Measurement of Cyclic Variation of the Air-to-Fuel Ratio of Exhaust Gas in an SI Engine by Laser-Induced Breakdown Spectroscopy
Abstract
:1. Introduction
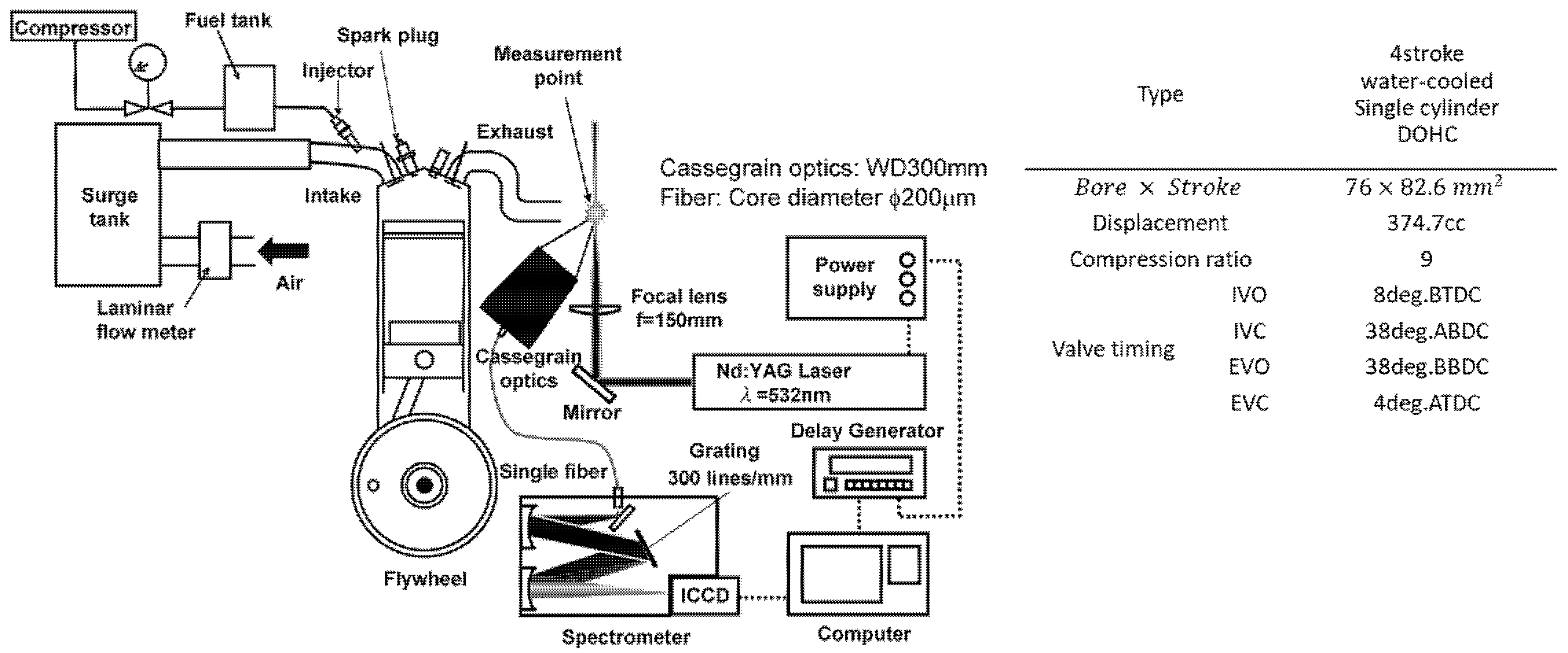
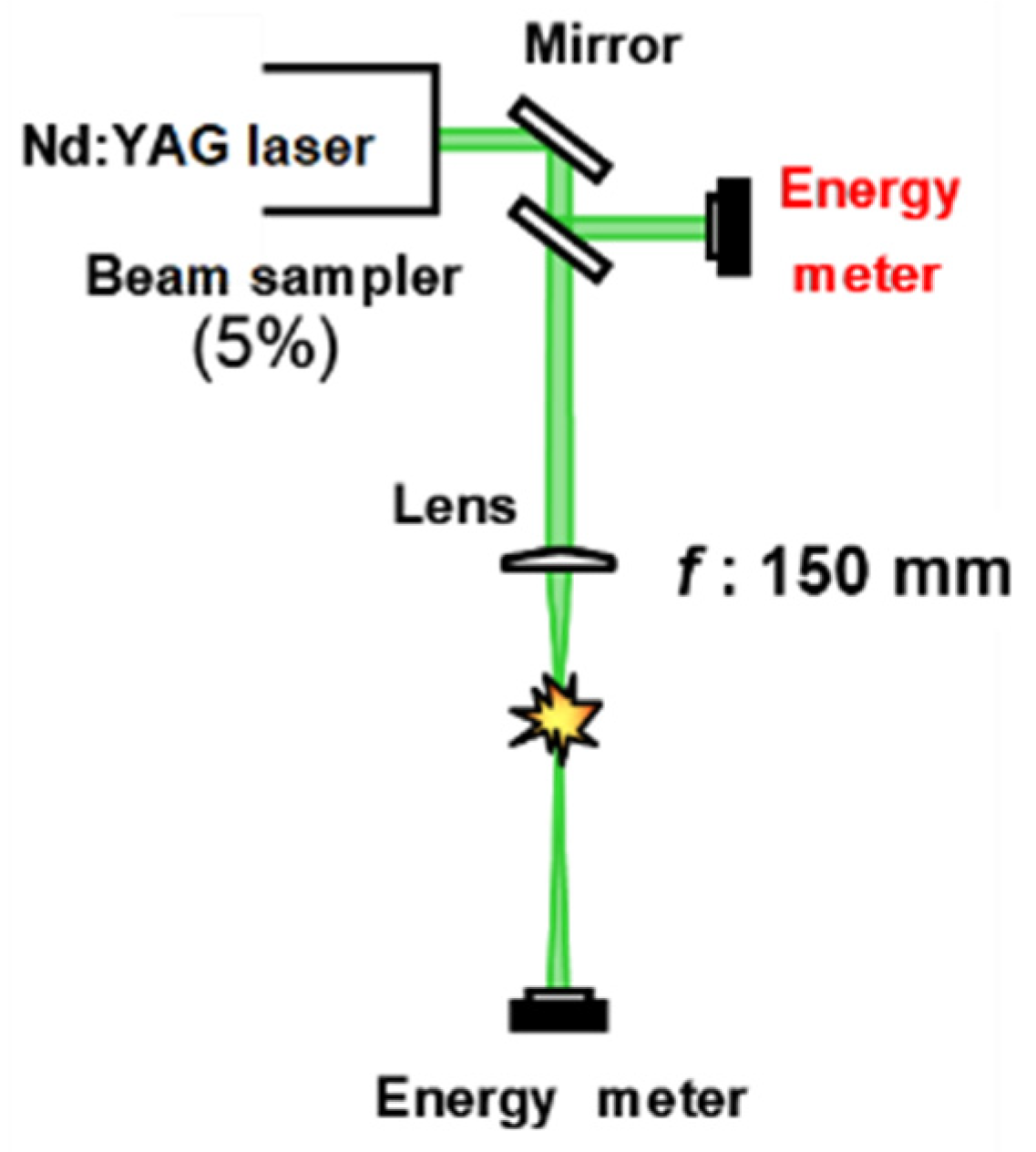
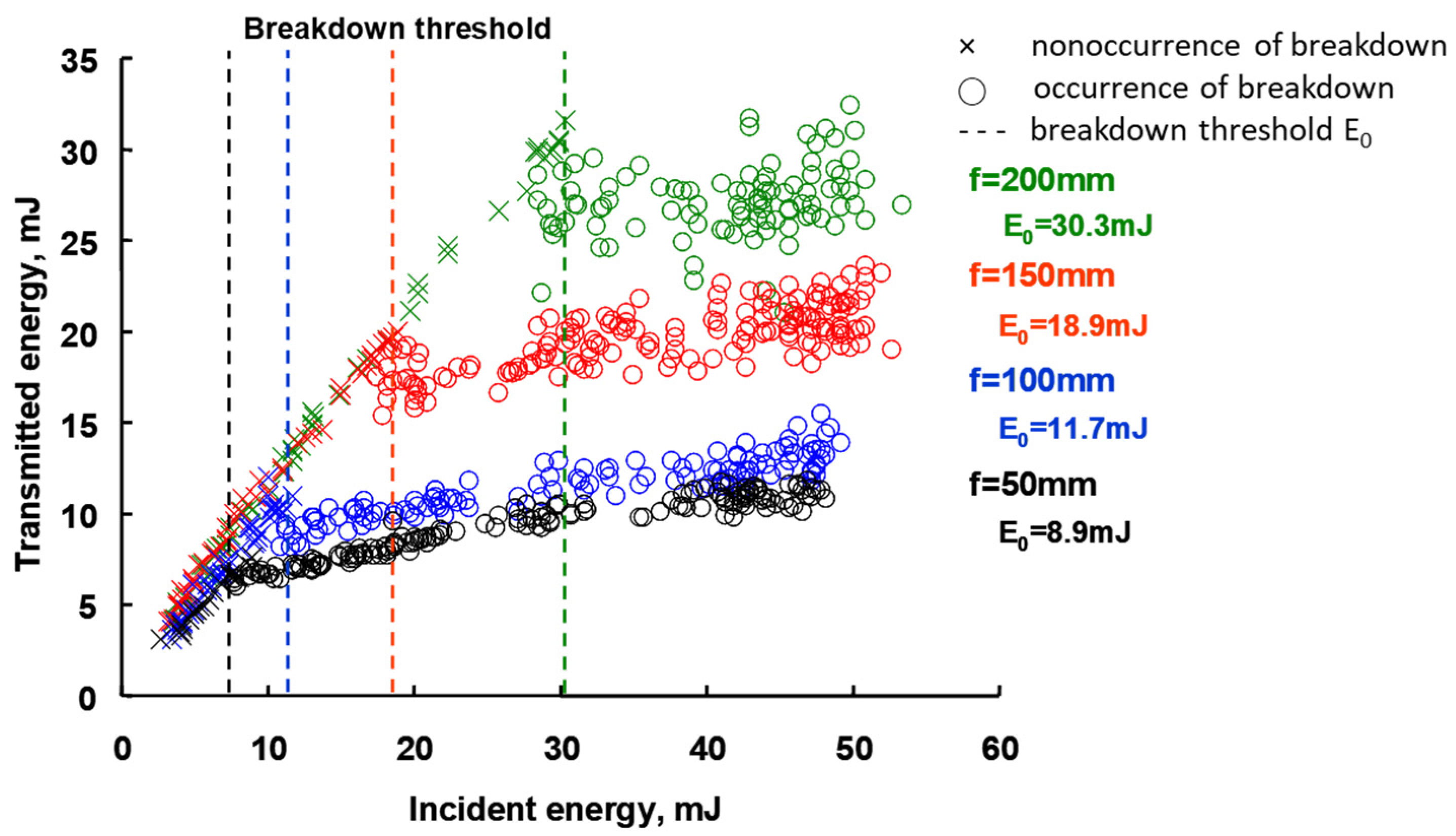
2. Materials and Methods
3. Result and Discussions
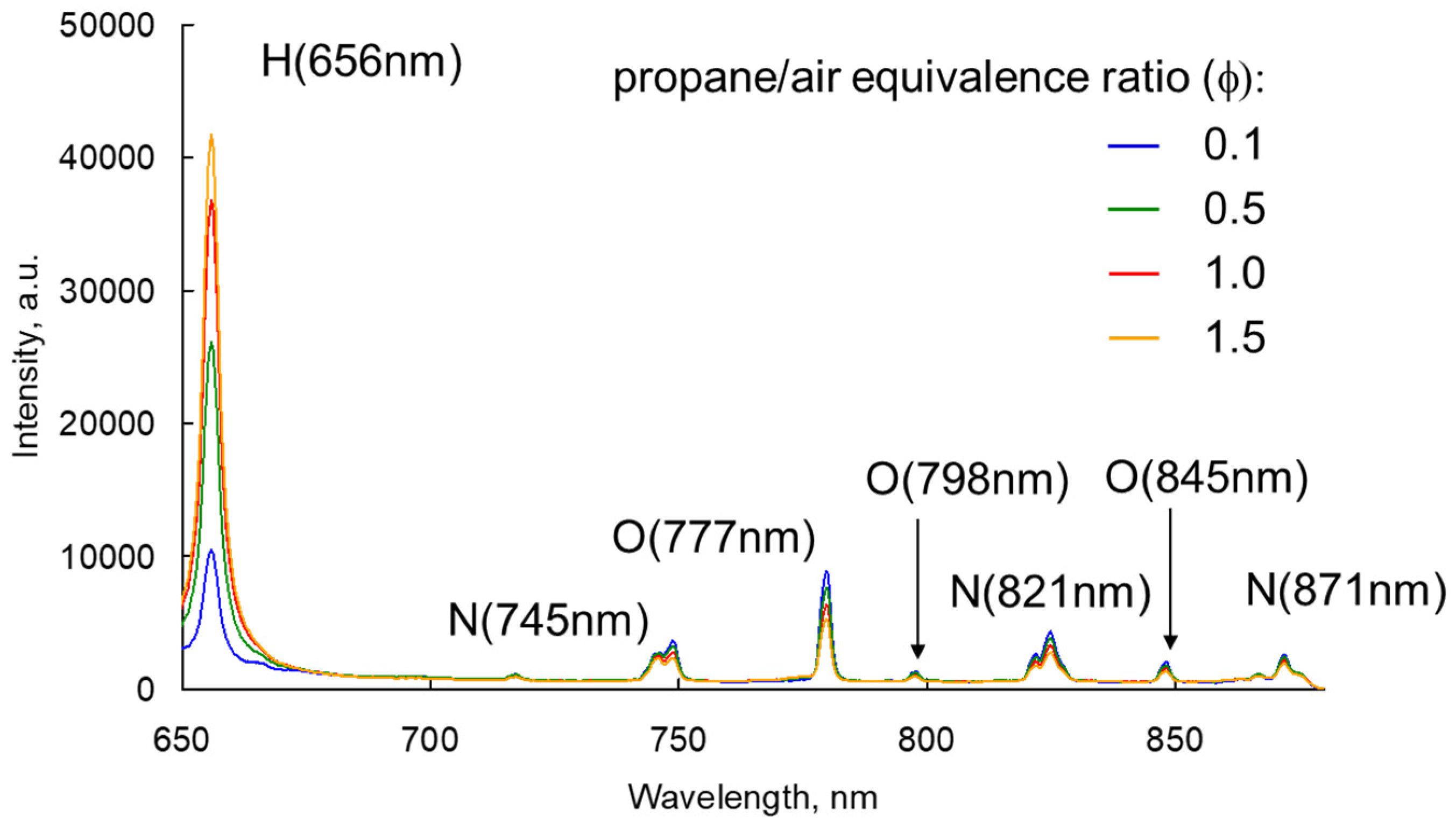
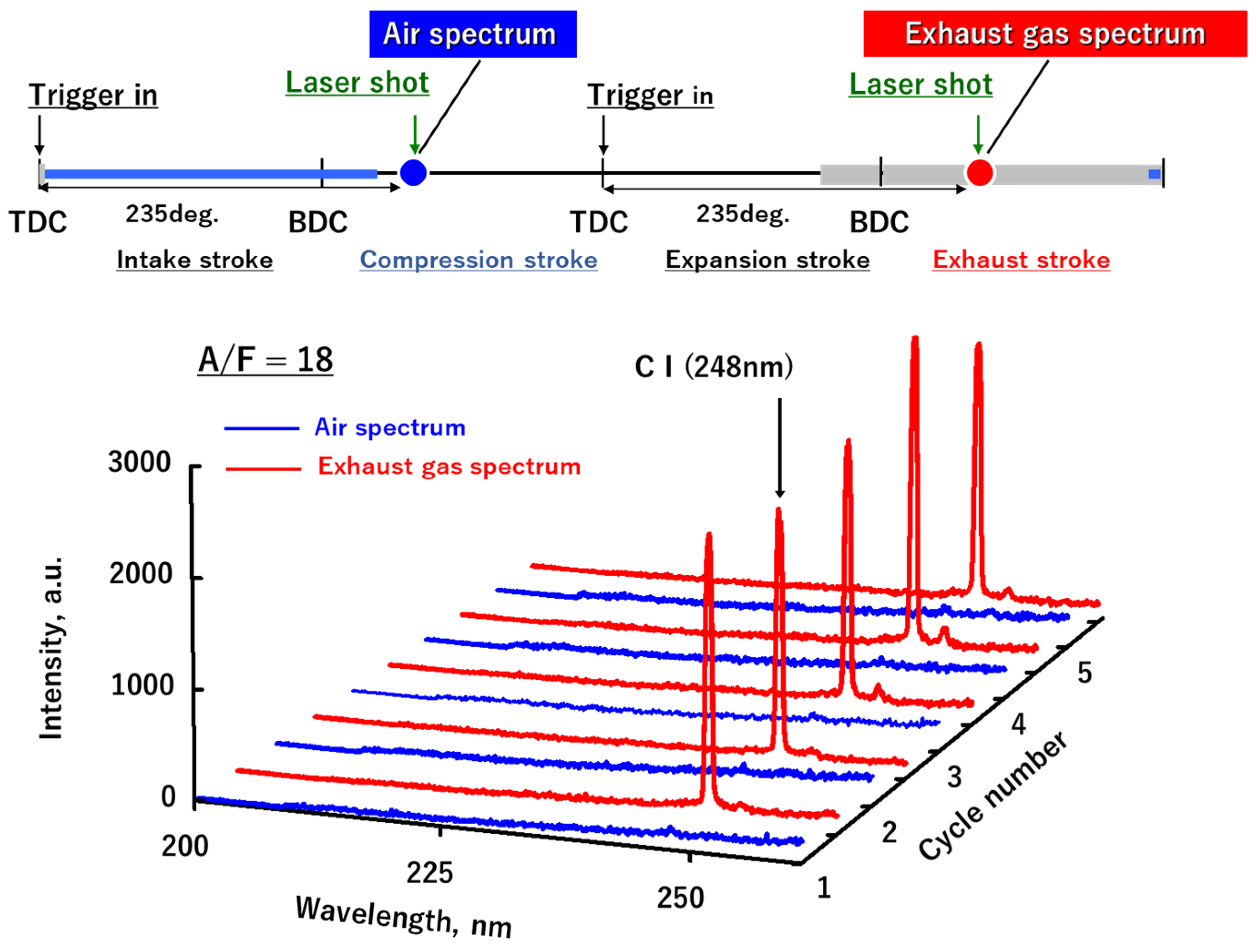
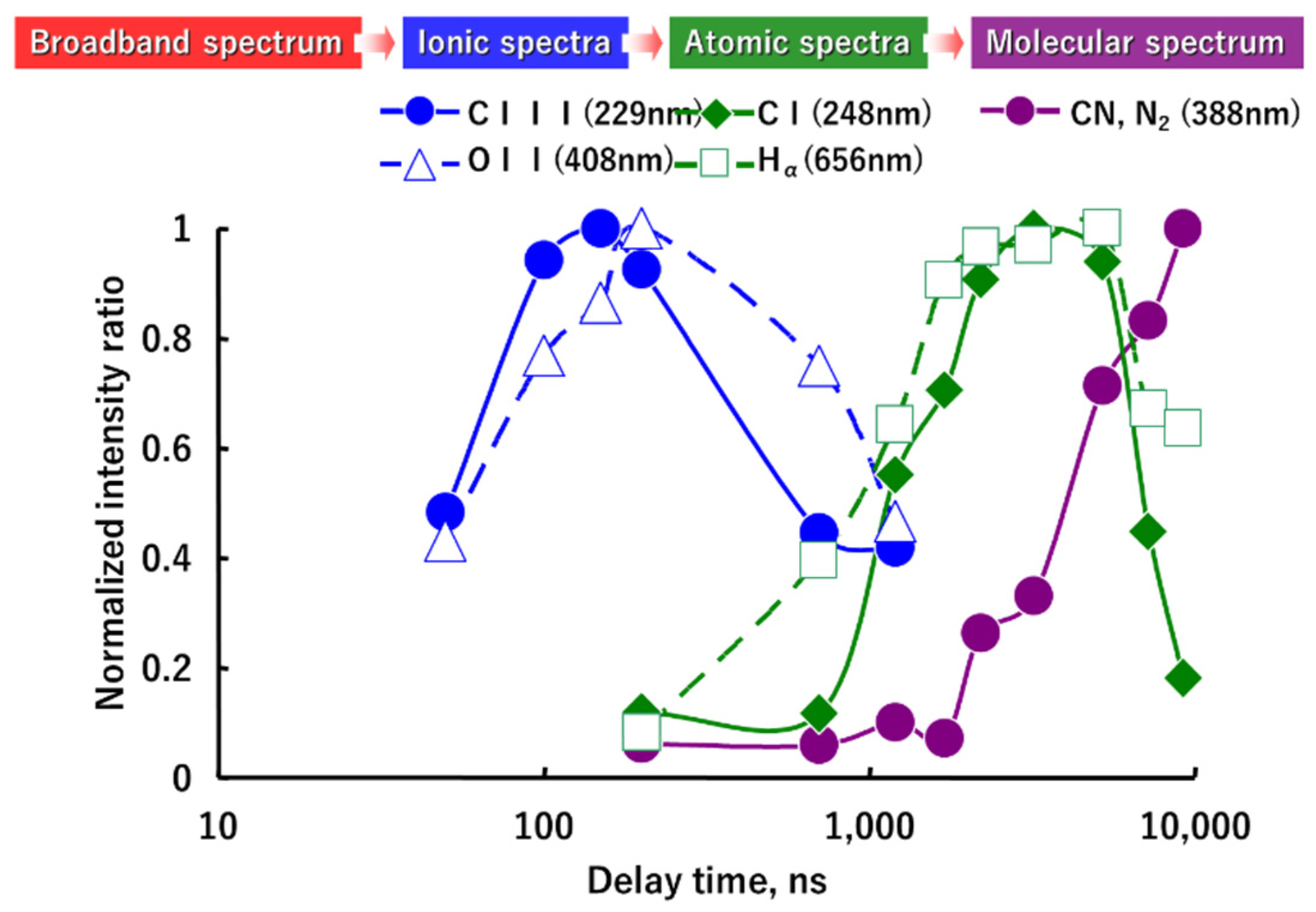
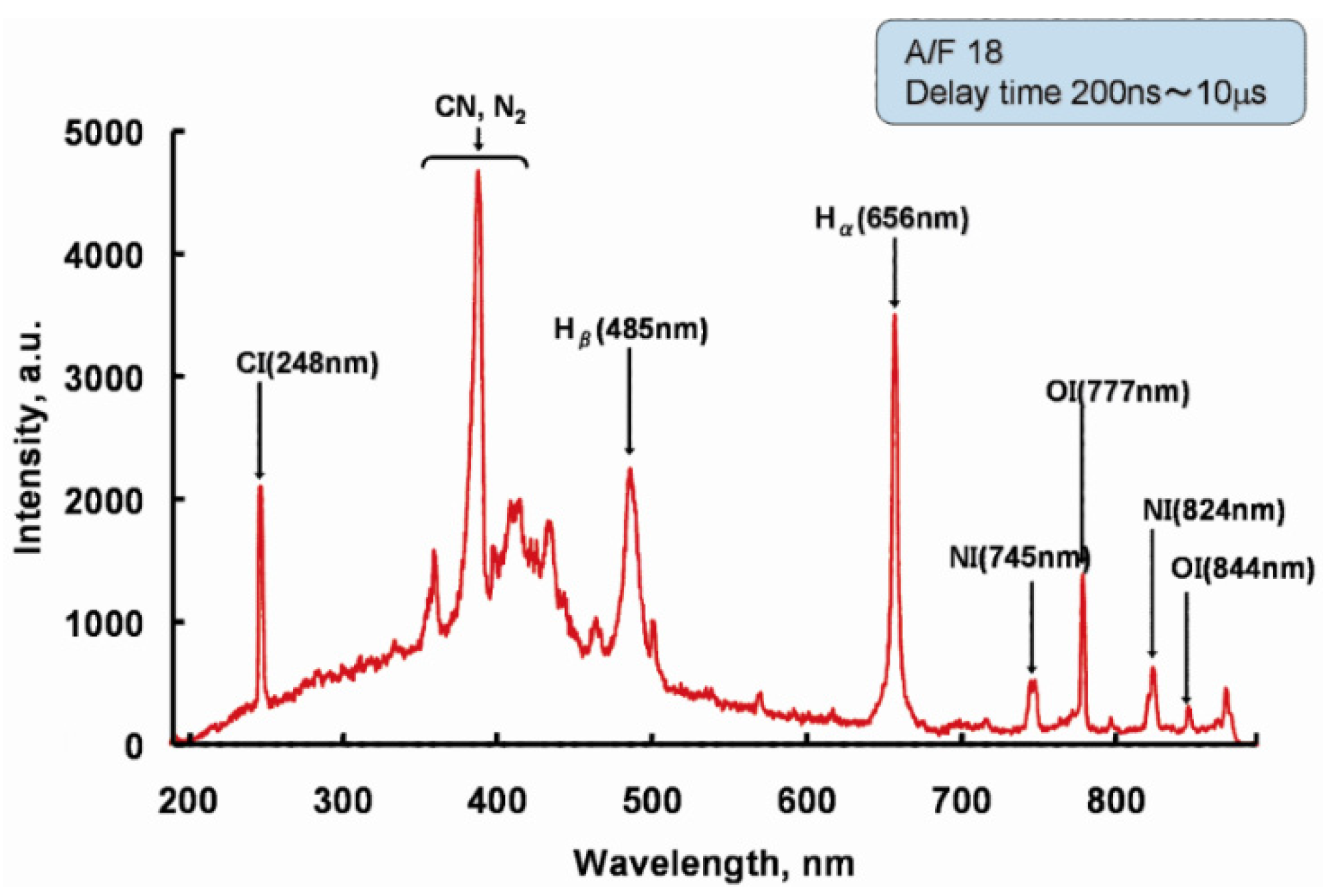
3.1. LIBS Spectrum of the Exhaust Gas
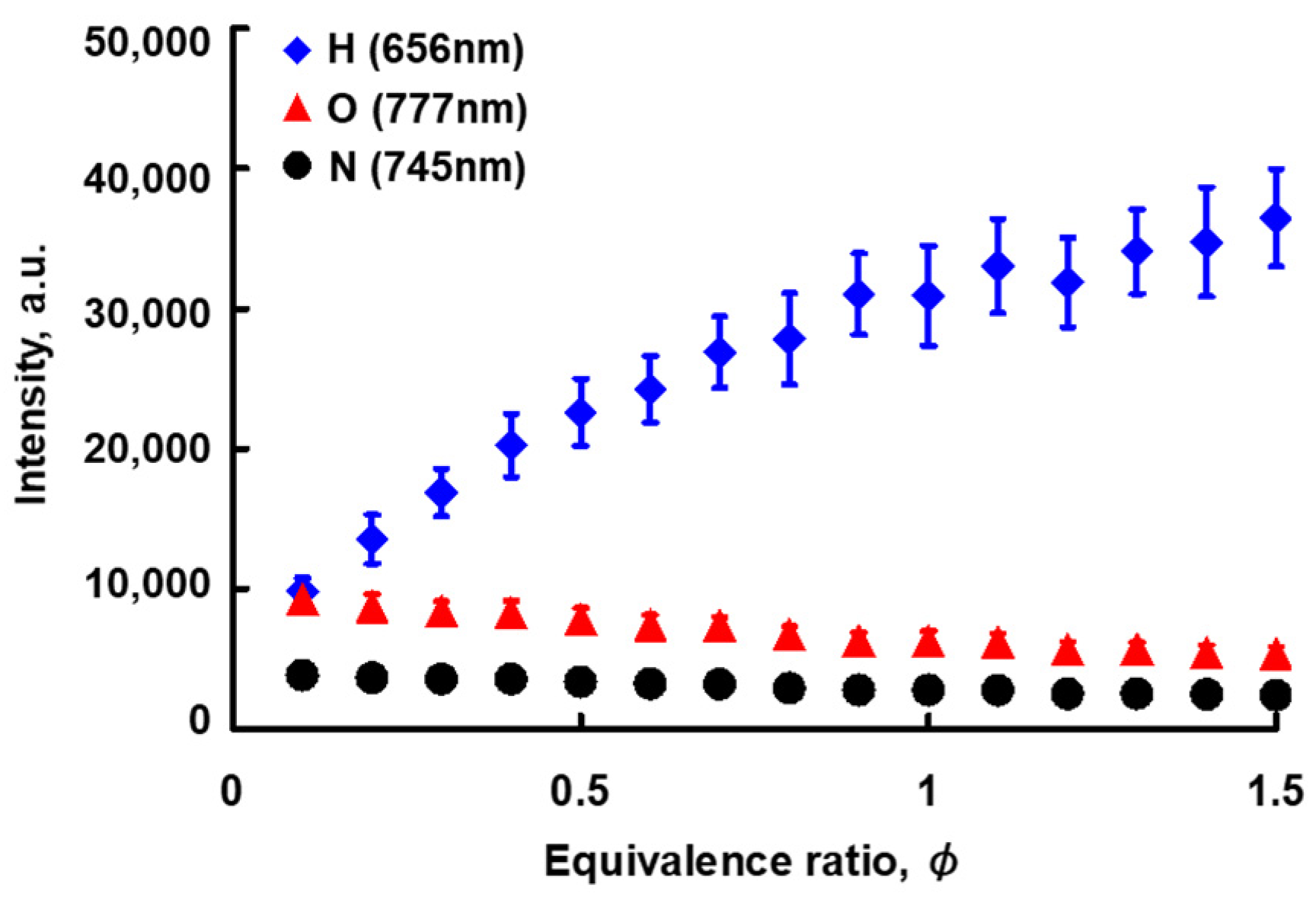
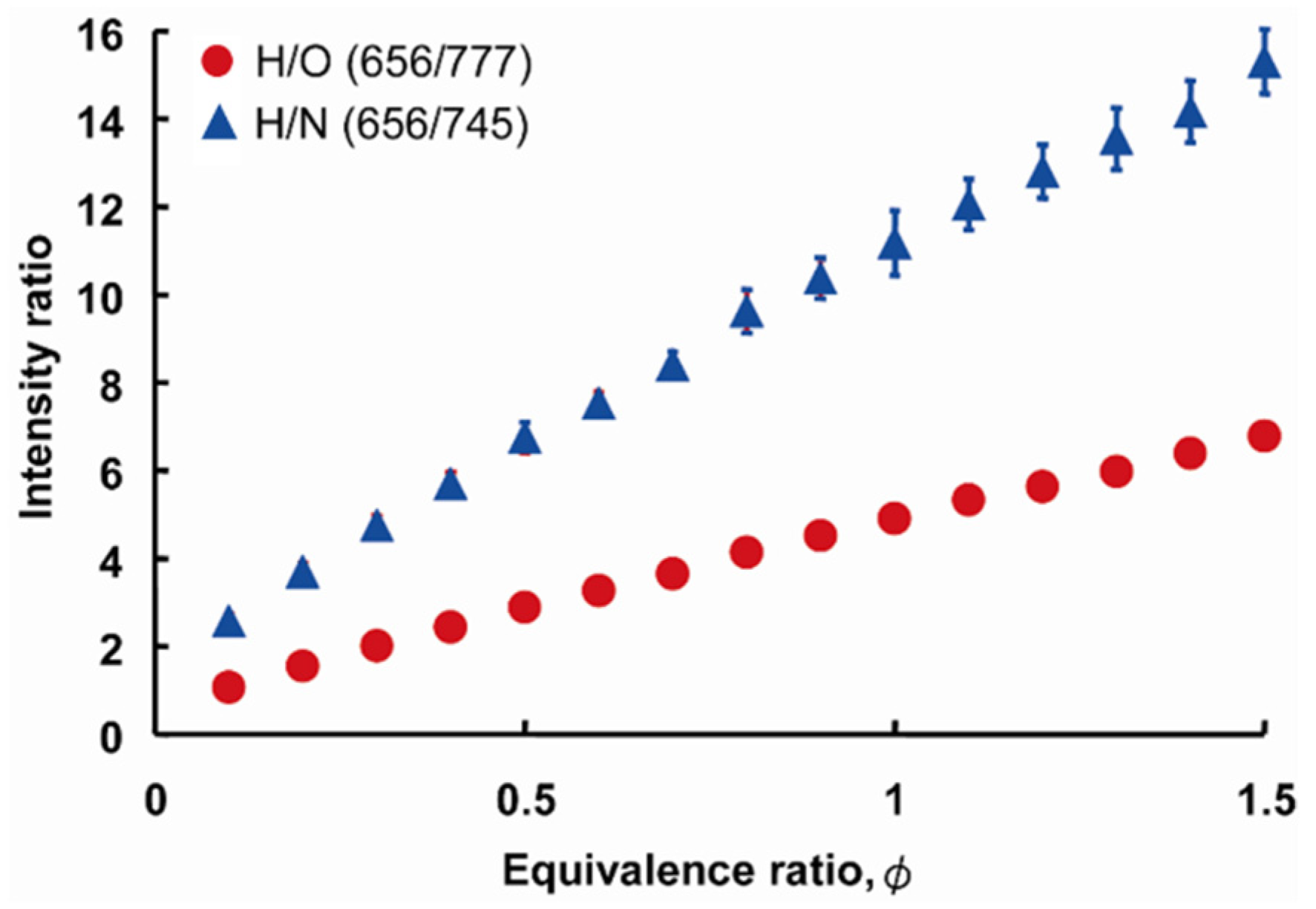
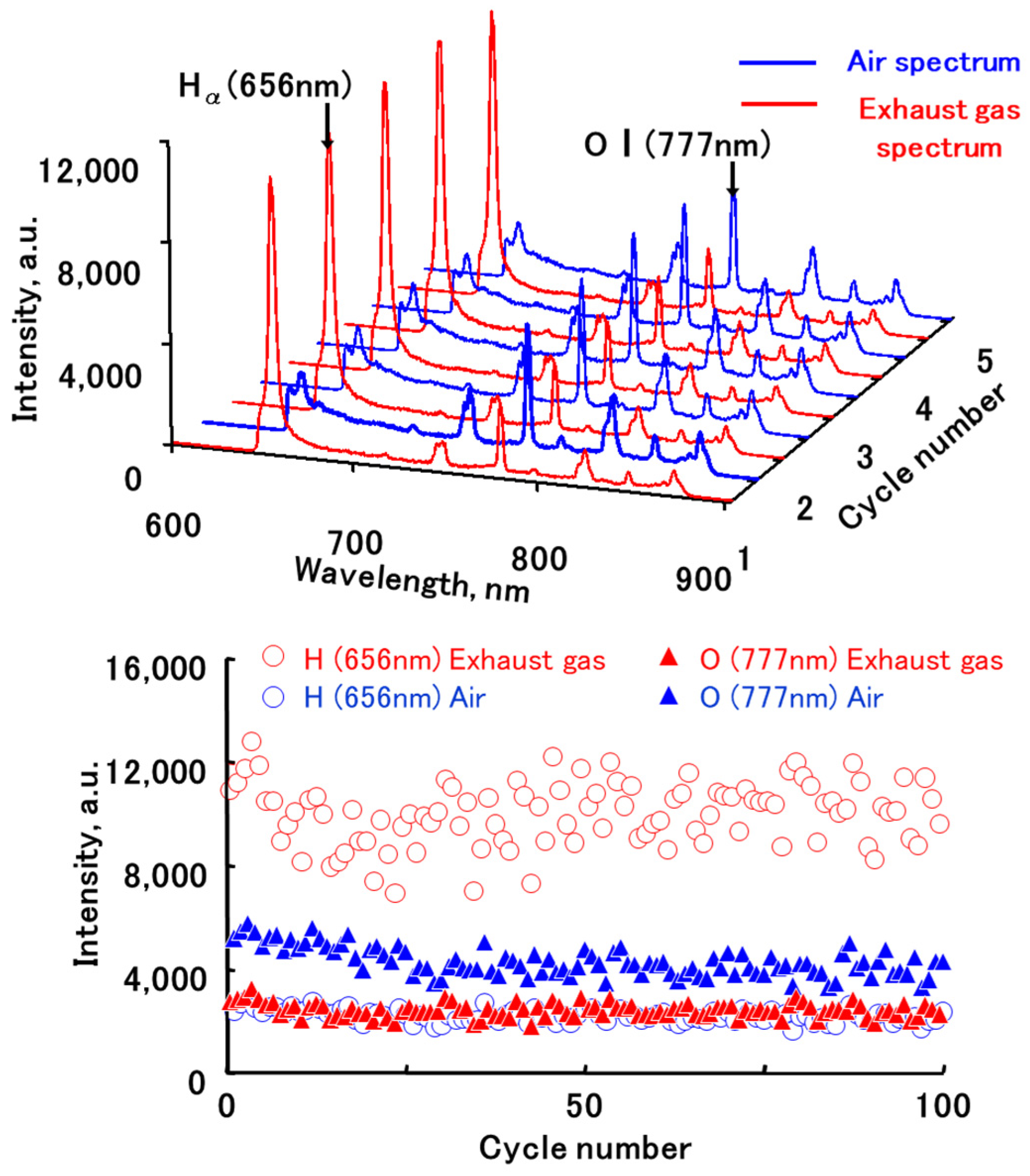
3.2. Relationship between the Atomic Spectrum Intensity and A/F
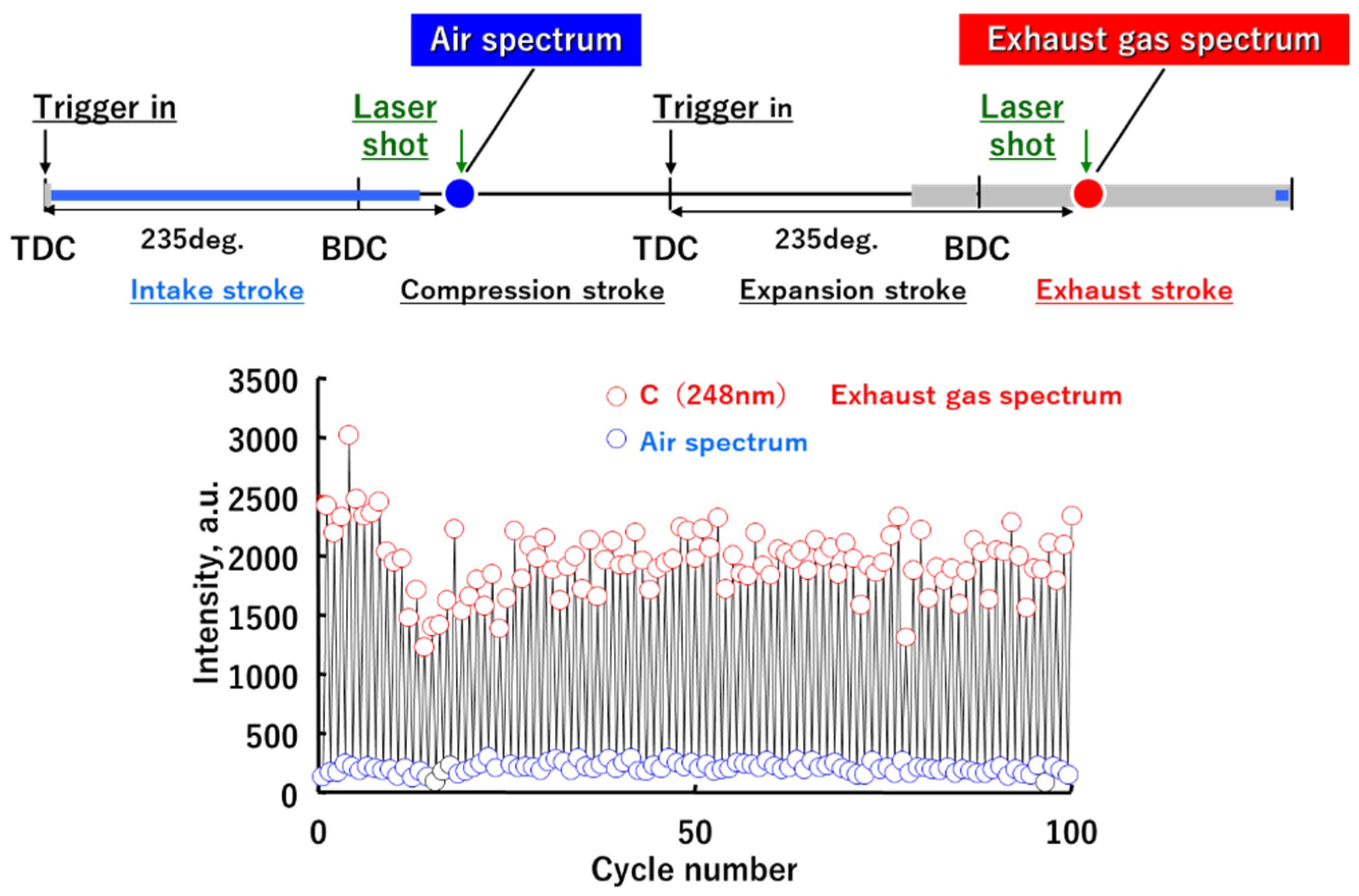
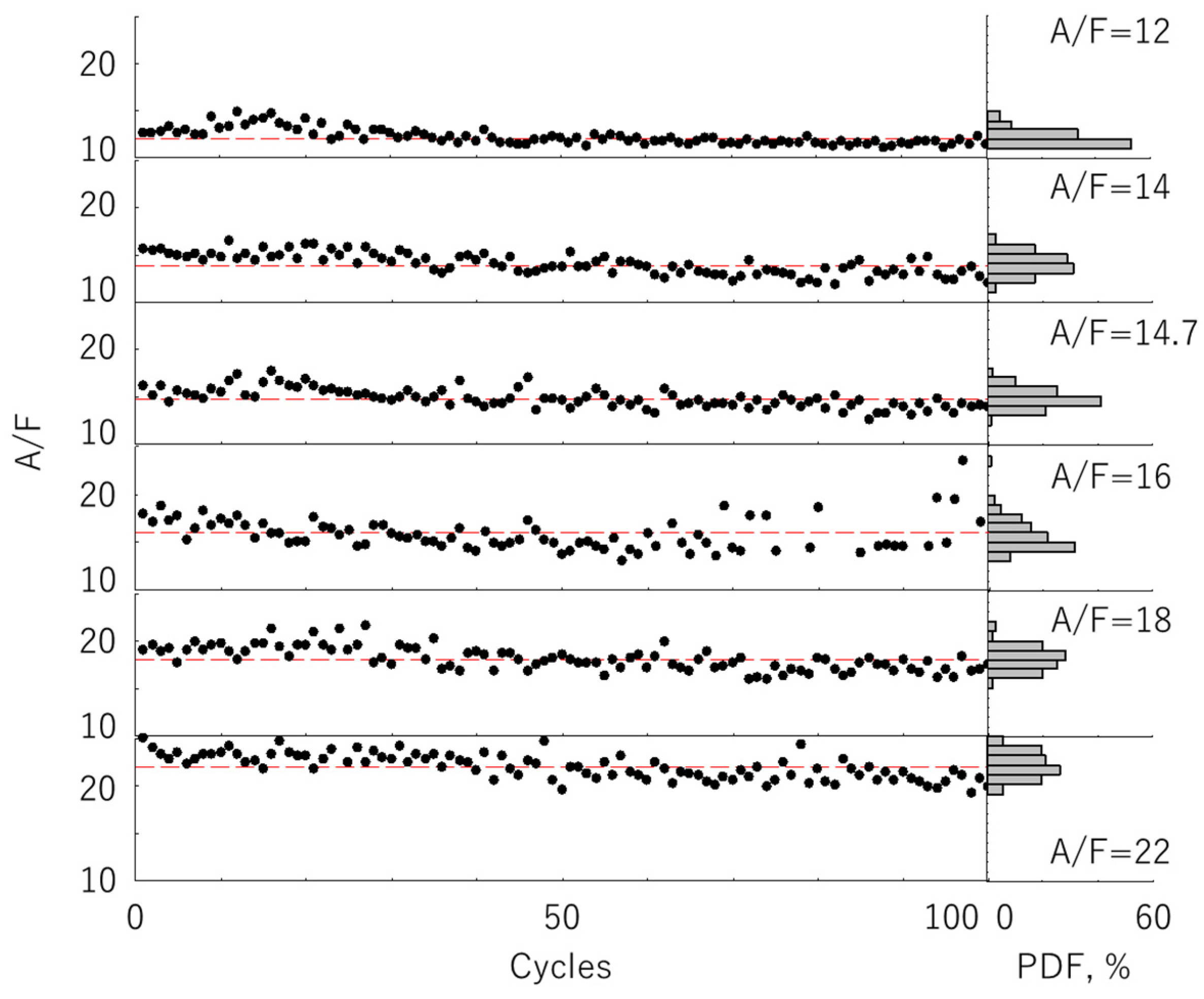
3.3. Cyclic Variation of the A/F
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heywood, J.B.; John, B. Internal Combustion Engine Fundamentals; McGraw-Hill Education: New York, NY, USA, 1988. [Google Scholar]
- Ramalingam, S.; Rajendran, S. Assessment of Performance, Combustion, and Emission Behavior of Novel Annona Biodiesel-Operated Diesel Engine. In Advances in Eco-Fuels for a Sustainable Environment; Woodhead Publishing: Sawston, UK, 2019; pp. 391–405. [Google Scholar] [CrossRef]
- Imamoto, T.; Kawahara, N.; Tomita, E. PREMIER Combustion Characteristics of a Pilot Fuel-Ignited Dual-Fuel Biogas Engine with Consideration of Cycle-to-Cycle Variations. Fuel 2022, 314, 123049. [Google Scholar] [CrossRef]
