Abstract
Microbial lipids produced from lignocellulosic biomass are sustainable alternative feedstock for biodiesel production. In this study, corn cobs were used as a carbon source for lipid production and growth of oleaginous yeast Trichosporon oleaginosus. Lignocellulosic biomass was subjected to alkali and acid pretreatment using sulfuric acid and sodium hydroxide under different temperatures, catalyst concentrations and treatment times. Pretreatment of corn cobs was followed by cellulase hydrolysis. Hydrolysis of alkali pretreated (2% NaOH at 50 °C for 6 h, 1% NaOH at 50 °C for 16 h, 2% NaOH at 121 °C for 1 h, 1% NaOH at 121 °C for 2 h) and acid pretreated (1% H2SO4 120 °C for 20 min, and 2% H2SO4 120 °C for 10 min) corn cobs resulted in more than 80% of the theoretical yield of glucose. The effect of substrate (5, 10, 15 and 20%, g g−1) and cellulase loading (15 and 30 Filter Paper Units per gram of glucan, FPU g−1) on fermentable sugar yield was also studied. The maximal glucose concentration of 81.64 g L−1 was obtained from alkali-pretreated corn cobs (2% NaOH at 50 °C for 6 h) at 20% substrate loading and 30 FPU of Cellic CTec2 g−1 of glucan. Enzymatic hydrolysates of pretreated biomasses and filtrates of lignocellulosic slurries obtained after pretreatment were used for growth and lipid synthesis by T. oleaginosus. The highest lipid concentration of 18.97 g L−1 was obtained on hydrolysate of alkali-pretreated corn cobs (with 1% NaOH at 50 °C for 16 h) using a 15% (g g−1) substrate loading and 15 FPU g−1 of cellulase loading. Significant lipid accumulation was also achieved using undetoxified filtrates of pretreated slurries as substrates. Results showed that pretreated corn cobs and undetoxified filtrates are suitable carbon sources for the growth and efficient accumulation of lipids in T. oleaginosus.
1. Introduction
The prices of plant oils, including palm and rapeseed oil, primary feedstocks for biodiesel production in the European Union, have steadily increased over the last ten years, reaching their highest level in January 2022 [1,2,3]. Biofuels policies implemented by several countries in the last two decades have encouraged first-generation biodiesel production and use. Growing demand for plant oils has increased the production of plant oil crops and aroused concerns regarding the sustainability of biofuel production and its environmental and social impact [4].
Lipids produced by oleaginous microorganisms have been considered an alternative feedstock to plant oils. Oleaginous microorganisms are a diverse group of organisms that include various strains of yeasts, molds, bacteria and microalgae. These microorganisms can accumulate more than 20% (g g−1) of lipids, while in some strains under specific growth conditions, lipid content can exceed 70% (g g−1) on a dry cell weight [5,6]. Lipid accumulation is triggered by cell growth limitation, most often by the shortage of nitrogen source, in the presence of a carbon source [5]. Except for nitrogen, a lack of other macro and micronutrients has been used to enhance lipid synthesis, e.g., sulfur, phosphate, iron, cobalt, zinc and vitamins [7,8,9]. Specifically, microalgae from the class Bacillariophyceae (diatoms) accumulate lipids during cultivation under silicon-limiting conditions [10]. High product yield requires optimization of two-phase cultivation that includes: (1) rapid exponential growth of microorganisms with high cell yield and (2) accumulation of lipids induced by nutrient deprivation (cells are in stationary phase). The microbial lipids, predominantly composed of triacylglycerols, accumulate in the form of lipid droplets intracellularly. Lipid profile varies depending on genus and species as well as growth conditions [11]. The most abundant fatty acids in oleaginous molds and yeasts are palmitic (C16:0), oleic (C18:1) and linoleic (C18:2), followed by stearic (C18:0) or palmitoleic acids (C16:1) at lower levels [12,13]. Some microalgal and mold strains also accumulate long-chain polyunsaturated fatty acids such as docosahexaenoic acid (C22:6), eicosapentaenoic acid (C20:5) and arachidonic acid (C20:4) [12,14].
Oleaginous yeasts are the most promising microorganisms for microbial lipids production due to their high growth rate, lipid productivity and lipid content. Moreover, a broad substrate range makes oleaginous yeasts suitable for industrial application. For the growth and lipid accumulation, oleaginous yeasts can use mono and disaccharides (glucose, fructose, arabinose, mannose, xylose, maltose, lactose, galactose, sucrose and cellobiose), polysaccharides (starch), sugar alcohols (glycerol and sorbitol) and organic acids (acetic acid, propionic and butyric) [5,11,15,16,17,18,19]. Oleaginous yeasts are robust microorganisms with an intrinsic tolerance level to acidic pH, different inhibitors and high ionic strength [20].
Microbial lipid production is still not feasible due to the high production costs, which are considerably higher than plant oils. According to several techno-economic studies, major production expenses are related to a substrate used for growth and lipid production, process equipment (bioreactors) and electricity costs for the operation of bioreactors (stirred tank reactors) [21]. The capacity of the plant also affects the production costs. Koutinas et al. predicted a decrease in production costs by half with a fivefold increase in the plant capacity of 20,000 MT per year [21]. The prices of microbial oils foreseen by several techno-economic studies significantly differed depending on the carbon source: 5.5 USD kg−1 of lipids produced on glucose by Rhodosporidium toruloides, 1.35 USD kg−1 of lipid produced by Cryptococcus curvatus on volatile fatty acids (obtained by anaerobic digestion of rice straw hydrolysate), 1.6 USD kg−1 of lipids produced by Cutaneotrichosporon oleaginosus on glucose and acetic acid mixture and 0.76 USD L−1 of lipids produced on sugarcane juice by Rhodosporidium toruloides [6,21,22,23]. Despite the high prices, the recent increase in plant oil price could boost the commercialization of microbial oil production. One way to reduce production costs is to replace pure chemicals usually used as carbon sources with renewable biomass, including wood processing and agricultural wastes, food wastes, industrial wastes and co-products, energy crops and sewage water. Several studies have shown that yeast lipids could be efficiently produced on inexpensive growth media, such as lignocellulosic and chitin hydrolysate, cheese whey, sugarcane juice, crude glycerol and potato processing wastewater [15,17,23,24,25]. Lignocellulosic biomass is an abundant and low-cost source of renewable carbohydrates. Recalcitrance nature of lignocellulosic biomass hampers hydrolysis of structural carbohydrates by enzymes and microorganisms. Therefore, lignocellulosic biomass is subjected to chemical, physical or biological pretreatment to improve the accessibility of structural carbohydrates and release fermentable sugars [12,24,26,27,28].
