Abstract
Solar–thermal conversion and migration characteristics of nanofluids have attracted intensive attention recently. Due to the strong absorption of solar energy, solar collectors with nanofluids have wide applications in many areas including desalination and power generation. Researchers have mainly focused on the macroscopic performance of nanofluids in solar collectors, but the nanoparticles’ migration characteristics with vapor during phase transformation have not been further investigated. Therefore, an experimental investigation on solar–thermal conversion characteristics of nanofluids and migration characteristics with vapor during phase transformation was conducted in this work, in order to verify the enhancement effect of nanoparticles on solar energy absorption and explore the nanoparticles’ migration behavior with vapor. It was found that part of Ag nanoparticles migrate out of the nanofluids with generated vapor by boiling nanofluids, and most of the nanoparticles remained in the nanofluids. In addition, more Ag nanoparticles migrated with vapor with the increased heating power. The concentration of migrated nanofluids was 20.58 ppm with a power of 16.2 W and 31.39 ppm with a power of 20 W. The investigation pointed out the potential danger of nanofluids in the process of utility and provided a reference for the standardized application of nanofluids.
1. Introduction
With the rapid development of society, the demand for energy is increasing sharply. The accelerated consumption of traditional fossil energy represented by oil, coal, and natural gas has caused a serious energy crisis and environmental pollution. The development and utilization of renewable energy have attracted more and more attention in industrial application and scientific research [1,2,3,4]. Solar energy, as one of the most abundant and cleanest renewable energy, could be directly absorbed into heat energy through the solar collector, the conversion efficiency of that is closely related to the absorption rate of the working medium [5,6,7,8,9,10,11,12,13]. However, how to improve the efficiency and extensive use of solar energy is still a huge challenge [14,15,16,17].
In 1995, Choi et al. [18] of Argonne National Laboratory in the United States first proposed the concept of nanofluid. Nanofluid is a stable and uniform medium with a high thermal conductivity formed by adding metallic or non-metallic nanoparticles into traditional heat transfer fluid media such as water, alcohol, or oil [19]. This concept brings new hope to the enhanced heat transfer technology. It was investigated that due to the small size effect and Brownian motion, the nanofluids have stronger suspension stability, excellent heat transport performance, and better spectral absorption performance than the traditional fluids [20,21,22]. Therefore, some researchers proposed to use nanofluids as a circulating working medium in direct absorption solar collectors in order to improve the solar–thermal conversion efficiency [23,24]. Traditional solar collectors face the questions that how to improve the absorption efficiency of solar energy and how to efficiently transmit the absorbed energy in the working medium. With excellent absorption properties and heat transfer characteristics, nanofluids can meet two requirements of the working medium in solar collectors, and greatly improve the conversion efficiency [25,26,27]. The solar–thermal conversion characteristics of nanofluids have been investigated, which is the key to efficient energy absorption and heat transfer of solar collectors [28,29,30]. Taylor et al. [31] applied nanoparticles in the field of solar energy and found that under the same laser intensity, nanofluids had lower temperatures and higher vapor production than liquid with black dyes. Otanicar et al. [32] analyzed the absorption property of different nanofluids, including carbon-based and metal-based nanoparticles. The results showed that the size, material, and volume concentration of nanoparticles had a certain impact on the solar energy collection efficiency, and the solar–thermal conversion efficiency was slightly improved compared with the base working medium (water). Zhang et al. [33] conducted experimental investigation on different nanofluids and found that gold nanoparticles had the highest solar–thermal conversion efficiency within the experimental range. The 0.15 ppm gold nanoparticles improved the solar–thermal conversion efficiency by 20%. However, the concentration of nanofluids used in the most of the experiments was lower than 1 ppm. Therefore, it is necessary to further study the solar–thermal conversion characteristics of nanofluids with high concentration. Chen et al. [34] found that gold nanoparticles with a mass fraction of 0.000008% could increase the temperature rise of pure water by 21.3%. Moreover, the solar–thermal conversion performance was improved with the decrease of the size of gold nanoparticles.
Nanofluids can greatly improve the solar–thermal conversion efficiency of solar collectors, which promotes the application of nanofluids in the field of efficient energy absorption and heat transfer. Meanwhile, the phenomenon of vapor generated after the absorption of solar energy by nanofluids also attracts much attention. In 2013, Neumann et al. [35] investigated that Au nanofluids could directly generate vapor under the irradiation of concentrated sunlight. The investigation indicated that 80% of the Au nanofluids’ absorbed solar energy was used to directly generate vapor, but only about 20% of that was used to increase the temperature. This phenomenon was also reported in the previous study by the corresponding author of this paper [5]. It was believed that the “thermal trapping” effect of nanoparticles caused uneven temperature distribution and the overheated and boiling in the nanofluids. The phenomenon will enable nanofluids to be used in new fields such as seawater desalination or vapor sterilization.
Although nanofluids have efficient energy absorption and heat transfer characteristics, they may still pose a threat to human life in the process of utility. Particularly, a number of nanoparticles will inevitably migrate during the process of nanofluids producing vapor under concentrated sunlight, which deserves more attention. In recent years, researchers have begun to pay attention to the possible harmful effects of rapidly developing nanoparticles and issued an early warning on the pollution of nanoparticles [36,37]. Studies indicated that nanofluids, including carbon nanotubes (CNT), metal, and metal oxides nanofluids, had adverse effects on plants, human cells, and microorganisms [38,39,40,41]. Therefore, it is very important to investigate the nanoparticles’ migration characteristics with vapor. However, few researchers have studied the migration characteristics of nanoparticles at present, which requires further research.
