1. Introduction
The International Energy Agency (IEA) has set a goal of carbon neutrality by 2100 for climate change mitigation. Additionally, the Paris Agreement has set a global initiative to reduce global warming to much less than 2 °C and to further engage efforts to reduce it to 1.5 °C [
1,
2,
3]. Despite the increasing use of renewable energy sources, their growth is still insufficient to switch from fossil fuel consumption by the end of the century. Further, the current trend will limit the rise in global temperature to 2.7 °C by 2100, which is not sufficient to meet the 2 °C reduction target. Carbon capture, utilization, and storage (CCUS) is a proposed solution to achieve carbon neutrality during the transition from fossil fuels to fully renewable energy generation. Carbon capture (CC) is an attractive option as it aims to achieve carbon neutrality, while simultaneously generating waste carbon dioxide (CO
2) that can be converted into products and sold for profit. Further, decentralized energy generation has been widely investigated as a possible developmental path for achieving carbon neutrality. Distributed power generation in the form of small, decentralized systems can support decrease in emissions and protection of grid capacity, while also offering options for renewable energy [
4,
5,
6,
7,
8].
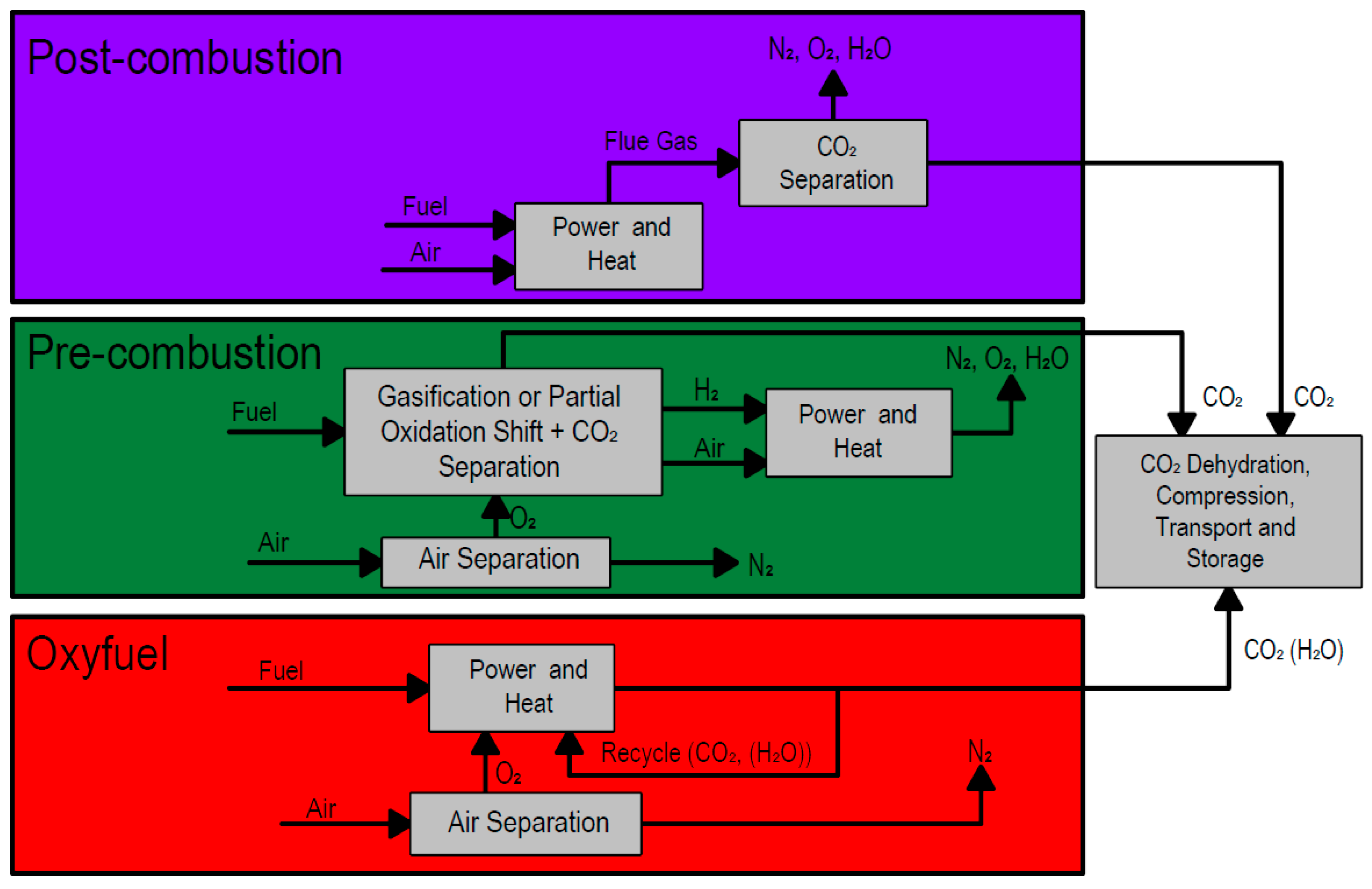
The available options for CC are grouped into either post-combustion, pre-combustion, or oxy-fuel combustion [
9,
10,
11,
12,
13,
14,
15]. The basic processes are summarized in
Figure 1.
