Recent Advances in Greener and Energy Efficient Alkene Epoxidation Processes
Abstract
:1. Introduction
2. Important Reactions of Epoxides
3. Energy Efficient Alkene Epoxidation Processes
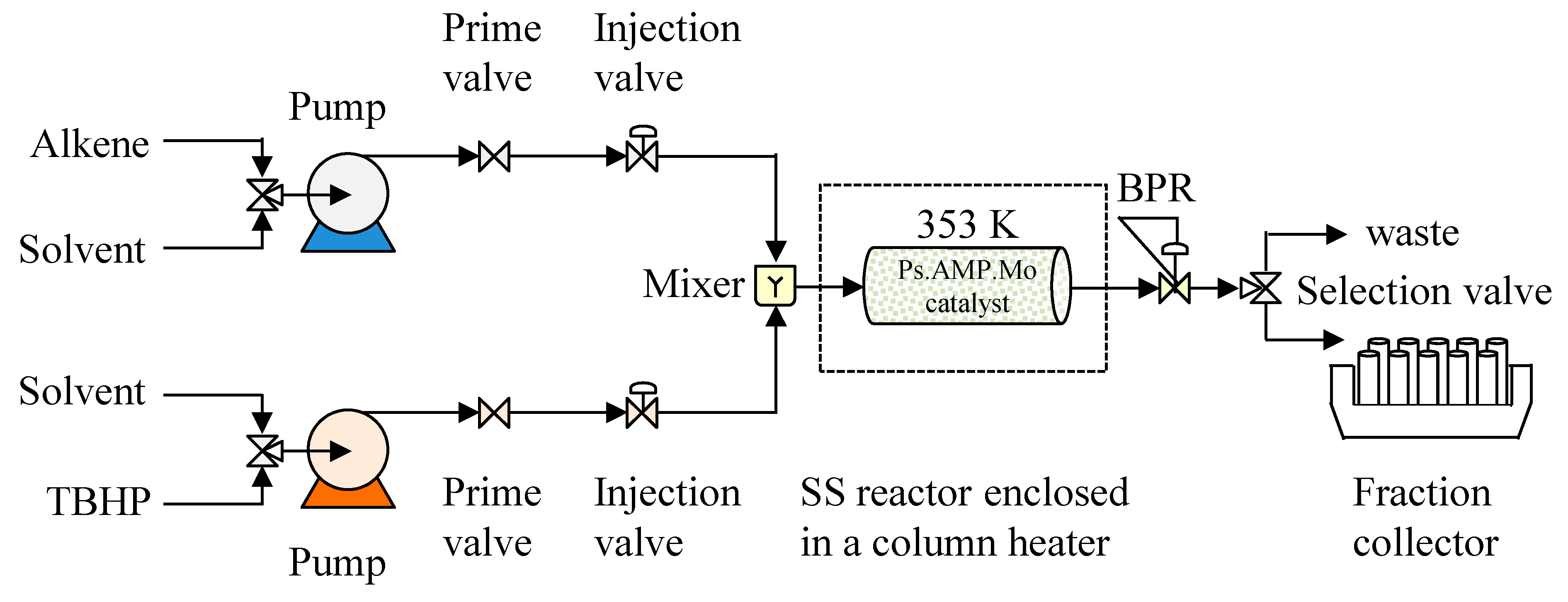
3.1. Continuous Flow Epoxidation Process
3.2. Epoxidation in a Reactive Distillation Column (RDC)
3.3. Microwave-Assisted Epoxidation
3.4. Epoxidation in Microreactors
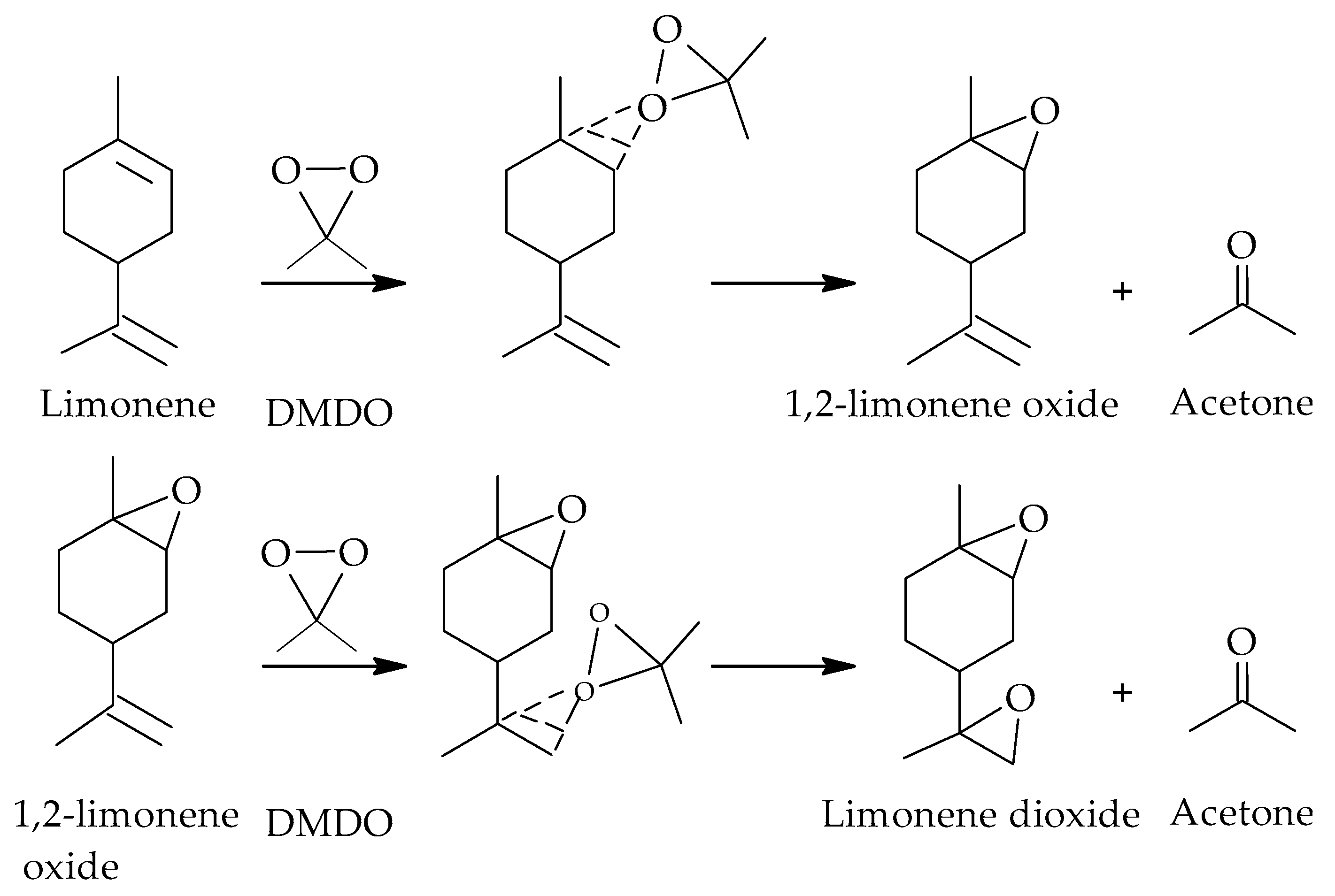
3.5. Sonochemical Synthesis of Epoxides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMP | 2-aminomethyl pyridine |
| CTF | Confined Taylor Flow |
| DBD | Dielectric Barrier Discharge |
| DMDO | Dimethyl dioxirane |
| DVB | divinylbenzene |
| MoO2(acac)2 | Molybdenyl acetylacetonate |
| Ps.AMP | Polystyrene 2-(aminomethyl) pyridine |
| Ps.AMP.Mo | Polystyrene 2-(aminomethyl) pyridine supported Mo(VI) complex |
| RDC | Reactive Distillation Column |
| ROS | Reactive Oxygen Species |
| TBHP | tert-butyl hydroperoxide |
| VBC | Vinylbenzyl chloride |
References
- Vooradi, R.; Anne, S.; Tula, A.; Eden, M.; Gani, R. Energy and CO2 management for chemical and related industries: Issues, opportunities and challenges. BMC Chem. Eng. 2019, 1, 7. [Google Scholar] [CrossRef]
- Tvaronavičienė, M.; Baublys, J.; Raudeliūnienė, J.; Jatautaitė, D. Global energy consumption peculiarities and energy sources: Role of renewables. In Energy Transformation towards Sustainability; Tvaronavičienė, M., Ślusarczyk, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–49. [Google Scholar]
- Bilgen, S. Structure and environmental impact of global energy consumption. Renew. Sustain. Energy Rev. 2014, 38, 890–902. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, X.; Xiao, J.; Liu, Z.; Xu, Y.; Tian, Y. Energy consumption and emission mitigation prediction based on data center traffic and PUE for global data centers. Glob. Energy Interconnect. 2020, 3, 272–282. [Google Scholar] [CrossRef]
- Rahman, M.S.; Noman, A.H.M.; Shahari, F.; Aslam, M.; Gee, C.S.; Isa, C.R.; Pervin, S. Efficient energy consumption in industrial sectors and its effect on environment: A comparative analysis between G8 and Southeast Asian emerging economies. Energy 2016, 97, 82–89. [Google Scholar] [CrossRef]
- Lesage, D.; Van de Graaf, T.; Westphal, K. G8+5 collaboration on energy efficiency and IPEEC: Shortcut to a sustainable future? Energy Policy 2010, 38, 6419–6427. [Google Scholar] [CrossRef]
- Tan, J.; Ji, Y.-N.; Deng, W.-S.; Su, Y.-F. Process intensification in gas/liquid/solid reaction in trickle bed reactors: A review. Petrol. Sci. 2021, 18, 1203–1218. [Google Scholar] [CrossRef]
- Žnidaršič-Plazl, P. Biocatalytic process intensification via efficient biocatalyst immobilization, miniaturization, and process integration. Curr. Opin. Green Sustain. Chem. 2021, 32, 100546. [Google Scholar] [CrossRef]
- Baharudin, L.; Watson, M.J.; Yip, A.C. Process intensification in multifunctional reactors: A review of multi-functionality by catalytic structures, internals, operating modes, and unit integrations. Chem. Eng. Process. 2021, 168, 108561. [Google Scholar] [CrossRef]
- Zhang, Y.; Goh, K.-L.; Ng, Y.L.; Chow, Y.; Wang, S.; Zivkovic, V. Process intensification in micro-fluidized bed systems: A review. Chem. Eng. Process. 2021, 164, 108397. [Google Scholar] [CrossRef]
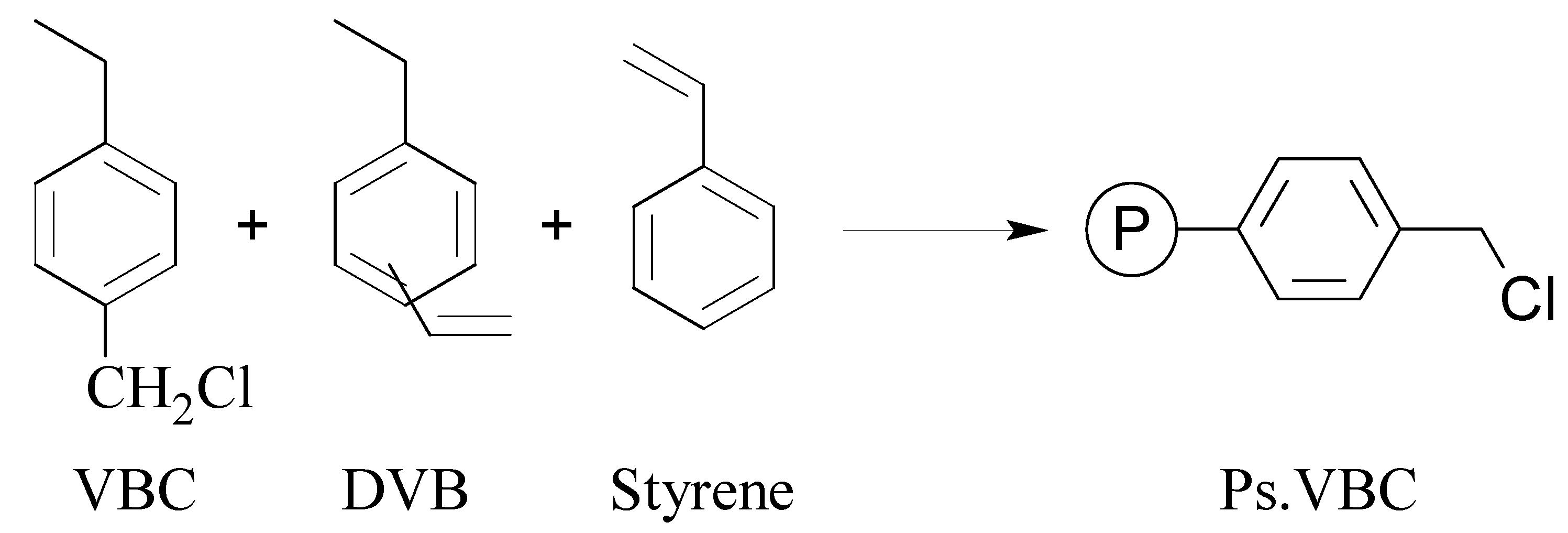
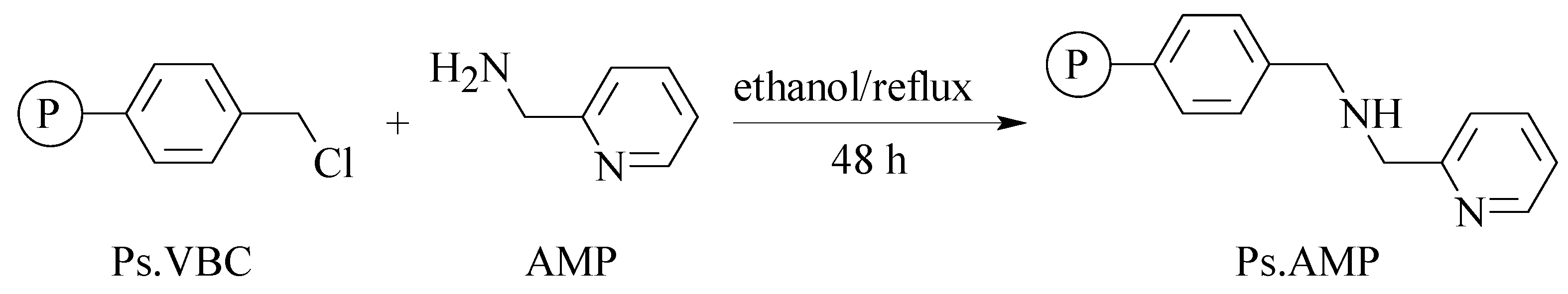