In summary, the solar–thermal conversion characteristics of nanofluids are the key to nanofluids in solar collectors. At present, researchers have mainly focused on the macroscopic performance of nanofluids in solar collectors, but the migration characteristics of nanoparticles with vapor during phase transformation have not been further investigated. Therefore, it is necessary to conduct experimental investigations on solar–thermal conversion characteristics of nanofluids and migration characteristics with vapor during phase transformation, in order to verify the enhancement effect of nanoparticles on solar energy absorption and explore the nanoparticles’ migration behavior with vapor. The originality of this paper lies in the study of the migration characteristics of nanoparticles, which is a special and rarely investigated scientific issue, pointing out the potential danger of nanofluids in the process of utility and providing a reference for the standardized application of nanofluids.
2. Synthesis of Nanofluids
2.1. One-Step Method
The one-step method consists of simultaneous nanoparticle manufacturing and nanofluid preparation in the fluid and avoids the processes of drying, storage, transportation, and dispersion of nanoparticles [42]. Gold nanofluids were prepared by a reduction reaction with citrate, which was described in our previous research in detail [5,25,43,44]. The chemical reaction formula [33] for preparing gold nanofluids by HAuCl4 is:
Figure 1 shows the synthesis steps of gold nanofluids which are as follows: (i) dissolving HAuCl4 and sodium citrate crystal to prepare solutions; (ii) stirring HAuCl4 solutions through magnetic heating stirrers with a stirring speed of 3000 rpm, and heating HAuCl4 solution to boiling; (iii) quickly mixing the sodium citrate solutions and increasing the stirring speed to 5000 rpm until the solution turns red and purple, which indicated that the preparation of gold nanofluids is completed. After the steps of synthesis, the prepared gold nanofluids were transferred to a clean storage bottle and placed in a cool and dark place. The gold nanofluids were photographed at 200 kV by TECNAI G2 F20 S-twin field emission transmission electron microscope (TEM), as shown in Figure 2a. It was found that gold nanoparticles were spherical and oval, with an average size of about 15 nm.
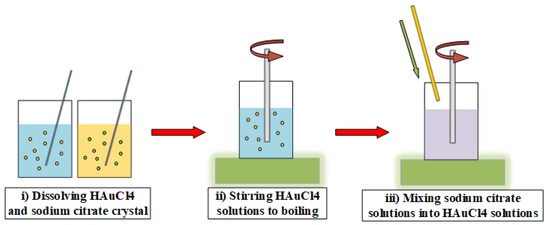
Figure 1.
One-step method for preparing the gold nanofluids.
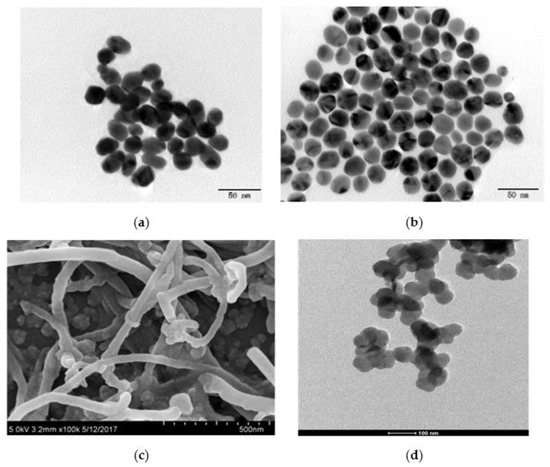
Figure 2.
TEM images of nanofluids. (a) Au; (b) Ag; (c) MWCNT; (d) SiO2.
2.2. Two-Step Method
The two-step method includes two steps: preparation and dispersion of nanoparticles by homogenizer instrument. Given that nanoparticle blends are already mass-produced, this simple and economical approach could expand the range of applications for nanofluids [42]. In this paper, Ag, SiO2, and MWCNT nanofluids were prepared with the two-step method, as shown in Figure 3. The specific method can be found in the relevant articles [45,46] and as follows: (i) weighing a certain amount of nanoparticles by a microbalance; (ii) mixing nanoparticles with a stabilizer in the base solution, and preliminarily dispersing the solution by ultrasonic water bath method; (iii) further dispersing the unstable dispersion by ultrasonic crusher with the controlled time to obtain stable nanofluids. The Ag, MWCNT, and SiO2 nanofluids were photographed at 200 kV by TECNAI G2 F20 S-twin field emission transmission electron microscope (TEM), as shown in Figure 2b–d.
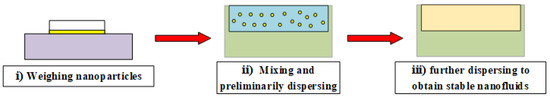
Figure 3.
Two-step method for preparing nanofluids.
3. Experimental Setup
3.1. Experimental Setup for Solar–Thermal Conversion Characteristics of Nanoparticles
The experimental setup is shown in Figure 4, including the solar simulator (Newport Oriel Sol AAA), digital mass balance, windshield, test container filled with nanofluids, thermocouple, and data acquisition system. The output light intensity was 1 sun (1000 W/m2) with a precision of ±0.5%. The mass of the test container filled with nanofluids was measured by the Ohaus EX224 digital mass balance with a precision of ±0.0005 g. The radium of the test container is 30 mm, which is measured by a caliper with a precision of ±0.05 mm. The initial mass of nanofluids is 50 g. The temperature of nanofluids at different positions was measured by four K-type thermocouples with a precision of ±0.5 °C and saved in the data acquisition system for subsequent analysis. Three thermocouples were placed in the test container to measure the temperature gradient from the surface to the bottom, named TC1 to TC3, and one thermocouple was placed in the environment to measure the ambient temperature, named TC0. The nanofluids in the experiment were Ag, Au, MWCNT, and SiO2 nanofluids. Except for the SiO2 nanofluids’ concentration of 1000 ppm, the other three nanofluids’ concentration were 50 ppm, 200 ppm, 500 ppm, and 1000 ppm.
