Dehydrogenation of Metal Hydride Reactor-Phase Change Materials Coupled with Light-Duty Fuel Cell Vehicles
Abstract
:1. Introduction
2. Mathematical Model
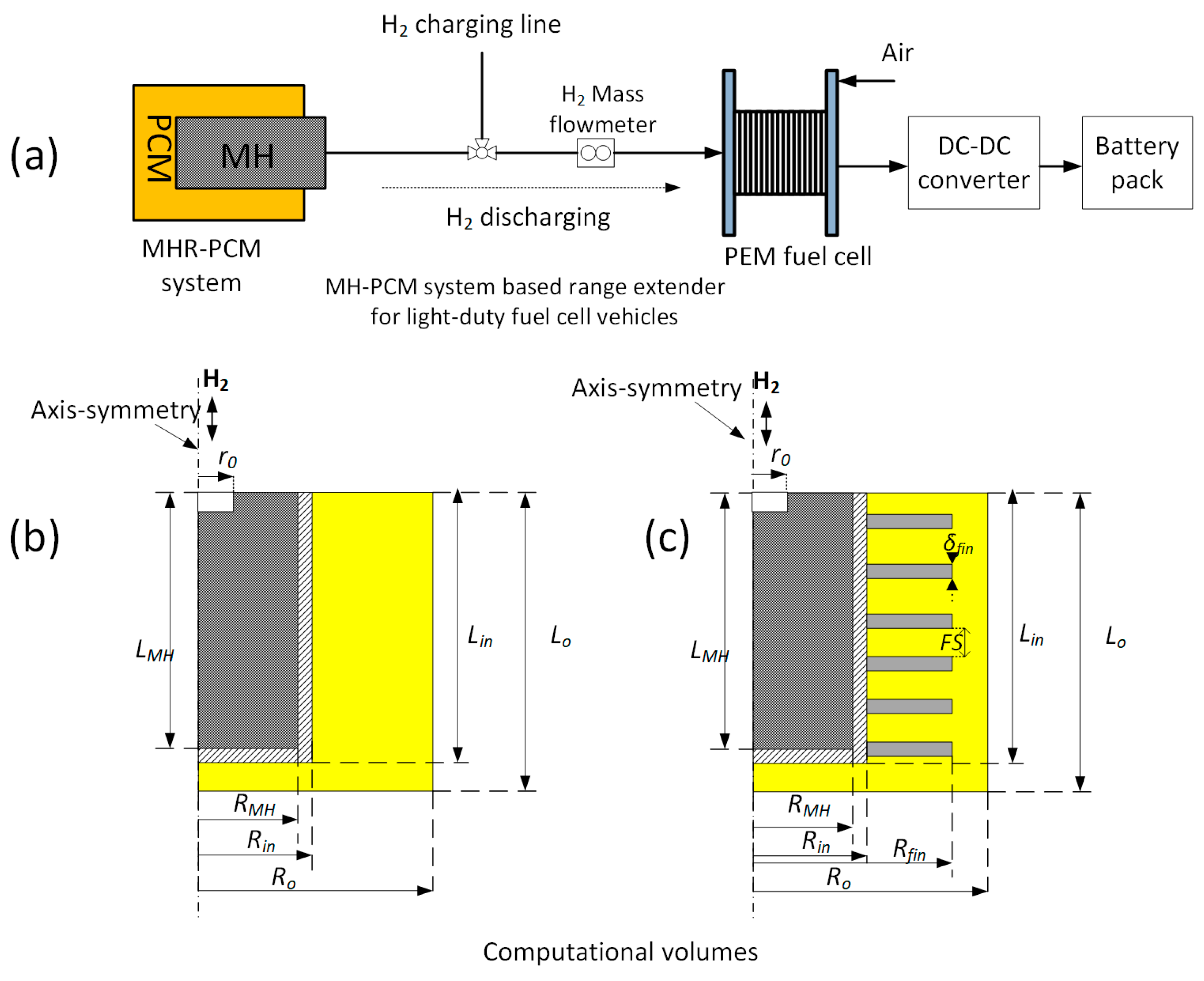
2.1. Problem Description
2.2. Governing Equations
- The thermophysical properties of hydride are independent of temperature and concentration.
- Hydrogen and metal hydrides are in thermal equilibrium.
- The phase change materials’ thermo-physical properties (density, solid-liquid specific heat, thermal conductivity) are uniform.
- The bed porosity is constant.
- The latent heat of phase change is temperature independent.
- The PCM jacket is perfectly insulated.