- Mbeleck, R.; Mohammed, M.L.; Ambroziak, K.; Sherrington, D.C.; Saha, B. Efficient epoxidation of cyclododecene and dodecene catalysed by polybenzimidazole supported Mo(VI) complex. Catal. Today 2015, 256, 287–293. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Mbeleck, R.; Patel, D.; Niyogi, D.; Sherrington, D.C.; Saha, B. Greener and efficient epoxidation of 4-vinyl-1-cyclohexene with polystyrene 2-(aminomethyl)pyridine supported Mo(VI) catalyst in batch and continuous reactors. Chem. Eng. Res. Des. 2015, 94, 194–203. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Patel, D.; Mbeleck, R.; Niyogi, D.; Sherrington, D.C.; Saha, B. Optimisation of alkene epoxidation catalysed by polymer supported Mo(VI) complexes and application of artificial neural network for the prediction of catalytic performances. Appl. Catal. A-Gen. 2013, 466, 142–152. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. 17-ethers and epoxides. In Organic Chemistry, 2nd ed.; Ouellette, R.J., Rawn, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 507–536. [Google Scholar]
- Wypych, A. 3.12-epoxides. In Databook of Plasticizers, 2nd ed.; Wypych, A., Ed.; ChemTec Publishing: Scarborough, ON, Canada, 2017; pp. 210–242. [Google Scholar]
- Dutia, P. Ethylene oxide: A techno-commercial profile. Chem. Wkly. 2010, 53, 199–203. [Google Scholar]
- Nijhuis, T.A.; Makkee, M.; Moulijn, J.A.; Weckhuysen, B.M. The production of propene oxide: catalytic processes and recent developments. Ind. Eng. Chem. Res. Res. 2006, 45, 3447–3459. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.Q.; Marks, M.J. Epoxy resins. In Ulmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Silvestre, A.J.D.; Gandini, A. Chapter 2—Terpenes: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 17–38. [Google Scholar]
- Santacesaria, E.; Tesser, R.; Di Serio, M.; Turco, R.; Russo, V.; Verde, D. A biphasic model describing soybean oil epoxidation with H2O2 in a fed-batch reactor. Chem. Eng. J. 2011, 173, 198–209. [Google Scholar] [CrossRef]
- Bechtold, K. Versatile and vexing: The many uses and hazards of peracetic acid. Synergist 2016. Available online: https://synergist.aiha.org/201612-peracetic-acid-uses-and-hazards (accessed on 26 January 2022).
- Shi, Z.-Q.; Jiao, L.-X.; Sun, J.; Chen, Z.-B.; Chen, Y.-Z.; Zhu, X.-H.; Zhou, J.-H.; Zhou, X.-C.; Li, X.-Z.; Li, R. Cobalt nanoparticles in hollow mesoporous spheres as a highly efficient and rapid magnetically separable catalyst for selective epoxidation of styrene with molecular oxygen. RSC Adv. 2014, 4, 47–53. [Google Scholar] [CrossRef]
- Vondran, J.; Pela, J.; Palczewski, D.; Skiborowski, M.; Seidensticker, T. Curse and blessing—The role of water in the homogeneously Ru-Catalyzed epoxidation of technical grade methyl oleate. ACS Sustain. Chem. Eng. 2021, 9, 11469–11478. [Google Scholar] [CrossRef]
- Thornburg, N.E.; Thompson, A.B.; Notestein, J.M. Periodic trends in highly dispersed Groups IV and V supported metal oxide catalysts for alkene epoxidation with H2O2. ACS Catal. 2015, 5, 5077–5088. [Google Scholar] [CrossRef]
- Lu, X.; Wu, H.; Jiang, J.; He, M.; Wu, P. Selective synthesis of propylene oxide through liquid-phase epoxidation of propylene with H2O2 over formed Ti-MWW catalyst. J. Catal. 2016, 342, 173–183. [Google Scholar] [CrossRef]
- Luttrell, W. Toxic tips: Sodium hypochlorite. J. Chem. Health Saf. 2001, 8, 24–26. [Google Scholar] [CrossRef]
- Bruch, M.K. Toxicity and safety of topical sodium hypochlorite. Contrib. Nephrol. 2007, 154, 24–38. [Google Scholar] [PubMed]
- Bregante, D.T.; Tan, J.Z.; Schultz, R.L.; Ayla, E.Z.; Potts, D.S.; Torres, C.; Flaherty, D.W. Catalytic consequences of oxidant, alkene, and pore structures on alkene epoxidations within titanium silicates. ACS Catal. 2020, 10, 10169–10184. [Google Scholar] [CrossRef]
