Pyrolysis Characteristics of Hailar Lignite in the Presence of Polyvinyl Chloride: Products Distribution and Chlorine Migration
Abstract
:1. Introduction
2. Experimental Section
2.1. Samples
2.2. Thermogravimetric Analysis
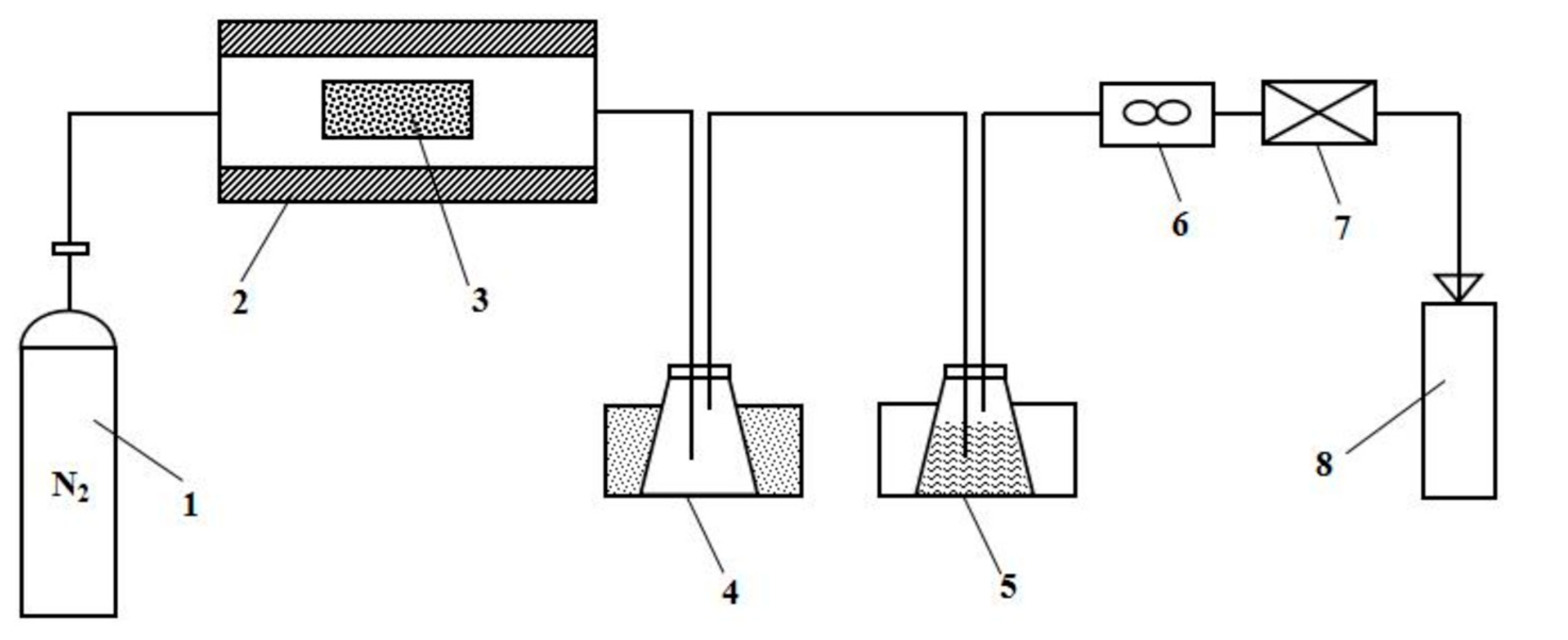
2.3. Co-Pyrolysis in PVC
2.4. Product Analysis and Characterization
2.5. Data Analysis
