Layer-by-Layer Materials for the Fabrication of Devices with Electrochemical Applications
Abstract
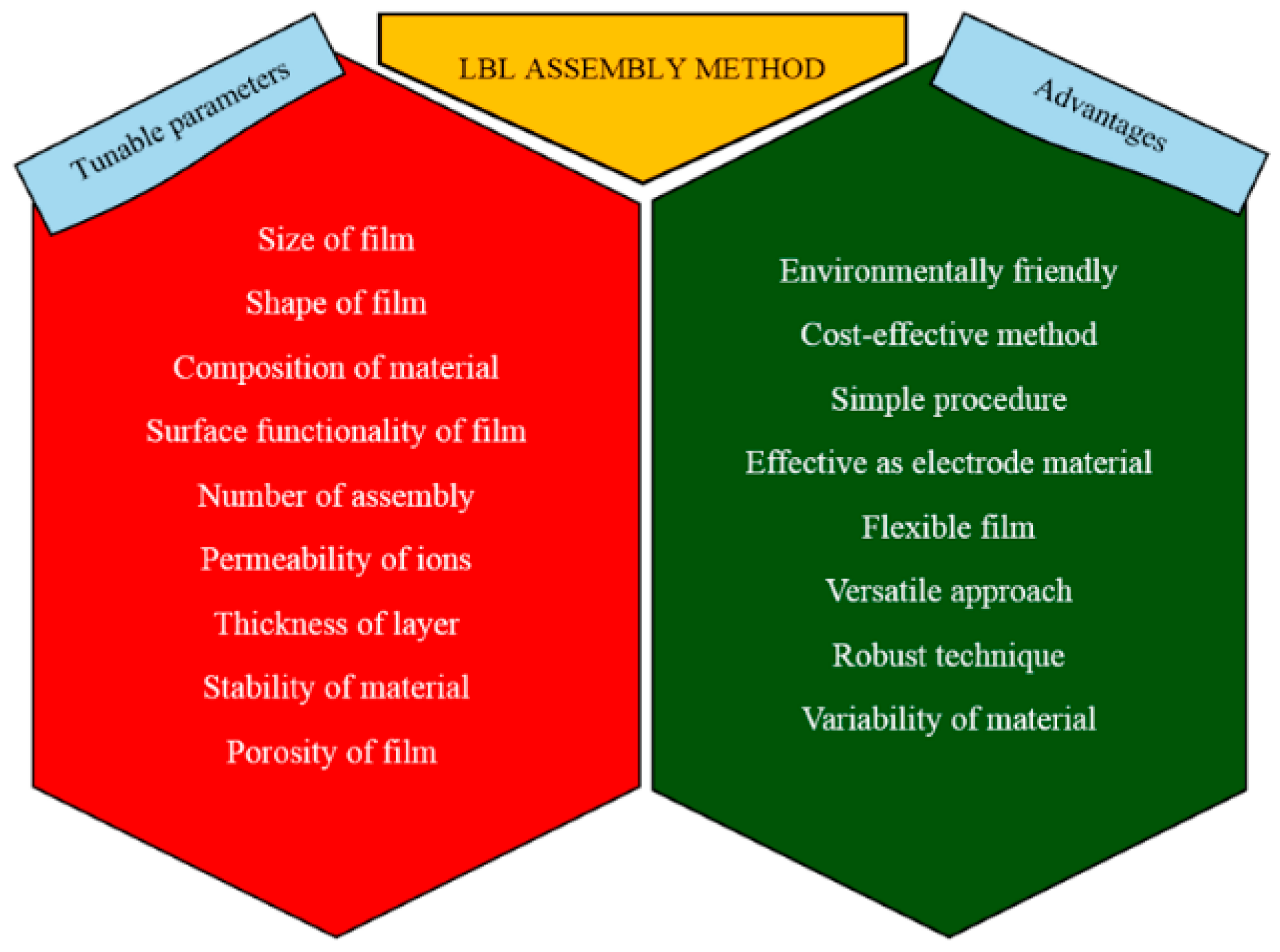
:1. Introduction
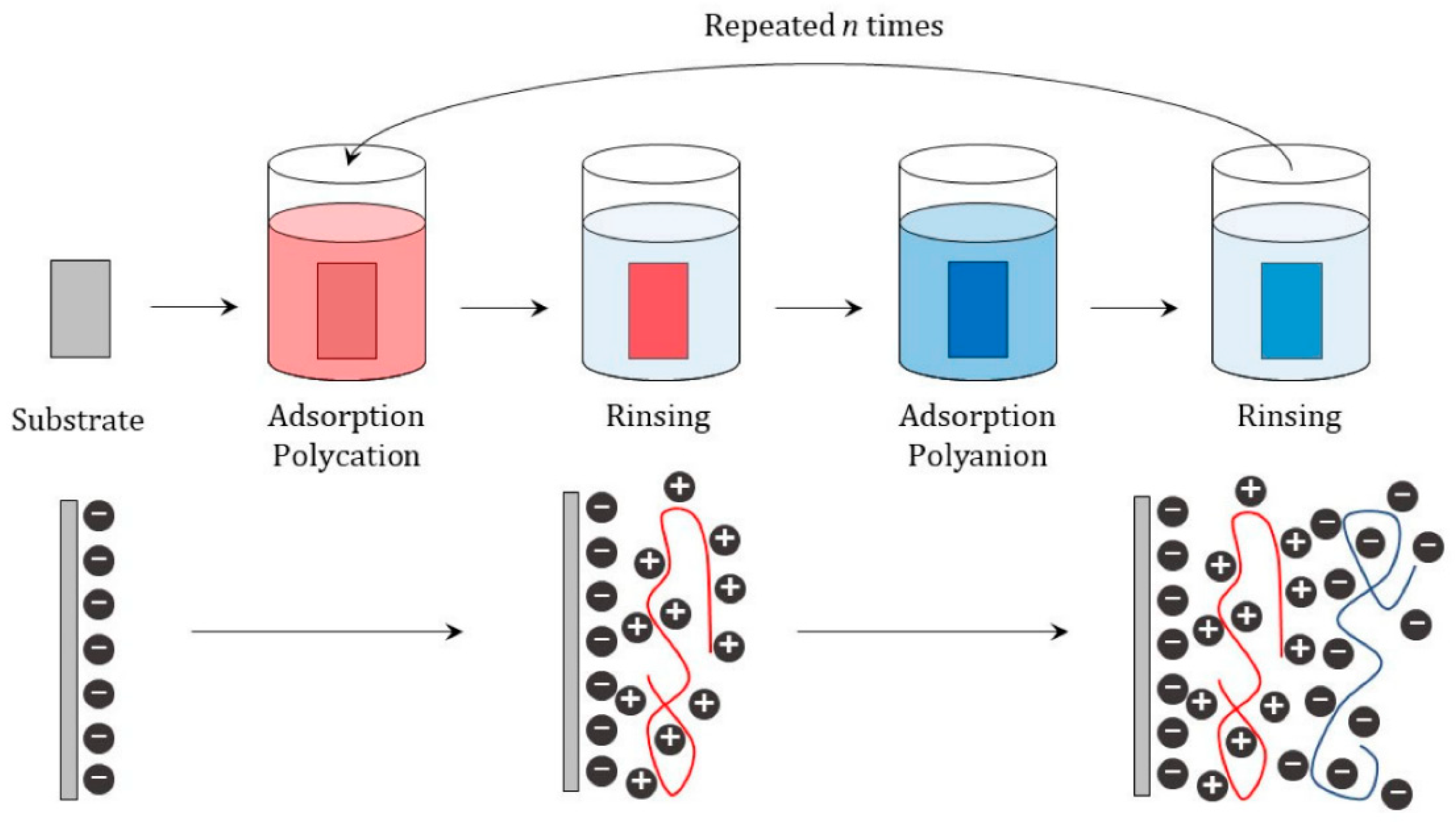
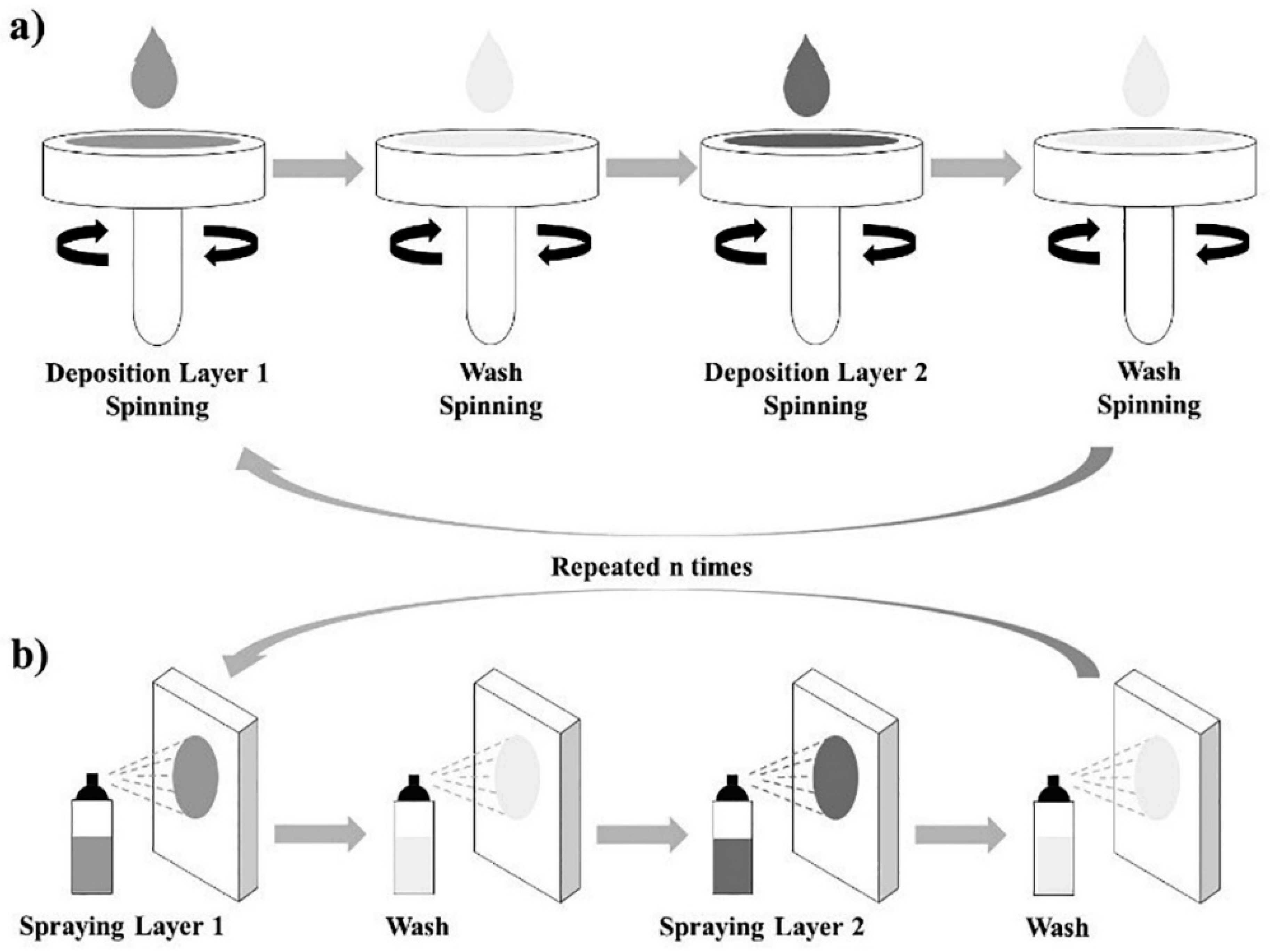
2. Approaches for the Assembly of LbL Materials for Energy Applications
3. LbL Nanomaterials in the Fabrication of Electrochemical Devices
3.1. LbL Electrochemical Devices Containing Conductive Polymers
3.2. LbL Electrochemical Devices Containing Carbon Nanomaterials
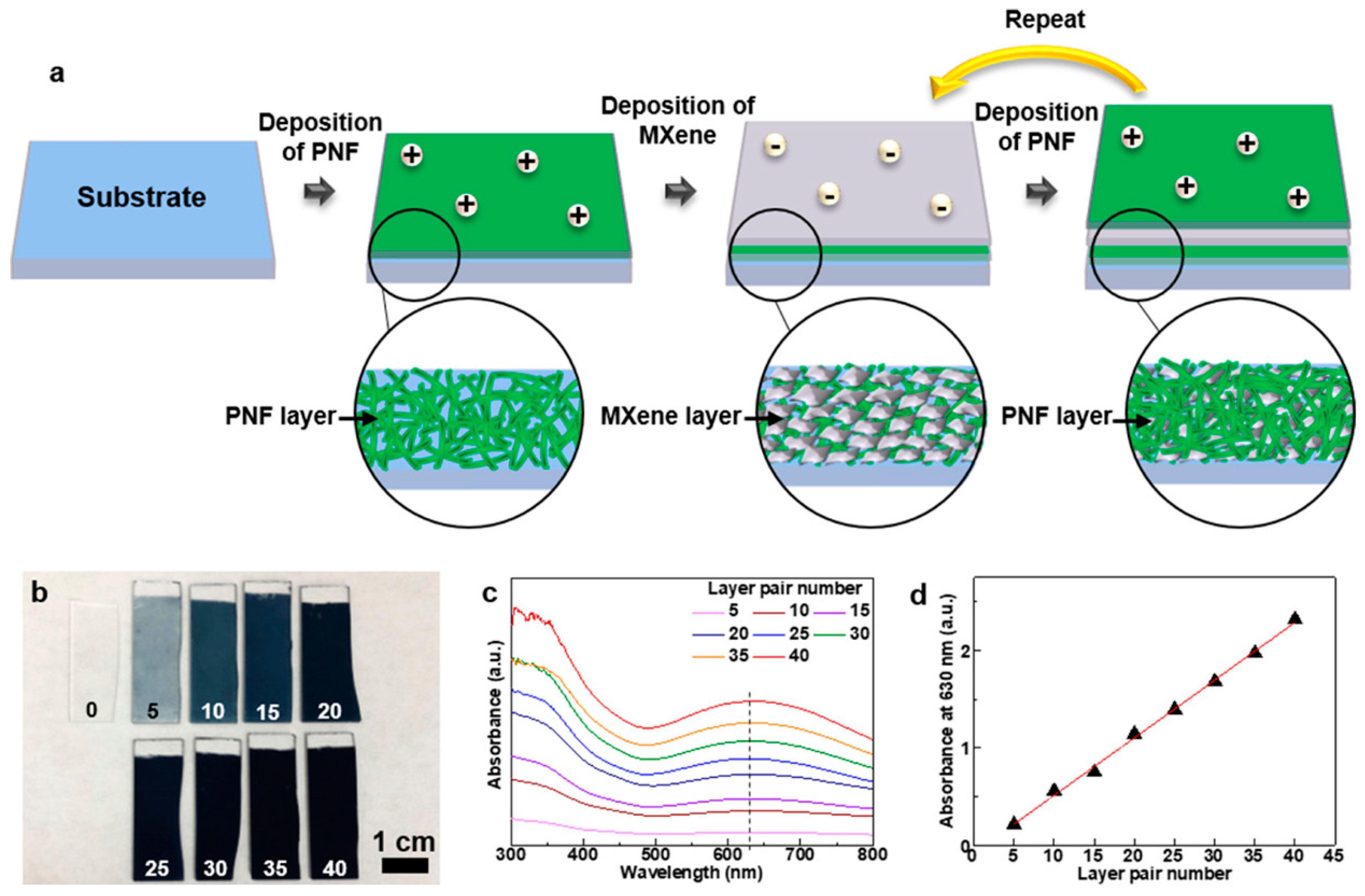
3.3. LbL Electrochemical Devices Containing MXene
3.4. LbL Electrochemical Devices Containing Pseudocapacitive Nanoparticles
3.5. LbL Electrochemical Devices Containing Layered Double Hydroxides
3.6. LbL Electrochemical Devices Containing Polyoxometalates
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni, J.; Dai, A.; Yuan, Y.; Li, L.; Lu, J. Three-Dimensional Microbatteries beyond Lithium Ion. Matter 2020, 2, 1366–1376. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, D.; Lee, Y.-H.; Chen, W.; Lee, S.-Y. Nanocellulose for Energy Storage Systems: Beyond the Limits of Synthetic Materials. Adv. Mat. 2019, 31, 1804826. [Google Scholar] [CrossRef] [PubMed]
- Dudney, N.J. Thin Film Microbatteries. Electrochem. Soc. Interface 2008, 17, 44–48. [Google Scholar] [CrossRef]
- Lee, S.W.; Gallant, B.M.; Byon, H.R.; Hammond, P.T.; Shao-Horn, Y. Nanostructured carbon-based electrodes: Bridging the gap between thin-film lithium-ion batteries and electrochemical capacitors. Energy Environ. Sci. 2011, 4, 1972–1985. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics: A navigator from materials to life. Mater. Chem. Front. 2017, 1, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Lutkenhaus, J.L.; Hammond, P.T. Electrochemically enabled polyelectrolyte multilayer devices: From fuel cells to sensors. Soft Matter 2007, 3, 804–816. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Film. 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Guzmán, E.; Ritacco, H.; Rubio, J.E.F.; Rubio, R.G.; Ortega, F. Salt-induced changes in the growth of polyelectrolyte layers of poly(diallyl-dimethylammonium chloride) and poly(4-styrene sulfonate of sodium). Soft Matter 2009, 5, 2130–2142. [Google Scholar] [CrossRef]
- Borges, J.; Mano, J.F. Molecular Interactions Driving the Layer-by-Layer Assembly of Multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.-D. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Rahdar, A. LbL Nano-Assemblies: A Versatile Tool for Biomedical and Healthcare Applications. Materials 2022, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, S.S.; Rubner, M.F. pH-Dependent Thickness Behavior of Sequentially Adsorbed Layers of Weak Polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Von Klitzing, R. Internal Structure of polyelectrolyte multilayer assemblies. Phys. Chem. Chem. Phys. 2006, 8, 5012–5033. [Google Scholar] [CrossRef]
- Guzmán, E.; San Miguel, V.; Peinado, C.; Ortega, F.; Rubio, R.G. Polyelectrolyte Multilayers Containing Triblock Copolymers of Different Charge Ratio. Langmuir 2010, 26, 11494–11502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Kim, Y.; Ko, Y.; Cheong, S.; Ryu, S.W.; Cho, J. Amphiphilic Layer-by-Layer Assembly Overcoming Solvent Polarity between Aqueous and Nonpolar Media. J. Am. Chem. Soc. 2014, 136, 17213–17223. [Google Scholar] [CrossRef] [PubMed]
- Dubas, S.T.; Schlenoff, J.B. Factors Controlling the Growth of Polyelectrolyte Multilayers. Macromolecules 1999, 32, 8153–8160. [Google Scholar] [CrossRef]
- Tuo, X.; Chen, D.; Cheng, H.; Wang, X. Fabricating Water-Insoluble Polyelectrolyte into Multilayers with Layer-by-layer Self-assembly. Polymer Bull. 2005, 54, 427–433. [Google Scholar] [CrossRef]
- Kamineni, V.K.; Lvov, Y.M.; Dobbins, T.A. Layer-by-Layer Nanoassembly of Polyelectrolytes Using Formamide as the Working Medium. Langmuir 2007, 23, 7423–7427. [Google Scholar] [CrossRef]
- Salomäki, M.; Laiho, T.; Kankare, J. Counteranion-Controlled Properties of Polyelectrolyte Multilayers. Macromolecules 2004, 37, 9585–9590. [Google Scholar] [CrossRef]
- Salomäki, M.; Tervasmäki, P.; Areva, S.; Kankare, J. The Hofmeister Anion Effect and the Growth of Polyelectrolyte Multilayers. Langmuir 2004, 20, 3679–3683. [Google Scholar] [CrossRef]
- Guzmán, E.; Cavallo, J.A.; Chuliá-Jordán, R.; Gómez, C.; Strumia, M.C.; Ortega, F.; Rubio, R.G. pH-Induced Changes in the Fabrication of Multilayers of Poly(acrylic acid) and Chitosan: Fabrication, Properties, and Tests as a Drug Storage and Delivery System. Langmuir 2011, 27, 6836–6845. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, P.; Gergely, C.; Cuisinier, F.J.G.; Decher, G.; Schaaf, P.; Voegel, J.C.; Picart, C. Comparison of the Structure of Polyelectrolyte Multilayer Films Exhibiting a Linear and an Exponential Growth Regime: An in Situ Atomic Force Microscopy Study. Macromolecules 2002, 35, 4458–4465. [Google Scholar] [CrossRef]
- Lavalle, P.; Voegel, J.-C.; Vautier, D.; Senger, B.; Schaaf, P.; Ball, V. Dynamic Aspects of Films Prepared by a Sequential Deposition of Species: Perspectives for Smart and Responsive Materials. Adv. Mat. 2011, 23, 1191–1221. [Google Scholar] [CrossRef] [PubMed]
