An Electrothermal Model to Predict Thermal Characteristics of Lithium-Ion Battery under Overcharge Condition
Abstract
:1. Introduction
2. Modeling of Thermal Behavior of LIB
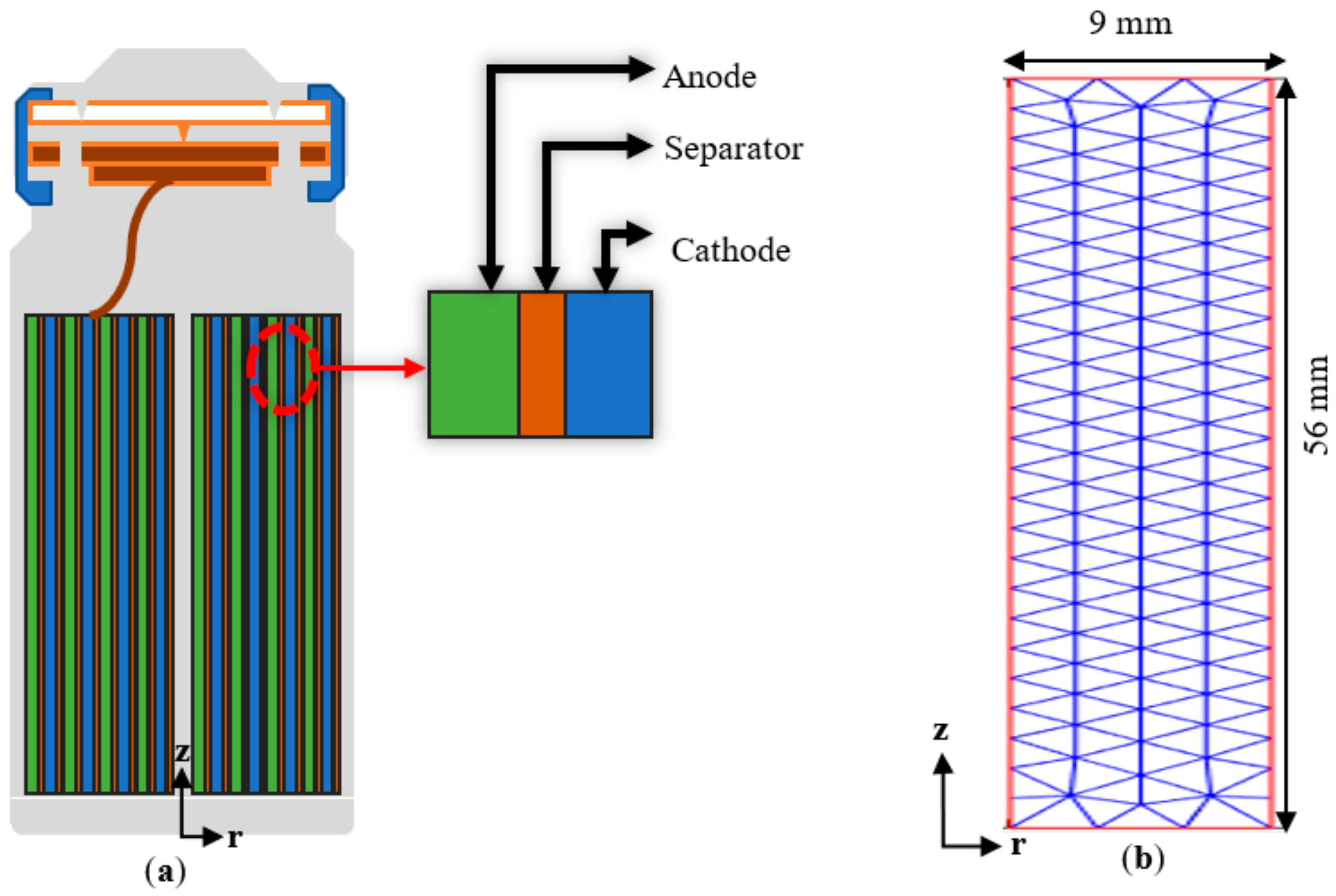
2.1. Physical Model
2.2. Heat Generation
2.3. Numerical Simulation Process
3. Experiment for Model Validation
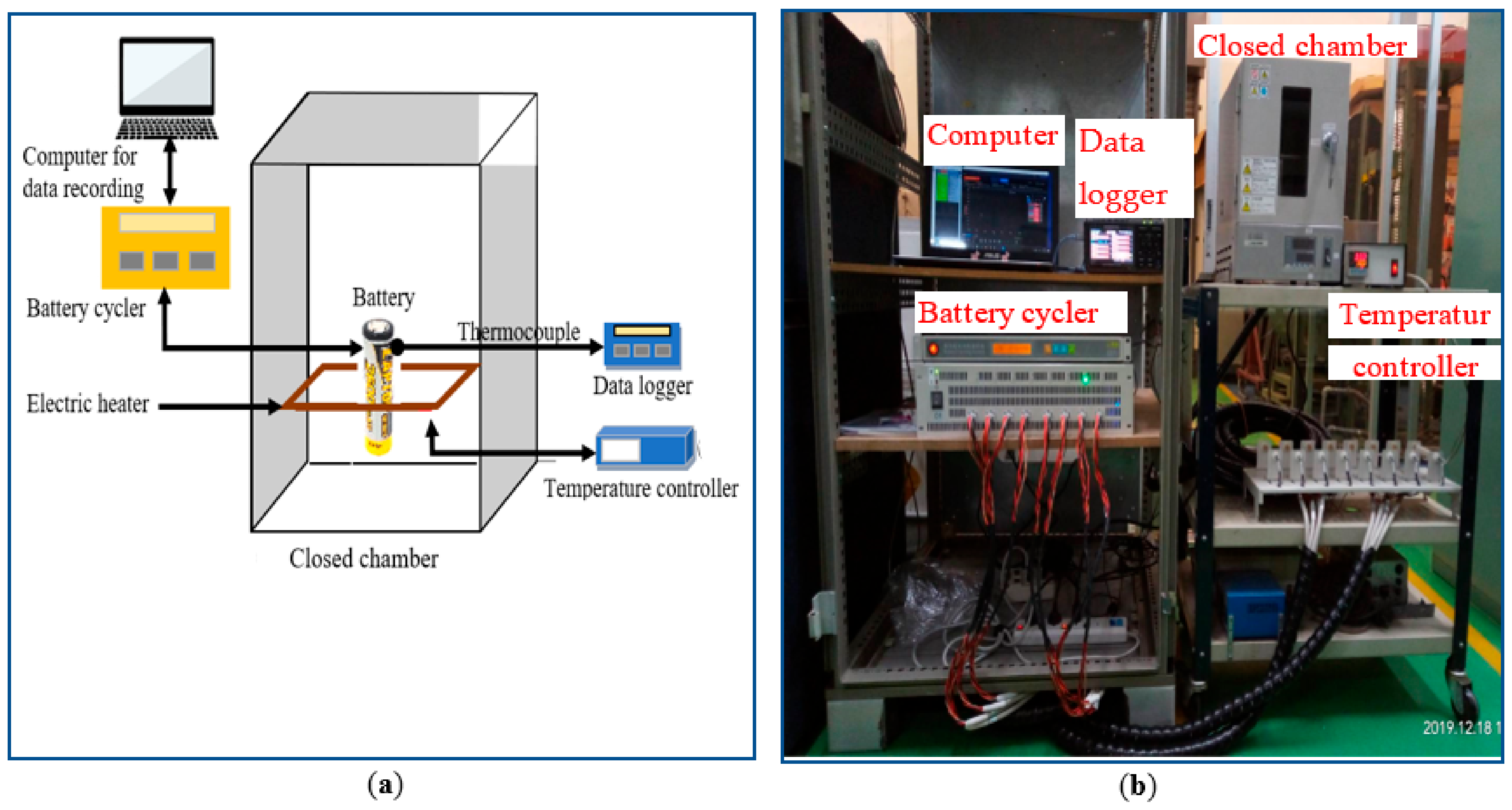
3.1. Experimental Setup
3.2. Observation of Battery Surface Temperature
4. Results and Discussions
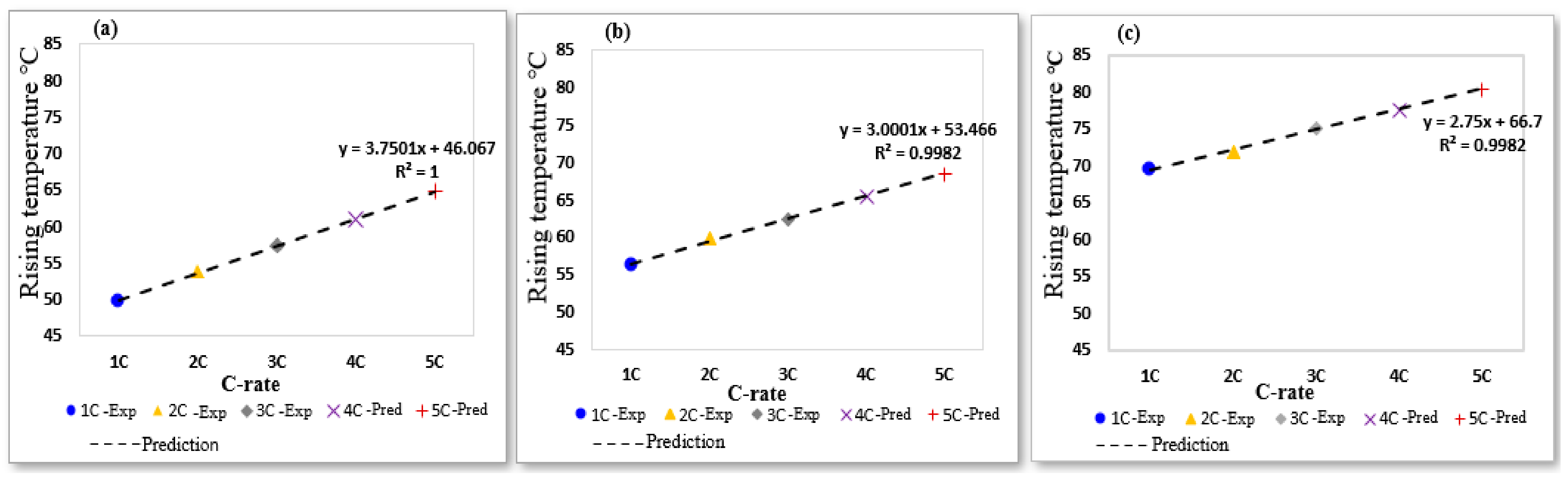
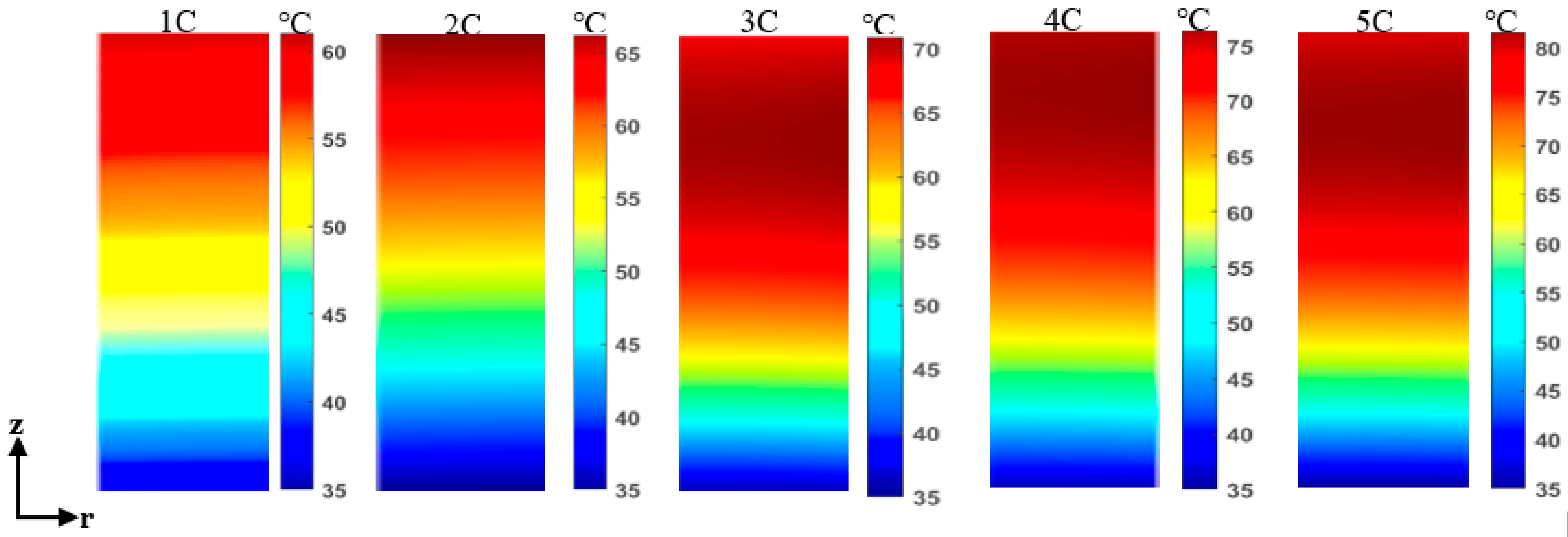
4.1. Thermal Behavior of Overcharged LIB at a Different Charging Current
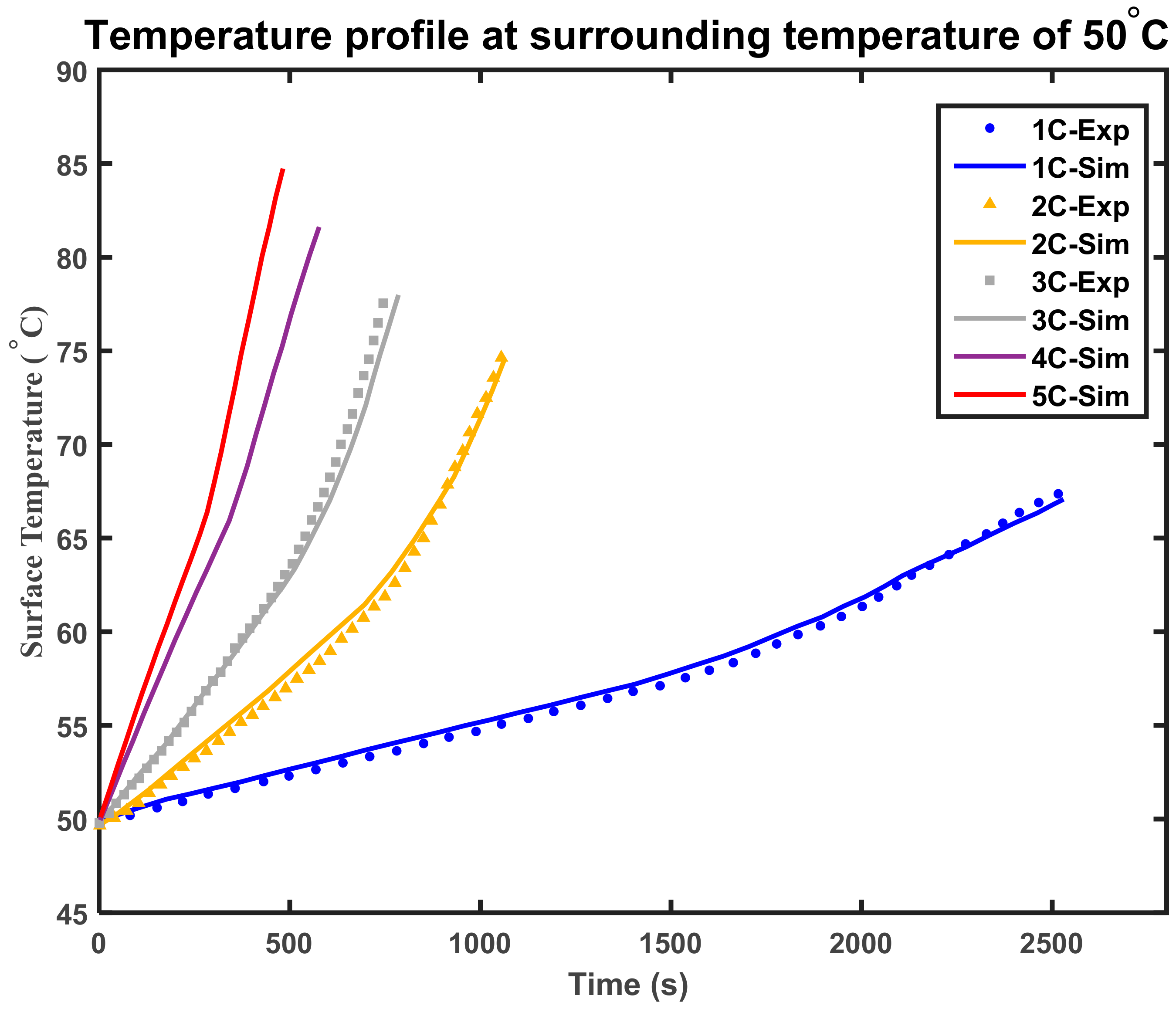
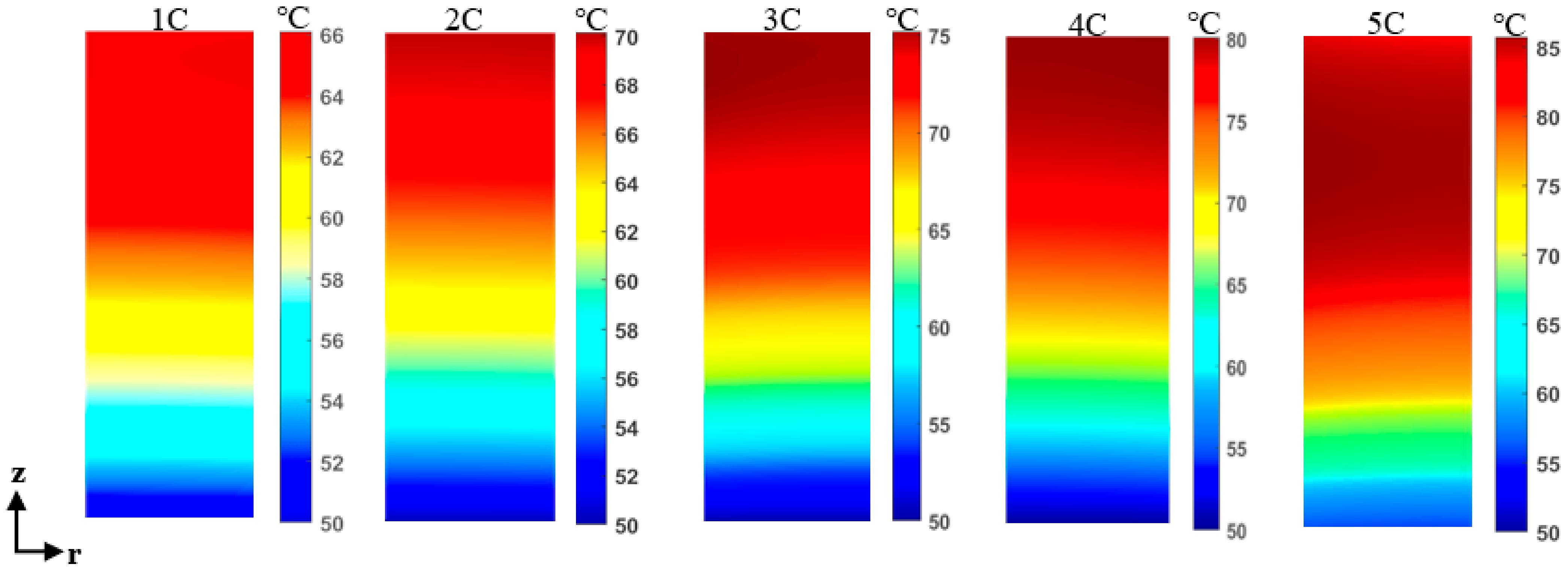
