A New Method for Capturing CO2 from Effluent Gases Using a Rice-Based Product
Abstract
:1. Introduction
2. Methodology and Experimental Procedure
2.1. Rice Cake and Grain Fabrication
2.2. Rice Cake Puffering Process
2.3. Fresh and Buffered Puffed Rice Cake Characterization
- -
- XRD analysis: The structural properties of samples were determined using an X-ray diffractometer by exposing them to Cu Kα radiation (λ = 1.54 Å) while applying a 30 mA tube current and a 40 kV target voltage. A 2θ scanning range of 5−70° with a scan speed of 2°/min was adopted to capture all significant diffraction peaks.
- -
- SEM analysis: The surface morphology, texture, and shape of both fresh and treated puffed rice samples were characterized using a scanning electron microscope. Samples were first coated with a 300 Å thick gold layer before subjecting three different areas to analysis.
- -
- FTIR analysis: The rice samples were subjected to FTIR analysis using an IRTrace-100 FTIR spectrophotometer (Shimadzu, Kyoto, Japan) to investigate the presence of effective functional groups. The spectral outcomes were documented within a 500−4000 cm−1 wavelength range using 4 cm−1 spectral resolution and 34 scans.
- -
- TGA analysis: TGA analysis was conducted in a thermogravimetric analyzer (Q500 series, TA Instruments) using samples of 2.0−5.0 mg weight while incrementing the temperature at a 15 °C/min rate. Nitrogen gas with a flow rate of 20 mL/min was used as a carrier gas.
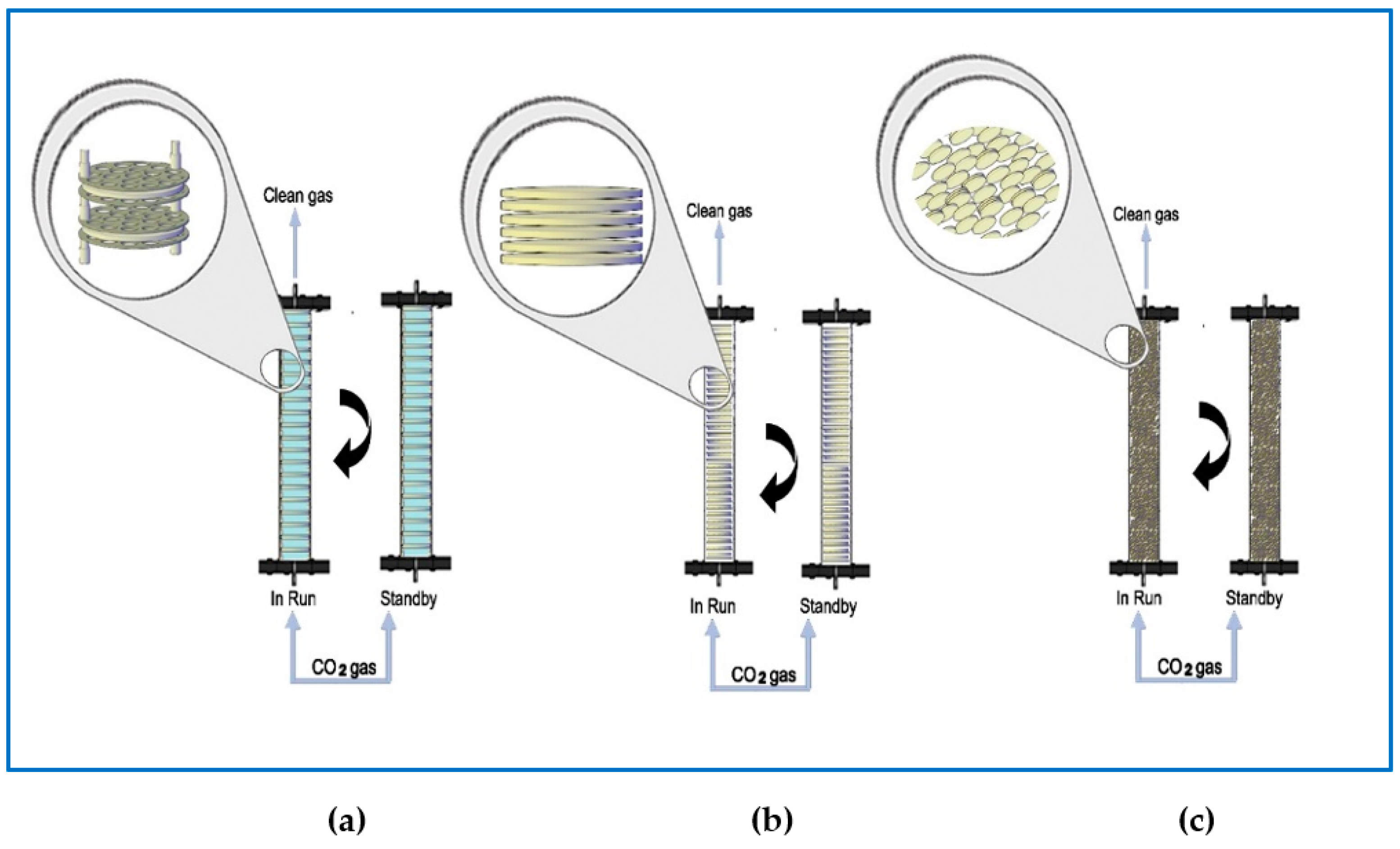
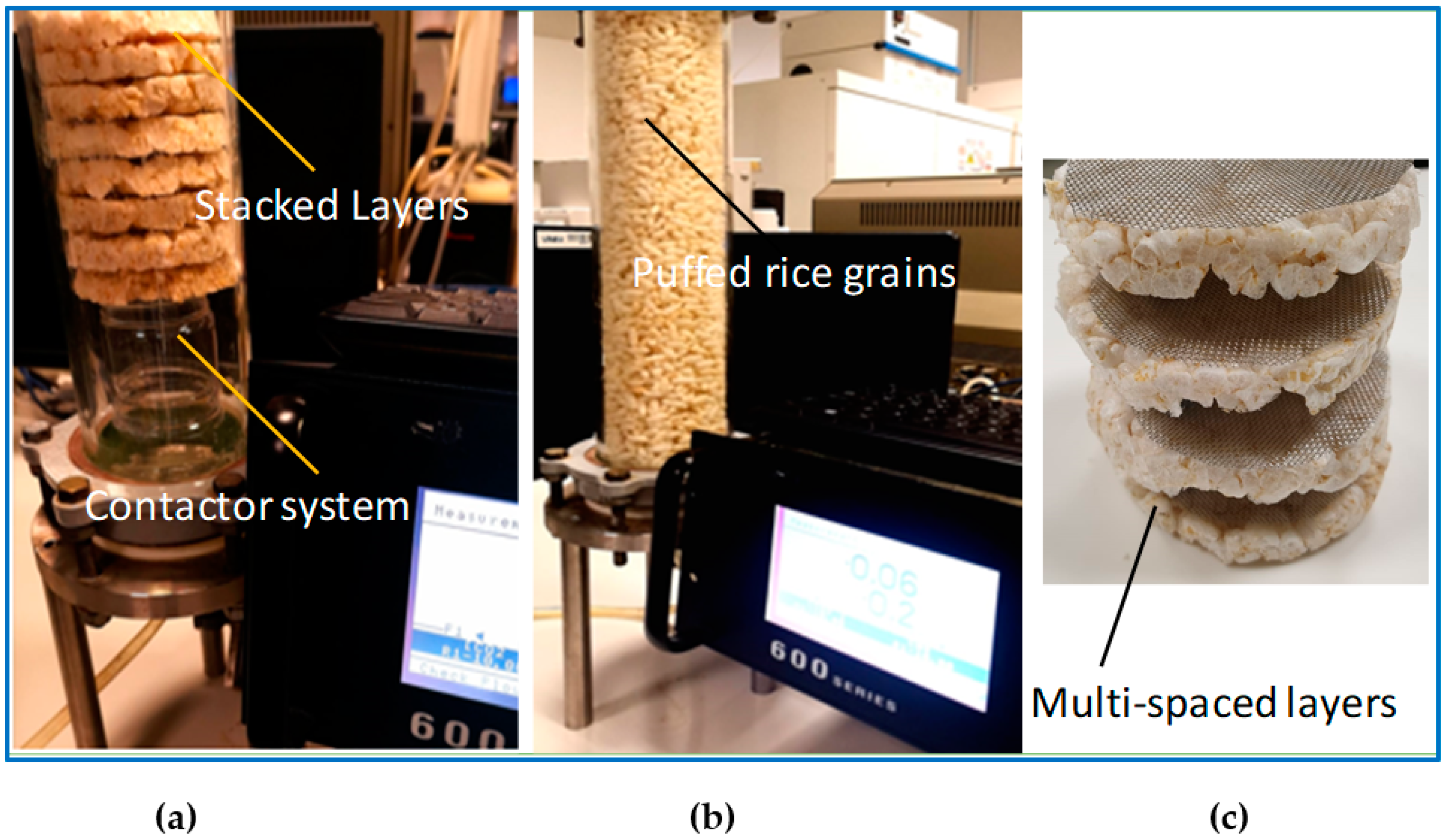
2.4. Gas Contact System
2.5. CO2 Capturing Process
3. Results and Discussion
3.1. Puffed Rice Cake
3.2. Fresh and Treated Rice Cake Characterization
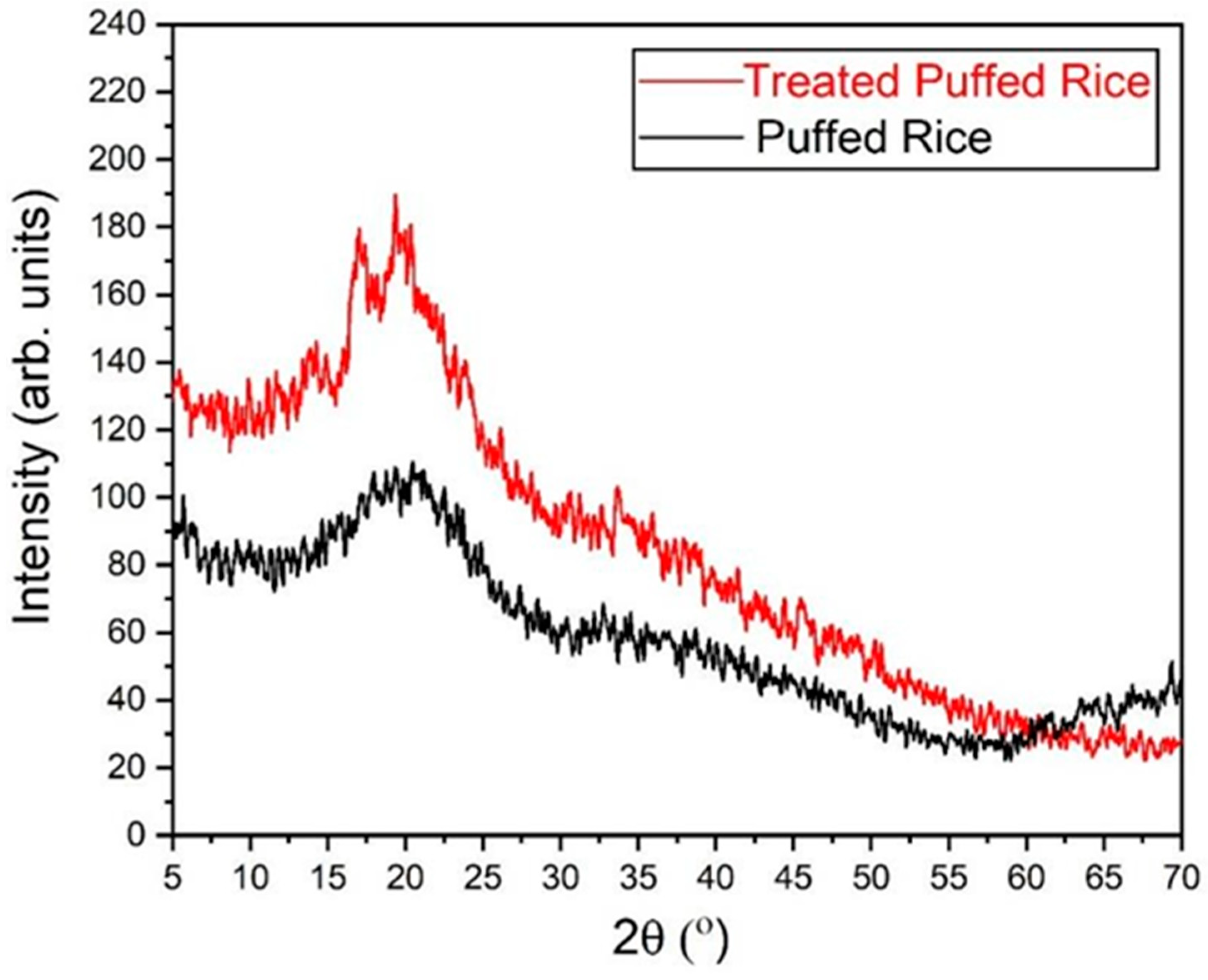
3.2.1. XRD Analysis of Fresh and Treated Puffed Rice Samples
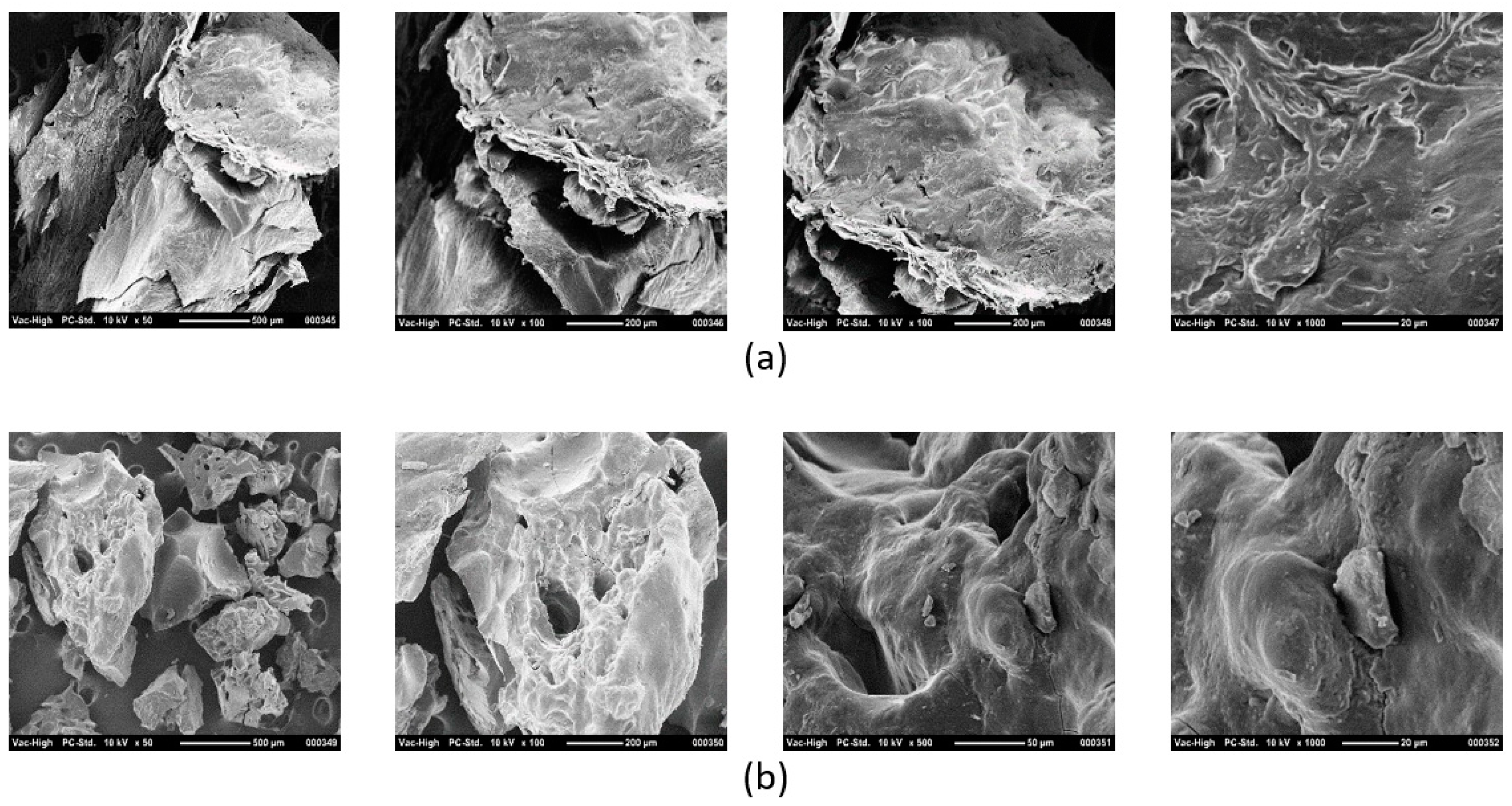
