Abstract
The chemical industry is considered to be one of the largest consumers of energy in the manufacturing sector. As the cost of energy is rising rapidly, coupled with the increasingly stringent standards for the release of harmful chemicals and gases into the environment, more attention is now focused on developing energy efficient chemical processes that could significantly reduce both operational costs and greenhouse gas emissions. Alkene epoxidation is an important chemical process as the resultant epoxides are highly reactive compounds that are used as platform chemicals for the production of commercially important products for flavours, fragrances, paints and pharmaceuticals. A number of epoxidation methods have been developed over the past decade with the ultimate aim of minimising waste generation and energy consumption. In this review paper, some of the recent advances in epoxides synthesis using energy efficient processes are discussed. The epoxidation methods may provide sustainability in terms of environmental impact and energy consumption.
1. Introduction
The rate of energy consumption, either for electricity, steam or heating purposes, has increased rapidly during the past decades due to rapid global developments in the transportation, industrial, agricultural, residential, commercial and other related sectors [1]. Fossil fuels including coal, oil, and natural gas are currently the largest sources of energy worldwide. However, the use of carbon-based fossil fuels has contributed significantly to an increase in the concentration of CO2 and related greenhouse gases in the atmosphere, with negative impacts on the environment [2,3,4]. Consequently, highly industrialised nations popularly known as the G8 have promulgated measures aimed at cutting greenhouse gas emissions associated with energy exploration and utilisation to prevent further deterioration of the environment [5,6]. In this respect, miniaturisation of chemical processes through intensification and integration of multiple operations plays a significant role by providing means of reducing energy consumption and waste generation [7,8,9,10].
Epoxides, also known as oxiranes, are mainly products of alkene epoxidation. They are key raw materials or intermediates in organic synthesis, particularly for the functionalisation of substrates and the production of a wide variety of chemicals such as pharmaceuticals, plastics, paints and adhesives [11,12,13,14,15]. The highest consumption of ethylene oxide (C2H4O) worldwide is in the production of ethylene glycol (C2H6O2), which is a building block in the production of pharmaceuticals, textiles, automobiles and detergents [16]. Similarly, propylene oxide (C3H6O) has found applications in the production of cosmetics, drugs, and plasticisers, and in the manufacture of unsaturated polyester resins used in the textile and construction industries [17]. Furthermore, epichlorohydrin (C3H5ClO) is an important epoxide that is widely employed in the manufacture of epoxy resins, glycerols, plastics and elastomers [18]. Epoxidation of limonene and α-pinene yields the corresponding 1,2-limonene oxide and α-pinene oxide, which are both vital intermediates for the production of fragrances, perfumes, food additives and pharmaceuticals [19].
The conventional epoxidation methods in the fine chemical industry employ in situ generated peracids such as peracetic (C2H4O3) or performic acid (CH2O3) as an oxidising reagent in liquid phase batch reactions in the presence of a mineral acid as a catalyst [20,21]. However, the employment of peracid for alkene epoxidation is not an environmentally benign method as an equivalent amount of acid waste is produced. In addition, there are safety issues associated with the handling and storage of peracid [21]. Molecular oxygen is probably the most suitable oxidant for alkene epoxidation in terms of environmental and economic considerations due to its high oxygen content and its ability to produce water as the only by-product. However, one of the major limitations of epoxidation with molecular oxygen is low product selectivity [22]. Similarly, hydrogen peroxide (H2O2) is another eco-friendly reagent for epoxidation because water is its only waste product [23]. As in the case with molecular oxygen, epoxidation with H2O2 can result in poor product selectivity [24], although researchers in recent years have developed catalytic systems that can activate the oxidant to yield up to 98% epoxide selectivity [25]. By comparison, there are limited applications of hypochlorites as oxygen sources for epoxidation due to their serious health and environmental hazards; these include the release of toxic gases such as chlorine when acidified or heated, and their reaction with ammonia or with substances that generate ammonia to yield chloramines, which are also toxic and have the explosive potential [26,27]. Alkyl hydroperoxides including tert-butyl hydroperoxide (C4H10O2) (TBHP), cumene hydroperoxide (C9H12O2) and ethylbenzyl hydroperoxide (C9H12O2) are commonly used as oxygen sources in epoxidation reactions since the reagents are readily available and inexpensive [28,29,30]. However, tert-butyl hydroperoxide (TBHP) has received considerable attention in recent times as an oxidant of choice for the reaction due to its numerous advantages including high thermal conductivity, good solubility in polar solvents, and neutral pH [31,32,33]. In addition, the oxidant is atom efficient since it yields tert-butanol, a major industrial feedstock, as a by-product [34,35,36].
There are numerous examples of epoxidation reactions carried out with a broad range of heterogeneous catalysts based on either transition metals or main group elements. Some of the catalysts tend to be more active on certain alkene substrates than others and the selectivity of a particular catalyst for epoxidation depends on the properties of the active component, the combination of ligands and the oxidant used. For instance, a number of heterogeneous catalysts have been developed for epoxidation reaction by immobilisation of catalytically active metal species on organic or inorganic materials, such as alumina [37,38], silica [39,40], zeolites [41,42], polymers [43], ion-exchange resins [44,45] and metal organic frameworks [46]. However, polymers have gained more attention as a suitable support for transition metal catalysts as they are inert, non-toxic, insoluble and often recyclable [11,12,13,31,34,47].
The main purpose of this review paper is to highlight some of the techniques being developed to achieve intensification of the alkene epoxidation process by reducing the volume of hazardous material and replacing large, energy-intensive equipment and stages with those that are smaller, less expensive and energy efficient.
2. Important Reactions of Epoxides
Epoxides are highly reactive cyclic ethers having a three-membered ring structure with an oxygen atom connected to two neighbouring carbon atoms by single bonds. The high reactivity of epoxides is due to their polarity and the strain of the three-membered ring, which weakens the carbon-oxygen (C-O) bond [48]. As shown in Scheme 1, epoxides can readily undergo ring-opening reactions and form a variety of products that can serve as a starting material or intermediate in the production of commercially important products for flavours, adhesives, fragrances, paints and pharmaceuticals [14,48,49]. The reactions involving epoxides are usually catalysed by many acidic and basic substances, and by acceptor-donor compounds [50,51].

Scheme 1.
Schemes of some important reactions of epoxide.
The reaction of epoxides and CO2 yields carbonates and polycarbonates as two major valuable products [52]. However, the selectivity toward either of the products depends on the reaction conditions and the type of catalysts used [53,54]. A number of transition metal-based catalysts have shown good activity in the solvent-free synthesis of propylene carbonate by cycloaddition reaction of propylene oxide and CO2 under mild reaction conditions [55,56].
Epoxides can be cleaved in hydrolysis by either aqueous base (NaOH/H2O) or acid (H2SO4/H2O) to form vicinal diol in the SN2 or SN1 mechanism [57,58]. In the presence of an aqueous base, the nucleophile (H2O) attacks the least substituted carbon atom based on an SN2-like reaction leading to ring-opening of the epoxide, whereas in the aqueous acid solution, the more substituted carbon is the site of nucleophilic attack according to the SN1 displacement mechanism [59]. Similarly, epoxides undergo cleavage of the ether bond in the presence of anhydrous acids including HCl, HI, HBr to form halohydrin in the SN1- or SN2-like reactions, depending on whether the carbon attacked by the halogen anion is a primary, secondary or tertiary carbon [60,61].
