A.; Baron, G.V.; Perreault, P.; Lenaerts, S.; Ciocarlan, R.-G.G.; Cool, P.; Mileo, P.G.M.; Rogge, S.; Van Speybroeck, V.; Watson, G.; et al. Hydrogen Clathrates: Next Generation Hydrogen Storage Materials. Energy Storage Mater. 2021, 41, 69–107. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Ren, B.; Li, H.-Y.; Yang, Y.-Z.; Wang, Z.-Q.; Wang, B.; Xu, J.-C.; Agarwal, R. CO2 Storage with Enhanced Gas Recovery (CSEGR): A Review of Experimental and Numerical Studies. Pet. Sci. 2022, 19, 594–607. [Google Scholar] [CrossRef]
- Duc, N.H.; Chauvy, F.; Herri, J.M. CO2 Capture by Hydrate Crystallization—A Potential Solution for Gas Emission of Steelmaking Industry. Energy Convers. Manag. 2007, 48, 1313–1322. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; von Solms, N. Screening of Amino Acids and Surfactant as Hydrate Promoter for CO2 Capture from Flue Gas. Processes 2020, 8, 124. [Google Scholar] [CrossRef]
- Rezvani, S.; Huang, Y.; McIlveen-Wright, D.; Hewitt, N.; Mondol, J.D. Comparative Assessment of Coal Fired IGCC Systems with CO2 Capture Using Physical Absorption, Membrane Reactors and Chemical Looping. Fuel 2009, 88, 2463–2472. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-Combustion CO2 Capture with Chemical Absorption: A State-of-the-Art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The Path towards Sustainable Energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.S.; Lively, R.P. Seven Chemical Separations to Change the World. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging Trends in Porous Materials for CO2 Capture and Conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-S.; Snurr, R.Q. Development and Evaluation of Porous Materials for Carbon Dioxide Separation and Capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Castellani, B.; Morini, E.; Filipponi, M.; Nicolini, A.; Palombo, M.; Cotana, F.; Rossi, F. Clathrate Hydrates for Thermal Energy Storage in Buildings: Overview of Proper Hydrate-Forming Compounds. Sustainability 2014, 6, 6815–6829. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, J.; Kim, H.; Seo, Y.; Linga, P. Hydrates for Cold Energy Storage and Transport: A Review. Adv. Appl. Energy 2021, 2, 100022. [Google Scholar] [CrossRef]
- Filarsky, F.; Schmuck, C.; Schultz, H.J. Development of a Gas Hydrate Absorption for Energy Storage and Gas Separation—Proof of Concept Based on Natural Gas. Energy Procedia 2019, 158, 5367–5373. [Google Scholar] [CrossRef]
- Wang, P.; Teng, Y.; Zhu, J.; Bao, W.; Han, S.; Li, Y.; Zhao, Y.; Xie, H. Review on the Synergistic Effect between Metal–Organic Frameworks and Gas Hydrates for CH4 Storage and CO2 Separation Applications. Renew. Sustain. Energy Rev. 2022, 167, 112807. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A. V “Nanoreactors” for Boosting Gas Hydrate Formation toward Energy Storage Applications. ACS Nano 2022, 16, 11504–11515. [Google Scholar] [CrossRef]
- Both, A.K.; Gao, Y.; Zeng, X.C.; Cheung, C.L. Gas Hydrates in Confined Space of Nanoporous Materials: New Frontier in Gas Storage Technology. Nanoscale 2021, 13, 7447–7470. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Albero, J. Clathrate-Mediated Gas Storage in Nanoporous Materials. In Green Energy and Technology; Springer: Singapore, 2019; pp. 383–403. ISBN 9789811335037. [Google Scholar]
- Sloan, E.D., Jr.; Koh, C.A.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780429129148. [Google Scholar]
- Sloan, E.D. Fundamental Principles and Applications of Natural Gas Hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Strobel, T.A.; Hester, K.C.; Koh, C.A.; Sum, A.K.; Sloan, E.D. Properties of the Clathrates of Hydrogen and Developments in Their Applicability for Hydrogen Storage. Chem. Phys. Lett. 2009, 478, 97–109. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Goh, M.N.; Arumuganainar, S.E.K.K.; Zhang, Y.; Linga, P. Ultra-Rapid Uptake and the Highly Stable Storage of Methane as Combustible Ice. Energy Environ. Sci. 2020, 13, 4946–4961. [Google Scholar] [CrossRef]