- Valipour Berenjestanaki, A.; Kawahara, N.; Tsuboi, K.; Tomita, E. End-Gas Autoignition Characteristics of PREMIER Combustion in a Pilot Fuel-Ignited Dual-Fuel Biogas Engine. Fuel 2019, 254, 115634. [Google Scholar] [CrossRef]
- Kim, J.; Chun, K.M.; Song, S.; Baek, H.K.; Lee, S.W. Hydrogen Effects on the Combustion Stability, Performance and Emissions of a Turbo Gasoline Direct Injection Engine in Various Air/Fuel Ratios. Appl. Energy 2018, 228, 1353–1361. [Google Scholar] [CrossRef]
- Zhou, L.; Hua, J.; Wei, H.; Han, Y. An Experimental Investigation on Low Load Combustion Stability and Cold-Firing Capacity of a Gasoline Compression Ignition Engine. Engineering 2019, 5, 558–567. [Google Scholar] [CrossRef]
- Han, X.; Yu, S.; Tjong, J.; Zheng, M. Study of an Innovative Three-Pole Igniter to Improve Efficiency and Stability of Gasoline Combustion under Charge Dilution Conditions. Appl. Energy 2020, 257, 113999. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a Fuel for Internal Combustion Engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Cheng, K.; Qin, J.; Shao, J.; Huang, H. Performance Comparison of Three Chemical Precooled Turbine Engine Cycles Using Methanol and N-Decane as the Precooling Fuels. Energy 2022, 249, 123606. [Google Scholar] [CrossRef]
- Li, X.; Ai, Y.; Ge, Y.; Qi, J.; Feng, Q.; Hu, J.; Porter, W.C.; Miao, Y.; Mao, H.; Jin, T. Integrated Effects of SCR, Velocity, and Air-Fuel Ratio on Gaseous Pollutants and CO2 Emissions from China V and VI Heavy-Duty Diesel Vehicles. Sci. Total Environ. 2022, 811, 152311. [Google Scholar] [CrossRef]
