Numerical Simulation on Heating Effects during Hydrogen Absorption in Metal Hydride Systems for Hydrogen Storage
Abstract
:1. Introduction
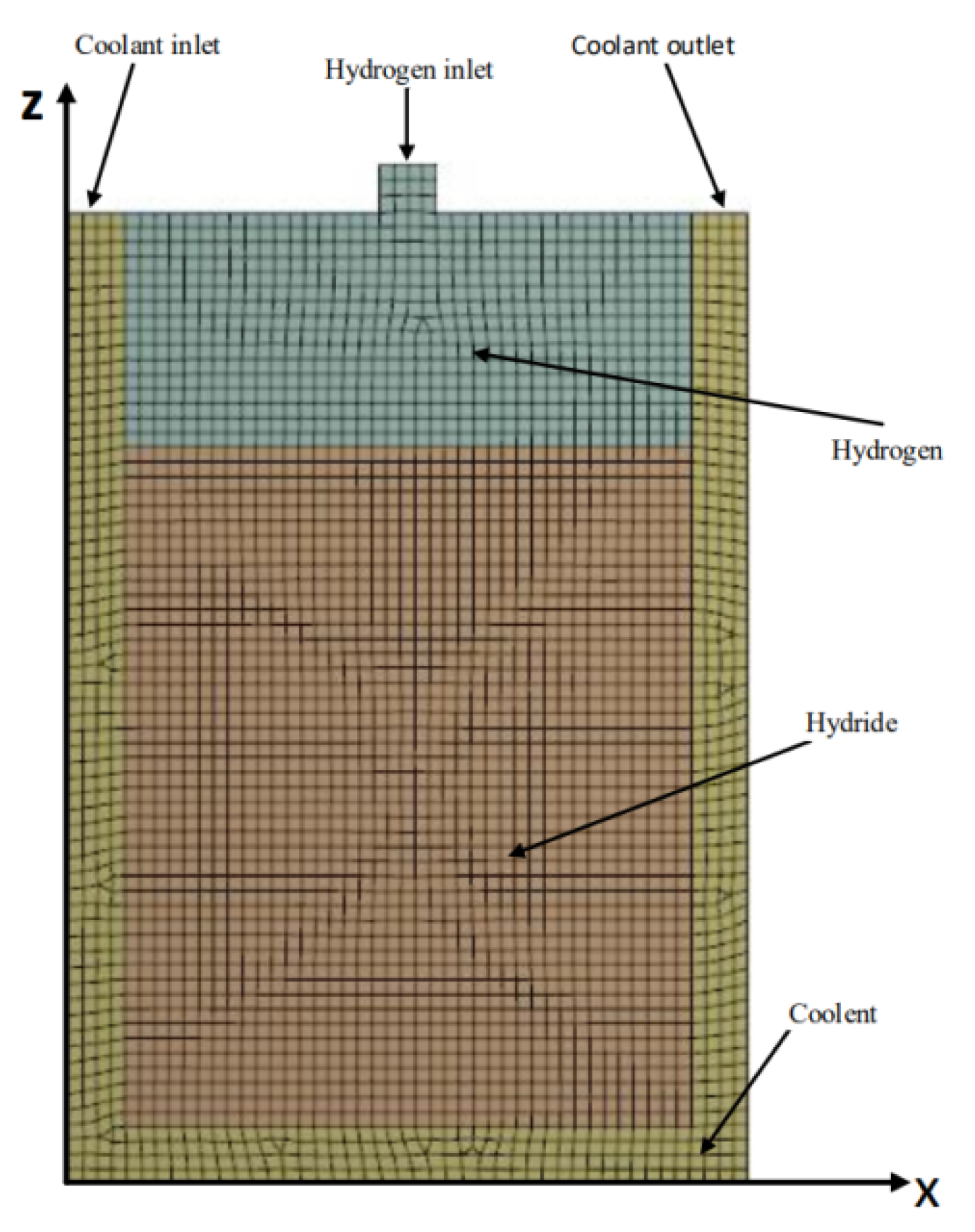
2. Numerical Model Building
- (1)
- Hydrogen is assumed to be an ideal gas, and relevant physical parameters conform to the ideal gas law;
- (2)
- Local thermal equilibrium is assumed to exist between solid metal and hydrogen;
- (3)
- Metal hydride volume expansion during hydrogen absorption is assumed to be negligible;
- (4)
- Metal powder is assumed to possess uniform pore characteristics (e.g., pore size and porosity);
- (5)
- Properties such as porosity, permeability, and heat conductivity coefficient, etc., of the metal hydride are assumed to remain unchanged in the hydrogen absorption process.