- Adeyemo, A.; Kumar, R.; Linga, P.; Ripmeester, J.; Englezos, P. Capture of Carbon Dioxide from Flue or Fuel Gas Mixtures by Clathrate Crystallization in a Silica Gel Column. Int. J. Greenh. Gas Control 2010, 4, 478–485. [Google Scholar] [CrossRef]
- Moon, S.; Lee, Y.; Seo, D.; Lee, S.; Hong, S.; Ahn, Y.-H.; Park, Y. Critical Hydrogen Concentration of Hydrogen-Natural Gas Blends in Clathrate Hydrates for Blue Hydrogen Storage. Renew. Sustain. Energy Rev. 2021, 141, 110789. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Vasheghani Farahani, M.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas Hydrates in Sustainable Chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef]
- Xu, C.-G.; Li, X.-S. Research Progress on Methane Production from Natural Gas Hydrates. RSC Adv. 2015, 5, 54672–54699. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of Metal–Organic Frameworks and Coordination Polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A Historical Overview of the Activation and Porosity of Metal–Organic Frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lim, H.-K.; Ro, H.; Kim, H.; Lee, H. Unexpected Carbon Dioxide Inclusion in Water-Saturated Pores of Metal-Organic Frameworks with Potential for Highly Selective Capture of CO2. Chem. Eur. J. 2015, 21, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Soubeyrand-Lenoir, E.; Vagner, C.; Yoon, J.W.; Bazin, P.; Ragon, F.; Hwang, Y.K.; Serre, C.; Chang, J.-S.; Llewellyn, P.L. How Water Fosters a Remarkable 5-Fold Increase in Low-Pressure CO2 Uptake within Mesoporous MIL-100(Fe). J. Am. Chem. Soc. 2012, 134, 10174–10181. [Google Scholar] [CrossRef]
- Yazaydın, A.Ö.; Benin, A.I.; Faheem, S.A.; Jakubczak, P.; Low, J.J.; Willis, R.R.; Snurr, R.Q. Enhanced CO2 Adsorption in Metal-Organic Frameworks via Occupation of Open-Metal Sites by Coordinated Water Molecules. Chem. Mater. 2009, 21, 1425–1430. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Benin, A.I.; Jakubczak, P.; Willis, R.R.; LeVan, M.D. CO2/H2O Adsorption Equilibrium and Rates on Metal−Organic Frameworks: HKUST-1 and Ni/DOBDC. Langmuir 2010, 26, 14301–14307. [Google Scholar] [CrossRef]
- De Toni, M.; Jonchiere, R.; Pullumbi, P.; Coudert, F.-X.X.; Fuchs, A.H. How Can a Hydrophobic MOF Be Water-Unstable? Insight into the Hydration Mechanism of IRMOFs. ChemPhysChem 2012, 13, 3497–3503. [Google Scholar] [CrossRef]
- Horike, S.; Nagarkar, S.S.; Ogawa, T.; Kitagawa, S. A New Dimension for Coordination Polymers and Metal–Organic Frameworks: Towards Functional Glasses and Liquids. Angew. Chem. Int. Ed. 2020, 59, 6652–6664. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous Metal-Organic Frameworks for Gas Storage and Separation: Status and Challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Zhou, W.; Chen, B. Porous Metal-Organic Frameworks for Gas Storage and Separation: What, How, and Why? J. Phys. Chem. Lett. 2014, 5, 3468–3479. [Google Scholar] [CrossRef]
- Bastin, L.; Bárcia, P.S.; Hurtado, E.J.; Silva, J.A.C.; Rodrigues, A.E.; Chen, B. A Microporous Metal−Organic Framework for Separation of CO2/N2 and CO2/CH4 by Fixed-Bed Adsorption. J. Phys. Chem. C 2008, 112, 1575–1581. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H. Phase Behavior of Gas Hydrates in Nanoporous Materials: Review. Korean J. Chem. Eng. 2016, 33, 1977–1988. [Google Scholar] [CrossRef]
- Angelini, P.; Armstrong, T.; Counce, R.; Griffith, W.; Klasson, T.; Muralidharan, G.; Closset, G.; Keller, G.; Watson Disclaimer, J. Materials for Separation Technologies: Energy and Emission Reduction Opportunities; BCS Inc.: Laurel, MD, USA, 2005. [Google Scholar] [CrossRef]
- Stroganov, Y.N.; Khakimov, R.T.; Ognev, O.G.; Kulev, M.V.; Belinskaia, I.V. Influence of Natural Gas Composition on Working Process of Gas Power Plant Efficiency. AIP Conf. Proc. 2022, 2456, 030039. [Google Scholar]