2.3. Numerical Settings and Validation
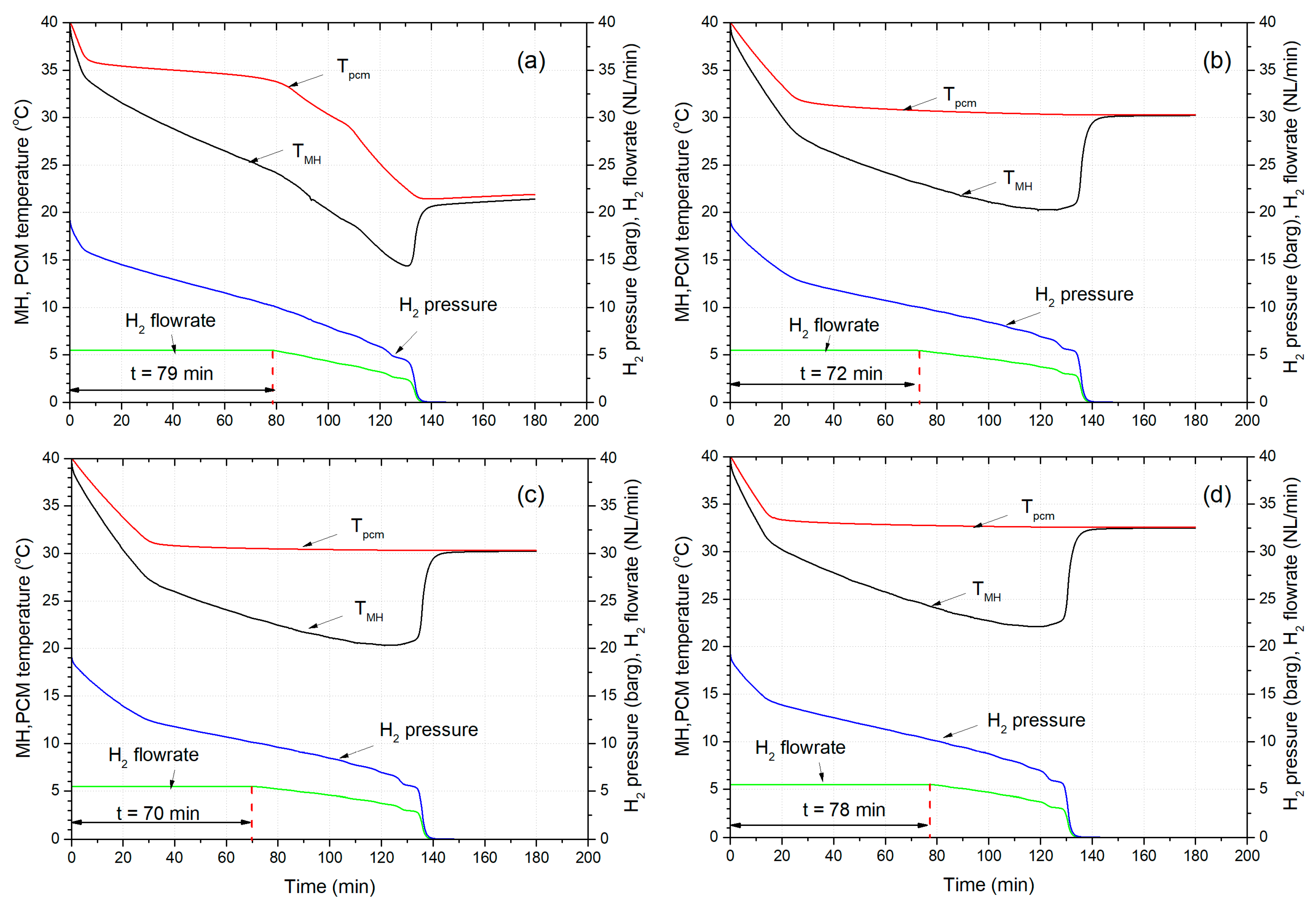
3. Results and Discussion
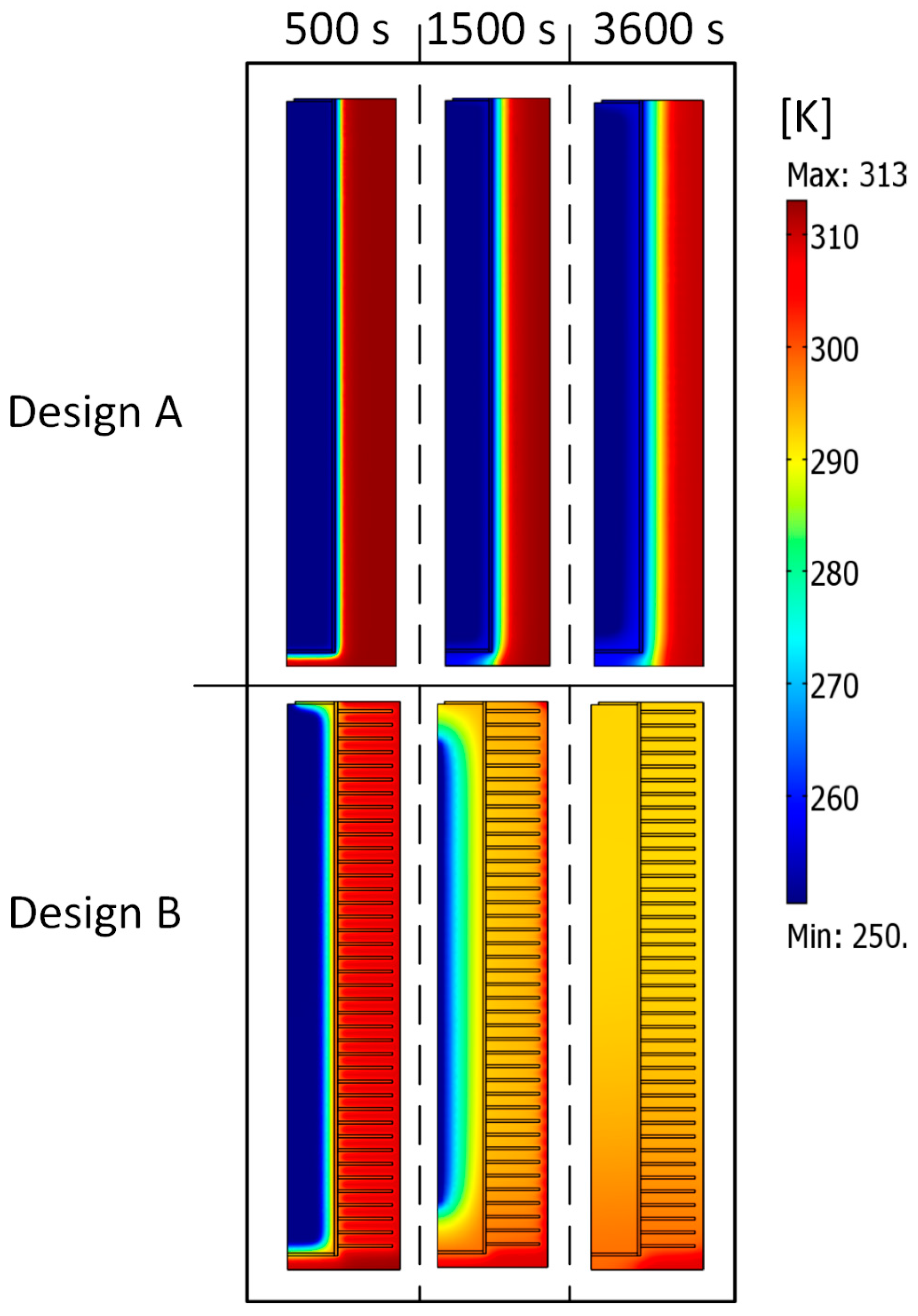
3.1. Designs Comparison
3.2. Dehydrogenation Performance under Constant Flowrate Demand (H2 Velocity)
3.3. The Effect of PCM’s Freezing Point on the Dehydrogenation Performance
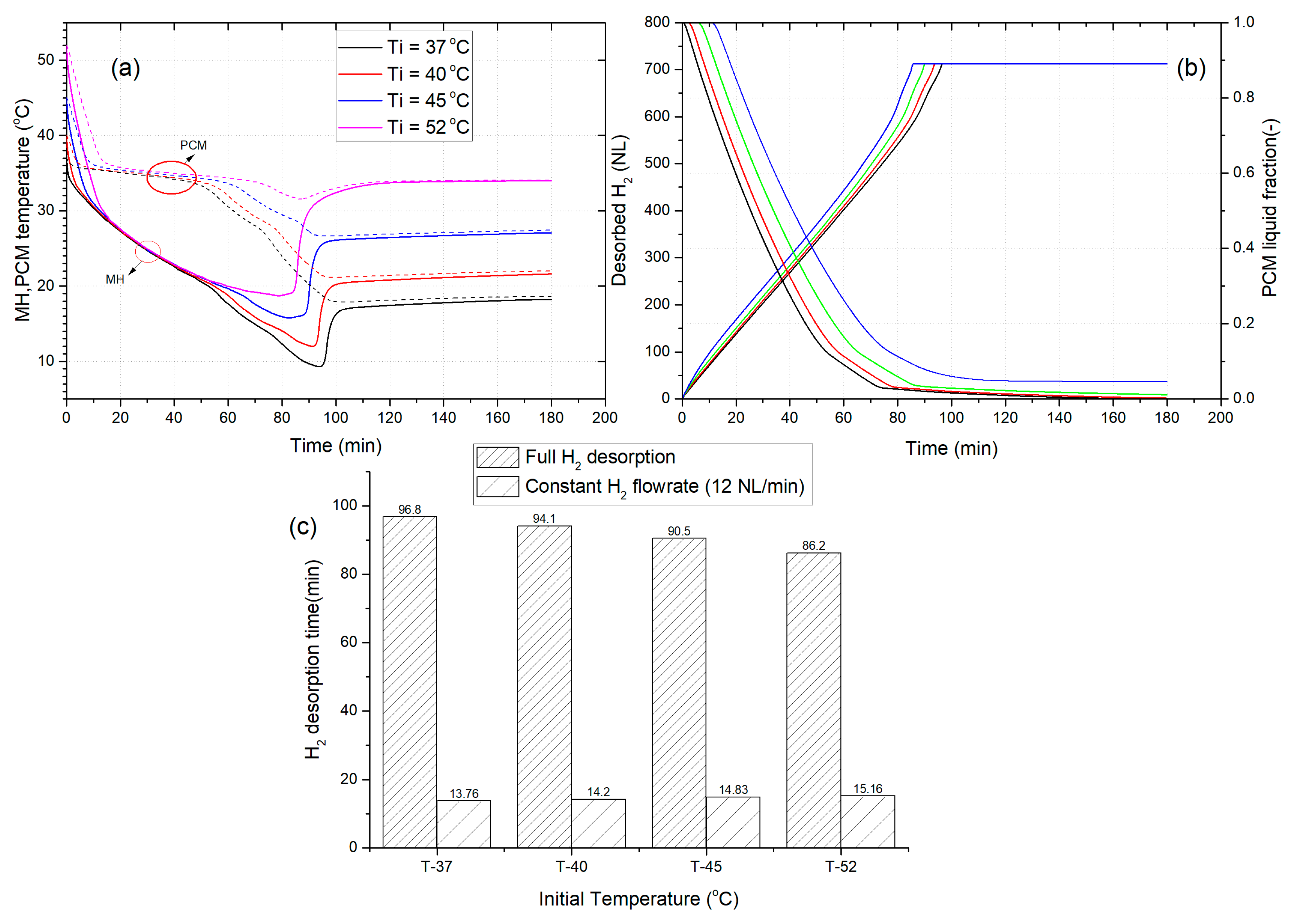
3.4. The Effect of Initial Temperature of MHR-PCM on the Dehydrogenation Performance
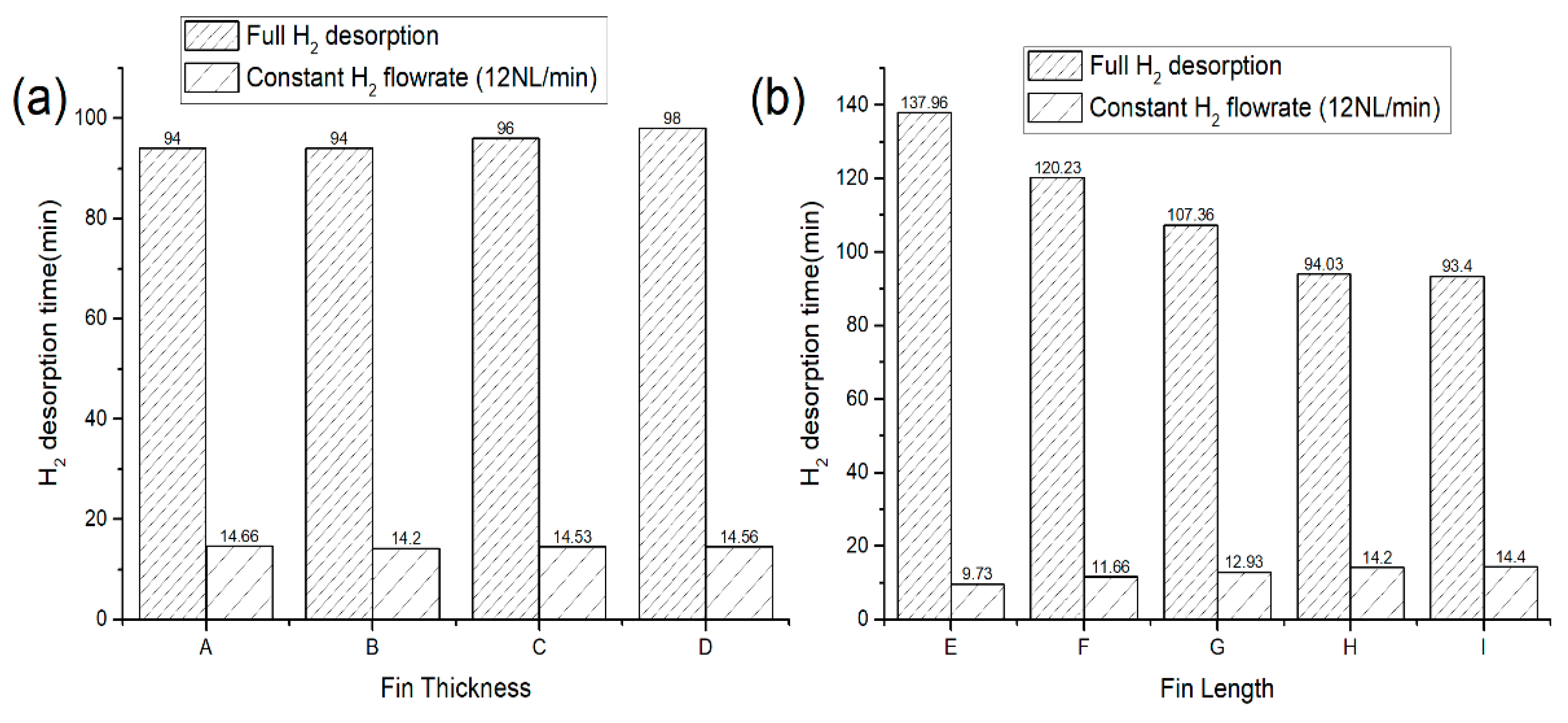
3.5. The Effects of Fin’s Geometrical Parameters on the Dehydrogenation Time
3.6. The Effect of PCM Selection on Dehydrogenation Performance
4. Conclusions
- The reactor design of the MHR-PCM systems plays a crucial role in their dehydrogenation performance. For example, the H2 desorption time of an MHR-PCM equipped with an annular fin is strongly reduced compared to that of MHR-PCM without fins.
- For a given initial temperature of the desorption process, the increase of the freezing point can positively improve by up to 42%, the duration of H2 supply at a constant flow rate of 12 NL/min.
- The PCM thermal conductivity enhancement with the addition of different fin volume fractions either by changing the fin length or thickness (8 to 43.6%) showed little to no improvement in the H2 desorption performance.
- Finally, among different PCMs such as salt hydrates, paraffin, and eutectic, paraffin wax RT35 showed the best dehydrogenation performance by providing hydrogen to a fuel cell for 79 min. Moreover, the gravimetric energy density for MHR-PCM systems using paraffin is superior to salt hydrates given their low density.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Cp | Heat capacity (J·kg−1·K−1) |
| Ed | Activation energy (J·mol−1) |
| f | Phase change material’s liquid fraction |
| ΔH | Reaction heat (J·mol−1 H2), latent heat (J·kg−1) |