2.1. Conservation Equations for the Reaction Zone
2.2. Subsection
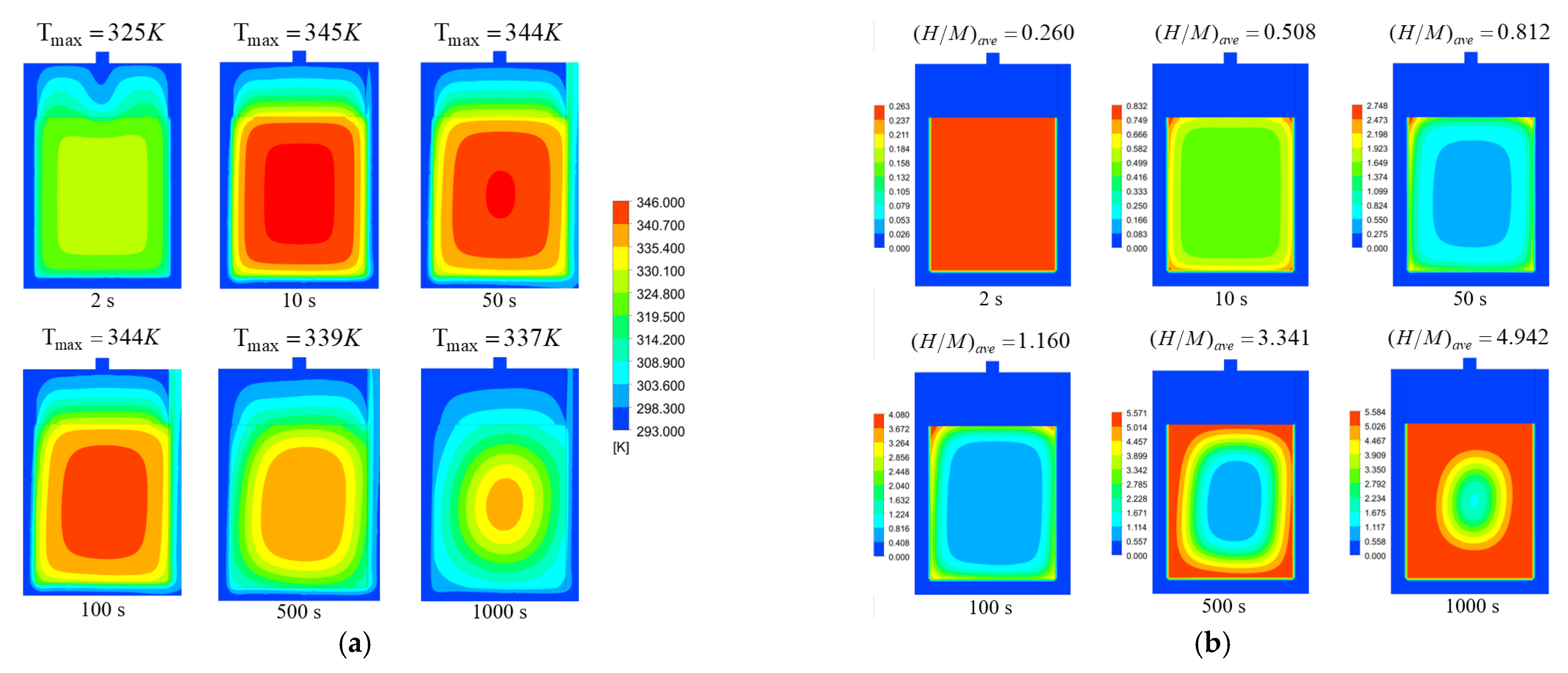
3. Results and Discussions
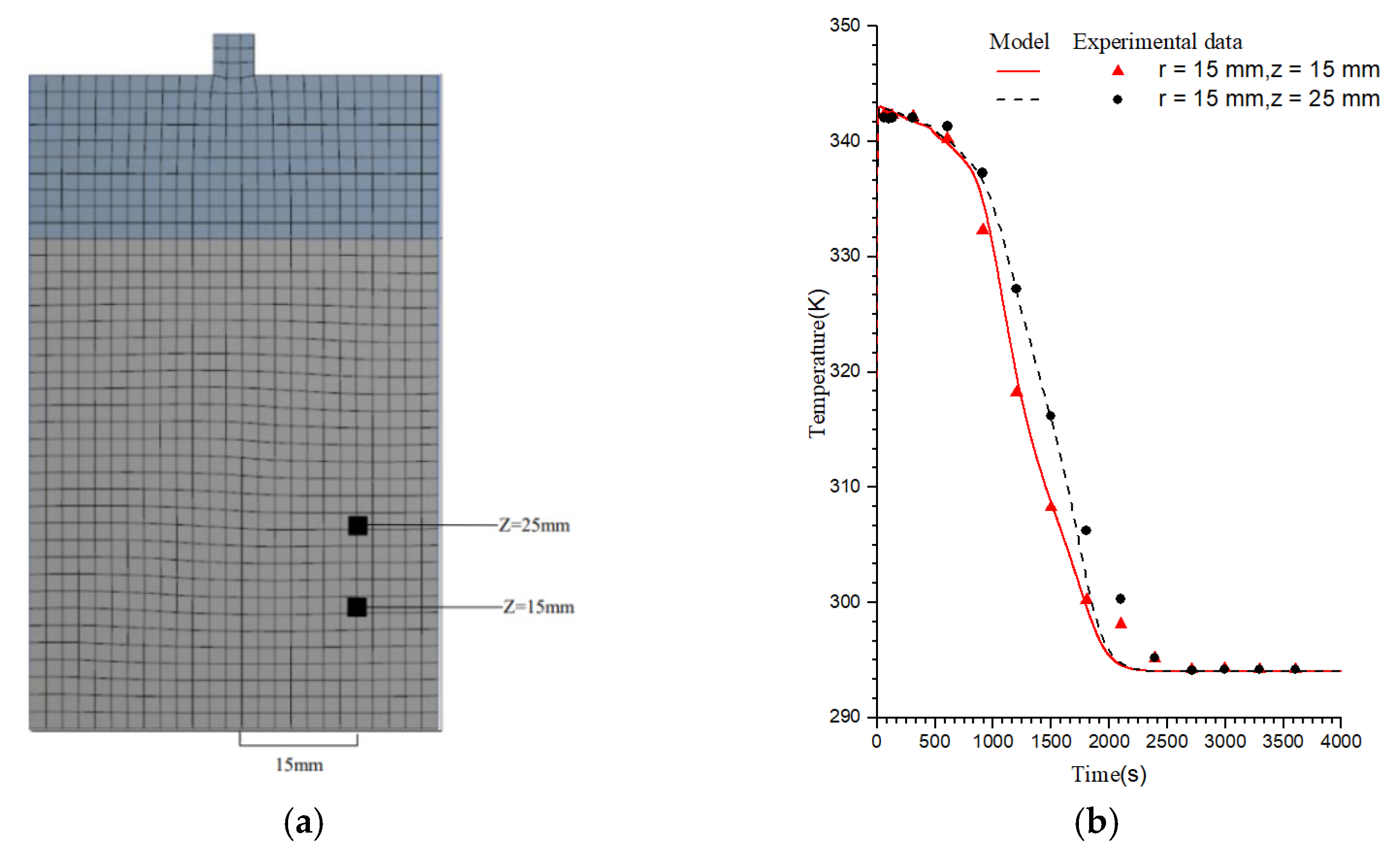
3.1. Model Validation
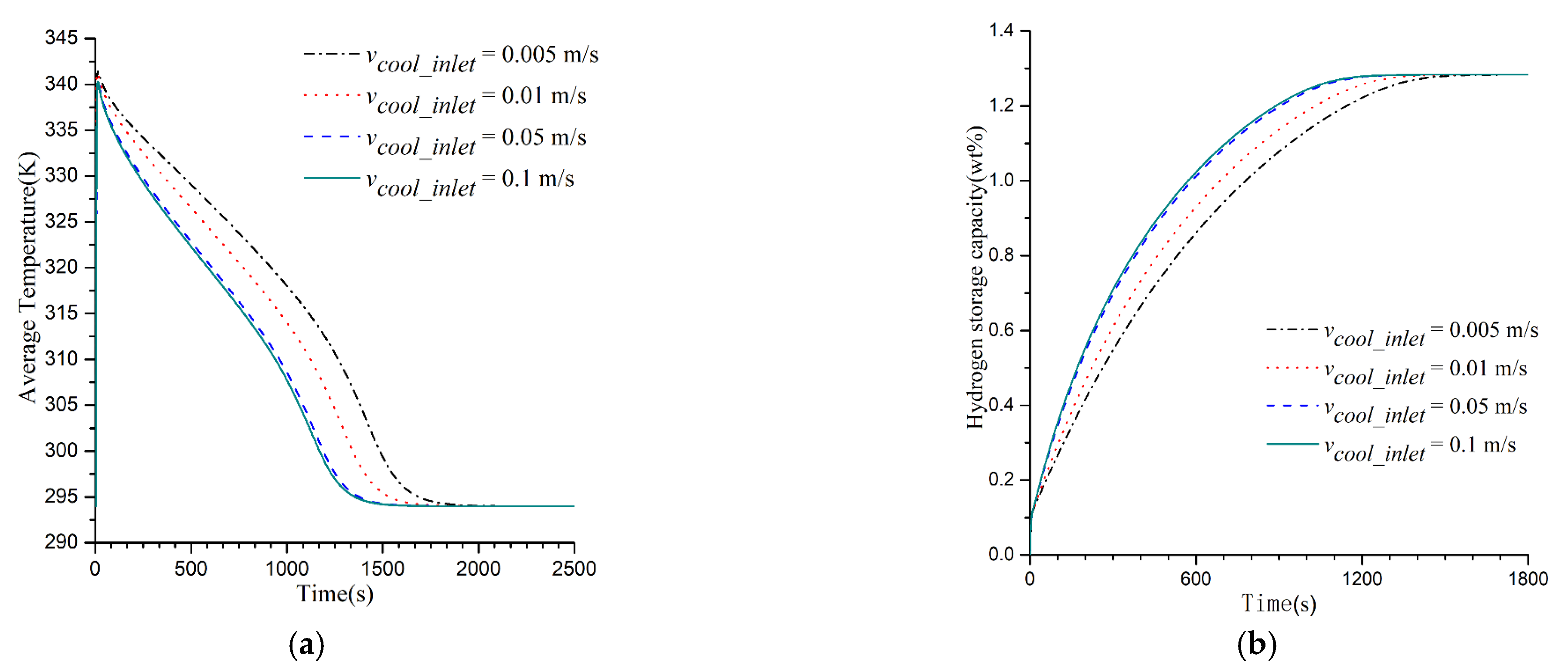
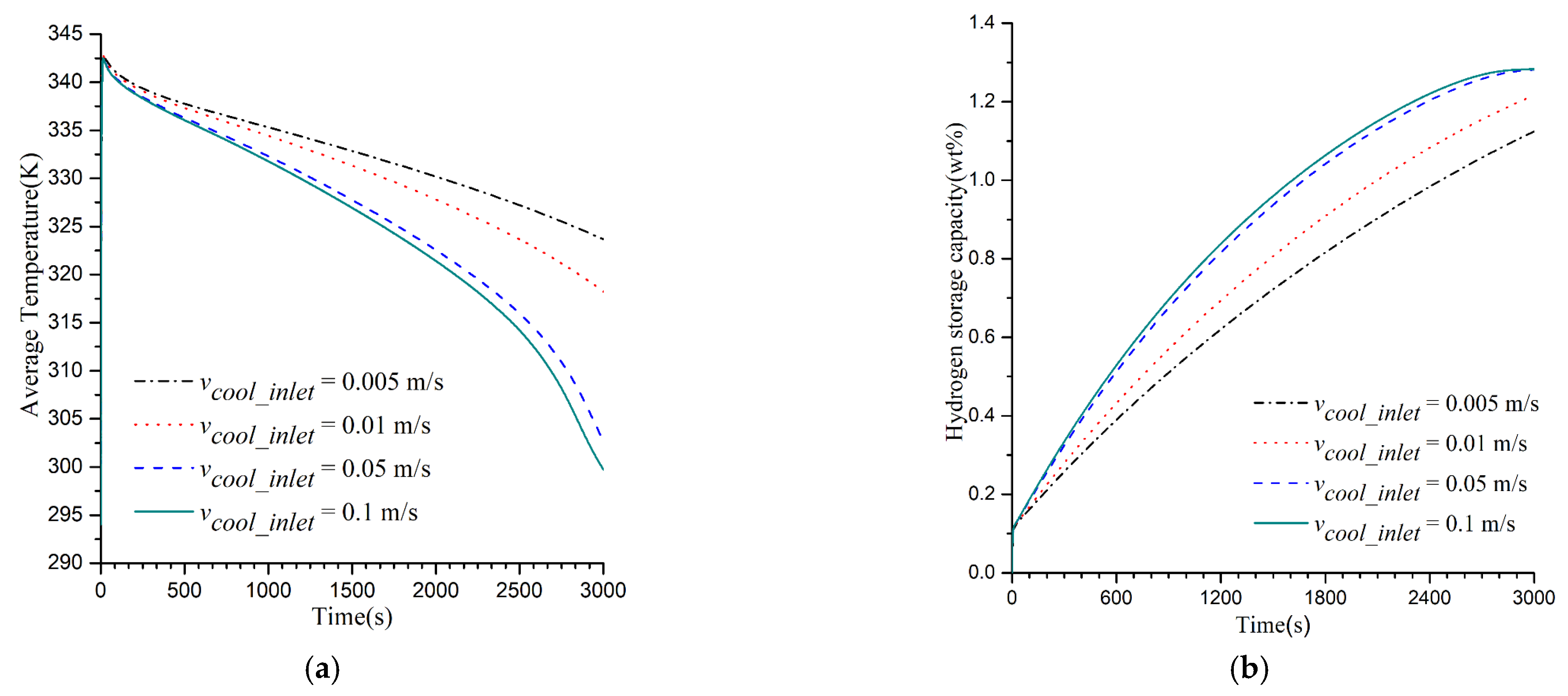
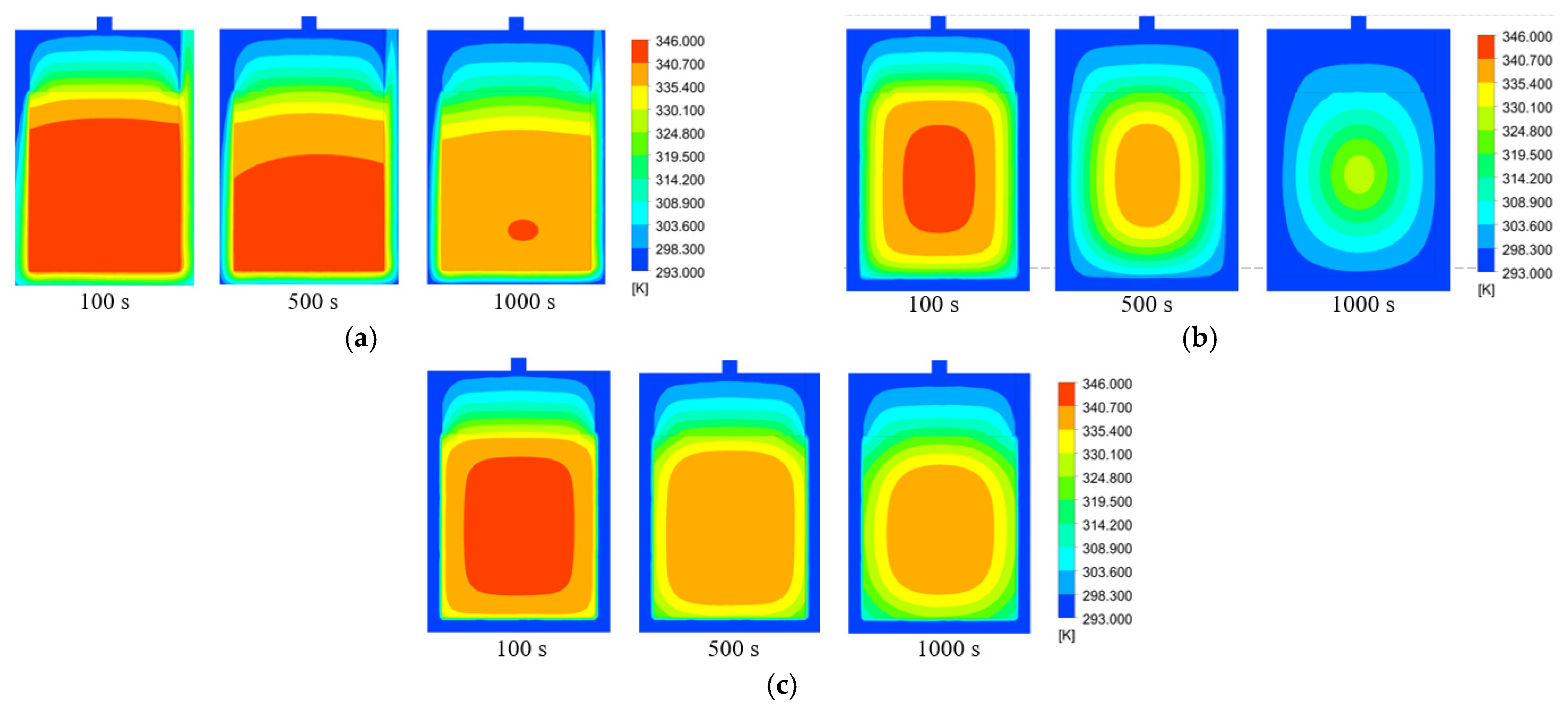
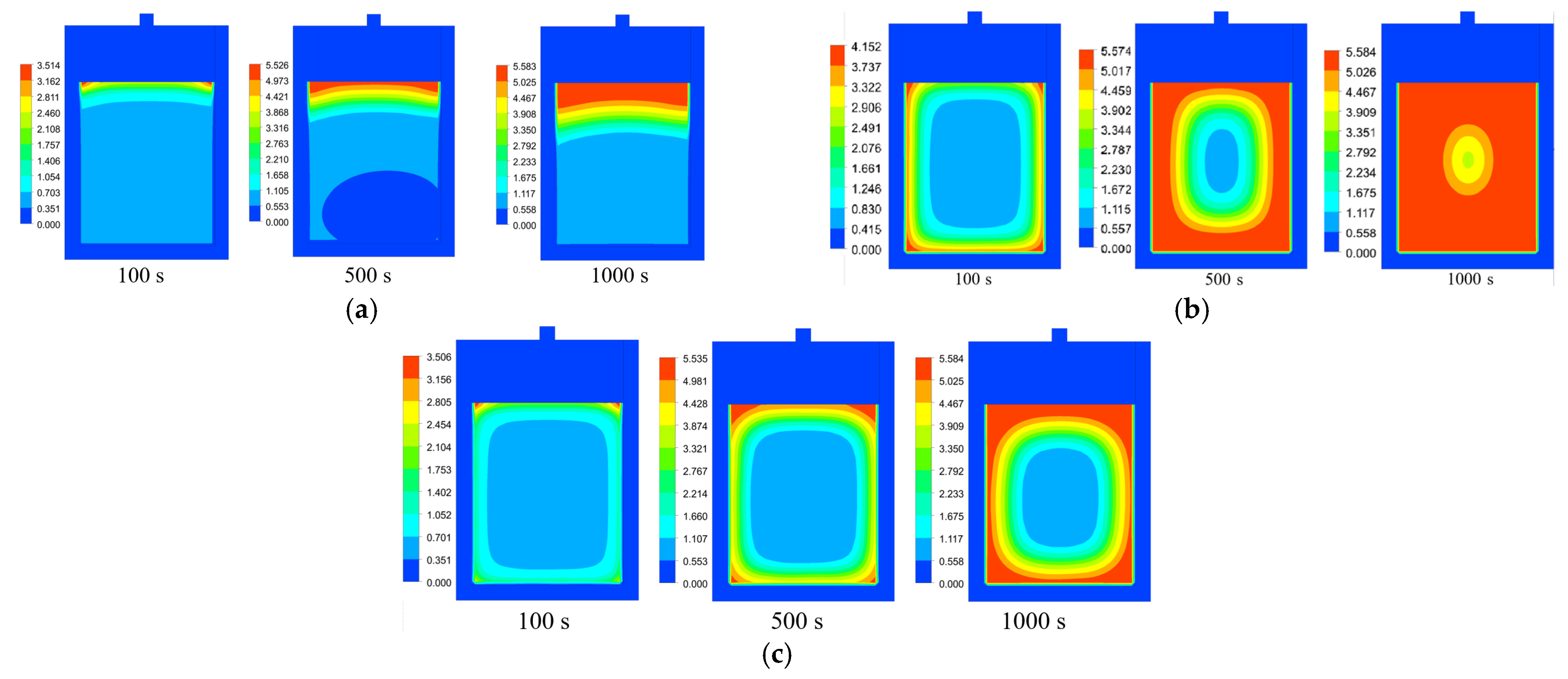
3.2. Impacts of Cooling Medium’s Flow Rate
3.3. Impacts of Different Cooling Media
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Briki, C.; Rango, P.D.; Belkhiria, S.; Dhaou, M.H.; Jemni, A. Measurements of expansion of LaNi5 compacted powder during hydrogen absorption/desorption cycles and their influences on the reactor wall. Int. J. Hydrogen Energy 2019, 44, 13647–13654. [Google Scholar] [CrossRef]
- Nasrallah, S.B.; Jemni, A. Heat and mass transfer models in metal-hydrogen reactor. Int. J. Hydrogen Energy 1997, 22, 67–76. [Google Scholar] [CrossRef]
- Jemni, A.; Nasrallah, S.B.; Lamloumi, J. Experimental and theoretical study of ametal–hydrogen reactor. Int. J. Hydrogen Energy 1999, 24, 631–644. [Google Scholar] [CrossRef]
