The Inhibitory Effect of Free Nitrous Acid and Free Ammonia on the Anoxic Phosphorus Uptake Rate of Polyphosphate-Accumulating Organisms
Abstract
:1. Introduction
2. Materials and Methods
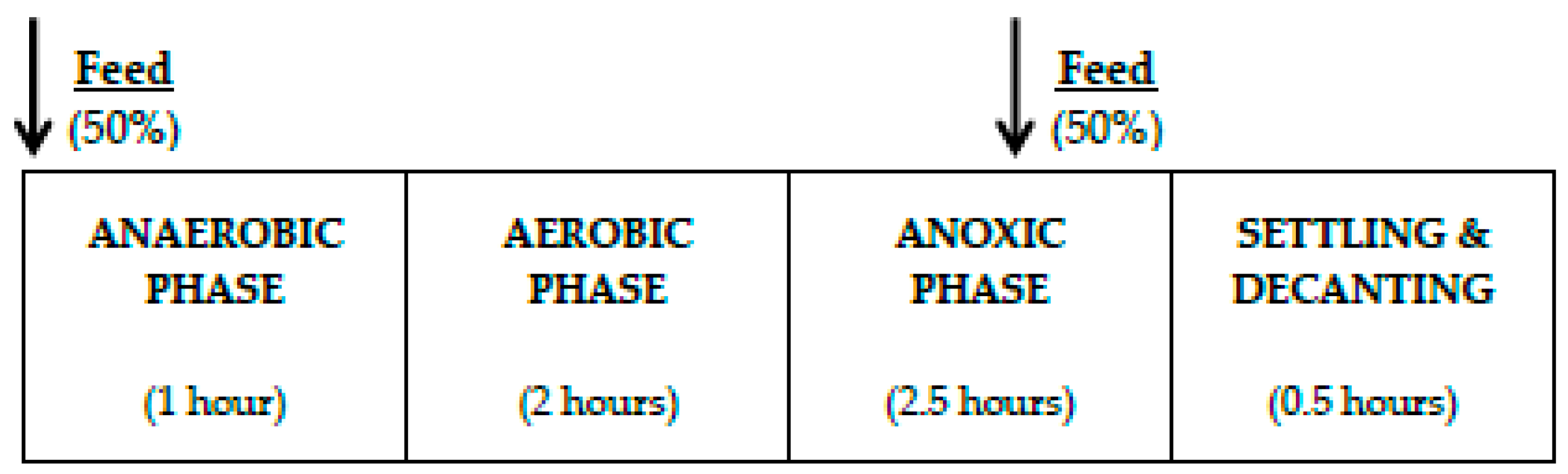
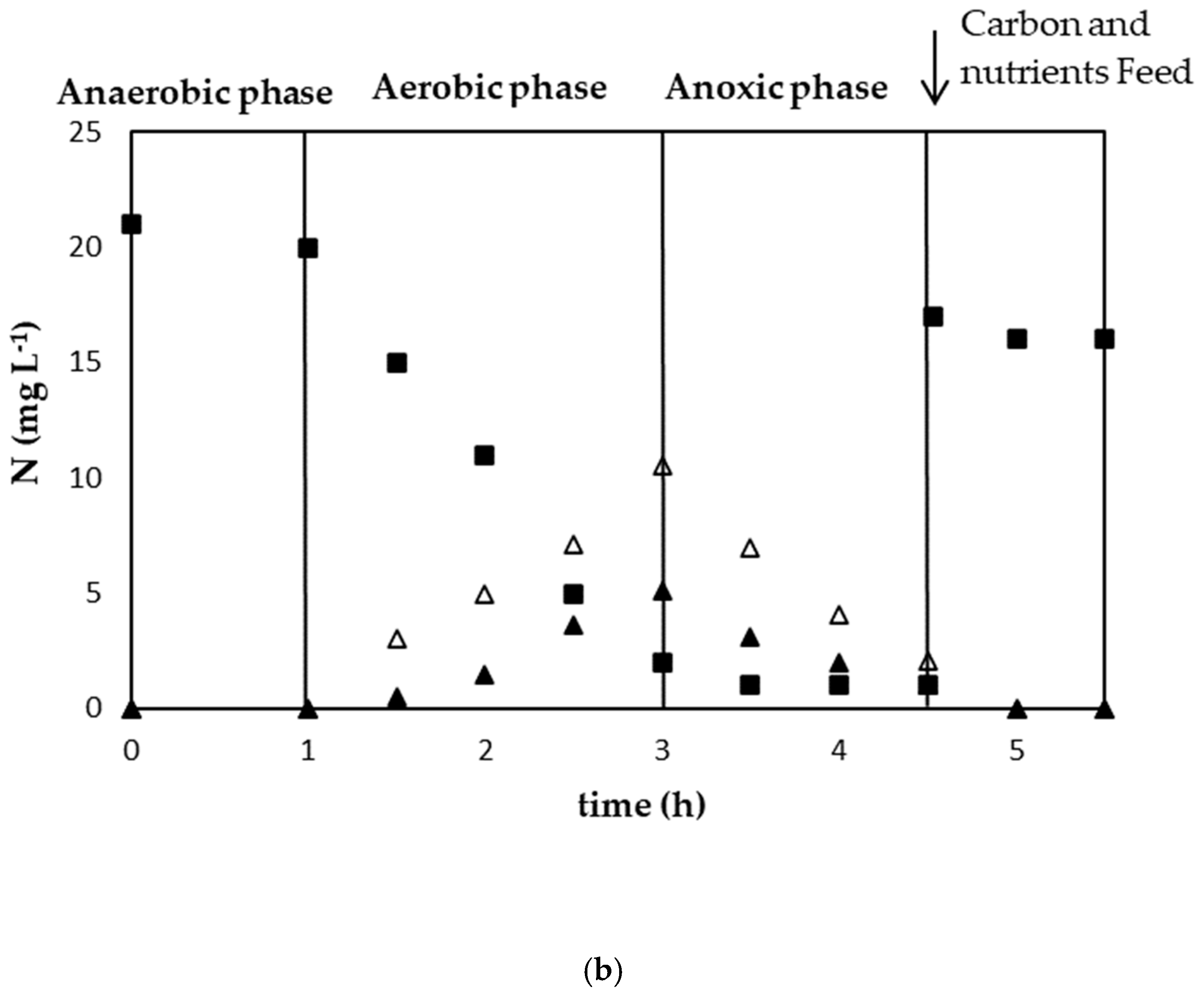
2.1. Experimental Set-Up
2.2. Batch Experiments
2.2.1. Investigation of the Effect of FNA on Anoxic PUR
2.2.2. Investigation of the Effect of FA on Anoxic PUR
2.3. Chemical and Microbiological Analyses
2.4. Modelling Approach
- PUR: the phosphorus uptake rate observed in the anoxic batch tests;
- S: the initial phosphate concentration;
- : the maximum apparent phosphorus uptake rate, and
- : the half saturation apparent concentration, which accounts for the toxic effect of either free ammonia or free nitrous acid.
3. Results
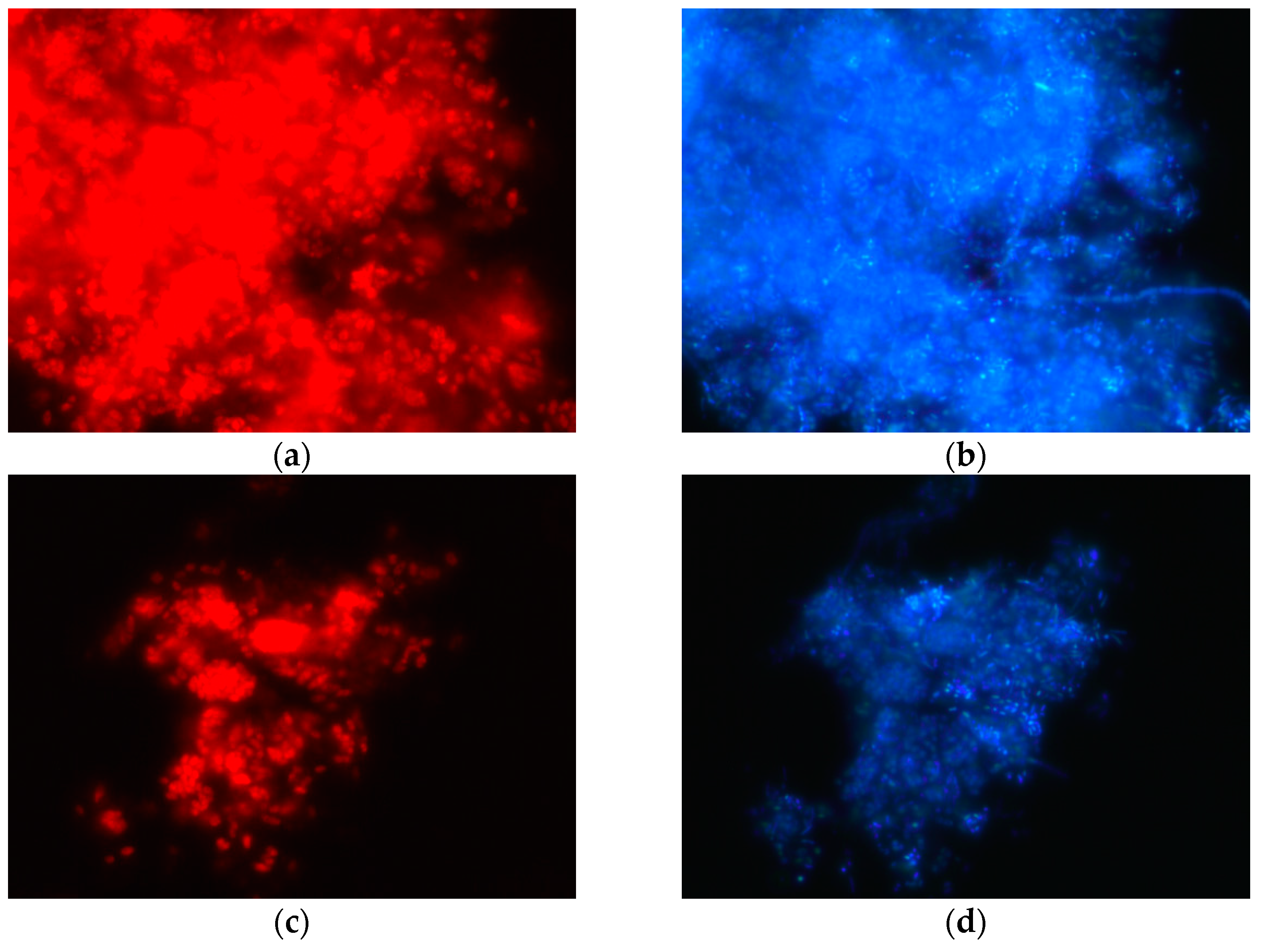
3.1. Establishment of a Strong PAO Culture
3.2. Effect of FNA
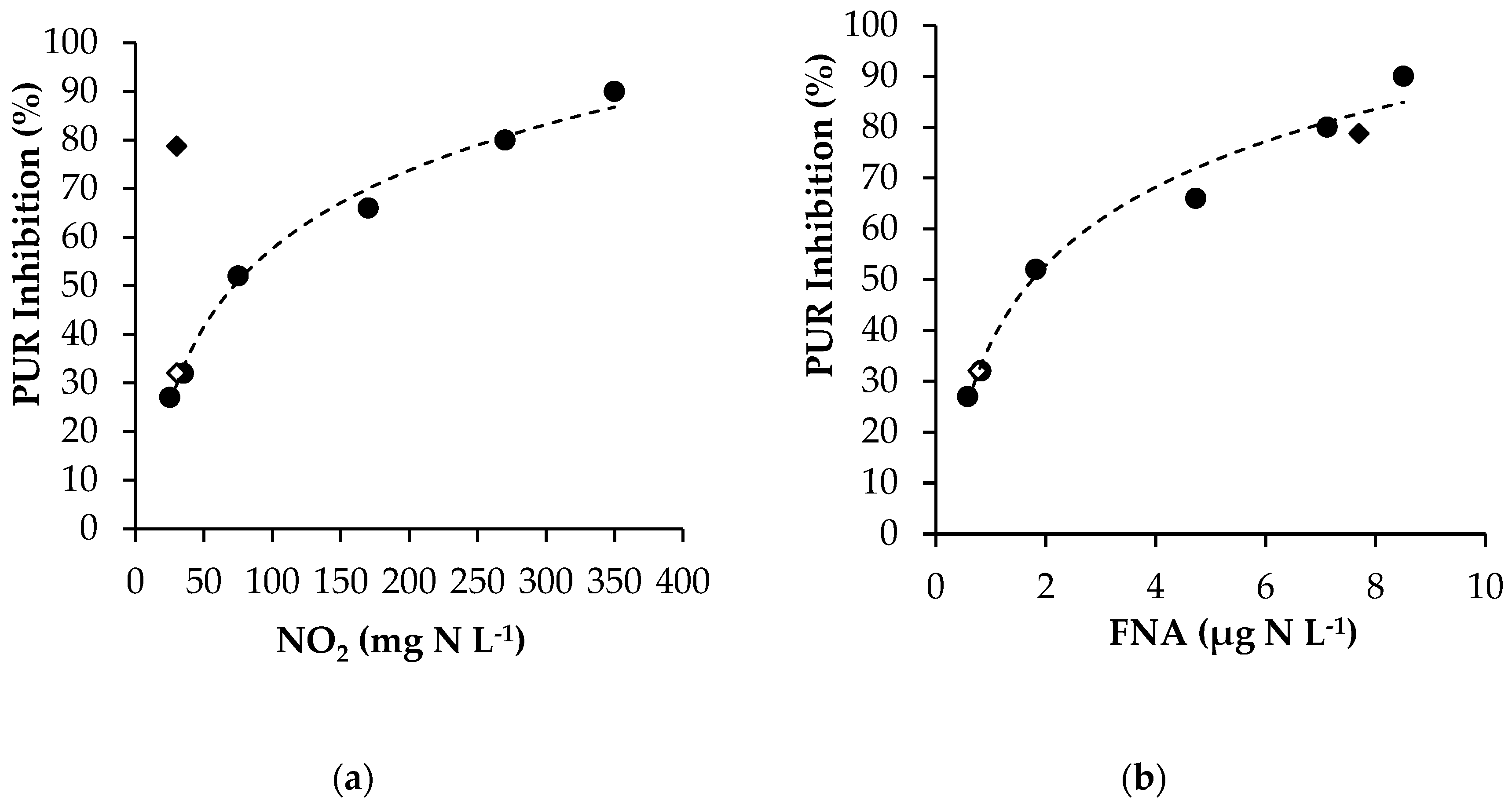
3.2.1. Experimental Results
3.2.2. Modelling FNA Inhibition on Anoxic PUR
- PURmax: the non-inhibited (maximum) anoxic phosphorus uptake rate (which essentially may be regarded as the PUR displayed by the control reactor of each batch series);
- SFNA: the concentration of FNA;
- KiFNA: the FNA concentration inhibiting anoxic PUR by 50%.
- SFNA*: the FNA concentration above which PUR is completely inhibited;
