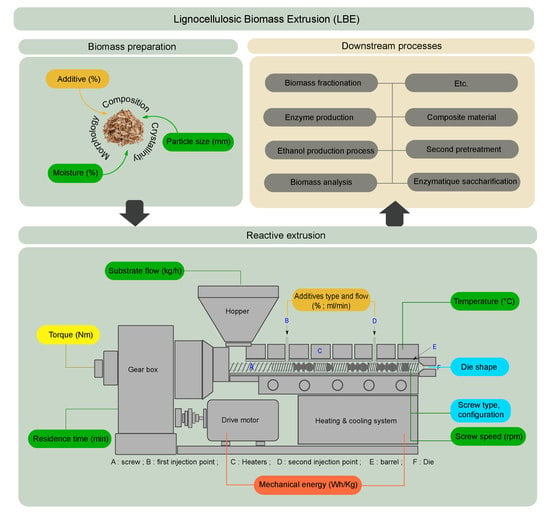
An Overview of Extrusion as a Pretreatment Method of Lignocellulosic Biomass
Abstract
:1. Introduction
2. Lignocellulosic Biomass
2.1. Biomass Composition
2.2. Crystallinity
| Biomass | Substrate Crystallinity (%) | Extrudate Crystallinity (%) | References |
|---|---|---|---|
| Banana fibers | 39 | - | [50] |
| Sugarcane bagasse | 48 | - | [50] |
| Sponge gourd fibers | 50 | - | [50] |
| Sweet corn | 41 ± 3 | 47 ± 6 | [45] |
| Barley straw | 44 ± 8 | 46 ± 2 | [45] |
| Blue agave bagasse | 27 ± 7 | 52 ± 1 | [45] |
| OPEFB | 50 ± 8 | 51 ± 7 | [45] |
| Corn stover | 48 ± 4 | 51.2 ± 3.4 | [46] |
| Sugarcane bagasse | 57.3 ± 1.3 | 54.0 ± 0.23 | [44] |
2.3. Particle Size
2.4. Morphology
2.5. Moisture
2.6. Biomass Preparation before Pretreatment
| Substrate | Source | Steps | Size (mm) | Storage Time | Tempera | Moisture (%) | Additives before Extrusion | Reference |
|---|---|---|---|---|---|---|---|---|
| Barleystraw | Research centre | [b]-[c]-[d]-[g] | 5 | - | - | - | No | [45] |
| Barley straw | Research centre | [c]-[d]-[f]-[g] | 5 | Stored until use | - | 6 | No | [61] |
| Big bluestem | Farm | [c]-[d]-[f]-[g] | 0.4–0.8 | Stored until use | RT | - | [63] | |
| Big bluestem | Farm | [c]-[d]-[e]/[f]-[g] | 2, 4, 6, 8, 10 | ~8 h | RT | 10, 20, 30, 40, 50 | Water | [64] |