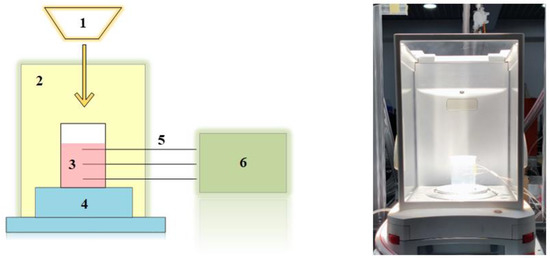
Figure 4.
Experimental setup for solar–thermal conversion characteristics of nanoparticles. 1. Solar simulator. 2. Windshield. 3. Test container filled with nanofluids. 4. Digital mass balance. 5. Thermocouple. 6. Data acquisition system.
3.2. Experimental Setup for Migration Characteristics of Nanoparticles
The experimental setup of migration thermal properties of nanofluids under solar concentrating conditions is shown in Figure 5. The sunlight passed through the Fresnel lens and focused on the glass tube containing Ag nanofluids, which absorbed the solar energy and boiled to generate vapor. The generated vapor was led out by the tube and condenses into liquid in the condenser, which was piped into the collection bottle. In the experiment, the concentration change of nanofluids was judged by the spectral absorptivity of nanofluids before and after the migration of nanoparticles, which was measured with the UV spectrophotometer, in order to explore the migration characteristics of nanoparticles during fluid evaporation.
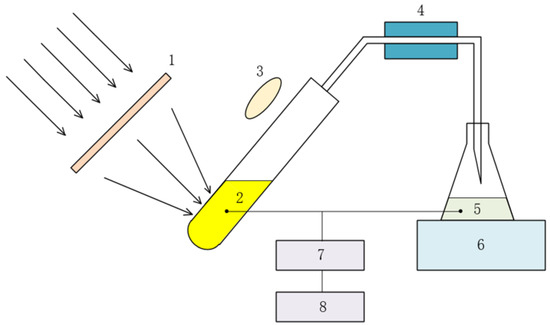
Figure 5.
Experimental setup for migration characteristics of nanoparticles. 1. Fresnel lens. 2. Nanofluids. 3. Solar radiation meter. 4. Condenser. 5. Condensed nanofluids. 6. Balance. 7. UV spectrophotometer. 8. Computer.
4. Results and Discussion
4.1. Solar–Thermal Conversion Characteristics
The temperature and mass change in 30 min of illumination were recorded in the experiment, as shown in Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10. The temperature of the nanofluids increased slowly with sunlight illumination. There was a temperature difference of about 3 °C between T1 and T3, as shown in Figure 8d. The temperature difference between T1 and T3 was about 3 °C, which was caused by the decrease of absorbed sunlight intensity with the increase of optical depth. A similar phenomenon called “thermal trapping” also occurred in our previous work [26]. In addition, the temperature increase of the four kinds of nanofluids with higher concentration was significantly faster than that of nanofluids with a lower concentration, e.g., the average temperature increase of 500 ppm Ag nanofluids after 30 min of irradiation was 16.7 °C, and about 7 °C higher than that of 50 ppm Ag nanofluids, as shown in Figure 6. In addition, a similar phenomenon is also found in Figure 7 and Figure 8 that the temperature rise of Au and MWCNT nanofluids also increased with the increase of concentration. In other words, the temperature of nanofluids changes faster with increasing concentration. Moreover, it was found in Figure 6, Figure 7, Figure 8 and Figure 9 that at the same concentration of 1000 ppm, the temperature rise of the four kinds of nanofluids from high to low was MWCNT, Au, Ag, and SiO2.
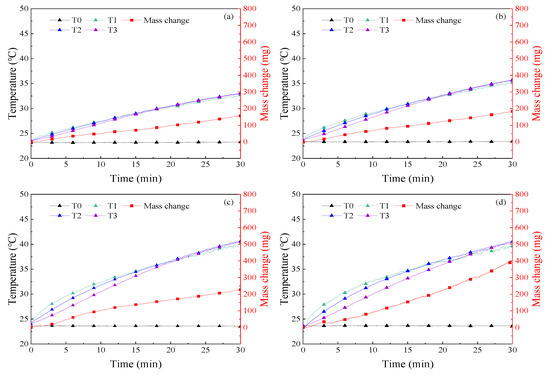
Figure 6.
Curves of temperature and mass change of Ag nanofluids in experiments on solar–thermal conversion characteristics (a) 50 ppm; (b) 200 ppm; (c) 500 ppm; (d) 1000 ppm.
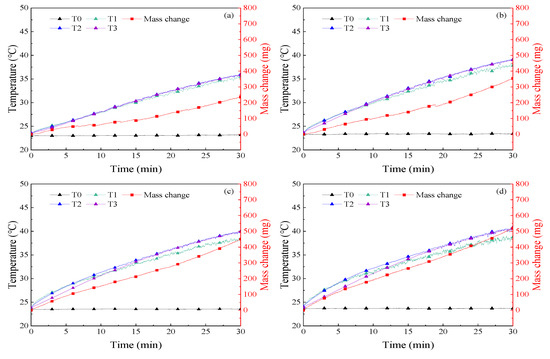
Figure 7.