Corn cobs are one of the most abundant agricultural wastes in Southern Europe that can be used as a substrate for the large-scale production of microbial lipids. This paper aimed to optimize acid and alkali pretreatment of corn cobs regarding catalyst concentration, temperature and treatment time. The efficiency of the pretreatment process was assessed by hydrolysis of the biomass using commercial cellulase Celluclast 1.5 L and Cellic CTec2 (Novozymes). The effect of enzyme and substrate loading on glucose and xylose yield was also studied. Two lignocellulose-based substrates were used as a carbon source for growth and lipid synthesis by yeast Trichocopron oleaginosus: the filtrate of pretreatment slurry and enzymatic hydrolysate of pretreated biomass. In addition, fatty acid profiles of yeast lipids grown from corn cob hydrolysates were also analyzed. Previous studies showed that the genus Trichosporon efficiently accumulates lipids during the growth on hydrolysates of different lignocellulosic feedstocks, such as corn cobs, corn stover, sugarcane bagasse and rice straw [14,26,27,28].
2. Materials and Methods
2.1. Pretreatment of Corn Cobs
Corn cobs were harvested in the northern part of Croatia (latitude/longitude: 46°15′00″ N/16°36′36″ E) in autumn 2019. As described previously by Ivancic Santek et al., corn cobs were milled to a particle size smaller than 5 mm and stored dry in plastic containers in the dark at room temperature [24].
Corn cobs were subjected to alkali and acid pretreatment at different temperatures, catalyst concentrations and pretreatment times (Table 1). Acid pretreatment was performed using a 20 L High-Pressure reactor. The reactor was heated using oil circulating in external heating jackets without mixing a slurry. Five hundred grams of corn cobs were mixed with 10 L of diluted acid at a liquid to solid ratio (L:S) of 20:1 (mL g−1). Alkali pretreatment at 121 °C was performed in an autoclave. Corn cobs were soaked with an alkali solution at L:S of 8:1 (mL g−1). After the pretreatment, lignocellulosic slurries were vacuum filtered through the Buechner funnel. Spent liquors were stored at −20 °C and the filter cakes were washed with deionized water to neutral pH. Corn cobs were dried at 50 °C to constant weight. Water usage for washing the biomass and biomass recovery is presented in Table 1.

Table 1.
Pretreatment conditions, biomass recovery and water usage.
2.2. Enzymatic Digestibility of Corn Cobs
The digestibility of raw and pretreated corn cobs was evaluated at low substrate concentration to avoid the effect of product inhibition. The enzymatic reaction was performed at fixed substrate loading of 1% (gram of glucan per gram of lignocellulosic slurry, g g−1) and Celluclast 1.5 L (Novozymes) loading of 25 filter paper units per gram of glucan (FPU g−1) in 50 mmol L−1 citrate buffer (pH = 4.8). The hydrolysis was carried out in 100 mL Erlenmeyer flasks containing 50 g of the reaction mixture. Reactions were conducted in duplicates. The lignocellulosic slurry was mixed on a magnetic stirrer at 125 rpm and 50 °C. Significant loss of water by evaporation was observed during the hydrolysis. Evaporated water was compensated by the addition of sterile water daily. During reaction time, samples of lignocellulosic slurry were withdrawn from the flasks, and the enzyme was deactivated by incubating the sample in boiling water for 15 min. Samples were centrifuged to remove solid particles (20,100× g for 5 min). Supernatants were analyzed for glucose and xylose concentration.
2.3. Analysis of Sugar Concentration
The concentration of monosaccharides (glucose and xylose) was determined by HPLC (Shimadzu, Japan) using a Supelcogel C610H column (300 mm × 7.8 mm; Supelco Analytical, Bellefonte, PA, USA) and matching guard column (Supelcogel H Guard Column, 50 mm × 4.6 mm). Analytes were eluted isocratically with 0.1% phosphoric acid at a flow rate of 0.5 mL min−1 and 55 °C. Sugars were detected and quantified by a refractive index detector (Shimadzu RID-10) and UV/Vis detector (SPD-M10Avp Diode Array Detector, Shimadzu, Kyoto, Japan).
2.4. Effect of Substrate and Enzyme Loading on Sugar Yield
The efficiency of cellulase hydrolysis was studied at substrate loadings 5, 10, 15 and 20% (g g−1) in 50 mmol L−1 citrate buffer (pH = 4.8) using the enzyme Cellic CTec2 kindly provided by Novozymes (Denmark). The enzyme loadings were 5, 10, 15 and 30 FPU g−1 glucan and a hydrolysis time of 72 h. Raw and pretreated corn cobs with 2% NaOH at 50 °C for 6 h, 2% NaOH at 121 °C for 1 h and 1% H2SO4 at 121 °C for 20 min were used as substrate. Hydrolysis experiments were performed in 20 mL crimped glass vials (total reaction weight 10 g) at 50 °C. The glass vials were placed on a rotary shaker, rotating at 50 rpm and incubated at 50 °C. Hydrolyses were performed in duplicate.
2.5. Cultivation of T. oleaginosus
Trichosporon oleaginosus DSM 11815 was maintained on YPD agar slants (10 g L−1 yeast extract, 20 g L−1 glucose, 20 g L−1 peptone and 20 g L−1 agar) at 30 °C and subcultured monthly. The inoculum was cultivated in 100 mL of liquid YPD medium at 30 °C for 48 h on a rotary shaker at 180 min−1.
Batch cultivations of T. oleaginosus on lignocellulosic biomass were performed using corn cobs pretreated with acid and alkali and filtrates of lignocellulosic slurries obtained by pretreatment. The pretreatment filtrate pH was set at 5.5 using sodium hydroxide and sulfuric acid. Pretreated corn cobs loading was 15%, and Cellic CTec2 (Novozymes) loading 15 and 30 FPU g−1. Cultivations were performed in 500 mL Erlenmeyer flasks. Growth media also contained trace elements and a nutrient solution. The final concentration of macro and micronutrients were: (trace elements solution) 40 mg L−1 CaCl2·2H2O, 5.5 mg L−1 FeSO4·7H2O, 0.52 mg L−1 citric acid monohydrate, 1 mg L−1 ZnSO4·7H2O, 0.76 mg L−1 MnSO4·H2O and 200 µL 9 M H2SO4; and (nutrient solution), 1.0 g L−1 yeast extract 0.95 g L−1 Na2HPO4, 2.7 g L−1 H2PO4, 0.2 g L−1 MgSO4·7H2O and 0.1 g L−1 EDTA. The initial carbon to nitrogen ratio was set to 207.4 mol mol−1 to enhance lipid yield [5]. The concentration of ammonium chloride was calculated based on C:N and initial carbon content in lignocellulosic slurry. Cultivations on pretreated corn cobs were started with hydrolysis at 50 °C using gentle mixing on a rotary shaker (approximately 50 rpm). Approximately 25% of water evaporated during the hydrolysis step, and this water was not replaced afterwards. After 72 h, slurries were cooled down to room temperature, centrifuged at 3500× g for 10 min to remove the solids and inoculated with a culture of T. oleaginosus grown on YPD (10%, L L−1). The initial and final concentration of sugars was determined in the growth media containing enzymatic hydrolysate. Due to the high ionic strength of the samples that impair sugar separation on a chromatographic column, it was not possible to determine the concentration of sugars in the filtrates from pretreated lignocellulosic slurries by HPLC. Cultivations for each pretreated biomass/filtrate were performed in duplicates at 30 °C on an orbital shaker at 180 min−1. After five days of cultivation, solids were separated by centrifugation (at 8000× g for 30 min), freeze died and lipid content and fatty acid composition were determined.