| Blue agave | Manufacture | [b]-[c]-[d]-[g] | 2 | - | - | - | No | [45] |
| Corn cob | Farm | [b]-[c]-[d]-[g] | 2 | - | RT | - | No | [13] |
| Corn stover | Farm | [c]-[b]-[e]-[g] | 2 | - | RT | 22.5, 25, 27.5 | No | [49] |
| Corn stover | Farm | [c]-[b]-[e]-[f]-[g] | 2 | 8 h | RT | 50 | NaOH | [15] |
| Corn stover | Farm | [c]-[g] | 2–5 | 0 h | - | - | No | [62] |
| Corn stover | Farm | [c]-[d]-[e]/[f]-[g] | 2, 4, 6, 8, 10 | ~8 h | RT | 10, 20, 30, 40, 50 | Water | [64] |
| Eucalyptus trees | Research centre | [c]-[b]/[f]-[d]-[g] | 60–190 | 2 months | - | 20 | No | [32] |
| Hardwood biomass (oak, fir, and pine sawdust) | - | [e]-[g] | 1 | - | RT | 21–28 | NaOH | [55] |
| Miscanthus | Farm | [c]-[d]-[g] | 3 | - | 7 | - | No | [65] |
| Olive tree pruning | Farm | [b]-[c]-[e]-[g] | 1–4 | - | RT | 10 | No | [66] |
| OPEFB | Manufacture | [b]-[c]-[d]-[f] | 2 | - | - | - | No | [45] |
| Prairie cordgrass | Farm | [c]-[d]-[e]/[f]-[g] | 2, 4, 6, 8, 10 | ~8 h | RT | 10, 20, 30, 40, 50 | Water | [64] |
| Rape straw | Research centre | [c]-[d]-[g] | 1.4–2.36 | 24 h | 45 ± 5 | 6.44 | No | [67] |
| Rice hull | Manufacture | [a]-[b]-[c]-[d]-[g] | 25.4 | 24 h | 60 | - | No | [53] |
| Soybean hulls | Manufacture | [b]-[d]-[g] | 1.041 | 24 h | RT | 40, 45, 50 | No | [68] |
| Sugarcane bagasse | Mill | [b]-[c]-[d]-[e]-[f]-[g] | 0.2–2 | 24 h | Cold room | 10.4 ± 0.36 8.9 ± 0.30 | Water, Glycol, Ethylene glycol, Tween 80 | [44] |