Curves of temperature and mass change of Au nanofluids in experiments on solar–thermal conversion characteristics (a) 50 ppm; (b) 200 ppm; (c) 500 ppm; (d) 1000 ppm.
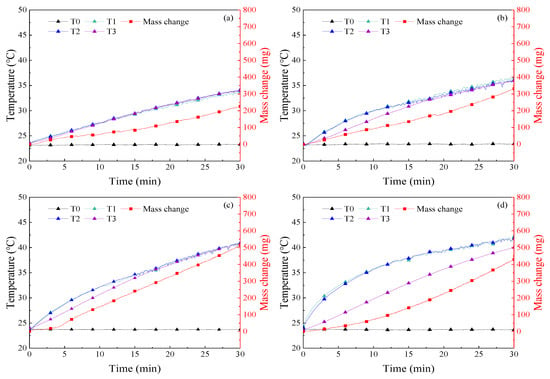
Figure 8.
Curves of temperature and mass change of MWCNT nanofluids in experiments on solar–thermal conversion characteristics (a) 50 ppm; (b) 200 ppm; (c) 500 ppm; (d) 1000 ppm.
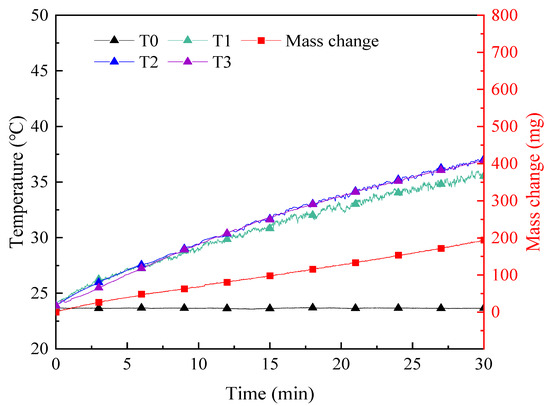
Figure 9.
Curves of temperature and mass change of SiO2 nanofluids of 1000 ppm in experiments on solar–thermal conversion characteristics.
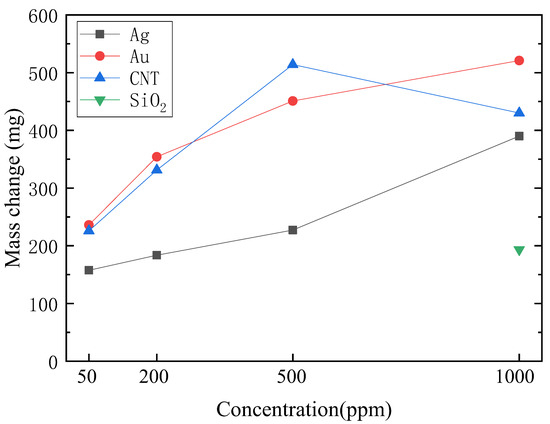
Figure 10.
Mass change of different nanofluids in 30 min of illumination.
Figure 10 shows the evaporation of the four kinds of nanofluids in 30 min under the sunlight of 1 sun. It was found that at the same concentration, the water evaporation of MWCNT and Au nanofluids was similar, which was higher than that of Ag and SiO2. This order was consistent with the depth of color of the four kinds of nanofluids. Moreover, it showed that the mass of nanofluids changed rapidly with increasing concentration by comparing the water evaporation velocity of Au, Ag, and MWCNT nanofluids with different concentrations. However, a special case contrary to the rule is shown in Figure 10 that the evaporation of 1000 ppm MWCNT nanofluids was lower than that of 500 ppm. The reason was that the temperature increase of the bottom and middle measuring points T1 and T2 of the MWCNT nanofluids were faster than that of the top measuring point T3 as shown in Figure 8d, i.e., the sunlight was not fully absorbed in the bottom of nanofluids, which was consistent with previous analyses. It can be concluded that due to the higher concentration and darker color of the 1000 ppm MWCNT nanofluids, more solar radiation was absorbed by the top of nanofluids rather than the bottom, leading to the reduction of mass change and evaporation rate.
The evaporation rates of different nanofluids were in the range of 0.2 to 0.4 mg/s, as shown in Figure 11. The results of the four kinds of nanofluids indicated that there was no significant difference except for the SiO2 nanofluids. Comparing the evaporation rate of different nanofluids, it was found that Au nanofluids had higher evaporation rates and better solar–thermal conversion characteristics.
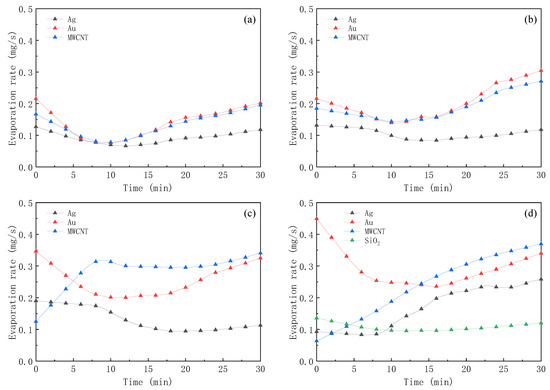
Figure 11.
Evaporation rate of Ag, Au, MWCNT, and SiO2 nanofluids in 30 min of illumination (a) 50 ppm; (b) 200 ppm; (c) 500 ppm; (d) 1000 ppm.