The capture methods that fall within each classification, as well as storage and utilization options, are summarized in
Figure 2. Among the methods shown, absorption by mono-ethanolamine (MEA) for post-combustion capture is the most developed. Because of the high thermal energy requirement for solvent regeneration, this process is well suited to combined heat and power (CHP) applications, which supply recoverable heat [
10]. Post-combustion absorption plants with an MEA-based solvent is the more commonly utilized method for small-scale carbon capture [
16].
There exist numerous reviews and studies on large-scale carbon dioxide capture, utilization and storage [
16,
17,
18,
19,
20,
21,
22,
23], carbon capture and storage (CCS) [
24,
25,
26,
27], demonstration and deployment of CCS systems [
28,
29,
30,
31,
32,
33], cost of CCS [
34,
35], CCS applied in industry [
36,
37,
38], CC [
39,
40], integration of CC in power generation plants [
41,
42] and in community scale energy systems [
43,
44], CC in pre-combustion, post-combustion and oxy-combustion in thermal power plants [
45], CC post-combustion by chemical-looping [
46], chemical absorption [
46,
47,
48,
49,
50,
51] or physical adsorption [
52,
53,
54], membrane-based CC [
55,
56,
57], and, finally, CC and separation technologies for end-of-pipe applications [
58]. Although there is a myriad of dedicated research studies on large-scale CCUS-CCS or CC systems, available literature reviews on small-scale CCS for building applications are very limited.
Therefore, this review will focus on the current technological developments in carbon capture for decentralized, small-scale CHP generation applications. This is to provide a thorough review of the current technologies in development, and the associated results and challenges, as identified in the literature. For this purpose, the review is structured in the following manner: First the relevant system configurations studied in the literature are presented, and the associated studies introduced (
Section 2). Subsequent performance and optimization sections (
Section 3 and
Section 4) then provide detail of the results, challenges, and optimal design parameters from the studies considered. A brief overview for current avenues of storage and utilization is presented, followed by an overview of commercially available small-scale and large-scale technologies (
Section 5). Further, general costs of CC systems are briefly reported (
Section 6). The next section provides a concise overview of current developments in direct air capture (DAC) technology. Following carbon capture and compression, the next step in the process chain is either storage or utilization (
Section 7), while conclusions are drawn in the last section (
Section 8).
5. Overview of Available CCUS Technologies
Several storage options are being considered for carbon capture applications, with varying levels of development. Injecting CO
2 into geologic formations could be considered as it is a mature technology already in use in the oil and gas industry. The main concern associated with geological storage is leakage of the concentrated CO
2 stream and associated environmental damage. However, annual leakage rates in the literature range from 0.00001% to 1%. The three main types of geological formation for carbon storage are depleted oil and gas reserves, deep saline reservoirs, and unmineable coal seams [
10,
11]. The captured CO
2 can also be sold for profit, which is one avenue to be explored in the interest of offsetting the high costs of CCS. Examples of utilization are direct utilization, enhanced oil recovery (EOR), carbonation, and conversion into chemicals and fuels. Direct uses include the food and drink industry, such as for drink carbonation, the decaffeination process, etc. CO
2 can also be used directly for pharmaceutical applications. Direct utilization is only possible for sources with high purity CO
2 waste streams, such as ammonia production. EOR is the process of using CO
2 to extract crude oil from an oil field or natural gas from unmineable coal deposits. As applied to natural gas, this process is still under development and not yet commercially available. However, EOR for crude oil extraction has been used for many years already in both Canada and the USA. For EOR, CO
2 is injected into otherwise unrecoverable oil reserves for increased oil extraction. Most of the CO
2 is pumped back to the surface and recycled, although some is released into the atmosphere. Under certain conditions, the CO
2 injected for EOR could remain underground as in geological storage. As mentioned, captured CO
2 can also be transformed into fuels and useful chemicals, or used as feedstock for fuel production. Unfortunately, using CO
2 for feedstock results in a highly energy intensive process. Further, fuels and chemicals have a short lifespan, and are quickly released back into the atmosphere. Therefore, the benefits of capture are undermined [
28,
35,
42].