2.6. Lignocellulose Biomass Analysis
The carbohydrate composition and lignin content of raw and pretreated feedstock was determined using two-step acid hydrolysis according to the National Renewable Energy Laboratory (NREL) standard protocol [29].
2.7. Lipid Analysis
The content of total lipids in cell biomass was determined by organic solvent extraction according to the Schneiter and Daum modified protocol [5,30]. Biomass was harvested by centrifugation, washed with deionized water and freeze-dried. Before solvent extraction, biomass was ground using mortar and pestle.
2.8. Fatty Acid Methyl Esters (FAMEs) Analysis
The composition of the lipid extracts was analyzed by gas chromatography (GC) using a CP-3800 device (Varian, Palo Alto, CA, USA) equipped with a flame ionization detector and split/splitless injector as described before [5]. Methyl esters were obtained by transesterification of cell lipids with potassium hydroxide.
3. Results
3.1. Composition and Enzyme Digestibility of Untreated and Pretreated Corn Cobs
To improve hydrolysis by cellulase, corn cobs were subjected to thermochemical pretreatment with sulfuric acid or sodium hydroxide at different catalysts concentrations, temperatures and treatment times (Table 1). The pretreatment conditions and catalyst were chosen based on previously published studies that improve the digestibility of the lignocellulosic biomass with moderate loss of the structural polysaccharides [31,32,33]. Pretreatment with acid breaks glycosidic linkages of hemicellulose, improving the accessibility of cellulose to cellulases. Alkali pretreatment enhances lignin breakdown by depolymerization and cleavage of lignin-carbohydrate linkages. Additionally, alkali solubilizes xylan by saponifying the intermolecular ester bonds (e.g., acetyl and uronic acid substitutions) [14,24,31,32,33]. Lower temperatures of pretreatments (50, 120 and 121 °C) and lower catalyst concentrations were chosen to minimize the formation of inhibitors, such as furfural and HMF, especially under acidic conditions [34,35]. In this study carbohydrate composition of lignocellulosic biomass was calculated from monosaccharides concentrations obtained by two-step acid hydrolysis according to the NREL [29]. The compositional analysis of raw and pretreated corn cobs is listed in Table 2. The raw and pretreated lignocellulosic biomass contained mainly glucan and xylan, while the content of remaining polysaccharides (arabinose, mannose and galactose) was below 3% (g g−1). The composition of raw corn cobs was in good agreement with published data [36]. As expected, acid and alkali pretreatments significantly affected the composition of lignocellulosic biomass. The removal of lignin by alkali pretreatment enriched two major polysaccharides in feedstock, i.e., glucan and xylan, under all pretreatment conditions (Figure 1). The higher temperature of pretreatment (121 °C) dissolved more lignin and enriched biomass with glucan and xylan, regardless of the sodium hydroxide concentration. On the other hand, acid pretreated corn cobs were significantly more abundant in glucan and lignin than the alkali pretreated corn cobs (Table 2 and Figure 1). Lignin and glucan content in acid-pretreated biomass was almost doubled compared to raw lignocellulosic biomass. According to published studies, an acidic environment favours hydrolysis of amorphous hemicellulose lowering xylan content in pretreated lignocellulosic biomass [24].

Table 2.
Composition of raw and pretreated corn cobs.
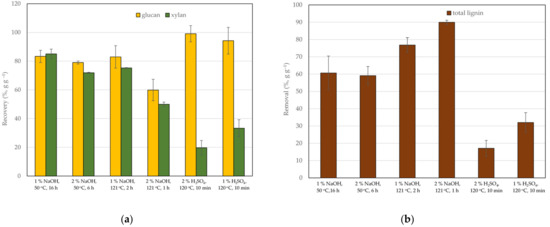
Figure 1.
Recovery of glucan and xylan (a) and lignin removal (b) from pretreated corn cobs relative to the raw corn cobs. The error bars present the standard deviation of values from two independent experiments.
Differences in composition and structural characteristics of biomass directly affect the yield of fermentative sugars by enzymatic hydrolysis. Optimal pretreatment conditions were chosen based on two criteria: high glucan and xylan recovery in the pretreated biomass and high glucose and xylose yield by enzymatic hydrolysis. Therefore, raw and pretreated corn cobs were tested for enzymatic digestibility in terms of glucose released by hydrolysis reaction. Efficient hydrolysis of cellulose to glucose depends on the synergistic action of three cellulolytic enzymes: endoglucanase or ß-1,4-endoglucan hydrolase (EC 3.2.1.4, breaks down glucan chains at random positions), exoglucanase or cellulose 1,4-ß-cellobiosidase (EC 3.2.1.91 and EC 3.2.1.176, which cleave off cellobiose from non-reducing and reducing cellulose chain ends, respectively) and ß- glucosidase or ß-D-glucoside glucohydrolase (EC 3.2.1.21, which converts cellobiose into glucose) [37,38,39,40,41]. Commercial cellulase preparation Celluclast 1.5 L derived from the fungus Trichoderma reesei has high cellobiohydrolase and endoglucanase activity but insufficiently low ß-glucosidase activity. In order to avoid end-product inhibition by cellobiose and, to a lesser extent, by glucose, the enzymatic reaction was conducted using high Celluclast 1.5 L loading (25 FPU g−1) and low substrate loading (1% glucan) (Figure 2). Glucan conversion during the enzymatic reaction was calculated based on 100% glucan hydrolysis (theoretical value). Glucan conversion curves displayed characteristic profiles with high initial rates during the first 6 h, followed by a gradual decrease in hydrolysis rate. Similar profiles were already observed in the hydrolysis of various lignocellulosic biomasses [14,24,42,43].
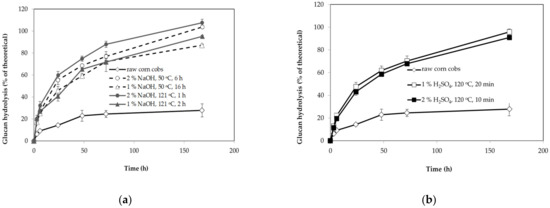
Figure 2.
