Current Trends in Waste Plastics’ Liquefaction into Fuel Fraction: A Review
Abstract
:1. Introduction
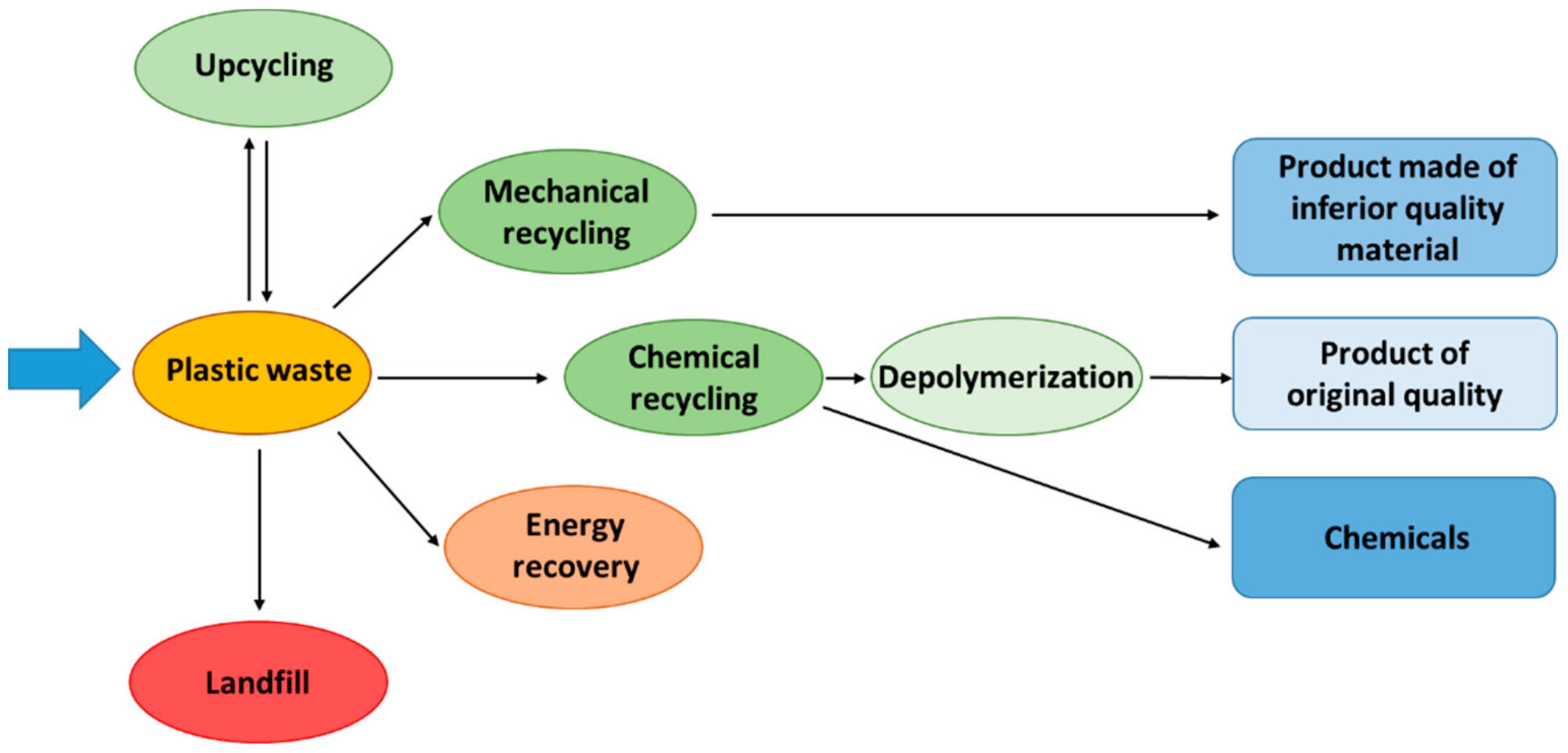
2. Plastic Waste Management
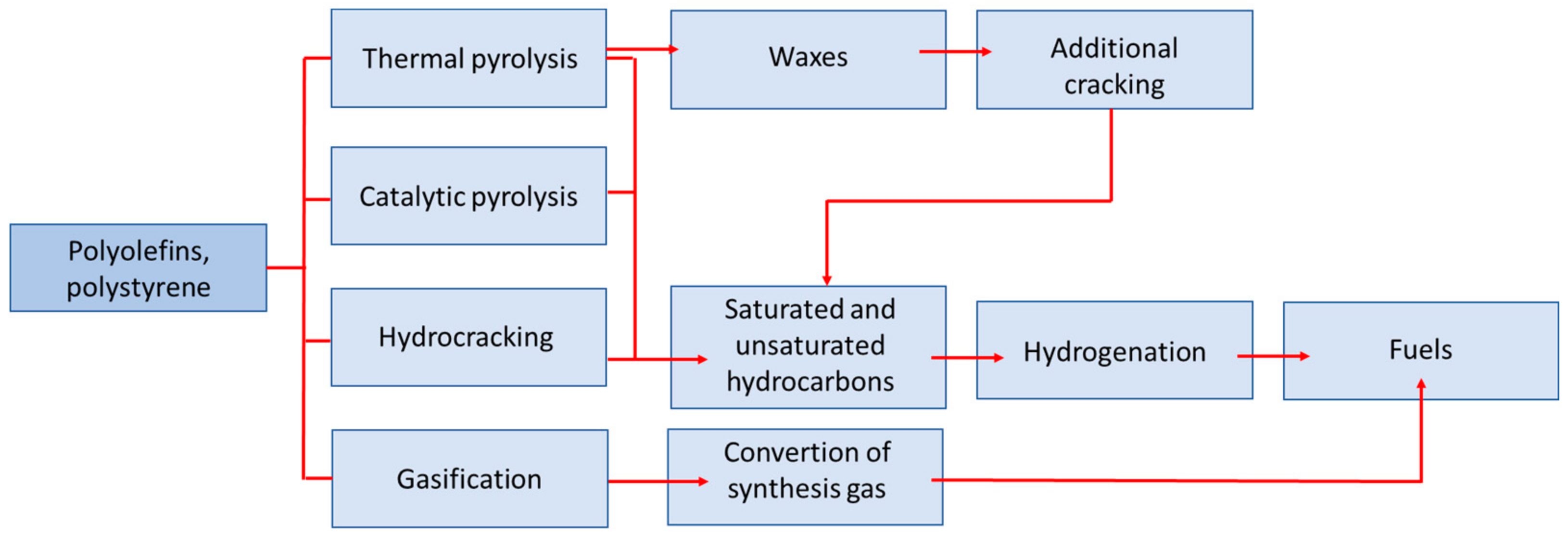
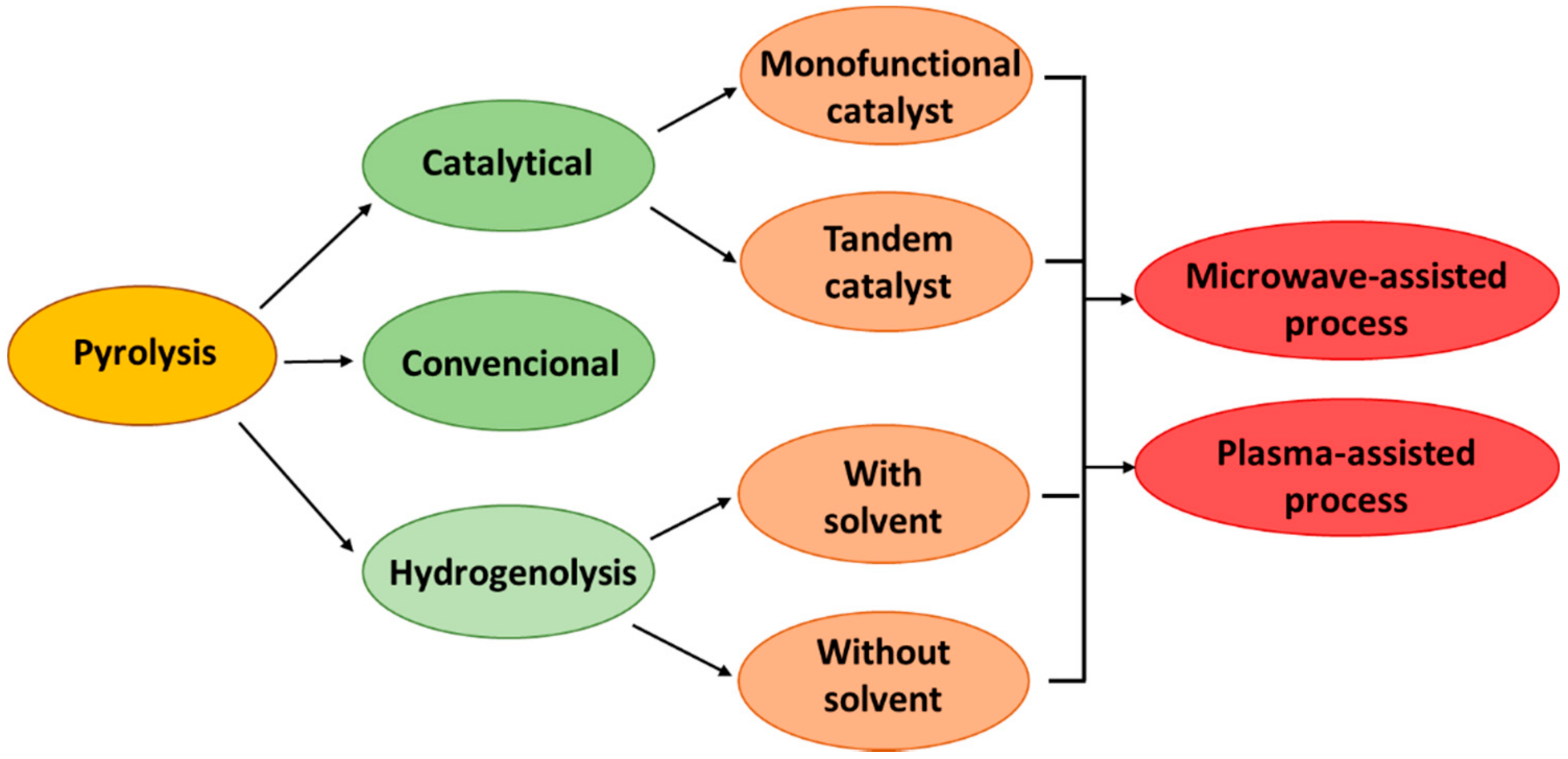
3. Pyrolysis
- Slow pyrolysis—process temperature from 350 °C to 550 °C, heating rate from 1 to 10 °C/min, extended steam residence time, where char is the main product’s first bullet;
- Fast pyrolysis—the process temperature usually ranges from 500 °C to 700 °C, the raw material heating rate is above 100 °C/min, and the vapor residence time is usually within a few seconds; the main product is the liquid fraction, and in the case of polyolefin, pyrolysis also waxes;
- Flash pyrolysis—the process temperature usually exceeds 700 °C, the heating rate of the raw material >200 °C/s, and the vapor residence time is in the millisecond range.