Epoxides are capable of undergoing intramolecular rearrangement often initiated by Lewis or Brꝋnsted acids to form aldehydes or ketones [62,63,64]. Ring-opening of epoxide with Ketene Silyl Acetals is regarded as the best approach for the preparation of an important class of extensive biologically active compounds, γ-lactones [65,66,67,68]. Furthermore, the reduction of epoxide by lithium aluminium hydride (LiAlH4) occurs at the least sterically hindered side of the epoxide to give the corresponding alcohol [69,70]. The nucleophilic addition reaction of epoxides with carboxylic acids such as pivalic acid (C5H10O2) results in acidolysis ring-opening to form hydroxyalkyl esters [71]. However, when epichlorohydrin (C3H5ClO) is used as the epoxy compound, the hydroxyalkyl ester formed can further undergo the ring-closure reaction with alkali by dehydrochlorination to yield vital intermediates for the coating and polymer industries, such as glycidyl methacrylate [72,73] and glycidyl neodecanoate [74,75]. The reaction schemes of the notable reactions of epoxides and their products are presented in Scheme 1.
3. Energy Efficient Alkene Epoxidation Processes
The search for an energy efficient method for the preparation of epoxide has been an active research area in recent years due to the significance of the compound, not only as a synthetic end product but as a versatile intermediate in organic synthesis. To date, several methods of epoxide synthesis have been developed and most of these methods employ alkene as a starting material, which is subsequently oxidised by a suitable reagent to yield the desired product. However, some alternative methods that utilise aldehydes and imines for the production of epoxides have also been reported.
3.1. Continuous Flow Epoxidation Process
The development of a continuous flow approach to synthetic organic chemistry has received considerable interest over the last few decades [76,77,78]. The inherently closed nature of continuous flow reactors enhances the reaction safety due to proper containment of harmful or toxic reagents, and allows rapid optimisation of reaction conditions from a small quantity of reactants under different reaction conditions with minimal energy consumption. The FlowSyn reactor (supplied by Uniqsis Ltd.) is one of the energy efficient continuous flow reactors currently employed for laboratory-scale synthesis of various organic products [79]. We earlier employed the FlowSyn reactor for continuous epoxidation of 1-hexene (C6H12) and 4-vinyl-1-cyclohexene (C8H12) using tert-butyl hydroperoxide (TBHP) and a polystyrene 2-(aminomethyl)pyridine supported molybdenum(VI) complex (Ps.AMP.Mo) as a catalyst (Scheme 2) [12,31]. The catalyst was prepared by immobilising the molybdenum metal species derived from molybdenyl acetylacetonate (MoO2(acac)2) on an already synthesised cross-linked polystyrene-based resin code-named polystyrene 2-aminomethyl(pyridine) (Ps.AMP), as shown in Scheme 3, Scheme 4 and Scheme 5.

Scheme 2.
Reaction schemes for epoxidation of (a) 1-hexene; (b) 4-vinyl-1-cyclohexene with TBHP catalysed by Ps.AMP.Mo complex.

Scheme 3.
Synthesis of poly (divinylbenzene-co-vinylbenzyl chloride-co-styrene) (Ps.VBC) resin.

Scheme 4.
Synthesis of polystyrene 2-(aminomethyl)pyridine (Ps.AMP) beads.
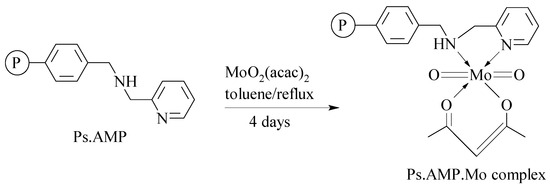
Scheme 5.
Loading of Mo(VI) into polystyrene 2-(aminomethyl)pyridine (Ps.AMP) beads to produce polystyrene 2-(aminomethyl)pyridine supported Mo(VI) (Ps.AMP.Mo) complex.
Continuous flow epoxidation has enabled rapid evaluation of the catalytic performance of Ps.AMP.Mo from a small quantity of reactants under different reaction conditions. For instance, the catalyst has demonstrated high catalytic performance in the continuous epoxidation of 4-vinyl-1-cyclohexene (C8H12) as compared to 1-hexene (C6H12). An experiment carried out at a feed molar ratio of 4-vinyl-1-cyclohexene (C8H12) to TBHP of 5:1, reaction temperature of 353 K and feed flow rate of 0.1 mL/min resulted in ~95% conversion of TBHP and ~82% yield of 4-vinyl-1-cyclohexane 1,2-epoxide (C8H12O) at steady state. However, continuous epoxidation of 1-hexene (C6H12) at similar conditions gave ~79% conversion of TBHP and ~64% yield of 1,2-epoxyhexane. The continuous epoxidation in a FlowSyn reactor has shown considerable time savings, high reproducibility and selectivity, along with remarkable improvements in catalyst stability compared with the reactions carried out in a batch reactor [13,80]. The schematic of the experimental set-up for the continuous epoxidation process in the FlowSyn reactor is shown in Figure 1.

Figure 1.
Schematic representation of continuous epoxidation of 4-vinyl-1-cyclohexene with TBHP using a FlowSyn reactor.
3.2. Epoxidation in a Reactive Distillation Column (RDC)
Reactive distillation (RD) is a unit operation that combines chemical reaction and distillation in the same vessel in a single step. By conducting chemical reaction and product separation simultaneously, equilibrium can be shifted to achieve almost complete conversion of the feedstock [81,82,83,84,85,86,87]. Thus, RD technology has many key advantages, such as reduced investment and operating costs, and significant energy savings, as it can simplify complex separation and purification processes [88]. Saha and co-workers [81,82,83,84,85,86,87] designed an RDC for the continuous epoxidation process using molybdenum-based complexes as catalysts for epoxidation of alkenes/terpenes using tert-butyl hydroperoxide (TBHP) as an oxidant. The RDC consists of three distinct parts: a catalytic section containing the catalyst, packed in a well-structured “rolled belt” type catalyst packing. The catalytic section is enclosed between a non-reactive enriching section and a non-reactive stripping section, both packed with ceramic Raschig rings, as shown in Figure 2.

Figure 2.
Schematic of an RDC set-up.
The epoxidation process developed by Saha and co-workers [81,82,83,84,85,86,87] may be termed as atom efficient since it forms tert-butanol, which is a valuable industrial feedstock as a co-product of the reaction. In addition, the process achieved nearly 100% conversion of cyclohexene [81,82,83,84,85,86,87] and 4-vinyl-1-cyclohexene (C8H12) [31] to cyclohexene oxide (C6H10O) and 4-vinyl-1-cyclohexane, 1-2-epoxide (C8H12O), respectively, under mild reaction conditions.
