Effects of Heating Temperature on the Isothermal Performance of a Potassium Concentric Annular Heat Pipe
Abstract
:1. Introduction and Literature Review
2. Experimental Methods
2.1. Experimental System and Procedure
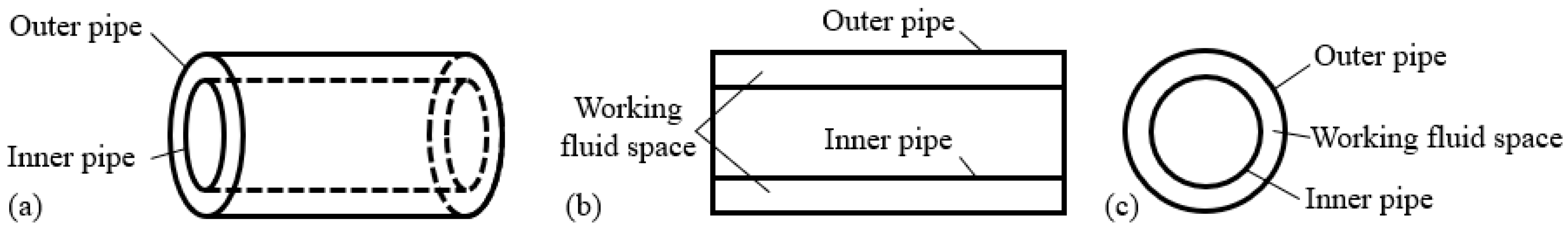
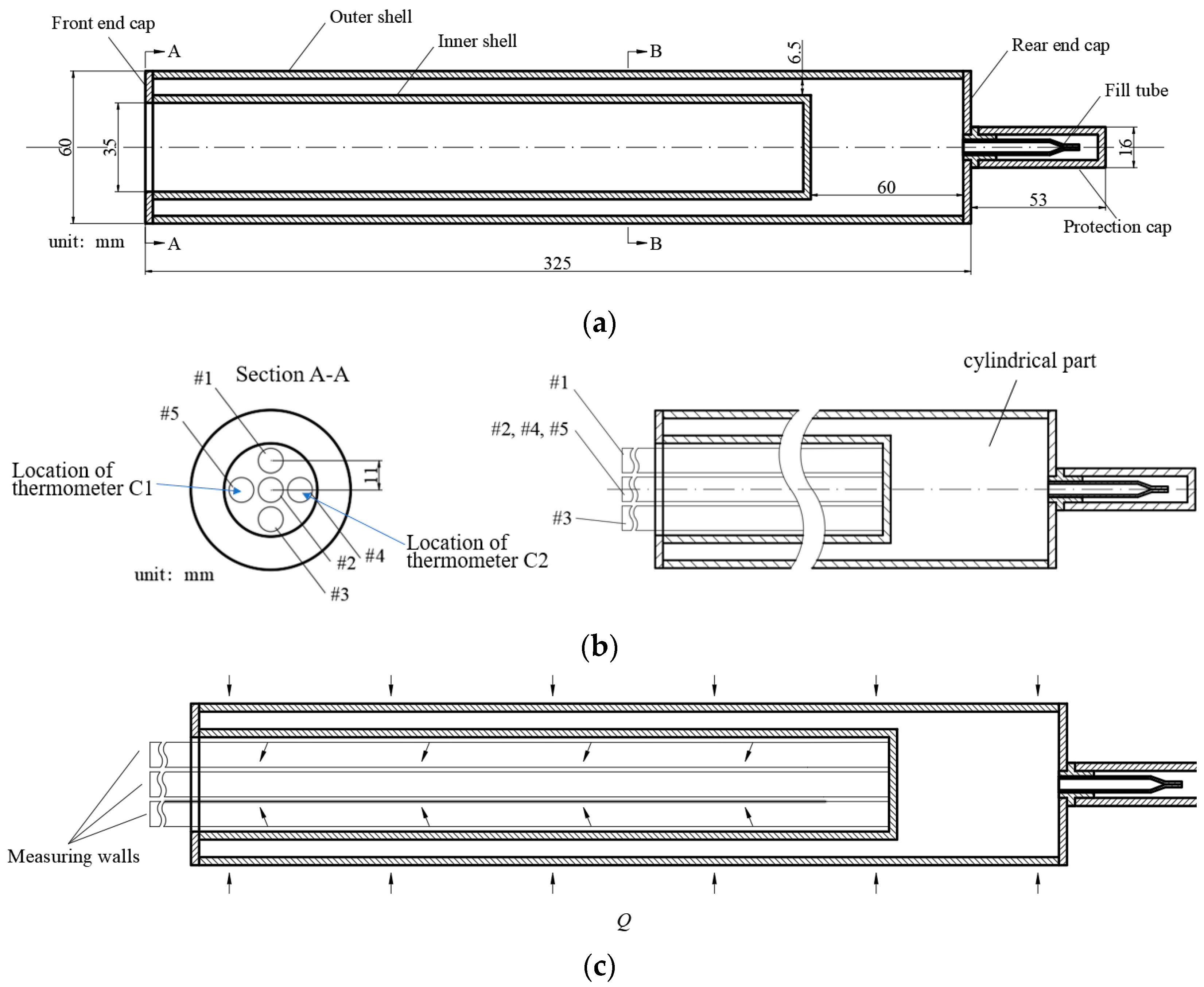
2.2. Potassium CAHP
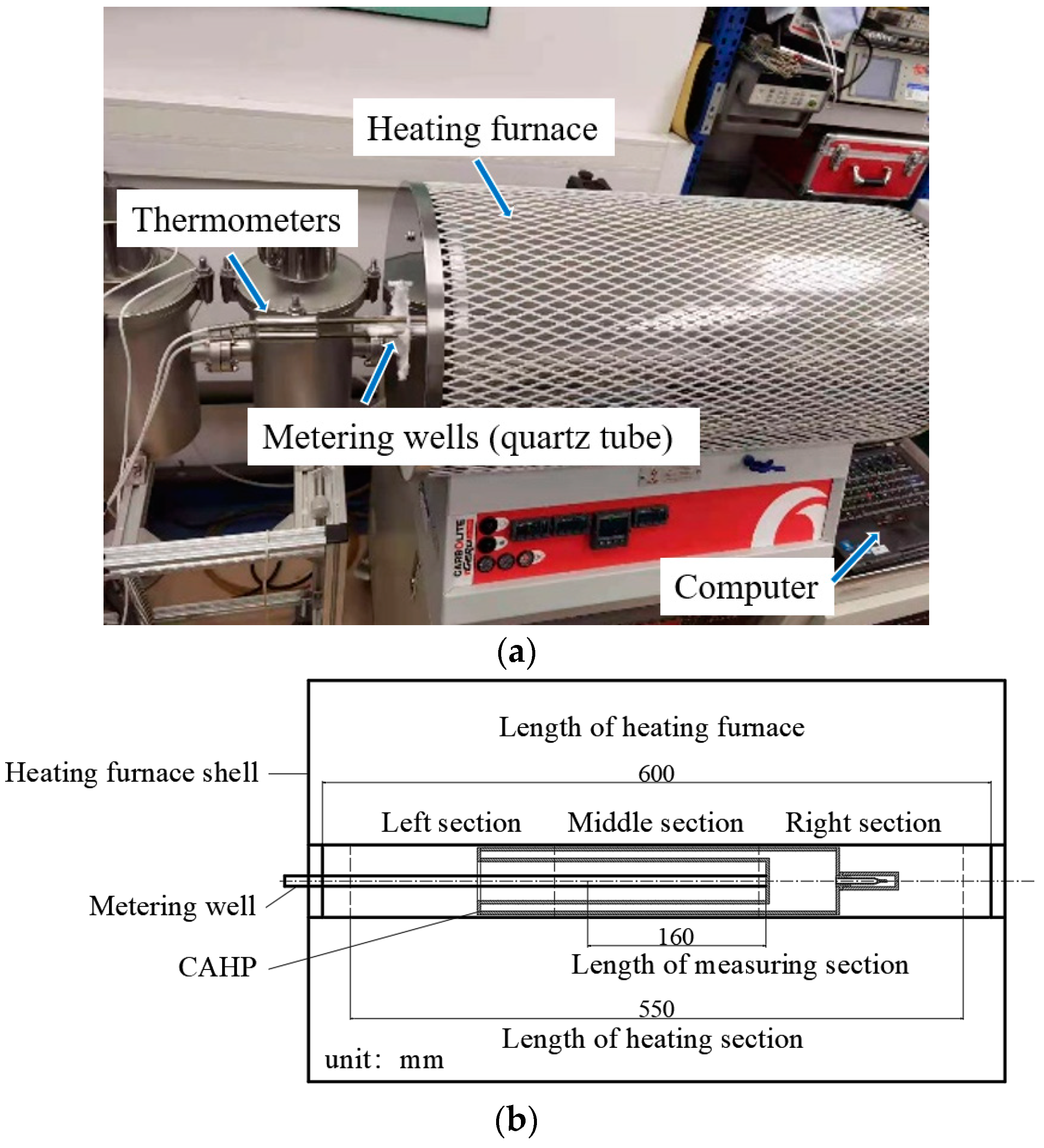
2.3. Heating Furnace
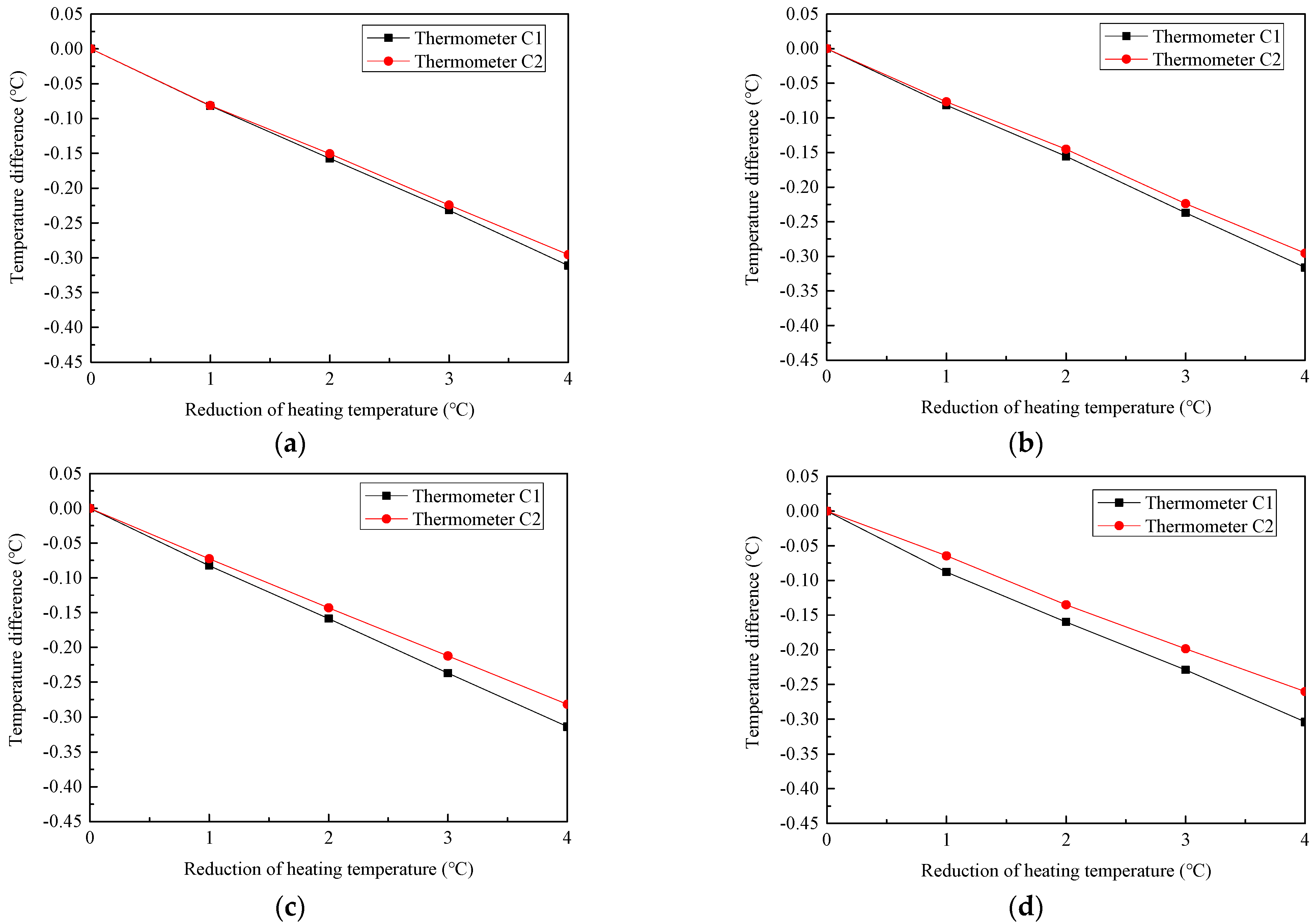
2.4. Temperature Stability and Reproducibility
3. Results and Discussions
3.1. Effect of Heating Mode on Isothermal Performance of CAHP
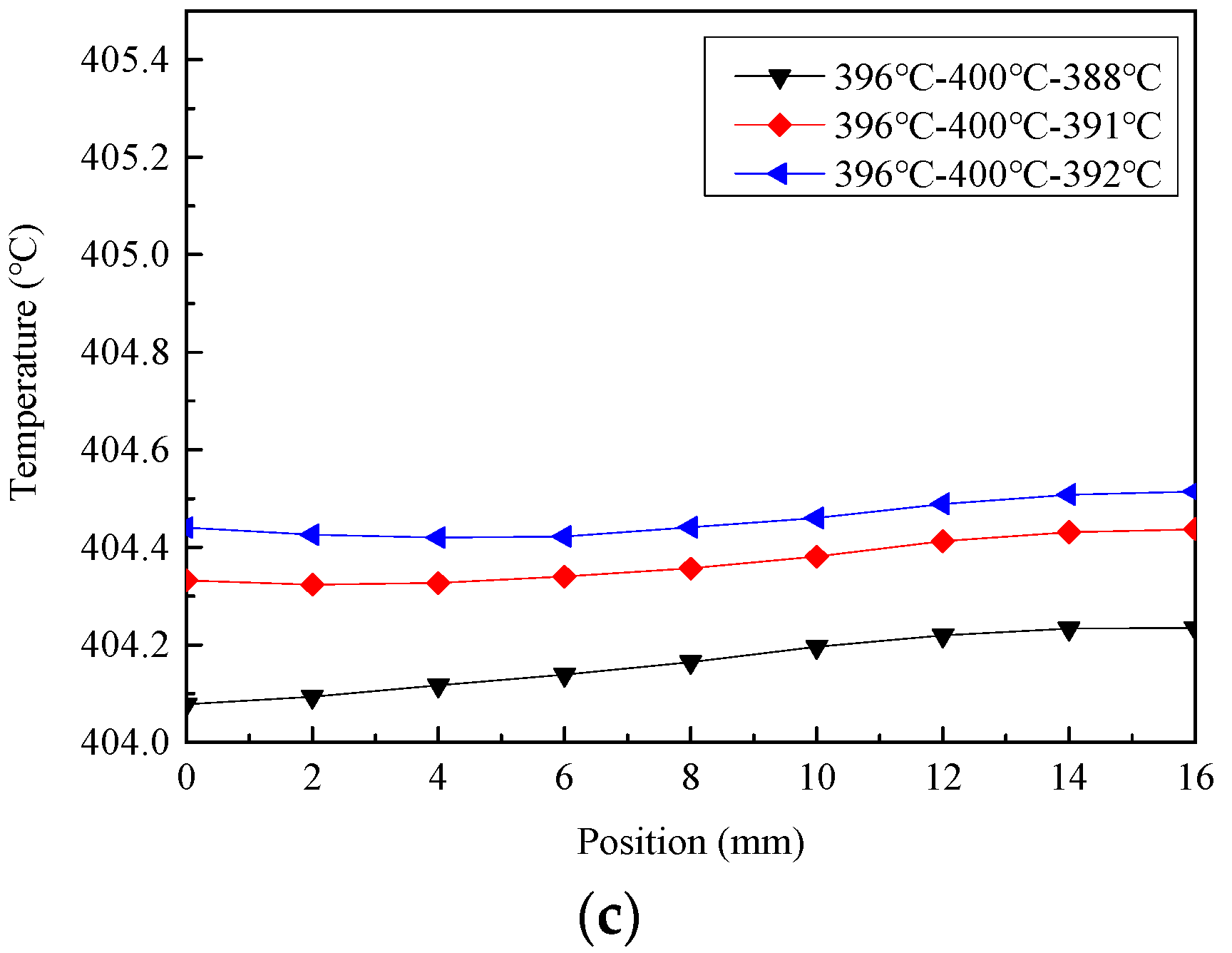
3.2. Influence of Temperature Fluctuation under Different Heating Temperature
3.3. Influence of Heating Temperature Fluctuation on Temperature Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotter, T.P. Theory of Heat Pipes; Los Alamos Scientific Laboratory: Los Alamos, NM, USA, 1965. [Google Scholar]
- Boo, J.H.; Kim, S.M.; Kang, Y.H. An experimental study on a sodium loop-type heat pipe for thermal transport from a high-temperature solar receiver. Energy Procedia 2015, 69, 608–617. [Google Scholar] [CrossRef] [Green Version]