- Ni, X.-L.; Liu, J.; Liu, Y.-Y.; Leus, K.; Depauw, H.; Wang, A.-J.; Van Der Voort, P.; Zhang, J.; Hu, Y.-K. Synthesis, characterization and catalytic performance of Mo based metal- organic frameworks in the epoxidation of propylene by cumene hydroperoxide. Chin. Chem. Lett. 2017, 28, 1057–1061. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Sainz-Pardo, J.; de Frutos, P.; Blázquez, S. Agglomeration of Ti-SBA-15 with clays for liquid phase olefin epoxidation in a continuous fixed bed reactor. Chem. Eng. J. 2008, 139, 631–641. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Saha, B. Greener and sustainable approach for the synthesis of commercially important epoxide building blocks using polymer-supported Mo(VI) complexes as catalysts. In Ion Exchange and Solvent Extraction, 1st ed.; SenGupta, A.K., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group LLC: Milton Park, UK, 2016; p. 33. [Google Scholar]
- Fadhli, M.; Khedher, I.; Fraile, J.M. Enantioselective epoxidation of styrene with TBHP catalyzed by bis(oxazoline)–vanadyl–laponite materials. Catal. Commun. 2018, 117, 90–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Gao, R.; Dai, W.-L. Manganese-doped CeO2 nanocubes as highly efficient catalysts for styrene epoxidation with TBHP. Appl. Surf. Sci. 2019, 471, 767–775. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Mbeleck, R.; Saha, B. Efficient and selective molybdenum based heterogeneous catalyst for alkene epoxidation using batch and continuous reactors. Polym. Chem. 2014, 6, 7308–7319. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Mbeleck, R.; Patel, D.; Sherrington, D.C.; Saha, B. Greener route to 4-vinyl-1-cyclohexane 1,2-epoxide synthesis using batch and continuous reactors. Green Process Synth. 2014, 3, 411–418. [Google Scholar] [CrossRef]
- Singh, B.; Rana, B.; Sivakumar, L.; Bahuguna, G.; Sinha, A. Efficient catalytic epoxidation of olefins with hierarchical mesoporous TS-1 using TBHP as an oxidant. J. Porous Mat. 2012, 20, 397–405. [Google Scholar] [CrossRef]
- Lueangchaichaweng, W.; Singh, B.; Mandelli, D.; Carvalho, W.A.; Fiorilli, S.; Pescarmona, P.P. High surface area, nanostructured boehmite and alumina catalysts: Synthesis and application in the sustainable epoxidation of alkenes. Appl. Catal. A-Gen. 2019, 571, 180–187. [Google Scholar] [CrossRef]
- Mikolajska, E.; Calvino-Casilda, V.; Bañares, M.A. Real-time Raman monitoring of liquid-phase cyclohexene epoxidation over alumina-supported vanadium and phosphorous catalysts. Appl. Catal. A-Gen. 2012, 421–422, 164–171. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, P.; Zhang, J.; Bian, G.; Zhang, P.; Dong, Y.; Zhang, W. Highly dispersed molybdenum incorporated hollow mesoporous silica spheres as an efficient catalyst on epoxidation of olefins. Mol. Catal. 2017, 433, 212–223. [Google Scholar] [CrossRef]
- Bisio, C.; Gallo, A.; Psaro, R.; Tiozzo, C.; Guidotti, M.; Carniato, F. Tungstenocene-grafted silica catalysts for the selective epoxidation of alkenes. Appl. Catal. A-Gen. 2019, 581, 133–142. [Google Scholar] [CrossRef]
- Cai, L.; Chen, C.; Wang, W.; Gao, X.; Kuang, X.; Jiang, Y.; Li, L.; Wu, G. Acid-free epoxidation of soybean oil with hydrogen peroxide to epoxidized soybean oil over titanium silicalite-1 zeolite supported cadmium catalysts. J. Ind. Eng. Chem. 2020, 91, 191–200. [Google Scholar] [CrossRef]
- Wu, Z.; He, Z.; Zhou, D.; Yang, Y.; Lu, X.; Xia, Q. One-step synthesis of bi-functional zeolite catalyst with highly exposed octahedral Co for efficient epoxidation of bulky cycloalkenes. Mater. Lett. 2020, 280, 128549. [Google Scholar] [CrossRef]