- Mimachi, H.; Takeya, S.; Yoneyama, A.; Hyodo, K.; Takeda, T.; Gotoh, Y.; Murayama, T. Natural Gas Storage and Transportation within Gas Hydrate of Smaller Particle: Size Dependence of Self-Preservation Phenomenon of Natural Gas Hydrate. Chem. Eng. Sci. 2014, 118, 208–213. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Wong, A.J.H.; Babu, P.; Kumar, R.; Kulprathipanja, S.; Rangsunvigit, P.; Linga, P. Rapid Methane Hydrate Formation to Develop a Cost Effective Large Scale Energy Storage System. Chem. Eng. J. 2016, 290, 161–173. [Google Scholar] [CrossRef]
- Wang, W.; Bray, C.L.; Adams, D.J.; Cooper, A.I. Methane Storage in Dry Water Gas Hydrates. J. Am. Chem. Soc. 2008, 130, 11608–11609. [Google Scholar] [CrossRef]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and Applications of Gas Hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef]
- Farrando-Perez, J.; Balderas-Xicohtencatl, R.; Cheng, Y.; Daemen, L.; Cuadrado-Collados, C.; Martinez-Escandell, M.; Ramirez-Cuesta, A.J.; Silvestre-Albero, J. Rapid and Efficient Hydrogen Clathrate Hydrate Formation in Confined Nanospace. Nat. Commun. 2022, 13, 5953. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, L.; Casco, M.E.; Silvestre-Albero, J. Methane Hydrate in Confined Spaces: An Alternative Storage System. ChemPhysChem 2018, 19, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Casco, M.E.; Silvestre-Albero, J.; Ramírez-Cuesta, A.J.; Rey, F.; Jordá, J.L.; Bansode, A.; Urakawa, A.; Peral, I.; Martínez-Escandell, M.; Kaneko, K.; et al. Methane Hydrate Formation in Confined Nanospace Can Surpass Nature. Nat. Commun. 2015, 6, 6432. [Google Scholar] [CrossRef] [PubMed]
- Casco, M.E.; Rey, F.; Jordá, J.L.; Rudić, S.; Fauth, F.; Martínez-Escandell, M.; Rodríguez-Reinoso, F.; Ramos-Fernández, E.V.; Silvestre-Albero, J. Paving the Way for Methane Hydrate Formation on Metal–Organic Frameworks (MOFs). Chem. Sci. 2016, 7, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, L.; Nickel, W.; Casco, M.; Senkovska, I.; Bon, V.; Wallacher, D.; Grimm, N.; Krause, S.; Silvestre-Albero, J. Illuminating Solid Gas Storage in Confined Spaces—Methane Hydrate Formation in Porous Model Carbons. Phys. Chem. Chem. Phys. 2016, 18, 20607–20614. [Google Scholar] [CrossRef]
- Casco, M.E.; Jordá, J.L.; Rey, F.; Fauth, F.; Martinez-Escandell, M.; Rodríguez-Reinoso, F.; Ramos-Fernández, E.V.; Silvestre-Albero, J. High-Performance of Gas Hydrates in Confined Nanospace for Reversible CH4/CO2 Storage. Chem. A Eur. J. 2016, 22, 10028–10035. [Google Scholar] [CrossRef]
- Chari, V.D.; Prasad, P.S.R.; Murthy, S.R. Structural Stability of Methane Hydrates in Porous Medium: Raman Spectroscopic Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 636–641. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.; Lee, Y.; Seo, Y. Thermodynamic and 13C NMR Spectroscopic Verification of Methane–Carbon Dioxide Replacement in Natural Gas Hydrates. Chem. Eng. J. 2013, 225, 636–640. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Metal–Organic Framework HKUST-1 Promotes Methane Hydrate Formation for Improved Gas Storage Capacity. ACS Appl. Mater. Interfaces 2020, 12, 53510–53518. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Methane Hydrate Growth Promoted by Microporous Zeolitic Imidazolate Frameworks ZIF-8 and ZIF-67 for Enhanced Methane Storage. ACS Sustain. Chem. Eng. 2021, 9, 9001–9010. [Google Scholar] [CrossRef]
- Denning, S.; Lucero, J.M.; Majid, A.A.A.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Porous Organic Cage CC3: An Effective Promoter for Methane Hydrate Formation for Natural Gas Storage. J. Phys. Chem. C 2021, 125, 20512–20521. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.; Crawford, J.M.; Wells, J.D.; Carreon, M.A.; Koh, C.A. Methane Storage Scale-up Using Hydrates & Metal Organic Framework HKUST-1 in a Packed Column. Fuel 2022, 325, 124920. [Google Scholar] [CrossRef]