- Valipour Berenjestanaki, A.; Kawahara, N.; Tsuboi, K.; Tomita, E. Performance, Emissions and End-Gas Autoignition Characteristics of PREMIER Combustion in a Pilot Fuel-Ignited Dual-Fuel Biogas Engine with Various CO2 Ratios. Fuel 2021, 286, 119330. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Y.; Zhen, X.; Liu, Z. The Effect of Methanol Production and Application in Internal Combustion Engines on Emissions in the Context of Carbon Neutrality: A Review. Fuel 2022, 320, 123902. [Google Scholar] [CrossRef]
- Ikeda, Y.; Furui, T.; Le, M.K.; Nishiyama, A. In-Cylinder Flow Measurement in SI Engine under Firing Conditions by High Speed PIV. In Proceedings of the 18th International Symposium on the Application of Laser and Imaging Techniques to Fluid Mechanics, Lisboa, Portugal, 14–17 July 2016. [Google Scholar]
- Kawahara, N.; Kim, Y.; Wadahama, H.; Tsuboi, K.; Tomita, E. Differences between PREMIER Combustion in a Natural Gas Spark-Ignition Engine and Knocking with Pressure Oscillations. Proc. Combust. Inst. 2019, 37, 4983–4991. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, R.; Wang, S.; Xu, H.; Yuan, Z. Fuel Economy Improvement of a Turbocharged Gasoline SI Engine through Combining Cooled EGR and High Compression Ratio. Energy 2022, 239, 122353. [Google Scholar] [CrossRef]
- Wu, Y.; Gragston, M.; Zhang, Z.; Hsu, P.S.; Jiang, N.; Patnaik, A.K.; Roy, S.; Gord, J.R. High-Pressure 1D Fuel/Air-Ratio Measurements with LIBS. Combust. Flame 2018, 198, 120–129. [Google Scholar] [CrossRef]
- Behnke, L.; Monroe, E.; Nguyen, B.; Landera, A.; George, A.; Yang, Z.; Heyne, J.; Davis, R.W. Maximizing Net Fuel Economy Improvement from Fusel Alcohol Blends in Gasoline Using Multivariate Optimization. Fuel Commun. 2022, 11, 100059. [Google Scholar] [CrossRef]