3.1. Catalytical Pyrolysis
- Classical hydrogenation;
- Hydro-pyrolysis (as well as hydrocracking and hydrogenolysis).
3.2. Stabilization of Pyrolysis Oil with Hydrogen
3.3. Microwave Pyrolysis
- The general principles of microwave pyrolysis;
- The influence of parameters on the process efficiency (e.g., microwave power, time and temperature, type of material (absorber, reactor, catalyst));
- Co-pyrolysis possibilities;
- Kinetic models of the process;
- The properties of the obtained products (fractions).
3.4. Plasma Pyrolysis
- High-temperature (thermal), where electrons, ions and particles are in thermodynamic equilibrium and have the same temperature, at approx. 106–108 °C; this is method used to obtain mainly gaseous products;
- Cold (non-thermal) electrons, ions and particles are not in thermodynamic equilibrium and have different temperatures of approx. 104–106 °C, while the gas is at room temperature).
4. Patents Review
- The use of various types of waste polyolefins, including mixed and contaminated;
- Obtaining hydrocarbons with a small mass distribution and short carbon chains (up to C20–C22—in the case of catalytic processes, the process with the recycling of heavy hydrocarbon vapors or the process using reactive distillation), which is possible to use in the production of liquid fuel components;
- The possibility of running the process continuously (in some cases);
- Obtaining chemically stable fractions thanks to the application of a cheap hydrogenation process (in the case of one patent).
- The manual loading of the feed into the reactor;
- Conducting the process in batch mode;
- Poor-quality product from the conventional pyrolysis process (e.g., high wax content, large dispersion of hydrocarbon masses);
- No hydrogenation process that chemically stabilizes the product and allows it to be used as a fuel component;
- High costs related to the use and regeneration of catalysts and product purification;
- High complexity of some processes.
5. Industrial Examples of Polyolefins Waste Conversion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Energy Outlook 2021. Report of International Energy Agency. October 2021. Available online: https://iea.blob.core.windows.net/assets/4ed140c1-c3f3-4fd9-acae-789a4e14a23c/WorldEnergyOutlook2021.pdf (accessed on 10 January 2022).
- Short-Term Energy Outlook. Report of U.S. Energy Information Adsministration. December 2021. Available online: https://www.eia.gov/outlooks/steo/pdf/steo_full.pdf (accessed on 10 January 2022).
- Umar, M.; Farid, S.; Naeem, M.A. Time-frequency connectedness among clean-energy stocks and fossil fuel markets: Comparison between financial, oil and pandemic crisis. Energy 2022, 240, 122702. [Google Scholar] [CrossRef]
- Tang, C.; Aruga, K. Relationships among the Fossil Fuel and Financial Markets during the COVID-19 Pandemic: Evidence from Bayesian DCC-MGARCH Models. Sustainability 2022, 14, 51. [Google Scholar] [CrossRef]
- Strielkowski, W.; Civín, L.; Tarkhanova, E.; Tvaronavičienė, M.; Petrenko, Y. Renewable Energy in the Sustainable Development of Electrical Power Sector: A Review. Energies 2021, 14, 8240. [Google Scholar] [CrossRef]
- Pisciotta, M.; Pilorgé, H.; Feldmann, J.; Jacobson, R.; Davids, J.; Swett, S.; Sasso, Z.; Wilcox, J. Current state of industrial heating and opportunities for decarbonization. Prog. Energy Combust. Sci. 2022, 91, 100982. [Google Scholar] [CrossRef]
- Special Report. Global Warming of 1.5 °C. Available online: https://www.ipcc.ch/sr15/ (accessed on 10 January 2022).
- Shooshtarian, S.; Maqsood, T.; Caldera, S.; Ryley, T. Transformation towards a circular economy in the Australian construction and demolition waste management system. Sustain. Prod. Consum. 2022, 30, 89–106. [Google Scholar] [CrossRef]
- Sikdar, S. Circular economy: Is there anything new in this concept? Clean Technol. Environ. Policy 2019, 21, 1173–1175. [Google Scholar] [CrossRef] [Green Version]
- Dittenber, D.B.; GangaRao, H.V.S. Critical review of recent publications on use of natural composites in infrastructure. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1419–1429. [Google Scholar] [CrossRef]
- Plastics—The Facts 2021—Report Plastics Europe. The Association of Plastics Manufacturers in Europe. Available online: https://plasticseurope.org/pl/knowledge-hub/plastics-the-facts-2021/ (accessed on 13 January 2022).
- Thakur, V.K.; Thakur, M.K. Sustainable Polymers: A Perspective to the Future. In Handbook of Sustainable Polymers. Structure and Chemistry; Thakur, V.K., Thakur, M.K., Eds.; Pan Stanford Publishing: Singapore, 2016; pp. 1–18. [Google Scholar]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 6010012. [Google Scholar] [CrossRef]
- Jalaluddin, M. Use of Plastic Waste in Civil Constructions and Innovative Decorative Material (Eco-Friendly). MedCrave Online J. Civ. Eng. 2017, 3, 00082. [Google Scholar] [CrossRef] [Green Version]
- Manžuch, Z.; Akelytė, R.; Camboni, M.; Carlander, D. Chemical Recycling of Polymeric Materials from Waste in the Circular Economy. RPA Europe Final Report. August 2021. Available online: https://echa.europa.eu/documents/10162/1459379/chem_recycling_final_report_en.pdf/887c4182-8327-e197-0bc4-17a5d608de6e?t=1636708465520, (accessed on 14 January 2022).
- Plastics—The Facts 2020—Report Plastics Europe. The Association of Plastics Manufacturers in Europe. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed on 15 January 2022).