4.2. Thermal Behavior of Overcharged LIB at High Surrounding Temperature
4.3. Effect of Heat Transfer Coefficient on Battery Surface Temperature
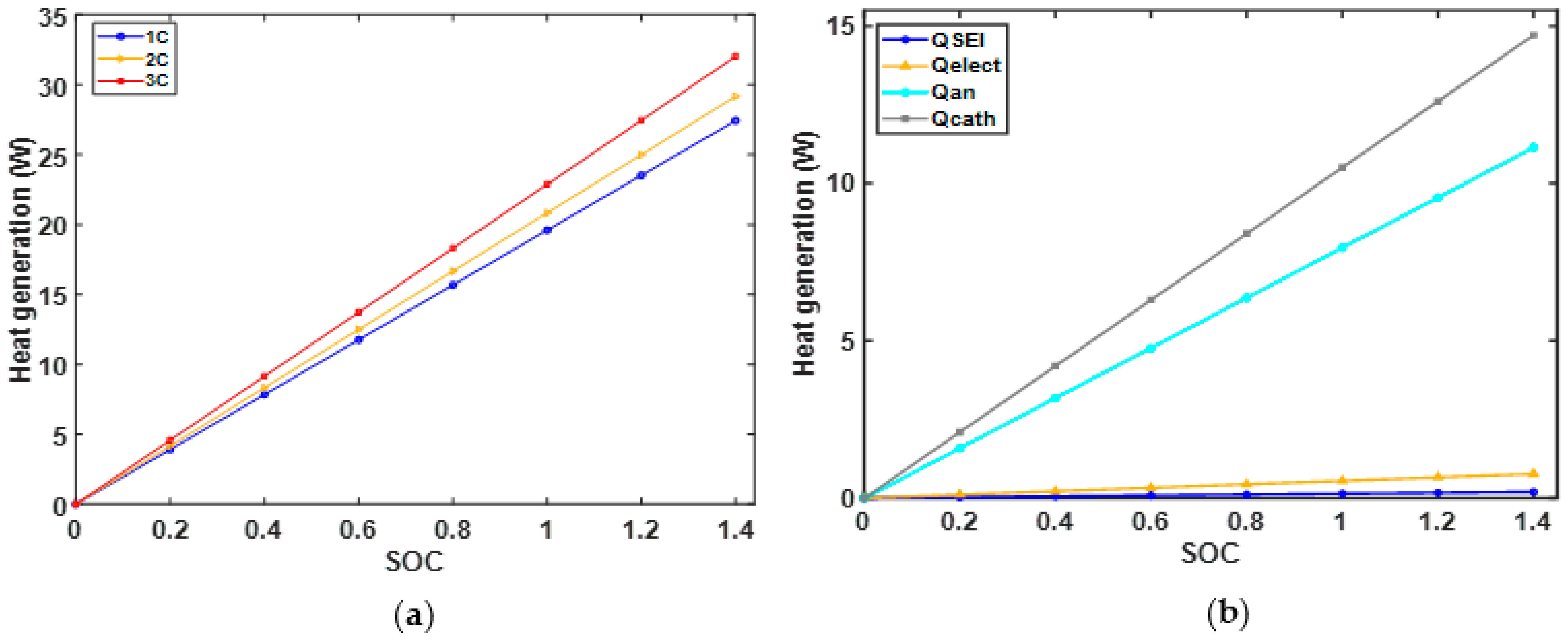
4.4. Analysis of Heat Generation Rates of Battery Overcharged at the Various Current Rate
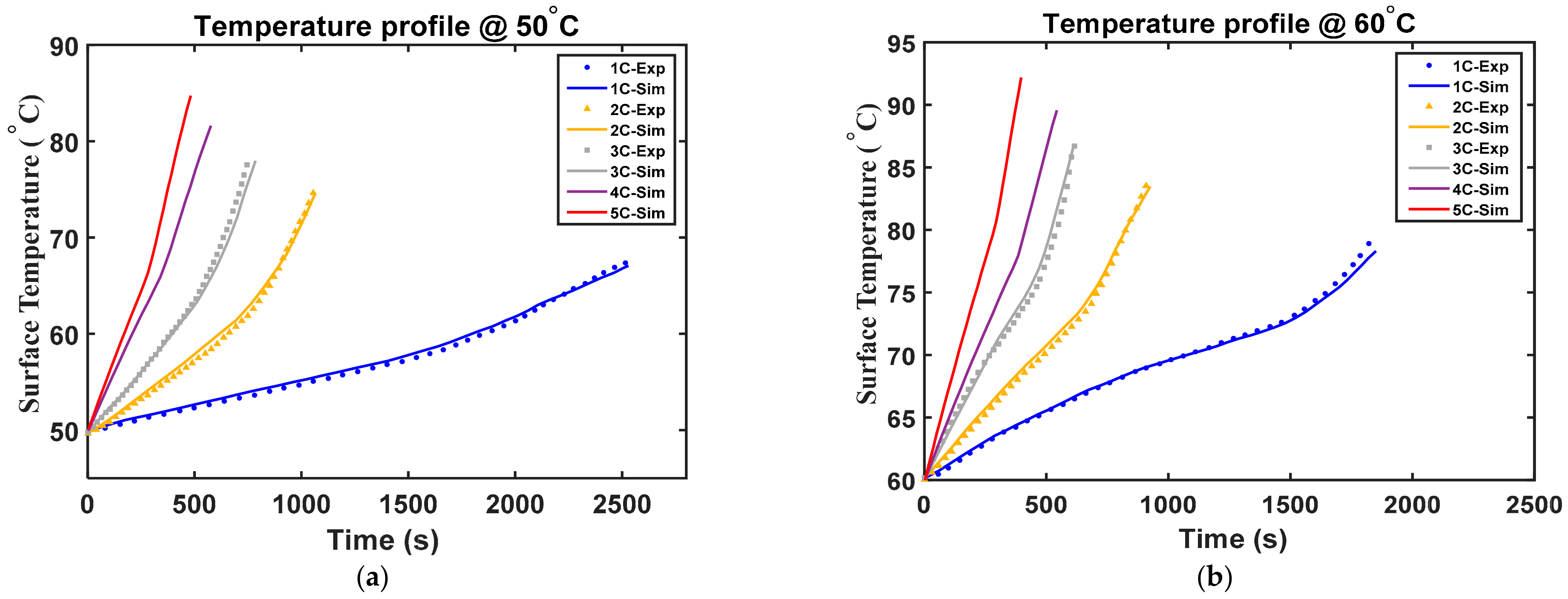
4.5. Model Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Aan-so | anode–solvent decomposition frequency factor (s−1) |
| Aca-so | cathode–solvent decomposition frequency factor (s−1) |
| Ae | electrolyte decomposition frequency factor (s−1) |
| ASEI | SEI decomposition frequency factor (s−1) |
| can | amount of lithium within the anode (dimensionless) |