The efficiency of enzymatic hydrolysis of glucan from raw and pretreated corn cobs with sodium hydroxide: ((a) 1% NaOH, 50 °C, 16 h; 2% NaOH, 50 °C, 6 h; 1% NaOH, 121 °C, 2 h and 2% NaOH, 121 °C, 1 h) and sulfuric acid; ((b) 2% H2SO4, 120 °C, 10 min and 1% H2SO4, 120 °C, 20 min). Glucan hydrolysis efficiency was calculated based on the total conversion of glucan from pretreated corn cobs. The error bars present the standard deviation of values from two independent experiments.
During the first phase, easily accessible and amorphous glucan was rapidly hydrolyzed, leaving recalcitrant and less accessible glucan, which was hydrolyzed in the second phase at a lower rate [44]. During the last 72–172 h, only 10–20% of the substrate was hydrolyzed regardless of the pretreatment conditions. Final glucan hydrolysis was more than 80% of the theoretical value for all pretreated lignocellulosic biomasses. Slightly higher glucan conversion rates were obtained with corn cobs pretreated with higher sodium hydroxide concentration (2%) and temperatures of 121 and 50 °C. Obtained results suggest that the concentration of sodium hydroxide concentration had a more significant effect on cellulose digestibility than the temperature of pretreatment. The conversion rate of xylan was also analyzed since most oleaginous microorganisms assimilate xylose as a carbon source [14,27,45,46,47].
Xylan hydrolysis curves (Figure 3) of pretreated corn cobs followed the characteristic trend already observed for glucan hydrolysis (Figure 2). The initial rapid rate of xylan hydrolysis during the first six hours was followed by its gradual decline. Pretreatment of corn cobs improved enzyme digestibility of xylan compared to untreated corn cobs. The highest xylose yields of more than 70% of theoretical value were obtained with biomass pretreated with 2% sodium hydroxide at 121 °C for 1 h and at 50 °C for 6 h. However, the xylan yield obtained for corn cobs pretreated with 1% (at 121 °C for 20 min) and 2% (at 121 °C for 10 min) sulfuric acid was significantly lower than with alkali pretreated corn cobs. Low xylose concentration is the result of the low xylanase activity of enzyme preparation.
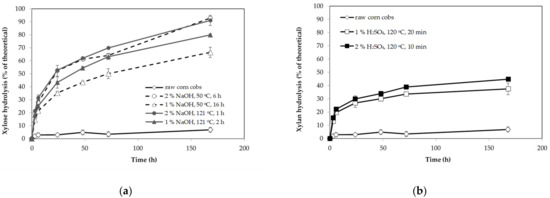
Figure 3.
Efficiency of enzymatic hydrolysis of xylan from raw and pretreated corn cobs with sodium hydroxide: ((a) 1% NaOH, 50 °C, 16 h; 2% NaOH, 50 °C, 6 h; 1% NaOH, 121 °C, 2 h and 2% NaOH, 121 °C, 1 h) and sulfuric acid; ((b) 2% H2SO4, 120 °C, 10 min and 1% H2SO4, 120 °C, 20 min) condition. Xylan hydrolysis efficiency was calculated based on the total conversion of xylan from pretreated corn cobs. The error bars present the standard deviation of values from two independent experiments.
A high product concentration is one of the essential prerequisites for achieving economically cost-effective production, especially in the brewing and bioethanol industry. Increased substrate loading efficiently improves bioethanol productivity without major modifications to existing facilities and reduces water and energy input in bioprocess, ensuing lower operational and capital costs [48]. The higher the product concentration entails greater substrate concentration in cultivation media. However, implementing this strategy introduces several challenges related to the mixing and mass transfer in lignocellulosic slurry, enzyme inhibition by end-products, the effect of osmotic stress on the microorganism viability and inhibition of enzyme and microorganism by side products of lignocellulosic pretreatment.
3.2. Effect of Enzyme and Substrate Loading
The effect of substrate loading (5%, 10%, 15% and 20%) and enzyme loadings (5, 10, 15 and 30 FPU g−1 glucan) were investigated by performing an enzymatic reaction with the commercial cellulase Cellic CTec2 for 72 h. Three pretreated biomasses were selected based on the total sugar yield (glucose + xylose) obtained by the digestibility test. Biomasses treated under the following conditions were chosen for further hydrolysis: 2% sodium hydroxide at 50 °C for 6 h, 2% sodium hydroxide at 121 °C for 1 h and 1% sulfuric acid at 120 °C for 20 min. The glucose and xylose concentrations were determined after 72 h and the efficiencies of glucan and xylan hydrolysis were also calculated (Supplementary Material). The monosaccharide levels of the three biomasses differed depending on the catalyst type and conditions applied in pretreatment. Obtained results were consistent with previous studies investigating acid and alkaline pretreatment of corn cobs [31,32,49]. As shown in Figure 4, the highest glucose and xylose concentrations were obtained by hydrolysis of lignocellulosic biomass pretreated with 2% alkali, suggesting that alkali-pretreated biomasses are in general more digestible than acid-pretreated biomass, regardless of pretreatment conditions. As expected, glucose and xylose levels consistently increased with substrate and enzyme loading for all three pretreated lignocellulosic biomasses. The increase in substrate and enzyme loading positively affected sugar concentration.
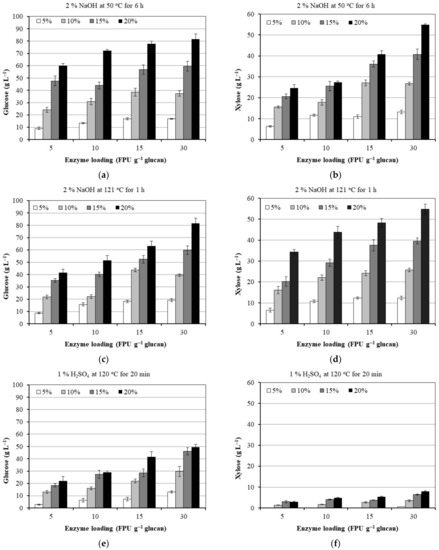
Figure 4.
Effect of a substrate (5, 10, 15 and 20%) and enzyme loading (5, 10, 15 and 30 FPU of Cellic CTec2 g−1 glucan of pretreated corn cobs) on glucose and xylose yield using pretreated corn cobs with 1% sodium hydroxide 50 °C for 6 h (a,b), 2% sodium hydroxide at 121 °C for 1 h (c,d) and 1% sulfuric acid at 120 °C for 20 min (e,f). The error bars present the standard deviation of values from two independent experiments.
Furthermore, the glucose and xylose yields were less affected by the enzyme loading than the substrate loading regardless of the pretreatment method used, which is in accordance with results obtained on enzymatic hydrolysis of different lignocellulosic feedstocks [50]. Conversions of glucan to glucose for alkaline pretreated corn cobs exceeded 70% theoretical values at higher cellulase loading (15 and 30 FPU g−1 glucan) in the studied range of substrate loadings (5–20%). However, an increase in enzyme loading from 15 to 30 FPU g−1 glucan only moderately increased or slightly decreased glucan conversion, suggesting irregular mixing and decreased mass transfer in lignocellulosic slurry, possible inhibition of cellulase by the end-product of enzymatic reaction or lignocellulose-derived inhibitors present in pretreated corn cobs, especially at higher substrate loadings (Figure 4, Supplementary Materials Table S1).