- Ladam, G.; Schaad, P.; Voegel, J.C.; Schaaf, P.; Decher, G.; Cuisinier, F. In Situ Determination of the Structural Properties of Initially Deposited Polyelectrolyte Multilayers. Langmuir 2000, 16, 1249–1255. [Google Scholar] [CrossRef]
- Guzmán, E.; Maestro, A.; Llamas, S.; Álvarez-Rodríguez, J.; Ortega, F.; Maroto-Valiente, Á.; Rubio, R.G. 3D solid supported inter-polyelectrolyte complexes obtained by the alternate deposition of poly(diallyldimethylammonium chloride) and poly(sodium 4-styrenesulfonate). Beilstein J. Nanotech. 2016, 7, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Comment on “Formation of polyelectrolyte multilayers: Ionic strengths and growth regimes” by K. Tang and A. M. Besseling, Soft Matter, 2016, 12, 1032. Soft Matter 2016, 12, 8460–8463. [Google Scholar] [CrossRef]
- Guzmán, E.; Ritacco, H.A.; Ortega, F.; Rubio, R.G. Growth of Polyelectrolyte Layers Formed by Poly(4-styrenesulfonate sodium salt) and Two Different Polycations: New Insights from Study of Adsorption Kinetics. J. Phys. Chem. C 2012, 116, 15474–15483. [Google Scholar] [CrossRef]
- Guzmán, E.; Ritacco, H.; Ortega, F.; Rubio, R.G. Evidence of the influence of adsorption kinetics on the internal reorganization of polyelectrolyte multilayers. Colloids Surf. A 2011, 384, 274–281. [Google Scholar] [CrossRef]
- Guzmán, E.; Ritacco, H.; Ortega, F.; Svitova, T.; Radke, C.J.; Rubio, R.G. Adsorption Kinetics and Mechanical Properties of Ultrathin Polyelectrolyte Multilayers: Liquid-Supported versus Solid-Supported Films. J. Phys. Chem. B 2009, 113, 7128–7137. [Google Scholar] [CrossRef]
- Guzmán, E.; Chuliá-Jordán, R.; Ortega, F.; Rubio, R.G. Influence of the percentage of acetylation on the assembly of LbL multilayers of poly(acrylic acid) and chitosan. Phys. Chem. Chem. Phys. 2011, 13, 18200–18207. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Donath, E.; Lichtenfeld, H.; Knippel, E.; Knippel, M.; Budde, A.; Möhwald, H. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surf. A 1998, 137, 253–266. [Google Scholar] [CrossRef]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel Hollow Polymer Shells by Colloid-Templated Assembly of Polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Caruso, F.; Donath, E.; Möhwald, H. Influence of Polyelectrolyte Multilayer Coatings on Förster Resonance Energy Transfer between 6-Carboxyfluorescein and Rhodamine B-Labeled Particles in Aqueous Solution. J. Phys. Chem. B 1998, 102, 2011–2016. [Google Scholar] [CrossRef]
- Ferri, J.K.; Dong, W.-F.; Miller, R.; Mohwald, H. Elastic Moduli of Asymmetric Ultrathin Free-Standing Polyelectrolyte Nanocomposites. Macromolecules 2006, 39, 1532–1537. [Google Scholar] [CrossRef]
- Ferri, J.K.; Dong, W.-F.; Miller, R. Ultrathin Free-Standing Polyelectrolyte Nanocomposites: A Novel Method for Preparation and Characterization of Assembly Dynamics. J. Phys. Chem. B 2005, 109, 14764–14768. [Google Scholar] [CrossRef] [PubMed]
- Shchukina, E.M.; Shchukin, D.G. Layer-by-layer coated emulsion microparticles as storage and delivery tool. Curr. Opin. Colloid Interface Sci. 2012, 17, 281–289. [Google Scholar] [CrossRef]
- Cuomo, F.; Lopez, F.; Miguel, M.G.; Lindman, B. Vesicle-Templated Layer-by-Layer Assembly for the Production of Nanocapsules. Langmuir 2010, 26, 10555–10560. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Zavgorodnya, O.; Chen, Y.; Ellis, K.; Tse, H.M.; Cui, W.; Thompson, J.A.; Kharlampieva, E. Ultrathin Polymeric Coatings Based on Hydrogen-Bonded Polyphenol for Protection of Pancreatic Islet Cells. Adv. Funct. Mater. 2012, 22, 3389–3398. [Google Scholar] [CrossRef]
- Ruano, M.; Mateos-Maroto, A.; Ortega, F.; Ritacco, H.; Rubio, J.E.F.; Guzmán, E.; Rubio, R.G. Fabrication of Robust Capsules by Sequential Assembly of Polyelectrolytes onto Charged Liposomes. Langmuir 2021, 37, 6189–6200. [Google Scholar] [CrossRef]
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-Layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid Interface Sci. 2017, 249, 209–307. [Google Scholar] [CrossRef]
- Guzmán, E.; Rubio, R.G.; Ortega, F. A closer physico-chemical look to the Layer-by-Layer electrostatic self-assembly of polyelectrolyte multilayers. Adv. Colloid Interface Sci. 2020, 282, 102197. [Google Scholar] [CrossRef] [PubMed]
- Kulandaivalu, S.; Sulaiman, Y. Recent Advances in Layer-by-Layer Assembled Conducting Polymer Based Composites for Supercapacitors. Energies 2019, 12, 2107. [Google Scholar] [CrossRef] [Green Version]
- Marmisollé, W.A.; Azzaroni, O. Recent developments in the layer-by-layer assembly of polyaniline and carbon nanomaterials for energy storage and sensing applications. From synthetic aspects to structural and functional characterization. Nanoscale 2016, 8, 9890–9918. [Google Scholar] [CrossRef] [PubMed]
- Hammond, P.T. Engineering materials layer-by-layer: Challenges and opportunities in multilayer assembly. AIChE J. 2011, 57, 2928–2940. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Gogotsi, Y. Two-Dimensional Heterostructures for Energy Storage. Nat. Energy 2017, 2, 17089. [Google Scholar] [CrossRef]
- Islam, M.M.; Aboutalebi, S.H.; Cardillo, D.; Liu, H.K.; Konstantinov, K.; Dou, S.X. Self-Assembled Multifunctional Hybrids: Toward Developing High-Performance Graphene-Based Architectures for Energy Storage Devices. ACS Cent. Sci. 2015, 1, 206–216. [Google Scholar] [CrossRef]