| ce | amount of lithium within the electrolyte (dimensionless) |
| cSEI | amount of lithium containing meta-stable species in the SEI (dimensionless) |
| Ea,an-so | anode–solvent decomposition activation energy (J/mol) |
| Ea,ca-so | cathode–solvent decomposition activation energy (J/mol) |
| Ea,SEI | SEI decomposition activation energy (J/mol) |
| Han-so | anode–solvent heat (J/kg) |
| Hca-so | cathode–solvent heat (J/kg) |
| He | electrolyte decomposition heat (J/kg) |
| HSEI | SEI decomposition heat (J/kg) |
| me | reaction order for ce |
| mSEI | reaction order for SEI |
| Ran-so | anode–solvent decomposition rate (s−1) |
| Rca-so | cathode–solvent decomposition rate (s−1) |
| Ea,-so | the anode decomposition activation energy (J/mol) |
| Re | electrolyte decomposition rate (s−1) |
| RSEI | SEI decomposition rate (s−1) |
| ΔHx | enthalpy of the chemical reaction of layer x |
| heat rate dissipated due to convection (W) | |
| heat rate due to exothermic reaction (W) | |
| heat rate due to electrochemical reaction (W) | |
| heat rate dissipated due to radiation (W) | |
| PVDF | polyvinylidene fluoride |
| SEI | solid electrolyte interphase layer |
| Subscripts | |
| x | electrochemical layers |
| 0 | initial condition |
| r | radial direction |
| z | axial direction |
| Greek symbols | |
| ρ | density of air (kg/m3) |
| k | thermal conductivity (W/m·K) |