The maximal glucose concentration of 81.64 g L−1 was obtained at 20% substrate loading and 30 FPU g−1 glucan with corn cobs pretreated with 2% NaOH at 50 °C for 6 h. Similar glucose concentrations were obtained under the same reaction conditions in an enzymatic reaction with corn cobs pretreated with 2% NaOH at 121 °C for 121 h. No significant difference between xylose concentrations was found for both alkali-pretreated lignocellulosic biomasses. The maximal xylose concentration was 56.42 g L−1 for corn cobs pretreated with 2% NaOH at 121 °C for 121 h. Significantly lower xylose and glucose concentrations obtained with acid pretreated lignocellulosic biomass could be related to the higher lignin content in this feedstock (Table 1). Lignin interferes with cellulase and hemicellulase activity in several ways. For example, lignin forms a physical barrier that restricts the access of the enzymes to a substrate and irreversibly binds the hydrolytic enzymes decreasing the available enzymes for hydrolysis. Moreover, lignin-degradation products inhibit the cellulases [34,51,52].
3.3. Cultivation of T. oleaginosus on Pretreated Lignocellulosic Biomasses and Filtrates of Lignocellulosic Slurries
Due to the natural resistance of plant cell walls to cellulase and hemicellulase, lignocellulose biomass has to be pretreated under severe conditions. Disruption of the cell wall matrix releases structural carbohydrates for enzymatic hydrolysis. Severe pretreatment conditions, especially at higher acid concentration and temperature, produce a series of products from the degradation of sugars and lignin that could inhibit cell growth and cellulases [16,17]. Therefore, the main purpose of this study was to investigate whether enzymatic hydrolysates of pretreated lignocellulosic biomasses and filtrates of lignocellulosic slurries after pretreatment could support the growth of T. oleaginosus and synthesis of lipids. Enzymatic hydrolysis of pretreated lignocellulosic biomass was performed at two enzyme loadings (15 and 30 FPU g−1 glucan). Based on results from the previous experiment, the chosen substrate loading was 15% (g g−1). Higher substrate loading of 20% did not significantly enhance the sugar concentration, which could compensate for increased enzyme usage and production costs. As expected, glucose and xylose concentrations depended on pretreatment conditions of biomass and less on enzyme loading. The highest sugar concentrations were obtained with biomass pretreated with sodium hydroxide regardless of treatment conditions. Concentrations of glucose and xylose exceeded 60 and 40 g L−1, respectively. Comparable glucose concentrations were obtained by hydrolysis of lignocellulosic biomass pretreated with 1% sulfuric acid (for 20 min), while the xylose concentration was considerably lower. The lowest sugar yield was obtained with lignocellulosic biomass pretreated with 2% sulfuric acid. The glucose and xylose concentrations were lower than in the control culture that contained raw corn cobs. The low product yield was probably the inhibition of cellulase and hemicellulase by lignocellulose degradation products generated in the presence of acid catalysts at higher temperatures. These inhibitors include lignin derivatives (vanillin, syringaldehyde and 4-hydroxybenzaldehyde), sugar degradation products (furfural and 5-hydroxymethylfurfural and HMF) and organic acids (acetic and formic acid) [53,54]. Jing et al. showed that lignin degradation products had the highest inhibition effect on cellulase activity, followed by furan derivates and organic acids [53]. Furthermore, lignin, which was present at higher levels in this biomass, could reduce the available cellulase by adsorption and form a physical barrier on the cellulose surface that prevents contact of the cellulase to its substrate [55]. Prepared lignocellulosic hydrolysates of raw and pretreated lignocellulosic biomass were further used as a carbon source for growth and lipid synthesis. The batch cultivations were performed under nitrogen-limited conditions that support lipid accumulation [14,31]. The initial molar carbon to nitrogen ratio (C:N) of 207.4 mol mol−1 supported high cell yield and effective lipid synthesis upon the exhaustion of the nitrogen from the growth medium (stationary phase) [14,31]. Unexpectedly, the highest lipid concentrations were achieved on hydrolysates obtained from pretreated lignocellulosic biomass using lower cellulase loading of 15 FPU g−1 of glucan that contained less fermentable sugars. The highest lipid concentration of 18.97 g L−1 was obtained on hydrolysate (15 FPU g−1) of lignocellulosic biomass pretreated with 1% (g g−1) NaOH at 50 °C for 16 h. According to the study by Ivancic et al., cells of T. oleaginosus are weakly inhibited by glucose (glucose concentration in the range from 35 to 100 g L−1) and xylose (xylose concentration of 35 and 75 g L−1) at the present concentration [5]. These results suggest that enzymes at a higher dosage (30 FPU g−1) probably impair cell growth and viability due to the preservatives or stabilizers present in commercial cellulase cocktails [56]. Tomás-Pejó et al. observed a significant decrease in the growth rate of yeast Kluyveromyces marxianus CECT 10875 in a growth medium with a high dosage of commercial cellulase Celluclast 1.5 L during alcohol fermentation. Nevertheless, no effect on the ethanol production rate was detected [57]. Faria et al. observed a significant decrease in glycolipid production rate in yeasts Pseudozyma antarctica PYCC 5048T and Pseudozyma aphidis PYCC 5535T grown in the presence of Celluclast 1.5 L and Novozyme 188 at medium (1.75 and 0.25% vol vol−1, respectively) and high (5.25 and 0.75% vol vol−1, respectively) levels. However, medium enzyme dosage did not affect cell growth [56]. Similarly, a decrease in lipid synthesis was observed during fed-batch cultivation of T. oleaginosus on lignocellulosic hydrolysate at high enzyme loading [14]. Based on obtained lipid yield and lipid concentration (Table 3), pretreatment with 1% NaOH at 50 °C for 16 h was chosen as the most suitable one for the production of lipids with T. oleaginosus. Compared to other alkali pretreatments, this is the most feasible due to the lowest energy requirement (temperature of pretreatment of 50 °C) and catalyst quantity (1% NaOH). The lipid yield of 0.126 g g−1 was significantly higher compared to that obtained by Ivancic Santek et al. with the same yeast strain grown on the hydrolysate of corn cobs pretreated with 3% NaOH at 121 °C for 30 min and cultivated under similar conditions [14]. A similar concentration of lipids (18.12 g L−1) was obtained by yeast Rhodotorula babjevae DVBPG 805. Wheat straw, used as a substrate for growth and lipid production, was presoaked with 1% acetic acid overnight, treated by steam explosion and hydrolyzed by Cellic CTec3 (10 FPU g−1 of lignocellulosic biomass) [58].

Table 3.