The conventional method for the production of epichlorohydrin (C3H5ClO) uses energy-intensive multistep processes involving the addition of chlorine to propene (C3H6) at high temperatures to give allyl chloride (C3H5Cl) and, subsequently, reacting the product with hypochlorous acid (HOCl) to form two dichlorohydrins isomers. Finally, dichlorohydrins are reacted with sodium hydroxide (NaOH) to yield epichlorohydrin (C3H5ClO) [89,90]. In addition to the high energy requirements, this method has major drawbacks, including the application of hazardous chlorine, low atom efficiency of the chlorine and a large number of by-products for disposal [91]. However, a more energy efficient and sustainable process for the production of epichlorohydrin (C3H5ClO) can be achieved by utilising glycerol (C3H8O3) based on two reaction steps: catalytic chlorination of liquid glycerol (C3H8O3) with gaseous hydrochloric acid (HCl), and then, reacting the dichlorinated compounds formed with an inorganic base in an RDC to form the product (Scheme 6) [92,93]. Although the stoichiometric bases used in the second step of the reaction may be soluble in the reaction mixture, thus increasing the difficulty in product isolation and purification, this problem can be surmounted when a heterogeneous catalyst is applied in the first step, and can be easily recovered from the reaction by filtration [94].

Scheme 6.
Reaction scheme for the conversion of glycerol into epichlorohydrin [93].
3.3. Microwave-Assisted Epoxidation
The microwave offers energy-efficient heating by the interaction of the generated electromagnetic radiations with the molecules (polar), thereby generating heat for the reaction without direct contact with the reaction mixture. The microwave provides substantial energy savings due to rapid and uniform heating during the chemical reaction in contrast to conventional heating by the conduction and convection mechanisms [95,96,97].
The epoxidation of oleic acid (C18H34O2) by peracetic acid carried out using microwave heating has recorded significant energy savings by enhancing the perhydrolysis step in the aqueous phase (Scheme 7) and reducing the reaction time by up to 50% compared with the reaction carried out using conventional heating with a heat exchanger [98]. The combination of microwave-induced heating and catalytic monolithic reactors has recorded higher energy efficiencies in the ethylene (C2H4) epoxidation to ethylene oxide (C2H4O) due to selective heating of the catalyst by the microwave compared to conventional electrically induced heating [99]. It was observed that the catalyst was heated rapidly under microwave irradiation, while the gas stream remains at a lower temperature. Thus, the gas temperature in the case of microwave heating was ~150 °C, about 70 °C lower in comparison with conventional heating conditions for conversion of 9% [99]. Epoxidation of α-pinene with molecular oxygen yields three products (Scheme 8) and the selectivity to the epoxide depends on the catalyst used and operating conditions [100].

Scheme 7.
Epoxidation of oleic acid using acetic acid by the Prilezhaev method [98].

Scheme 8.
Possible products of α-pinene epoxidation with air.
Cobalt(II) acetate tetrahydrate (C4H6CoO4.4H2O) catalyst supported on Mordenite (zeolite), prepared by impregnation with microwave heating, resulted in up to 92.7 mol% conversion of α-pinene in 4 h at 90 °C in the epoxidation with air, which is about 1.5 times higher than the conversion obtained using the catalyst prepared by conventional impregnation heating [101]. The higher activity and selectivity recorded by the catalysts was attributed to the ability of the microwave heating to produce and uniformly disperse the CoOx nanoparticles in the mordenite support [101]. A comparison of microwave-assisted epoxidation of α-pinene and styrene with air and the traditional heating method under atmospheric pressure showed that using a microwave heating method may yield an excellent efficiency of epoxidation. For instance, when using microwaves to heat the epoxidation reaction, the conversion of α-pinene and styrene reaches 85.3% and 86.5%, respectively. However, under the same conditions, the reaction conducted using the traditional heating method shows that the substrates were substantially unreacted, resulting in only 5.2% and 3.4% conversion of α-pinene and styrene, respectively [102].
3.4. Epoxidation in Microreactors
Microreactors are chemical reactors having extremely small dimensions and made from silicon, polymers, glass, metal and other materials, employing a variety of fabrication techniques often requiring special equipment and skills to fit the intended reaction [103,104]. The small channel sizes provide a high surface area to volume ratio, which results in uniform heating across the reaction site, in addition to efficient heat and mass transfer characteristics [105,106]. Microreactors have helped to minimise reagent and energy consumption in chemical synthesis due to their small dimensions, and allow safe handling of hazardous or highly exothermic reactions [107,108].
A microreactor coated with a catalytic TiO2 layer has achieved higher oxirane selectivity (92%) in the epoxidation of methyl oleate (C19H36O2) with H2O2 as oxidant and recorded a higher reaction rate of epoxide production (23 times) compared with the reaction carried out in a conventional batch reactor with an average yield of 75% (Scheme 9) [109]. The higher epoxide selectivity obtained in the microreactor was attributed to the efficient mixing of reactants, high surface to volume ratio and accurate control of the substrate to oxidant ratio [109]. Moreover, a microfluidic device that generates dielectric barrier discharge (DBD) plasma at the gas–liquid interface has efficiently diffused the reactive oxygen species (ROS) to the liquid phase via microbubbles in trans-stilbene (C14H12) epoxidation to a trans-stilbene epoxide (C14H12O) [106]. The highest epoxide yield of ~94% was obtained at the optimum operating conditions of short bubble–liquid contact times (~2 s), with frequent exposure to freshly generated microbubbles containing reactive oxygen species by continuous liquid recirculation [106].
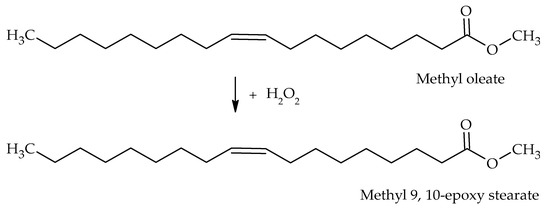
Scheme 9.
Epoxidation of methyl oleate with H2O2.
Monolithic microreactors are continuous unitary structures consisting of parallel channels, with the catalyst either incorporated into a thin layer of a porous oxide deposited on the channel wall (acting as support for the catalyst) or into the wall of the reactor itself [110,111]. A catalytic monolith reactor offers high catalytic efficiency in the chemical reaction due to high concentrations of active sites per unit volume, and provides better heat and mass transfer, and very low pressure drop along the channels [112,113,114]. A flow reactor used for the continuous epoxidation of styrene (C8H8) with TBHP as an oxidant in the presence of a monolith catalyst resulted in good conversion (55%) and excellent selectivity (74%) towards styrene oxide (C8H8O) within 50 min residence time, and allowed easy separation of the catalyst and recyclability of more than seven cycles without a significant loss in catalytic activity [115]. A comparison of a monolith reactor and a Confined Taylor Flow (CTF) reactor in the heterogeneous catalytic epoxidation of propene (C3H6) to propene oxide (C3H6O) with hydrogen peroxide revealed that the production rate of propene oxide (C3H6O) was higher in the monolith reactor in the entire range of operating pressures due to its larger catalyst coating area, larger mass-transfer surface area and more frequent recycling of liquid flow [116].