- Zeng, Y.; Tan, X.; Gu, B.; Guo, J.; Jiang, J.; Wang, D.; Tang, J. Cost-Effectiveness Analysis on Improving Fuel Economy and Promoting Alternative Fuel Vehicles: A Case Study of Chongqing, China. J. Clean. Prod. 2021, 323, 129075. [Google Scholar] [CrossRef]
- Odunlami, O.A.; Oderinde, O.K.; Akeredolu, F.A.; Sonibare, J.A.; Obanla, O.R.; Ojewumi, M.E. The Effect of Air-Fuel Ratio on Tailpipe Exhaust Emission of Motorcycles. Fuel Commun. 2022, 11, 100040. [Google Scholar] [CrossRef]
- Yang, J.; Tao, W.; Wang, M.; Feng, C.; Yin, C. Stochastic Air-Fuel Ratio Control with Cylinder Pressure Measurement Inaccuracy of Gas Engines. IFAC-PapersOnline 2021, 54, 84–89. [Google Scholar] [CrossRef]
- Woo, S.; Lee, J.; Lee, K. Experimental Study on the Performance of a Liquefied Petroleum Gas Engine According to the Air Fuel Ratio. Fuel 2021, 303, 121330. [Google Scholar] [CrossRef]
- Moonsammy, S.; Oyedotun, T.D.T.; Oderinde, O.; Durojaiye, M.; Durojaye, A. Exhaust Determination and Air-to-Fuel Ratio Performance of End-of-Life Vehicles in a Developing African Country: A Case Study of Nigeria. Transp. Res. Part D Transp. Environ. 2021, 91, 102705. [Google Scholar] [CrossRef]
- Musculus, M.P.B.; Pickett, L.M. In-Cylinder Spray, Mixing, Combustion, and Pollutant-Formation Processes in Conventional and Low-Temperature-Combustion Diesel Engines. Adv. Direct Inject. Combust. Eng. Technol. Dev. 2010, 241, 644–675. [Google Scholar] [CrossRef]