| Sugarcane bagasse | Manufacture | [c]-[d]-[e]-[g] | 0.425–1.000 | - | 40 | 10 | [EMIM]Ac | [69] |
| Sugarcane straw | Mill | [b]-[c]-[d]-[e]-[f]-[g] | 0.2–2 | 24 h | Cold room | 12.05 ± 0.36 10.34 ± 0.26 | Water, Glycol, Ethylene glycol, Tween 80 | [44] |
| Sweet corn | Manufacture | [b]-[c]-[d]-[g] | 6 | - | - | - | No | [45] |
| Switchgrass | Farm | [c]-[d]-[e]/[f]-[g] | 2, 4, 6, 8, 10 | ~8 h | RT | 10, 20, 30, 40, 50 | water | [64] |
| Switchgrass (matured) | Farm | [c]-[d]-[f]-[g] | 0.3–1.2 | Stored until use | RT | - | [63] | |
| Wheat straw | - | [c]-[f]-[g] | 5 | Stored until use | 40 | 6 | No | [37] |
| Wood powder of pussy willow | - | [d]-[b]-[f]-[e]-[g] | 25.4 | 24 h | 40 | - | [EMIM]Ac, DMSO, [EMIM]Ac/DMSO | [59] |
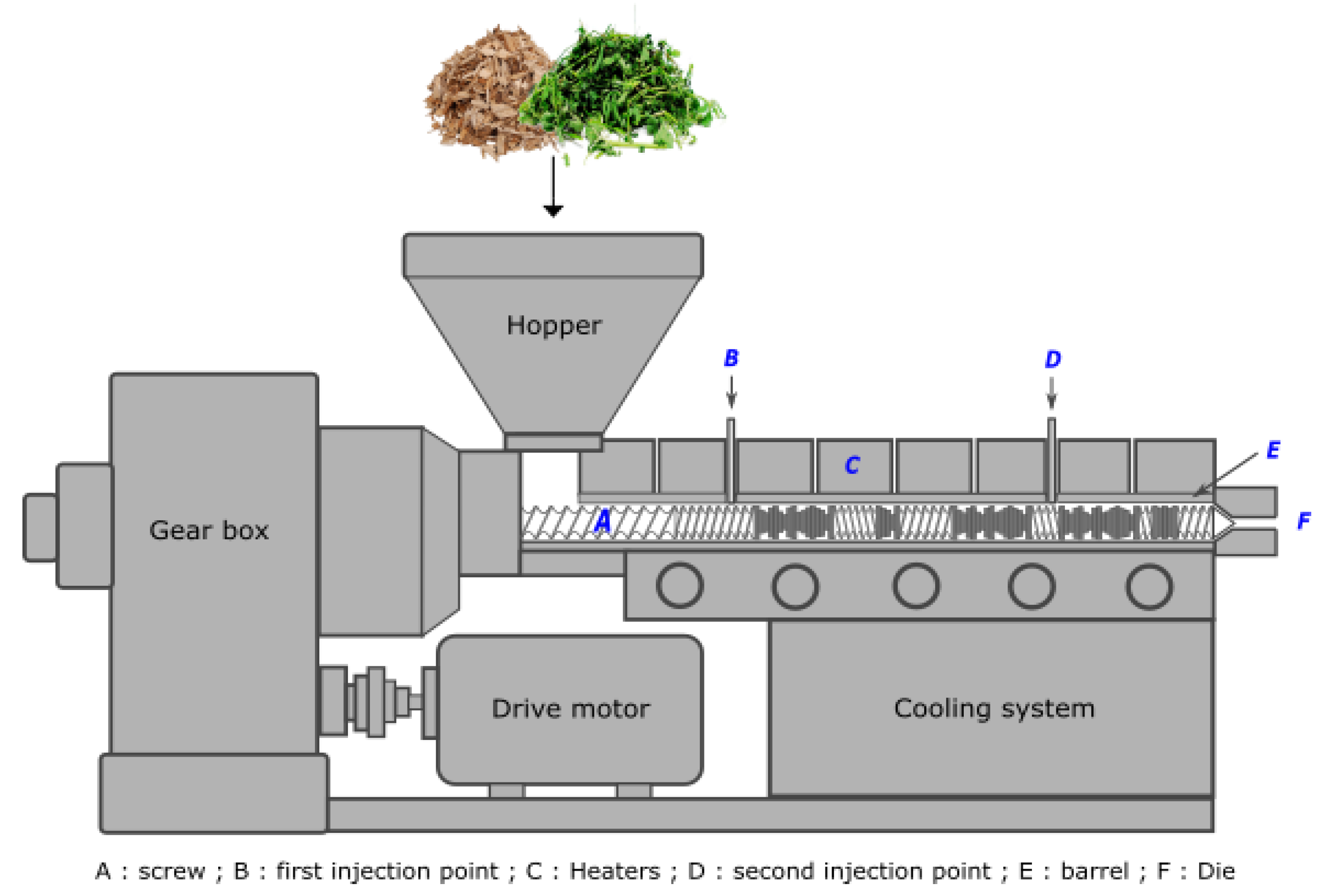
3. Extruder
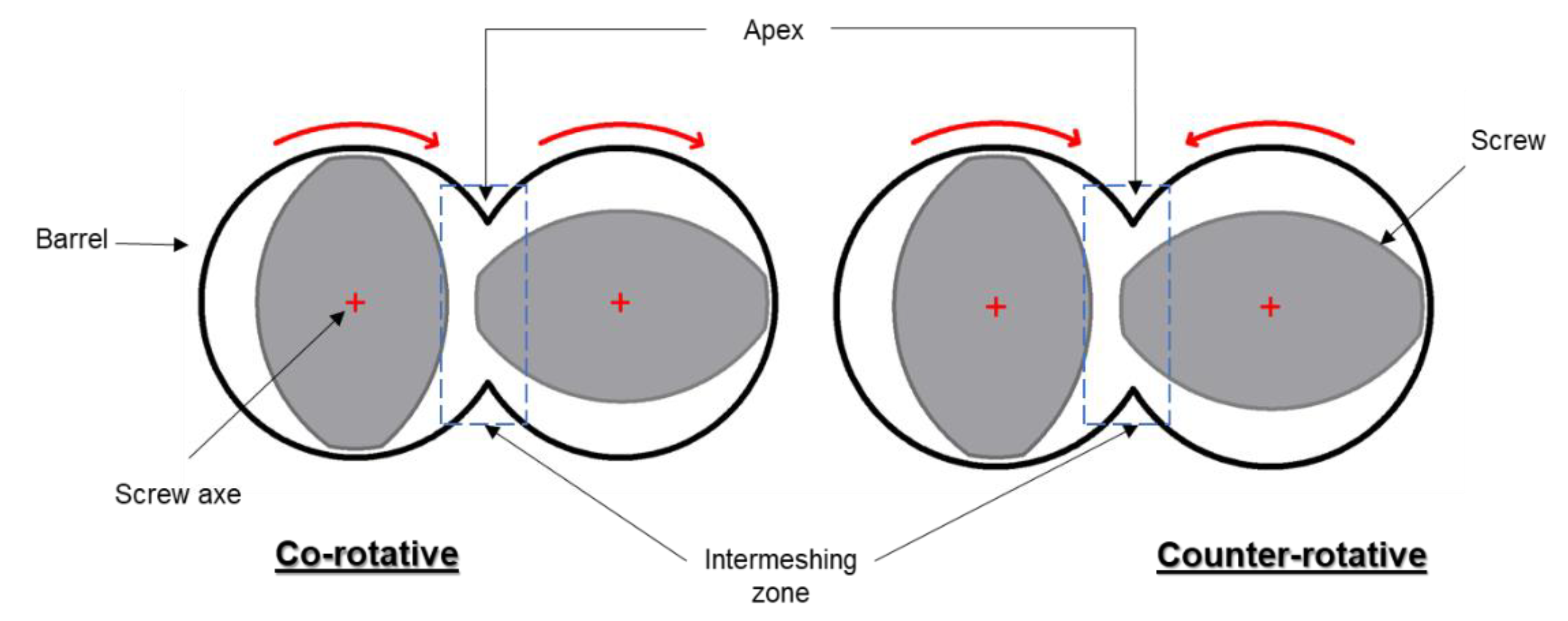
3.1. Screw Type
3.2. Screw Configuration
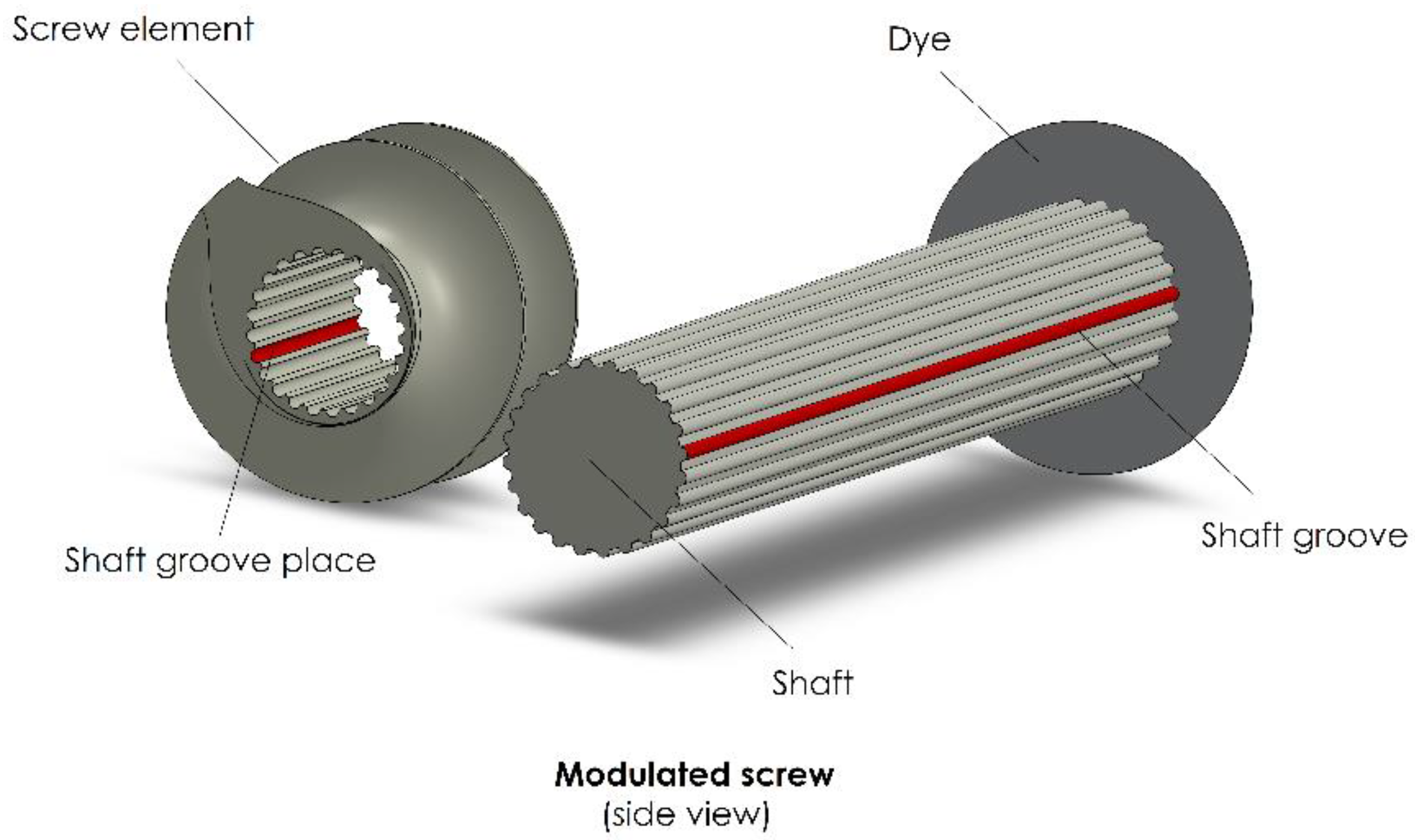
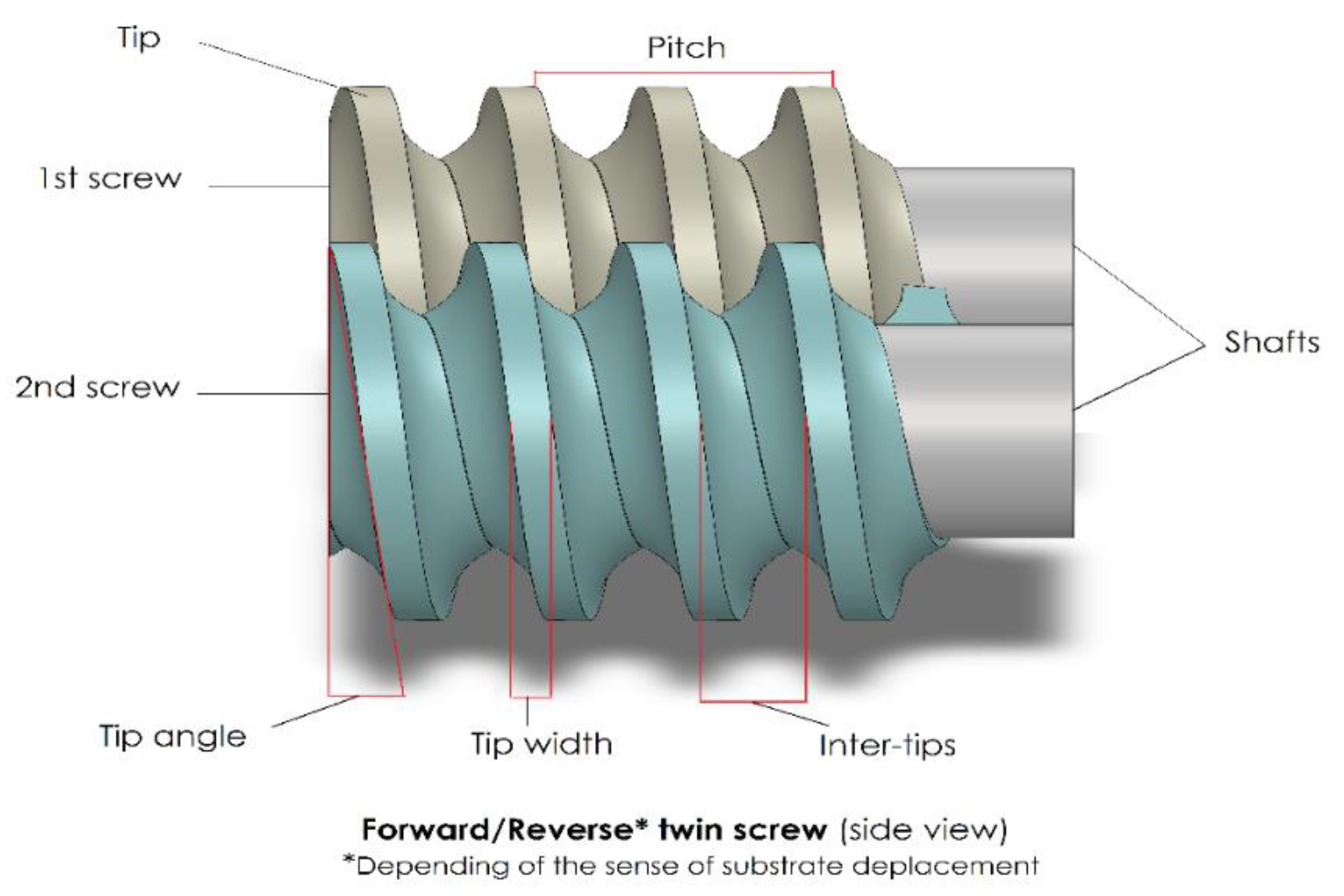
3.3. Screw Elements
3.3.1. Forward Screw Element
3.3.2. Reverse Screw Element
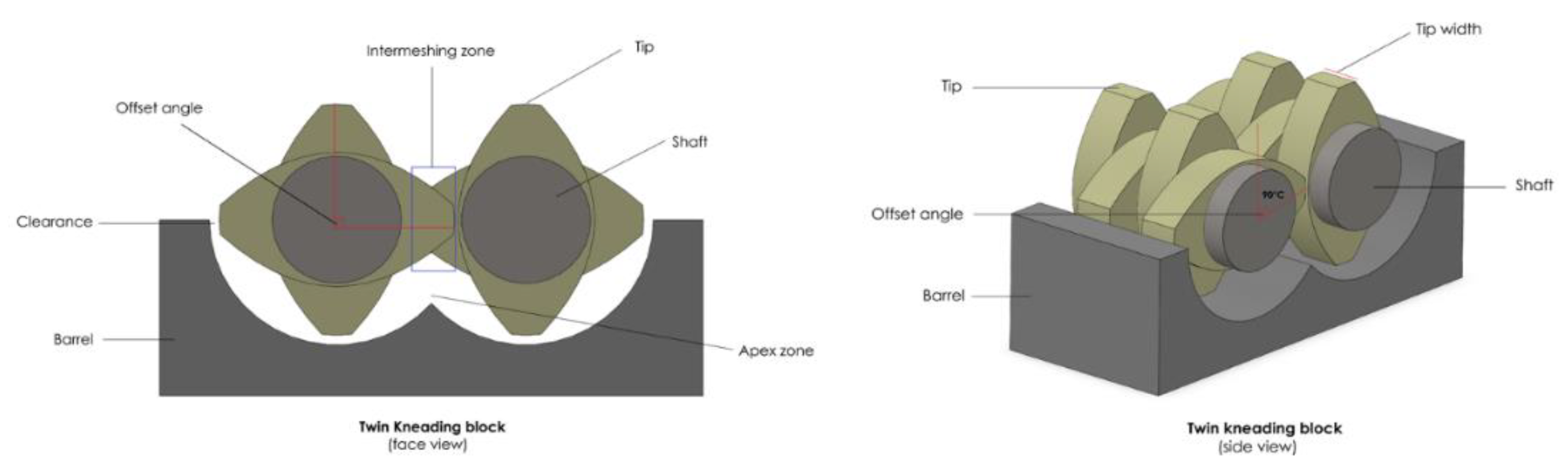
3.3.3. Kneading Element
3.4. Die Shape
3.5. Torque
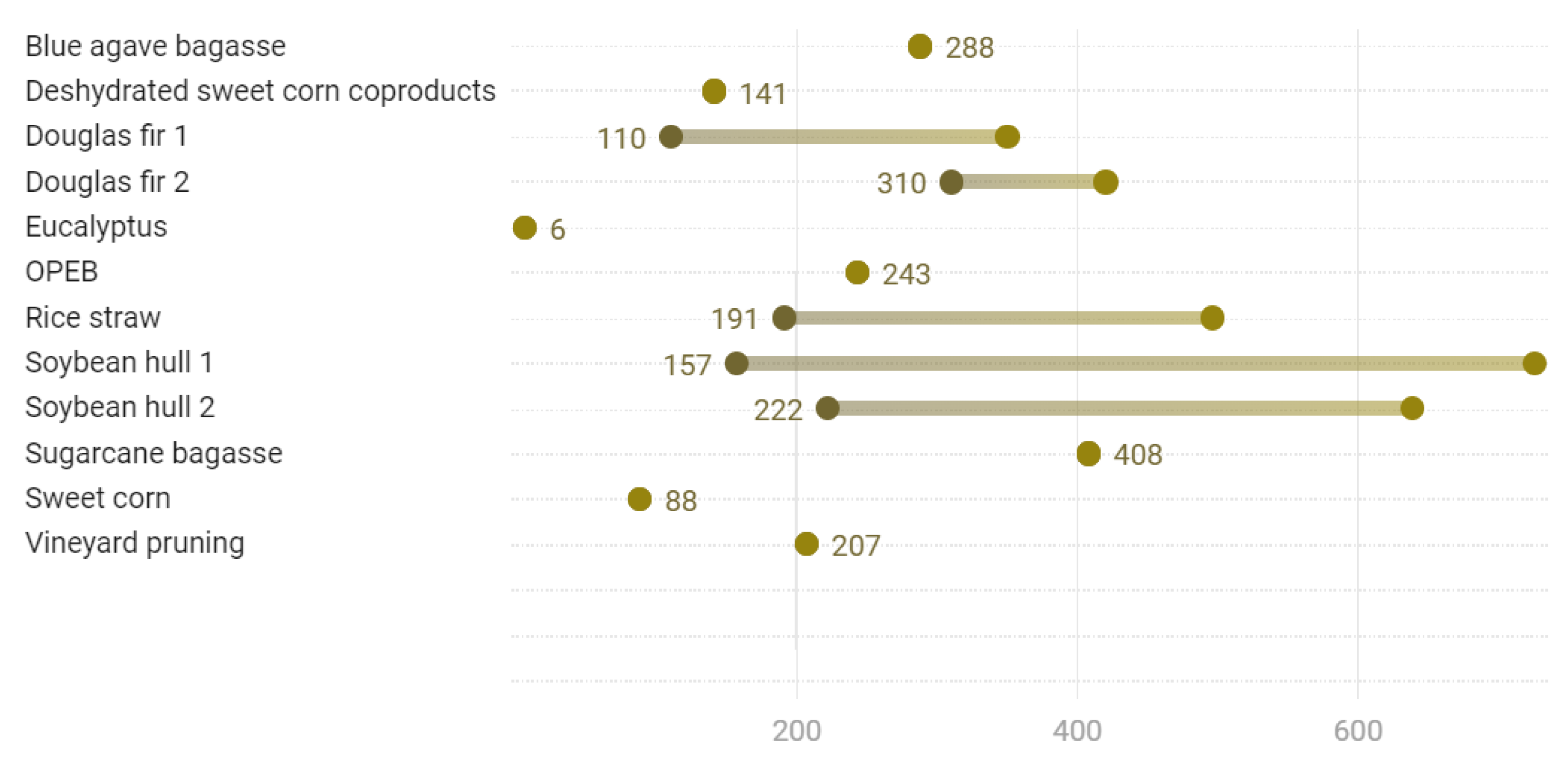
3.6. Specific Mechanical Energy
- Avoid the use of a thermal source during biomass preparation, instead privilege room temperature or solar heat.