- Kumar, N.A.; Baek, J.-B. Electrochemical supercapacitors from conducting polyaniline–graphene platforms. Chem. Comm. 2014, 50, 6298–6308. [Google Scholar] [CrossRef]
- Hyder, M.N.; Kavian, R.; Sultana, Z.; Saetia, K.; Chen, P.-Y.; Lee, S.W.; Shao-Horn, Y.; Hammond, P.T. Vacuum-Assisted Layer-by-Layer Nanocomposites for Self-Standing 3D Mesoporous Electrodes. Chem. Mater. 2014, 26, 5310–5318. [Google Scholar] [CrossRef]
- Ren, H.; Pyo, S.; Lee, J.-I.; Park, T.-J.; Gittleson, F.S.; Leung, F.C.C.; Kim, J.; Taylor, A.D.; Lee, H.-S.; Chae, J.A. High Power Density Miniaturized Microbial Fuel Cell Having Carbon Nanotube Anodes. J. Power Sources 2015, 273, 823–830. [Google Scholar] [CrossRef]
- Beidaghi, M.; Gogotsi, Y. Capacitive energy storage in micro-scale devices: Recent advances in design and fabrication of microsupercapacitors. Energy Environ. Sci. 2014, 7, 867–884. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, H.; Lei, Y. Emerging smart design of electrodes for micro-supercapacitors: A review. SmartMat 2022. [Google Scholar] [CrossRef]
- Lvov, Y.; Essler, F.; Decher, G. Combination of polycation/polyanion self-assembly and Langmuir-Blodgett transfer for the construction of superlattice films. J. Phys. Chem. 1993, 97, 13773–13777. [Google Scholar] [CrossRef]
- Decher, G.; Schmitt, J. Fine-Tuning of the film thickness of ultrathin multilayer films composed of consecutively alternating layers of anionic and cationic polyelectrolytes. Prog. Colloid Polym. Sci. 1992, 89, 160–164. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, Y.; Gong, K.; Mao, L.; Guo, Z.; Chen, Y. Electrostatic Layer-by-Layer Assembled Carbon Nanotube Multilayer Film and Its Electrocatalytic Activity for O2 Reduction. Langmuir 2004, 20, 8781–8785. [Google Scholar] [CrossRef]
- Zhan, M.; Su, L.; Mao, L. Surfactant functionalization of carbon nanotubes (CNTs) for layer-by-layer assembling of CNT multi-layer films and fabrication of gold nanoparticle/CNT nanohybrid. Carbon 2005, 44, 276–283. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Ji, Q. Layer-by-Layer Assembly as a Versatile Bottom-up Nanofabrication Technique for Exploratory Research and Realistic Application. Phys. Chem. Chem. Phys. 2007, 9, 2319–2340. [Google Scholar] [CrossRef]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Technology-Driven Layer-by-Layer Assembly of Nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [Green Version]
- Decher, G.; Hong, J.D. Buildup of Ultrathin Multilayer Films by a Self-Assembly Process: II. Consecutive Adsorption of Anionic and Cationic Bipolar Amphiphiles and Polyelectrolytes on Charged Surfaces. Ber. Bunsenges. Phys. Chem. 1991, 95, 1430–1434. [Google Scholar] [CrossRef]
- Iler, R.K. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Mateos-Maroto, A.; Fernández-Peña, L.; Abelenda-Núñez, I.; Ortega, F.; Rubio, R.G.; Guzmán, E. Polyelectrolyte Multilayered Capsules as Biomedical Tools. Polymers 2022, 14, 479. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, S.; Jiang, S.P. Layer-by-layer self-assembly in the development of electrochemical energy conversion and storage devices from fuel cells to supercapacitors. Chem. Soc. Rev. 2012, 41, 7291–7321. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Toniazzo, V.; Ruch, D.; Ball, V. Deposition Mechanisms in Layer-by-Layer or Step-by-Step Deposition Methods: From Elastic and Impermeable Films to Soft Membranes with Ion Exchange Properties. Int. Sch. Res. Notices 2012, 2012, 701695. [Google Scholar] [CrossRef] [Green Version]
- Mateos-Maroto, A.; Abelenda-Núñez, I.; Ortega, F.; Rubio, R.G.; Guzmán, E. Polyelectrolyte Multilayers on Soft Colloidal Nanosurfaces: A New Life for the Layer-By-Layer Method. Polymers 2021, 13, 1221. [Google Scholar] [CrossRef]
- Shim, B.S.; Podsiadlo, P.; Lilly, D.G.; Agarwal, A.; Lee, J.; Tang, Z.; Ho, S.; Ingle, P.; Paterson, D.; Lu, W.; et al. Nanostructured thin films made by dewetting method of layer-by-layer assembly. Nano Lett. 2007, 7, 3266–3273. [Google Scholar] [CrossRef]
- Fu, Y.; Li, S.-J.; Xu, J.; Yang, M.; Zhang, J.-D.; Jiao, Y.-H.; Zhang, J.-C.; Zhang, K.; Jia, Y.-G. Facile and Efficient Approach to Speed up Layer-by-Layer Assembly: Dipping in Agitated Solutions. Langmuir 2011, 27, 672–677. [Google Scholar] [CrossRef]
- Dubal, D.P.; Dhawale, D.S.; Salunkhe, R.R.; Jamdade, V.S.; Lokhande, C.D. Fabrication of copper oxide multilayer nanosheets for supercapacitor application. J. Alloys Compd. 2010, 492, 26–30. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Dubal, D.P.; Joo, O.-S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011, 11, 255–270. [Google Scholar] [CrossRef]
- Pathan, H.M.; Lokhande, C.D. Deposition of metal chalcogenide thin films by successive ionic layer adsorption and reaction (SILAR) method. Bull. Mater. Sci. 2004, 27, 85–111. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Pusawale, S.N.; Shinde, N.M.; Lokhande, C.D. Growth of polyaniline nanofibers for supercapacitor applications using successive ionic layer adsorption and reaction (SILAR) method. J. Korean Phys. Soc. 2014, 65, 80–86. [Google Scholar] [CrossRef]