3.2.2. Scanning Electron Microscopy (SEM)
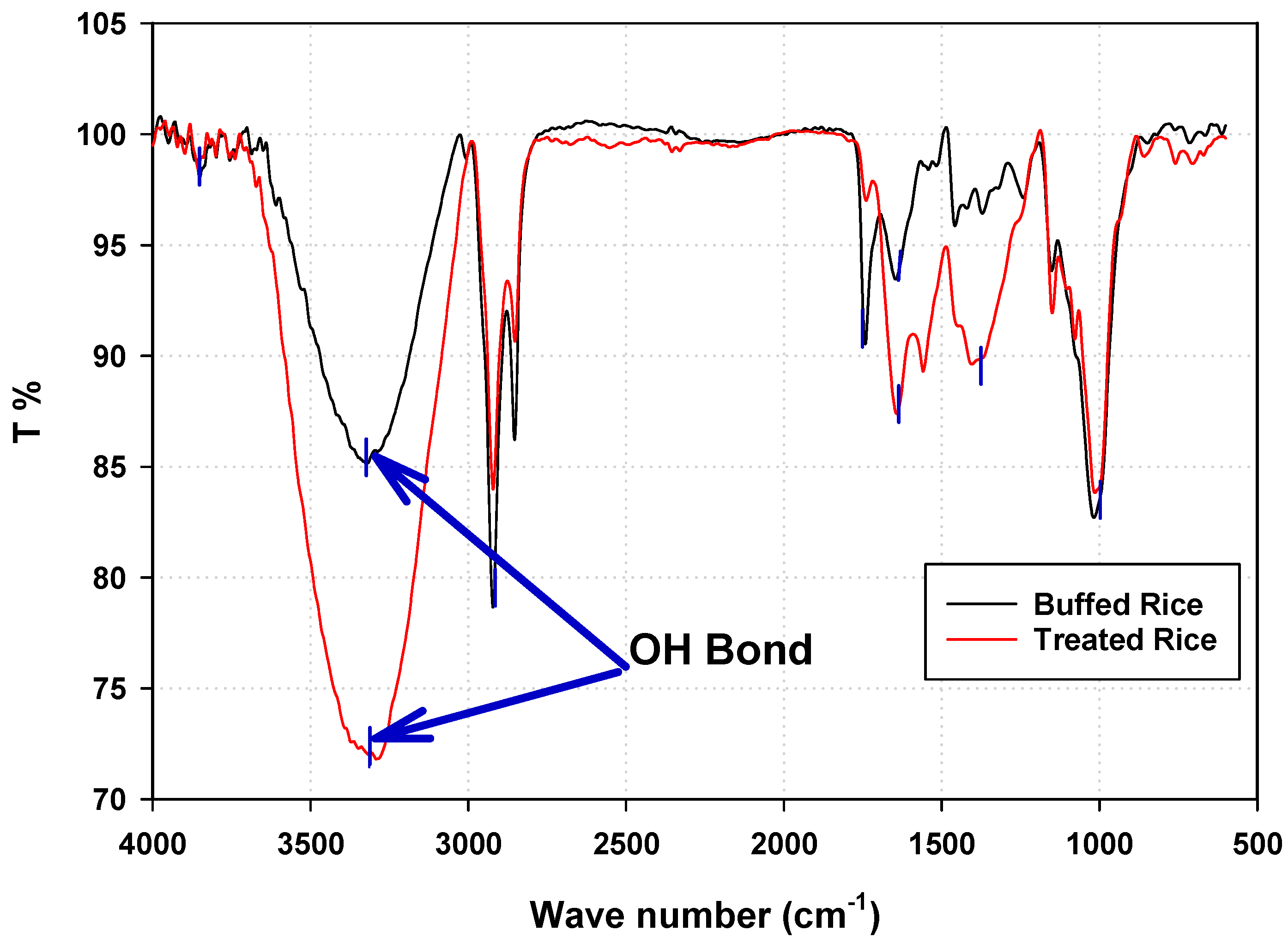
3.2.3. Fourier Transform Infrared (FTIR) Spectroscopy
3.2.4. Thermogravimetric Analysis
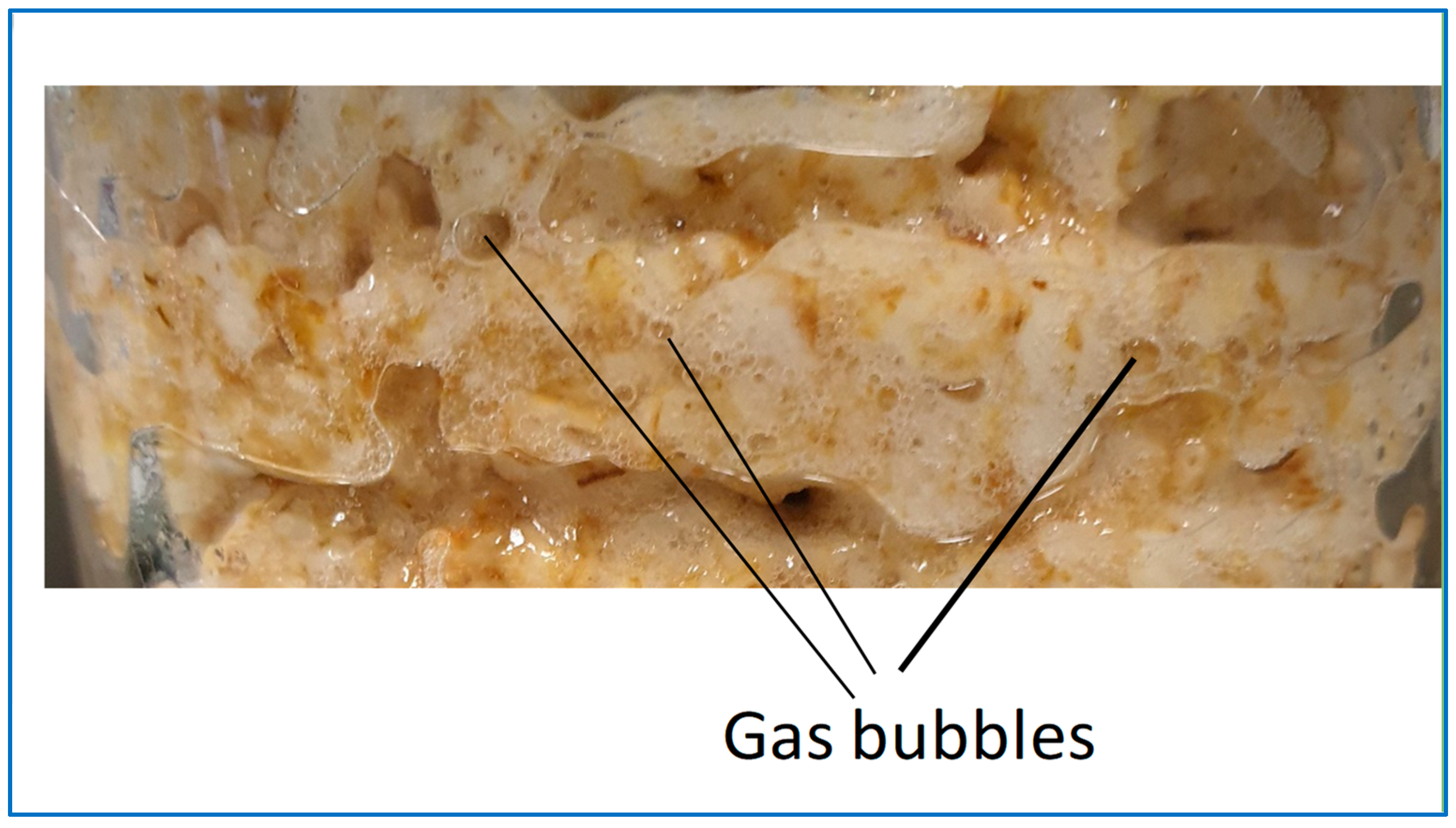
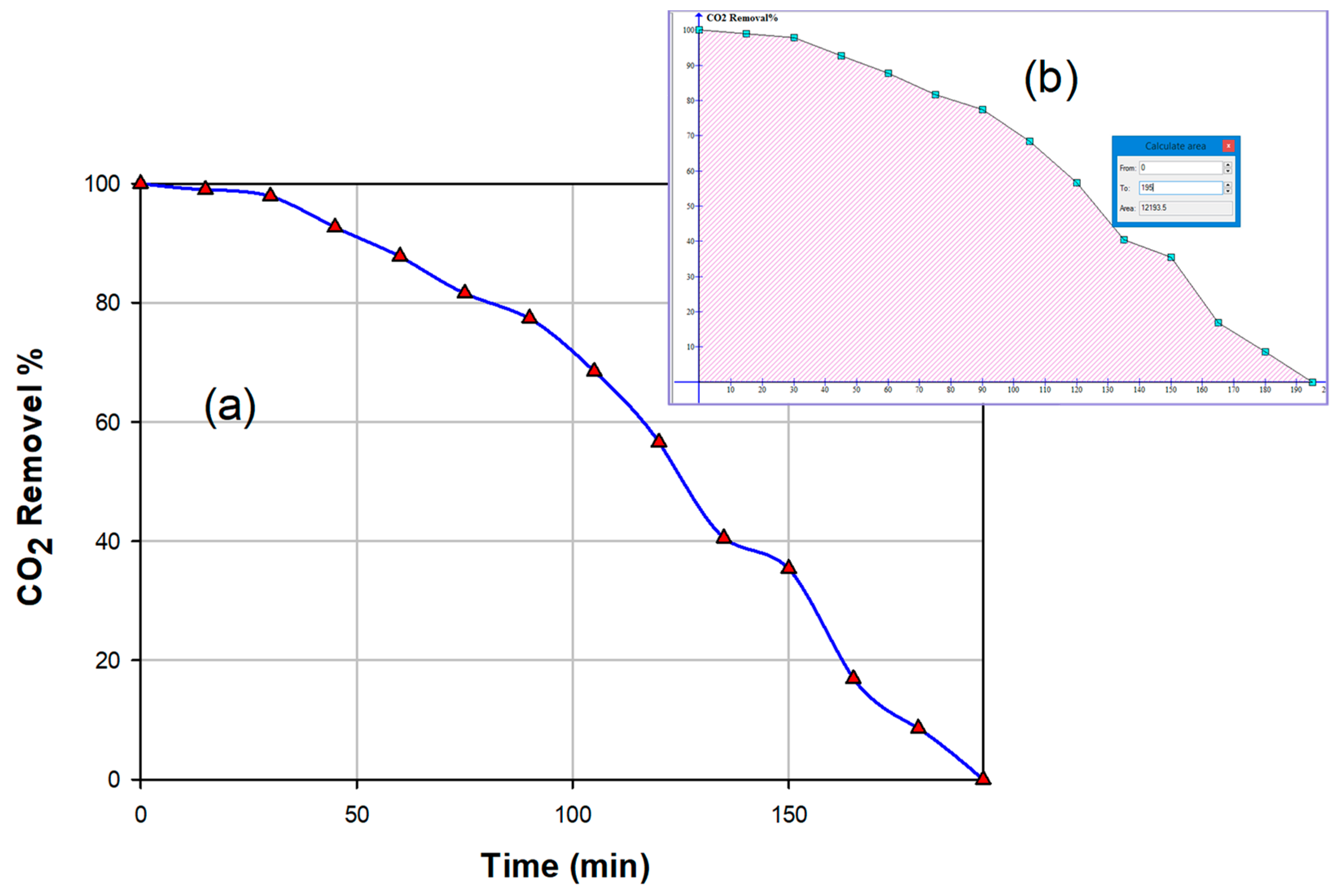
3.2.5. CO2 Capturing Efficiency
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction. Rome. 2019, Licence: CC BY-NC-SA 3.0 IGO. Available online: http://www.fao.org/3/ca6030en/ca6030en.pdf (accessed on 8 July 2021).
- Social Enterprise UK OLIO, the Problem of Food Waste Report 2019. Available online: https://olioex.com/food-waste/the-problem-of-foodwaste/ (accessed on 8 July 2021).