- Askri, F.; Salah, M.B.; Jemni, A.; Nasrallah, S.B. Optimization of hydrogen storage in metal-hydride tanks. Int. J. Hydrogen Energy 2009, 34, 897–905. [Google Scholar] [CrossRef]
- Jemni, A.; Nasrallah, S.B. Study of two-dimensional heat and mass transfer during absorption in a metal-hydrogen reactor. Int. J. Hydrogen Energy 1995, 20, 43–52. [Google Scholar] [CrossRef]
- Gkanas, E.I.; Grant, D.M.; Khzouz, M.; Stuart, A.D.; Manickam, K.; Walker, G.S. Efficient hydrogen storage in up-scale metal hydride tanks as possible metal hydride compression agents equipped with aluminium extended surfaces. Int. J. Hydrogen Energy 2016, 41, 10795–10810. [Google Scholar] [CrossRef]
- Mohan, G.; Maiya, M.P.; Murthy, S.S. Performance simulation of metal hydride hydrogen storage device with embedded filters and heat exchanger tubes—Science Direct. Int. J. Hydrogen Energy 2007, 32, 4978–4987. [Google Scholar] [CrossRef]
- Nam, J.; Ko, J.; Ju, H. Three-dimensional modeling and simulation of hydrogen absorption in metal hydride hydrogen storage vessels. Appl. Energy 2012, 89, 164–175. [Google Scholar] [CrossRef]