- Mohammadikish, M.; Yarahmadi, S.; Molla, F. A new water-insoluble coordination polymer as efficient dye adsorbent and olefin epoxidation catalyst. J. Environ. Manag. 2020, 254, 109784. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Epoxidation of castor oil fatty acid methyl esters (COFAME) as a Lubricant base stock using heterogeneous ion-exchange resin (IR-120) as a catalyst. Energy Procedia 2014, 54, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Lu, X.H.; Ma, X.T.; Shen, Y.; Wei, C.C.; He, J.; Zhou, D.; Xia, Q.H. Highly efficient epoxidation of cyclohexene with aqueous H2O2 over powdered anion-resin supported solid catalysts. J. Mol. Catal. A-Chem. 2016, 423, 393–399. [Google Scholar] [CrossRef]
- Otake, K.-i.; Ahn, S.; Knapp, J.; Hupp, J.T.; Notestein, J.M.; Farha, O.K. Vapor-phase cyclohexene epoxidation by single-ion Fe(III) sites in metal–organic frameworks. Inorg. Chem. 2021, 60, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Abbasi, A.; Masteri-Farahani, M.; Rodrigues, V.H.N. Synthesis, characterization and crystal structure of a copper molybdate coordination polymer as an epoxidation catalyst. Inorg. Chim. Acta. 2015, 433, 21–25. [Google Scholar]
- McMurry, J. Organic Chemistry; Brooks/Cole Cengage Learning: Southbank, VIC, Australia, 2012. [Google Scholar]
- Parod, R.J. Ethylene oxide. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 535–538. [Google Scholar]
- Meng, Y.; Taddeo, F.; Aguilera, A.F.; Cai, X.; Russo, V.; Tolvanen, P.; Leveneur, S. The lord of the chemical rings: Catalytic synthesis of important industrial epoxide compounds. Catalysts 2021, 11, 765. [Google Scholar] [CrossRef]
- Kück, J.W.; Reich, R.M.; Kühn, F.E. Molecular epoxidation reactions catalyzed by rhenium, molybdenum, and iron complexes. Chem. Rec. 2016, 16, 349–364. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchionia, F.; Pescarmona, P.P. CO2 -fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef] [Green Version]
- Cuesta-Aluja, L.; Castilla, J.; Masdeu-Bultó, A.M.; Henriques, C.A.; Calvete, M.J.F.; Pereira, M.M. Halogenated meso-phenyl Mn(III) porphyrins as highly efficient catalysts for the synthesis of polycarbonates and cyclic carbonates using carbon dioxide and epoxides. J Mol. Catal. A-Chem. 2016, 423, 489–494. [Google Scholar] [CrossRef]
- Mandal, M. Group 4 complexes as catalysts for the transformation of CO2 into polycarbonates and cyclic carbonates. J. Organomet. Chem. 2020, 907, 121067. [Google Scholar] [CrossRef]
- Onyenkeadi, V.; Kellici, S.; Saha, B. Greener synthesis of 1,2-butylene carbonate from CO2 using graphene-inorganic nanocomposite catalyst. Energy 2018, 165, 867–876. [Google Scholar] [CrossRef]
- Adeleye, A.I.; Kellici, S.; Heil, T.; Morgan, D.; Vickers, M.; Saha, B. Greener synthesis of propylene carbonate using graphene-inorganic nanocomposite catalysts. Catal. Today 2015, 256, 347–357. [Google Scholar] [CrossRef]
- Cavdar, H.; Saracoglu, N. Ring opening of epoxides with NaHSO4: Isolation of β-hydroxy sulfate esters and an effective synthesis for trans-diols. Tetrahedron 2009, 65, 985–989. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wu, M.-D.; Zhu, X.-X.; Zhang, X.-D.; Zhang, C.; Xu, Y.-H.; Wu, M.-C. Remarkable improvement in the regiocomplementarity of a Glycine max epoxide hydrolase by reshaping its substrate-binding pocket for the enantioconvergent preparation of (R)-hexane-1,2-diol. Mol. Catal. 2021, 514, 111851. [Google Scholar] [CrossRef]
- Wade, L.G. Organic Chemistry, 8th ed.; Pearson: Glenview, IL, USA, 2013. [Google Scholar]
- Delost, M.D.; Njardarson, J.T. Oxiranes and oxirenes: Monocyclic. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Gras, E.; Sadek, O. Oxiranes and oxirenes: Fused-ring derivatives. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Davies, T.E.; Kondrat, S.A.; Nowicka, E.; Kean, J.L.; Harris, C.M.; Socci, J.M.; Apperley, D.C.; Taylor, S.H.; Graham, A.E. Nanoporous alumino- and borosilicate-mediated Meinwald rearrangement of epoxides. Appl. Catal. A-Gen. 2015, 493, 17–24. [Google Scholar] [CrossRef]