- Sustainable Rice Platform New York, Sustainable Rice Platform Calls upon Rice Industry to Halve Rice Loss and Waste Worldwide Food Loss and Waste Is a Global Problem of Epic Proportions 2019. World Resources Institute. Available online: https://champions123.org/release-major-food-retailers-providers-rice-industry-announce-new-food-loss-and-waste-efforts (accessed on 8 July 2021).
- United Nations Environment Programme. Food Waste Index Report 2021; UNEP: Nairobi, Kenya, 2021. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U. The Swedish Institute for Food and Biotechnology, Global Food Losses and Food Waste 2011. Available online: http://www.madr.ro/docs/ind-alimentara/risipa_alimentara/presentation_food_waste.pdf (accessed on 9 July 2021).
- Kuo, L. QUARTZ, Wasted rice in Asia Emits Over 600 Million Tonnes of Greenhouse Gases a Year 2013. Available online: https://qz.com/123456/wasted-rice-in-asia-emits-over-600-million-tonnes-of-greenhouse-gases-a-year/ (accessed on 8 July 2021).
- Shahbandari, S. GULF NEWS, UAE Among the World’s Biggest Rice Consumers Report 2014. Available online: https://gulfnews.com/goingout/society/uae-among-worlds-biggest-rice-consumers-1.1350656# (accessed on 10 July 2021).