| kd | Reaction rate constant (s−1) |
| K | Permeability (m2) |
| L | Length (m) |
| Mg | H2 molecular weight (kg·mol−1) |
| p | Pressure (Pa) |
| PEMFC | Proton exchange membrane fuel cell |
| H2 flow rate (kg·s−1) | |
| R | Radius (m) |
| Rg | Universal gas constant (J·mol−1·K−1) |
| S | Surface (m2) |
| ΔS | Entropy change of the reaction (J·mol−1·K−1) |
| Δt | Time step (s) |
| ΔT | Transition interval/mushy zone (K) |
| t | Time (s) |
| T | Temperature (K) |
| Velocity (m·s−1) | |
| wt | H2 weight capacity |
| Greek letters | |
| α | Reacted fraction |
| ε | Bed porosity |
| δ | Fin thickness |
| λ | Thermal conductivity (W·m−1·K−1) |
| µ | Dynamic viscosity (Pa·s) |
| ρ | Density (kg·m−3) |
| Subscript | |
| d | desorption |
| eq | equilibrium |
| eff | effective |
| fin | fin |
| g | gas |
| i | initial |
| in | inner |
| m | Melting/freezing point |
| MH | Metal hydride |
| pcm | Phase change material |
| o | outer |
| tr | transition |
| w | wall |
References
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Zhang, J.; Fisher, T.S.; Ramachandran, P.V.; Gore, J.P.; Mudawar, I. A review of heat transfer issues in hydrogen storage technologies. J. Heat Trans.—Trans. ASME 2005, 127, 1391–1399. [Google Scholar] [CrossRef]
- Afzal, M.; Mane, R.; Sharma, P. Heat transfer techniques in metal hydride hydrogen storage: A review. Int. J. Hydrogen Energy 2017, 42, 30661–30682. [Google Scholar] [CrossRef]
- Laurencelle, F.; Goyette, J. Simulation of heat transfer in a metal hydride reactor with aluminium foam. Int. J. Hydrogen Energy 2007, 32, 2957–2964. [Google Scholar] [CrossRef]
- Mellouli, S.; Dhaou, H.; Askri, F.; Jemni, A.; Ben Nasrallah, S. Hydrogen storage in metal hydride tanks equipped with metal foam heat exchanger. Int. J. Hydrogen Energy 2009, 34, 9393–9401. [Google Scholar] [CrossRef]