- Use kneading screw elements and reverse screw elements sparingly in the screw configuration. As highlighted in Section 3.2, these two elements enhance the disruptive effect of screws on biomass, but at the same time, they increase the energy consumption [66,80]. The operator must find a compromise according to the objectives of their extrusion pretreatment.
- Opt for continuous extrusion to avoid unnecessary energy consumption and also because starting up the extruder is time-consuming and energy-intensive. Therefore, plan each extrusion well and prepare everything before starting.
- Make sure the moisture of the substrate is sufficient to ensure smooth transport of the substrate in the barrel, as dry matter content and extruder electricity consumption are strongly linked (R2 = 0.73) [99]. This practice also helps to avoid the overloading of the barrel and the jamming of the screws.
- Limit to the strict minimum the number of passes of the biomass in the extruder. This number may vary from one type of biomass to another. For this, preliminary tests are necessary. As highlighted in Section 5.2, several studies have shown that beyond a certain number of passes, there is no longer any significant improvement in the sugar recovery rate [44,69].
4. Additives
4.1. Addition before Extrusion
4.2. Addition during Extrusion
5. Working Parameters
5.1. Temperature
5.2. Residence Time
5.3. Screw Speed
6. Challenges, Limitations, and Future Prospects
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Substrate | Value or Range (Wh/kg) | References |
|---|---|---|
| Blue agave bagasse | 288 | [84] |
| Deshydrated sweet corn coproducts | 141 | [84] |
| Douglas fir 1 | 110–350 | [78] |
| Douglas fir 2 | 310–420 | [47] |
| Eucalyptus | 6 | [84] |
| OPEB | 243 | [84] |
| Rice straw | 191–496 | [92] |
| Soybean hull 1 | 157–726 | [68] |
| Soybean hull 2 | 222–639 | [79] |
| Sugarcane bagasse | 408 | [84] |
| Sweet corn | 88 | [84] |
| Vineyard pruning | 207 | [84] |
Appendix B
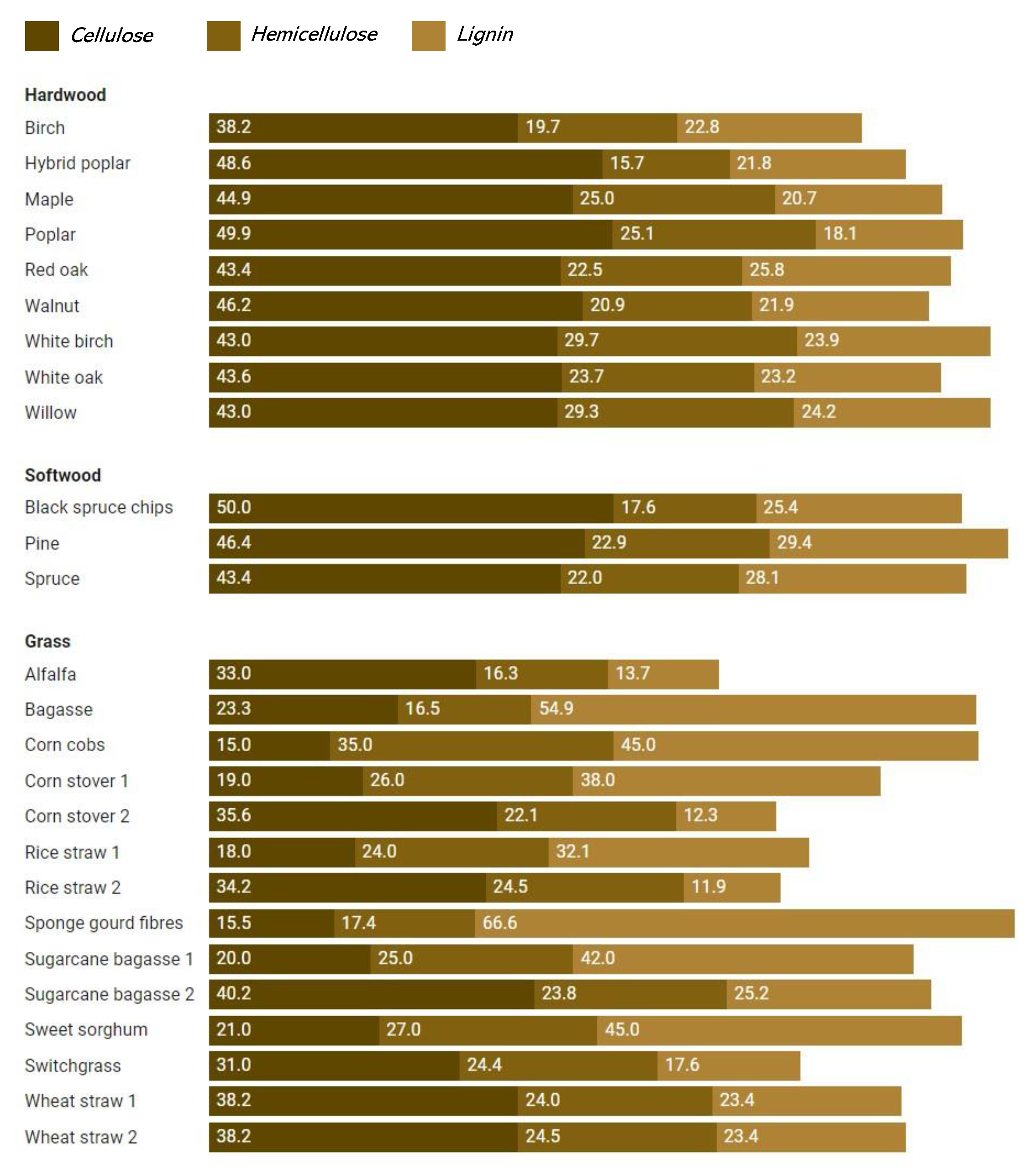
| N° | Substrate | Composition (%) | References | ||
|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | |||
| Hardwood | |||||
| 1. | Birch | 38.2 | 19.7 | 22.8 | [124] |
| 2. | Hybrid poplar | 48.6 | 15.7 | 21.8 | [42] |
| 3. | Maple | 44.9 | 25 | 20.7 | [42] |
| 4. | Poplar | 49.9 | 25.1 | 18.1 | [42] |
| 5. | Red oak | 43.4 | 22.5 | 25.8 | [42] |
| 6. | Walnut | 46.2 | 20.9 | 21.9 | [42] |
| 7. | White birch | 43 | 29.7 | 23.9 | [39] |
| 8. | White oak | 43.6 | 23.7 | 23.2 | [42] |
| 9. | Willow | 43 | 29.3 | 24.2 | [124] |
| Softwood | |||||
| 1. | Black spruce chips | 50 | 17.6 | 25.4 | [39] |
| 2. | Pine | 46.4 | 22.9 | 29.4 | [124] |
| 3. | Spruce | 43.4 | 22 | 28.1 | [124] |
| Grasses | |||||
| 1. | Alfalfa | 33 | 16.3 | 13.7 | [39] |
| 2. | Bagasse | 23.33 | 16.52 | 54.87 | [50] |
| 3. | Corn cobs | 15 | 35 | 45 | [125] |
| 4. | Corn stover 1 | 19 | 26 | 38 | [126] |
| 5. | Corn stover 2 | 35.6 | 22.1 | 12.3 | [124] |
| 6. | Rice straw 1 | 18 | 24 | 32.1 | [125] |
| 7. | Rice straw 2 | 34.2 | 24.5 | 11.9 | [124] |
| 8. | Sponge gourd fibres | 15.46 | 17.44 | 66.59 | [50] |
| 9. | Sugarcane bagasse 1 | 20 | 25 | 42 | [127] |
| 10. | Sugarcane bagasse 2 | 40.2 | 23.8 | 25.2 | [42] |
| 11. | Sweet sorghum | 21 | 27 | 45 | [127] |
| 12. | Switchgrass | 31.0 | 24.4 | 17.6 | [42] |
| 13. | Wheat straw 1 | 38.2 | 24 | 23.4 | [124] |
| 14. | Wheat straw 2 | 38.2 | 24.5 | 23.4 | [42] |
References
- Gupta, R.; Sharma, M.; Jangir, V.; Gautam, A.K.; Chandra, A.; Arya, R.K. A theoretical and mathematical study on the importance of petroleum derivatives in the production of sanitizer based products. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Al-Samhan, M.; Al-Fadhli, J.; Al-Otaibi, A.M.; Al-Attar, F.; Bouresli, R.; Rana, M.S. Prospects of refinery switching from conventional to integrated: An opportunity for sustainable investment in the petrochemical industry. Fuel 2022, 310, 122161. [Google Scholar] [CrossRef]
- Braga, A.; Faria, N. Biotechnological production of specialty aromatic and aromatic-derivative compounds. World J. Microbiol. Biotechnol. 2022, 38, 80. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Paulose, T.A.P.; Philip, E.; Thomas, D.; Madhavan, A.; Sirohi, R.; Binod, P.; Kumar Awasthi, M.; Pandey, A.; Sindhu, R. Updates on high value products from cellulosic biorefinery. Fuel 2022, 308, 122056. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, H.; Zhang, M.; Zhang, R.; Li, H.; Xie, H.; Zhang, X.; Ma, M.; et al. Recent advances in cellulose and its derivatives for oilfield applications. Carbohydr. Polym. 2021, 259, 117740. [Google Scholar] [CrossRef]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Nanocellulose and its derivative materials for energy and environmental applications. J. Mater. Sci. 2022, 57, 6835–6880. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nizetic, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.F.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere 2021, 281, 130878. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, D.; Singh, S.; Cheng, G. Recent developments in ionic liquid pretreatment of lignocellulosic biomass for enhanced bioconversion. Sustain. Energy Fuels 2021, 5, 1655–1667. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.; Peng, J.; Song, X.; Liu, Y.; Su, Z.; Li, B.; Gao, C.; Tian, W. Comprehensive analysis of important parameters of choline chloride-based deep eutectic solvent pretreatment of lignocellulosic biomass. Bioresour. Technol. 2021, 319, 124209. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment of lignocellulosic materials for efficient bioethanol production. Adv. Biochem. Eng. Biotechnol. 2007, 108, 41–65. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, N.; García-Bernet, D.; Domínguez, J.M. Extrusion and enzymatic hydrolysis as pretreatments on corn cob for biogas production. Renew. Energy 2017, 107, 597–603. [Google Scholar] [CrossRef]
- Gatt, E.; Rigal, L.; Vandenbossche, V. Biomass pretreatment with reactive extrusion using enzymes: A review. Ind. Crops Prod. 2018, 122, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Keshwani, D.R.; Xu, Y.; Hanna, M.A. Alkali combined extrusion pretreatment of corn stover to enhance enzyme saccharification. Ind. Crops Prod. 2012, 37, 352–357. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Hernández-Meléndez, O.; Hernández-Luna, M.G.; Manero, O.; Bárzana, E.; Vivaldo-Lima, E. An experimental and modeling study on the pretreatment and alkaline hydrolysis of blue agave bagasse in twin-screw extruders. Ind. Eng. Chem. Res. 2021, 60, 12449–12460. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, W.; Roslan, I.I.; Jaenicke, S.; Chuah, G.-K. A combo Zr-HY and Al-HY zeolite catalysts for the one-pot cascade transformation of biomass-derived furfural to γ-valerolactone. J. Catal. 2019, 375, 56–67. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Z.; Zhang, D.; Wang, Z.; Cong, W. Lipid extraction from nannochloropsis oceanica biomass after extrusion pretreatment with twin-screw extruder: Optimization of processing parameters and comparison of lipid quality. Bioprocess Biosyst. Eng. 2020, 43, 655–662. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Iamtham, S. Physical extraction and extrusion entrapment of C-phycocyanin from Arthrospira platensis. J. King Saud Univ.—Sci. 2019, 31, 1535–1542. [Google Scholar] [CrossRef]