| Cp | specific heat (J/kg·K) |
References
- Victor Chombo, P.; Laoonual, Y. Investigation of the Thermal Hazard of Fault of Li-Ion Battery under External Heating. In Proceedings of the International Conference on Energy, Environment and Climate Change (International Conference on Utilities and Exhibition)-ICUE 2020, Bangkok, Thailand, 20–22 October 2020. [Google Scholar]
- Zhenpo, W.; Jun, M.A.; Lei, Z. Finite element thermal model and simulation for a cylindrical Li-ion battery. IEEE Access 2017, 5, 15372–15379. [Google Scholar] [CrossRef]
- Victor Chombo, P.; Laoonual, Y.; Wongwises, S. Lessons from the electric vehicle crashworthiness leading to battery fire. Energies 2021, 14, 4802. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Y.; Zou, C.; Yao, K.; Xia, Y.; Gao, F. A novel framework for Lithium-ion battery modeling considering uncertainties of temperature and aging. Energy Convers. Manag. 2019, 180, 162–170. [Google Scholar] [CrossRef]
- Dongxu, O.; Liu, J.H.; Mingyi, C.; Jian, W. Investigation into the fire hazards of lithium-ion batteries under overcharging. Appl. Sci. 2017, 7, 1314. [Google Scholar] [CrossRef] [Green Version]
- Motor1.com. A Tesla Model S Burns to the Ground while at Charging Station in Norway. Available online: https://www./news/60408/tesla-model-s-burns-to-the-ground-while-at-charging-station/ (accessed on 25 February 2022).