- Küsgens, P.; Rose, M.; Senkovska, I.; Fröde, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of Metal-Organic Frameworks by Water Adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Bordiga, S.; Regli, L.; Bonino, F.; Groppo, E.; Lamberti, C.; Xiao, B.; Wheatley, P.S.; Morris, R.E.; Zecchina, A. Adsorption Properties of HKUST-1 toward Hydrogen and Other Small Molecules Monitored by IR. Phys. Chem. Chem. Phys. 2007, 9, 2676. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Latroche, M.; Serre, C.; Millange, F.; Loiseau, T.; Percheron-Guégan, A. Hydrogen Adsorption in the Nanoporous Metal-Benzenedicarboxylate M(OH)(O2C–C6H4–CO2) (M = Al3+, Cr3+), MIL-53. Chem. Commun. 2003, 3, 2976–2977. [Google Scholar] [CrossRef]
- Babaei, H.; DeCoster, M.E.; Jeong, M.; Hassan, Z.M.; Islamoglu, T.; Baumgart, H.; McGaughey, A.J.H.; Redel, E.; Farha, O.K.; Hopkins, P.E.; et al. Observation of Reduced Thermal Conductivity in a Metal-Organic Framework Due to the Presence of Adsorbates. Nat. Commun. 2020, 11, 4010. [Google Scholar] [CrossRef]
- Gunatilleke, W.D.C.B.; Wei, K.; Niu, Z.; Wojtas, L.; Nolas, G.; Ma, S. Thermal Conductivity of a Perovskite-Type Metal–Organic Framework Crystal. Dalt. Trans. 2017, 46, 13342–13344. [Google Scholar] [CrossRef]
- Cui, B.; Audu, C.O.; Liao, Y.; Nguyen, S.T.; Farha, O.K.; Hupp, J.T.; Grayson, M. Thermal Conductivity of ZIF-8 Thin-Film under Ambient Gas Pressure. ACS Appl. Mater. Interfaces 2017, 9, 28139–28143. [Google Scholar] [CrossRef]
- Huang, B.L.; Ni, Z.; Millward, A.; McGaughey, A.J.H.; Uher, C.; Kaviany, M.; Yaghi, O. Thermal Conductivity of a Metal-Organic Framework (MOF-5): Part II. Measurement. Int. J. Heat Mass Transf. 2007, 50, 405–411. [Google Scholar] [CrossRef]
- Dongliang, L.; Hao, P.; Deqing, L. Thermal Conductivity Enhancement of Clathrate Hydrate with Nanoparticles. Int. J. Heat Mass Transf. 2017, 104, 566–573. [Google Scholar] [CrossRef]
- Wei, R.; Shi, K.; Guo, X.; Wang, T.; Lv, X.; Li, Q.; Zhang, Y.; Zhao, J.; Yang, L. Evolving Thermal Conductivity upon Formation and Decomposition of Hydrate in Natural Marine Sediments. Fuel 2021, 302, 121141. [Google Scholar] [CrossRef]
- Li, X.-Y.; Feng, J.-C.; Li, X.-S.; Wang, Y.; Hu, H.-Q. Experimental Study of Methane Hydrate Formation and Decomposition in the Porous Medium with Different Thermal Conductivities and Grain Sizes. Appl. Energy 2022, 305, 117852. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, Y.-H.; Lee, H. Phase Equilibria of CO2 and CH4 Hydrates in Intergranular Meso/Macro Pores of MIL-53 Metal Organic Framework. J. Chem. Eng. Data 2015, 60, 2178–2185. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, S.; Guo, P.; Fan, S.; Zhang, S. Understanding the Characteristic of Methane Hydrate Equilibrium in Materials and Its Potential Application. Chem. Eng. J. 2018, 349, 775–781. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Mouchaham, G.; Daemen, L.; Cheng, Y.; Ramirez-Cuesta, A.; Aggarwal, H.; Missyul, A.; Eddaoudi, M.; Belmabkhout, Y.; Silvestre-Albero, J. Quest for an Optimal Methane Hydrate Formation in the Pores of Hydrolytically Stable Metal–Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 13391–13397. [Google Scholar] [CrossRef]
- Mu, L.; Liu, B.; Liu, H.; Yang, Y.; Sun, C.; Chen, G. A Novel Method to Improve the Gas Storage Capacity of ZIF-8. J. Mater. Chem. 2012, 22, 12246. [Google Scholar] [CrossRef]
- He, Z.; Zhang, K.; Jiang, J. Formation of CH 4 Hydrate in a Mesoporous Metal–Organic Framework MIL-101: Mechanistic Insights from Microsecond Molecular Dynamics Simulations. J. Phys. Chem. Lett. 2019, 10, 7002–7008. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, J.; Chen, S.; Fu, Y.; Zhang, Y.; Wang, D.; Pei, J.; Liu, D. Molecular Insights into Hybrid CH4 Physisorption-Hydrate Growth in Hydrophobic Metal–Organic Framework ZIF-8: Implications for CH4 Storage. Chem. Eng. J. 2022, 430, 132901. [Google Scholar] [CrossRef]