Cultivation of T. oleaginosus on enzymatic hydrolysate of raw and pretreated corn cobs. Lignocellulosic biomass was hydrolyzed using 15 and 30 FPU of Cellic CTec2 per gram of glucan. The concentration of glucose, xylose and lipids, lipid content in solid residue and fatty acid composition of extracted lipids were determined at the end of cultivation.
Yeast T. oleaginosus was also cultivated on filtrate of lignocellulosic slurry obtained through different types of pretreatments without previous detoxification (Table 4). The concentration of sugars was not determined due to the analytical problem with the separation of sugars on the chromatographic column from the samples with high ionic strength. The lipid yield is affected by the concentration of sugars and the concentration of degradation products. Based on the composition and recovery of glucan and xylan of lignocellulosic biomass (Table 1), higher sugar concentrations were expected in the filtrates of lignocellulosic slurries obtained by pretreatment with an acid catalyst. Without removing the degradation by-products, the highest lipid yields of 2.48 and 2.38 g L−1 were obtained on lignocellulosic filtrates from pretreatment using 2% (for 10 min) and 1% (for 20 min) sulfuric acid, respectively. The concentration of lipids on filtrates from alkali pretreated biomass was much lower, probably due to lower sugar yield and higher concentration of lignocellulose degradation products. Under alkaline conditions, structural carbohydrates were hydrolyzed to a lesser extent (Table 2), especially at lower pretreatment temperatures.

Table 4.
Cultivation of T. oleaginosus on filtrate of lignocellulose slurry obtained after pretreatment. The concentration of lipids, lipid content in solid residue and fatty acid composition of extracted lipids were determined at the end of cultivation.
Yeast lipids were extracted from cell biomass and analyzed for fatty acid composition (Table 3 and Table 4). The fatty acids produced by T. oleaginosus grown on lignocellulosic filtrates and hydrolysates contained predominantly oleic acid (C18:1), followed by palmitic (C16:0), stearic (C18:0) and linoleic (C18:2) acids. Obtained results were in accordance with published data for several oleaginous yeast strains [23,59,60]. The fatty acid profile of T. oleaginosus grown on different lignocellulosic hydrolysates varied probably due to inhibitors in lignocellulosic hydrolysates and filtrates. The most significant effect on major fatty acids content was observed in lipids produced on enzymatic hydrolysate of corn cobs pretreated with 2% sulfuric acid at 120 °C for 10 min. Under these pretreatment conditions, furfural and 5-hydroxymethylfurfural (5-HMF) are the major inhibitors generated by the degradation of sugars pentoses and hexoses, respectively [54]. These inhibitors significantly affect cell growth as well as fatty acid composition. Zhao et al. observed an increase in unsaturated (oleic acid, C18:1 and linoleic, C18:2) and a decrease in saturated fatty acids (palmitic C16:0 and stearic, C18:0) in the biomass of yeast Rhodosporidium toruloides grown in the presence of furfural [46]. On the contrary, growth in the presence of different aldehydes (furfural, HMF, 4-hydroxybenzaldehyde, syringaldehyde and vanillin) did not significantly change the composition of fatty acids in Trichosporon fermentans. Similar observations were reported by Yu et al., who cultivated Cryptococcus curvatus in a growth medium with mixtures of main lignocellulosic inhibitors (vanillin, furfural, p-hydroxybenzaldehyde and syringaldehyde) [61]. This considerable disagreement between the published studies can be attributed to the difference in important factors such as cultivation conditions, the resistance of yeast strains toward specific inhibitors, inhibitor concentrations, and the synergistic effect of several inhibitors in the mixture [46,61,62].
A similar fatty acid profile to conventional plant oils makes T. oleaginosus lipids an alternative feedstock for sustainable biodiesel production. Soybean oil contains mostly C18:1 (23.7%) and C18:2 (53.8%), while rapeseeds consist mainly of the same fatty acids at 59.5% and 21.5%, respectively [63]. Compared to rapeseed oil, which is commonly used as feedstock for biodiesel production in European Union, the FAME profiles of T. oleaginosus lipids were more saturated regardless of the substrate used for growth and lipid production (Table 3 and Table 4). The oleic acid (18:1) content was lower than that found in rapeseed oil and several other oleaginous yeasts [23,60,63]. Ivancic Santek et al. analyzed the chemical and physical characteristics of biodiesel by the chemical composition of fatty acids produced by T. oleaginosus grown on glucose under nitrogen-limited conditions. According to the analysis, yeast biodiesel meets the requirements of EN14214 standard specifications for B100 (100% FAME). Since the fatty acid profile was similar to those obtained in this study, yeast lipids produced from different pretreated biomasses and filtrates of lignocellulosic slurries could be used as feedstock for sustainable production of microbial biodiesel, except for biomass pretreated with 2% H2SO4 (at 121 °C for 20 min and hydrolyzed with 30 FPU g−1).
4. Conclusions
The yeast T. oleaginosus is a promising candidate for the sustainable production of lipids on corn cob hydrolysate. Acid and alkali pretreatments under different conditions efficiently improved the digestibility of the lignocellulosic biomass. High lipid yields were obtained on hydrolysates from alkali pretreated corn cobs. Due to intrinsic tolerance to lignocellulose-derived inhibitors, this yeast grew and produced the lipids on undetoxified filtrates from acid and alkali pretreatment of corn cobs. Fatty acid composition similar to rapeseed oil makes T. oleaginosus lipids a favourable feedstock for green production of second-generation biodiesel.
Obtained lipid concentrations are not sufficiently high for cost-effective production. Therefore, further research should be focused on improving lipid concentration by applying a fed-batch mode of cultivation using the whole slurry obtained by pretreatment of the lignocellulosic biomass. Increased concentration of lignocellulose-derived inhibitors in the slurry may interfere with lignocellulose hydrolysis and microorganism growth, requiring a detoxification step to be included in the process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en15093208/s1, Table S1. Effect of enzyme and substrate loading on glucan and xylan conversion of corn cobs pretreated with sodium hydroxide and sulfuric acid at different pretreatment conditions.
Author Contributions
Conceptualization, M.I.Š.; methodology, K.M.; validation, M.G.; formal analysis, M.G., I.P. and S.B.; investigation, M.G. and M.G.P.; writing—original draft preparation, M.G. and K.M.; writing—review and editing, M.I.Š.; supervision, M.I.Š.; project administration, B.Š.; funding acquisition, B.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research received was funded by the Croatian Science Foundation under the project “Sustainable production of biochemicals from waste lignocellulose containing feedstocks” (Croatian Science Foundation no. 9717).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO Vegetable Oil Price Index Hits 13-Year High. Available online: https://www.ofimagazine.com/news/fao-vegetable-oil-price-index-hits-13-year-high (accessed on 25 March 2022).
- Rapeseed Oil-Monthly Price-Commodity Prices-Price Charts, Data, and News-IndexMundi. Available online: https://www.indexmundi.com/commodities/?commodity=rapeseed-oil&months=120 (accessed on 29 March 2022).