- Coventry, J.; Andraka, C.; Pye, J.; Blanco, M.; Fisher, J. A review of sodium receiver technologies for central receiver solar power plants. Solar Energy 2015, 122, 749–762. [Google Scholar] [CrossRef] [Green Version]
- McGlen, R.J.; Jachuck, R.; Lin, S. Integrated thermal management techniques for high power electronic devices. Appl. Therm. Eng. 2004, 24, 1143–1156. [Google Scholar] [CrossRef]
- Boukhanouf, R.; Haddad, A.; North, M.T.; Buffone, C. Experimental investigation of a flat heat pipe performance using IR thermal imaging camera. Appl. Therm. Eng. 2006, 26, 2148–2156. [Google Scholar] [CrossRef]
- Mukhsinun, H.K.; Nandy, P.; Anhar, R.A.; Raldi, A.K.; Surip, W.; Sri, I.; Brilian, T.V. Passive cooling system in a nuclear spent fuel pool using a vertical straight wickless-heat pipe. Int. J. Therm. Sci. 2018, 126, 162–171. [Google Scholar]
- Liu, M.H.; Zhang, D.L.; Wang, C.L.; Qiu, S.Z.; Su, G.H.; Tian, W.X. Experimental study on heat transfer performance between fluoride salt and heat pipes in the new conceptual passive residual heat removal system of molten salt reactor. Nucl. Eng. Des. 2018, 339, 215–224. [Google Scholar] [CrossRef]