The solar–thermal conversion efficiency can be calculated by the energy conservation equation. Ignoring the heat loss, the energy conservation equation is:
where is sensible heat, is latent heat, and are the mass of base fluid and nanoparticle respectively, and are the specific heat capacity of the base fluid and nanoparticle respectively, is the average temperature increase of T1, T2, and T3 of nanofluids, is the heating time, is the solar–thermal conversion efficiency to be calculated, L is the latent heat of evaporation of water, I is the irradiation intensity of sunlight, and A is the irradiation area. Due to the low concentration of nanofluids, the influence of sensible heat increase of nanoparticles can be ignored.
The energy conservation has been calculated according to the equation below:
where is 50 g, is J/(kg·°C), I is 1000 W/m2, L is 2260 kJ/kg, is 1800 s, and A is m2. The solar–thermal conversion efficiency is shown in Figure 12, indicating that with the increase of nanofluid concentration, the solar–thermal conversion efficiency gradually increased, but the growth rate of solar–thermal conversion efficiency gradually slowed down.
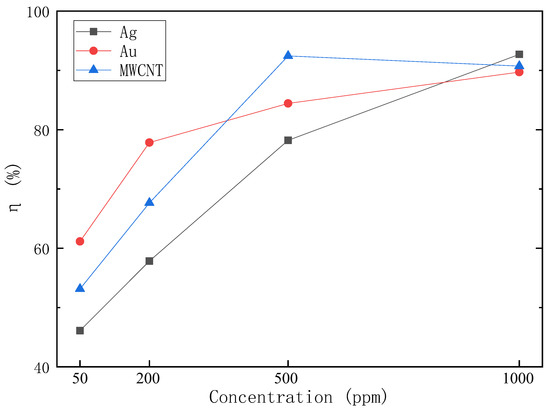
Figure 12.
Solar–thermal conversion efficiency of Ag, Au, MWCNT, and SiO2 nanofluids.
4.2. Uncertainty Analysis
In this paper, the uncertainty is analyzed according to the Type B evaluation of measurement uncertainty, which is important for understanding the quality of the final result.
According to the experimental setup in Section 3.1, the accuracy of the thermal couples, digital mass balance, solar simulator, and caliper are ±0.5 °C, ±0.0005 g, ±0.5%, and ±0.05 mm respectively. Therefore, the uncertainty of temperature UT = 0.5 °C, the uncertainty of temperature Um = 0.0005 g, the uncertainty of intensity UI is 5 W/m2, and the uncertainty of container radium Ur is 0.05 mm. According to Equations (3) and (4), the uncertainty of sensible heat, latent heat, and irradiation area are as follows:
The maximum uncertainty of sensible heat and latent heat are: , , .
According to Equation (2), the uncertainty of solar–thermal conversion efficiency can be calculated by the following equation:
The maximum uncertainty of solar–thermal conversion efficiency is .
Moreover, the concentration of nanofluids by the one-step method and two-step methods was decided by the mass of the solute and solution. The nanofluids with a concentration of 1000 ppm and a mass of 2 kg were prepared and a solution with a lower concentration and a mass of 1 kg is diluted with that. In this experiment, 3.452 g of HAuCl4 and 3.228 g of sodium citrate crystal were used to prepare the nanofluids by the one-step method, and 2 g of Ag, SiO2, and MWCNT particles were used to prepare the nanofluids by the two-step method, which were measured by the Ohaus EX224 digital mass balance with a precision of ±0.0005 g. Therefore, according to the Type B evaluation of measurement uncertainty, the uncertainty of nanofluids is calculated as follows in Table 1:

Table 1.
The uncertainty of nanofluids.
4.3. Migration Characteristics with Vapor
A total of 200 ppm silver nanofluids were investigated in the experiment. The boiling phenomenon of silver nanofluids under the focused sunlight of 220 suns and with the heating of resistance wire are respectively shown in Figure 13a,b. Bubbles can be observed to form on the surface of the glass tube, indicating that the nucleation site on the tube wall is the birthplace of bubbles. The bubbles grew larger and eventually rose to the surface and detach from the nanofluids.
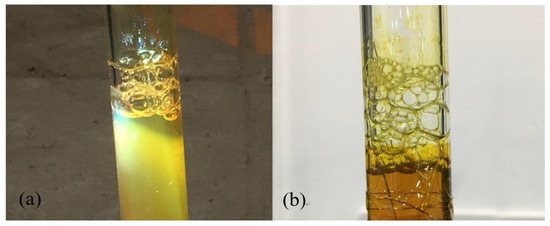
Figure 13.
Boiling of Ag nanofluids (a) under the focused sunlight of 220 suns, (b) with the heating of resistance wire.
The mass of the flushing water was 31.19 g, and the total mass of the mixed nanofluids after flushing was 34.40 g. It could be calculated that the mass of the vapor migrated by the nanofluids was 3.21 g, indicating that the dilution ratio was 10.72. The absorbance was measured with a UV spectrophotometer, as shown in Figure 14. Figure 14 shows that the absorption peak of mixed nanofluids after flushing was 410 nm and its absorbance was 0.287. Since the concentration of nanofluids is directly proportional to the absorbance, the absorbance of a specific concentration can be converted to calculate the concentration of nanofluids before dilution, that is, the concentration of nanofluids migrating with vapor. A total of 20 ppm silver nanofluids were chosen as a reference for calculation, and their absorbance was 3.225. The concentration of nanofluids after dilution could be calculated as 1.78 ppm, and the concentration of nanofluids before dilution was 19.08 ppm. It could be concluded that the Ag nanoparticles migrated with vapor, but the concentration was lower than that of the original liquid, indicating that only part of the Ag nanoparticles migrated with vapor, and most of the nanoparticles remained in the nanofluids.