- Benzinga.com. Opel Ampera-e EV Catches Fire while Charging in Germany. Available online: https://www.benzinga.com/tech/20/11/18206721/opel-ampera-e-ev-catches-fire-while-charging-in-germany-video#:~:text=An%20Opel%20Ampera%2De%20caught,shows%20firefighters%20battling%20the%20blaze (accessed on 25 February 2022).
- Gizmochina.com. Xpeng Car Catches Fire in a Charging Station in China. Available online: https://www.gizmochina.com/2021/04/15/xpeng-car-catch-fire-charging-station-in-china/ (accessed on 25 February 2022).
- Rushlane.com. Electric Car in Southern China Explodes While Charging. Available online: https://www.rushlane.com/electric-car-explodes-while-charging-12374231.html (accessed on 25 February 2022).
- Insideevs.com. Electric Car Fire and Explosion at a Charging Station. Available online: https://insideevs.com/news/423581/severe-electric-car-fire-explosion-charging/ (accessed on 25 February 2022).
- Jiang, F.; Peng, P.; Sun, Y. Thermal analyses of LiFePO4/graphite battery discharge process. J. Power Sources 2013, 243, 181–194. [Google Scholar] [CrossRef]
- Mahshid, N.; Farschad, T. A computationally efficient model for performance prediction of lithium-ion batteries. Sustain. Energy Technol. Assess. 2021, 43, 100938. [Google Scholar] [CrossRef]
- Melcher, A.; Ziebert, C.; Rohde, M.; Seifert, H.J. Modeling, and simulation of the thermal runaway behavior of cylindrical li-ion cells-computing of critical parameters. Energies 2016, 9, 292. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Feng, X.; Languang, L.; Ouyang, M.; Zheng, S.; Li, J.; He, X. An electrochemical-thermal coupled overcharge-to-thermal-runaway model for lithium-ion battery. J. Power Sources 2017, 364, 328–340. [Google Scholar] [CrossRef]
- Park, Y.; Kim, N.H.; Baek, S.; Kim, J.M.; Cheol, G.; Kim, M.S.; Man, S.; Eom, Y.; Choi, H.C.; Jung, Y.M. Characterization of the passivating layer on Li[Ni0.31 Co0.32Mn0.28 Al0.09]O2 cathode in the overcharge state. J. Mol. Struct. 2010, 974, 139–143. [Google Scholar] [CrossRef]
- Zeng, G.; Bai, Z.; Huang, P.; Wang, Q. Thermal safety study of Li-ion batteries under limited overcharge abuse based on coupled electrochemical thermal model. Int. J. Energy Res. 2020, 44, 1–19. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Wang, Y.; Wang, H.; Wang, C.; Tong, L.; Yi, M. Overcharge investigation of large-format lithium-ion pouch cells with Li(Ni0.6Co0.2Mn0.2)O2 cathode for electric vehicles thermal runaway features and safety management method. Energy 2019, 169, 868–880. [Google Scholar] [CrossRef]
- Mao, N.; Wang, Z.; Chung, Y.H.; Shu, C. Overcharge cycling effect on the thermal behavior, structure, and material of lithium-ion batteries. Appl. Therm. Eng. 2019, 163, 114147. [Google Scholar] [CrossRef]
- Manohar, G.; Taedong, G.; Anshul, K.; Shashishekar, P.; Sagar, B.; Rajkumar, S.; Daehyun, K.; Youngho, R. Fully coupled simplified electrochemical and thermal model for series-parallel configured battery pack. J. Energy Storage 2021, 36, 102424. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, C.; Gao, F.; Yang, K.; Jia, Q. Mathematical model for thermal behavior of lithium-ion battery pack under overcharge. Heat Mass Transf. 2018, 124, 552–563. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Li, Z.; Ren, Y.; Xie, J.; He, H.; Xu, F. Failure study of commercial LiFePO4 cells in over-discharge conditions using electrochemical impedance spectroscopy. J. Electrochem. Soc. 2014, 161, 620–632. [Google Scholar] [CrossRef]