- Boo, J.H.; Park, S.Y. Isothermal characteristics of a rectangular parallelepiped sodium heat pipe. J. Mech. Sci. Technol. 2005, 19, 1044–1051. [Google Scholar] [CrossRef]
- Hack, N.; Unz, S.; Beckmann, M. Ceramic heat pipes for high temperature application. In Proceedings of the INFUB-11th European Conference on Industrial Furnaces and Boiler, Algarve, Portugal, 18–21 April 2017; pp. 140–148. [Google Scholar]
- Diver, R.B.; Fish, J.D.; Levitan, R.; Levy, M.; Meirovitch, E.; Rosin, H.; Paripatyadar, S.A.; Richardson, J.T. Solar test of an integrated sodium reflux heat pipe receiver/reactor for thermochemical energy transport. Sol. Energy 1992, 48, 21–30. [Google Scholar] [CrossRef]
- Ahmad, M.; Adam, H.; David, R. Melting of phase change material assisted by expanded metal mesh. Appl. Therm. Eng. 2015, 90, 1052–1060. [Google Scholar]
- Vadiraj, A.H.; Ashish, G.; Sameer, K. Thermal radiators with embedded pulsating heat pipes: Infra-red thermography and simulations. Appl. Therm. Eng. 2011, 31, 1332–1346. [Google Scholar]
- Reyes, M.; Alonso, D.; Arias, J.R.; Velazquez, A. Experimental and theoretical study of a vapor chamber based heat spreader for avionics applications. Appl. Therm. Eng. 2012, 37, 51–59. [Google Scholar] [CrossRef]