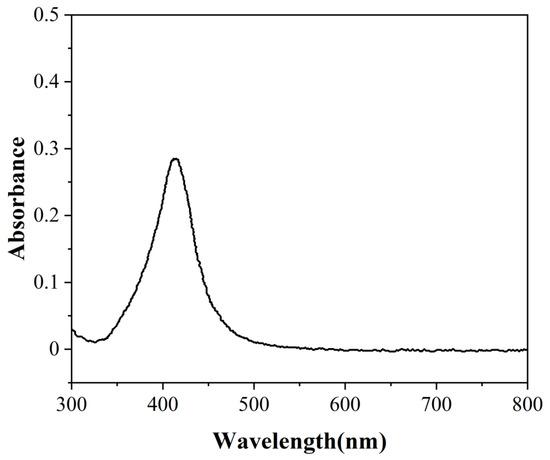
Figure 14.
The absorbance of mixed nanofluids after flushing under the focused sunlight.
In order to study the influence of different heating powers on the migration of nanoparticles with vapor, the nanofluids were heated with resistance wires. Figure 15 shows the absorbance of migrated nanofluids after flushing with powers of 16.2 W and 20 W. By the previous concentration calculation method, the concentration of nanofluids before dilution was 20.58 ppm with a power of 16.2 W, and the concentration of nanofluids before dilution was 31.39 ppm with a power of 20 W. It showed that more Ag nanoparticles migrated with vapor with increasing power. The reason was that the higher heating power reduction caused nanofluids to boil more violently in the tube, leading to more nanoparticles detaching from nanofluids with vapor generated by the boiling.
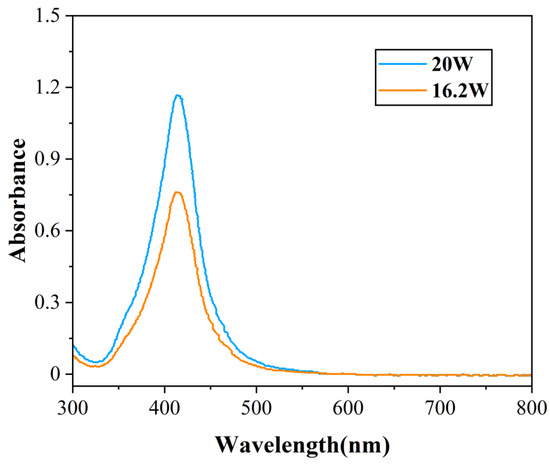
Figure 15.
The absorbance of mixed nanofluids after flushing with the heating of resistance wire.
5. Conclusions
In this paper, stable Au, Ag, MWCNT, and SiO2 nanofluids were prepared by the one-step method and two-step method, and the solar–thermal conversion and migration characteristics were investigated. The conclusions are as follows:
- (1)
- Under the sunlight of one sun by a sun simulator, the temperature of nanofluids changed faster with increasing concentration. Moreover, the water evaporation of the four kinds of nanofluids from high to low was MWCNT, Au, Ag, and SiO2 at the same concentration of 1000 ppm.
- (2)
- More sunlight is absorbed by the top of nanofluids with a high concentration and a dark color rather than the bottom of that, leading to the reduction of mass change and evaporation rate.
- (3)
- With generated vapor by boiling nanofluids, Ag nanoparticles would migrate out of the nanofluids. However, the concentration was lower than that of the original liquid, indicating that only part of the Ag nanoparticles migrated with vapor, and most of the nanoparticles remained in the nanofluids. In addition, more Ag nanoparticles migrated with vapor with the increased heating power.