- García-Muñoz, R.A.; Serrano, D.P.; Vicente, G.; Linares, M.; Vitvarova, D.; Čejka, J. Remarkable catalytic properties of hierarchical zeolite-Beta in epoxide rearrangement reactions. Catal. Today 2015, 243, 141–152. [Google Scholar] [CrossRef]
- Hoang, P.H.; Dien, L.Q. Synthesis of magnetically recyclable ZSM-5 zeolite for styrene epoxide rearrangement reaction. Chem. Eng. J. 2015, 262, 140–145. [Google Scholar] [CrossRef]
- Keshavarz, M.; Zarei Ahmady, A.; Mostoufi, A.; Mohtasham, N. One-pot green regioselesctive synthesis of γ-lactones from epoxides and ketene silyl acetals using 1,3-dimethylimidazolium fluoride as a recoverable metal-free catalyst. Molecules 2017, 22, 1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonollo, S.; Ahmady, A.Z.; Petrucci, C.; Marrocchi, A.; Pizzo, F.; Vaccaro, L. A catalytic approach to the metal-free reaction of epoxides with ketene silyl acetals for accessing γ-lactones. Org. Lett. 2014, 16, 5721–5723. [Google Scholar] [CrossRef]
- Chen, X.; Sumoto, K.; Mitani, S.; Yamagami, T.; Yokoyama, K.; Wang, P.; Hirao, S.; Nishiwaki, N.; Kobiro, K. One-step and non-catalytic intramolecular redox reactions of conjugated all E-dienals to non-conjugated Z-enoic acids in subcritical water. J. Supercrit. Fluids. 2012, 62, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Sancineto, L.; Mangiavacchi, F.; Tidei, C.; Bagnoli, L.; Marini, F.; Gioiello, A.; Scianowski, J.; Santi, C. Selenium-catalyzed oxacyclization of alkenoic acids and alkenols. Asian J. Org. Chem. 2017, 6, 988–992. [Google Scholar] [CrossRef]
- Vidović, D. Aluminum complexes in organic. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Florio, S.; Perna, F.M.; Salomone, A.; Vitale, P. 8.29 reduction of epoxides. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1086–1122. [Google Scholar]
- Yan, Z.; Ma, Z.; Deng, J.; Luo, G. Mechanism and kinetics of epoxide ring-opening with carboxylic acids catalyzed by the corresponding carboxylates. Chem. Eng. Sci. 2021, 242, 116746. [Google Scholar] [CrossRef]
- Desport, J.S.; Mantione, D.; Moreno, M.; Sardón, H.; Barandiaran, M.J.; Mecerreyes, D. Synthesis of three different galactose-based methacrylate monomers for the production of sugar-based polymers. Carbohydr. Res. 2016, 432, 50–54. [Google Scholar] [CrossRef]
- Matsuzaki, T.N. Method of Producing Glycidyl Methacrylate. U.S. Patent 7176328, 13 February 2007. [Google Scholar]
- Yan, Z.; Deng, J.; Chen, Y.; Luo, G. Preparation of 2,3-epoxypropyl neodecanoate: Process optimization and mechanism discussion. Ind. Eng. Chem. Res. 2020, 59, 19168–19176. [Google Scholar] [CrossRef]
- Yan, Z.; Du, C.; Luo, G.; Deng, J. Remarkable improvement of epoxide ring-opening reaction efficiency and selectivity with water as a green regulator. React. Chem. Eng. 2021, 6, 2159–2169. [Google Scholar] [CrossRef]
- Tanbouza, N.; Ollevier, T.; Lam, K. Bridging lab and industry with flow electrochemistry. iScience 2020, 23, 101720. [Google Scholar] [CrossRef] [PubMed]
- Aguillón, A.R.; Bezerra, M.A.d.M.; Gomez, M.R.B.P.; de Souza, R.O.M.A. Continuous-flow chemistry toward sustainable chemical synthesis. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Boddula, R., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–69. [Google Scholar]
- Hartman, R.L. Flow chemistry remains an opportunity for chemists and chemical engineers. Curr. Opin. Chem. Eng. 2020, 29, 42–50. [Google Scholar] [CrossRef]
- UNIQSIS, FlowSyn—Flow Chemistry Made Simple. Available online: https://www.uniqsis.com/paProducts.aspx (accessed on 13 September 2021).