- Tamba, J.; Yamazawa, K.; Masuyama, S.; Ogura, H.; Izuchi, M. Evaluating the inhomogeneity of thermocouples using a pressure-controlled water heat pipe. Int. J. Thermophys. 2011, 32, 2436–2451. [Google Scholar] [CrossRef]
- Bertiglia, F.; Iacomini, L.; Moro, F.; Merlone, A. Comparison of two potassium-filled gas-controlled heat pipes. Int. J. Thermophys. 2015, 36, 3393–3403. [Google Scholar] [CrossRef]
- Zhao, J.; Yuana, D.Z.; Tang, D.W.; Jiang, Y.Y. Heat transfer characteristics of a concentric annular high temperature heat pipe under anti-gravity conditions. Appl. Therm. Eng. 2019, 148, 817–824. [Google Scholar] [CrossRef]
- Boo, J.H.; Park, S.Y. An experimental study on the thermal performance of a concentric annular heat pipe. J. Mech. Sci. Technol. 2005, 19, 1036–1043. [Google Scholar] [CrossRef]
- Mustaffar, A.; Phan, A.N.; Reay, D.; Boodhoo, K. Concentric annular heat pipe characterisation analysis for a drying application. Appl. Therm. Eng. 2019, 149, 275–286. [Google Scholar] [CrossRef]
- Choi, J.; Yuan, Y.; Borca-Tasciuc, D.A.; Kang, H. Design, construction and performance testing of an isothermal naphthalene heat pipe furnace. Rev. Sci. Instrum. 2014, 85, 95–105. [Google Scholar] [CrossRef]