3. Results and Discussion
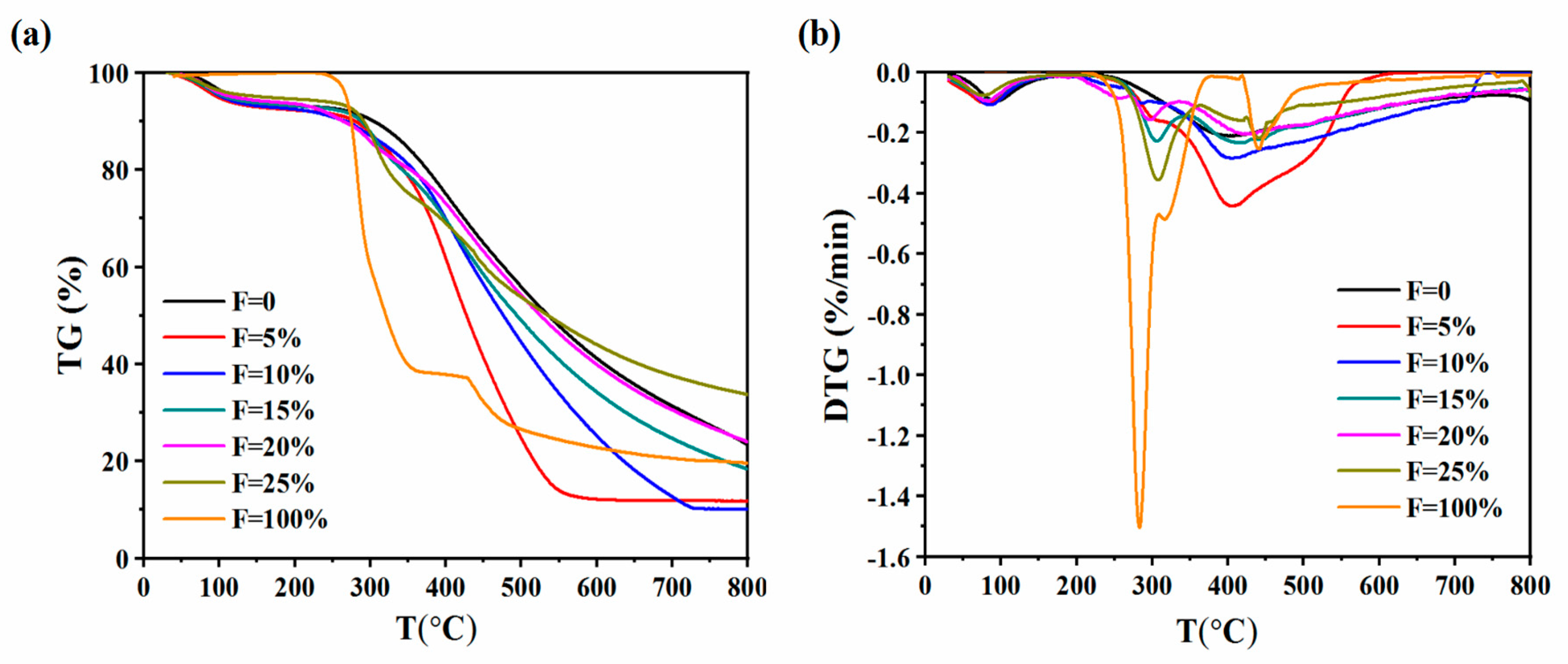
3.1. TG and DTG Analyses
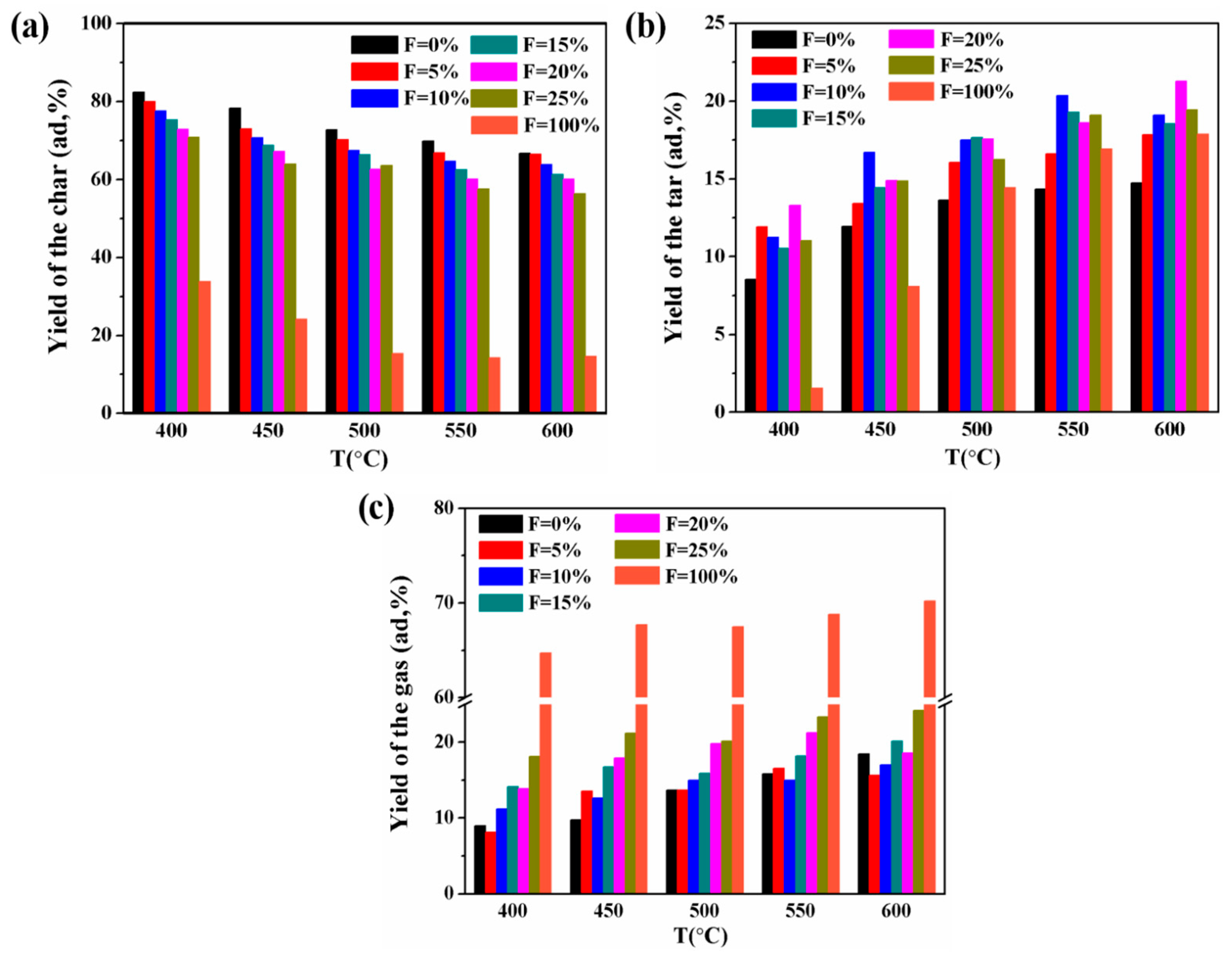
3.2. Yield Analyses of Pyrolysis Product
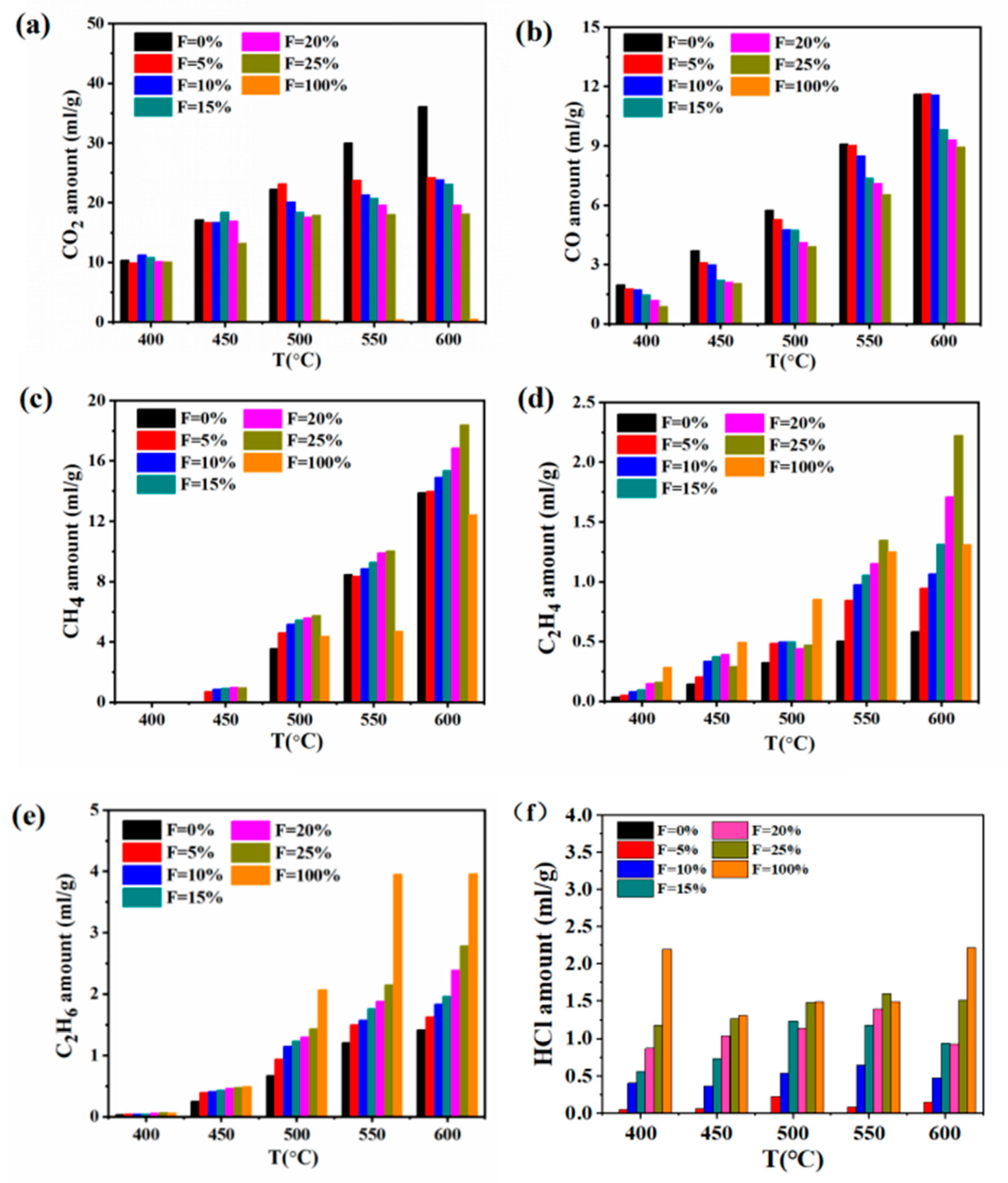
3.3. Gas Composition Analyses in Different PVC Additions
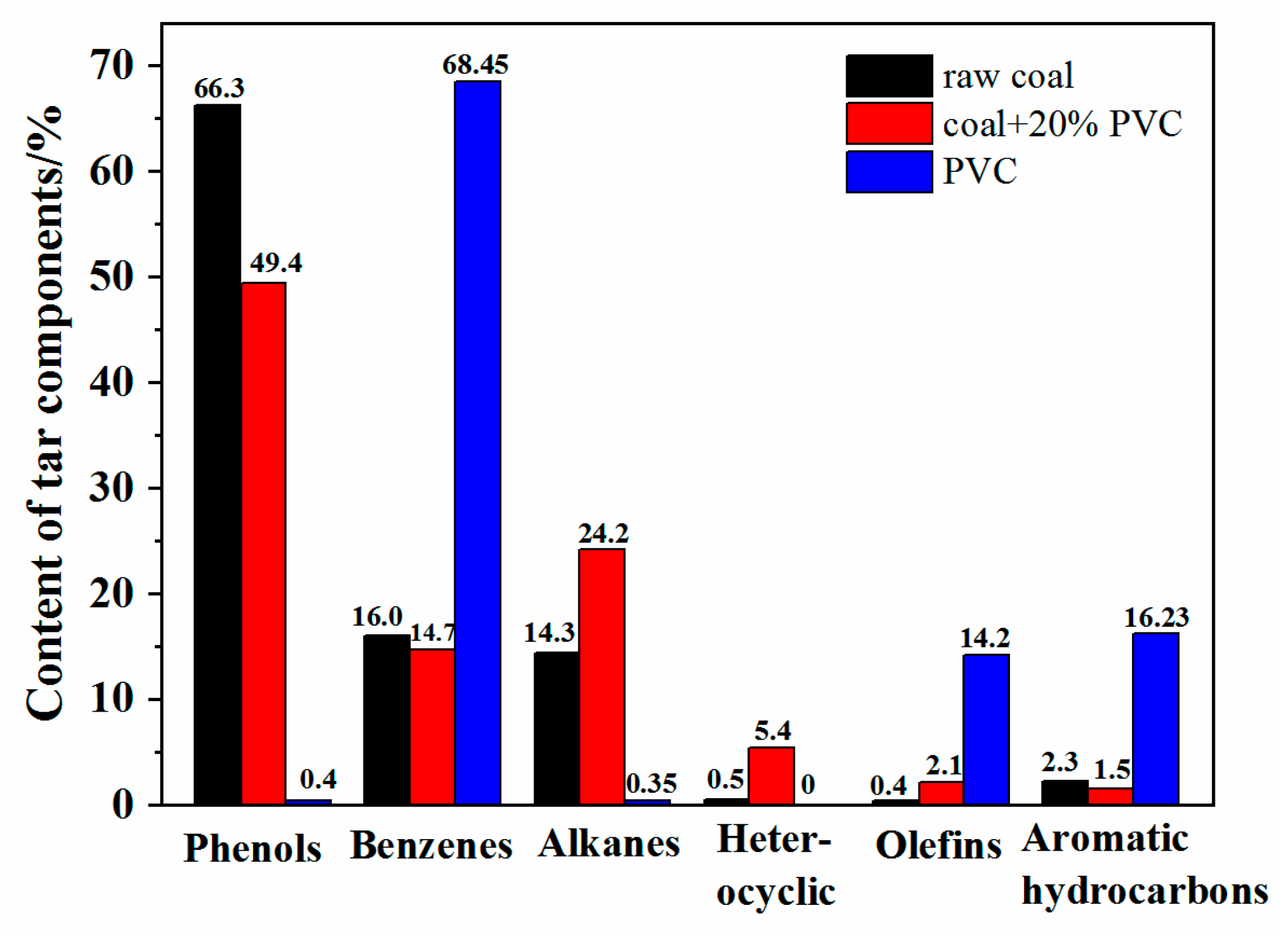
3.4. Tar Composition Analyses with Different PVC Additions