- Sarker, T.R.; Azargohar, R.; Stobbs, J.; Karunakaran, C.; Meda, V.; Dalai, A.K. Complementary effects of torrefaction and pelletization for the production of fuel pellets from agricultural residues: A comparative study. Ind. Crops Prod. 2022, 181, 114740. [Google Scholar] [CrossRef]
- Ahiduzzaman, M.; Islam, A.S. Development of biomass stove for heating up die barrel of rice husk briquette machine. Procedia Eng. 2013, 56, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Rowell, R.M. Challenges in biomass–Thermoplastic composites. J. Polym. Environ. 2007, 15, 229–235. [Google Scholar] [CrossRef]
- Guiao, K.S.; Tzoganakis, C.; Mekonnen, T.H. Green mechano-chemical processing of lignocellulosic biomass for lignin recovery. Chemosphere 2022, 293, 133647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, S.; Wu, R.; Fu, Y.; Qin, M.; Willför, S.; Xu, C. Green fractionation approaches for isolation of biopolymers and the critical technical challenges. Ind. Crops Prod. 2022, 177, 114451. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wagberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, V. Bioethanol Production From Corn. In Corn; AACC International Press: Washington, DC, USA, 2019; pp. 615–631. [Google Scholar]
- Niu, N.; Feng, L.; Lin, Y.; Li, X.; Zhang, D.; Yao, S. The sources of dechlorane plus (DP) in surface sediment from Bohai sea and the northern part of the yellow sea, China: Evidence from the fractional abundance of anti-DP (fanti) combined with lignin biomarker. Reg. Stud. Mar. Sci. 2020, 39, 101437. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Erdocia, X.; Sánchez, C.; González Alriols, M.; Labidi, J. Lignin depolymerization for phenolic monomers production by sustainable processes. J. Energy Chem. 2017, 26, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Ahvazi, B.; Wojciechowicz, O.; Ton-That, T.M.; Hawari, J. Preparation of lignopolyols from wheat straw soda lignin. J. Agric. Food Chem. 2011, 59, 10505–10516. [Google Scholar] [CrossRef] [Green Version]
- Garlapati, V.K.; Chandel, A.K.; Kumar, S.P.J.; Sharma, S.; Sevda, S.; Ingle, A.P.; Pant, D. Circular economy aspects of lignin: Towards a lignocellulose biorefinery. Renew. Sustain. Energy Rev. 2020, 130, 109977. [Google Scholar] [CrossRef]
- Yağcı, S.; Sutay Kocabaş, D.; Çalışkan, R.; Özbek, H.N. Statistical investigation of the bioprocess conditions of alkali combined twin-screw extrusion pretreatment to enhance fractionation and enzymatic hydrolysis of bulgur bran. J. Sci. Food Agric. 2022. early view. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; González, A.; Ballesteros, M. Study of the application of alkaline extrusion to the pretreatment of eucalyptus biomass as first step in a bioethanol production process. Energies 2018, 11, 2961. [Google Scholar] [CrossRef] [Green Version]
- Doménech, P.; Duque, A.; Higueras, I.; Iglesias, R.; Manzanares, P. Biorefinery of the olive tree—Production of sugars from enzymatic hydrolysis of olive stone pretreated by alkaline extrusion. Energies 2020, 13, 4517. [Google Scholar] [CrossRef]
- Duque, A.; Doménech, P.; Álvarez, C.; Ballesteros, M.; Manzanares, P. Study of the bioprocess conditions to produce bioethanol from barley straw pretreated by combined soda and enzyme-catalyzed extrusion. Renew. Energy 2020, 158, 263–270. [Google Scholar] [CrossRef]
- Wang, Z.; He, X.; Yan, L.; Wang, J.; Hu, X.; Sun, Q.; Zhang, H. Enhancing enzymatic hydrolysis of corn stover by twin-screw extrusion pretreatment. Ind. Crops Prod. 2020, 143, 111960. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. A green approach based on reactive extrusion to produce nanofibrillated cellulose from oat hull. Waste Biomass Valoriz. 2021, 12, 1051–1060. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Duque, A.; Saéz, F.; Manzanares, P.; Garcia-Cruz, C.H.; Ballesteros, M. Sugar production from wheat straw biomass by alkaline extrusion and enzymatic hydrolysis. Renew. Energy 2016, 86, 1060–1068. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Adjallé, K.; Lai, T.T.; Barnabé, S.; Perrier, M.; Paris, J. Effect of mechanical pretreatment for enzymatic hydrolysis of woody residues, corn stover and alfalfa. Waste Biomass Valorization 2019, 11, 5847–5856. [Google Scholar] [CrossRef]
- Lee, S.H.; Teramoto, Y.; Endo, T. Enzymatic saccharification of woody biomass micro/nanofibrillated by continuous extrusion process I--effect of additives with cellulose affinity. Bioresour. Technol. 2009, 100, 275–279. [Google Scholar] [CrossRef]
- Ansell, M.P.; Mwaikambo, L.Y. The structure of cotton and other plant fibres. In Handbook of Textile Fibre Structure; Woodhead Publishing: Sawston, UK, 2009; pp. 62–94. [Google Scholar]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Bioeng. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Ghaemi, F.; Abdullah, L.C.; Ariffin, H. Lignocellulose structure and the effect on nanocellulose production. In Lignocellulose for Future Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–30. [Google Scholar]
- Moro, M.K.; Teixeira, R.S.S.; da Silva, A.S.; Fujimoto, M.D.; Melo, P.A.; Secchi, A.R.; da Silva Bon, E.P. Continuous pretreatment of sugarcane biomass using a twin-screw extruder. Ind. Crops Prod. 2017, 97, 509–517. [Google Scholar] [CrossRef]