- Chippar, P.; Lewis, S.D.; Rai, S.; Sircar, A. Numerical investigation of hydrogen absorption in a stackable metal hydride reactor utilizing compartmentalization. Int. J. Hydrogen Energy 2018, 43, 8007–8017. [Google Scholar] [CrossRef]
- Chandra, S.; Sharma, P.; Muthukumar, P.; Tatiparti, S.S.V. Modeling and numerical simulation of a 5kg LaNi 5 -based hydrogen storage reactor with internal conical fins. Int. J. Hydrogen Energy 2020, 45, 8794–8809. [Google Scholar] [CrossRef]
- Mghari, H.E.; Huot, J.; Xiao, J. Analysis of hydrogen storage performance of metal hydride reactor with phase change materials. Int. J. Hydrogen Energy 2019, 44, 28893–28908. [Google Scholar] [CrossRef]
- Chibani, A.; Merouani, S.; Bougriou, C.; Hamadi, L. Heat and mass transfer during the storage of hydrogen in LaNi5 -based metal hydride: 2D simulation results for a large scale, multi-pipes fixed-bed reactor. Int. J. Heat Mass Transf. 2020, 147, 118939. [Google Scholar] [CrossRef]
- Lewis, S.D.; Chippar, P. Numerical investigation of hydrogen absorption in a metal hydride reactor with embedded embossed plate heat exchanger. Energy 2020, 194, 116942. [Google Scholar] [CrossRef]
- Gwak, G.; Kim, M.; Oh, K.; Kyoung, S.; Ferekh, S.; Ju, H. Analyzing effects of volumetric expansion of uranium during hydrogen absorption. Int. J. Hydrogen Energy 2017, 42, 3723–3730. [Google Scholar] [CrossRef]
- Elhamshri, F.; Kayfeci, M.; Matik, U.; Alous, S. Thermoflui dynamic modelling of hydrogen absorption in metal hydride beds by using multiphysics software. Int. J. Hydrogen Energy 2020, 45, 34956–34971. [Google Scholar] [CrossRef]
- Gwak, G.; Yun, S.; Ju, H. Multi-dimensional modeling and large-scale simulation of hydrogen absorption/desorption phenomena in metal hydride vessels (MHVs). Fusion Eng. Des. 2018, 130, 107–113. [Google Scholar] [CrossRef]
- Freni, A.; Cipiti, F.; Cacciola, G. Finite element-based simulation of a metal hydride-based hydrogen storage tank. Int. J. Hydrogen Energy 2009, 34, 8574–8582. [Google Scholar] [CrossRef]