- Alkekhia, D.; Hammond, P.T.; Shukla, A. Layer-by-Layer Biomaterials for Drug Delivery. Annu. Rev. Biomed. Eng. 2020, 22, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.; Mitzscherling, S.; Leitenberger, W.; Santer, S.; Tiersch, B.; Sievers, T.K.; Möhwald, H.; Bargheer, M. Structural Characterization of a Spin-Assisted Colloid−Polyelectrolyte Assembly: Stratified Multilayer Thin Films. Langmuir 2010, 26, 18499–18502. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Char, K.; Hong, J.-D.; Lee, K.-B. Fabrication of highly ordered multilayer films using a spin self-assembly method. Adv. Mater. 2001, 13, 1076–1078. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Kozlovskaya, V.; Chan, J.; Ankner, J.F.; Tsukruk, V.V. Spin-assisted layer-by-layer assembly: Variation of stratification as studied with neutron reflectivity. Langmuir 2009, 25, 14017–14024. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cheng, M.; Jia, G.; Wang, Y.; An, Q.; Zeng, X.; Shen, Z.; Zhang, Y.; Shi, F. Layer-by-Layer Self-Assembly under High Gravity Field. Langmuir 2012, 28, 9849–9856. [Google Scholar] [CrossRef] [PubMed]
- Schlenoff, J.B.; Dubas, S.T.; Farhat, T. Sprayed Polyelectrolyte Multilayers. Langmuir 2000, 16, 9968–9969. [Google Scholar] [CrossRef]
- Félix, O.; Zheng, Z.; Cousin, F.; Decher, G. Are sprayed LbL-films stratified? A first assessment of the nanostructure of spray-assembled multilayers by neutron reflectometry. Comptes Rendus Chim. 2009, 12, 225–234. [Google Scholar] [CrossRef]
- Elosua, C.; Lopez-Torres, D.; Hernaez, M.; Matias, I.R.; Arregui, F.J. Comparative study of layer-by-layer deposition techniques for poly(sodium phosphate) and poly(allylamine hydrochloride). Nanosc. Res. Lett. 2013, 8, 539. [Google Scholar] [CrossRef] [Green Version]
- Dierendonck, M.; Koker, S.D.; Rycke, R.D.; Geest, B.G.D. Just spray it—LbL assembly enters a new age. Soft Matter 2014, 10, 804–807. [Google Scholar] [CrossRef]
- Schaaf, P.; Voegel, J.-C.; Jierry, L.; Boulmedais, F. Spray-assisted polyelectrolyte multilayer buildup: From step-by-step to single-step polyelectrolyte film constructions. Adv. Mater. 2012, 24, 1001–1016. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, A.; Ono, S.S.; Voegel, J.C.; Schaaf, P.; Decher, G. Dipping versus spraying: Exploring the deposition conditions for speeding up layer-by-layer assembly. Langmuir 2005, 21, 7558–7567. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Carosio, F.; Frache, A.; Malucelli, G. Layer by Layer coatings assembled through dipping, vertical or horizontal spray for cotton flame retardancy. Carbohydr. Polym. 2013, 92, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Mulhearn, W.D.; Kim, D.D.; Gu, Y.; Lee, D. Facilitated transport enhances spray layer-by-layer assembly of oppositely charged nanoparticles. Soft Matter 2012, 8, 10419–10427. [Google Scholar] [CrossRef]
- Ariga, K.; Ahn, E.; Park, M.; Kim, B.-S. Layer-by-Layer Assembly: Recent Progress from Layered Assemblies to Layered Nanoarchitectonics. Chem. Asian J. 2019, 14, 2553–2566. [Google Scholar] [CrossRef]
- Sui, Z.; Salloum, D.; Schlenoff, J.B. Effect of Molecular Weight on the Construction of Polyelectrolyte Multilayers: Stripping versus Sticking. Langmuir 2003, 19, 2491–2495. [Google Scholar] [CrossRef]
- Krogman, K.C.; Cohen, R.E.; Hammond, P.T.; Rubner, M.F.; Wang, B.N. Industrial-scale spray layer-by-layer assembly for production of biomimetic photonic systems. Bioinspir. Biomim. 2013, 8, 045005. [Google Scholar] [CrossRef]
- Michel, M.; Izquierdo, A.; Decher, G.; Voegel, J.C.; Schaaf, P.; Ball, V. Layer by Layer Self-Assembled Polyelectrolyte Multilayers with Embedded Phospholipid Vesicles Obtained by Spraying: Integrity of the Vesicles. Langmuir 2005, 21, 7854–7859. [Google Scholar] [CrossRef]
- Lefort, M.; Boulmedais, F.; Jierry, L.; Gonthier, E.; Voegel, J.C.; Hemmerl, J.; Lavalle, P.; Ponche, A.; Schaaf, P. Simultaneous Spray Coating of Interacting Species: General Rules Governing the Poly(styrene sulfonate)/Poly(allylamine) System. Langmuir 2011, 27, 4653–4660. [Google Scholar] [CrossRef] [Green Version]
- Llamas, S.; Fernández-Peña, L.; Akanno, A.; Guzmán, E.; Ortega, V.; Ortega, F.; Csaky, A.G.; Campbell, R.A.; Rubio, R.G. Towards understanding the behavior of polyelectrolyte–surfactant mixtures at the water/vapor interface closer to technologically-relevant conditions. Phys. Chem. Chem. Phys. 2018, 20, 1395–1407. [Google Scholar] [CrossRef]
- Llamas, S.; Guzmán, E.; Akanno, A.; Fernández-Peña, L.; Ortega, F.; Campbell, R.A.; Miller, R.; Rubio, R.G. Study of the Liquid/Vapor Interfacial Properties of Concentrated Polyelectrolyte–Surfactant Mixtures Using Surface Tensiometry and Neutron Reflectometry: Equilibrium, Adsorption Kinetics, and Dilational Rheology. J. Phys. Chem. C 2018, 122, 4419–4427. [Google Scholar] [CrossRef]
- Merrill, M.H.; Sun, C.T. Fast, simple and efficient assembly of nanolayered materials and devices. Nanotechnology 2009, 20, 075606. [Google Scholar] [CrossRef] [PubMed]
- Akanno, A.; Guzmán, E.; Ortega, F.; Rubio, R.G. Behavior of the water/vapor interface of chitosan solutions with an anionic surfactant: Effect of the polymer-surfactant interactions. Phys. Chem. Chem. Phys. 2020, 22, 23360–23373. [Google Scholar] [CrossRef] [PubMed]