- Lee, D.; Jeoung, S.; Moon, H.R.; Seo, Y. Recoverable and Recyclable Gas Hydrate Inhibitors Based on Magnetic Nanoparticle-Decorated Metal–Organic Frameworks. Chem. Eng. J. 2020, 401, 126081. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic Imidazolate Framework Materials: Recent Progress in Synthesis and Applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Erkartal, M.; Erkilic, U.; Tam, B.; Usta, H.; Yazaydin, O.; Hupp, J.T.; Farha, O.K.; Sen, U. From 2-Methylimidazole to 1,2,3-Triazole: A Topological Transformation of ZIF-8 and ZIF-67 by Post-Synthetic Modification. Chem. Commun. 2017, 53, 2028–2031. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, K.A.; Matzger, A.J. Water Stability of Microporous Coordination Polymers and the Adsorption of Pharmaceuticals from Water. Langmuir 2010, 26, 17198–17202. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Wannapaiboon, S.; Khaletskaya, K.; Fischer, R.A. Engineering Zeolitic-Imidazolate Framework (ZIF) Thin Film Devices for Selective Detection of Volatile Organic Compounds. Adv. Funct. Mater. 2015, 25, 4470–4479. [Google Scholar] [CrossRef]
- Zhang, K.; Lively, R.P.; Zhang, C.; Koros, W.J.; Chance, R.R. Investigating the Intrinsic Ethanol/Water Separation Capability of ZIF-8: An Adsorption and Diffusion Study. J. Phys. Chem. C 2013, 117, 7214–7225. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Düren, T. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [PubMed]
- Cladek, B.R.; Everett, S.M.; McDonnell, M.T.; Tucker, M.G.; Keffer, D.J.; Rawn, C.J. Guest–Host Interactions in Mixed CH4–CO2 Hydrates: Insights from Molecular Dynamics Simulations. J. Phys. Chem. C 2018, 122, 19575–19583. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ryu, B.J.; Yun, T.S.; Lee, J.; Cho, G.C. Review on the Gas Hydrate Development and Production as a New Energy Resource. KSCE J. Civ. Eng. 2011, 15, 689–696. [Google Scholar] [CrossRef]
- Xu, C.-G.; Chen, Z.-Y.; Cai, J.; Li, X.-S. Study on Pilot-Scale CO2 Separation from Flue Gas by the Hydrate Method. Energy Fuels 2014, 28, 1242–1248. [Google Scholar] [CrossRef]
- Kumar, R.; Englezos, P.; Moudrakovski, I.; Ripmeester, J.A. Structure and Composition of CO2/H2 and CO2/H2/C3H8 Hydrate in Relation to Simultaneous CO2 Capture and H2 Production. AIChE J. 2009, 55, 1584–1594. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3 (TMA)2(H2O)3] N. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Fujimori, T.; Morelos-Gómez, A.; Zhu, Z.; Muramatsu, H.; Futamura, R.; Urita, K.; Terrones, M.; Hayashi, T.; Endo, M.; Young Hong, S.; et al. Conducting Linear Chains of Sulphur inside Carbon Nanotubes. Nat. Commun. 2013, 4, 2162. [Google Scholar] [CrossRef] [PubMed]
- Urita, K.; Shiga, Y.; Fujimori, T.; Iiyama, T.; Hattori, Y.; Kanoh, H.; Ohba, T.; Tanaka, H.; Yudasaka, M.; Iijima, S.; et al. Confinement in Carbon Nanospace-Induced Production of KI Nanocrystals of High-Pressure Phase. J. Am. Chem. Soc. 2011, 133, 10344–10347. [Google Scholar] [CrossRef] [PubMed]
- Alcoutlabi, M.; McKenna, G.B. Effects of Confinement on Material Behaviour at the Nanometre Size Scale. J. Phys. Condens. Matter 2005, 17, R461–R524. [Google Scholar] [CrossRef]
- Derouane, E. Surface Curvature Effects in Physisorption and Catalysis by Microporous Solids and Molecular Sieves. J. Catal. 1988, 110, 58–73. [Google Scholar] [CrossRef]
- Sastre, G.; Corma, A. The Confinement Effect in Zeolites. J. Mol. Catal. A Chem. 2009, 305, 3–7. [Google Scholar] [CrossRef]
- Handa, Y.P.; Stupin, D.Y. Thermodynamic Properties and Dissociation Characteristics of Methane and Propane Hydrates in 70-.ANG.-Radius Silica Gel Pores. J. Phys. Chem. 1992, 96, 8599–8603. [Google Scholar] [CrossRef]
- Handa, Y.P.; Zakrzewski, M.; Fairbridge, C. Effect of Restricted Geometries on the Structure and Thermodynamic Properties of Ice. J. Phys. Chem. 1992, 96, 8594–8599. [Google Scholar] [CrossRef]
- Alba-Simionesco, C.; Coasne, B.; Dosseh, G.; Dudziak, G.; Gubbins, K.E.; Radhakrishnan, R.; Sliwinska-Bartkowiak, M. Effects of Confinement on Freezing and Melting. J. Phys. Condens. Matter 2006, 18, R15–R68. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, D.W.; Lim, H.-K.; Jeon, J.; Kim, H.; Jung, H.-T.; Lee, H. Inhibited Phase Behavior of Gas Hydrates in Graphene Oxide: Influences of Surface and Geometric Constraints. Phys. Chem. Chem. Phys. 2014, 16, 22717–22722. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, S.; Cha, I.; Lee, J.D.; Kang, S.P. Phase Equilibria of Ethane and Propane Hydrates in Porous Silica Gels. Chem. Eng. Trans. 2009, 17, 1479–1484. [Google Scholar] [CrossRef]