- Ks: the affinity coefficient constant;
- m, α: constants (constant m describes how the observed rate decreases as the inhibitor concentration increases, α is an empirical constant);
- n: the Hill coefficient representing the number of bound ligands.
3.3. Effect of FA
3.3.1. Experimental Results
3.3.2. Modelling FA Inhibition on Anoxic PUR
- PURmax: the non-inhibited (maximum) anoxic phosphorus uptake rate (which essentially may be regarded as the PUR displayed by the control reactor of each batch series);
- SFA: the free ammonia concentration;
- S: the concentration of phosphates;
- KS: the half saturation coefficient for phosphates;
- KiFA: the FA concentration that results in 50% inhibition of the process.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Mino, T.; van Loosdrecht, M.C.M.; Heijnen, J.J. Microbiology and Biochemistry of the Enhanced Biological Phosphate Removal Process. Water Res. 1998, 32, 3193–3207. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Huang, H.; Wang, S.; Ge, S.; Zhang, J.; Wang, Z. Short- and Long-Term Effects of Temperature on Partial Nitrification in a Sequencing Batch Reactor Treating Domestic Wastewater. J. Hazard. Mater. 2010, 179, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Noutsopoulos, C.; Mamais, D.; Statiris, E.; Lerias, E.; Malamis, S.; Andreadakis, A. Reject Water Characterization and Treatment through Short-Cut Nitrification/Denitrification: Assessing the Effect of Temperature and Type of Substrate: Reject Water Characterization and Treatment via Nitritation/Denitritation. J. Chem. Technol. Biotechnol. 2018, 93, 3638–3647. [Google Scholar] [CrossRef]
- Gu, A.Z.; Pedros, P.B.; Kristiansen, A.; Onnis-Hayden, A.; Schramm, A. Nitrifying Community Analysis in a Single Submerged Attached-Growth Bioreactor for Treatment of High-Ammonia Waste Stream. Water Environ. Res. 2007, 79, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Pijuan, M.; Ye, L.; Yuan, Z. Free Nitrous Acid Inhibition on the Aerobic Metabolism of Poly-Phosphate Accumulating Organisms. Water Res. 2010, 44, 6063–6072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ganda, L.; Lim, M.; Yuan, Z.; Ng, W.J. Response of Poly-Phosphate Accumulating Organisms to Free Nitrous Acid Inhibition under Anoxic and Aerobic Conditions. Bioresour. Technol. 2012, 116, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Andreadakis, D.; Noutsopoulos, C.; Fragkiskatos, G.; Mamais, D.; Misirli, T.; Argyropoulou, K.; Themeli, E.; Malamis, S. Inhibition of Free Nitrous Acid and Free Ammonia on Polyphosphate Accumulating Organisms: Evidence of Insufficient Phosphorus Removal through Nitritation-Denitritation. J. Environ. Manag. 2021, 297, 113390. [Google Scholar] [CrossRef]