- Menyuka, N.N.; Sibanda, M.; Bob, U. Perceptions of the Challenges and Opportunities of Utilising Organic Waste through Urban Agriculture in the Durban South Basin. Int. J. Environ. Res. Public Health 2020, 17, 1158. [Google Scholar] [CrossRef] [Green Version]
- Jha, K.K.; Kannan, T.T.M. Recycling of plastic waste into fuel by pyrolysis—A review. Mater. Today Proc. 2021, 37, 3718–3720. [Google Scholar]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Balema, V.P.; Hlova, I.Z.; Carnahan, S.L.; Seyedi, M.; Dolotko, O.; Rossini, A.J.; Luzinov, I. Depolymerization of polystyrene under ambient conditions. New J. Chem. 2021, 45, 2935–2938. [Google Scholar] [CrossRef]
- Dos Santos, P.B.; da Silva Ribeiro, H.J.; Ferreira, A.C.; Ferreira, C.C.; Bernar, L.P.; da Costa Assunção, F.P.; de Castro, D.A.R.; Santos, M.C.; Duvoisin, S.; Borges, L.E.P.; et al. Process Analysis of PMMA-Based Dental Resins Residues Depolymerization: Optimization of Reaction Time and Temperature. Energies 2022, 15, 91. [Google Scholar] [CrossRef]
- Lahtela, V.; Hyvärinen, M.; Kärki, T. Composition of Plastic Fractions in Waste Streams: Toward More Efficient Recycling and Utilization. Polymers 2019, 11, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jubinville, D.; Esmizadeh, E.; Saikrishnan, S.; Tzoganakis, C.; Mekonnen, T. A comprehensive review of global production and recycling methods of polyolefin (PO) based products and their post-recycling applications. Sustain. Mater. Technol. 2020, 25, e00188. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E. Chapter 13—Plastic Recycling. In Handbook of Recycling. State-of-the-Art for Practitioners, Analysts, and Scientists; Worrell, E., Reuter, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 179–190. [Google Scholar]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of Polymers: A Review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef]
- Schwabl, D.; Bauer, M.; Lehner, M. Advancing Plastic Recycling by Wet-Mechanical Processing of Mixed Waste Fractions. Processes 2021, 9, 493. [Google Scholar] [CrossRef]
- Ragaert, K.; Huysveld, S.; Vyncke, G.; Hubo, S.; Veelaert, L.; Dewulf, J.; Du Bois, E. Design from recycling: A complex mixed plastic waste case study. Resour. Conserv. Recycl. 2020, 155, 104646. [Google Scholar] [CrossRef]
- Solis, M.; Silveira, S. Technologies for chemical recycling of household plastics—A technical review and TRL assessment. Waste Manag. 2020, 105, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, H.X.; Cheng, Z.N.; Yan, B.B.; Chen, G.Y.; Wang, S.B. Conversion of plastic waste into fuels: A critical review. J. Hazard. Mater. 2022, 424, 127460. [Google Scholar] [CrossRef] [PubMed]
- Palos, R.; Gutiérrez, A.; Vela, F.J.; Maña, J.A.; Hita, I.; Asueta, A.; Arnaiz, S.; Arandes, J.M.; Bilbao, J. Assessing the potential of the recycled plastic slow pyrolysis for the production of streams attractive for refineries. J. Anal. Appl. Pyrolysis 2019, 142, 104668. [Google Scholar] [CrossRef]
- Prajapati, R.; Kohli, K.; Maity, S.K.; Sharma, B.K. Potential Chemicals from Plastic Wastes. Molecules 2021, 26, 3175. [Google Scholar] [CrossRef] [PubMed]
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. ChemistryOpen 2021, 10, 1202–1226. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Cao, Y.; Lu, X.; Ding, Z.; Zhou, W. Review of Typical Municipal Solid Waste Disposal Status and Energy Technology. Energy Procedia 2016, 88, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Arandes, J.M.; Torre, I.; Castaño, P.; Olazar, M.; Bilbao, J. Catalytic Cracking of Waxes Produced by the Fast Pyrolysis of Polyolefins. Energy Fuels 2007, 21, 561–569. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Vela, F.J.; Arandes, J.M.; Bilbao, J. Fuel production by cracking of polyolefins pyrolysis waxes under fluid catalytic cracking (FCC) operating conditions. Waste Manag. 2019, 93, 162–172. [Google Scholar] [CrossRef]
- Fivga, A.; Dimitriou, I. Pyrolysis of plastic waste for production of heavy fuel substitute: A techno-economic assessment. Energy 2018, 149, 865–874. [Google Scholar] [CrossRef]
- Fekhar, B.; Zsinka, V.; Miskolczi, N. Value added hydrocarbons obtained by pyrolysis of contaminated waste plastics in horizontal tubular reactor: In situ upgrading of the products by chlorine capture. J. Clean. Prod. 2019, 241, 118166. [Google Scholar] [CrossRef]
- Kaminsky, W. Chemical recycling of plastics by fluidized bed pyrolysis. Fuel Commun. 2021, 8, 100023. [Google Scholar] [CrossRef]
- Syamsiro, M.; Saptoadi, H.; Norsujianto, T.; Noviasri, P.; Cheng, S.; Alimuddin, Z.; Yoshikawa, K. Fuel Oil Production from Municipal Plastic Wastes in Sequential Pyrolysis and Catalytic Reforming Reactors. Energy Procedia 2014, 47, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Bezergianni, S.; Dimitriadis, A.; Faussone, G.C.; Karonis, D. Alternative Diesel from Waste Plastics. Energies 2017, 10, 1750. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.; Berruti, F. Thermochemical conversion of plastic waste to fuels: A review. Environ. Chem. Lett. 2021, 19, 123–148. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.I.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86. [Google Scholar] [CrossRef]
- Fahim, I.; Mohsen, O.; ElKayaly, D. Production of Fuel from Plastic Waste: A Feasible Business. Polymers 2021, 13, 915. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. Development and Validation of a Process Model to DescribePyrolysis of Forestry Residues in an Auger Reactor. Energy Fuels 2017, 31, 10833–10841. [Google Scholar] [CrossRef]
- Suresh, A.; Alagusundaram, A.; Kumar, P.S.; Vo, D.V.N.; Christopher, F.C.; Balaji, B.; Viswanathan, V.; Sankar, S. Microwave pyrolysis of coal, biomass and plastic waste: A review. Environ. Chem. Lett. 2021, 19, 3609–3629. [Google Scholar] [CrossRef]
- Miandad, R.; Nizami, A.S.; Rehan, M.; Barakat, M.A.; Khan, M.I.; Mustafa, A.; Ismail, I.M.I.; Murphy, J.D. Influence of temperature and reaction time on the conversion of polystyrene waste to pyrolysis liquid oil. Waste Manag. 2016, 58, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Silvarrey, L.S.; Zhang, K.; Phan, A.N. Monomer recovery through advanced pyrolysis of waste high density polyethylene (HDPE). Green Chem. 2018, 20, 1813–1823. [Google Scholar] [CrossRef] [Green Version]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.M.I.; Nizami, A.S. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 239–252. [Google Scholar] [CrossRef]
- Grabda, M.; Oleszek, S.; Shibata, E.; Nakamura, T. Study on simultaneous recycling of EAF dust and plastic waste containing TBBPA. J. Hazard. Mater. 2014, 278, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Fekhar, B.; Gombor, L.; Miskolczi, N. Pyrolysis of chlorine contaminated municipal plastic waste: In-situ upgrading of pyrolysis oils by Ni/ZSM-5, Ni/SAPO-11, red mud and Ca(OH)2 containing catalysts. J. Energy Inst. 2019, 92, 1270–1283. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Oleszek, S.; Kumagai, S.; Grabda, M.; Shiota, K.; Yoshioka, T.; Takaoka, M. Mitigation of bromine-containing products during pyrolysis of polycarbonate-based tetrabromobisphenol A in the presence of copper(I) oxide. J. Hazard. Mater. 2021, 409, 124972. [Google Scholar] [CrossRef]
- Ma, C.; Yu, J.; Wang, B.; Song, Z.; Xiang, J.; Hu, S.; Su, S.; Sun, L. Chemical recycling of brominated flame retarded plastics from e-waste for clean fuels production: A review. Renew. Sustain. Energy Rev. 2016, 61, 433–450. [Google Scholar] [CrossRef]
- Terakado, O.; Ohhashi, R.; Hirasawa, M. Bromine fixation by metal oxide in pyrolysis of printed circuit board containing brominated flame retardant. J. Anal. Appl. Pyrolysis 2013, 103, 216–221. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, S.J.; Kim, J.S. Thermal degradation of acrylonitrile–butadiene–styrene (ABS) containing flame retardants using a fluidized bed reactor: The effects of Ca-based additives on halogen removal. Fuel Process Technol. 2012, 96, 265–270. [Google Scholar] [CrossRef]
- Brebu, M.; Bhaskar, T.; Murai, K.; Muto, A.; Sakata, Y.; Uddin, M.A. Removal of nitrogen, bromine, and chlorine from PP/PE/PS/PVC/ABS–Br pyrolysis liquid products using Fe- and Ca-based catalysts. Polym. Degrad. Stab. 2005, 87, 225–230. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Kalogiannis, K.G.; Lappas, A.A.; Achilias, D.S. Novel trends in the thermo-chemical recycling of plastics from WEEE containing brominated flame retardants. Environ. Sci. Pollut. Res. 2021, 28, 59190–59213. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef] [Green Version]