- Anderson, R.; Llamedo, M.; Tohidi, B.; Burgass, R.W. Experimental Measurement of Methane and Carbon Dioxide Clathrate Hydrate Equilibria in Mesoporous Silica. J. Phys. Chem. B 2003, 107, 3507–3514. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Majid, A.A.A.; Martínez-Escandell, M.; Daemen, L.L.; Missyul, A.; Koh, C.; Silvestre-Albero, J. Freezing/Melting of Water in the Confined Nanospace of Carbon Materials: Effect of an External Stimulus. Carbon N. Y. 2020, 158, 346–355. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Galib, M.; Nguyen, A.V. Critical Review on Gas Hydrate Formation at Solid Surfaces and in Confined Spaces—Why and How Does Interfacial Regime Matter? Energy Fuels 2020, 34, 6751–6760. [Google Scholar] [CrossRef]
- Mohammadi, A.A.H.; Manteghian, M.; Haghtalab, A.; Mohammadi, A.A.H.; Rahmati-Abkenar, M. Kinetic Study of Carbon Dioxide Hydrate Formation in Presence of Silver Nanoparticles and SDS. Chem. Eng. J. 2014, 237, 387–395. [Google Scholar] [CrossRef]
- Jacobson, L.C.; Hujo, W.; Molinero, V. Amorphous Precursors in the Nucleation of Clathrate Hydrates. J. Am. Chem. Soc. 2010, 132, 11806–11811. [Google Scholar] [CrossRef]
- Miyawaki, J.; Kanda, T.; Suzuki, T.; Okui, T.; Maeda, Y.; Kaneko, K. Macroscopic Evidence of Enhanced Formation of Methane Nanohydrates in Hydrophobic Nanospaces. J. Phys. Chem. B 1998, 102, 2187–2192. [Google Scholar] [CrossRef]
- Perrin, A.; Celzard, A.; Marêché, J.F.; Furdin, G. Methane Storage within Dry and Wet Active Carbons: A Comparative Study. Energy Fuels 2003, 17, 1283–1291. [Google Scholar] [CrossRef]
- Müller, E.A.; Rull, L.F.; Vega, L.F.; Gubbins, K.E. Adsorption of Water on Activated Carbons: A Molecular Simulation Study. J. Phys. Chem. 1996, 100, 1189–1196. [Google Scholar] [CrossRef]
- Casco, M.E.; Zhang, E.; Grätz, S.; Krause, S.; Bon, V.; Wallacher, D.; Grimm, N.; Többens, D.M.; Hauß, T.; Borchardt, L. Experimental Evidence of Confined Methane Hydrate in Hydrophilic and Hydrophobic Model Carbons. J. Phys. Chem. C 2019, 123, 24071–24079. [Google Scholar] [CrossRef]
- Andersen, R.; Llamedo, M.; Tohidi, B.; Burgass, R.W. Characteristics of Clathrate Hydrate Equilibria in Mesopores and Interpretation of Experimental Data. J. Phys. Chem. B 2003, 107, 3500–3506. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A.V.; Steel, K.M.; Dang, L.X.; Galib, M. Interfacial Gas Enrichment at Hydrophobic Surfaces and the Origin of Promotion of Gas Hydrate Formation by Hydrophobic Solid Particles. J. Phys. Chem. C 2017, 121, 3830–3840. [Google Scholar] [CrossRef]
- Mahboub, M.J.D.; Ahmadpour, A.; Rashidi, H. Improving Methane Storage on Wet Activated Carbons at Various Amounts of Water. J. Fuel Chem. Technol. 2012, 40, 385–389. [Google Scholar] [CrossRef]
- Siangsai, A.; Rangsunvigit, P.; Kitiyanan, B.; Kulprathipanja, S.; Linga, P. Investigation on the Roles of Activated Carbon Particle Sizes on Methane Hydrate Formation and Dissociation. Chem. Eng. Sci. 2015, 126, 383–389. [Google Scholar] [CrossRef]
- Mukherjee, S.; Datta, K.K.R.; Fischer, R.A. Hydrophobicity: A Key Factor En Route to Applications of Metal–Organic Frameworks. Trends Chem. 2021, 3, 911–925. [Google Scholar] [CrossRef]
- Bai, D.; Chen, G.; Zhang, X.; Wang, W. Microsecond Molecular Dynamics Simulations of the Kinetic Pathways of Gas Hydrate Formation from Solid Surfaces. Langmuir 2011, 27, 5961–5967. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, W.; Pu, W.; Hu, J.; Zhao, J.; Zhang, L. CH 4 Nanobubbles on the Hydrophobic Solid–Water Interface Serving as the Nucleation Sites of Methane Hydrate. Langmuir 2018, 34, 10181–10186. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the Driving Force of Hydrophobic Assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef]