- Palm oil-Monthly Price-Commodity Prices-Price Charts, Data, and News-IndexMundi. Available online: https://www.indexmundi.com/commodities/?commodity=palm-oil&months=240 (accessed on 29 March 2022).
- Ravindranath, N.H.; Manuvie, R.; Fargione, J.; Canadell, J.G.; Berndes, G. Greenhouse Gas Implications of Land Use and Land Conversion to Biofuel Crops; Cornell University: Ithaca, NY, USA, 2009; pp. 111–125. [Google Scholar]
- Ivančić Šantek, M.; Miškulin, E.; Petrović, M.; Beluhan, S.; Šantek, B. Effect of carbon and nitrogen source concentrations on the growth and lipid accumulation of yeast Trichosporon oleaginosus in continuous and batch culture. J. Chem. Technol. Biotechnol. 2017, 92, 1620–1629. [Google Scholar] [CrossRef]
- Masri, M.A.; Garbe, D.; Mehlmer, N.; Brück, T.B. A sustainable, high-performance process for the economic production of waste-free microbial oils that can replace plant-based equivalents. Energy Environ. Sci. 2019, 12, 2717–2732. [Google Scholar] [CrossRef]
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.Y.S.; Salley, S.O. Bioresource Technology Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Zhu, Z.; Shen, H.; Lin, X.; Jin, X.; Jiao, X.; Zhao, Z.K. Biotechnology for Biofuels Systems analysis of phosphate-limitation-induced lipid accumulation by the oleaginous yeast Rhodosporidium toruloides. Biotechnol. Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafari, M.; Rashidi, B.; Haznedaroglu, B.Z. Effects of macro and micronutrients on neutral lipid accumulation in oleaginous microalgae. Biofuels 2018, 9, 147–156. [Google Scholar] [CrossRef]
- Sabu, S.; Sarojini, I.; Singh, B.; Joseph, V. Improved lipid production in oleaginous brackish diatom Navicula phyllepta MACC8 using two-stage cultivation approach. 3 Biotech 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Bellou, S.; Moustogianni, A.; Makri, A.; Aggelis, G. Lipids Containing Polyunsaturated Fatty Acids Synthesized by Zygomycetes Grown on Glycerol. Appl. Biochem. Biotechnol. 2012, 166, 146–158. [Google Scholar] [CrossRef]
- Valdés, G.; Mendonça, R.T.; Aggelis, G. Lignocellulosic biomass as a substrate for oleaginous microorganisms: A review. Appl. Sci. 2020, 10, 7698. [Google Scholar] [CrossRef]
- Vasconcelos, B.; Teixeira, J.C.; Dragon, G.; Teixeira, J.A. Oleaginous yeasts for sustainable lipid production—from biodiesel to surf boards, a wide range of “green” applications. Appl. Microbiol. Biotechnol. 2019, 103, 3651–3667. [Google Scholar] [CrossRef] [Green Version]
- Grubišić, M.; Mihajlovski, K.; Gruičić, A.M.; Beluhan, S.; Šantek, B.; Ivančić Šantek, M. Strategies for Improvement of Lipid Production by Yeast Trichosporon oleaginosus from Lignocellulosic Biomass. J. Fungi 2021, 7, 934. [Google Scholar] [CrossRef]
- Bao, R.; Wu, X.; Liu, S. Efficient Conversion of Fructose-Based Biomass into Lipids with Trichosporon fermentans Under Phosphate-Limited Conditions. Appl. Biochem. Biotechnol. 2018, 184, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Awad, D.; Bohnen, F.; Mehlmer, N.; Brueck, T. Multi-Factorial-Guided Media Optimization for Enhanced Biomass and Lipid Formation by the Oleaginous Yeast Cutaneotrichosporon oleaginosus. Front. Bioeng. Biotechnol. 2019, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwig, S.; Conte, C.; De Andrade, P.; Ghiselli, G.; Maugeri, F. Bioresource Technology Exploration of Brazilian biodiversity and selection of a new oleaginous yeast strain cultivated in raw glycerol. Bioresour. Technol. 2013, 138, 377–381. [Google Scholar]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioresource Technology Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Muniraj, I.; Zappi, M.; El-Ghonemy, D.H. Microbial lipid production from potato processing wastewater using oleaginous filamentous fungi Aspergillus oryzae. Water Res. 2013, 47, 3477–3483. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Yaguchi, A.; Blenner, M. Oleaginous yeast for biofuel and oleochemical production. Curr. Opin. Biotechnol. 2019, 57, 73–81. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Park, G.W.; Chang, H.N.; Jung, K.; Seo, C.; Kim, Y.; Choi, J.H.; Woo, H.C.; Hwang, I. Production of microbial lipid by Cryptococcus curvatus on rice straw hydrolysates. Process Biochem. 2017, 56, 147–153. [Google Scholar] [CrossRef]
- Ricardo, C.; José, C.; Neto, D.; Thomaz, V.; Bittencourt, E.; Scopel, E.; Bianchi, A.; Medeiros, P.; Porto, L.; Vandenberghe, D.S. Pilot scale biodiesel production from microbial oil of Rhodosporidium toruloides DEBB 5533 using sugarcane juice: Performance in diesel engine and preliminary economic study. Bioresour. Technol. 2017, 223, 259–268. [Google Scholar]
- Ivančić Šantek, M.; Grubišić, M.; Galić Perečinec, M.; Beluhan, S.; Šantek, B. Lipid production by Mortierella isabellina from pretreated corn cobs and effect of lignocellulose derived inhibitors on growth and lipid synthesis. Process Biochem. 2021, 109, 46–58. [Google Scholar] [CrossRef]
- Tang, M.; Wang, Y.; Zhou, W.; Yang, M.; Liu, Y.; Gong, Z. Efficient conversion of chitin-derived carbon sources into microbial lipid by the oleaginous yeast Cutaneotrichosporon oleaginosum. Bioresour. Technol. 2020, 315, 123897. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zong, M.; Wu, H.; Liu, Q. Bioresource Technology Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresour. Technol. 2009, 100, 4535–4538. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Yu, Z.; Bao, J. Simultaneous saccharification and microbial lipid fermentation of corn stover by oleaginous yeast Trichosporon cutaneum. Bioresour. Technol. 2012, 118, 13–18. [Google Scholar] [CrossRef]
- Brar, K.K.; Sarma, A.K.; Aslam, M.; Polikarpov, I.; Chadha, B.S. Technology Potential of oleaginous yeast Trichosporon sp., for conversion of sugarcane bagasse hydrolysate into biodiesel. Bioresour. Technol. 2017, 242, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in Biomass—NREL/TP-510-42618. Lab. Anal. Proced. 2012, 1617, 1–16. [Google Scholar]
- Schneiter, R.; Daum, G. Extraction of yeast lipids. Methods Mol. Biol. 2006, 313, 41–45. [Google Scholar] [PubMed]
- Šantek, M.I.; Lisičar, J.; Mušak, L.; Špoljarić, I.V.; Beluhan, S.; Šantek, B. Lipid Production by Yeast Trichosporon oleaginosus on the Enzymatic Hydrolysate of Alkaline Pretreated Corn Cobs for Biodiesel Production. Energy Fuels 2018, 32, 12501–12513. [Google Scholar] [CrossRef]
- Gao, K.; Rehmann, L. ABE fermentation from enzymatic hydrolysate of NaOH-pretreated corncobs. Biomass Bioenergy 2014, 66, 110–115. [Google Scholar] [CrossRef]
- Baadhe, R.R.; Potumarthi, R.; Mekala, N.K. Influence of dilute acid and alkali pretreatment on reducing sugar production from corncobs by crude enzymatic method: A comparative study. Bioresour. Technol. 2014, 162, 213–217. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eylen, D.; Van Dongen, F.; Kabel, M.; De Bont, J. Corn fiber, cobs and stover: Enzyme-aided saccharification and co-fermentation after dilute acid pretreatment. Bioresour. Technol. 2011, 102, 5995–6004. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Chuan, J. Microbial cellulases: Engineering, production and applications. Renew. Sustain. Energy Rev. 2014, 33, 188–203. [Google Scholar] [CrossRef]
- Expasy. EC 3.2.1.21 ß—Glucosidase. Available online: https://enzyme.expasy.org/EC/3.2.1.21 (accessed on 21 March 2022).