3.5. Sonochemical Synthesis of Epoxides
Sonochemical synthesis (sonochemistry) involves chemical reactions between molecules due to the application of powerful ultrasound radiation usually in the range of 20 kHz–10 MHz [117]. The chemical reaction is derived from a physical phenomenon referred to as acoustic cavitation, which is responsible for the formation, growth, and collapse of tiny acoustic bubbles inside a liquid, thereby inducing high temperatures (up to 5000 K) and very high pressures (up to 1000 atm) inside such cavities, in addition to a shock wave at the interface and bulk liquids leading to enhanced heat and mass transfer, reduced reaction times and lower energy consumption [118,119,120,121].
A catalytic system consisting of a combination of mild mixing resulting from high-frequency ultrasonic irradiation (800 kHz) with precise temperature regulation of the double jacketed sonochemical reactor has achieved a higher yield of cyclooctene oxide (C8H14O) (96%) and selectivity (98%) within 30 min in the epoxidation of 1-octene mediated by H2O2 and H2WO4 compared with silent conditions (Scheme 10) [122]. Similarly, ultrasonic-assisted limonene epoxidation using in situ generated dimethyl dioxirane, C3H6O2 (DMDO), as the oxidising agent, achieved 100% yield of the limonene dioxide product within 4.5 min, compared to 97% yield obtained in the reaction conducted using conventional agitation with a magnetic stirrer after 1.5 h [123]. A proposed two-step mechanism for the reaction is presented in Scheme 11. Furthermore, epoxidation of α-pinene (C10H16) to α-pinene oxide (C10H16O) under ultrasound conditions by DMDO yielded 100% after 4 min, and took 60 min to achieve a similar yield using the traditional method [123].

Scheme 10.
Epoxidation of 1-octene with H2O2.

Scheme 11.
Epoxidation mechanism of limonene to limonene dioxide by dimethyl dioxirane (DMDO) [123].
Chavan and co-workers [124] observed a substantial reduction in the reaction time for soybean oil epoxidation using the ultrasonic horn in the presence of tetra-n-butyl ammonium bromide as a phase transfer catalyst for similar levels of conversion compared with the conventional stirring approach. It was found that the relative percentage conversion to epoxide using the conventional method was about 87% in 10 h, whereas the reaction carried out in an ultrasound horn under optimised conditions (20 kHz with pulse of 5 s ON and 5 s OFF) achieved almost 83% in 4 h of reaction time.
4. Conclusions
It can be deduced from the foregoing that the technologies used for the synthesis of epoxides are continuously being improved with a view to reducing the environmental impact, energy consumption and overall costs. The recent advances in this respect are geared towards the process intensification approaches, by utilising equipment and processes that are smaller, safer, less expensive and more energy efficient. Thus, industrial application of the energy efficient epoxidation techniques may significantly reduce the cost associated with energy consumption, waste generation and product purification in epoxide production. For instance, the continuous flow epoxidation we previously carried out in a FlowSyn reactor shows substantial benefits, which include fast heat and mass transfer, short setup and reaction times, the flexibility of scaling-up reactions, complete non-attended operation and remarkable catalyst stability compared with the experiments carried out in a classical batch reactor under similar reaction conditions.
Although epoxidation processes described in this review have been successfully carried out at the laboratory scale, the development of scaled-up processes is recommended first of all to move the chemistry on from small-scale laboratory reactions to large-scale industrial production. The energy-efficient epoxidation techniques, including continuous flow chemistry and reactive distillation, in addition to microwave, microreactor and sonochemical syntheses, can significantly reduce the cost associated with energy consumption, waste generation and product purification in epoxide production.
Author Contributions
M.L.M.: Conceptualization, methodology, writing—original draft preparation, formal analysis; B.S.: Conceptualization, methodology, writing—review and editing, formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMP | 2-aminomethyl pyridine |
| CTF | Confined Taylor Flow |
| DBD | Dielectric Barrier Discharge |
| DMDO | Dimethyl dioxirane |
| DVB | divinylbenzene |
| MoO2(acac)2 | Molybdenyl acetylacetonate |
| Ps.AMP | Polystyrene 2-(aminomethyl) pyridine |
| Ps.AMP.Mo | Polystyrene 2-(aminomethyl) pyridine supported Mo(VI) complex |
| RDC | Reactive Distillation Column |
| ROS | Reactive Oxygen Species |
| TBHP | tert-butyl hydroperoxide |
| VBC | Vinylbenzyl chloride |
References
- Vooradi, R.; Anne, S.; Tula, A.; Eden, M.; Gani, R. Energy and CO2 management for chemical and related industries: Issues, opportunities and challenges. BMC Chem. Eng. 2019, 1, 7. [Google Scholar] [CrossRef]
- Tvaronavičienė, M.; Baublys, J.; Raudeliūnienė, J.; Jatautaitė, D. Global energy consumption peculiarities and energy sources: Role of renewables. In Energy Transformation towards Sustainability; Tvaronavičienė, M., Ślusarczyk, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–49. [Google Scholar]
- Bilgen, S. Structure and environmental impact of global energy consumption. Renew. Sustain. Energy Rev. 2014, 38, 890–902. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, X.; Xiao, J.; Liu, Z.; Xu, Y.; Tian, Y. Energy consumption and emission mitigation prediction based on data center traffic and PUE for global data centers. Glob. Energy Interconnect. 2020, 3, 272–282. [Google Scholar] [CrossRef]
- Rahman, M.S.; Noman, A.H.M.; Shahari, F.; Aslam, M.; Gee, C.S.; Isa, C.R.; Pervin, S. Efficient energy consumption in industrial sectors and its effect on environment: A comparative analysis between G8 and Southeast Asian emerging economies. Energy 2016, 97, 82–89. [Google Scholar] [CrossRef]
- Lesage, D.; Van de Graaf, T.; Westphal, K. G8+5 collaboration on energy efficiency and IPEEC: Shortcut to a sustainable future? Energy Policy 2010, 38, 6419–6427. [Google Scholar] [CrossRef]