- Nishiyama, A.; Le, M.K.; Furui, T.; Ikeda, Y. Simultaneous In-Cylinder Flow Measurement and Flame Imaging in a Realistic Operating Engine Environment Using High-Speed PIV. Appl. Sci. 2019, 9, 2678. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, N.; Beduneau, J.L.; Nakayama, T.; Tomita, E.; Ikeda, Y. Spatially, temporally, and spectrally resolved measurement of laser-induced plasma in air. Appl. Phys. B 2007, 86, 605–614. [Google Scholar] [CrossRef]
- Kojima, J.; Ikeda, Y.; Nakajima, T. Spatially resolved measurement of OH *, CH * and C2 * chemiluminescence in the reaction zone of laminar methane/air premixed flames. Proc. Combust. Inst. 2000, 28, 1757–1764. [Google Scholar] [CrossRef]
- Kojima, J.; Ikeda, Y.; Nakajima, T. Multi-point time-series observation of optical emissions for flame-front motion analysis. Meas. Sci. Technol. 2003, 14, 1714–1724. [Google Scholar] [CrossRef]
- Ikeda, Y.; Kawahara, N.; Tomita, E. Time-series A/F analysis in a SI engine by micro-local chemiluminescence technique. In Proceedings of the 6th COMODIA 2004, Yokohama, Japan, 2–5 August 2004. [Google Scholar]
- Ikeda, Y.; Nishiyama, A.; Kawahara, N.; Tomita, E.; Arimoto, S.; Takeuch, A. In-spark-plug sensor for analyzing the initial flame and its structure in an SI engine. In SAE Technical Papers; MDPI: Basel, Switzerland, 2005. [Google Scholar] [CrossRef]
- Ikeda, Y.; Nishiyama, A.; Kim, S.M.; Takeuchi, A.; Winklhofer, E.; Baritaud, T. Cyclic variation of local A/F and mixture quality in SI engine using local chemiluminescence. In Proceedings of the 7th international symposium on internal combustion diagnostics, Baden, Germany, 18–19 May 2006. [Google Scholar]