- Sanchez, F.S.; Carvajal, I.M.; Morino, A.E.; Diez, P.Q.; Velazquez, M.T. Study of an annular two-phase thermosyphon used as an isothermal source in thermometry. J. Mech. Eng. 2015, 61, 273–282. [Google Scholar] [CrossRef]
- Gam, K.S. A stable microcomputer-controlled heat pipe furnace and test of new noble metal thermocouples. Measurement 1996, 18, 101–108. [Google Scholar] [CrossRef]
- Yan, X.K.; Duan, Y.N.; Ma, C.F.; Lv, Z.F. Construction of sodium heat-pipe furnaces and the isothermal characteristics of the furnaces. Int. J. Thermophys. 2011, 32, 494–504. [Google Scholar] [CrossRef]
- Merlone, A.; Giunta, S.; Tiziani, A. A new mercury gas-controlled heat pipe for temperature amplifier and as calibration facility. Int. J. Thermophys. 2008, 29, 1876–1886. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, J.T.; Merlone, A.; Duan, Y.; Wang, W. NIM gas controlled sodium heat pipe. In Proceedings of the 9th International Temperature Symposium on Temperature—Its Measurement and Control in Science and Industry, Los Angeles, CA, USA, 19–23 March 2012; pp. 834–839. [Google Scholar]