- Nagel, M.; Komazaki, Y.; Suda, S. Effective thermal-conductivity of a metal hydride bed augmented with a copper wire-net matrix. J. Less-Common Met. 1987, 131, 426. [Google Scholar] [CrossRef]
- Mohan, G.; Maiya, M.P.; Murthy, S.S. Performance simulation of metal hydride hydrogen storage device with embedded filters and heat exchanger tubes. Int. J. Hydrogen Energy 2007, 32, 4978–4987. [Google Scholar] [CrossRef]
- Askri, F.; Salah, M.B.; Jemni, A.; Nasrallah, S.B. Optimization of hydrogen storage in metal-hydride tanks. Int. J. Hydrogen Energy 2009, 34, 897–905. [Google Scholar] [CrossRef]
- Nyamsi, S.N.; Yang, F.S.; Zhang, Z.X. An optimization study on the finned tube heat exchanger used in hydride hydrogen storage system-analytical method and numerical simulation. Int. J. Hydrogen Energy 2012, 37, 16078–16092. [Google Scholar] [CrossRef]
- Jensen, J.O.; Vestbø, A.P.; Li, Q.; Bjerrum, N.J. The energy efficiency of onboard hydrogen storage. J. Alloy. Compd. 2007, 446–447, 723–728. [Google Scholar] [CrossRef] [Green Version]
- Garrier, S.; Delhomme, B.; de Rango, P.; Marty, P.; Fruchart, D.; Miraglia, S. A new MgH2 tank concept using a phase-change material to store the heat of reaction. Int. J. Hydrogen Energy 2013, 38, 9766–9771. [Google Scholar] [CrossRef]
- Mellouli, S.; Ben Khedher, N.; Askri, F.; Jemni, A.; Ben Nasrallah, S. Numerical analysis of metal hydride tank with phase change material. Appl. Therm. Eng. 2015, 90, 674–682. [Google Scholar] [CrossRef]
- Darzi, A.A.R.; Afrouzi, H.H.; Moshfegh, A.; Farhadi, M. Absorption and desorption of hydrogen in long metal hydride tank equipped with phase change material jacket. Int. J. Hydrogen Energy 2016, 41, 9595–9610. [Google Scholar] [CrossRef]
- Tong, L.; Xiao, J.; Bénard, P.; Chahine, R. Thermal management of metal hydride hydrogen storage reservoir using phase change materials. Int. J. Hydrogen Energy 2019, 44, 21055–21066. [Google Scholar] [CrossRef]