- Vandenbossche, V.; Brault, J.; Vilarem, G.; Hernández-Meléndez, O.; Vivaldo-Lima, E.; Hernández-Luna, M.; Barzana, E.; Duque, A.; Manzanares, P.; Ballesteros, M.; et al. A new lignocellulosic biomass deconstruction process combining thermo-mechano chemical action and bio-catalytic enzymatic hydrolysis in a twin-screw extruder. Ind. Crops Prod. 2014, 55, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Marone, A.; Trably, E.; Carrère, H.; Prompsy, P.; Guillon, F.; Joseph-Aimé, M.; Barakat, A.; Fayoud, N.; Bernet, N.; Escudié, R. Enhancement of corn stover conversion to carboxylates by extrusion and biotic triggers in solid-state fermentation. Appl. Microbiol. Biotechnol. 2018, 103, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Gu, B.-J.; Wang, J.; Gao, J.; Ganjyal, G.M.; Wolcott, M.P. Novel micronized woody biomass process for production of cost-effective clean fermentable sugars. Bioresour. Technol. 2018, 260, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Dhumal, G.S.; Wolcott, M.P.; Ganjyal, G.M. Disruption of lignocellulosic biomass along the length of the screws with different screw elements in a twin-screw extruder. Bioresour. Technol. 2019, 275, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, Y.; Hanna, M.A. Pretreatment of corn stover with twin-screw extrusion followed by enzymatic saccharification. Appl. Biochem. Biotechnol. 2012, 166, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.L.; Frollini, E.; da Silva, C.G.; Wypych, F.; Satyanarayana, K.G. Characterization of banana, sugarcane bagasse and sponge gourd fibers of Brazil. Ind. Crops Prod. 2009, 30, 407–415. [Google Scholar] [CrossRef]
- Zheng, J.; Choo, K.; Rehmann, L. The effects of screw elements on enzymatic digestibility of corncobs after pretreatment in a twin-screw extruder. Biomass Bioenergy 2015, 74, 224–232. [Google Scholar] [CrossRef]
- Kang, K.E.; Han, M.; Moon, S.-K.; Kang, H.-W.; Kim, Y.; Cha, Y.-L.; Choi, G.-W. Optimization of alkali-extrusion pretreatment with twin-screw for bioethanol production from miscanthus. Fuel 2013, 109, 520–526. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Shen, Y.; Wang, L.; Zhang, H.; Qian, H.; Qi, X. Extrusion followed by ultrasound as a chemical-free pretreatment method to enhance enzymatic hydrolysis of rice hull for fermentable sugars production. Ind. Crops Prod. 2020, 149, 112356. [Google Scholar] [CrossRef]
- Rashed, G.I.; Chong, L.; Zhiyuan, S.; Hongzhen, F.; Chen, C.; Junjie, L.; Qiao, L.; Jianhui, D.; Huazheng, C.; Hongmei, W.; et al. Effect of a promising CSESE pretreatment on the morphological structure and properties of jute fibers. E3S Web Conf. 2021, 252, 02049. [Google Scholar] [CrossRef]
- Senturk-Ozer, S.; Gevgilili, H.; Kalyon, D.M. Biomass pretreatment strategies via control of rheological behavior of biomass suspensions and reactive twin screw extrusion processing. Bioresour. Technol. 2011, 102, 9068–9075. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.A.; Gaugler, M.; Smith, D.A. Green route to modification of wood waste, cellulose and hemicellulose using reactive extrusion. Carbohydr. Polym. 2016, 136, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. Nanofibrillated cellulose obtained from soybean hull using simple and eco-friendly processes based on reactive extrusion. Cellulose 2019, 27, 1975–1988. [Google Scholar] [CrossRef]
- Byun, J.; Cha, Y.-L.; Park, S.-M.; Kim, K.-S.; Lee, J.-E.; Kang, Y.-G. Lignocellulose pretreatment combining continuous alkaline single-screw extrusion and ultrasonication to enhance biosugar production. Energies 2020, 13, 5636. [Google Scholar] [CrossRef]
- Han, S.-Y.; Park, C.-W.; Endo, T.; Febrianto, F.; Kim, N.-H.; Lee, S.-H. Extrusion process to enhance the pretreatment effect of ionic liquid for improving enzymatic hydrolysis of lignocellulosic biomass. Wood Sci. Technol. 2020, 54, 599–613. [Google Scholar] [CrossRef]
- Karunanithy, C.; Muthukumarappan, K. Thermo-mechanical pretreatment of feedstocks. In Green Biomass Pretreatment for Biofuels Production; Gu, T., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 31–65. [Google Scholar]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Negro, M.J.; Oliva, J.M.; Saez, F.; Ballesteros, M. Optimization of integrated alkaline–extrusion pretreatment of barley straw for sugar production by enzymatic hydrolysis. Process Biochem. 2013, 48, 775–781. [Google Scholar] [CrossRef]
- Liu, C.; Van Der Heide, E.; Wang, H.; Li, B.; Yu, G.; Mu, X. Alkaline twin-screw extrusion pretreatment for fermentable sugar production. Biotechnol. Biofuels 2013, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Karunanithy, C.; Muthukumarappan, K.; Gibbons, W.R. Sequential extrusion-microwave pretreatment of switchgrass and big bluestem. Bioresour. Technol. 2014, 153, 393–398. [Google Scholar] [CrossRef]
- Karunanithy, C.; Muthukumarappan, K. Influence of extruder and feedstock variables on torque requirement during pretreatment of different types of biomass—A response surface analysis. Biosyst. Eng. 2011, 109, 37–51. [Google Scholar] [CrossRef]
- Cha, Y.-L.; Yang, J.; Seo, S.-i.; An, G.H.; Moon, Y.-H.; You, G.-D.; Lee, J.-E.; Ahn, J.-W.; Lee, K.-B. Alkaline twin-screw extrusion pretreatment of Miscanthus with recycled black liquor at the pilot scale. Fuel 2016, 164, 322–328. [Google Scholar] [CrossRef]
- Negro, M.J.; Duque, A.; Manzanares, P.; Sáez, F.; Oliva, J.M.; Ballesteros, I.; Ballesteros, M. Alkaline twin-screw extrusion fractionation of olive-tree pruning biomass. Ind. Crops Prod. 2015, 74, 336–341. [Google Scholar] [CrossRef]
- Choi, C.H.; Oh, K.K. Application of a continuous twin screw-driven process for dilute acid pretreatment of rape straw. Bioresour. Technol. 2012, 110, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Alavi, S.; Vadlani, P.; Amanor-Boadu, V. Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to fermentable sugars. Bioresour. Technol. 2011, 102, 7583–7590. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.S.A.; Teixeira, R.S.S.; Endo, T.; Bon, E.P.S.; Lee, S.-H. Continuous pretreatment of sugarcane bagasse at high loading in an ionic liquid using a twin-screw extruder. Green Chem. 2013, 15, 1991. [Google Scholar] [CrossRef]
- Shah, A.; Gupta, M. Comparision of the flow in co-rotating and counter-rotating twinscrew extruders. ANTEC 2004, 5, 443–447. [Google Scholar]
- Cantine, O. PEO Hot Melt Extrudates for Controlled Drug Delivery. Ph.D. Thesis, Universite Lille, Lille Cedex, France, 2017. [Google Scholar]
- Solano, D.; Vinyes, P.; Arranz, P. The Biomass Briquetting Process: A Guideline Report; UNDP-CEDRO Publication: Beirut, Lebanon, 2016. [Google Scholar]
- Zhang, X.; Zhang, W.; Su, J.; Wang, M.; Lu, C. Preparation, characterization, and properties of polyethylene composites highly filled with calcium carbonate through co-rotating conical twin-screw extrusion. J. Vinyl Addit. Technol. 2014, 20, 108–115. [Google Scholar] [CrossRef]
- Leng, J.; Wu, J.; Chen, N.; Xu, X.; Zhang, J. The development of a conical screw-based extrusion deposition system and its application in fused deposition modeling with thermoplastic polyurethane. Rapid Prototyp. J. 2019, 26, 409–417. [Google Scholar] [CrossRef]
- Vera-Sorroche, J.; Kelly, A.L.; Brown, E.C.; Gough, T.; Abeykoon, C.; Coates, P.D.; Deng, J.; Li, K.; Harkin-Jones, E.; Price, M. The effect of melt viscosity on thermal efficiency for single screw extrusion of HDPE. Chem. Eng. Res. Des. 2014, 92, 2404–2412. [Google Scholar] [CrossRef] [Green Version]