For perspective, the following section will provide a brief overview of the large-scale CC projects currently in operation. CCS technology functioning at large-scale has existed since the 1970s. Government subsidized projects to aid develop and commercialize CC plants have been principally concentrated on electricity generation. The major part of CO
2 injection from CC is in EOR in the USA that offers extra revenue to partially offset capture costs. In Canada, there are three capture plants in operation and two in construction as of 2019. The Boundary Dam Carbon Capture and Storage facility in Saskatchewan has been in operation since 2014 with a capture capacity of one million tons of CO
2 on an annual basis [
24,
101]. The post-combustion capture facility is coupled to a fossil-fueled electricity generating plant, to which CO
2 is largely transported by pipeline and used for EOR at the Weyburn Oil Unit. The remaining CO
2 is transported, also via pipeline, to the nearby Aquistore project for geological storage [
22]. The Petra Nova plant in Texas is another commercial large-scale fossil-fueled power plant integrated with CCS technology, with annual CO
2 capture of one million tons. Both plants sell captured CO
2 for EOR, which partially offsets the cost introduced by CCS [
107,
108,
109].
The micro cogeneration systems with integrated CC discussed thus far are purely in the development stage and are not yet commercially available. For instance, Clean O
2 Carbon Capture has developed the first commercial unit for decentralized CC applications [
110]. The product provides direct capture to by-product utilization with minimal processing. The process is described as follows: A portion of the flue gases, having a CO
2 concentration of 40,000 ppm or greater, pass through the reaction chamber where caustic soda reacts with the carbon dioxide to create soda ash and water. The flue gases then pass through the reaction chamber into the heat exchanger where waste heat is recovered by heating the municipal water supply for domestic hot water. The caustic soda must be replenished weekly. The Clean O
2 Carbon Capture unit is currently installed in concentrated residential, commercial, and single residential applications. Depending on the use, either a residential or commercial unit may be installed. For concentrated residential applications, a commercial pilot is installed at Garrison Woods and Marda Loop in Calgary. For commercial use, a commercial unit is installed at Westjet airlines in Calgary. Both are projected to produce 6.5 tonnes of by-product, the equivalent of 3 metric tonnes of captured CO
2 per year. In terms of single residential use, a residential pilot is installed in Calgary and projected to produce 630 kg of by-product, the equivalent of 320 kg of captured CO
2 per year. The cost per tonne of CO
2 captured is approximately
$14.94. However, by selling the soda ash by-product, the cost of capture is actually negative. The advantages of using the Clean O
2 Carbon Capture unit are notable, comprising savings of up to 20% on energy charges per annum, and, reflecting ecological concerns, inhibition of GHG emissions into the atmosphere [
110].
6. Cost Analysis
This section presents a brief overview of the cost of carbon capture at large scale, as the literature on the small-scale is scarce. This will be to provide a general sense of the costs of CC, which can provide context for evaluating potential costs at a small scale. Further, an economic evaluation of the impact of CC on the biomass-fired ORC cycle for mCHP applications is presented.
For large scale power generation plants, CC costs are typically defined based on the separation and compression costs at a single facility independent of the costs of transport, storage, or further conversion steps [
111,
112,
113,
114,
115]. Rubin et al. [
35] presented an updated CCS cost estimate and compared it to the CCS costs reported in the 2005 Intergovernmental Panel on Climate Change (IPCC) Special Report on Carbon Dioxide Capture and Storage (SRCCS) [
15]. This analysis was based on natural gas combined cycle plants with MEA-based post-combustion CC. The previous research details only separation and compression costs based on the addition of the capture plant only, without consideration of transport and storage. The Robin et al. [
35] study included the costs of transport and storage to provide an estimate of CCS costs.
All planned large-scale transport of CO
2 continues with pipelines. However, according to Pieri et al. [
111], the use of trucks is likely to be more economical for short distance and small-scale CO
2 transport than branch pipelines. Pipelines are advantageous as they can handle large flowrates but impose an economic challenge in the case of low flowrates. For small quantities, truck tankers and railroad tankers are favorable, and provide lower capital costs. Nonetheless, large scale trunk pipelines would need to be constructed for both large and small-scale capture [
111]. The current costs of CO
2 pipeline transport taken from the SRCCS [
15], IPCC [
15], ZEP [
112], and USDOE [
113], are summarized in
Table 11 and
Table 12 for onshore and offshore pipelines, respectively [
35]. The values for SRCCS are reported in 2002 USD/tCO
2/250 km and the values for all other studies are in 2013 USD/tCO
2/250 km. These results are derived for “normal” terrain; however, given that the cost is highly dependent upon the given terrain, actual costs may be much higher. The costs were adjusted to a common basis of 2013 USD/tCO
2/250 km using the Chemical Engineering Plant Cost Index (CEPCI) escalation factors. From 2002 to 2013 the CEPCI rose more than the US general inflation index, CPI (44% vs. 29%, respectively), showing “real” cost escalation during that time. The Power Capital Cost Index (PCCI, an index particular to the capital cost of non-nuclear power plants) increased further, at 64%. As the study of Rubin et al. [
35] involved the cost of power plants with and without CCS, they applied the PCCI to escalate the capital cost of power plants from 2002 to 2013 dollars. To adjust transport and storage costs, they utilized the CEPCI, as these services are usually supplied to power plants by distinct companies largely from the oil and gas industry.