- Tan, J.; Ji, Y.-N.; Deng, W.-S.; Su, Y.-F. Process intensification in gas/liquid/solid reaction in trickle bed reactors: A review. Petrol. Sci. 2021, 18, 1203–1218. [Google Scholar] [CrossRef]
- Žnidaršič-Plazl, P. Biocatalytic process intensification via efficient biocatalyst immobilization, miniaturization, and process integration. Curr. Opin. Green Sustain. Chem. 2021, 32, 100546. [Google Scholar] [CrossRef]
- Baharudin, L.; Watson, M.J.; Yip, A.C. Process intensification in multifunctional reactors: A review of multi-functionality by catalytic structures, internals, operating modes, and unit integrations. Chem. Eng. Process. 2021, 168, 108561. [Google Scholar] [CrossRef]
- Zhang, Y.; Goh, K.-L.; Ng, Y.L.; Chow, Y.; Wang, S.; Zivkovic, V. Process intensification in micro-fluidized bed systems: A review. Chem. Eng. Process. 2021, 164, 108397. [Google Scholar] [CrossRef]
- Mbeleck, R.; Mohammed, M.L.; Ambroziak, K.; Sherrington, D.C.; Saha, B. Efficient epoxidation of cyclododecene and dodecene catalysed by polybenzimidazole supported Mo(VI) complex. Catal. Today 2015, 256, 287–293. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Mbeleck, R.; Patel, D.; Niyogi, D.; Sherrington, D.C.; Saha, B. Greener and efficient epoxidation of 4-vinyl-1-cyclohexene with polystyrene 2-(aminomethyl)pyridine supported Mo(VI) catalyst in batch and continuous reactors. Chem. Eng. Res. Des. 2015, 94, 194–203. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Patel, D.; Mbeleck, R.; Niyogi, D.; Sherrington, D.C.; Saha, B. Optimisation of alkene epoxidation catalysed by polymer supported Mo(VI) complexes and application of artificial neural network for the prediction of catalytic performances. Appl. Catal. A-Gen. 2013, 466, 142–152. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. 17-ethers and epoxides. In Organic Chemistry, 2nd ed.; Ouellette, R.J., Rawn, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 507–536. [Google Scholar]
- Wypych, A. 3.12-epoxides. In Databook of Plasticizers, 2nd ed.; Wypych, A., Ed.; ChemTec Publishing: Scarborough, ON, Canada, 2017; pp. 210–242. [Google Scholar]
- Dutia, P. Ethylene oxide: A techno-commercial profile. Chem. Wkly. 2010, 53, 199–203. [Google Scholar]
- Nijhuis, T.A.; Makkee, M.; Moulijn, J.A.; Weckhuysen, B.M. The production of propene oxide: catalytic processes and recent developments. Ind. Eng. Chem. Res. Res. 2006, 45, 3447–3459. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.Q.; Marks, M.J. Epoxy resins. In Ulmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Silvestre, A.J.D.; Gandini, A. Chapter 2—Terpenes: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 17–38. [Google Scholar]
- Santacesaria, E.; Tesser, R.; Di Serio, M.; Turco, R.; Russo, V.; Verde, D. A biphasic model describing soybean oil epoxidation with H2O2 in a fed-batch reactor. Chem. Eng. J. 2011, 173, 198–209. [Google Scholar] [CrossRef]
- Bechtold, K. Versatile and vexing: The many uses and hazards of peracetic acid. Synergist 2016. Available online: https://synergist.aiha.org/201612-peracetic-acid-uses-and-hazards (accessed on 26 January 2022).
- Shi, Z.-Q.; Jiao, L.-X.; Sun, J.; Chen, Z.-B.; Chen, Y.-Z.; Zhu, X.-H.; Zhou, J.-H.; Zhou, X.-C.; Li, X.-Z.; Li, R. Cobalt nanoparticles in hollow mesoporous spheres as a highly efficient and rapid magnetically separable catalyst for selective epoxidation of styrene with molecular oxygen. RSC Adv. 2014, 4, 47–53. [Google Scholar] [CrossRef]
- Vondran, J.; Pela, J.; Palczewski, D.; Skiborowski, M.; Seidensticker, T. Curse and blessing—The role of water in the homogeneously Ru-Catalyzed epoxidation of technical grade methyl oleate. ACS Sustain. Chem. Eng. 2021, 9, 11469–11478. [Google Scholar] [CrossRef]
- Thornburg, N.E.; Thompson, A.B.; Notestein, J.M. Periodic trends in highly dispersed Groups IV and V supported metal oxide catalysts for alkene epoxidation with H2O2. ACS Catal. 2015, 5, 5077–5088. [Google Scholar] [CrossRef]
- Lu, X.; Wu, H.; Jiang, J.; He, M.; Wu, P. Selective synthesis of propylene oxide through liquid-phase epoxidation of propylene with H2O2 over formed Ti-MWW catalyst. J. Catal. 2016, 342, 173–183. [Google Scholar] [CrossRef]
- Luttrell, W. Toxic tips: Sodium hypochlorite. J. Chem. Health Saf. 2001, 8, 24–26. [Google Scholar] [CrossRef]
- Bruch, M.K. Toxicity and safety of topical sodium hypochlorite. Contrib. Nephrol. 2007, 154, 24–38. [Google Scholar] [PubMed]
- Bregante, D.T.; Tan, J.Z.; Schultz, R.L.; Ayla, E.Z.; Potts, D.S.; Torres, C.; Flaherty, D.W. Catalytic consequences of oxidant, alkene, and pore structures on alkene epoxidations within titanium silicates. ACS Catal. 2020, 10, 10169–10184. [Google Scholar] [CrossRef]
- Ni, X.-L.; Liu, J.; Liu, Y.-Y.; Leus, K.; Depauw, H.; Wang, A.-J.; Van Der Voort, P.; Zhang, J.; Hu, Y.-K. Synthesis, characterization and catalytic performance of Mo based metal- organic frameworks in the epoxidation of propylene by cumene hydroperoxide. Chin. Chem. Lett. 2017, 28, 1057–1061. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Sainz-Pardo, J.; de Frutos, P.; Blázquez, S. Agglomeration of Ti-SBA-15 with clays for liquid phase olefin epoxidation in a continuous fixed bed reactor. Chem. Eng. J. 2008, 139, 631–641. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Saha, B. Greener and sustainable approach for the synthesis of commercially important epoxide building blocks using polymer-supported Mo(VI) complexes as catalysts. In Ion Exchange and Solvent Extraction, 1st ed.; SenGupta, A.K., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group LLC: Milton Park, UK, 2016; p. 33. [Google Scholar]
- Fadhli, M.; Khedher, I.; Fraile, J.M. Enantioselective epoxidation of styrene with TBHP catalyzed by bis(oxazoline)–vanadyl–laponite materials. Catal. Commun. 2018, 117, 90–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Gao, R.; Dai, W.-L. Manganese-doped CeO2 nanocubes as highly efficient catalysts for styrene epoxidation with TBHP. Appl. Surf. Sci. 2019, 471, 767–775. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Mbeleck, R.; Saha, B. Efficient and selective molybdenum based heterogeneous catalyst for alkene epoxidation using batch and continuous reactors. Polym. Chem. 2014, 6, 7308–7319. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Mbeleck, R.; Patel, D.; Sherrington, D.C.; Saha, B. Greener route to 4-vinyl-1-cyclohexane 1,2-epoxide synthesis using batch and continuous reactors. Green Process Synth. 2014, 3, 411–418. [Google Scholar] [CrossRef]