- Gopal, M.R.; Murthy, S.S. Studies on heat and mass transfer in metal hydride beds. Int. J. Hydrogen Energy 1995, 20, 911–917. [Google Scholar] [CrossRef]
- Muthukumar, P.; Madhavakrishna, U.; Dewan, A. Parametric studies on a metal hydride based hydrogen storage device. Int. J. Hydrogen Energy 2007, 32, 4988–4997. [Google Scholar] [CrossRef]
- Brown, T.M.; Brouwer, J.; Samuelsen, G.S.; Holcomb, F.H.; King, J. Accurate simplified dynamic model of a metal hydride tank. Int. J. Hydrogen Energy 2008, 33, 5596–5605. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, P.; Ramana, S.V. Numerical simulation of coupled heat and mass transfer in metal hydride-based hydrogen storage reactor. J. Alloy. Compd. 2009, 472, 466–472. [Google Scholar] [CrossRef]
- Mellouli, S.; Askri, F.; Dhaou, H.; Jemni, A.; Nasrallah, S.B. Numerical simulation of heat and mass transfer in metal hydride hydrogen storage tanks for fuel cell vehicles. Int. J. Hydrogen Energy 2010, 35, 1693–1705. [Google Scholar] [CrossRef]
- Askri, F.; Jemni, A.; Nasrallah, S.B. Dynamic behavior of metal–hydrogen reactor during hydriding process. Int. J. Hydrogen Energy 2004, 29, 635–647. [Google Scholar] [CrossRef]