Rubin et al. [
35] also investigated the cost of storage through a survey of the available literature on the topic. They focused only on storage through injection into geologic formations as this technology is well established and has been practiced for many years in different contexts. However, in the context of storage, more research must be done, and several uncertainties remain. For example, it is unknown how future regulations may impact costs in terms of monitoring and liability, as well as the impact of public acceptance on project economics. As discussed, several types of geologic storage reservoirs are available, with varying costs depending on the reservoir type. Based on this, the various studies examined by Rubin et al. [
35] provide cost ranges. In the SRCCS, the cost of geologic storage was reported to range from 0.5 to 8.0 2002 USD/tCO
2, with an added monitoring cost of 0.1–0.3 2002 USD/tCO
2. The typical ranges of onshore storage costs evaluated on a standard basis are reported in
Table 13 from four different studies.
Table 14 displays the levelized cost ranges for amine-based post-combustion capture integrated with a natural gas combined cycle (NGCC) plant based on the literature, as reviewed and reported by Rubin et al. [
35]. From the analysis, it is clear that the total cost of CCS can be decreased considerably if CO
2 is sold for EOR as well as geologic storage. However, it is worth pointing out that due to the volatility of oil prices, the value of the EOR credits is quite uncertain.
Carbon capture is the most costly phase of a CCUS supply chain, due to high capture cost from sources, which are diluted in CO
2. This is especially true at a small-scale, as CO
2 content in flue gases are significantly lower than those from large scale energy generation. The values of the cost of CO
2 captured and the cost of CO
2 avoided reported by Rubin et al. [
35] would be much lower than those for small-scale generation due to the diluted CO
2. For this application, the greatest challenge to be overcome is making MEA-based post combustion economically feasible through lowering of the high energy requirement for regeneration, solvent degradation and loss, and corrosion issues.
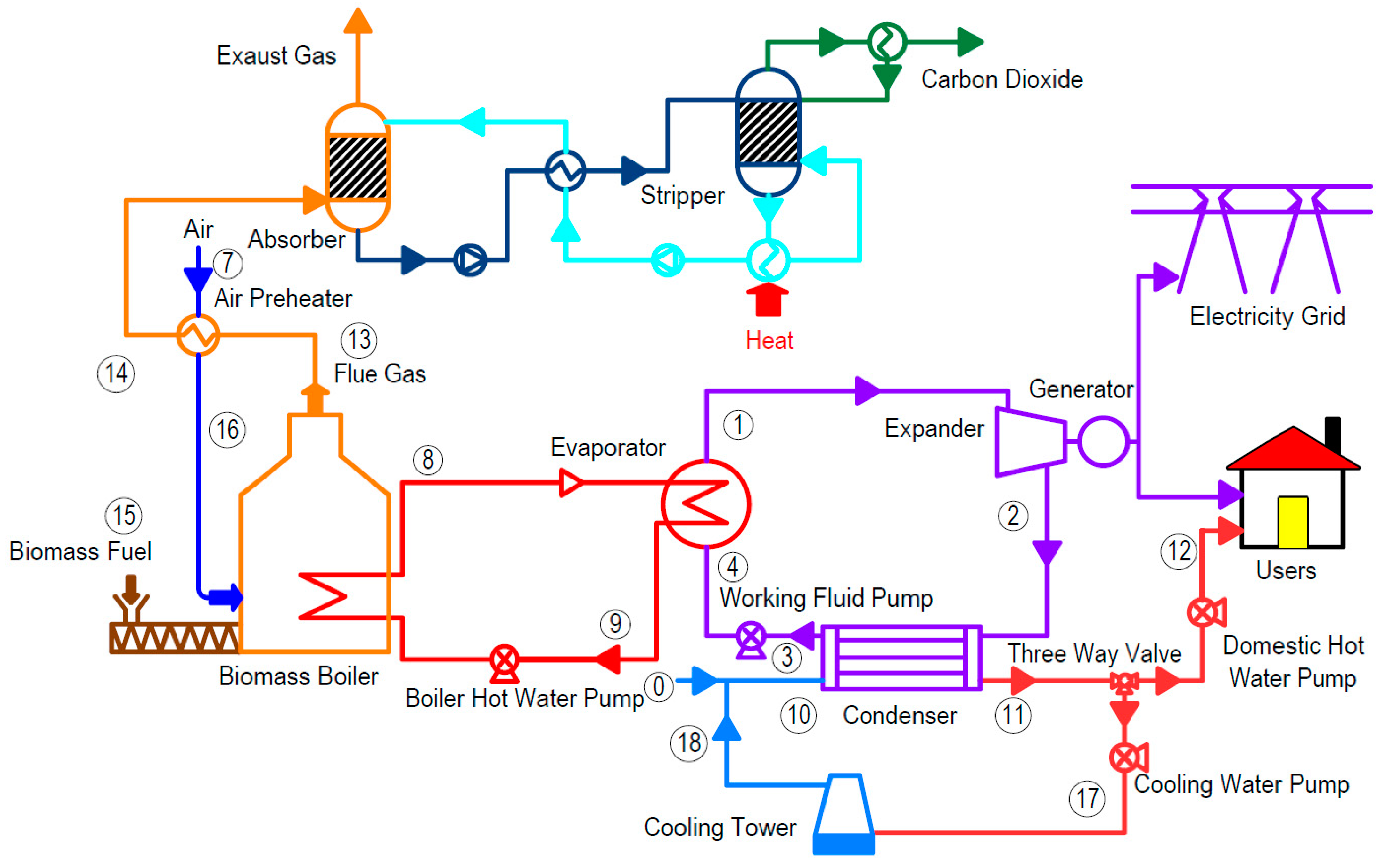
For an ORC-based micro-CHP system, at optimal conditions using R245fa as a working fluid, Zhu et al. [
102] performed a thermo-economic analysis to assess the cost effects of CO
2 capture integration, with the results summarized in
Table 15. It was found that the total investment (INV
tot) increases from 31.48 to 82.9 × 10
4 USD and the annual operation and maintenance costs (C
O&M) increase from 39.26 to 62.36 × 10
4 USD. Further the net annual income (NAI) and net present value (NPV) decrease, while the dynamic payback period (DPP), net power index (NPI), and levelized energy cost (LEC) increase, according to
Table 15. This leads to the conclusion that the economic performance is decreased with MEA-based CC. It is important to mention that the biomass fuel cost represents a substantial portion of the annual operation and maintenance costs, followed by the cost of input heat for MEA absorbent regeneration.
Following the previous study by Zhu et al. [