- Mbeleck, R.; Ambroziak, K.; Saha, B.; Sherrington, D.C. Stability and recycling of polymer-supported Mo(VI) alkene epoxidation catalysts. React. Funct. Polym. 2007, 67, 1448–1457. [Google Scholar] [CrossRef]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. Epoxidation Process. European Patent Number EP2459545B1, 28 February 2019. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. A Continuous Process for the Liquid Phase Epoxidation of an Olefinic Compound. Indian Patent No. 295846, 17 April 2018. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. A Permeable Particle Container. Chinese Patent No. ZL 201410840925.7, 7 December 2016. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. Liquid Phase Epoxidation Process. US Patent Number US 9,248,942 B2, 2 February 2016. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. A Continuous Process for the Liquid Phase Epoxidation of an Olefinic Compound with an Oxidant. Chinese Patent Number ZL201080044175.2, 11 February 2015. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. Liquid Phase Epoxidation Process. US Patent Number US 8,759,552 B2, 24 June 2014. [Google Scholar]
- Ambroziak, K.; Mbeleck, R.; He, Y.; Saha, B.; Sherrington, D.C. Investigation of batch alkene epoxidations catalyzed by polymer-supported Mo(VI) complexes. Ind. Eng. Chem. Res. 2009, 48, 3293–3302. [Google Scholar] [CrossRef]
- Kiss, A.A.; Jobson, M.; Gao, X. Reactive distillation: Stepping up to the next level of process intensification. Ind. Eng. Chem. Res. 2019, 58, 5909–5918. [Google Scholar] [CrossRef] [Green Version]
- Okhlopkova, E.A.; Serafimov, L.A.; Frolkova, A.V. Methods of preparing epichlorohydrin. Theor. Found. Chem. Eng. 2019, 53, 864–870. [Google Scholar] [CrossRef]
- Bell, B.; Briggs, J.; Campbell, R.; Chambers, S.; Gaarenstroom, P.; Hippler, J.; Hook, B.; Kearns, K.; Kenney, J.; Kruper, W.; et al. Glycerin as a renewable feedstock for epichlorohydrin production. The GTE process. Clean-Soil Air Water 2008, 36, 657–661. [Google Scholar] [CrossRef]
- Almena, A.; Martín, M. Technoeconomic analysis of the production of epichlorohydrin from glycerol. Ind. Eng. Chem. Res. 2016, 55, 3226–3238. [Google Scholar] [CrossRef]
- Santacesaria, E.; Tesser, R.; Di Serio, M.; Casale, L.; Verde, D. New process for producing epichlorohydrin via glycerol chlorination. Ind. Eng. Chem. Res. 2009, 49, 964–970. [Google Scholar] [CrossRef]
- Lari, G.M.; Pastore, G.; Haus, M.; Ding, Y.; Papadokonstantakis, S.; Mondelli, C.; Pérez-Ramírez, J. Environmental and economical perspectives of a glycerol biorefinery. Energy Environ. Sci. 2018, 11, 1012–1029. [Google Scholar] [CrossRef]
- Lari, G.M.; Pastore, G.; Mondelli, C.; Pérez-Ramírez, J. Towards sustainable manufacture of epichlorohydrin from glycerol using hydrotalcite-derived basic oxides. Green Chem. 2018, 20, 148–159. [Google Scholar] [CrossRef]
- Gupta, D.; Jamwal, D.; Rana, D.; Katoch, A. 26-Microwave synthesized nanocomposites for enhancing oral bioavailability of drugs. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 619–632. [Google Scholar]
- Ewis, D.; Hameed, B.H. A review on microwave-assisted synthesis of adsorbents and its application in the removal of water pollutants. J. Water Process. Eng. 2021, 41, 102006. [Google Scholar] [CrossRef]
- Zeng, X.; Hu, X.; Song, H.; Xia, G.; Shen, Z.-Y.; Yu, R.; Moskovits, M. Microwave synthesis of zeolites and their related applications. Micropor. Mesopor. Mat. 2021, 323, 111262. [Google Scholar] [CrossRef]