- Zhou, Y.; Ganda, L.; Lim, M.; Yuan, Z.; Kjelleberg, S.; Ng, W.J. Free Nitrous Acid (FNA) Inhibition on Denitrifying Poly-Phosphate Accumulating Organisms (DPAOs). Appl. Microbiol. Biotechnol. 2010, 88, 359–369. [Google Scholar] [CrossRef]
- Yoshida, Y.; Takahashi, K.; Saito, T.; Tanaka, K. The Effect of Nitrite on Aerobic Phosphate Uptake and Denitrifying Activity of Phosphate-Accumulating Organisms. Water Sci. Technol. 2006, 53, 21–27. [Google Scholar] [CrossRef]
- Saito, T.; Brdjanovic, D.; van Loosdrecht, M.C.M. Effect of Nitrite on Phosphate Uptake by Phosphate Accumulating Organisms. Water Res. 2004, 38, 3760–3768. [Google Scholar] [CrossRef]
- Meinhold, J.; Arnold, E.; Isaacs, S. Effect of Nitrite on Anoxic Phosphate Uptake in Biological Phosphorus Removal Activated Sludge. Water Res. 1999, 33, 1871–1883. [Google Scholar] [CrossRef]
- Frison, N.; Katsou, E.; Malamis, S.; Fatone, F. A Novel Scheme for Denitrifying Biological Phosphorus Removal via Nitrite from Nutrient-Rich Anaerobic Effluents in a Short-Cut Sequencing Batch Reactor: Via-Nitrite Phosphorus Removal in a Sequencing Batch Reactor. J. Chem. Technol. Biotechnol. 2016, 91, 190–197. [Google Scholar] [CrossRef]