- Lefort, M.; Popa, G.; Seyrek, E.; Szamocki, R.; Felix, O.; Hemmerlé, J.; Vidal, L.; Voegel, J.-C.; Boulmedais, F.; Decher, G.; et al. Spray-On Organic/Inorganic Films: A General Method for the Formation of Functional Nano- to Microscale Coatings. Angew. Chem. Int. Ed. 2010, 49, 10110–10113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.; Li, J.; Wang, M.; Xu, J.; Guo, W.; Li, J.; Bai, Y.; Li, T. Fabrication of Magnetic Luminescent Nanocomposites by a Layer-by-Layer Self-assembly Approach. Chem. Mat. 2004, 16, 4022–4027. [Google Scholar] [CrossRef]
- Sun, J.; Gao, M.; Feldmann, J. Electric Field Directed Layer-by-Layer Assembly of Highly Fluorescent CdTe Nanoparticles. J. Nanosci. Nanotech. 2001, 1, 133–136. [Google Scholar] [CrossRef]
- Shi, L.; Lu, Y.; Sun, J.; Zhang, J.; Sun, C.; Liu, J.; Shen, J. Site-Selective Lateral Multilayer Assembly of Bienzyme with Polyelectrolyte on ITO Electrode Based on Electric Field-Induced Directly Layer-by-Layer Deposition. Biomacromolecules 2003, 4, 1161–1167. [Google Scholar] [CrossRef]
- Richardson, J.J.; Ejima, H.; Lörcher, S.L.; Liang, K.; Senn, P.; Cui, J.; Caruso, F. Preparation of Nano- and Microcapsules by Electrophoretic Polymer Assembly. Angew. Chem. Int. Ed. 2013, 52, 6455–6458. [Google Scholar] [CrossRef]
- Van Tassel, P.R. Polyelectrolyte adsorption and layer-by-layer assembly: Electrochemical control. Curr. Opin. Colloid Interface Sci. 2012, 17, 106–113. [Google Scholar] [CrossRef]
- Ko, Y.H.; Kim, Y.H.; Park, J.; Nam, K.T.; Park, J.H.; Yoo, P.J. Electric-Field-Assisted Layer-by-Layer Assembly of Weakly Charged Polyelectrolyte Multilayers. Macromolecules 2011, 44, 2866–2872. [Google Scholar] [CrossRef]
- Salas, I.M.D.l.F.; Sudhakar, Y.N.; Selvakumar, M. High performance of symmetrical supercapacitor based on multilayer films of graphene oxide/polypyrrole electrodes. Appl. Surf. Sci. 2014, 296, 195–203. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Gu, J.; Yang, H.; Nie, J.; Ma, G. Electrodeposition of alginate/chitosan layer-by-layer composite coatings on titanium substrates. Carbohydr. Polym. 2014, 103, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Liu, P.; Du, P.; Dong, Y.; Lu, C. Magnetic-Targeted pH-Responsive Drug Delivery System via Layer-by-Layer Self-Assembly of Polyelectrolytes onto Drug-Containing Emulsion Droplets and Its Controlled Release. J. Polym. Sci. A Polym. Chem. 2011, 49, 1969–1976. [Google Scholar] [CrossRef]
- Dey, S.; Mohanta, K.; Pal, A.J. Magnetic-Field-Assisted Layer-by-Layer Electrostatic Assembly of Ferromagnetic Nanoparticles. Langmuir 2010, 26, 9627–9631. [Google Scholar] [CrossRef] [PubMed]
- Nyström, G.; Marais, A.; Karabulut, E.; Wågberg, L.; Cui, Y.; Hamed, M.M. Self-assembled three-dimensional and compressible interdigitated thin-film supercapacitors and batteries. Nat. Commun. 2015, 6, 7259. [Google Scholar] [CrossRef] [Green Version]
- Aradilla, D.; Estrany, F.; Alemán, C. Symmetric Supercapacitors Based on Multilayers of Conducting Polymers. J. Phys. Chem. C 2011, 115, 8430–8438. [Google Scholar] [CrossRef]
- Cheng, F.; Tang, W.; Li, C.; Chen, J.; Liu, H.; Shen, P.; Dou, S. Conducting Poly(aniline) Nanotubes and Nanofibers: Controlled Synthesis and Application in Lithium/Poly(aniline) Rechargeable Batteries. Chem. Eur. J. 2006, 12, 3082–3088. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.; Chen, R.; Wu, S.; Li, L.; Chen, S.; Zhao, T. Sulfur/Polythiophene with a Core/Shell Structure: Synthesis and Electrochemical Properties of the Cathode for Rechargeable Lithium Batteries. J. Phys. Chem. C 2011, 115, 6057–6063. [Google Scholar] [CrossRef]
- Yun, J.; Echols, I.; Flouda, P.; Wang, S.; Easley, A.; Zhao, X.; Tan, Z.; Prehn, E.; Zi, G.; Radovic, M.; et al. Layer-by-Layer Assembly of Polyaniline Nanofibers and MXene Thin-Film Electrodes for Electrochemical Energy Storage. ACS Appl. Mat. Interfaces 2019, 11, 47929–47938. [Google Scholar] [CrossRef]
- Shao, L.; Jeon, J.-W.; Lutkenhaus, J.L. Polyaniline/Vanadium Pentoxide Layer-by-Layer Electrodes for Energy Storage. Chem. Mater. 2012, 24, 181–189. [Google Scholar] [CrossRef]
- Shao, L.; Jeon, J.-W.; Lutkenhaus, J.L. Porous polyaniline nanofiber/vanadium pentoxide layer-by-layer electrodes for energy storage. J. Chem. Mat. A 2013, 1, 7648–7656. [Google Scholar] [CrossRef]
- Lee, J.; Ryu, J.; Youn, H.J. Conductive paper through LbL multilayering with conductive polymer: Dominant factors to increase electrical conductivity. Cellulose 2012, 19, 2153–2164. [Google Scholar] [CrossRef]
- Agarwal, M.; Xing, Q.; Shim, B.S.; Kotov, N.; Varahramyan, K.; Lvov, Y. Conductive paper from lignocellulose wood microfibers coated with a nanocomposite of carbon nanotubes and conductive polymers. Nanotechnology 2009, 20, 215602. [Google Scholar] [CrossRef] [PubMed]
- Hyder, M.N.; Lee, S.W.; Cebeci, F.Ç.; Schmidt, D.J.; Shao-Horn, Y.; Hammond, P.T. Layer-by-Layer Assembled Polyaniline Nanofiber/Multiwall Carbon Nanotube Thin Film Electrodes for High-Power and High-Energy Storage Applications. ACS Nano 2011, 5, 8552–8561. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Gas sensing properties of layer-by-layer self-assembled ultrathin film of polyaniline/titanium dioxide. Synth. Met. 2012, 162, 2242–2249. [Google Scholar] [CrossRef]
- Easley, A.D.; Shaligram, S.V.; Echols, I.J.; Nixon, K.; Regen, S.L.; Lutkenhaus, J.L. Layer-by-Layer Nanoarchitectonics of Electrochemically Active Thin Films Comprised of Radical-Containing Polymers. J. Electrochem. Soc. 2022, 169, 020510. [Google Scholar] [CrossRef]