- Singh, B.; Rana, B.; Sivakumar, L.; Bahuguna, G.; Sinha, A. Efficient catalytic epoxidation of olefins with hierarchical mesoporous TS-1 using TBHP as an oxidant. J. Porous Mat. 2012, 20, 397–405. [Google Scholar] [CrossRef]
- Lueangchaichaweng, W.; Singh, B.; Mandelli, D.; Carvalho, W.A.; Fiorilli, S.; Pescarmona, P.P. High surface area, nanostructured boehmite and alumina catalysts: Synthesis and application in the sustainable epoxidation of alkenes. Appl. Catal. A-Gen. 2019, 571, 180–187. [Google Scholar] [CrossRef]
- Mikolajska, E.; Calvino-Casilda, V.; Bañares, M.A. Real-time Raman monitoring of liquid-phase cyclohexene epoxidation over alumina-supported vanadium and phosphorous catalysts. Appl. Catal. A-Gen. 2012, 421–422, 164–171. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, P.; Zhang, J.; Bian, G.; Zhang, P.; Dong, Y.; Zhang, W. Highly dispersed molybdenum incorporated hollow mesoporous silica spheres as an efficient catalyst on epoxidation of olefins. Mol. Catal. 2017, 433, 212–223. [Google Scholar] [CrossRef]
- Bisio, C.; Gallo, A.; Psaro, R.; Tiozzo, C.; Guidotti, M.; Carniato, F. Tungstenocene-grafted silica catalysts for the selective epoxidation of alkenes. Appl. Catal. A-Gen. 2019, 581, 133–142. [Google Scholar] [CrossRef]
- Cai, L.; Chen, C.; Wang, W.; Gao, X.; Kuang, X.; Jiang, Y.; Li, L.; Wu, G. Acid-free epoxidation of soybean oil with hydrogen peroxide to epoxidized soybean oil over titanium silicalite-1 zeolite supported cadmium catalysts. J. Ind. Eng. Chem. 2020, 91, 191–200. [Google Scholar] [CrossRef]
- Wu, Z.; He, Z.; Zhou, D.; Yang, Y.; Lu, X.; Xia, Q. One-step synthesis of bi-functional zeolite catalyst with highly exposed octahedral Co for efficient epoxidation of bulky cycloalkenes. Mater. Lett. 2020, 280, 128549. [Google Scholar] [CrossRef]
- Mohammadikish, M.; Yarahmadi, S.; Molla, F. A new water-insoluble coordination polymer as efficient dye adsorbent and olefin epoxidation catalyst. J. Environ. Manag. 2020, 254, 109784. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Epoxidation of castor oil fatty acid methyl esters (COFAME) as a Lubricant base stock using heterogeneous ion-exchange resin (IR-120) as a catalyst. Energy Procedia 2014, 54, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Lu, X.H.; Ma, X.T.; Shen, Y.; Wei, C.C.; He, J.; Zhou, D.; Xia, Q.H. Highly efficient epoxidation of cyclohexene with aqueous H2O2 over powdered anion-resin supported solid catalysts. J. Mol. Catal. A-Chem. 2016, 423, 393–399. [Google Scholar] [CrossRef]
- Otake, K.-i.; Ahn, S.; Knapp, J.; Hupp, J.T.; Notestein, J.M.; Farha, O.K. Vapor-phase cyclohexene epoxidation by single-ion Fe(III) sites in metal–organic frameworks. Inorg. Chem. 2021, 60, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Abbasi, A.; Masteri-Farahani, M.; Rodrigues, V.H.N. Synthesis, characterization and crystal structure of a copper molybdate coordination polymer as an epoxidation catalyst. Inorg. Chim. Acta. 2015, 433, 21–25. [Google Scholar]
- McMurry, J. Organic Chemistry; Brooks/Cole Cengage Learning: Southbank, VIC, Australia, 2012. [Google Scholar]
- Parod, R.J. Ethylene oxide. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 535–538. [Google Scholar]
- Meng, Y.; Taddeo, F.; Aguilera, A.F.; Cai, X.; Russo, V.; Tolvanen, P.; Leveneur, S. The lord of the chemical rings: Catalytic synthesis of important industrial epoxide compounds. Catalysts 2021, 11, 765. [Google Scholar] [CrossRef]
- Kück, J.W.; Reich, R.M.; Kühn, F.E. Molecular epoxidation reactions catalyzed by rhenium, molybdenum, and iron complexes. Chem. Rec. 2016, 16, 349–364. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchionia, F.; Pescarmona, P.P. CO2 -fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef] [Green Version]
- Cuesta-Aluja, L.; Castilla, J.; Masdeu-Bultó, A.M.; Henriques, C.A.; Calvete, M.J.F.; Pereira, M.M. Halogenated meso-phenyl Mn(III) porphyrins as highly efficient catalysts for the synthesis of polycarbonates and cyclic carbonates using carbon dioxide and epoxides. J Mol. Catal. A-Chem. 2016, 423, 489–494. [Google Scholar] [CrossRef]
- Mandal, M. Group 4 complexes as catalysts for the transformation of CO2 into polycarbonates and cyclic carbonates. J. Organomet. Chem. 2020, 907, 121067. [Google Scholar] [CrossRef]
- Onyenkeadi, V.; Kellici, S.; Saha, B. Greener synthesis of 1,2-butylene carbonate from CO2 using graphene-inorganic nanocomposite catalyst. Energy 2018, 165, 867–876. [Google Scholar] [CrossRef]
- Adeleye, A.I.; Kellici, S.; Heil, T.; Morgan, D.; Vickers, M.; Saha, B. Greener synthesis of propylene carbonate using graphene-inorganic nanocomposite catalysts. Catal. Today 2015, 256, 347–357. [Google Scholar] [CrossRef]
- Cavdar, H.; Saracoglu, N. Ring opening of epoxides with NaHSO4: Isolation of β-hydroxy sulfate esters and an effective synthesis for trans-diols. Tetrahedron 2009, 65, 985–989. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wu, M.-D.; Zhu, X.-X.; Zhang, X.-D.; Zhang, C.; Xu, Y.-H.; Wu, M.-C. Remarkable improvement in the regiocomplementarity of a Glycine max epoxide hydrolase by reshaping its substrate-binding pocket for the enantioconvergent preparation of (R)-hexane-1,2-diol. Mol. Catal. 2021, 514, 111851. [Google Scholar] [CrossRef]
- Wade, L.G. Organic Chemistry, 8th ed.; Pearson: Glenview, IL, USA, 2013. [Google Scholar]
- Delost, M.D.; Njardarson, J.T. Oxiranes and oxirenes: Monocyclic. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Gras, E.; Sadek, O. Oxiranes and oxirenes: Fused-ring derivatives. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Davies, T.E.; Kondrat, S.A.; Nowicka, E.; Kean, J.L.; Harris, C.M.; Socci, J.M.; Apperley, D.C.; Taylor, S.H.; Graham, A.E. Nanoporous alumino- and borosilicate-mediated Meinwald rearrangement of epoxides. Appl. Catal. A-Gen. 2015, 493, 17–24. [Google Scholar] [CrossRef]
- García-Muñoz, R.A.; Serrano, D.P.; Vicente, G.; Linares, M.; Vitvarova, D.; Čejka, J. Remarkable catalytic properties of hierarchical zeolite-Beta in epoxide rearrangement reactions. Catal. Today 2015, 243, 141–152. [Google Scholar] [CrossRef]