- Giunta, S.; Merlone, A.; Marenco, S.; Marcarino, P.; Tiziani, A. Capabilities of a new pressure controller for gas-controlled heat pipes. Int. J. Thermophys. 2008, 29, 1887–1895. [Google Scholar] [CrossRef]
- Jahan, F.; Ballico, M.J. Calibration of radiation thermometry fixed points using Au/Pt thermocouples. Int. J. Thermophys. 2011, 32, 361–371. [Google Scholar] [CrossRef]
- Zhao, G.C.; Zhao, C.L.; Song, L.P.; Tong, X.Y. Research on and applications of Au/Pt thermocouples. Appl. Mech. Mater. 2014, 483, 105–109. [Google Scholar] [CrossRef]






| Case 1 | Case 2 | Case 3 |
|---|---|---|
| 400 °C-400 °C-400 °C | 500 °C-500 °C-500 °C | 600 °C-600 °C-600 °C |
| 400 °C-400 °C-399 °C | 500 °C-500 °C-499 °C | 600 °C-600 °C-599 °C |
| 400 °C-400 °C-398 °C | 500 °C-500 °C-498 °C | 600 °C-600 °C-598 °C |
| 400 °C-400 °C-397 °C | 500 °C-500 °C-497 °C | 600 °C-600 °C-597 °C |
| Properties | Values |
|---|---|
| Boiling temperature (0.1 MPa) | 756.5 °C |
| Density (500 °C) | 726.7 kg/m3 |
| Specific heat (500 °C) | 0.77 kJ/(kg·K) |
| Viscosity (500 °C) | 1.5 × 10−3 Pa·s |
| Latent heat of vaporization (0.1 MPa) | 1983.7 kJ/kg |
| Heating Temperature | Temperature Change Rate |