102], a comparison of the equipment cost ratio for a biomass-fired ORC-CHP system with and without CO
2 capture investment, showed the following differences: evaporator (19.65%), condenser (18.14%), expander (16.04%), and biomass boiler (15.79%). Further, the cooling water loop, which consists of the cooling water pump (7.33%) and cooling tower (4.26%), accounted for 11.59% of the total system investment. When the CO
2 capture system was integrated, it accounted for 62.03% of the total investment. Further, the comparison of the combined ratio for biomass-fired ORC-CHP system annual operation and maintenance costs (C
O&M) were also provided without and with CO
2 capture, showing that biomass-fuel (CA
bf) accounted for 58.07% of the annual operation and maintenance costs. This was followed by the thermal heat expenditures for MEA solvent regeneration in the stripper (27.84%), which resulted from the significant energy penalty.
7. Other Applications of CCS
With the goal of net zero emissions, negative emissions technology (NET) is required for the purpose of recapturing greenhouse gases emitted in the past. Direct air capture is the process of removing CO
2 from the air and generating a concentrated stream of carbon dioxide for sequestration or utilization [
116,
117,
118,
119]. If the carbon dioxide is stored, DAC contributes to the group of negative emission technologies. A challenge that must be addressed during the development of DAC systems is the large energy input that is required to remove and concentrate CO
2 to a pure stream (>90%) from air (390 ppm). The thermodynamic minimum energy required is 250 kWh per ton of CO
2, which is significantly higher than that for concentrated sources, such as flue gases from power plants. The current cost per ton of CO
2 is estimated to be
$200–
$1000, whereas the cost estimate for concentrated point sources is within the range of
$40–
$60 [
108].
Several DAC technologies are currently in developmental stages; however, there is no agreement on the processes of CO
2 separation from air. Several processes are under development as reported in the literature—these include solid sorbent adsorption, vacuum swing adsorption, and calcium (carbonate-bicarbonate) looping absorption cycles [
120,
121,
122,
123,
124,
125,
126].
Due to the highly dilute concentration of CO
2 in ambient air, the energy input is far greater than that for point source CCUS. However, net negative global emissions are necessary to accomplish the current climate change prevention goals [
4,
108].
An interesting application for DAC is a ventilation approach for buildings using CO
2 capture. Kim et al. [
120,
121] proposed a CO
2 adsorption capture device and its use as a ventilation strategy in buildings in 2015, and further examined the system moisture performance in 2020. The goal was to provide a more efficient means of indoor air recirculation. Through numerical calculations, they found that during the tropical summer and central European winter seasons, 30–60% of air ventilation energy for cooling and heating can be saved when compared to the traditional air ventilation system and can save building energy at peak energy load times.