- Fang, Y.; Yuan, B.; Jiang, Y.; Song, R.-B.; Zhang, J.-R.; Zhu, J.-J. Layer-by-layer construction of in situ formed polypyrrole and bacterial cells as capacitive bioanodes for paper-based microbial fuel cells. J. Mater. Chem. A 2022, 10, 4915–4925. [Google Scholar] [CrossRef]
- Delgado, J.L.; Herranz, M.Á.; Martín, N. The nano-forms of carbon. J. Mat. Sci. 2008, 18, 1417–1426. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X. Self-Assembled Graphene/Azo Polyelectrolyte Multilayer Film and Its Application in Electrochemical Energy Storage Device. Langmuir 2011, 27, 2007–2013. [Google Scholar] [CrossRef]
- Bulakhe, R.N.; Nguyen, V.H.; Shim, J.-J. Layer-structured 3D Nanohybrid MoS2@rGO on Nickel Foam for High Performance Energy Storage Applications. New J. Chem. 2017, 41, 1473–1482. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, J.; Chen, S.; Hammond, P.T.; Shao-Horn, Y. Carbon nanotube/manganese oxide ultrathin film electrodes for electrochemical capacitors. ACS Nano 2010, 4, 3889–3896. [Google Scholar] [CrossRef]
- Zhou, Z.; Panatdasirisuk, W.; Mathis, T.S.; Anasori, B.; Lu, C.; Zhang, X.; Liao, Z.; Gogotsi, Y.; Yang, S. Layer-by-layer assembly of MXene and carbon nanotubes on electrospun polymer films for flexible energy storage. Nanoscale 2018, 19, 6005–6013. [Google Scholar] [CrossRef] [PubMed]
- Weng, G.-M.; Li, J.; Alhabeb, M.; Karpovich, C.; Wang, H.; Lipton, J.; Maleski, K.; Kong, J.; Shaulsky, E.; Elimelech, M.; et al. Layer-by-Layer Assembly of Cross-Functional Semi-transparent MXene-Carbon Nanotubes Composite Films for Next-Generation Electromagnetic Interference Shielding. Adv. Funct. Mat. 2018, 28, 1803360. [Google Scholar] [CrossRef]
- Gittleson, F.S.; Hwang, D.; Ryu, W.-H.; Hashmi, S.M.; Hwang, J.; Goh, T.; Taylor, A.D. Ultrathin Nanotube/Nanowire Electrodes by SpinSpray Layer-by-Layer Assembly: A Concept for Transparent Energy Storage. ACS Nano 2015, 9, 10005–10017. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z.; Jiang, W.; Zou, Y.; Wang, Y.; Lu, C. Sandwich-like CNTs@SnO2/SnO/Sn anodes on three-dimensional Ni foam substrate for lithium ion batteries. J. Electroanal. Chem. 2016, 767, 49–55. [Google Scholar] [CrossRef]
- Wanga, Y.; Pub, Y.; Ma, Z.; Pan, Y.; Sun, C.Q. Interfacial adhesion energy of lithium-ion battery electrodes. Extreme Mech. Lett. 2016, 9, 226–236. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Z.; Wang, Y.; Lu, C. Failure Prediction of High-Capacity Electrode Materials in Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1157. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.S.; Xie, Z.C.; Yang, Y.; Zhang, P.P.; Pan, Y.; Zhou, Y.C.; Lu, C. Failure modes of hollow core–shell structural active materials during the lithiation–delithiation process. J. Power Sources 2015, 290, 114–122. [Google Scholar] [CrossRef]
- Camic, B.T.; Vapaavuori, J.; Basarir, F. Transparent conductive electrode based on LBL deposition of graphene oxide and copper nanowires. Mat. Lett. 2022, 311, 131632. [Google Scholar] [CrossRef]
- Neuber, S.; Sill, A.; Efthimiopoulos, I.; Nestler, P.; Fricke, K.; Helm, C.A. Influence of molecular weight of polycation polydimethyldiallylammonium and carbon nanotube content on electric conductivity of layer-by-layer films. Thin Solid Films 2022, 745, 139103. [Google Scholar] [CrossRef]
- Shakir, I. High Energy Density based Flexible Electrochemical Supercapacitors from Layer-by-Layer Assembled Multiwall Carbon Nanotubes and Graphene. Electrochim. Acta 2014, 129, 396–400. [Google Scholar] [CrossRef]
- Wan, K.; Li, Y.; Wang, Y.; Wei, G. Recent Advance in the Fabrication of 2D and 3D Metal Carbides-Based Nanomaterials for Energy and Environmental Applications. Nanomaterials 2021, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Sambyal, P.; Koo, C.M. 2D MXenes for Electromagnetic Shielding: A Review. Adv. Funct. Mat. 2020, 30, 2000883. [Google Scholar] [CrossRef]
- Tian, W.; VahidMohammadi, A.; Wang, Z.; Ouyang, L.; Beidaghi, M.; Hamedi, M.M. Layer-by-layer self-assembly of pillared two-dimensional multilayers. Nat. Commun. 2019, 10, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Wang, J.; Dai, L.; Liu, X.; Li, L.; Yang, Y.; Cao, Y.; Wang, W.; Wu, H.; Guo, S. Flame-retardant poly(vinyl alcohol)/MXene multilayered films with outstanding electromagnetic interference shielding and thermal conductive performances. Chem. Eng. J. 2020, 380, 122475. [Google Scholar] [CrossRef]
- Lipton, J.; Weng, G.-M.; Alhabeb, M.; Maleski, K.; Antonio, F.; Kong, J.; Gogotsi, Y.; Taylor, A.D. Mechanically strong and electrically conductive multilayer MXene nanocomposites. Nanoscale 2019, 11, 20295–20300. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-X.; Chen, W.; Zhang, H.-B.; Wang, Q.-W.; Guan, F.; Yu, Z.-Z. Flexible and Multifunctional Silk Textiles with Biomimetic Leaf-Like MXene/Silver Nanowire Nanostructures for Electromagnetic Interference Shielding, Humidity Monitoring, and Self-Derived Hydrophobicity. Adv. Funct. Mat. 2019, 29, 1905197. [Google Scholar] [CrossRef]
- An, H.; Habib, T.; Shah, S.; Gao, H.; Patel, A.; Echols, I.; Zhao, X.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. Water Sorption in MXene/Polyelectrolyte Multilayers for Ultrafast Humidity Sensing. ACS App. Nano Mat. 2019, 2, 948–955. [Google Scholar] [CrossRef]