- Rasul, S.B.; Som, U.; Hossain, S.; Rahman, W. Liquid fuel oil produced from plastic based medical wastes by thermal cracking. Sci. Rep. 2021, 11, 17048. [Google Scholar] [CrossRef]
- Lee, K.H.; Shin, D.H. Characteristics of liquid product from the pyrolysis of waste plastic mixture at low and high temperatures: Influence of lapse time of reaction. Waste Manag. 2007, 27, 168–176. [Google Scholar] [CrossRef]
- Pan, R.; Martins, M.F.; Debenest, G. Pyrolysis of waste polyethylene in a semi-batch reactor to produce liquid fuel: Optimization of operating conditions. Energy Convers. Manag. 2021, 237, 114114. [Google Scholar] [CrossRef]
- Torres, D.; Jiang, Y.; Sanchez Monsalve, D.A.; Leeke, G.A. Chlorine removal from the pyrolysis of urban polyolefinic waste in a semi-batch reactor. J. Environ. Chem. Eng. 2021, 9, 104920. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Yang, Y.; Wang, J.; Leeke, G.A. Pyro-Oil and Wax Recovery from Reclaimed Plastic Waste in a Continuous Auger Pyrolysis Reactor. Energies 2020, 13, 2040. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Dai, L.; Lv, Y.; Li, H.; Deng, W.; Guo, F.; Chen, P.; Lei, H.; Ruan, R. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production. Chem. Eng. J. 2021, 418, 129412. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Boadu, E.K.; Dapaah, S. Plastic waste to fuel via pyrolysis: A key way to solving the severe plastic waste problem in Ghana. Therm. Sci. Eng. Prog. 2019, 11, 417–424. [Google Scholar] [CrossRef]
- Papuga, S.V.; Gvero, P.M.; Vukić, L.M. Temperature and time influence on the waste plastics pyrolysis in the fixed bed reactor. Therm. Sci. 2016, 20, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Mašek, O.; Harper, A.; Ocone, R. Kinetic study of pyrolysis of high-density polyethylene (HDPE) waste at different bed thickness in a fixed bed reactor. Can. J. Chem. Eng. 2021, 99, 1733–1744. [Google Scholar] [CrossRef]
- Milne, B.J.; Behie, L.A.; Berruti, F. Recycling of waste plastics by ultrapyrolysis using an internally circulating fluidized bed reactor. J. Anal. Appl. Pyrolysis 1999, 51, 157–166. [Google Scholar] [CrossRef]
- Jung, S.H.; Cho, M.H.; Kang, B.S.; Kim, J.S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Mastral, J.F.; Berrueco, C.; Ceamanos, J. Modelling of the pyrolysis of high density polyethylene: Product distribution in a fluidized bed reactor. J. Anal. Appl. Pyrolysis 2007, 79, 313–322. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237. [Google Scholar] [CrossRef]
- Orozco, S.; Alvarez, J.; Lopez, G.; Artetxe, M.; Bilbao, J.; Olazar, M. Pyrolysis of plastic wastes in a fountain confined conical spouted bed reactor: Determination of stable operating conditions. Energy Convers. Manag. 2021, 229, 113768. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Elordi, G.; Amutio, M.; Bilbao, J.; Olazar, M. Production of Light Olefins from Polyethylene in a Two-Step Process: Pyrolysis in a Conical Spouted Bed and Downstream High-Temperature Thermal Cracking. Ind. Eng. Chem. Res. 2012, 51, 13915–13923. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Qian, M.; Yadavalli, G.; Wu, J.; Chen, S. From plastics to jet fuel range alkanes via combined catalytic conversions. Fuel 2017, 188, 28–38. [Google Scholar] [CrossRef]
- De Marco, I.; Caballero, B.M.; López, A.; Laresgoiti, M.F.; Torres, A.; Chomón, M.J. Pyrolysis of the rejects of a waste packaging separation and classification plant. J. Anal. Appl. Pyrolysis 2009, 85, 384–391. [Google Scholar] [CrossRef]
- Dwivedi, P.; Mishra, P.K.; Mondal, M.K.; Srivastava, N. Non-biodegradable polymeric waste pyrolysis for energy recovery. Heliyon 2019, 5, e02198. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Johnston, P.; Bai, X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers. Manag. 2017, 142, 441–451. [Google Scholar] [CrossRef]
- Colantonio, S.; Cafiero, L.; De Angelis, D.; Ippolito, N.M.; Tuffi, R.; Ciprioti, S.V. Thermal and catalytic pyrolysis of a synthetic mixture representative of packaging plastics residue. Front. Chem. Sci. Eng. 2020, 14, 288–303. [Google Scholar] [CrossRef]
- Milato, J.V.; França, R.J.; Rocha, A.S.; Calderari, M.R.C.M. Catalytic co-pyrolysis of oil sludge with HDPE to obtain paraffinic products over HUSY zeolites prepared by dealumination and desilication. J. Anal. Appl. Pyrolysis 2020, 151, 104928. [Google Scholar] [CrossRef]
- Zeaiter, J. A process study on the pyrolysis of waste polyethylene. Fuel 2014, 133, 276–282. [Google Scholar] [CrossRef]
- Kasar, P.; Sharma, D.K.; Ahmaruzzaman, M. Thermal and catalytic decomposition of waste plastics and its co-processing with petroleum residue through pyrolysis process. J. Clean. Prod. 2020, 265, 121639. [Google Scholar] [CrossRef]
- Orozco, S.; Artetxe, M.; Lopez, G.; Suarez, M.; Bilbao, J.; Olazar, M. Conversion of HDPE into Value Products by Fast Pyrolysis Using FCC Spent Catalysts in a Fountain Confined Conical Spouted Bed Reactor. ChemSusChem 2021, 14, 4291–4300. [Google Scholar] [CrossRef]
- De la Puente, G.; Klocker, C.; Sedran, U. Conversion of waste plastics into fuels: Recycling polyethylene in FCC. Appl. Catal. B Environ. 2002, 36, 279–285. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Gonzalez, R.M.; Meirer, F.; Weckhuysen, B.M. Plastic Waste Conversion over a Refinery Waste Catalyst. Angew. Chem. Int. Ed. 2021, 60, 16101–16108. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.; Devan, P.K.; Pitchandi, K. Production of pyrolytic oil from ULDP plastics using silica-alumina catalyst and used as fuel for DI diesel engine. RSC Adv. 2020, 10, 37266–37279. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M. Fuels from waste plastics by thermal and catalytic processes: A review. Ind. Eng. Chem. Res. 2008, 47, 7982–7992. [Google Scholar] [CrossRef]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J. Anal. Appl. Pyrolysis 2017, 127, 31–37. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Lv, Y.; Cobb, K.; Cheng, Y.; Wang, Y.; Liu, Y.; Chen, P.; Zou, R.; Lei, H.; et al. Pyrolysis-catalysis for waste polyolefin conversion into low aromatic naphtha. Energy Convers. Manag. 2021, 245, 114578. [Google Scholar] [CrossRef]
- Rahimi, S.; Rostamizadeh, M. Novel Fe/B-ZSM-5 nanocatalyst development for catalytic cracking of plastic to valuable products. J. Taiwan Inst. Chem. Eng. 2021, 118, 131–139. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J. Energy 2019, 92, 195–202. [Google Scholar] [CrossRef]
- Anene, A.F.; Fredriksen, S.B.; Sætre, K.A.; Tokheim, L.A. Experimental Study of Thermal and Catalytic Pyrolysis of Plastic Waste Components. Sustainability 2018, 10, 3979. [Google Scholar] [CrossRef] [Green Version]
- Damodharan, D.; Sathiyagnanam, A.P.; Rana, D.; Kumar, B.R.; Saravanan, S. Combined influence of injection timing and EGR on combustion, performance and emissions of DI diesel engine fueled with neat waste plastic oil. Energy Convers. Manag. 2018, 161, 294–305. [Google Scholar] [CrossRef]
- Mangesh, V.L.; Padmanabhan, S.; Tamizhdurai, P.; Rameshe, A. Experimental investigation to identify the type of waste plastic pyrolysis oil suitable for conversion to diesel engine fuel. J. Clean. Prod. 2020, 246, 119066. [Google Scholar] [CrossRef]
- Quesada, L.; Calero, M.; Martín-Lara, M.A.; Pérez, A.; Blázquez, G. Characterization of fuel produced by pyrolysis of plastic film obtained of municipal solid waste. Energy 2019, 186, 115874. [Google Scholar] [CrossRef]
- Saha, D.; Sinha, A.; Roy, B. Critical insights into the effects of plastic pyrolysis oil on emission and performance characteristics of CI engine. Environ. Sci. Pollut. Res. 2021, 28, 44598–44621. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, D.; Sathiyagnanam, A.P.; Kumar, B.R.; Ganesh, K.C. Cleaner emissions from a DI diesel engine fueled with waste plastic oil derived from municipal solid waste under the influence of n-pentanol addition, cold EGR, and injection timing. Environ. Sci. Pollut. Res. 2018, 25, 13611–13625. [Google Scholar] [CrossRef]