- Kelly, A.L.; Brown, E.C.; Coates, P.D. The effect of screw geometry on melt temperature profile in single screw extrusion. Polym. Eng. Sci. 2006, 46, 1706–1714. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Negro, M.J.; Oliva, J.M.; Gonzalez, A.; Ballesteros, M. Sugar production from barley straw biomass pretreated by combined alkali and enzymatic extrusion. Bioresour. Technol. 2014, 158, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Choo, K.; Rehmann, L. Xylose removal from lignocellulosic biomass via a twin-screw extruder: The effects of screw configurations and operating conditions. Biomass Bioenergy 2016, 88, 10–16. [Google Scholar] [CrossRef]
- Lamsal, B.; Yoo, J.; Brijwani, K.; Alavi, S. Extrusion as a thermo-mechanical pre-treatment for lignocellulosic ethanol. Biomass Bioenergy 2010, 34, 1703–1710. [Google Scholar] [CrossRef]
- Wahid, R.; Hjorth, M.; Kristensen, S.; Møller, H.B. Extrusion as pretreatment for boosting methane production: Effect of screw configurations. Energy Fuels 2015, 29, 4030–4037. [Google Scholar] [CrossRef]
- Djuric, D.; Kleinebudde, P. Impact of screw elements on continuous granulation with a twin-screw extruder. J. Pharm. Sci. 2008, 97, 4934–4942. [Google Scholar] [CrossRef]
- Fülöp, G.; Domokos, A.; Galata, D.; Szabó, E.; Gyürkés, M.; Szabó, B.; Farkas, A.; Madarász, L.; Démuth, B.; Lendér, T.; et al. Integrated twin-screw wet granulation, continuous vibrational fluid drying and milling: A fully continuous powder to granule line. Int. J. Pharm. 2021, 594, 120126. [Google Scholar] [CrossRef]
- Kohlgrüber, K.; Ullrich, M.; Hepperle, J.; Rudolf, R.; König, T.; Liesenfelder, U.; Bierdel, M.; Kirchhoff, J.; Lechner, F.; Sämann, H.-J.; et al. Co-Rotating Twin-Screw Extruders Fundamentals, Technology, and Applications; Hanser Publications: Munich, Germany, 2008. [Google Scholar] [CrossRef] [Green Version]
- Vandenbossche, V.; Brault, J.; Vilarem, G.; Rigal, L. Bio-catalytic action of twin-screw extruder enzymatic hydrolysis on the deconstruction of annual plant material: Case of sweet corn co-products. Ind. Crops Prod. 2015, 67, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, G.S.; Gautam, A. Screw configuration effects on macroscopic characteristics of extradates produced by twin-screw extrusion of rice flour. J. Food Sci. 1999, 64, 479–487. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, M. Extrusion as a pretreatment for lignocellulosic biomass: Fundamentals and applications. Renew. Energy 2017, 114, 1427–1441. [Google Scholar] [CrossRef]
- Abeykoon, C.; Kelly, A.L.; Brown, E.C.; Coates, P.D. The effect of materials, process settings and screw geometry on energy consumption and melt temperature in single screw extrusion. Appl. Energy 2016, 180, 880–894. [Google Scholar] [CrossRef] [Green Version]
- Patil, P.D.; Feng, J.J.; Hatzikiriakos, S.G. Constitutive modeling and flow simulation of polytetrafluoroethylene (PTFE) paste extrusion. J. Non-Newton. Fluid Mech. 2006, 139, 44–53. [Google Scholar] [CrossRef]
- Ai, B.; Li, W.; Woomer, J.; Li, M.; Pu, Y.; Sheng, Z.; Zheng, L.; Adedeji, A.; Ragauskas, A.J.; Shi, J. Natural deep eutectic solvent mediated extrusion for continuous high-solid pretreatment of lignocellulosic biomass. Green Chem. 2020, 22, 6372–6383. [Google Scholar] [CrossRef]
- Guha, M.; Ali, S.Z.; Bhattacharya, S. Twin-screw extrusion of rice flour without a die: Effect of barrel temperature and screw speed on extrusion and extrudate characteristics. J. Food Eng. 1997, 32, 251–267. [Google Scholar] [CrossRef]
- Akdogan, H. Pressure, torque, and energy responses of a twin screw extruder at high moisture contents. Food Res. Int. 1996, 29, 423–429. [Google Scholar] [CrossRef]
- Chen, W.H.; Xu, Y.Y.; Hwang, W.S.; Wang, J.B. Pretreatment of rice straw using an extrusion/extraction process at bench-scale for producing cellulosic ethanol. Bioresour. Technol. 2011, 102, 10451–10458. [Google Scholar] [CrossRef]
- Lei, H.; Fulcher, R.G.; Ruan, R.; van Lengerich, B. Empirical modeling of die pressure, shaft torque, sme, and product temperature of rice flour in a corotating twin-screw extruder. Cereal Chem. J. 2005, 82, 582–587. [Google Scholar] [CrossRef]
- Harmann, D.V.; Harper, J.M. Effect of extruder geometry on torque and flow. Trans. ASAE 1973, 16, 1175–1178. [Google Scholar] [CrossRef]
- Godavarti, S.; Karwe, M.V. Determination of specific mechanical energy distribution on a twin-screw extruder. J. Agric. Eng. Res. 1997, 67, 277–287. [Google Scholar] [CrossRef]
- Kazi, F.K.; Fortman, J.A.; Anex, R.P.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Yoo, J. Technical And Economical Assessment of Thermo-Mechanical Extrusion Pretreatment for Cellulosic Ethanol Production; Kansas State University: Manhattan, KS, USA, 2011. [Google Scholar]
- Zheng, J.; Rehmann, L. Extrusion pretreatment of lignocellulosic biomass: A review. Int. J. Mol. Sci. 2014, 15, 18967–18984. [Google Scholar] [CrossRef] [Green Version]
- Hjorth, M.; Gränitz, K.; Adamsen, A.P.S.; Møller, H.B. Extrusion as a pretreatment to increase biogas production. Bioresour. Technol. 2011, 102, 4989–4994. [Google Scholar] [CrossRef]
- Montiel, C.; Hernández-Meléndez, O.; Vivaldo-Lima, E.; Hernández-Luna, M.; Bárzana, E. Enhanced bioethanol production from blue agave bagasse in a combined extrusion–saccharification process. BioEnergy Res. 2016, 9, 1005–1014. [Google Scholar] [CrossRef]
- Beisl, S.; Biermair, F.; Friedl, A.; Mundigler, N.; Miltner, A. Sequential extrusion and organosolv pretreatment for wheat straw valorization. Chem. Eng. Trans. 2017, 61, 853–858. [Google Scholar]
- Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.A.; Mali, S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res. Int. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Appels, L.; Degreve, J.; Van der Bruggen, B.; Van Impe, J.; Dewil, R. Influence of low temperature thermal pre-treatment on sludge solubilisation, heavy metal release and anaerobic digestion. Bioresour. Technol. 2010, 101, 5743–5748. [Google Scholar] [CrossRef]
- Formela, K.; Zedler, L.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites—Current trends and future developments. Express Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Karunanithy, C.; Muthukumarappan, K.; Gibbons, W.R. Extrusion pretreatment of pine wood chips. Appl. Biochem. Biotechnol. 2012, 167, 81–99. [Google Scholar] [CrossRef]
- Formela, K.; Wołosiak, M.; Klein, M.; Wang, S. Characterization of volatile compounds, structural, thermal and physico-mechanical properties of cross-linked polyethylene foams degraded thermo-mechanically at variable times. Polym. Degrad. Stab. 2016, 134, 383–393. [Google Scholar] [CrossRef]