- Aguilera, A.F.; Tolvanen, P.; Eränen, K.; Wärnå, J.; Leveneur, S.; Marchant, T.; Salmi, T. Kinetic modelling of Prileschajew epoxidation of oleic acid under conventional heating and microwave irradiation. Chem. Eng. Sci. 2019, 199, 426–438. [Google Scholar] [CrossRef]
- Ramírez, A.; Hueso, J.L.; Mallada, R.; Santamaría, J. Ethylene epoxidation in microwave heated structured reactors. Catal. Today 2016, 273, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.H.; Lei, J.; Wei, X.L.; Ma, X.T.; Zhang, T.J.; Hu, W.; Zhou, D.; Xia, Q.H. Selectively catalytic epoxidation of α-pinene with dry air over the composite catalysts of Co–MOR(L) with Schiff-base ligands. J. Mol. Catal. A Chem. 2015, 400, 71–80. [Google Scholar] [CrossRef]
- Tao, P.; Lu, X.; Zhang, H.; Jing, R.; Huang, F.; Wu, S.; Zhou, D.; Xia, Q. Enhanced activity of microwave-activated CoOx/MOR catalyst for the epoxidation of α-pinene with air. Mol. Catal. 2019, 463, 8–15. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Tao, P.; Lu, X.; Li, X.; Wang, C.; Wang, B.; Yue, F.; Zhou, D.; Xia, Q. Microwave-assisted air epoxidation of mixed biolefins over a spherical bimetal ZnCo-MOF catalyst. ACS Appl. Mater. Interfaces 2021, 13, 8474–8487. [Google Scholar] [CrossRef]
- Wirth, T. Microreactors in Organic Chemistry and Catalysis, 2nd ed.; Willey-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Domínguez, M.I.; Centeno, M.A.; Martínez, T.M.; Bobadilla, L.F.; Laguna, Ó.H.; Odriozola, J.A. Current scenario and prospects in manufacture strategies for glass, quartz, polymers and metallic microreactors: A comprehensive review. Chem. Eng. Res. Des. 2021, 171, 13–35. [Google Scholar] [CrossRef]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K. Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792–821. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Du, L.; Liu, J.; Yao, J. Review of the applications of microreactors. Renew. Sustain. Energy Rev. 2015, 47, 519–539. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Fanelli, F.; Parisi, G.; Degennaro, L.; Luisi, R. Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis. Beilstein J. Org. Chem. 2017, 13, 520–542. [Google Scholar] [CrossRef] [Green Version]
- Phimsen, S.; Yamada, H.; Tagawa, T.; Kiatkittipong, W.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Epoxidation of methyl oleate in a TiO2 coated-wall capillary microreactor. Chem. Eng. J. 2017, 314, 594–599. [Google Scholar] [CrossRef]
- Ogunyinka, O.; Iza, F.; Buckley, B.; Bandulasena, H.C.H. Epoxidation of trans-stilbene in a microfluidic plasma reactor. Chem. Eng. Sci. 2021, 240, 116665. [Google Scholar] [CrossRef]
- Ciemięga, A.; Maresz, K.; Malinowski, J.J.; Mrowiec-Białoń, J. Continuous-flow monolithic silica microreactors with arenesulphonic acid groups: Structure—Catalytic activity relationships. Catalysts 2017, 7, 255. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Gamliel, D.P.; Valla, J.A.; Bollas, G.M. Fischer-tropsch synthesis in monolith catalysts coated with hierarchical ZSM-5. Appl. Catal. B-Environ. 2021, 284, 119719. [Google Scholar] [CrossRef]
- García-Moncada, N.; Navarro, J.C.; Odriozola, J.A.; Lefferts, L.; Faria, J.A. Enhanced catalytic activity and stability of nanoshaped Ni/CeO2 for CO2 methanation in micro-monoliths. Catal. Today 2022, 383, 205–215. [Google Scholar] [CrossRef]