- Kawahara, N.; Tomita, E.; Takeuchi, A.; Arimoto, S.; Ikeda, Y.; Nishiyama, A. Measurement of flame propagation characteristics in an SI engine using micro-local chemiluminescence technique. In SAE Technical Papers; MDPI: Basel, Switzerland, 2005. [Google Scholar] [CrossRef]
- Radziemski, L.J. From LASER to LIBS, the path of technology development. Spectrochim. Acta B 2002, 57, 1109–1113. [Google Scholar] [CrossRef]
- Phuoc, T.X.; White, F.P. Laser-induced spark for measurements of the fuel-to-air ratio of a combustible mixture. Fuel 2002, 81, 1761–1765. [Google Scholar] [CrossRef]
- Stavropoulos, P.; Michalakou, A.; Skevis, G.; Couris, S. Quantitative local equivalence ratio determination in laminar premixed methane-air flames by laser induced breakdown spectroscopy (LIBS). Chem. Phys. Lett. 2005, 404, 309–314. [Google Scholar] [CrossRef]
- Stavropoulos, P.; Michalakou, A.; Skevis, G.; Couris, S. Laser-induced breakdown spectroscopy as an analytical tool for equivalence ratio measurement in methane-air premixed flames. Spectrochim. Acta Part B At. Spectrosc. 2005, 60, 1092–1097. [Google Scholar] [CrossRef]
- Sturm, V.; Noll, R. Laser-induced breakdown spectroscopy of gas mixture of air, CO2, N2, and C3H8 for simultaneous C, H, O, and N measurement. Appl. Opt. 2003, 42, 6221–6225. [Google Scholar] [CrossRef]
- Ferioli, F.; Buckley, S.G. Measurements of hydrocarbons using laser-induced breakdown spectroscopy. Combust. Flame 2006, 144, 435–447. [Google Scholar] [CrossRef]
- Hohreiter, V.; Carranza, J.E.; Hahn, D.W. Temporal analysis of laser-induced plasma properties as related to laser-induced breakdown spectroscopy. Spectrochim. Acta Part B 2004, 59, 327–333. [Google Scholar] [CrossRef]