- Peer, M.S.; Peer, M.N. Experimental investigation on engine characteristics fueled with waste HDPE oil and study on NOx emission variation using thermal imager. Environ. Sci. Pollut. Res. 2019, 26, 3436–3446. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sankaranarayanan, G. Investigation on environmental factors of waste plastics into oil and its emulsion to control the emission in DI diesel engine. Ecotoxicol. Environ. Saf. 2016, 134, 440–444. [Google Scholar] [CrossRef]
- Khoo, H.H. LCA of plastic waste recovery into recycled materials, energy and fuels in Singapore Resources. Conserv. Recycl. 2019, 145, 67–77. [Google Scholar] [CrossRef]
- Li, C.; Yuan, X.; Sun, Z.; Suvarna, M.; Hu, X.; Wang, X.; Ok, Y.S. Pyrolysis of waste surgical masks into liquid fuel and its life-cycle assessment. Bioresour. Technol. 2022, 346, 126582. [Google Scholar] [CrossRef]
- Damodharan, D.; Sathiyagnanam, A.P.; Rana, D.; Kumar, B.R.; Saravanan, S. Extraction and characterization of waste plastic oil (WPO) with the effect of n-butanol addition on the performance and emissions of a DI diesel engine fueled with WPO/diesel blends. Energy Convers. Manag. 2017, 131, 117–126. [Google Scholar] [CrossRef]
- Kaimal, V.K.; Vijayabalan, P. A detailed study of combustion characteristics of a DI diesel engine using waste plastic oil and its blends. Energy Convers. Manag. 2015, 105, 951–956. [Google Scholar] [CrossRef]
- Matuszewska, A.; Hańderek, A.; Paczuski, M.; Biernat, K. Hydrocarbon Fractions from Thermolysis of Waste Plastics as Components of Engine Fuels. Energies 2021, 14, 7245. [Google Scholar] [CrossRef]
- Mangesh, V.L.; Padmanabhan, S.; Tamizhdurai, P.; Narayanan, S.; Ramesh, A. Combustion and emission analysis of hydrogenated waste polypropylene pyrolysis oil blended with diesel. J. Hazard. Mater. 2020, 386, 121453. [Google Scholar] [CrossRef] [PubMed]
- Scharnagl, F.K.; Hertrich, M.F.; Ferretti, F.; Kreyenschulte, C.; Lund, H.; Jackstell, R.; Beller, M. Hydrogenation of terminal and internal olefins using a biowaste-derived heterogeneous cobalt catalyst. Sci. Adv. 2018, 4, eaau1248. [Google Scholar] [CrossRef] [Green Version]
- Léonard, N.G.; Chirik, P.J. Air-Stable α-diimine nickel precatalysts for the hydrogenation of hindered, unactivated alkenes. ACS Catal. 2018, 8, 342–348. [Google Scholar] [CrossRef]
- Büschelberger, P.; Gärtner, D.; Reyes-Rodriguez, E.; Kreyenschmidt, F.; Koszinowski, K.; von Wangelin, A.J.; Wolf, R. Alkene Metalates as Hydrogenation Catalysts. Chemistry 2017, 23, 3139–3151. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef] [Green Version]
- Jia, C.; Xie, S.; Zhang, W.; Intan, N.N.; Sampath, J.; Pfaendtner, J.; Lin, H. Deconstruction of high-density polyethylene into liquid hydrocarbon fuels and lubricants by hydrogenolysis over Ru catalyst. Chem. Catal. 2021, 1, 437–455. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Beckham, G.T.; Román-Leshkov, Y. Conversion of Polyolefin Waste to Liquid Alkanes with Ru-Based Catalysts under Mild Conditions. JACS Au 2021, 1, 8–12. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Troyano-Valls, C.; Beckham, G.T.; Román-Leshkov, Y. Hydrogenolysis of Polypropylene and Mixed Polyolefin Plastic Waste over Ru/C to Produce Liquid Alkanes. ACS Sustain. Chem. Eng. 2021, 9, 11661–11666. [Google Scholar] [CrossRef]
- Wang, C.; Xie, T.J.; Kots, P.A.; Vance, B.C.; Yu, K.W.; Kumar, P.; Fu, J.Y.; Liu, S.B.; Tsilomelekis, G.; Stach, E.A.; et al. Polyethylene Hydrogenolysis at Mild Conditions over Ruthenium on Tungstated Zirconia. JACS Au 2021, 1, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; LaPointe, A.M.; Ammal, S.C.; Heyden, A.; Perras, F.A.; et al. Upcycling Single-Use Polyethylene into High-Quality Liquid Products. ACS Cent. Sci. 2019, 5, 1795–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaji, Y.; Tamura, M.; Miyaoka, S.; Kumagai, S.; Tanji, M.; Nakagawa, Y.; Yoshioka, T.; Tomishige, K. Low-temperature catalytic upgrading of waste polyolefinic plastics into liquid fuels and waxes. Appl. Catal. B Environ. 2021, 285, 119805. [Google Scholar] [CrossRef]
- Lee, W.T.; Bobbink, F.D.; van Muyden, A.P.; Lin, K.H.; Corminboeuf, C.; Zamani, R.R.; Dyson, P.J. Catalytic hydrocracking of synthetic polymers into grid-compatible gas streams. Cell Rep. Phys. Sci. 2021, 2, 100332. [Google Scholar] [CrossRef]
- Tennakoon, A.; Wu, X.; Paterson, A.L.; Patnaik, S.; Pei, Y.; LaPointe, A.M.; Ammal, S.C.; Hackler, R.A.; Heyden, A.; Slowing, I.I.; et al. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 2020, 3, 893–901. [Google Scholar] [CrossRef]
- Liu, S.; Kots, P.A.; Vance, B.; Danielson, A.; Vlachos, D.G. Plastic waste to fuels by hydrocracking at mild conditions. Sci. Adv. 2021, 7, eabf8283. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef]
- Whajah, B.; da Silva Moura, N.; Blanchard, J.; Wicker, S.; Gandar, K.; Dorman, J.A.; Dooley, K.M. Catalytic Depolymerization of Waste Polyolefins by Induction Heating: Selective Alkane/Alkene Production. Ind. Eng. Chem. Res. 2021, 60, 15141–15150. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Qian, H.; Nie, Y.; Bai, X.; Xia, T.; Rehman, M.L.U.; Li, Q.; Ju, M. Upcycling and catalytic degradation of plastic wastes. Cell Rep. Phys. Sci. 2021, 2, 100514. [Google Scholar] [CrossRef]
- Matuszewska, A.; Hańderek, A.; Biernat, K.; Bukrejewski, P. Thermolytic Conversion of Waste Polyolefins into Fuels Fraction with the Use of Reactive Distillation and Hydrogenation with the Syngas under Atmospheric Pressure. Energy Fuels 2019, 33, 1363–1371. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q. Review on microwave-matter interaction fundamentals and efficient microwave-associated heating strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Xie, Q.; Addy, M.; Zhou, W.; Liu, Y.; Wang, Y.; Cheng, Y.; Li, K.; Ruan, R. Utilization of municipal solid and liquid wastes for bioenergy and bioproducts production. Bioresour. Technol. 2016, 215, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.N.; Chen, P.; Liu, S.Y.; Peng, P.; Min, M.; Cheng, Y.L.; Anderson, E.; Zhou, N.; Fan, L.L.; Liu, C.H.; et al. Effects of feedstock characteristics on microwave-assisted pyrolysis—A review. Bioresour. Technol. 2017, 230, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Liu, S.; Huang, Y.; Liu, S.; Zhou, N.; Peng, P.; Wang, Y.; Chen, P.; Ruan, R. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manag. 2019, 196, 1316–1325. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, W.; Zhang, X.; Qiao, J. Chemical Recycling of Plastics by Microwave-Assisted High-Temperature Pyrolysis. Glob. Chall. 2020, 4, 1900074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putra, P.H.M.; Rozali, S.; Patah, M.F.A.; Idris, A. A review of microwave pyrolysis as a sustainable plastic waste management technique. J. Environ. Manag. 2022, 303, 114240. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, K.M.; Hussain, K. Microwave–metal interaction pyrolysis of polystyrene. J. Anal. Appl. Pyrolysis 2010, 89, 39–43. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Basak, T. Susceptor-assisted enhanced microwave processing of ceramics—A review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 433–469. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T. A review on the microwave-assisted pyrolysis technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew. Sustain. Energy Rev. 2015, 52, 1580–1595. [Google Scholar] [CrossRef]
- Martín, M.T.; Aguirre, J.L.; Baena-González, J.; González, S.; Pérez-Aparicio, R.; Saiz-Rodríguez, L. Influence of Specific Power on the Solid and Liquid Products Obtained in the Microwave-Assisted Pyrolysis of End-of-Life Tires. Energies 2022, 15, 2128. [Google Scholar] [CrossRef]
- Fan, L.L.; Zhang, Y.N.; Liu, S.Y.; Zhou, N.; Chen, P.; Liu, Y.H.; Wang, Y.P.; Peng, P.; Cheng, Y.L.; Addy, M.; et al. Ex-situ catalytic upgrading of vapors from microwave-assisted pyrolysis of low-density polyethylene with MgO. Energy Convers. Manag. 2017, 149, 432–441. [Google Scholar] [CrossRef]
- Zhang, X.S.; Lei, H.W.; Yadavalli, G.; Zhu, L.; Wei, Y.; Liu, Y.P. Gasoline-range hydrocarbons produced from microwave-induced pyrolysis of low-density polyethylene over ZSM-5. Fuel 2015, 144, 33–42. [Google Scholar] [CrossRef]
- Rosi, L.; Bartoli, M.; Frediani, M. Microwave assisted pyrolysis of halogenated plastics recovered from waste computers. Waste Manag. 2018, 73, 511–522. [Google Scholar] [CrossRef]