Author Contributions
Conceptualization, G.L. and H.J.; methodology, H.J.; software, K.M.; validation, K.M.; formal analysis, K.M.; investigation, H.S. and Y.Z.; resources, Y.Z.; data curation, K.M.; writing—original draft preparation, H.S.; writing—review and editing, G.L. and H.J.; visualization, H.S.; supervision, H.J.; project administration, H.J.; funding acquisition, G.L. and H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 51906010 and No. 52106067), Beihang University Youth Talent Support Program (No. YWF-21-BJ-J-1111), and the Beijing Municipal Science and Technology Commission.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bozorgan, N.; Shafahi, M. Performance evaluation of nanofluids in solar energy: A review of the recent literature. Micro Nano Syst. Lett. 2015, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Mourad, A.; Aissa, A.; Said, Z.; Younis, O.; Iqbal, M.; Alazzam, A. Recent advances on the applications of phase change materials for solar collectors, practical limitations, and challenges: A critical review. J. Energy Storage 2022, 49, 104186. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Khalid, M.; Vaka, M.; Walvekar, R.; Numan, A.; Rasheed, A.K.; Mubarak, N.M. Recent progress in solar water heaters and solar collectors: A comprehensive review. Therm. Sci. Eng. Prog. 2021, 25, 100981. [Google Scholar] [CrossRef]
- Olfian, H.; Ajarostaghi, S.S.M.; Ebrahimnataj, M. Development on evacuated tube solar collectors: A review of the last decade results of using nanofluids. Sol. Energy 2020, 211, 265–282. [Google Scholar] [CrossRef]
- Jin, H.; Lin, G.; Bai, L.; Zeiny, A.; Wen, D. Steam generation in a nanoparticle-based solar receiver. Nano Energy 2016, 28, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Alshukri, M.J.; Hussein, A.K.; Eidan, A.A.; Alsabery, A.I. A review on applications and techniques of improving the performance of heat pipe-solar collector systems. Sol. Energy 2022, 236, 417–433. [Google Scholar] [CrossRef]
- Murugan, M.; Saravanan, A.; Elumalai, P.; Kumar, P.; Saleel, C.A.; Samuel, O.D.; Setiyo, M.; Enweremadu, C.C.; Afzal, A. An overview on energy and exergy analysis of solar thermal collectors with passive performance enhancers. Alex. Eng. J. 2022, 61, 8123–8147. [Google Scholar] [CrossRef]
- Mund, C.; Rathore, S.K.; Sahoo, R.K. A review of solar air collectors about various modifications for performance enhancement. Sol. Energy 2021, 228, 140–167. [Google Scholar] [CrossRef]
- Alawi, O.A.; Kamar, H.M.; Mallah, A.; Mohammed, H.A.; Kazi, S.; Sidik, N.A.C.; Najafi, G. Nanofluids for flat plate solar collectors: Fundamentals and applications. J. Clean. Prod. 2020, 291, 125725. [Google Scholar] [CrossRef]
- Verma, S.K.; Gupta, N.K.; Rakshit, D. A comprehensive analysis on advances in application of solar collectors considering design, process and working fluid parameters for solar to thermal conversion. Sol. Energy 2020, 208, 1114–1150. [Google Scholar] [CrossRef]
- Pal, R.K.; Kumar, R. Investigations of thermo-hydrodynamics, structural stability, and thermal energy storage for direct steam generation in parabolic trough solar collector: A comprehensive review. J. Clean. Prod. 2021, 311, 127550. [Google Scholar] [CrossRef]
- Ituna-Yudonago, J.-F.; Galindo-Luna, Y.-R.; García-Valladares, O.; Brown, R.B.Y.; Shankar, R.; Ibarra-Bahena, J. Review of solar-thermal collectors powered autoclave for the sterilization of medical equipment. Alex. Eng. J. 2021, 60, 5401–5417. [Google Scholar] [CrossRef]
- Sharma, A.; Chauhan, R. Integrated and separate collector storage type low-temperature solar water heating systems with latent heat storage: A review. Sustain. Energy Technol. Assess. 2022, 51, 101935. [Google Scholar] [CrossRef]
- Bouich, A.; Marí-Guaita, J.; Bouich, A.; Pradas, I.G.; Marí, B. Towards Manufacture Stable Lead Perovskite APbI3 (A = Cs, MA, FA) Based Solar Cells with Low-Cost Techniques. In Proceedings of the 1st International Conference on Energy, Power and Environment, Gujrat, Pakistan, 11–12 November 2021. [Google Scholar]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Marí-Guaita, J.; Bouich, A.; Marí, B. Shedding Light on Phase Stability and Surface Engineering of Formamidinium Lead Iodide (FaPbI3) Thin Films for Solar Cells. In Proceedings of the 1st International Conference on Energy, Power and Environment, Gujrat, Pakistan, 11–12 November 2021. [Google Scholar]
- Kumar, L.; Hasanuzzaman, M.; Rahim, N. Global advancement of solar thermal energy technologies for industrial process heat and its future prospects: A review. Energy Convers. Manag. 2019, 195, 885–908. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids With Nanoparticles. In Proceedings of the 1995 International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995; pp. 99–105. [Google Scholar]
- Kakaç, S.; Pramuanjaroenkij, A. Review of convective heat transfer enhancement with nanofluids. Int. J. Heat Mass Transf. 2009, 52, 3187–3196. [Google Scholar] [CrossRef]
- He, Y.; Jin, Y.; Chen, H.; Ding, Y.; Cang, D.; Lu, H. Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int. J. Heat Mass Transf. 2007, 50, 2272–2281. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Smith, D.S.; Singh, D.; Timofeeva, E.V.; Routbort, J.L. Heat transfer to a silicon carbide/water nanofluid. Int. J. Heat Mass Transf. 2009, 52, 3606–3612. [Google Scholar] [CrossRef]