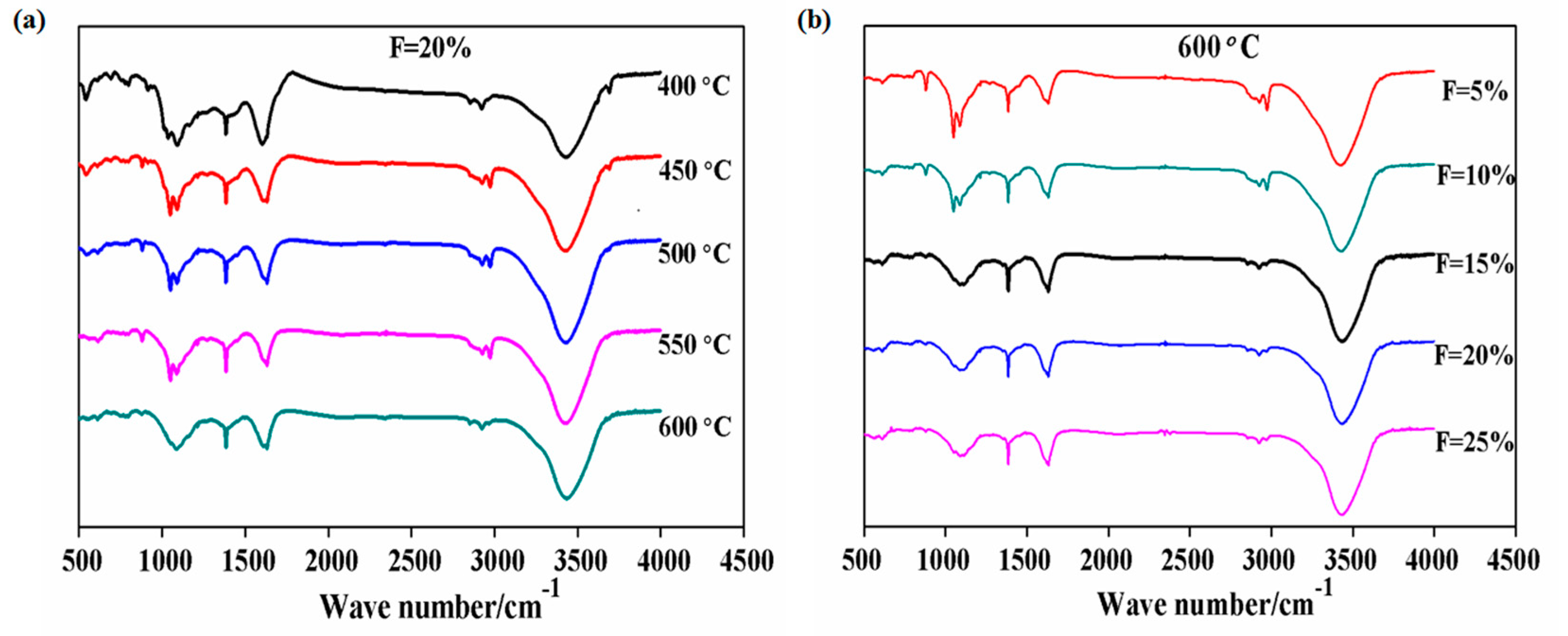
3.5. Effect of the PVC Additions on the Co-Pyrolysis Char
3.6. Effect of Co-Pyrolysis on the Distribution of Chlorine
4. Conclusions
- The study of the co-pyrolysis products distribution characteristics showed that the addition of PVC had a significant increasing effect on the tar yield and an inhibitory effect on the release of gas by comparing the actual value of the pyrolysis product yield with the theoretical value. At 600 °C, the tar yield was the highest with 20 wt% PVC additions, reaching 21.3%. The gas-phase composition analysis showed that the release amount of CO2 and CO decreased and that the release amount of CH4, C2H4, and C2H6 increased significantly when PVC was added. In particular, the maximum increase of CH4 could reach 8.33 mL/g at 600 °C. The content of tar components changed greatly during the co-pyrolysis of raw coal and PVC. Compared with raw coal pyrolysis, the alkanes, olefins, and heterocyclic compounds increased by 6.9%, 1.7%, and 10.9%, respectively.