|---|---|
| 400 °C | 0.0942 °C/°C |
| 500 °C | 0.0790 °C/°C |
| 600 °C | 0.0705 °C/°C |
| Position | Temperature Change Rate |
|---|---|
| 0 cm | 0.0790 °C/°C |
| 2 cm | 0.0764 °C/°C |
| 4 cm | 0.0739 °C/°C |
| 6 cm | 0.0705 °C/°C |
| 8 cm | 0.0650 °C/°C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ye, F.; Guo, H.; Yan, X. Effects of Heating Temperature on the Isothermal Performance of a Potassium Concentric Annular Heat Pipe. Energies 2022, 15, 2367. https://doi.org/10.3390/en15072367
Zhang H, Ye F, Guo H, Yan X. Effects of Heating Temperature on the Isothermal Performance of a Potassium Concentric Annular Heat Pipe. Energies. 2022; 15(7):2367. https://doi.org/10.3390/en15072367
Chicago/Turabian StyleZhang, Hongzhe, Fang Ye, Hang Guo, and Xiaoke Yan. 2022. "Effects of Heating Temperature on the Isothermal Performance of a Potassium Concentric Annular Heat Pipe" Energies 15, no. 7: 2367. https://doi.org/10.3390/en15072367
APA StyleZhang, H., Ye, F., Guo, H., & Yan, X. (2022). Effects of Heating Temperature on the Isothermal Performance of a Potassium Concentric Annular Heat Pipe. Energies, 15(7), 2367. https://doi.org/10.3390/en15072367