- Hsieh, F.; Huff, H.E.; Peng, I.C.; Marek, S.W. Puffing of Rice Cakes as Influenced by Tempering and Heating Conditions. J. Food Sci. 1989, 54, 1310–1312. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, M.H.; Yang, S.A.; Choi, Y.S.; Jegal, S.A.; Sung, C.K.; Mo, E.K. Physicochemical Characteristics and Antioxidant Capacity of Rice Cake (Sulgitteok) Supplemented with Lyophilized Sedum sarmentosum (Dolnamul) Powder. Prev. Nutr. Food Sci. 2012, 17, 152–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennaceur, K. Chapter 26—CO2 Capture and Sequestration. In Future Energy, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 583–611. [Google Scholar]
- Hashimoto, K.M.; Yamasaki, K.; Fujimura, T.; Matsui, K.; Izumiya, M.; Komori, A.; El-Moneim, A.; Akiyama, E.; Habazaki, H.; Kumagai, N.; et al. Global CO2 recycling—Novel materials and prospect for prevention of global warming and abundant energy supply. Mater. Sci. Eng. A 1999, 267, 200–206. [Google Scholar] [CrossRef]
- Carroll, A.; Przeslawski, R.; Radke, L.; Black, J.; Picard, K.; Moreau, J.W.; Haese, R.; Nichol, S. Environmental considerations for subseabed geological storage of CO2: A review. Cont. Shelf Res. 2014, 83, 116–128. [Google Scholar] [CrossRef]
- Calvo, L.M.; Domingo, R. Influence of process operating parameters on CO2 emissions in continuous industrial plants. J. Clean. Prod. 2015, 96, 253–262. [Google Scholar] [CrossRef]
- Abanades, J.C. Calcium Looping for CO2 Capture in Combustion Systems, Fluidized Bed Technologies for Near Zero Emission Combustion and Gasification; Woodhead Publishing: Sawston, UK, 2013; Volume 21, pp. 931–970. [Google Scholar]
- Sipöcz, N.; Hernandez-Nogales, A.; Gonzalez-Salazar, M.A.; Shisler, R.; Lissianski, V. Low-Temperature CO2 Capture for Near-term Applications. Energy Procedia 2013, 37, 1228–1238. [Google Scholar] [CrossRef] [Green Version]
- Sema, T.; Naami, A.; Fu, K.; Edali, M.; Liu, H.; Shi, H.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P. Comprehensive mass transfer and reaction kinetics studies of CO2 absorption into aqueous solutions of blended MDEA–MEA. Chem. Eng. J. 2012, 209, 501–512. [Google Scholar] [CrossRef]
- Den Elzen, M.; Meinshausen, M.; Van Vuuren, D. Multi-gas emission envelopes to meet greenhouse gas concentration targets: Costs versus certainty of limiting temperature increase. Glob. Environ. Chang. 2007, 17, 260–280. [Google Scholar] [CrossRef] [Green Version]
- Holtz-Eakin, D.; Selden, T.M. Stoking the fires? CO2 emissions and economic growth. J. Public Econ. 1995, 57, 85–101. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; De Lasa, H.I. Chemical-looping combustion (CLC) for inherent separations—A review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Kunze, C.; Spliethoff, H. Assessment of oxy-fuel, pre-and post-combustion-based carbon capture for future IGCC plants. Appl. Energy 2012, 94, 109–116. [Google Scholar] [CrossRef]
- Al-Marzouqi, M.; El-Naas, M.; Marzouk, S.; Abdullatif, N. Modeling of chemical absorption of CO2 in membrane contactors. Sep. Purif. Technol. 2008, 62, 499–506. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A.; Cho, P. The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. Fuel 2001, 80, 953–1962. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Perspectives on microalgal CO2-emission mitigation systems—A review. Biotechnol. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Membrane Permeation Processes. In Gas Purification, 5th ed.; Kohl, A.L., Nielsen, R.B., Eds.; Gulf Professional Publishing: Houston, TX, USA, 1997; Chapter 15; pp. 1238–1295. [Google Scholar]
- Herzog, H.; Caldeira, K.; Adams, E. Carbon Sequestration Via Direct Injection. In Encyclopedia of Ocean Sciences; Steele, J.H., Ed.; Academic Press: Oxford, UK, 2001; pp. 408–414. [Google Scholar]
- Afkhamipour, M.; Mofarahi, M. Sensitivity analysis of the rate-based CO2 absorber model using amine solutions (MEA, MDEA and AMP) in packed columns. Int. J. Greenh. Gas Control 2014, 25, 9–22. [Google Scholar] [CrossRef]
- Spector, N.A.; Dodge, B.F. Removal of carbon dioxide from atmospheric air. Trans. Am. Inst. Chem. Eng. 1946, 42, 827–848. [Google Scholar]
- Lackner, K.S.; Grimes, P.; Ziock, H.J. Capturing carbon dioxide from the air. In Proceedings of the 24th Annual Technical Conference on Coal Utilization, Clearwater, FL, USA, 8–11 March 1999. [Google Scholar]
- Baciocchi, R.; Storti, G.; Mazzotti, M. Process design, and energy requirement or the capture of carbon dioxide from the air. Chem. Eng. Process. 2006, 45, 1047–1058. [Google Scholar] [CrossRef]
- Zeman, F. Energy and material balance of CO2 capture from ambient air. Environ. Sci. Technol. 2007, 41, 7558–7563. [Google Scholar] [CrossRef]