- Espert, A.; de las Heras, L.A.; Karlsson, S. Emission of possible odourous low molecular weight compounds in recycled biofibre/polypropylene composites monitored by head-space SPME-GC–MS. Polym. Degrad. Stab. 2005, 90, 555–562. [Google Scholar] [CrossRef]
- Seifert, S. Comparison of parallel and conical twin screw extruders from the processing point of view. Plast. Rubber Compos. 2013, 34, 134–142. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Narayan, R.; Dubois, P. Recent advances in reactive extrusion processing of biodegradable polymer-based compositions. Macromol. Mater. Eng. 2008, 293, 447–470. [Google Scholar] [CrossRef]
- Gogoi, B.K.; Oswalt, A.J.; Choudhury, G.S. Reverse screw element(s) and feed composition effects during twin-screw extrusion of rice flour and fish muscle blends. J. Food Sci. 1996, 61, 590–595. [Google Scholar] [CrossRef]
- da Silva, A.S.; Sobral Teixeira, R.S.; de Oliveira Moutta, R.; Santana, V.; de Barros, R.D.R.O.; Antonieta, M.; da Silva Bo, E.P. Sugarcane and woody biomass pretreatments for ethanol production. In Sustainable Degradation of Lignocellulosic Biomass—Techniques, Applications and Commercialization; Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Duque, A.; Álvarez, C.; Doménech, P.; Manzanares, P.; Moreno, A.D. Advanced bioethanol production: From novel raw materials to integrated biorefineries. Processes 2021, 9, 206. [Google Scholar] [CrossRef]
- Guo, Y.; Chung, C.I. Dependence of melt temperature on screw speed and size in extrusion. Polym. Eng. Sci. 1989, 29, 415–419. [Google Scholar] [CrossRef]
- Chung, C.I. Maximum pressure developed by solid conveying force in screw extruders. Polym. Eng. Sci. 1975, 15, 29–34. [Google Scholar] [CrossRef]
- Choi, W.-I.; Oh, K.-K.; Park, J.-Y.; Lee, J.-S. Continuous sodium hydroxide-catalyzed pretreatment of empty fruit bunches (EFB) by continuous twin-screw-driven reactor (CTSR). J. Chem. Technol. Biotechnol. 2014, 89, 290–296. [Google Scholar] [CrossRef]
- Endersen, P.G.; Lechner, F. Co-rotating fully intermeshing twin-screw compounding: Advancements for improved performance and productivity. Plast. Eng. 2012, 64, 32–38. [Google Scholar] [CrossRef]
- Karunanithy, C.; Muthukumarappan, K. Effect of extruder parameters and moisture content of switchgrass, prairie cord grass on sugar recovery from enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2010, 162, 1785–1803. [Google Scholar] [CrossRef]
- Heredia-Olea, E.; Pérez-Carrillo, E.; Serna-Saldívar, S.O. Effect of extrusion conditions and hydrolysis with fiber-degrading enzymes on the production of C5 and C6 sugars from brewers’ spent grain for bioethanol production. Biofuel Res. J. 2015, 2, 203–208. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.-F.; Zhang, C.-C.; Nan, J.; Ren, N.-Q.; Lee, D.-J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef]
- Brudecki, G.; Cybulska, I.; Rosentrater, K. Integration of extrusion and clean fractionation processes as a pre-treatment technology for prairie cordgrass. Bioresour. Technol. 2013, 135, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Shen, F.; Yang, G.; Deng, S.; Long, L.; He, J.; Zhang, J.; Huang, C.; Luo, L. Liquid hot water extraction followed by mechanical extrusion as a chemical-free pretreatment approach for cellulosic ethanol production from rigid hardwood. Fuel 2019, 252, 589–597. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Fan, X.-G.; Qiu, X.-L.; Zhang, Q.-X.; Wang, W.-Y.; Li, S.-X.; Deng, L.-H.; Koffas, M.A.G.; Wei, D.-S.; Yuan, Q.-P. A novel cleaning process for industrial production of xylose in pilot scale from corncob by using screw-steam-explosive extruder. Bioprocess Biosyst. Eng. 2014, 37, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Nastaj, A.; Wilczyński, K. Optimization and Scale-Up for Polymer Extrusion. Polymers 2021, 13, 1547. [Google Scholar] [CrossRef]
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod. Bioeng. 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, A.; Joshi, H.C. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycl. 2007, 50, 1–39. [Google Scholar] [CrossRef]
- Zhu, Y.; Lee, Y.Y.; Elander, R.T. Optimization of dilute-acid pretreatment of corn stover using a high-solids percolation reactor. Appl. Biochem. Biotechnol. 2005, 124, 1045–1054. [Google Scholar] [CrossRef]
- Kim, M.; Day, D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 2011, 38, 803–807. [Google Scholar] [CrossRef]


| Biomass | Compounds | Composition (%) | References | |
|---|---|---|---|---|
| Before Extrusion | After Extrusion | |||
| Bulgur bran | Glucose | 36.38 ± 0.32 | 30.86 ± 0.64 | [31] |
| Hemicellulose | 29.42 ± 0.13 | 33.18 ± 0.53 | ||
| Total lignin | 12.54 ± 0.14 | 16.24 ± 0.31 | ||
| Eucalyptus | Cellulose | 46.90 ± 1.21 | 44.90 ± 1.86 | [32] |
| Hemicellulose | 12.87 ± 0.35 | 13.71 ± 0.32 | ||
| Lignin | 31.15 ± 0.40 | 32.97 ± 0.86 | ||
| Ash | 0.86 ± 0.00 | 0.57 ± 0.05 | ||
| Olive stone | Cellulose | 20.8 ± 0.2 | 18.3 ± 2.8 | [33] |
| Hemicellulose | 25.9 ± 0.1 | 22.4 ± 0.4 | ||
| Lignin | 35.5 ± 0.6 | 39.0 ± 0.2 | ||
| Barley straw | Glucose | 32.9 | 32.80 | [34] |
| Hemicellulose | 26.1 | 15.53 | ||
| Lignin | 18.8 | 15.71 | ||
| Ash | 3.9 | 2.17 | ||
| Corn stover | Cellulose | 32.75 ± 0.32 | 33.98 ± 0.14 | [35] |
| Hemicellulose | 31.08 ± 0.57 | 30.20 ± 0.28 | ||
| Lignin | 10.07 ± 0.91 | 9.89 ± 0.43 | ||
| Oat hull | Cellulose | 31.16 ± 1.15 | 34.32 ± 2.06 | [36] |
| Hemicellulose | 28.72 ± 0.25 | 26.40 ± 0.53 | ||
| Lignin | 18.12 ± 0.63 | 15.00 ± 1.30 | ||
| Wheat straw | Cellulose | 37.8 ± 1.9 | 46.9 ± 0.1 | [37] |
| Hemicellulose | 28.2 ± 0.5 | 28.7 ± 0.1 | ||
| Lignin | 19.8 ± 0.3 | 15.4 ± 0.1 | ||
| Ash | 3.7 ± 0.0 | 3.3 ± 0.0 | ||
| Corn cob | Cellulose | 42.0 ± 0.15 | 34.8 ± 0.23 | [13] |
| Hemicellulose | 45.9 ± 0.90 | 38.9 ± 0.52 | ||
| Neutral detergent soluble | 9.3 ± 0.95 | 19.0 ± 0.60 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konan, D.; Koffi, E.; Ndao, A.; Peterson, E.C.; Rodrigue, D.; Adjallé, K. An Overview of Extrusion as a Pretreatment Method of Lignocellulosic Biomass. Energies 2022, 15, 3002. https://doi.org/10.3390/en15093002
Konan D, Koffi E, Ndao A, Peterson EC, Rodrigue D, Adjallé K. An Overview of Extrusion as a Pretreatment Method of Lignocellulosic Biomass. Energies. 2022; 15(9):3002. https://doi.org/10.3390/en15093002
Chicago/Turabian StyleKonan, Delon, Ekoun Koffi, Adama Ndao, Eric Charles Peterson, Denis Rodrigue, and Kokou Adjallé. 2022. "An Overview of Extrusion as a Pretreatment Method of Lignocellulosic Biomass" Energies 15, no. 9: 3002. https://doi.org/10.3390/en15093002
APA StyleKonan, D., Koffi, E., Ndao, A., Peterson, E. C., Rodrigue, D., & Adjallé, K. (2022). An Overview of Extrusion as a Pretreatment Method of Lignocellulosic Biomass. Energies, 15(9), 3002. https://doi.org/10.3390/en15093002