- Cifuentes, B.; Cifuentes, A.; Bustamante, F.; Soler, L.; Llorca, J.; Cobo, M. Monoliths washcoated with AuCu catalysts for CO removal in an ethanol fuel processor: Effect of CeO2–SiO2 dual support on the catalytic performance and reactor cost. Int. J. Hydrogen Energy 2021, 46, 2166–2181. [Google Scholar] [CrossRef]
- Alimi, O.A.; Ncongwane, T.B.; Meijboom, R. Design and fabrication of a monolith catalyst for continuous flow epoxidation of styrene in polypropylene printed flow reactor. Chem. Eng. Res. Des. 2020, 159, 395–409. [Google Scholar] [CrossRef]
- Shin, S.B.; Chadwick, D. Comparison of a monolith and a confined Taylor flow (CTF) reactor for propene epoxidation. Chem. Eng. Process 2018, 125, 173–182. [Google Scholar] [CrossRef]
- Qiao, S.Z.; Liu, J.; Max Lu, G.Q. Chapter 21—Synthetic Chemistry of Nanomaterials. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 613–640. [Google Scholar]
- Schiel, M.A.; Chopa, A.B.; Silbestri, G.F.; Alvarez, M.B.; Lista, A.G.; Domini, C.E. Use of ultrasound in the synthesis of heterocycles of medicinal interest. In Green Synthetic Approaches for Biologically Relevant Heterocycles; Brahmachari, G., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 571–601. [Google Scholar]
- Pokhrel, N.; Vabbina, P.K.; Pala, N. Sonochemistry: Science and engineering. Ultrason. Sonochem. 2016, 29, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Penteado, F.; Monti, B.; Sancineto, L.; Perin, G.; Jacob, R.G.; Santi, C.; Lenardão, E.J. Ultrasound-assisted multicomponent reactions, organometallic and organochalcogen chemistry. Asian J. Org. Chem. 2018, 7, 2368–2385. [Google Scholar] [CrossRef]
- Savun-Hekimoğlu, B. A review on sonochemistry and its environmental applications. Acoustics 2020, 2, 766–775. [Google Scholar] [CrossRef]
- Cousin, T.; Chatel, G.; Kardos, N.; Andrioletti, B.; Draye, M. High frequency ultrasound as a tool for elucidating mechanistic elements of cis-cyclooctene epoxidation with aqueous hydrogen peroxide. Ultrason. Sonochem. 2019, 53, 120–125. [Google Scholar] [CrossRef]
- Charbonneau, L.; Foster, X.; Kaliaguine, S. Ultrasonic and Catalyst-Free Epoxidation of Limonene and Other Terpenes Using Dimethyl Dioxirane in Semibatch Conditions. ACS Sustain. Chem. Eng. 2018, 6, 12224–12231. [Google Scholar] [CrossRef]
- Chavan, V.P.; Patwardhan, A.V.; Gogate, P.R. Intensification of epoxidation of soybean oil using sonochemical reactors. Chem. Eng. Process. 2012, 54, 22–28. [Google Scholar] [CrossRef]

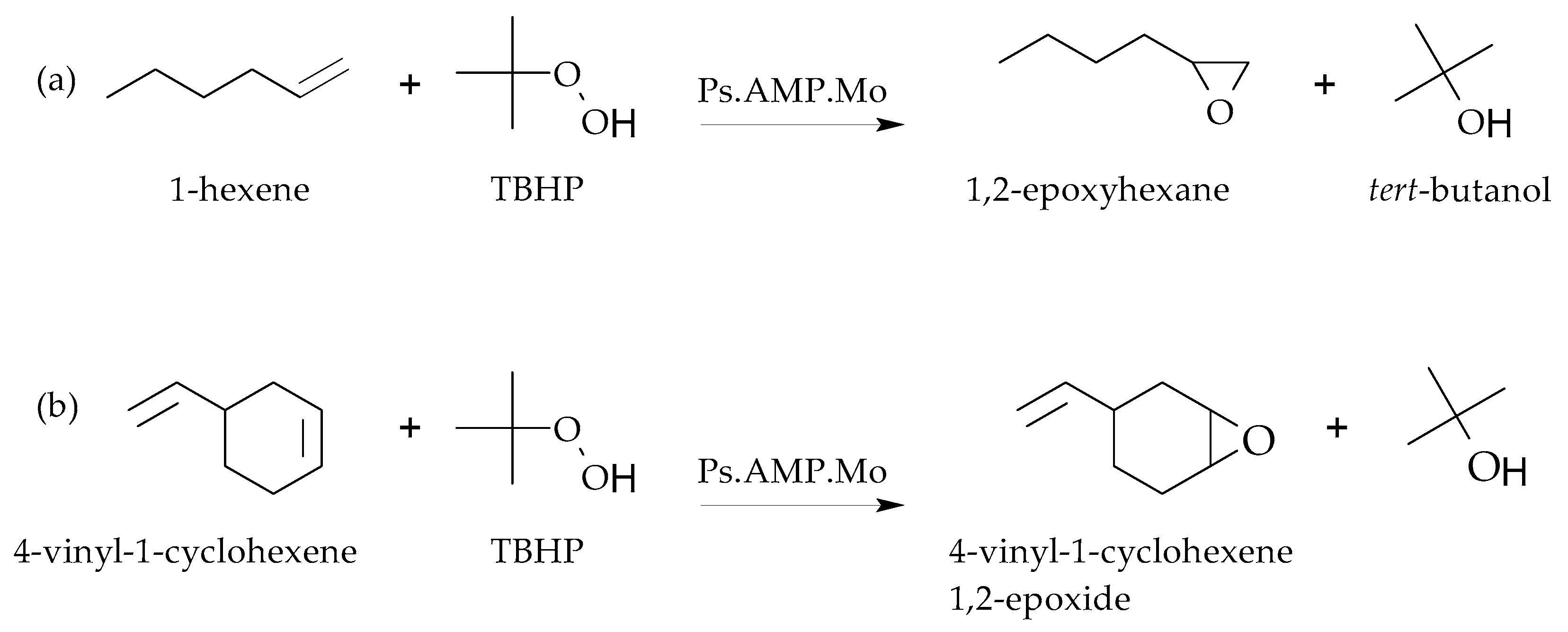
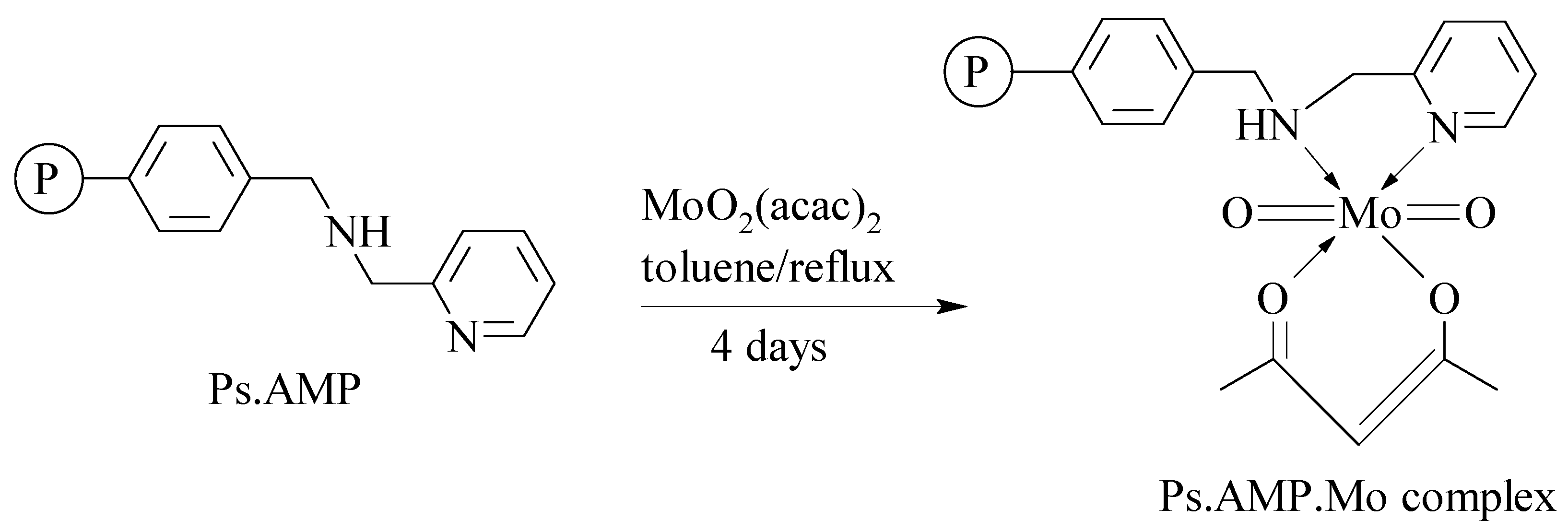





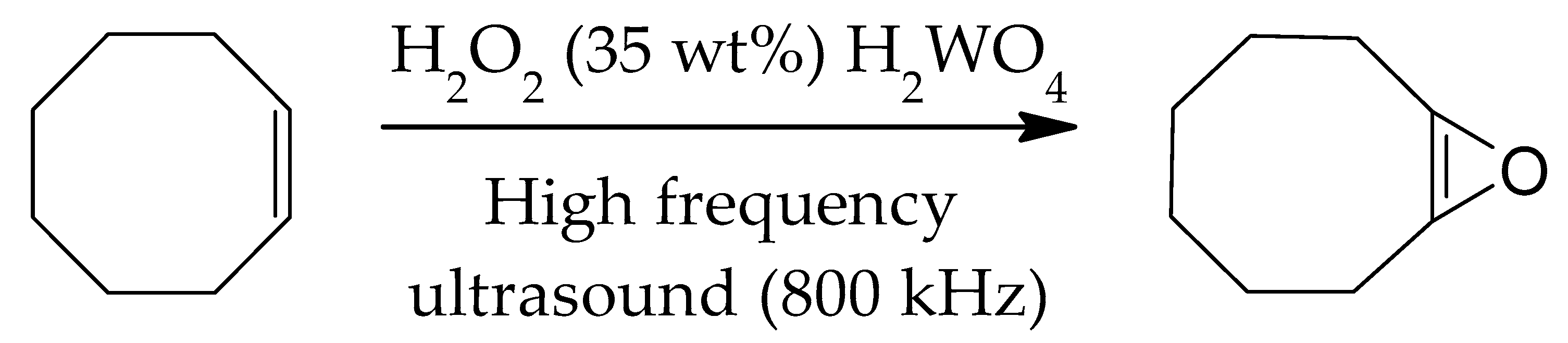
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, M.L.; Saha, B. Recent Advances in Greener and Energy Efficient Alkene Epoxidation Processes. Energies 2022, 15, 2858. https://doi.org/10.3390/en15082858
Mohammed ML, Saha B. Recent Advances in Greener and Energy Efficient Alkene Epoxidation Processes. Energies. 2022; 15(8):2858. https://doi.org/10.3390/en15082858
Chicago/Turabian StyleMohammed, Misbahu Ladan, and Basudeb Saha. 2022. "Recent Advances in Greener and Energy Efficient Alkene Epoxidation Processes" Energies 15, no. 8: 2858. https://doi.org/10.3390/en15082858
APA StyleMohammed, M. L., & Saha, B. (2022). Recent Advances in Greener and Energy Efficient Alkene Epoxidation Processes. Energies, 15(8), 2858. https://doi.org/10.3390/en15082858