- Mâad, H.B.; Miled, A.; Askri, F.; Nasrallah, S.B. Numerical simulation of absorption-desorption cyclic processes for metal hydrogen reactor with heat recovery using phase-change material. Appl. Therm. Eng. 2016, 96, 267–276. [Google Scholar] [CrossRef]
- Nyamsi, N.S.; Tolj, I.; Lototskyy, M. Metal hydride beds-phase change materials: Dual mode thermal energy storage for medium-high temperature industrial waste heat recovery. Energies 2019, 12, 3949. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Lu, J.; Ding, J.; Wang, W.; Yan, J. Numerical simulation on the storage performance of a phase change materials based metal hydride hydrogen storage tank. Appl. Energy 2020, 278, 115682. [Google Scholar] [CrossRef]
- Tong, L.; Yuan, Y.; Yang, T.; Bénard, P.; Yuan, C.; Xiao, J. Hydrogen release from a metal hydride tank with phase change material jacket and coiled-tube heat exchanger. Int. J. Hydrogen Energy 2021, 46, 32135–32148. [Google Scholar] [CrossRef]
- Facci, A.L.; Lauricella, M.; Succi, S.; Villani, V.; Falcucci, G. Optimized Modeling and Design of a PCM-Enhanced H2 Storage. Energies 2021, 14, 1554. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Shabani, B. Metal hydride thermal management using phase change material in the context of a standalone solar-hydrogen system. Energy Convers. Manag. 2020, 224, 113352. [Google Scholar] [CrossRef]
- Alqahtani, T.; Bamasag, A.; Mellouli, S.; Askri, F.; Phelan, P.E. Cyclic behaviors of a novel design of a metal hydride reactor encircled by cascaded phase change materials. Int. J. Hydrogen Energy 2020, 45, 32285–32297. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, J.; Xu, T.; Fang, X.; Gao, X.; Zhang, Z. Thermal conductivity of an organic phase change material/expanded graphite composite across phase change temperature range and a novel thermal conductivity model. Energy Convers. Manag. 