- Echols, I.J.; An, H.; Yun, J.; Sarang, K.T.; Oh, J.-H.; Habib, T.; Zhao, X.; Cao, H.; Holta, D.E.; Radovic, M.; et al. Electronic and Optical Property Control of Polycation/MXene Layer-by-Layer Assemblies with Chemically Diverse MXenes. Langmuir 2021, 37, 11338–11350. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.-E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.; Ko, Y.; Huh, J.; Kim, Y.; Yeom, B.; Moon, J.H.; Cho, J. Charge-Transfer Effects of Organic Ligands on Energy Storage Performance of Oxide Nanoparticle-Based Electrodes. Adv. Funct. Mat. 2022, 32, 2106438. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kavyashree; Parveen, S.; Pandey, S.N. High performance binary composite (Sr(OH)2/CoO(OH)) thin film for solid state supercapacitor. J. Energy Storage 2022, 51, 104328. [Google Scholar] [CrossRef]
- Nam, D.; Kwon, M.; Ko, Y.; Huh, J.; Lee, S.W.; Cho, J. Aluminum textile-based binder-free nanostructured battery cathodes using a layer-by-layer assembly of metal/metal oxide nanoparticles. Appl. Phys. Rev. 2021, 8, 011405. [Google Scholar] [CrossRef]
- Yun, J.; Song, Y.; Cho, I.; Ko, Y.; Kwon, C.H.; Cho, J. High-performance electrochromic films with fast switching times using transparent/conductive nanoparticle-modulated charge transfer. Nanoscale 2019, 11, 17815–17830. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Nam, D.; Shin, D.; Song, Y.; Kwon, C.H.; Cho, I.; Lee, S.W.; Cho, J. Charge-Transfer-Modulated Transparent Supercapacitor Using Multidentate Molecular Linker and Conductive Transparent Nanoparticle Assembly. ACS Nano 2019, 13, 12719–12731. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Zhang, R.; Li, Z.; Wei, M.; Evans, D.G.; Duan, X. Layered double hydroxides toward electrochemical energy storage and conversion: Design, synthesis and applications. Chem. Comm. 2015, 51, 15880. [Google Scholar] [CrossRef]
- Shao, M.; Han, J.; Shi, W.; Wei, M.; Duan, X. Layer-by-layer assembly of porphyrin/layered double hydroxide ultrathin film and its electrocatalytic behavior for H2O2. Electrochem. Comm. 2010, 12, 1077–1080. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Zhou, L.; Li, Z.; Shao, M.; Wei, M. Layer-by-layer assembly of exfoliated layered double hydroxide nanosheets for enhanced electrochemical oxidation of water. J. Mater. Chem. A 2016, 4, 11516–11523. [Google Scholar] [CrossRef]
- Kong, X.; Zhao, J.; Han, J.; Zhang, D.; Wei, M.; Duan, X. Fabrication of Naphthol green B/layered double hydroxide nanosheets ultrathin film and its application in electrocatalysis. Electrochim. Acta 2011, 56, 1123–1129. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, S.; Shi, W.; Sun, Z.; Kong, X.; Wei, M.; Duan, X. Assembly of ruthenium(II) complex/layered double hydroxide ultrathin film and its application as an ultrasensitive electrochemiluminescence sensor. Electrochim. Acta 2012, 67, 133–139. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Yan, D.; Wei, M. Electrochemiluminescence resonance energy transfer (ERET) towards trinitrotoluene sensor based on layer-by-layer assembly of luminol-layered double hydroxides and CdTe quantum dots. J. Mater. Chem. C 2017, 5, 3473–3479. [Google Scholar] [CrossRef]
- Han, J.; Xu, X.; Rao, X.; Wei, M.; Evans, D.G.; Duan, X. Layer-by-layer assembly of layered double hydroxide/cobalt phthalocyanine ultrathin film and its application for sensors. J. Mater. Chem. 2011, 21, 2126–2130. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Aramesh, N.; Chen, J.; Liu, W.; Shen, W.; Tang, S.; Lee, H.K. Polyoxometalate-based materials in extraction, and electrochemical and optical detection methods: A review. Anal. Chim. Acta 2022, 339509. [Google Scholar] [CrossRef]
- Genovese, M.; Foong, Y.W.; Lian, K. The unique properties of aqueous polyoxometalate (POM) mixtures and their role in the design of molecular coatings for electrochemical energy storage. Electrochim. Acta 2016, 199, 261–269. [Google Scholar] [CrossRef]
- Akter, T.; Hu, K.; Lian, K. Investigations of multilayer polyoxometalates-modified carbon nanotubes for electrochemical capacitors. Electrochim. Acta 2011, 56, 4966–4971. [Google Scholar] [CrossRef]
- Xing, R.; Tong, L.; Zhao, X.; Liu, H.; Ma, P.; Zhao, J.; Liu, X.; Liu, S. Rapid and sensitive electrochemical detection of myricetin based on polyoxometalates/SnO2/gold nanoparticles ternary nanocomposite film electrode. Sens. Actuators B 2019, 283, 35–41. [Google Scholar] [CrossRef]
- Park, S.; Lian, K.; Gogotsi, Y. Pseudocapacitive Behavior of Carbon Nanoparticles Modified by Phosphomolybdic Acid. J. Electrochem. Soc. 2009, 156, A921–A926. [Google Scholar] [CrossRef]
- Salimi, A.; Korani, A.; Hallaj, R.; Soltanian, S.; Hadadzadeh, H. Deposition of α–SiΜο12O404−-[Ru(bipyridine)(terpyridine)Cl]+ multilayer film on single wall carbon nanotube modified glassy carbon electrode: Improvement of the electrochemical properties and chemical stability. Thin Solid Films 2010, 518, 5304–5310. [Google Scholar] [CrossRef]