Baus and Nehr [
126] proposed a coupling of HVAC-systems with DAC-technology to separate CO
2 in the exhaust air of buildings and recirculate the CO
2-depleted air back into the building. Based on a theoretical method, the possibilities and drawbacks of the recommended HVAC/DAC-coupling in recirculation mode were evaluated. They concluded that the system can decrease the energy demand of buildings while also enabling access to unutilized CO
2-resources transported in the building. In addition, the system presents the possibility of enhancing indoor air quality. However, the authors pointed out that an adequate DAC unit for operation in indoor air is not yet commercially available.
8. Conclusions
This paper has reviewed the latest advances related to small-scale carbon capture systems and their application, focusing mainly on micro-combined heat and power cogeneration systems for use in buildings. As can be seen, the use of CC on small-scale distributed combined heat and power sources has the potential to reduce carbon emissions and achieve the carbon neutral goal. This review discussed in detail some of the main concerns and proposed several key findings that can aid in assessing the feasibility of CC when applied to the small-scale.
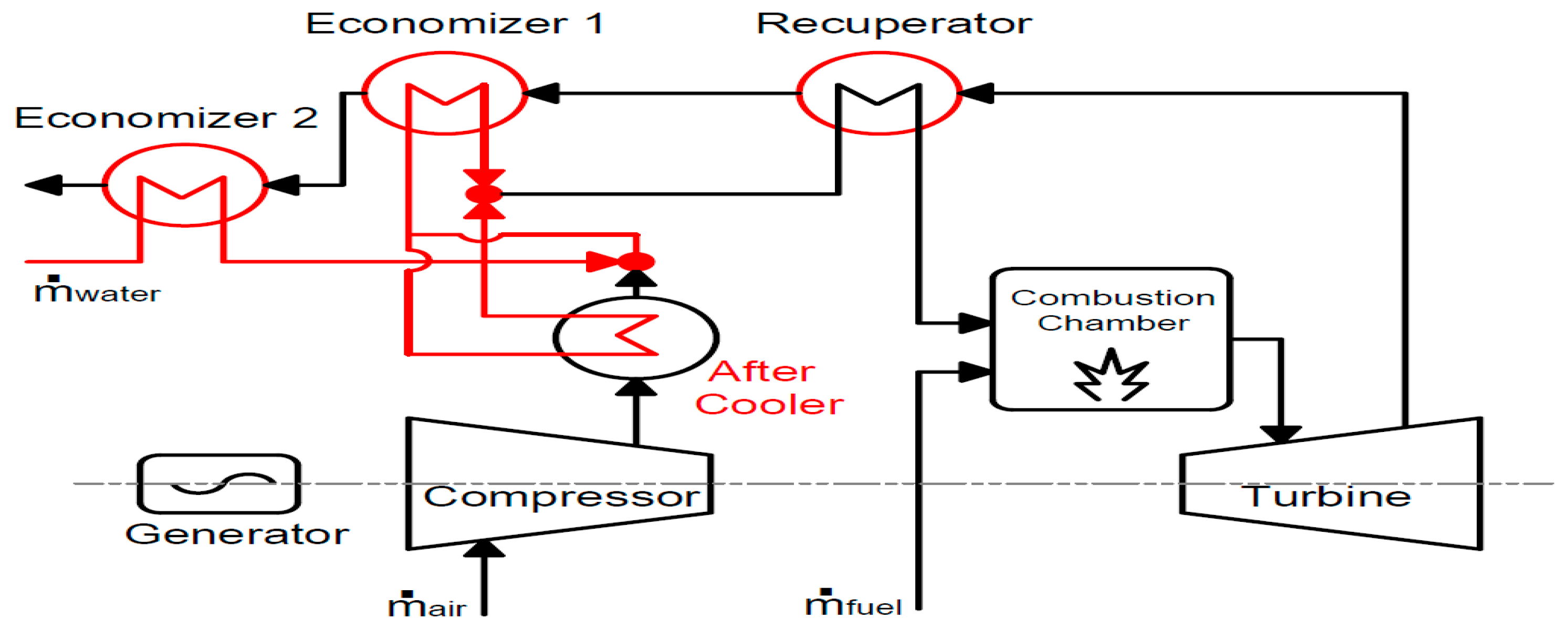
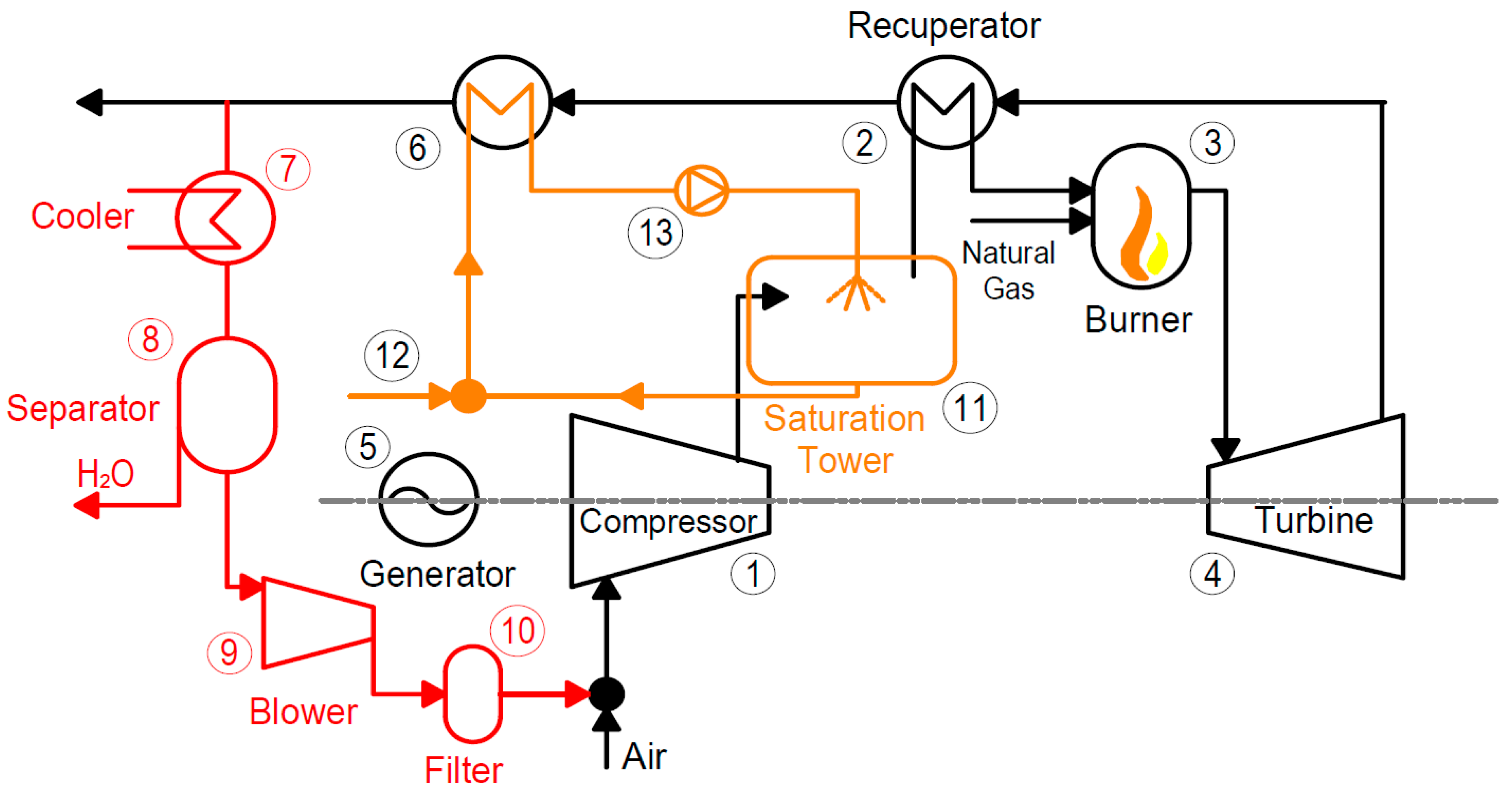
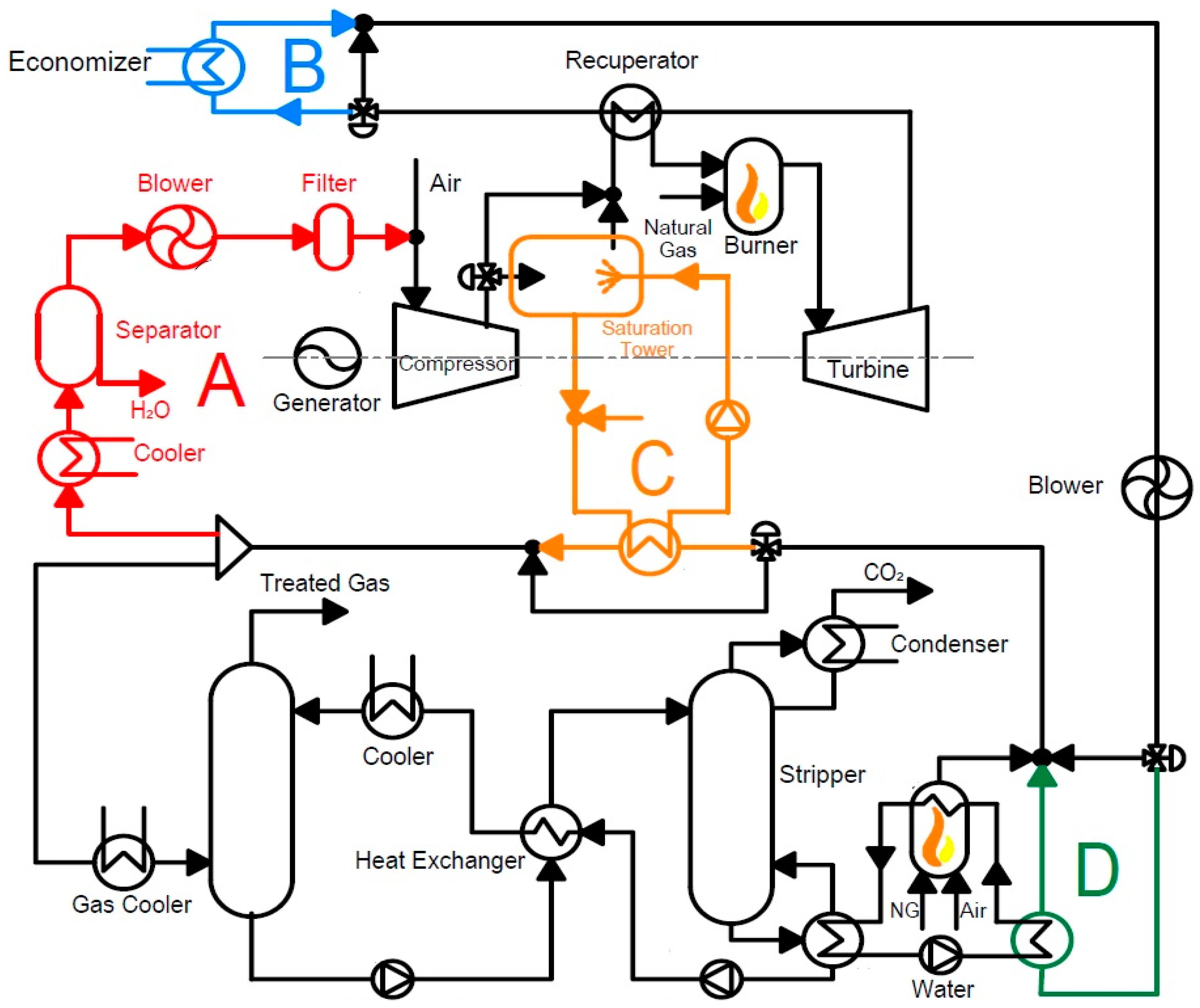