2015, 102, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Warzoha, R.J.; Weigand, R.M.; Fleischer, A.S. Temperature-dependent thermal properties of a paraffin phase change material embedded with herringbone style graphite nanofibers. Appl. Energy 2015, 137, 716–725. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, C.; Liu, Q.; Tian, Z.; Fan, X. Thermal performance of copper foam/paraffin composite phase change material. Energy Convers. Manag. 2018, 157, 372–381. [Google Scholar] [CrossRef]
- Erek, A.; Ilken, Z.; Acar, M.A. Experimental and numerical investigation of thermal energy storage with a finned tube. Int. J. Energy Res. 2005, 29, 283–301. [Google Scholar] [CrossRef] [Green Version]
- Rathod, M.K.; Banerjee, J. Thermal performance enhancement of shell and tube Latent Heat Storage Unit using longitudinal fins. Appl. Therm. Eng. 2015, 75, 1084–1092. [Google Scholar] [CrossRef]
- Yao, J.; Zhu, P.; Guo, L.; Duan, L.; Zhang, Z.; Kurko, S.; Wu, Z. A continuous hydrogen absorption/desorption model for metal hydride reactor coupled with PCM as heat management and its application in the fuel cell power system. Int. J. Hydrogen Energy 2020, 45, 28087–28099. [Google Scholar] [CrossRef]
- Lewis, S.D.; Chippar, P. Analysis of heat and mass transfer during charging and discharging in metal hydride-phase change material reactor. J. Energy Storage 2021, 33, 102108. [Google Scholar] [CrossRef]
- de Rango, P.; Marty, P.; Fruchart, D. Hydrogen storage systems based on magnesium hydride: From laboratory tests to fuel cell integration. Appl. Phys. A 2016, 122, 126. [Google Scholar] [CrossRef]
- Apostolou, D. Assessing the operation and different refuelling cost scenarios of a fuel cell electric bicycle under low pressure hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 23587–23602. [Google Scholar] [CrossRef]