- Rex, P.; Masilamani, I.P.; Miranda, L.R. Microwave pyrolysis of polystyrene and polypropylene mixtures using different activated carbon from biomass. J. Energy Inst. 2020, 93, 1819–1832. [Google Scholar] [CrossRef]
- Aishwarya, K.N.; Sindhu, N. Microwave Assisted Pyrolysis of Plastic Waste. Procedia Technol. 2016, 25, 990–997. [Google Scholar] [CrossRef] [Green Version]
- Bu, Q.; Chen, K.; Xie, W.; Liu, Y.; Cao, M.; Kong, X.; Chu, Q.; Mao, H. Hydrocarbon rich bio-oil production, thermal behavior analysis and kinetic study of microwave-assisted co-pyrolysis of microwave-torrefied lignin with low density polyethylene. Bioresour. Technol. 2019, 291, 121860. [Google Scholar] [CrossRef]
- Bu, Q.; Liu, Y.; Liang, J.; Morgan, H.M.; Yan, L.; Xu, F.; Mao, H. Microwave-assisted co-pyrolysis of microwave torrefied biomass with waste plastics using ZSM-5 as a catalyst for high quality bio-oil. J. Anal. Appl. Pyrolysis 2018, 134, 536–543. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R.; Shukla, A.; Haldar, S. Effective deoxygenation for the production of liquid biofuels via microwave assisted co-pyrolysis of agro residues and waste plastics combined with catalytic upgradation. Bioresour. Technol. 2020, 302, 122775. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.P.; Liu, Y.H.; Dai, L.L.; Wu, Q.H.; Xia, M.L.; Zhang, S.M.; Ke, L.Y.; Zou, R.G.; Ruan, R. Microwave catalytic co-pyrolysis of waste cooking oil and low-density polyethylene to produce monocyclic aromatic hydrocarbons: Effect of different catalysts and pyrolysis parameters. Sci. Total Environ. 2022, 809, 152182. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Wan Mahari, W.A.; Ok, Y.S.; Peng, W.X.; Chong, C.T.; Ma, N.L.; Chase, H.A.; Liew, Z.; Yusup, S.; Kwon, E.E.; et al. Microwave vacuum pyrolysis of waste plastic and used cooking oil for simultaneous waste reduction and sustainable energy conversion: Recovery of cleaner liquid fuel and techno-economic analysis. Renew. Sustain. Energy Rev. 2019, 115, 109359. [Google Scholar] [CrossRef]
- Jing, X.D.; Dong, J.Q.; Huang, H.L.; Deng, Y.X.; Wen, H.; Xu, Z.H.; Ceylan, S. Interaction between feedstocks, absorbers and catalysts in the microwave pyrolysis process of waste plastics. J. Clean. Prod. 2021, 291, 125857. [Google Scholar] [CrossRef]
- Fan, L.L.; Su, Z.Y.; Wu, J.B.; Xiao, Z.G.; Huang, P.; Liu, L.; Jiang, H.W.; Zhou, W.G.; Liu, S.Y.; Ruan, R. Integrating continuous-stirred microwave pyrolysis with ex-situ catalytic upgrading for linear low-density polyethylene conversion: Effects of parameter conditions. J. Anal. Appl. Pyrolysis 2021, 157, 105213. [Google Scholar] [CrossRef]
- Russell, A.D.; Antreou, E.I.; Lam, S.S.; Ludlow-Palafox, C.; Chase, H.A. Microwave-assisted pyrolysis of HDPE using an activated carbon bed. R. Soc. Chem. 2012, 2, 6756–6760. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Frediani, M.; Undri, A.; Frediani, P. Depolymerization of polystyrene at reduced pressure through a microwave assisted pyrolysis. J. Anal. Appl. Pyrolysis 2015, 113, 281–287. [Google Scholar] [CrossRef]
- Undri, A.; Frediani, M.; Rosi, L.; Frediani, P. Reverse polymerization of waste polystyrene through microwave assisted pyrolysis. J. Anal. Appl. Pyrolysis 2014, 105, 35–42. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H. Synthesis of high-density jet fuel from plastics via catalytically integral processes. RSC Adv. 2016, 6, 6154–6163. [Google Scholar] [CrossRef]
- Undri, A.; Rosi, L.; Frediani, M.; Frediani, P. Microwave assisted pyrolysis of corn derived plastic bags. J. Anal. Appl. Pyrolysis 2014, 108, 86–97. [Google Scholar] [CrossRef]
- Duan, D.; Feng, Z.; Dong, X.; Chen, X.; Zhang, Y.; Wan, K.; Wang, Y.; Wang, Q.; Xiao, G.; Liu, H.; et al. Improving bio-oil quality from low-density polyethylene pyrolysis: Effects of varying activation and pyrolysis parameters. Energy 2021, 232, 121090. [Google Scholar] [CrossRef]
- Prathiba, R.; Shruthi, R.; Miranda, L.R. Pyrolysis of polystyrene waste in the presence of activated carbon in conventional and microwave heating using modified thermocouple. Waste Manag. 2018, 76, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Juliastuti, S.; Hendrianie, N.; Ramadhan, P.; Satria, D. Microwave Pyrolysis of Multilayer Plastic Waste (LDPE) Using Zeolite Catalyst. AIP Conf. Proc. 2017, 1840, 110001. [Google Scholar]
- Undri, A.; Rosi, L.; Frediani, M.; Frediani, P. Efficient disposal of waste polyolefins through microwave assisted pyrolysis. Fuel 2014, 116, 662–671. [Google Scholar] [CrossRef]
- Khaghanikavkani, E.M.F.M.; Holdem, J.; Williamson, A. Microwave pyrolysis of plastic. J. Chem. Eng. Process Technol. 2013, 4, 1000150. [Google Scholar] [CrossRef] [Green Version]
- Arshad, H.; Sulaiman, S.A.; Hussain, Z.; Naz, M.Y.; Moni, M.N.Z. Effect of input power and process time on conversion of pure and mixed plastics into fuels through microwave-metal interaction pyrolysis. Waste Biomass Valorization 2021, 12, 3443–3457. [Google Scholar] [CrossRef]
- Fan, L.; Chen, P.; Zhang, Y.; Liu, S.; Liu, Y.; Wang, Y.; Dai, L.; Ruan, R. Fast microwave-assisted catalytic co-pyrolysis of lignin and low-density polyethylene with HZSM-5 and MgO for improved bio-oil yield and quality. Bioresour. Technol. 2017, 225, 199–205. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Chong, C.T.; Lam, W.H.; Anuar, T.N.S.T.; Ma, N.L.; Ibrahim, M.D.; Lam, S.S. Microwave co-pyrolysis of waste polyolefins and waste cooking oil: Influence of N2 atmosphere versus vacuum environment. Energy Convers. Manag. 2018, 171, 1292–1301. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Yerrayya, A.; Nagababu, G.; Guduru, R.K.; Kumar, T.H. Recovery of renewable aromatic and aliphatic hydrocarbon resources from microwave pyrolysis/co-pyrolysis of agro-residues and plastics wastes. Bioresour. Technol. 2020, 318, 124277. [Google Scholar] [CrossRef]
- Rayaan, M.B.A. Recent advancements of thermochemical conversion of plastic waste to biofuel—A review. Clean. Eng. Technol. 2021, 2, 100062. [Google Scholar] [CrossRef]
- Tavares, R.; Ramos, A.; Rouboa, A. A theoretical study on municipal solid waste plasma gasification. Waste Manag. 2019, 90, 37–45. [Google Scholar] [CrossRef]
- Sharma, B.; Goswami, Y.; Sharma, S.; Shekhar, S. Inherent roadmap of conversion of plastic waste into energy and its life cycle assessment: A frontrunner compendium. Renew. Sustain. Energy Rev. 2021, 146, 111070. [Google Scholar] [CrossRef]
- Tamosiunas, A.; Valatkevicius, P.; Valincius, V.; Grigaitiene, V. Production of synthesis gas from propane using thermal water vapor plasma. Int. J. Hydrogen Energy 2014, 39, 2078–2086. [Google Scholar] [CrossRef]
- Ruj, B.; Ghosh, S. Technological aspects for thermal plasma treatment of municipal solid waste—A review. Fuel Process. Technol. 2014, 126, 298–308. [Google Scholar] [CrossRef]
- Silvarrey, L.S.D. Advanced Pyrolysis of Plastic Waste for Chemicals, Fuel and Materials. Ph.D. Thesis, School of Engineering, Newcastle University, Newcastle upon Tyne, UK, 2019. [Google Scholar]
- Fabry, F.; Rehmet, C.; Rohani, V.; Fulcheri, L. Waste Gasification by Thermal Plasma: A Review. Waste Biomass Valorization 2013, 4, 421–439. [Google Scholar] [CrossRef] [Green Version]
- Muvhiiwa, R.; Kuvarega, A.; Llana, E.M.; Muleja, A. Study of biochar from pyrolysis and gasification of wood pellets in a nitrogen plasma reactor for design of biomass processes. J. Environ. Chem. Eng. 2019, 7, 103391. [Google Scholar] [CrossRef]
- Fan, Y.; Xiong, Y.; Zhu, L.; Fan, L.; Jin, L.; Chen, Y.; Zhao, W. Comparison of the one-step and two-step plasma-catalytic upgrading of biomass pyrolysis volatiles to bio-fuel. Chem. Eng. Process. Process Intensif. 2019, 135, 53–62. [Google Scholar] [CrossRef]
- Vaidyanathan, A.; Mulholland, J.; Ryu, J.; Smith, M.S.; Circeo, L.J., Jr. Characterization of fuel gas products from the treatment of solid waste streams with a plasma arc torch. J. Environ. Manag. 2007, 82, 77–82. [Google Scholar] [CrossRef]
- Pak, A.Y.; Larionov, K.B.; Kolobova, E.N.; Slyusarskiy, K.V.; Bolatova, J.; Yankovsky, S.A.; Stoyanovskii, V.O.; Vassilyeva, Y.Z.; Gubin, V.E. A novel approach of waste tires rubber utilization via ambient air direct current arc discharge plasma. Fuel Process. Technol. 2022, 227, 107111. [Google Scholar] [CrossRef]
- Cai, X.; Du, C. Thermal Plasma Treatment of Medical Waste. Plasma Chem. Plasma Process. 2021, 41, 1–46. [Google Scholar] [CrossRef]