- Lum, K.; Chandler, D.; Weeks, J.D. Hydrophobicity at Small and Large Length Scales. J. Phys. Chem. B 1999, 103, 4570–4577. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Deng, S.; Zhao, R.; Nie, X.; Liu, Y. Effect of Nanobubble Evolution on Hydrate Process: A Review. J. Therm. Sci. 2019, 28, 948–961. [Google Scholar] [CrossRef]
- Le Donne, A.; Tinti, A.; Amayuelas, E.; Kashyap, H.K.; Camisasca, G.; Remsing, R.C.; Roth, R.; Grosu, Y.; Meloni, S. Intrusion and Extrusion of Liquids in Highly Confining Media: Bridging Fundamental Research to Applications. Adv. Phys. X 2022, 7, 2052353. [Google Scholar] [CrossRef]
- Ortiz, G.; Nouali, H.; Marichal, C.; Chaplais, G.; Patarin, J. Energetic Performances of the Metal–Organic Framework ZIF-8 Obtained Using High Pressure Water Intrusion–Extrusion Experiments. Phys. Chem. Chem. Phys. 2013, 15, 4888. [Google Scholar] [CrossRef] [PubMed]
- Khay, I.; Chaplais, G.; Nouali, H.; Marichal, C.; Patarin, J. Water Intrusion–Extrusion Experiments in ZIF-8: Impacts of the Shape and Particle Size on the Energetic Performances. RSC Adv. 2015, 5, 31514–31518. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y. Stabilization of Amine-Containing CO2 Adsorbents: Dramatic Effect of Water Vapor. J. Am. Chem. Soc. 2010, 132, 6312–6314. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Babu, P.; Yee, D.; Linga, P.; Palmer, A.; Khoo, B.C.; Tan, T.S.; Rangsunvigit, P. Morphology of Methane Hydrate Formation in Porous Media. Energy Fuels 2013, 27, 3364–3372. [Google Scholar] [CrossRef]
- Jung, J.W.; Santamarina, J.C. Hydrate Formation and Growth in Pores. J. Cryst. Growth 2012, 345, 61–68. [Google Scholar] [CrossRef]
- Yan, L.; Chen, G.; Pang, W.; Liu, J. Experimental and Modeling Study on Hydrate Formation in Wet Activated Carbon. J. Phys. Chem. B 2005, 109, 6025–6030. [Google Scholar] [CrossRef]
- Govindaraj, V.; Mech, D.; Pandey, G.; Nagarajan, R.; Sangwai, J.S. Kinetics of Methane Hydrate Formation in the Presence of Activated Carbon and Nano-Silica Suspensions in Pure Water. J. Nat. Gas Sci. Eng. 2015, 26, 810–818. [Google Scholar] [CrossRef]
- Ganji, H.; Manteghian, M.; Sadaghiani, K.; Omidkhah, M.R.; Mofrad, H.R. Effect of Different Surfactants on Methane Hydrate Formation Rate, Stability and Storage Capacity. Fuel 2007, 86, 434–441. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; Von Solms, N. Methane Hydrate Formation, Storage and Dissociation Behavior InUnconsolidated Sediments in the Presence of Environment-FriendlyPromoters. In Proceedings of the SPE Europec Featured at 82nd EAGE Conference and Exhibition, Amsterdam, The Netherlands, 18–21 October 2021. [Google Scholar] [CrossRef]
- Shanker Pandey, J.; Jouljamal Daas, Y.; Paul Karcz, A.; Solms, N. Von Methane Hydrate Formation Behavior in the Presence of Selected Amino Acids. J. Phys. Conf. Ser. 2020, 1580, 012003. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; Karcz, A.P.; von Solms, N. Enhanced Hydrate-Based Geological CO2 Capture and Sequestration as a Mitigation Strategy to Address Climate Change. Energies 2020, 13, 5661. [Google Scholar] [CrossRef]
- Celzard, A.; Marêché, J.F. Optimal Wetting of Active Carbons for Methane Hydrate Formation. Fuel 2006, 85, 957–966. [Google Scholar] [CrossRef]
- Li, J.; Liang, D.; Guo, K.; Wang, R.; Fan, S. Formation and Dissociation of HFC134a Gas Hydrate in Nano-Copper Suspension. Energy Convers. Manag. 2006, 47, 201–210. [Google Scholar] [CrossRef]
- Casco, M.E.; Cuadrado-Collados, C.; Martínez-Escandell, M.; Rodríguez-Reinoso, F.; Silvestre-Albero, J. Influence of the Oxygen-Containing Surface Functional Groups in the Methane Hydrate Nucleation and Growth in Nanoporous Carbon. Carbon N. Y. 2017, 123, 299–301. [Google Scholar] [CrossRef]
- Liu, C.-C.; Chou, H.-J.; Lin, C.-Y.; Janmanchi, D.; Chung, P.-W.; Mou, C.-Y.; Yu, S.S.F.; Chan, S.I. The Oversolubility of Methane Gas in Nano-Confined Water in Nanoporous Silica Materials. Microporous Mesoporous Mater. 2020, 293, 109793. [Google Scholar] [CrossRef]
- Muñoz-Santiburcio, D.; Marx, D. Chemistry in Nanoconfined Water. Chem. Sci. 2017, 8, 3444–3452. [Google Scholar] [CrossRef]