- Davids, M.W.; Lototskyy, M.; Malinowski, M.; van Schalkwyk, D.; Parsons, A.; Pasupathi, S.; Swanepoel, D.; van Niekerk, T. Metal hydride hydrogen storage tank for light fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 29263–29272. [Google Scholar] [CrossRef]
- Hwang, J.J.; Chang, W.R. Characteristic study on fuel cell/battery hybrid power system on a light electric vehicle. J. Power Sources 2012, 207, 111–119. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Davids, M.W.; Tolj, I.; Klochko, Y.V.; Sekhar, B.S.; Chidziva, S.; Smith, F.; Dana Swanepoel, D.; Pollet, B.G. Metal hydride systems for hydrogen storage and supply for stationary and automotive low temperature PEM fuel cell power modules. Int. J. Hydrogen Energy 2015, 40, 11491–11497. [Google Scholar] [CrossRef]
- El Mghari, H.; Huot, J.; Tong, L.; Xiao, J.S. Selection of phase change materials, metal foams and geometries for improving metal hydride performance. Int. J. Hydrogen Energy 2020, 45, 14922–14939. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tolj, I.; Parsons, A.; Smith, F.; Sita, C.; Linkov, V. Performance of electric forklift with low-temperature polymer exchange membrane fuel cell power module and metal hydride hydrogen storage extension tank. J. Power Sources 2016, 316, 239–250. [Google Scholar] [CrossRef]
- Visaria, M.; Mudawar, I. Experimental investigation and theoretical modeling of dehydriding process in high-pressure metal hydride hydrogen storage systems. Int. J. Hydrogen Energy 2012, 37, 5735–5749. [Google Scholar] [CrossRef]
- Visaria, M.; Issam Mudawar, I.; Pourpoint, T. Enhanced heat exchanger design for hydrogen storage using high-pressure metal hydride: Part 1. Design methodology and computational results. Int. J. Heat Mass Transf. 2011, 54, 413–423. [Google Scholar] [CrossRef]
- ASM Material Data Sheet. Available online: matweb.com (accessed on 13 January 2022).