- Younes, H.; Mao, M.; Murshed, S.S.; Lou, D.; Hong, H.; Peterson, G. Nanofluids: Key parameters to enhance thermal conductivity and its applications. Appl. Therm. Eng. 2022, 207, 118202. [Google Scholar] [CrossRef]
- Yousefi, T.; Shojaeizadeh, E.; Veysi, F.; Zinadini, S. An experimental investigation on the effect of pH variation of MWCNT–H2O nanofluid on the efficiency of a flat-plate solar collector. Sol. Energy 2011, 86, 771–779. [Google Scholar] [CrossRef]
- Lu, L.; Liu, Z.-H.; Xiao, H.-S. Thermal performance of an open thermosyphon using nanofluids for high-temperature evacuated tubular solar collectors: Part 1: Indoor experiment. Sol. Energy 2011, 85, 379–387. [Google Scholar] [CrossRef]
- Jin, H.; Lin, G.; Bai, L.; Amjad, M.; Filho, E.P.B.; Wen, D. Photothermal conversion efficiency of nanofluids: An experimental and numerical study. Sol. Energy 2016, 139, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Lin, G.; Zeiny, A.; Bai, L.; Wen, D. Nanoparticle-based solar vapor generation: An experimental and numerical study. Energy 2019, 178, 447–459. [Google Scholar] [CrossRef]
- Khullar, V.; Tyagi, H.; Hordy, N.; Otanicar, T.P.; Hewakuruppu, Y.; Modi, P.; Taylor, R.A. Harvesting solar thermal energy through nanofluid-based volumetric absorption systems. Int. J. Heat Mass Transf. 2014, 77, 377–384. [Google Scholar] [CrossRef]
- Huq, T.; Ong, H.C.; Chew, B.T.; Leong, K.Y.; Kazi, S.N. Review on aqueous graphene nanoplatelet Nanofluids: Preparation, Stability, thermophysical Properties, and applications in heat exchangers and solar thermal collectors. Appl. Therm. Eng. 2022, 210, 118342. [Google Scholar] [CrossRef]
- Xiong, Q.; Hajjar, A.; Alshuraiaan, B.; Izadi, M.; Altnji, S.; Shehzad, S.A. State-of-the-art review of nanofluids in solar collectors: A review based on the type of the dispersed nanoparticles. J. Clean. Prod. 2021, 310, 127528. [Google Scholar] [CrossRef]
- Kumar, S.; Chander, N.; Gupta, V.K.; Kukreja, R. Progress, challenges and future prospects of plasmonic nanofluid based direct absorption solar collectors—A state-of-the-art review. Sol. Energy 2021, 227, 365–425. [Google Scholar] [CrossRef]
- Taylor, R.A.; Phelan, P.E.; Adrian, R.J.; Gunawan, A.; Otanicar, T.P. Characterization of light-induced, volumetric steam generation in nanofluids. Int. J. Therm. Sci. 2012, 56, 1–11. [Google Scholar] [CrossRef]
- Otanicar, T.P.; Phelan, P.E.; Prasher, R.S.; Rosengarten, G.; Taylor, R.A. Nanofluid-based direct absorption solar collector. J. Renew. Sustain. Energy 2010, 2, 033102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, H.-J.; Du, X.; Wen, D. Photothermal conversion characteristics of gold nanoparticle dispersions. Sol. Energy 2014, 100, 141–147. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Zhu, J.; Kim, D.R. Enhancement of photo-thermal conversion using gold nanofluids with different particle sizes. Energy Convers. Manag. 2016, 112, 21–30. [Google Scholar] [CrossRef]
- Oara, N.; Alexander, S.U.; Jared, D.; Surbhi, L.; Peter, N.; Naomi, J.H. Solar Vapor Generation Enabled by Nanoparticles. ACS Nano 2013, 7, 42–49. [Google Scholar]
- Ahmed, T.; Noman, M.; Manzoor, N.; Ali, S.; Rizwan, M.; Ijaz, M.; Allemailem, K.S.; BinShaya, A.S.; Alhumaydhi, F.A.; Li, B. Recent advances in nanoparticles associated ecological harms and their biodegradation: Global environmental safety from nano-invaders. J. Environ. Chem. Eng. 2021, 9, 106093. [Google Scholar] [CrossRef]
- Westerhoff, P.; Atkinson, A.; Fortner, J.; Wong, M.S.; Zimmerman, J.; Gardea-Torresdey, J.; Ranville, J.; Herckes, P. Low risk posed by engineered and incidental nanoparticles in drinking water. Nat. Nanotechnol. 2018, 13, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, R.; Wang, R.; Ju, Q.; Wang, Y.; Xu, J. Comparative Physiological and Transcriptomic Analyses Reveal the Toxic Effects of ZnO Nanoparticles on Plant Growth. Environ. Sci. Technol. 2019, 53, 4235–4244. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Shabbir, S.; Kulyar, M.F.-E.; Bhutta, Z.A.; Boruah, P.; Asif, M. Toxicological Consequences of Titanium Dioxide Nanoparticles (TiO2NPs) and Their Jeopardy to Human Population. BioNanoScience 2021, 11, 621–632. [Google Scholar] [CrossRef]
- Liu, J.; Williams, P.C.; Goodson, B.M.; Geisler-Lee, J.; Fakharifar, M.; Gemeinhardt, M.E. TiO2 nanoparticles in irrigation water mitigate impacts of aged Ag nanoparticles on soil microorganisms, Arabidopsis thaliana plants, and Eisenia fetida earthworms. Environ. Res. 2019, 172, 202–215. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Xiong, M.; Gao, C.; Ren, H.; Ma, L. Process intensification in gas-liquid mass transfer by nanofluids: Mechanism and current status. J. Mol. Liq. 2021, 346, 118268. [Google Scholar] [CrossRef]
- Jin, H.; Lin, G.; Guo, Y.; Bai, L.; Wen, D. Nanoparticles enabled pump-free direct absorption solar collectors. Renew. Energy 2019, 145, 2337–2344. [Google Scholar] [CrossRef]
- Jin, H.; Lin, G.; Zeiny, A.; Bai, L.; Cai, J.; Wen, D. Experimental study of transparent oscillating heat pipes filled with solar absorptive nanofluids. Int. J. Heat Mass Transf. 2019, 139, 789–801. [Google Scholar] [CrossRef]
- Chen, H.-J.; Wen, D. Ultrasonic-aided fabrication of gold nanofluids. Nanoscale Res. Lett. 2011, 6, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, D.; Ding, Y. Experimental investigation into convective heat transfer of nanofluids at the entrance region under laminar flow conditions. Int. J. Heat Mass Transf. 2004, 47, 5181–5188. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).