- Expasy. EC 3.2.1.4 Endoglucanase or ß-1,4-Endoglucan Hydrolase. Available online: https://enzyme.expasy.org/EC/3.2.1.4 (accessed on 21 March 2022).
- Expasy. EC 3.2.1.91 Exoglucanase. Available online: https://enzyme.expasy.org/EC/3.2.1.91 (accessed on 21 March 2022).
- Expasy. EC 3.2.1.176 Exoglucanase. Available online: https://enzyme.expasy.org/EC/3.2.1.176 (accessed on 21 March 2022).
- Kaar, W.E.; Holtzapple, M.T. Using lime pretreatment to facilitate the enzymic hydrolysis of corn stover. Biomass Bioenergy. 2000, 18, 189–199. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Zhu, Z.; Zhang, Y.P. Bioresource Technology Cellulose solvent- and organic solvent-based lignocellulose fractionation enabled efficient sugar release from a variety of lignocellulosic feedstocks. Bioresour. Technol. 2012, 117, 228–233. [Google Scholar] [CrossRef]
- Hu, F.; Ragauskas, A. Pretreatment and Lignocellulosic Chemistry. Bioenergy Resour. 2012, 5, 1043–1066. [Google Scholar] [CrossRef]
- Dai, X.; Shen, H.; Li, Q.; Rasool, K.; Wang, Q.; Yu, X.; Wang, L.; Bao, J.; Yu, D.; Zhao, Z.K. Microbial lipid production from corn stover by the oleaginous yeast Rhodosporidium toruloides using the presslp process. Energies 2019, 12, 1053. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Peng, F.; Du, W.; Liu, C.; Liu, D. Effects of some inhibitors on the growth and lipid accumulation of oleaginous yeast Rhodosporidium toruloides and preparation of biodiesel by enzymatic transesterification of the lipid. Bioprocess Biosyst. Eng. 2012, 35, 993–1004. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Mehtani, J.; Pruthi, V.; Pruthi, P.A. Assessment of fuel properties on the basis of fatty acid pro fi les of oleaginous yeast for potential biodiesel production. Renew. Sustain. Energy Rev. 2017, 77, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic ethanol production at high-gravity: Challenges and perspectives. Trends Biotechnol. 2014, 32, 46–53. [Google Scholar] [CrossRef]
- Sahare, P.; Singh, R.; Laxman, R.S.; Rao, M. Effect of Alkali Pretreatment on the Structural Properties and Enzymatic Hydrolysis of Corn Cob. Appl. Biochem. Biotechnol. 2012, 168, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Saddler, J.N. Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnol. Biofuels 2011, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhgren, K.; Bura, R.; Saddler, J.; Zacchi, G. Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour. Technol. 2007, 98, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, A.; Carlos, A.; Ximenes, E.; Carreira, C.; Boscolo, M.; Gomes, E.; Ladisch, R. Temperature Dependent Cellulase Adsorption on Lignin from Sugarcane Bagasse. Bioresour. Technol. 2017, 252, 143–149. [Google Scholar] [CrossRef]
- Jing, X.; Zhang, X.; Bao, J. Inhibition Performance of Lignocellulose Degradation Products on Industrial Cellulase Enzymes During Cellulose Hydrolysis. Appl. Biochem. Biotechnol. 2009, 159, 696–707. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Renewable Energy Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Renewable_energy_statistics&oldid=515129#of_renewable_energy_used_in_transport_activities_in_2019 (accessed on 24 September 2021).
- Faria, N.T.; Santos, M.; Ferreira, C.; Marques, S.; Ferreira, F.C. Conversion of cellulosic materials into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma spp. under SHF and SSF processes. Microb. Cell Fact. 2014, 13, 155. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Pejó, E.; Oliva, J.M.; González, A.; Ballesteros, I.; Ballesteros, M. Bioethanol production from wheat straw by the thermotolerant yeast Kluyveromyces marxianus CECT 10875 in a simultaneous saccharification and fermentation fed-batch process. Fuel 2009, 88, 2142–2147. [Google Scholar] [CrossRef]
- Brandenburg, J.; Blomqvist, J.; Shapaval, V.; Kohler, A.; Sampels, S. Biotechnology for Biofuels Oleaginous yeasts respond differently to carbon sources present in lignocellulose hydrolysate. Biotechnol. Biofuels 2021, 14, 124. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Yang, X.; Wang, Q.; Xie, H.; Zhao, Z.K. Lipid production from corn stover by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2014, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- Rane, D.V.; Pawar, P.P.; Odaneth, A.A.; Lali, A.M. Microbial oil production by the oleaginous red yeast, Rhodotorula glutinis NCIM 3168, using corncob hydrolysate. Biomass Convers. Biorefinery 2021, 1–11. [Google Scholar] [CrossRef]
- Yu, X.; Zeng, J.; Zheng, Y.; Chen, S. Effect of lignocellulose degradation products on microbial biomass and lipid production by the oleaginous yeast Cryptococcus curvatus. Process Biochem. 2014, 49, 457–465. [Google Scholar] [CrossRef]
- Poontawee, R.; Yongmanitchai, W.; Limtong, S. Efficient oleaginous yeasts for lipid production from lignocellulosic sugars and effects of lignocellulose degradation compounds on growth and lipid production. Process Biochem. 2017, 53, 44–60. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).