- Sharifi, N.; Bergman, T.L.; Faghri, A. Enhancement of PCM melting in enclosures with horizontally-finned internal surfaces. Int. J. Heat Mass Transf. 2011, 54, 4182–4192. [Google Scholar] [CrossRef]
- Tay, N.H.S.; Belusko, M.; Castell, A.; Cabeza, L.F.; Bruno, F. An effectiveness-NTU technique for characterising a finned tubes PCM system using a CFD model. Appl. Energy 2014, 131, 377–385. [Google Scholar] [CrossRef]
- Nyallang, N.S.; Lototskyy, M.; Tolj, I. Optimal design of combined two-tank latent and metal hydrides-based thermochemical heat storage systems for high-temperature waste heat recovery. Energies 2020, 13, 4216. [Google Scholar]
- Nyallang, N.S.; Tolj, I. The Impact of Active and Passive Thermal Management on the Energy Storage Efficiency of Metal Hydride Pairs Based Heat Storage. Energies 2021, 14, 3006. [Google Scholar]




| Ti0.85Zr0.15(FeCrMnNiV)2 [31,36,37] | Al (6061-T6) [38] | |
|---|---|---|
| Heat of reaction/kJ/mol | 26.62 | - |
| Entropy change/J/mol/K | 109.69 | - |
| Activation energy, des/kJ/mol | 16.5 | - |
| Rate constant, des/s−1 | 300 | - |
| Packed density/kg/m3 | 3100 | 2700 |
| Specific heat/J/kg/K | 500 | 897 |
| H2 capacity/wt.% | 1.5 | - |
| Effective thermal conductivity/W/mK | 1.5 | 166 |
| Bed permeability/m2 | 1.3 × 10−12 | - |
| Dynamic viscosity/Pa∙s | 8.4 × 10−6 | - |
| Parameter/Unit | RT35 | LiNO3∙3H2O | Na2SO4∙10H2O | RT(SP29Eu) |
|---|---|---|---|---|
| Density: solid–liquid/kg/m3 | 880–60 | 2140–1780 | 1485–1420 | 2000 |
| Freezing Point/°C | 34–35 | 30–31 | 32.5–33.5 | 29–30 |
| Latent heat/kJ/kg | 170 | 296 | 251 | 175 |
| Heat capacity: solid–liquid/J/(kg K) | 1800–2400 | 1730–2770 | 1440–2570 | 2500 |
| Thermal conductivity: solid–liquid/W/(m K) | 0.24–0.20 | 1.32–0.58 | 1.23–0.54 | 0.6 |
| Parameters/Unit | Design A | Design B |
|---|---|---|
| MH reactor, RMH/m | 0.03 | 0.03 |
| MH reactor height, Lin/m | 0.4 | 0.4 |
| Wall thickness, Rin − RMH/m | 0.002 | 0.002 |
| PCM thickness, Ro − Rin/m | 0.04 | 0.04 |
| PCM height, Lo/m | 0.412 | 0.412 |
| Fin length, Lfin/m | - | 0.035 |
| Fin spacing, FS/m | - | 0.01 |
| Fin thickness, δfin/m | - | 0.002 |
| H2 filter radius, r0/m | 0.005 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyamsi, S.N.; Tolj, I.; Gęca, M.J. Dehydrogenation of Metal Hydride Reactor-Phase Change Materials Coupled with Light-Duty Fuel Cell Vehicles. Energies 2022, 15, 2982. https://doi.org/10.3390/en15092982
Nyamsi SN, Tolj I, Gęca MJ. Dehydrogenation of Metal Hydride Reactor-Phase Change Materials Coupled with Light-Duty Fuel Cell Vehicles. Energies. 2022; 15(9):2982. https://doi.org/10.3390/en15092982
Chicago/Turabian StyleNyamsi, Serge Nyallang, Ivan Tolj, and Michał Jan Gęca. 2022. "Dehydrogenation of Metal Hydride Reactor-Phase Change Materials Coupled with Light-Duty Fuel Cell Vehicles" Energies 15, no. 9: 2982. https://doi.org/10.3390/en15092982
APA StyleNyamsi, S. N., Tolj, I., & Gęca, M. J. (2022). Dehydrogenation of Metal Hydride Reactor-Phase Change Materials Coupled with Light-Duty Fuel Cell Vehicles. Energies, 15(9), 2982. https://doi.org/10.3390/en15092982