- Toraloff, J.; Keith, D.W.; Lowry, G. Carbon dioxide capture from atmospheric air using hydroxide spray. Environ. Sci. Technol. 2008, 42, 2728–2735. [Google Scholar]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Budd, P.M.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; McKeown, N.B.; Fritsch, D. Gas separate on membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Singh, R.; Koros, W.J. Carbon molecular sieve membrane performance tuning by dual temperature secondary oxygen doping (DTSOD). J. Membr. Sci. 2013, 427, 472–478. [Google Scholar] [CrossRef]
- Krishna, R.; Van Baten, J.M. In silico screening of zeolite membranes for CO2 capture. J. Membr. Sci. 2010, 360, 323–333. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, Z.; Yuan, S.; Wang, J.; Wang, S. Preparation and characterization of small molecular amine-modified PVAm membranes for CO2/H2 separation. J. Membr. Sci. 2015, 475, 290–302. [Google Scholar] [CrossRef]
- Shao, L.; Samseth, J.; Hägg, M.B. Crosslinking, and stabilization of nanoparticle filled PMP nanocomposite membranes for gas separations. J. Membr. Sci. 2009, 326, 285–292. [Google Scholar] [CrossRef]
- Stewart, C.; Hessami, M.A. A study of methods of carbon dioxide capture and sequestration––the sustainability of a photosynthetic bioreactor approach. Energy Convers. Manag. 2005, 46, 403–420. [Google Scholar] [CrossRef]
- Berend, S.; Reimer, J.A.; Oldenburg, C.M.; Bourg, I.C. Introduction to Carbon Capture and Sequestration; Imperial College Press: London, UK, 2014; pp. 281–354. ISBN 978-1-78326-328-8. [Google Scholar]
- Khoukhi, M.; Dar Saleh, A.; Hassan, A.; Abdelbaqi, S. Thermal Characterization of a New Bio-Based Insulation Material Containing Puffed Rice. Energies 2021, 14, 5700. [Google Scholar] [CrossRef]
- Mohammad, A.F.; El-Naas, M.H.; Suleiman, M.I.; al Musharfy, M. Optimization of a solvay-based approach for CO2 capture. Int. J. Chem. Eng. Appl. 2016, 7, 230–234. [Google Scholar] [CrossRef] [Green Version]
- El-Naas, M.H.; Mohammad, A.F.; Suleiman, M.I.; al Musharfy, M.; Al-Marzouqi, A.H. A new process for the capture of CO2 and reduction of water salinity. Desalination 2017, 411, 69–75. [Google Scholar] [CrossRef]
- Muftah, E.-N. Process for Capture of Carbon Dioxide and Desalination. U.S. Patent No. 10,118,843, 6 November 2018. [Google Scholar]
- Mourad, A.A.H.I.; Mohammad, A.F.; Altarawneh, M.; Al-Marzouqi, A.H.; El-Naas, M.H.; Al-Marzouqi, M.H. Effects of potassium hydroxide and aluminum oxide on the performance of a modified solvay process for CO2 capture: A comparative study. Int. J. Energy Res. 2021, 45, 13952–13964. [Google Scholar] [CrossRef]
- Mourad, A.A.H.; Mohammad, A.F.; Al-Marzouqi, A.H.; El-Naas, M.H.; Al-Marzouqi, M.H.; Altarawneh, M. KOH-Based Modified Solvay Process for Removing Na Ions from High Salinity Reject Brine at High Temperatures. Sustainability 2021, 13, 10200. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Chew, T.L.; Ahmad, A.L.; Bhatia, S. Ordered mesoporous silica (OMS) as an adsorbent and membrane for separation of carbon dioxide (CO2). Adv. Colloid Interface Sci. 2010, 153, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Bosco, S.J.D.; Shah, M.A.; Mir, M.M.; Sunooj, K.V. Process optimization and characterization of popped Brown Rice. Int. J. Food Prop. 2016, 19, 2102–2112. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Galiwango, E.; Abdel Rahman, N.S.; Al-Marzouqi, A.H.; Abu-Omar, M.M.; Khaleel, A.A. Isolation and characterization of cellulose and α-cellulose from date palm biomass waste. Heliyon 2019, 5, e02937. [Google Scholar] [CrossRef] [Green Version]
- Nor, N.A.M.; Mustapha, W.A.W.; Hassan, O. Deep eutectic solvent (DES) as a pretreatment for oil palm empty fruit bunch (OPEFB) in sugar production. Procedia Chem. 2016, 18, 147–154. [Google Scholar] [CrossRef] [Green Version]






| Moisture [%] | Temperature [°C] | Thickness [mm] |
|---|---|---|
| 18 | 260 | 12 |
| 16 | 240 | 9 |
| 14 | 220 | 8 |
| 12 | 200 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, A.F.; Dar Saleh, A.F.; Khoukhi, M.; Al-Marzouqi, A.H. A New Method for Capturing CO2 from Effluent Gases Using a Rice-Based Product. Energies 2022, 15, 2287. https://doi.org/10.3390/en15062287
Mohammad AF, Dar Saleh AF, Khoukhi M, Al-Marzouqi AH. A New Method for Capturing CO2 from Effluent Gases Using a Rice-Based Product. Energies. 2022; 15(6):2287. https://doi.org/10.3390/en15062287
Chicago/Turabian StyleMohammad, Ameera F., Abeer F. Dar Saleh, Maatouk Khoukhi, and Ali H. Al-Marzouqi. 2022. "A New Method for Capturing CO2 from Effluent Gases Using a Rice-Based Product" Energies 15, no. 6: 2287. https://doi.org/10.3390/en15062287
APA StyleMohammad, A. F., Dar Saleh, A. F., Khoukhi, M., & Al-Marzouqi, A. H. (2022). A New Method for Capturing CO2 from Effluent Gases Using a Rice-Based Product. Energies, 15(6), 2287. https://doi.org/10.3390/en15062287