- Stavropoulos, P.; Palagas, C.; Angelopoulos, G.N.; Papamantellos, D.N.; Couris, S. Calibration measurement in laser-induced breakdown spectroscopy using nanosecond and picosecond lasers. Spectrochim. Acta Part B At. Spectrosc. 2004, 59, 1885–1892. [Google Scholar] [CrossRef]
- Ikeda, Y.; Nishiyama, A.; Kawahara, N.; Tomita, E.; Nakayama, T. Local equivalence ratio measurement of CH4/air and C3H8/air laminar flames by laser-induced breakdown. In Proceedings of the 44th AIAA Aerospace Sciences Meeting and Exhibit, Reno, Nevada, 12 January 2006; p. 965. [Google Scholar]
- Mansour, M.S.; Imam, H.; Elsayed, K.A.; Abbass, W. Local equivalence ratio measurements in turbulent partially premixed flames using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 1079–1084. [Google Scholar] [CrossRef]
- Joshi, S.; Olsen, D.B.; Dumitrescu, C.; Puzinauskas, P.V.; Yalin, A.P. Laser-induced breakdown spectroscopy for in-cylinder equivalence ratio measurements in laser-ignited natural gas engines. Appl. Spectrosc. 2009, 63, 549–554. [Google Scholar] [CrossRef]
- Eseller, K.E.; Yueh, F.Y.; Singh, J.P. Laser-induced breakdown spectroscopy measurement in methane and biodiesel flames using an ungated detector. Appl. Opt. 2008, 47, G144–G148. [Google Scholar] [CrossRef]
- Kim, Y.W. Fundamentals of analysis of solids by laser-produced plasmas. In Laser-Induced Plasmas and Applications; Radziemski, L.J., Cremers, D.A., Eds.; Marcel Dekker Inc: New York, NY, USA, 1989. [Google Scholar]
- Beduneau, J.L.; Kim, B.; Zimmer, L.; Ikeda, Y. Measurements of minimum ignition energy in premixed laminar methane/air flow by using laser induced spark. Combust. Flame 2003, 132, 653–665. [Google Scholar] [CrossRef]