- Hoang, P.H.; Dien, L.Q. Synthesis of magnetically recyclable ZSM-5 zeolite for styrene epoxide rearrangement reaction. Chem. Eng. J. 2015, 262, 140–145. [Google Scholar] [CrossRef]
- Keshavarz, M.; Zarei Ahmady, A.; Mostoufi, A.; Mohtasham, N. One-pot green regioselesctive synthesis of γ-lactones from epoxides and ketene silyl acetals using 1,3-dimethylimidazolium fluoride as a recoverable metal-free catalyst. Molecules 2017, 22, 1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonollo, S.; Ahmady, A.Z.; Petrucci, C.; Marrocchi, A.; Pizzo, F.; Vaccaro, L. A catalytic approach to the metal-free reaction of epoxides with ketene silyl acetals for accessing γ-lactones. Org. Lett. 2014, 16, 5721–5723. [Google Scholar] [CrossRef]
- Chen, X.; Sumoto, K.; Mitani, S.; Yamagami, T.; Yokoyama, K.; Wang, P.; Hirao, S.; Nishiwaki, N.; Kobiro, K. One-step and non-catalytic intramolecular redox reactions of conjugated all E-dienals to non-conjugated Z-enoic acids in subcritical water. J. Supercrit. Fluids. 2012, 62, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Sancineto, L.; Mangiavacchi, F.; Tidei, C.; Bagnoli, L.; Marini, F.; Gioiello, A.; Scianowski, J.; Santi, C. Selenium-catalyzed oxacyclization of alkenoic acids and alkenols. Asian J. Org. Chem. 2017, 6, 988–992. [Google Scholar] [CrossRef]
- Vidović, D. Aluminum complexes in organic. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Florio, S.; Perna, F.M.; Salomone, A.; Vitale, P. 8.29 reduction of epoxides. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1086–1122. [Google Scholar]
- Yan, Z.; Ma, Z.; Deng, J.; Luo, G. Mechanism and kinetics of epoxide ring-opening with carboxylic acids catalyzed by the corresponding carboxylates. Chem. Eng. Sci. 2021, 242, 116746. [Google Scholar] [CrossRef]
- Desport, J.S.; Mantione, D.; Moreno, M.; Sardón, H.; Barandiaran, M.J.; Mecerreyes, D. Synthesis of three different galactose-based methacrylate monomers for the production of sugar-based polymers. Carbohydr. Res. 2016, 432, 50–54. [Google Scholar] [CrossRef]
- Matsuzaki, T.N. Method of Producing Glycidyl Methacrylate. U.S. Patent 7176328, 13 February 2007. [Google Scholar]
- Yan, Z.; Deng, J.; Chen, Y.; Luo, G. Preparation of 2,3-epoxypropyl neodecanoate: Process optimization and mechanism discussion. Ind. Eng. Chem. Res. 2020, 59, 19168–19176. [Google Scholar] [CrossRef]
- Yan, Z.; Du, C.; Luo, G.; Deng, J. Remarkable improvement of epoxide ring-opening reaction efficiency and selectivity with water as a green regulator. React. Chem. Eng. 2021, 6, 2159–2169. [Google Scholar] [CrossRef]
- Tanbouza, N.; Ollevier, T.; Lam, K. Bridging lab and industry with flow electrochemistry. iScience 2020, 23, 101720. [Google Scholar] [CrossRef] [PubMed]
- Aguillón, A.R.; Bezerra, M.A.d.M.; Gomez, M.R.B.P.; de Souza, R.O.M.A. Continuous-flow chemistry toward sustainable chemical synthesis. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Boddula, R., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–69. [Google Scholar]
- Hartman, R.L. Flow chemistry remains an opportunity for chemists and chemical engineers. Curr. Opin. Chem. Eng. 2020, 29, 42–50. [Google Scholar] [CrossRef]
- UNIQSIS, FlowSyn—Flow Chemistry Made Simple. Available online: https://www.uniqsis.com/paProducts.aspx (accessed on 13 September 2021).
- Mbeleck, R.; Ambroziak, K.; Saha, B.; Sherrington, D.C. Stability and recycling of polymer-supported Mo(VI) alkene epoxidation catalysts. React. Funct. Polym. 2007, 67, 1448–1457. [Google Scholar] [CrossRef]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. Epoxidation Process. European Patent Number EP2459545B1, 28 February 2019. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. A Continuous Process for the Liquid Phase Epoxidation of an Olefinic Compound. Indian Patent No. 295846, 17 April 2018. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. A Permeable Particle Container. Chinese Patent No. ZL 201410840925.7, 7 December 2016. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. Liquid Phase Epoxidation Process. US Patent Number US 9,248,942 B2, 2 February 2016. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. A Continuous Process for the Liquid Phase Epoxidation of an Olefinic Compound with an Oxidant. Chinese Patent Number ZL201080044175.2, 11 February 2015. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. Liquid Phase Epoxidation Process. US Patent Number US 8,759,552 B2, 24 June 2014. [Google Scholar]
- Ambroziak, K.; Mbeleck, R.; He, Y.; Saha, B.; Sherrington, D.C. Investigation of batch alkene epoxidations catalyzed by polymer-supported Mo(VI) complexes. Ind. Eng. Chem. Res. 2009, 48, 3293–3302. [Google Scholar] [CrossRef]
- Kiss, A.A.; Jobson, M.; Gao, X. Reactive distillation: Stepping up to the next level of process intensification. Ind. Eng. Chem. Res. 2019, 58, 5909–5918. [Google Scholar] [CrossRef] [Green Version]
- Okhlopkova, E.A.; Serafimov, L.A.; Frolkova, A.V. Methods of preparing epichlorohydrin. Theor. Found. Chem. Eng. 2019, 53, 864–870. [Google Scholar] [CrossRef]
- Bell, B.; Briggs, J.; Campbell, R.; Chambers, S.; Gaarenstroom, P.; Hippler, J.; Hook, B.; Kearns, K.; Kenney, J.; Kruper, W.; et al. Glycerin as a renewable feedstock for epichlorohydrin production. The GTE process. Clean-Soil Air Water 2008, 36, 657–661. [Google Scholar] [CrossRef]
- Almena, A.; Martín, M. Technoeconomic analysis of the production of epichlorohydrin from glycerol. Ind. Eng. Chem. Res. 2016, 55, 3226–3238. [Google Scholar] [CrossRef]
- Santacesaria, E.; Tesser, R.; Di Serio, M.; Casale, L.; Verde, D. New process for producing epichlorohydrin via glycerol chlorination. Ind. Eng. Chem. Res. 2009, 49, 964–970. [Google Scholar] [CrossRef]
- Lari, G.M.; Pastore, G.; Haus, M.; Ding, Y.; Papadokonstantakis, S.; Mondelli, C.; Pérez-Ramírez, J. Environmental and economical perspectives of a glycerol biorefinery. Energy Environ. Sci. 2018, 11, 1012–1029. [Google Scholar] [CrossRef]
- Lari, G.M.; Pastore, G.; Mondelli, C.; Pérez-Ramírez, J. Towards sustainable manufacture of epichlorohydrin from glycerol using hydrotalcite-derived basic oxides. Green Chem. 2018, 20, 148–159. [Google Scholar] [CrossRef]
- Gupta, D.; Jamwal, D.; Rana, D.; Katoch, A. 26-Microwave synthesized nanocomposites for enhancing oral bioavailability of drugs. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 619–632. [Google Scholar]