- Erdogan, A.A.; Yilmazoglu, M.Z. Plasma gasification of the medical waste. Int. J. Hydrogen Energy 2021, 46, 29108–29125. [Google Scholar] [CrossRef]
- Zhovtyansky, V.A.; Kolesnikova, E.P.; Iakymovych, M.V. Plasma-assisted “waste-to-energy” processes. Probl. At. Sci. Technol. Ser. Plasma Phys. 2017, 23, 231–236. [Google Scholar]
- Paulino, R.F.S.; Essiptchouk, A.M.; Silveira, J.L. The use of syngas from biomedical waste plasma gasification systems for electricity production in internal combustion: Thermodynamic and economic issues. Energy 2020, 199, 117419. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Illathukandy, B.; Wu, W.; Chang, J.-S. Plasma gasification performances of various raw and torrefied biomass materials using different gasifying agents. Bioresour. Technol. 2020, 314, 123740. [Google Scholar] [CrossRef] [PubMed]
- Punčochář, M.; Ruj, B.; Chatterje, P.K. Development of process for disposal of plastic waste using plasma pyrolysis technology and option for energy recovery. Procedia Eng. 2012, 42, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Capodaglio, A.G.; Bolognesi, S. Ecofuel feedstocks and their prospects. In Advances in Eco-Fuels for a Sustainable Environment; Azad, K., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2019; pp. 15–51. [Google Scholar]
- Yang, L.; Wang, H.; Wang, H.; Wang, D.; Wang, Y. Solid waste plasma disposal plant. J. Electrost. 2011, 69, 411–413. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H.; Hao, H.; Zhao, K. Development of plasma pyrolysis/gasification systems for energy efficient and environmentally sound waste disposal. J. Electrost. 2013, 71, 839–847. [Google Scholar] [CrossRef]
- Mohsenian, S.; Esmaili, M.S.; Shokri, B.; Ghorbanalilu, M. Physical characteristics of twin DC thermal plasma torch applied to polymer waste treatment. J. Electrost. 2015, 76, 231–237. [Google Scholar] [CrossRef]
- Mohsenian, S.; Esmaili, S.S.; Fathi, J.; Shokri, B. Hydrogen and carbon black nano-spheres production via thermal plasma pyrolysis of polymers. Int. J. Hydrogen Energy 2016, 41, 16656–16663. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Aboughaly, M.; Stoute, C.A.B. DC Thermal Plasma Design and Utilization for the Low Density Polyethylene to Diesel Oil Pyrolysis Reaction. Energies 2017, 10, 784. [Google Scholar] [CrossRef]
- Yao, L.; King, J.; Wu, D.; Chuang, S.S.C.; Peng, Z. Non-thermal plasma-assisted hydrogenolysis of polyethylene to light hydrocarbons. Catal. Commun. 2021, 150, 106274. [Google Scholar] [CrossRef]
- Kumar, S.B.V. Process for the Conversion of Plastic Material to Fuels. U.S. Patent WO2021171313A1, 2 September 2021. [Google Scholar]
- McNamara, D.; Murray, M. Conversion of Waste Plastics Material to Fuel. U.S. Patent US20120261247A1, 18 October 2012. [Google Scholar]
- Daggupati, S.; Thakur, S.; Majhi, S.; Mandal, S.; Das, A.K.; Ajit, V.S. A Method for Catalytic Conversion of Waste Plastic into Liquid Fuel. U.S. Patent US20210348061A1, 11 November 2021. [Google Scholar]
- Baker, G. Process and Plant for Conversion of Waste Material to Liquid Fuel. European Patent EP1725633B1, 24 October 2018. [Google Scholar]
- Schabel, J.; Schwarz, R.A.; Grispin, C.W.; Gencer, M.A.; Hensel, J.D. Process, Apparatus, Controller and System for Producing Petroleum Products. U.S. Patent US20200199457A1, 25 June 2020. [Google Scholar]
- Handerek, A.; Kowalczyk, M.P.; Kiraga, J.; Biernat, K.; Matuszewska, A. Method of Preparation of Hydrocarbon Fuels from Polyolefin Waste Materials. U.S. Patent US20200080004, 12 March 2020. [Google Scholar]
- Dooley, B. Production of Hydrocarbon Fuels from Plastics. U.S. Patent US20180355256A1, 13 December 2018. [Google Scholar]
- Jamradloedluk, J.; Lertsatitthanakorn, C. Characterization and Utilization of Char Derived from Fast Pyrolysis of Plastic Waste. Procedia Eng. 2014, 69, 1437–1442. [Google Scholar] [CrossRef] [Green Version]
- Wijesekara, D.A.; Sargent, P.; Ennis, C.J.; Hughes, D. Prospects of using chars derived from mixed post waste plastic pyrolysis in civil engineering applications. J. Clean. Prod. 2021, 317, 128212. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, X.; Ge, X.; Chen, M. Chemical pyrolysis of E-waste plastics: Char characterization. J. Environ. Manag. 2018, 214, 94–103. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Subadra, S.P.; Striūgas, N. Functionalization of char derived from pyrolysis of metallised food packaging plastics waste and its application as a filler in fiberglass/epoxy composites. Process Saf. Environ. Prot. 2021, 147, 723–733. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef] [Green Version]
- Luu, P.; Daly, K.; Croke, B.; Sherwin, G.; Miñana, B. Transitioning to a Circular System for Plastics. Assessing Molecular Recycling Technologies in the United States and Canada; Closed Loop Partners: New York, NY, USA, 2021. [Google Scholar]
- Takada, H.; Bell, L. Plastic Waste Management Hazards; International Pollutants Elimination Network (IPEN): Gothenburg, Sweden, 2021. [Google Scholar]
- Plastic Energy Technology. Available online: https://plasticenergy.com/technology/ (accessed on 27 January 2022).
- Monreal, C. Chemical Recycling in Practice. Available online: https://www.basf.com/global/documents/en/sustainability/we-drive-sustainable-solutions/circular-economy/chemcycling/Plastic%20Energy_BASF%20Dialog%20Forum.pdf (accessed on 27 January 2022).
- Promote a Circular Economy with Clean Fuels and Chemicals Made from Waste. Available online: https://enerkem.com/process-technology/carbon-recycling/ (accessed on 27 January 2022).
- Ashley Plastics Renewal Facility. Available online: https://www.brightmark.com/plastics-renewal/projects/ashley-indiana (accessed on 27 January 2022).
- Yao, J.Y.; Wang, Y.W.; Muppaneni, T.; Shrestha, R.; Le Roy, J.; Figuly, G.D.; Freer, E. Methods for the Decomposition of Contaminated Plastic Waste. International Patent WO2019204687A1, 24 October 2019. [Google Scholar]
- From Plastic Waste to Virgin-Grade Products. Available online: https://www.basf.com/global/en/who-we-are/sustainability/we-drive-sustainable-solutions/circular-economy/mass-balance-approach/chemcycling.html (accessed on 27 January 2022).
- ReOil: Getting Crude Oil Back Out of Plastic. Available online: https://www.omv.com/en/blog/reoil-getting-crude-oil-back-out-of-plastic (accessed on 27 January 2022).
- OMV Presentation. Available online: https://www.voeb.at/fileadmin/user_upload/voeeb.at/Intern/2019/Schneider_Chemisches_Recycling.pdf (accessed on 27 January 2022).
- Case Study 3 Cynar Plastics to Diesel. 2013. Available online: https://www.greenindustries.sa.gov.au/resources/case-study-3-cynar-plastics-to-diesel-(2013) (accessed on 28 January 2022).
- Cynar Plc Presentation. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/13364/GLOCIIPresentationMurray.pdf?sequence=1&isAllowed=y (accessed on 28 January 2022).
- Process Description Alphakat—Update December 2010. Available online: https://www.scribd.com/document/118589743/Process-Description-Alphakat-Update-Dec-2010-2 (accessed on 28 January 2022).
- Koshi Village Project. Available online: https://alphakatholdings.com/koshi-village-project/ (accessed on 28 January 2022).
- Patrick Lowe, White Paper—Petrosynthesis—The Competition of the Industrial Revolution, 1 November 2021. Industrial Revolution. © 2021 Patrick Lowe, 15 November 2021. Available online: https://zeropetroleum.com/wp-content/uploads/2021/11/White-Paper-Petrosynthesis-15-11-21.pdf (accessed on 12 February 2022).
| Type of Reactor | Type of Feedstock Materials | References |
|---|---|---|
| Batch reactor | PS, PE, PP and PET | [15] |
| HDPE | [31] | |
| Plastic medical wastes | [65] | |
| Semi-batch reactor | PS, HDPE, LDPE, PP | [66] |
| PE | [67] | |
| Polyolefin | [68] | |
| Continuous reactor | Plastic waste from landfill | [69] |
| HDPE | [70] | |
| Fixed bed reactor | PP, HDPE, LDPE | [71,72] |
| HDPE | [73] | |
| Fluidized bed reactor | LDPE | [74] |
| PP, PE | [75] | |
| PE | [76] | |
| CSBR | HDPE, LDPE, PP | [77] |
| HDPE, LDPE, PP, PS, PET, PMMA | [78] | |
| PE | [79] |
| Type of Reactor | Advantages | Disadvantages |
|---|---|---|
| Batch reactor |
|
|
| Semi-batch reactor |
|
|
| Fixed-bed reactor |
|
|
| Fluidized-bed reactor |
|
|
| Rotary kiln reactor |
|
|
| Stirred tank reactor |
|
|
| Conical spouted bed reactor |
|
|
| Material | Catalyst | Conditions | Liquid Product | Reference |