- By analyzing the migration law of chlorine under different pyrolysis temperature and PVC additions, we identified that the content of chlorine in the gas phase increased first and then decreased with increasing pyrolysis temperature. At a terminal temperature of 600 °C, the chlorine in the gas phase began to decrease. At the same time, the chlorine in the PVC reacted with the metal oxide to form the metal chloride, such that the chlorine was fixed in the solid-phase product.
Author Contributions
Funding
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, O.; Kwak, S.-Y. Suppression of Dioxin Emission in Co-Incineration of Poly(vinyl Chloride) with TiO2-Encapsulating Polystyrene. Environ. Sci. Technol. 2007, 41, 5833–5838. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Fang, W.; Xu, W.; Cao, A.; Wang, R. Characteristics and the recovery potential of plastic wastes obtained from landfill mining. J. Clean. Prod. 2014, 80, 80–86. [Google Scholar] [CrossRef]
- Park, K.C.; Tomiyasu, H. Gasification reaction of organic compounds catalyzed by RuO2 in supercritical water. Chem. Commun. 2003, 6, 694–695. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2015, 48, 300–314. [Google Scholar] [CrossRef]
- Czégény, Z.; Jakab, E.; Bozi, J.; Blazsó, M. Pyrolysis of wood–PVC mixtures. Formation of chloromethane from lignocellulosic materials in the presence of PVC. J. Anal. Appl. Pyrolysis 2015, 113, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Özsin, G.; Pütün, A.E. TGA/MS/FT-IR study for kinetic evaluation and evolved gas analysis of a biomass/PVC co-pyrolysis process. Energy Convers. Manag. 2019, 182, 143–153. [Google Scholar] [CrossRef]
- Chen, B.; Wei, X.-Y.; Zong, Z.-M.; Yang, Z.-S.; Qing, Y.; Liu, C. Difference in chemical composition of supercritical methanolysis products between two lignites. Appl. Energy 2011, 88, 4570–4576. [Google Scholar] [CrossRef]
- Jangam, S.V.; Karthikeyan, M.; Mujumdar, A.S. A Critical Assessment of Industrial Coal Drying Technologies: Role of Energy, Emissions, Risk and Sustainability. Dry. Technol. 2011, 29, 395–407. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, T.; Huang, Q. Co-pyrolysis characteristics and kinetics of coal and plastic blends. Energy Convers. Manag. 2009, 50, 705–710. [Google Scholar] [CrossRef]
- Nabeel, A.; Alam Khan, T.; Sharma, D.K. Non-isothermal Kinetic Studies of Co-combustion and Co-cracking of Coal and Plastic Blends Using Thermogravimetric Analysis. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 722–732. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, K.M.; Basheer, N.; Hussain, K. Co-liquefaction of Makarwal coal and waste polystyrene by microwave–metal interaction pyrolysis in copper coil reactor. J. Anal. Appl. Pyrolysis 2010, 90, 53–55. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Y.; Zhou, L.; Huang, Q. Thermogravimetric analysis and kinetics of coal/plastic blends during co-pyrolysis in nitrogen atmosphere. Fuel Process. Technol. 2008, 89, 21–27. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, A.K. Study of kinetics of co-pyrolysis of coal and waste LDPE blends under argon atmosphere. Fuel 2010, 89, 3943–3951. [Google Scholar] [CrossRef]