- Dhaou, H.; Askri, F.; Salah, M.B.; Jemni, A.; Nasrallah, S.B.; Lamloumi, J. Measurement and modelling of kinetics of hydrogen sorption by LaNi and two related pseudobinary compounds. Int. J. Hydrogen Energy 2007, 32, 576–587. [Google Scholar] [CrossRef]






| Parameter | Unit | Value |
|---|---|---|
| Initial temperature, | °C | 20 |
| Reference temperature, | °C | 30 |
| Pressure intensity at the inlet, | bar | 10 |
| Reference pressure intensity, | bar | 10 |
| Hydrogen absorption speed constant, | 59.187 | |
| Activation energy, | 21,179.6 | |
| Hydrogen heat capacity, | 1489 | |
| Heat capacity of hydrogen storage alloy, | 419 | |
| Heat conductivity coefficient of hydrogen, | 0.167 | |
| Heat conductivity coefficient of alloy, | 3.18 | |
| Porosity of hydrogen storage alloy, | -- | 0.63 |
| Permeability of the pore area, | ||
| Cooling temperature, | °C | 20 |
| Alloy temperature, | 5300 | |
| Saturated alloy density, | 5369 | |
| a0 | -- | −0.34863 |
| a1 | -- | 10.1059 |
| a2 | -- | −14.2442 |
| a3 | -- | 10.3535 |
| a4 | -- | −4.20646 |
| a5 | -- | 0.962371 |
| a6 | -- | −0.115468 |
| a7 | -- | 0.00563776 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Chai, M.; He, K.; Chen, Y. Numerical Simulation on Heating Effects during Hydrogen Absorption in Metal Hydride Systems for Hydrogen Storage. Energies 2022, 15, 2673. https://doi.org/10.3390/en15072673
Tan J, Chai M, He K, Chen Y. Numerical Simulation on Heating Effects during Hydrogen Absorption in Metal Hydride Systems for Hydrogen Storage. Energies. 2022; 15(7):2673. https://doi.org/10.3390/en15072673
Chicago/Turabian StyleTan, Jiahui, Mu Chai, Kuanfang He, and Yong Chen. 2022. "Numerical Simulation on Heating Effects during Hydrogen Absorption in Metal Hydride Systems for Hydrogen Storage" Energies 15, no. 7: 2673. https://doi.org/10.3390/en15072673
APA StyleTan, J., Chai, M., He, K., & Chen, Y. (2022). Numerical Simulation on Heating Effects during Hydrogen Absorption in Metal Hydride Systems for Hydrogen Storage. Energies, 15(7), 2673. https://doi.org/10.3390/en15072673