|---|---|---|---|---|
| PE | Ultra-stable Y (USY) zeolite | Batch reactor Temperaturę 450 °C Polimer/catalyst ratio 10:1 Reaction time 50 min | Yield 71% C5–C39 compounds | [94] |
| PP | Ultra-stable Y (USY) zeolite | Batch reactor Temperaturę 450 °C Polimer/catalyst ratio 10:1 Reaction time 45 min | Yield 82% C5–C30 compounds | [94] |
| LDPE | Zeolite CAT-2 | Batch reactor Polimer/catalyst ratio 10:1 Temperaturę 460 °C Nitrogen atmosphere | Yield: -96% without catalyst (24% C7–C12, 30% C13–C20, 46% C21–C40) -52% with catalyst (100% C7–C12) | [98] |
| PP | Zeolite CAT-2 | Batch reactor Polimer/catalyst ratio 10:1 Temperaturę 460 °C Nitrogen atmosphere | Yield: -86% without catalyst (44% C7–C12, 33% C13–C20, 23% C21–C40) -58% with catalyst (100% C7–C12) | [98] |
| HDPE, LDPE, and PP | Zeolite CAT-2 | Batch reactor Polimer/catalyst ratio 10:1 Temperaturę 460 °C Nitrogen atmosphere | Yield (for 34% PP and 66% PE): -64% without catalyst (36% C7–C12, 32% C13–C20, 32% C21–C40) -68% with catalyst (100% C7–C12) Yield (for 66% PP and 34% PE): -84% without catalyst (46% C7–C12, 31% C13–C20, 23% C21–C40) -63% with catalyst (100% C7–C12) | [98] |
| HDPE | Y-zeolite impregnated with transition metal (Ni, Fe, Mo, Ga, Ru and Co) | Fixed bed reactor Two-stage process (pyrolysis/catalysis) Temperature both processes 600 °C Reaction time 30 min | Yield of oil: –without catalyst—69% –with Y-zeolit—45%–with metal/Y-zeolit—below 40% Y-zeolit with and without metal causes greater production of aromatic hydrocarbons | [97] |
| LDPE | FCC catalyst silica alumina | Semi-batch pyrolysis reactor Fluidizing gas—nitrogen Amount of catalyst 5% Temperature 500 °C Reaction time 60 min | Yield of oil—93.5% where: C6–C9—20.7% C10–C15—64.7% C16–C19—12.2% >C20—2.4% | [91] |
| Type of Plastic/Material | Type of Catalyst/Absorber | Process Paramethers | Pyrolysis Oil Yield (%) | Oil Composition | Reference |
|---|---|---|---|---|---|
| HDPE | AC, molecular sieves | HDPE to AC ratio from 100:60 to 100:330, heating power combinations (20–40% of the maximum power of 900 W) | 87.8 | 82.4% C7 and C20 | [151] |
| LLDPE | HZSM-5 | Catalyst to feedstock 5–20%, catalytic temperature 550 and 500 °C, | 84.1% | 98.0% gasoline-range hydrocarbons (70.4–72.3% mono-aromatics) | [152] |
| LDPE | ZSM-5 | Pyrolysis temperature 480 °C, catalytic temperature 250–500 °C, ZSM-5 to plastic ratio from 1:4.68 to 1:1.32, time 10 min, power 0.7 kW, N2 gas | 24.4–32.6 | >94% aromatics; 74.7–88.5% C8–C12 MAHs | [142] |
| HDPE | AC | Pyrolysis temperature 400–600 °C | 27.3–54.9 | >90% for C ≤ 21, ∼45–58% normal aliphatics; ∼35–45% aromatics | [153] |
| LDPE | Silicone carbide, MgO | Pyrolysis and catalytic temperature 350–550 °C, MgO to plastic ratio from 1:15 to 1:3, plastic to absorber ratio 0.03:1, time 20 min, vacuum | 24.2–38.5 | 79.5–96.0% gasoline fraction; ∼15–50% MAHs | [141] |
| PS | Carbon, silicon carbide | Time 28 min, temperature 574 °C, pressure N2 flux, power 3 kW, PS to absorber ratio 2.21 | 94.3 | 40.5–71.9% styrene, 5.9–18.4% α-methylstyrene, 6.2–10.9% toluene | [154] |
| Waste PS | Tire, carbonaceous char | Power 1.2–6.0 kW, PS to carbon ratio 2.0–0.5, reaction time 13–82 min, temperature 364–578 °C, anoxic | 86.5 | 93.9% single-ring aromatic compounds (C6–C10), 66.0% styrene | [155] |
| Waste: PS, PP, PS + PP | Carbon, AC | Power 0.18–0.9 kW, polymer to an absorbent ratio 10:0.5–10:2, time 10–20 min | 83.3–86.1 | 67.6% styrene | [144] |
| LDPE | NiO, zeolite HY, silicon carbide | LDPE to HY ratio 20:1, 15:1, 10:1, 5:1, pyrolysis temperatures 450–600 °C, catalysis temperatures 350–500 °C, HY to NiO 15:1, 10:1, 5:1, 3:1, time 20 min, power 1.8 kW, vacuum | 56.5 | >92% C5–C12 gasoline fraction; 34.6–46.6% aromatics; 26–30% isomerized aliphatics | [132] |
| LDPE | ZSM-5 | Pyrolysis and catalytic temperature 375 °C, ZSM-5 to plastic ratio 1:10 | 64.4 | 97% C8–C16 aromatics | [156] |
| LDPE, torrefied rice straw | ZSM-5 | Temperature 309–590 °C, ratio catalyst to feedstock from 3.9% to 11.04% | 24.5–29.8 | ∼80% gasoline fraction; 26.5–40.2% long-chain hydrocarbons and cyclic hydrocarbons | [147] |
| Corn derived plastic bags | Iron powder, carbon powder | Power 1.2–3 kW, temperature 617–808 K, plastic bags to absorber ratio 2.02–2.39 | 28.–47.5 | Mainly oxygen componds, 9.6–13.3% aromatic hydrocarbons, 2.2–7.1% alifatic hydrocarbons | [157] |
| HDPE | AC, molecular sieves | HDPE to AC ratio from 100:60 to 100:330, heating power combinations (20–40% of the maximum power of 900 W) | 87.8 | 82.4% C7–C20 | [151] |
| LDPE | Chestnut shell (CNSACC) | Carbonization temperature 850 °C, catalytic temperature 550 °C, CNSACC to LDPE ratio 1.0 | - | 95.9% aromatics | [158] |
| PP | Talk, ZSM-5 | Pyrolysis temperature 620 °C | 48.9 | 73.5% gasoline fraction (45.0% aromatic hydrocarbons and 24.6% isomerized aliphatic) | [70] |
| Wastes PS | AC | Power 450 W, PS to AC ratio 10:0.5–10:3, temperature 500 °C, time 5.5 min | 80.7–93.0 | 26.8% benzene derivatives, 23.2% condensed ring aromatics, 8.4% alkenes | [159] |
| LDPE | AC, zeolite catalyst | Atmospheric pressure, temperature 300–550 °C, time 45–90 min, plastic to absorber ratio 1:1, catalyst to plastic ratio 1:1, N2 gas | 28.1 | 19.0% hydrocarbons | [160] |
| Plastic waste from WEEE | Carbon, iron | Power 3 kW, in a nitrogen environment, plastic to absorber ratio 2:1, temperature 386–450 °C, time 30–60 min, N2 gas | 76.6 | 117.7 mg/mL of styrene and 25.6 mg/mL of xylenes | [143] |
| HDPE, PP | Chopped tire, carbon | Power 1.2–6 kW, time 33–260 min, temperature 702–872 K, plastic to absorber ratio from 0.5:1 to 2.5:1 | 27.6–83.9 | <88.4% aliphatic hydrocarbons, <20.32% aromatic hydrocarbons | [161] |
| HDPE | Carbon, silicon carbide powder | Temperature 400–550 °C, absorbent to HDPE ratio 10:1 and 3.3:1, power 3–5 kW, time 60–100 min, N2 gas | 34.7–73.4 | C8–C35 | [162] |
| PS, PP, LDPE | Iron | Power 500–2500 W, temperature 250–800 °C, time 19 min | 28.1–88.7 | Aromatic and aliphatic hydrocarbons (C8–C16) | [163] |
| LDPE, lignin | HZSM-5, MgO, silicon carbide | Power of 750 W, pyrolysis temperature 450–600 °C, lignin to LDPE ratio (only lignin, 3:1, 2:1, 1:1, 1:2 and only LDPE), MgO to HZSM-5 ratio (only MgO, 1:2, 1:1, 2:1 and only HZSM-5), feedstock to catalyst ratio (only feedstock, 1:2, 1:1 and 2:1), vacuum | 22.8–38.5 | ∼30–85% aromatic compounds, ∼15–55% phenolic compounds | [164] |
| Waste cooking oil (WCO), waste polyolefins (WP) | AC | Power 800 W, WP to WCO ratio 1:2, 1:1.5, 1:1, 1.5:1, 2:1, time 20 min, temperature <450 °C, N2 gas, vacuum | 24.0–62.0 | 50% C13–C24 hydrocarbons | [165] |
| Wheat straw, rice husk, expanded PS, waste PP | Graphite | Power of 600 W, biomass to plastic ratio 1:1, temperature 450 °C, N2 gas, | 51.5–64.9 | 50.5–93.7% aromatic hydrocarbons, <27.1% cycloalkanes and alkanes | [166] |
| Process | Drawback | Advantage |
|---|---|---|
| Conventional pyrolysis |
|
|
| Microwave-assisted pyrolysis |
|
|
| Plasma-assisted pyrolysis |
|
|
| Catalytical cracking |
|
|
| Hydrogenolysis |
|
|
| Substrate/Product | Conditions of Thermal Decomposition | Process Description | Reference |
|---|---|---|---|
| Waste hydrocarbon material such as plastics/fuel |
|
| [192] |
| Waste plastics PE, PP, PS, PET, EPDM |
|
| [192] |
| Plastic material/diesel fuel |
|
| [194] |
| Plastic material (PP, PE), light and heavy distillates/fuels |
|
| [191] |
| Plastic material/petroleum products |
|
| [195] |
| Plastic material (PP, PE, PS) |
|
| [196] |
| Plastic material |
|
| [197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matuszewska, A.; Owczuk, M.; Biernat, K. Current Trends in Waste Plastics’ Liquefaction into Fuel Fraction: A Review. Energies 2022, 15, 2719. https://doi.org/10.3390/en15082719
Matuszewska A, Owczuk M, Biernat K. Current Trends in Waste Plastics’ Liquefaction into Fuel Fraction: A Review. Energies. 2022; 15(8):2719. https://doi.org/10.3390/en15082719
Chicago/Turabian StyleMatuszewska, Anna, Marlena Owczuk, and Krzysztof Biernat. 2022. "Current Trends in Waste Plastics’ Liquefaction into Fuel Fraction: A Review" Energies 15, no. 8: 2719. https://doi.org/10.3390/en15082719
APA StyleMatuszewska, A., Owczuk, M., & Biernat, K. (2022). Current Trends in Waste Plastics’ Liquefaction into Fuel Fraction: A Review. Energies, 15(8), 2719. https://doi.org/10.3390/en15082719