- Beduneau, J.L.; Ikeda, Y. Spatial characterization of laser-induced sparks in air. J. Quant. Spectrosc. Radiat. Transf. 2004, 84, 123–139. [Google Scholar] [CrossRef]
- Beduneau, J.L.; Kawahara, N.; Nakayama, T.; Ikeda, Y. Laser-Induced radical generation and evolution to a self-sustaining flame. Combust. Flame 2009, 156, 642–656. [Google Scholar] [CrossRef]



| FTIR | FID | NDIR | LIBS | |
|---|---|---|---|---|
| Temporal resolution | 0.6 s | 1 ms | 8 ms | <1 ms |
| Spatial resolution | × | × | × | ○ |
| Species | CO, CO2, NO, NO2, H2O, etc. | THC | CO, CO2 | C, H, N, O, etc. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, Y.; Kawahara, N. Measurement of Cyclic Variation of the Air-to-Fuel Ratio of Exhaust Gas in an SI Engine by Laser-Induced Breakdown Spectroscopy. Energies 2022, 15, 3053. https://doi.org/10.3390/en15093053
Ikeda Y, Kawahara N. Measurement of Cyclic Variation of the Air-to-Fuel Ratio of Exhaust Gas in an SI Engine by Laser-Induced Breakdown Spectroscopy. Energies. 2022; 15(9):3053. https://doi.org/10.3390/en15093053
Chicago/Turabian StyleIkeda, Yuji, and Nobuyuki Kawahara. 2022. "Measurement of Cyclic Variation of the Air-to-Fuel Ratio of Exhaust Gas in an SI Engine by Laser-Induced Breakdown Spectroscopy" Energies 15, no. 9: 3053. https://doi.org/10.3390/en15093053
APA StyleIkeda, Y., & Kawahara, N. (2022). Measurement of Cyclic Variation of the Air-to-Fuel Ratio of Exhaust Gas in an SI Engine by Laser-Induced Breakdown Spectroscopy. Energies, 15(9), 3053. https://doi.org/10.3390/en15093053