- Samba, A.; Oma, N.; Gualous, H.; Van den Bossche, P.; Mierlo, J.; Boubekeur, V. Development of 2D thermal battery model for lithium-ion pouch cells. World Electr. Veh. J. 2013, 63, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Victor Chombo, P.; Laoonual, Y. A review of safety strategies of Lithium-ion battery. J. Power Sources 2020, 478, 228649–228668. [Google Scholar] [CrossRef]
- Yang, X.; Hu, X.; Chen, Z.; Chen, Y. Effect of ambient dissipation condition on the thermal behavior of a lithium-ion battery using a 3D multi-partition model. Appl. Therm. Eng. 2020, 178, 115634. [Google Scholar] [CrossRef]
- Feng, X.; Sun, J.; Ouyang, M.; He, X.; Lu, L.; Han, X. Characterization of large-format lithium-ion battery exposed to extremely high temperature. J. Power Sources 2014, 272, 457–467. [Google Scholar] [CrossRef]
- Li, J.; Sun, D.; Jin, X.; Shi, W.; Sun, C. Lithium-ion battery overcharging thermal characteristics analysis and an impedance-based electro-thermal coupled model simulation. Appl. Energy 2019, 254, 113574. [Google Scholar] [CrossRef]





| Battery Layers | Material | Thickness (μm) | Density (kg/m3) | Specific Heat (J/kg·K) | Thermal Conductivity (W/m·K) |
|---|---|---|---|---|---|
| Cathode | LiCoO2 | 55 | 2328.5 | 1269.2 | 1.58 |
| Anode | graphite | 55 | 1347.33 | 1437.4 | 1.04 |
| Separator | PVDF | 30 | 1008.98 | 1978 | 0.34 |
| Positive current collector | Cu | 10 | 8933 | 385 | 298.15 |
| Negative current collector | Al | 7 | 2700 | 875 | 170 |
| Reaction Parameter | Abbreviation | (J/kg) | (kg) | (s−1) | (J mol−1) | (-) | Ref. |
|---|---|---|---|---|---|---|---|
| SEI | SEI | 2.57 × 105 | 0.219 | 1.667 × 1015 | 1.3508 × 105 | 0.15 | [13] |
| Anode | an | 1.714 × 106 | 0.219 | 2.5 × 1013 | 1.3508 × 105 | 0.75 | [13] |
| Cathode | cath | 3.14 × 105 | 0.36198 | 6.667 × 1013 | 1.396 × 105 | 0.04 | [14] |
| Electrolyte | electro | 1.55 ×105 | 0.1708 | 5.14 × 1025 | 2.74 × 105 | 1 | [13] |
| Particular | Value |
|---|---|
| Nominal voltage (V) | 3.70 |
| Discharge end voltage (V) | 3.00 |
| Maximum charging voltage (V) | 4.2 |
| Maximum capacity (Ah) @ 25.5 °C, discharge 0.52 A, 2.75 V | 2.60 |
| Standard constant charging current (A) | 0.52 |
| Maximum charging current (A) | 1.30 |
| Internal impedance (Ω) | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamisi, C.M.; Chombo, P.V.; Laoonual, Y.; Wongwises, S. An Electrothermal Model to Predict Thermal Characteristics of Lithium-Ion Battery under Overcharge Condition. Energies 2022, 15, 2284. https://doi.org/10.3390/en15062284
Hamisi CM, Chombo PV, Laoonual Y, Wongwises S. An Electrothermal Model to Predict Thermal Characteristics of Lithium-Ion Battery under Overcharge Condition. Energies. 2022; 15(6):2284. https://doi.org/10.3390/en15062284
Chicago/Turabian StyleHamisi, Charles Mohamed, Pius Victor Chombo, Yossapong Laoonual, and Somchai Wongwises. 2022. "An Electrothermal Model to Predict Thermal Characteristics of Lithium-Ion Battery under Overcharge Condition" Energies 15, no. 6: 2284. https://doi.org/10.3390/en15062284
APA StyleHamisi, C. M., Chombo, P. V., Laoonual, Y., & Wongwises, S. (2022). An Electrothermal Model to Predict Thermal Characteristics of Lithium-Ion Battery under Overcharge Condition. Energies, 15(6), 2284. https://doi.org/10.3390/en15062284