- Meng, H.; Wang, S.; Chen, L.; Wu, Z.; Zhao, J. Investigation on Synergistic Effects and Char Morphology during Co-pyrolysis of Poly(vinyl chloride) Blended with Different Rank Coals from Northern China. Energy Fuels 2015, 29, 6645–6655. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Altarawneh, M. Catalytic de-chlorination of products from PVC degradation by magnetite (Fe3O4). Appl. Surf. Sci. 2019, 480, 792–801. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.; Adrados, A.; Laresgoiti, M. Deactivation and regeneration of ZSM-5 zeolite in catalytic pyrolysis of plastic wastes. Waste Manag. 2011, 31, 1852–1858. [Google Scholar] [CrossRef]
- Kakuta, Y.; Hirano, K.; Sugano, M.; Mashimo, K. Study on chlorine removal from mixture of waste plastics. Waste Manag. 2008, 28, 615–621. [Google Scholar] [CrossRef]
- Khoshnoud, P.; Abu-Zahra, N. Kinetics of thermal decomposition of PVC/fly ash composites. Int. J. Polym. Anal. Charact. 2017, 23, 170–180. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M. Fuels from Waste Plastics by Thermal and Catalytic Processes: A Review. Ind. Eng. Chem. Res. 2008, 47, 7982–7992. [Google Scholar] [CrossRef]
- Jiménez, A.; Iannoni, A.; Torre, L.; Kenny, J.M. Kinetic Modeling of the Thermal Degradation of Stabilized PVC Plastisols. J. Therm. Anal. 2000, 61, 483–491. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Wang, G.; Wang, H.; Sun, M.M. Thermal and Kinetic Analysis of Coal with Different Waste Plastics (PVC) in Cocombustion. Energy Fuels 2018, 32, 2145–2155. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Woszidlo, S.; Koehler, R.; Kopinke, F.-D. Hydrothermal carbonization of poly(vinyl chloride). Chemosphere 2015, 119, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Yamashita, Y.; Saito, I. Studies on structural changes of coal density-separated components during pyrolysis by means of solid-state 13C NMR spectra. J. Anal. Appl. Pyrolysis 2000, 53, 35–50. [Google Scholar] [CrossRef]
- Liu, Y.; Hodek, W.; Van Heek, K.H. Characterization of tar, char and gas from pyrolysis of coal asphaltenes. Fuel 1998, 77, 1099–1105. [Google Scholar] [CrossRef]
- Kaminsky, W.; Kim, J.-S. Pyrolysis of mixed plastics into aromatics. J. Anal. Appl. Pyrolysis 1999, 51, 127–134. [Google Scholar] [CrossRef]
- Uddin, M.A.; Sakata, Y.; Shiraga, Y.; Muto, A.A.; Murata, K. Dechlorination of Chlorine Compounds in Poly(vinyl chloride) Mixed Plastics Derived Oil by Solid Sorbents. Ind. Eng. Chem. Res. 1999, 38, 1406–1410. [Google Scholar] [CrossRef]
- Saito, K.; Narita, H. Studies on the dechlorination and oil-production technology of waste plastics. J. Mater. Cycles Waste Manag. 2001, 3, 93–98. [Google Scholar]
- Solomon, P.; Fletcher, T.; Pugmire, R. Progress in coal pyrolysis. Fuel 1993, 72, 587–597. [Google Scholar] [CrossRef]

| Sample | Proximate Analysis wt% | Ultimate Analysis wt%, daf | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vdaf | FCad | C | H | O * | N | S | Cl | |
| Coal | 6.93 | 16.20 | 42.99 | 33.88 | 70.95 | 4.27 | 23.59 | 0.79 | 0.29 | 0.11 |
| PVC | 0.02 | 0.05 | 99.46 | 0.47 | 37.89 | 4.69 | 1.62 | — | — | 55.8 |