The findings pertaining to the application of carbon capture in different energy systems in summary are as follows: For micro gas turbine systems, EGR can increase CO2 concentration by 2.2 times the baseline mGT cycle, and can be increased to a ratio of 0.55, significantly decreasing the volumetric flow rate to the capture plant. This leads to decreased capital and operating costs of the capture plant. S-EGR can enhance CO2 concentrations further than EGR while maintaining stable combustion. Conversion into the mHAT cycle can increase global efficiency to 25.8% compared to the baseline efficiency of 23.0%. Improved energy integration using turbine exhaust heat for reboiler duty can increase the cycle efficiency up to 26.8% when there is no heat demand. For the hybrid solid state fuel cell and micro gas turbine systems, the maximum net efficiency was found to be 51.4% LHV and a 100% carbon capture ratio can be achieved. The biomass-fired organic Rankine cycle systems allow for negative carbon emissions when integrated with CC. The maximum system efficiency was found to be 13.7%, not including the effects of CC. This must be further assessed. When the CO2 capture system is integrated, it accounts for 62.0% of the total system investment; thermal expenditures for MEA solvent regeneration account for 27.8% of the annual operating and maintenance costs. Moreover, direct air capture was also identified as a potential technology for negative carbon emissions. However, further development is required to reduce the substantial energy penalty and increase the economic feasibility.
Currently, carbon capture, as applied at small-scale in buildings applications, is in the developmental stages, and further research must be performed to reduce the energy penalty of the capture process for economic feasibility. Additionally, the majority of the results from the literature were obtained through numerical analyses. Therefore, it is necessary that more extensive experimental work be accomplished to validate the current findings. Further, there is a gap in the literature pertaining to the economic feasibility of small-scale carbon capture systems, and, in the future, a full cost analysis must be performed to address this.
Although large-scale CC/CCS/CCUS systems are currently deployed in a range of industrial applications, the integration of small-scale CC in micro-CHP systems for buildings in full-scale projects is required to advance the understanding and practice necessary for demonstration and deployment of this technology. To achieve this, as mentioned above, more research and development are required to develop innovative concepts that have the capacity to improve the operability, reliability and environmental performance, and to substantially decrease the costs of CC for use in new and existing residential, commercial and institutional buildings. Experimental pilot and demonstration scales are required to address these gaps.
The effective deployment of integrated small-scale CC in building energy systems requires the active involvement and engagement of government, building constructers, power utilities, CC manufacturers, policy makers and owners. It is anticipated that this review paper will be of interest to all concerned parties to enhance awareness of the challenges and issues, and to contribute more to this area.