- Ewis, D.; Hameed, B.H. A review on microwave-assisted synthesis of adsorbents and its application in the removal of water pollutants. J. Water Process. Eng. 2021, 41, 102006. [Google Scholar] [CrossRef]
- Zeng, X.; Hu, X.; Song, H.; Xia, G.; Shen, Z.-Y.; Yu, R.; Moskovits, M. Microwave synthesis of zeolites and their related applications. Micropor. Mesopor. Mat. 2021, 323, 111262. [Google Scholar] [CrossRef]
- Aguilera, A.F.; Tolvanen, P.; Eränen, K.; Wärnå, J.; Leveneur, S.; Marchant, T.; Salmi, T. Kinetic modelling of Prileschajew epoxidation of oleic acid under conventional heating and microwave irradiation. Chem. Eng. Sci. 2019, 199, 426–438. [Google Scholar] [CrossRef]
- Ramírez, A.; Hueso, J.L.; Mallada, R.; Santamaría, J. Ethylene epoxidation in microwave heated structured reactors. Catal. Today 2016, 273, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.H.; Lei, J.; Wei, X.L.; Ma, X.T.; Zhang, T.J.; Hu, W.; Zhou, D.; Xia, Q.H. Selectively catalytic epoxidation of α-pinene with dry air over the composite catalysts of Co–MOR(L) with Schiff-base ligands. J. Mol. Catal. A Chem. 2015, 400, 71–80. [Google Scholar] [CrossRef]
- Tao, P.; Lu, X.; Zhang, H.; Jing, R.; Huang, F.; Wu, S.; Zhou, D.; Xia, Q. Enhanced activity of microwave-activated CoOx/MOR catalyst for the epoxidation of α-pinene with air. Mol. Catal. 2019, 463, 8–15. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Tao, P.; Lu, X.; Li, X.; Wang, C.; Wang, B.; Yue, F.; Zhou, D.; Xia, Q. Microwave-assisted air epoxidation of mixed biolefins over a spherical bimetal ZnCo-MOF catalyst. ACS Appl. Mater. Interfaces 2021, 13, 8474–8487. [Google Scholar] [CrossRef]
- Wirth, T. Microreactors in Organic Chemistry and Catalysis, 2nd ed.; Willey-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Domínguez, M.I.; Centeno, M.A.; Martínez, T.M.; Bobadilla, L.F.; Laguna, Ó.H.; Odriozola, J.A. Current scenario and prospects in manufacture strategies for glass, quartz, polymers and metallic microreactors: A comprehensive review. Chem. Eng. Res. Des. 2021, 171, 13–35. [Google Scholar] [CrossRef]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K. Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792–821. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Du, L.; Liu, J.; Yao, J. Review of the applications of microreactors. Renew. Sustain. Energy Rev. 2015, 47, 519–539. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Fanelli, F.; Parisi, G.; Degennaro, L.; Luisi, R. Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis. Beilstein J. Org. Chem. 2017, 13, 520–542. [Google Scholar] [CrossRef] [Green Version]
- Phimsen, S.; Yamada, H.; Tagawa, T.; Kiatkittipong, W.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Epoxidation of methyl oleate in a TiO2 coated-wall capillary microreactor. Chem. Eng. J. 2017, 314, 594–599. [Google Scholar] [CrossRef]
- Ogunyinka, O.; Iza, F.; Buckley, B.; Bandulasena, H.C.H. Epoxidation of trans-stilbene in a microfluidic plasma reactor. Chem. Eng. Sci. 2021, 240, 116665. [Google Scholar] [CrossRef]
- Ciemięga, A.; Maresz, K.; Malinowski, J.J.; Mrowiec-Białoń, J. Continuous-flow monolithic silica microreactors with arenesulphonic acid groups: Structure—Catalytic activity relationships. Catalysts 2017, 7, 255. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Gamliel, D.P.; Valla, J.A.; Bollas, G.M. Fischer-tropsch synthesis in monolith catalysts coated with hierarchical ZSM-5. Appl. Catal. B-Environ. 2021, 284, 119719. [Google Scholar] [CrossRef]
- García-Moncada, N.; Navarro, J.C.; Odriozola, J.A.; Lefferts, L.; Faria, J.A. Enhanced catalytic activity and stability of nanoshaped Ni/CeO2 for CO2 methanation in micro-monoliths. Catal. Today 2022, 383, 205–215. [Google Scholar] [CrossRef]
- Cifuentes, B.; Cifuentes, A.; Bustamante, F.; Soler, L.; Llorca, J.; Cobo, M. Monoliths washcoated with AuCu catalysts for CO removal in an ethanol fuel processor: Effect of CeO2–SiO2 dual support on the catalytic performance and reactor cost. Int. J. Hydrogen Energy 2021, 46, 2166–2181. [Google Scholar] [CrossRef]
- Alimi, O.A.; Ncongwane, T.B.; Meijboom, R. Design and fabrication of a monolith catalyst for continuous flow epoxidation of styrene in polypropylene printed flow reactor. Chem. Eng. Res. Des. 2020, 159, 395–409. [Google Scholar] [CrossRef]
- Shin, S.B.; Chadwick, D. Comparison of a monolith and a confined Taylor flow (CTF) reactor for propene epoxidation. Chem. Eng. Process 2018, 125, 173–182. [Google Scholar] [CrossRef]
- Qiao, S.Z.; Liu, J.; Max Lu, G.Q. Chapter 21—Synthetic Chemistry of Nanomaterials. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 613–640. [Google Scholar]
- Schiel, M.A.; Chopa, A.B.; Silbestri, G.F.; Alvarez, M.B.; Lista, A.G.; Domini, C.E. Use of ultrasound in the synthesis of heterocycles of medicinal interest. In Green Synthetic Approaches for Biologically Relevant Heterocycles; Brahmachari, G., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 571–601. [Google Scholar]
- Pokhrel, N.; Vabbina, P.K.; Pala, N. Sonochemistry: Science and engineering. Ultrason. Sonochem. 2016, 29, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Penteado, F.; Monti, B.; Sancineto, L.; Perin, G.; Jacob, R.G.; Santi, C.; Lenardão, E.J. Ultrasound-assisted multicomponent reactions, organometallic and organochalcogen chemistry. Asian J. Org. Chem. 2018, 7, 2368–2385. [Google Scholar] [CrossRef]
- Savun-Hekimoğlu, B. A review on sonochemistry and its environmental applications. Acoustics 2020, 2, 766–775. [Google Scholar] [CrossRef]
- Cousin, T.; Chatel, G.; Kardos, N.; Andrioletti, B.; Draye, M. High frequency ultrasound as a tool for elucidating mechanistic elements of cis-cyclooctene epoxidation with aqueous hydrogen peroxide. Ultrason. Sonochem. 2019, 53, 120–125. [Google Scholar] [CrossRef]
- Charbonneau, L.; Foster, X.; Kaliaguine, S. Ultrasonic and Catalyst-Free Epoxidation of Limonene and Other Terpenes Using Dimethyl Dioxirane in Semibatch Conditions. ACS Sustain. Chem. Eng. 2018, 6, 12224–12231. [Google Scholar] [CrossRef]
- Chavan, V.P.; Patwardhan, A.V.; Gogate, P.R. Intensification of epoxidation of soybean oil using sonochemical reactors. Chem. Eng. Process. 2012, 54, 22–28. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).