| Ash Chemical Composition (wt%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | MgO | Na2O | SiO2 | P2O5 | SO3 | K2O | TiO2 | Fe2O3 | CaO | Cl− | Others | |
| Coal | 6.94 | 7.03 | 46.80 | 17.10 | 1.42 | 3.49 | 0.73 | 0.81 | 6.90 | 7.30 | 0.54 | 0.94 |
| PVC | — | — | — | — | — | — | — | — | — | — | ||
| Sample | Petrographic Analysis (vol%) | Rmax | |||
|---|---|---|---|---|---|
| Vitrinite | Inertinite | Exinite | Minerals | ||
| Coal | 70.4 | 22.1 | 1.8 | 5.7 | 0.986 |
| Products | T (°C) | PVC Addition (wt, d) | ||||
|---|---|---|---|---|---|---|
| 5% | 10% | 15% | 20% | 25% | ||
| char | 400 | −0.06 | −0.03 | 0.16 | 0.10 | 0.54 |
| 450 | −2.54 | −2.19 | −1.35 | −0.27 | −0.83 | |
| 500 | 0.42 | 0.54 | 2.31 | 1.42 | 5.26 | |
| 550 | −0.20 | 0.39 | 1.04 | 1.41 | 1.58 | |
| 600 | 2.27 | 1.38 | 1.45 | 1.98 | 1.85 | |
| tar | 400 | 3.71 | 3.38 | 3.04 | 6.16 | 4.23 |
| 450 | 1.64 | 5.11 | 3.07 | 3.73 | 3.88 | |
| 500 | 2.40 | 3.80 | 3.93 | 3.79 | 2.43 | |
| 550 | 2.15 | 3.26 | 4.57 | 3.76 | 4.10 | |
| 600 | 3.89 | 4.53 | 4.18 | 4.71 | 4.67 | |
| gas | 400 | −3.65 | −3.35 | −3.20 | −6.25 | −4.78 |
| 450 | 0.89 | −2.86 | −1.72 | −3.46 | −3.06 | |
| 500 | −2.82 | −4.33 | −5.24 | −5.21 | −6.69 | |
| 550 | −2.95 | −4.14 | −5.61 | −5.17 | −5.68 | |
| 600 | −5.21 | −5.91 | −5.63 | −6.69 | −6.52 | |
| Species | PVC Addition (wt, d) | |||||
|---|---|---|---|---|---|---|
| 0% | 5% | 10% | 15% | 20% | 25% | |
| Al2O3 | 6.94 | 7.41 | 7.01 | 6.62 | 6.31 | 6.22 |
| MgO | 7.03 | 5.65 | 5.42 | 5.04 | 5.55 | 5.28 |
| Na2O | 46.80 | 44.50 | 40.70 | 39.50 | 39.10 | 36.20 |
| CeO2 | 0.20 | 0.10 | 0.12 | 0.11 | 0.13 | 0.11 |
| La2O3 | 0.13 | 0.12 | 0.11 | 0.10 | 0.98 | 0.82 |
| SiO2 | 17.10 | 18.40 | 18.30 | 18.10 | 18.00 | 17.80 |
| SO3 | 3.49 | 2.48 | 2.31 | 2.30 | 2.25 | 2.14 |
| K2O | 0.73 | 0.72 | 0.70 | 0.69 | 0.67 | 0.67 |
| TiO2 | 0.81 | 0.65 | 0.71 | 0.67 | 0.71 | 0.69 |
| Fe2O3 | 6.90 | 7.81 | 7.69 | 7.06 | 7.73 | 7.14 |
| MnO | 0.24 | 0.28 | 0.27 | 0.26 | 0.27 | 0.26 |
| CaO | 7.30 | 6.66 | 6.68 | 6.28 | 6.16 | 6.00 |
| Cl− | 0.54 | 3.60 | 4.38 | 4.68 | 5.43 | 6.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Ren, M.; Feng, C.; Jiao, Y.; Bai, Y.; Ma, Q. Pyrolysis Characteristics of Hailar Lignite in the Presence of Polyvinyl Chloride: Products Distribution and Chlorine Migration. Energies 2022, 15, 3377. https://doi.org/10.3390/en15093377
Fan H, Ren M, Feng C, Jiao Y, Bai Y, Ma Q. Pyrolysis Characteristics of Hailar Lignite in the Presence of Polyvinyl Chloride: Products Distribution and Chlorine Migration. Energies. 2022; 15(9):3377. https://doi.org/10.3390/en15093377
Chicago/Turabian StyleFan, Hui, Menglin Ren, Caiyun Feng, Yue Jiao, Yonghui Bai, and Qingxiang Ma. 2022. "Pyrolysis Characteristics of Hailar Lignite in the Presence of Polyvinyl Chloride: Products Distribution and Chlorine Migration" Energies 15, no. 9: 3377. https://doi.org/10.3390/en15093377
APA StyleFan, H., Ren, M., Feng, C., Jiao, Y., Bai, Y., & Ma, Q. (2022). Pyrolysis Characteristics of Hailar Lignite in the Presence of Polyvinyl Chloride: Products Distribution and Chlorine Migration. Energies, 15(9), 3377. https://doi.org/10.3390/en15093377