- Qu, J.; Zou, X.; Liu, B.; Dong, S. Assembly of polyoxometalates on carbon nanotubes paste electrode and its catalytic behaviors. Anal. Chim. Acta 2007, 599, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.-X.; Ding, X.-M.; Chen, X.-W.; Wang, L. [(PEI/PPy)(PMo12/PPy)5] multilayer composite film modified electrode as a sensor for sensitive determination of tyrosinase in Penaeus vannamei. Inorg. Chim. Acta 2022, 530, 120673. [Google Scholar] [CrossRef]
- Xu, B.; Xu, L.; Gao, G.; Yang, Y.; Guo, W.; Liu, S.; Sun, Z. Multicolor electrochromic and pH-sensitive nanocomposite thin film based on polyoxometalates and polyviologen. Electrochim. Acta 2009, 54, 2246–2252. [Google Scholar] [CrossRef]


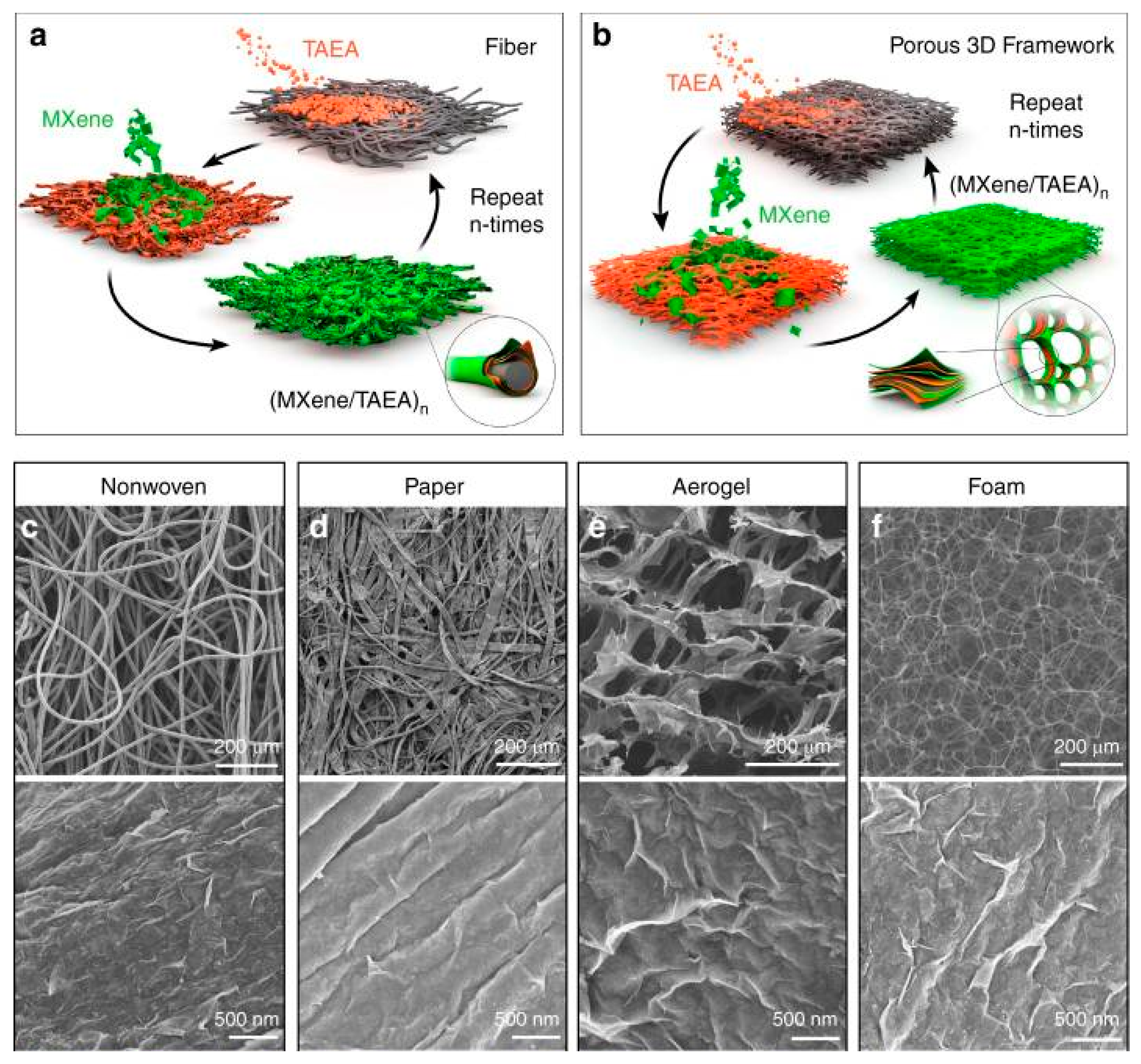
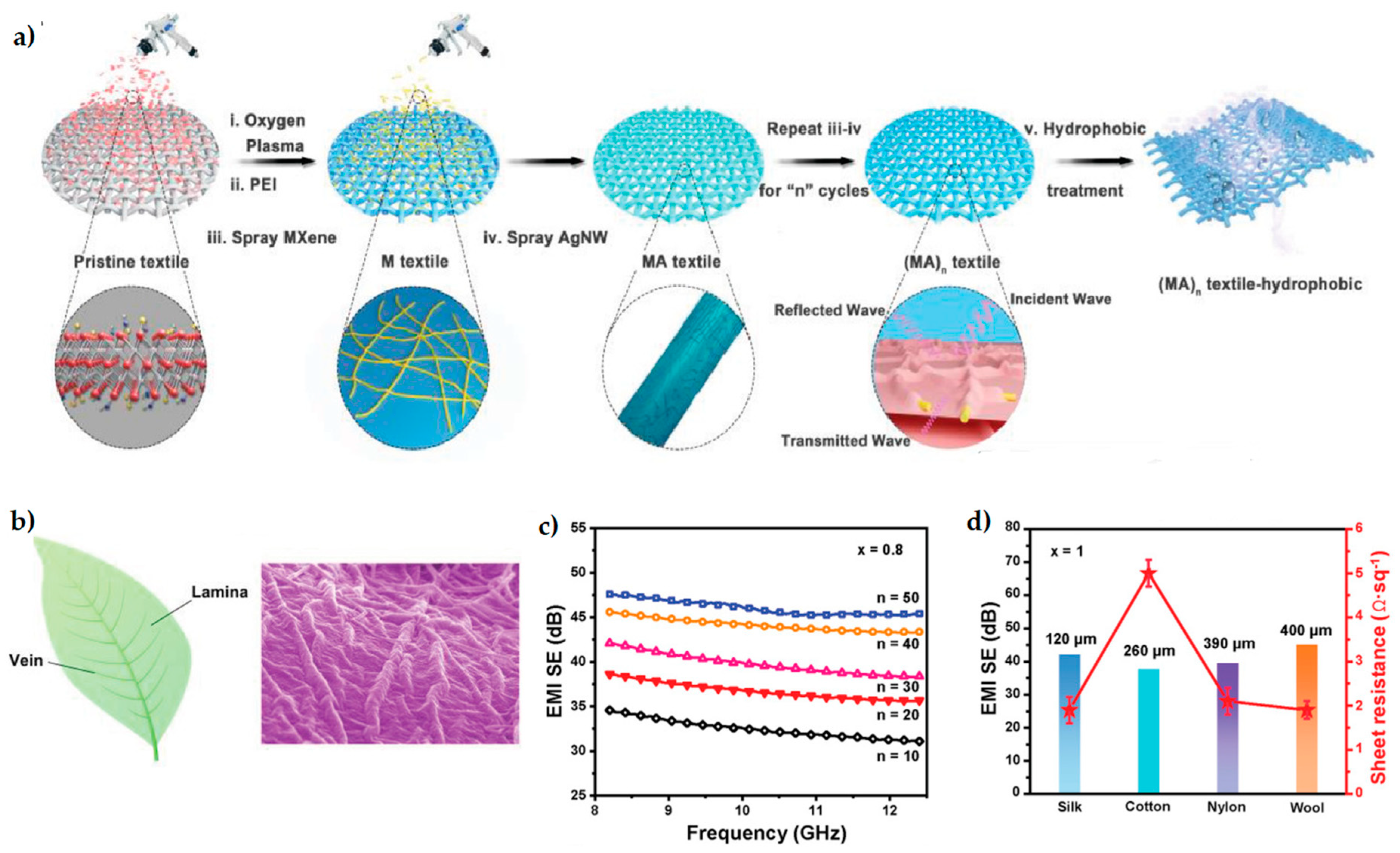
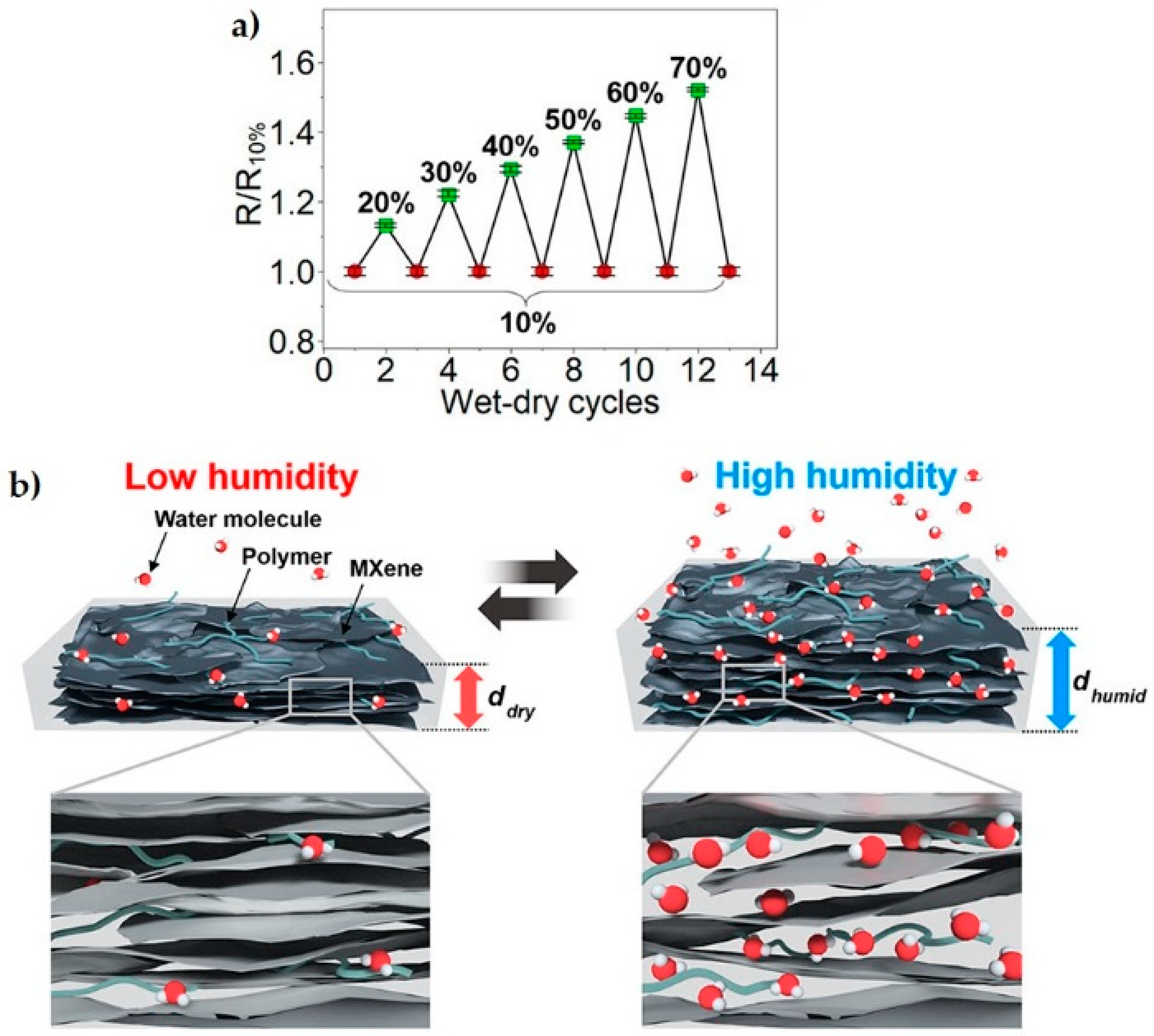
| System | Application | Reference |
|---|---|---|
| MXene nanosheet and PNF | Supercapacitor electrodes | Yun et al. [108] |
| Polyaniline and Vanadium pentoxide | Capacitor electrodes | Shao et al. [109,110] |
| PEDOT and poly(N-methylpyrrole) | Thin film electrodes (anodes or cathodes) | Aradilla et al. [107] |
| PEDOT:PSS and polyethyleneimine | Capacitor electrodes | Lee et al. [111] |
| PEDOT:PSS and Carbon nanotubes | Capacitor electrodes | Agarwal et al. [112] |
| Polyaniline and MWCNT | Thin film electrodes | Hyder et al. [113] |
| PANI-PSSA and titanium dioxide particles | Gas sensors | Lin et al. [114] |
| Polycation and polyanion capped with nitroxide groups | Electroactive coatings | Easley et al. [115] |
| Polypyrrole and bacterial layers | Fuel cell electrode | Fang et al. [116] |
| System | Application | Reference |
|---|---|---|
| Graphene nanosheets and azo-polymer | Capacitor electrodes | Wang and Wang [118] |
| Reduced graphene oxide nanosheets and molybdenum sulfide | Capacitor electrodes | Bulakhe et al. [119] |
| Alternate layers of MWCNT modified with ammonium and carboxylic group capped with a layer of MnO2 | Capacitor electrodes | Lee et al. [120] |
| MXene and MWCNT | Supercapacitor electrodes | Zhou et al. [121] |
| MXene and Carbon nanotubes | Thin films for electromagnetic interference shielding | Weng et al. [122] |
| SWCNT and PSS | Fuel cell anodes | Gittleson et al. [123] |
| Graphene oxide and copper nanowires | Transparent electrodes | Camic et al. [128] |
| PDADMAC and oxidized carbon nanotubes | Conductive layers for biological applications | Neuber et al. [129] |
| MWCNT and graphene | Thin film electrodes | Shakir [130] |
| System | Application | Reference |
|---|---|---|
| MXene and tris(2-aminoethyl) amine | Supercapacitor electrodes | Tian et al. [133] |
| MXene and poly(vinyl alcohol) | Electromagnetic interference shielding devices | Jin et al. [134] |
| MXene and nanoclays | Multifunctional materials | Lipton et al. [135] |
| MXene and silver nanowires | Electromagnetic interference shielding devices and Humidity sensors | Liu et al. [136] |
| MXene and PDADMAC | Humidity sensors | An et al. [137] |
| System | Application | Reference |
|---|---|---|
| Fe3O4 and ITO nanoparticles | Capacitor electrodes | Song et al. [140] |
| Sr(OH)2 and CoO(OH) nanoflakes | Supercapacitor electrodes | Sharma et al. [141] |
| ITO nanoparticles and LiFePO4 | Lithium-ion battery cathodes | Nam et al. [142] |
| Tungsten oxide nanorods and ITO nanoparticles | Electrochromic films | Yun et al. [143] |
| Manganese oxide and ITO nanoparticles | Supercapacitor electrodes | Choi et al. [144] |
| System | Application | Reference |
|---|---|---|
| Layered double hydroxide of cobalt and aluminum and iron (III) porphyrin | Electrodes for electrocatalysis | Shao et al. [146] |
| Layered double hydroxide of cobalt and aluminum and naphthol green B | Electrodes for electrocatalysis | Kong et al. [148] |
| Layered double hydroxide, PSS and Ru(bpy)32+ | Electrochemiluminescence sensor | Zhang et al. [149] |
| Layer double hydroxide of cobalt and aluminum capped with luminol and anionic quantum dots of CdSe | Electrochemiluminescence sensor | Li et al. [150] |
| Layer double hydroxide and cobalt phthalocyanine | Dopamine sensor | Han et al. [151] |
| System | Application | Reference |
|---|---|---|
| Polyoxometalates and MWCNT | Double layer capacitors | Genovese et al. [153] |
| Polyoxometalates, mesoporous SnO2 and gold nanoparticles | Pseudocapacitive systems | Akter et al. [154] |
| Polyoxometalates and different carbon materials | Capacitors | Park et al. [156] |
| Polyoxometalates and complex of Ruthenium | Electrodes | Salimi et al. [157] |
| Polyoxometalate of tungsten and poly(hexyl viologen) | Electrochromic devices | Xu et al. [160] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán, E.; Ortega, F.; Rubio, R.G. Layer-by-Layer Materials for the Fabrication of Devices with Electrochemical Applications. Energies 2022, 15, 3399. https://doi.org/10.3390/en15093399
Guzmán E, Ortega F, Rubio RG. Layer-by-Layer Materials for the Fabrication of Devices with Electrochemical Applications. Energies. 2022; 15(9):3399. https://doi.org/10.3390/en15093399
Chicago/Turabian StyleGuzmán, Eduardo, Francisco Ortega, and Ramón G. Rubio. 2022. "Layer-by-Layer Materials for the Fabrication of Devices with Electrochemical Applications" Energies 15, no. 9: 3399. https://doi.org/10.3390/en15093399
APA StyleGuzmán, E., Ortega, F., & Rubio, R. G. (2022). Layer-by-Layer Materials for the Fabrication of Devices with Electrochemical Applications. Energies, 15(9), 3399. https://doi.org/10.3390/en15093399