- Findenegg, G.H.; Jähnert, S.; Akcakayiran, D.; Schreiber, A. Freezing and Melting of Water Confined in Silica Nanopores. ChemPhysChem 2008, 9, 2651–2659. [Google Scholar] [CrossRef]
- Mallamace, F.; Broccio, M.; Corsaro, C.; Faraone, A.; Majolino, D.; Venuti, V.; Liu, L.; Mou, C.-Y.; Chen, S.-H. Evidence of the Existence of the Low-Density Liquid Phase in Supercooled, Confined Water. Proc. Natl. Acad. Sci. USA 2007, 104, 424–428. [Google Scholar] [CrossRef]
- Ho, L.N.; Clauzier, S.; Schuurman, Y.; Farrusseng, D.; Coasne, B. Gas Uptake in Solvents Confined in Mesopores: Adsorption versus Enhanced Solubility. J. Phys. Chem. Lett. 2013, 4, 2274–2278. [Google Scholar] [CrossRef]
- Ho, L.N.; Schuurman, Y.; Farrusseng, D.; Coasne, B. Solubility of Gases in Water Confined in Nanoporous Materials: ZSM-5, MCM-41, and MIL-100. J. Phys. Chem. C 2015, 119, 21547–21554. [Google Scholar] [CrossRef]
- Coasne, B.; Farrusseng, D. Gas Oversolubility in Nanoconfined Liquids: Review and Perspectives for Adsorbent Design. Microporous Mesoporous Mater. 2019, 288, 109561. [Google Scholar] [CrossRef]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual High Throughput Screening Confirmed Experimentally: Porous Coordination Polymer Hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef] [PubMed]
- Domán, A.; Czakkel, O.; Porcar, L.; Madarász, J.; Geissler, E.; László, K. Role of Water Molecules in the Decomposition of HKUST-1: Evidence from Adsorption, Thermoanalytical, X-Ray and Neutron Scattering Measurements. Appl. Surf. Sci. 2019, 480, 138–147. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.; Li, Y.; Xu, F.; Xiao, J.; Xia, Q.; Li, Y.; Li, Z. A Novel Mechanochemical Method for Reconstructing the Moisture-Degraded HKUST-1. Chem. Commun. 2015, 51, 10835–10838. [Google Scholar] [CrossRef] [PubMed]
- Majano, G.; Martin, O.; Hammes, M.; Smeets, S.; Baerlocher, C.; Pérez-Ramírez, J. Solvent-Mediated Reconstruction of the Metal-Organic Framework HKUST-1 (Cu3(BTC)2). Adv. Funct. Mater. 2014, 24, 3855–3865. [Google Scholar] [CrossRef]
- Lin, J.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-nik, O.; Marx, S.; et al. A Scalable Metal-Organic Framework as a Durable Physisorbent for Carbon Dioxide Capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Silvestre-Albero, J. Methane Storage on Nanoporous Carbons. In Green Energy and Technology; Springer Nature: Singapore, 2019; pp. 209–226. ISBN 9789811335044. [Google Scholar]
- Cracknell, R.F.; Gordon, P.; Gubbins, K.E. Influence of Pore Geometry on the Design of Microporous Materials for Methane Storage. J. Phys. Chem. 1993, 97, 494–499. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.; Koh, C.A. Stability and Growth of Methane Hydrates in Confined Media for Carbon Sequestration. J. Phys. Chem. C 2022, 126, 11800–11809. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal Properties of Graphene and Nanostructured Carbon Materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Moon, S.; Koh, D.Y.; Hong, S.; Lee, H.; Lee, J.W.; Park, Y. One-Step Formation of Hydrogen Clusters in Clathrate Hydrates Stabilized via Natural Gas Blending. Energy Storage Mater. 2020, 24, 655–661. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Unusual Behavior of Propane as a Co-Guest during Hydrate Formation in Silica Sand: Potential Application to Seawater Desalination and Carbon Dioxide Capture. Chem. Eng. Sci. 2014, 117, 342–351. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A Review of Clathrate Hydrate Based Desalination to Strengthen Energy-Water Nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, V.; Barati Farimani, A. Water Desalination with Two-Dimensional Metal–Organic Framework Membranes. Nano Lett. 2019, 19, 8638–8643. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Metal-Organic Frameworks (MOFs) in Water Filtration Membranes for Desalination and Other Applications. Appl. Mater. Today 2018, 11, 219–230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).