- Yang, G.; Xu, Q.; Wang, D.; Tang, L.; Xia, J.; Wang, Q.; Zeng, G.; Yang, Q.; Li, X. Free Ammonia-Based Sludge Treatment Reduces Sludge Production in the Wastewater Treatment Process. Chemosphere 2018, 205, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sun, P.; Lou, J.; Cai, J.; Song, Y.; Yu, S.; Lu, X. Inhibition of Free Ammonia to the Granule-Based Enhanced Biological Phosphorus Removal System and the Recoverability. Bioresour. Technol. 2013, 148, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Tayà, C.; Garlapati, V.K.; Guisasola, A.; Baeza, J.A. The Selective Role of Nitrite in the PAO/GAO Competition. Chemosphere 2013, 93, 612–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of Nitrification by Ammonia and Nitrous Acid. J. (Water Pollut. Control Fed.) 1976, 48, 835–852. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Amann, R.I. Fluorescently Labelled, RRNA-Targeted Oligonucleotide Probes in the Study of Microbial Ecology. Mol. Ecol. 1995, 4, 543–554. [Google Scholar] [CrossRef]
- Crocetti, G.R.; Hugenholtz, P.; Bond, P.L.; Schuler, A.; Keller, J.; Jenkins, D.; Blackall, L.L. Identification of Polyphosphate-Accumulating Organisms and Design of 16S RRNA-Directed Probes for Their Detection and Quantitation. Appl. Environ. Microbiol. 2000, 66, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Daims, H.; Brühl, A.; Amann, R.; Schleifer, K.-H.; Wagner, M. The Domain-Specific Probe EUB338 Is Insufficient for the Detection of All Bacteria: Development and Evaluation of a More Comprehensive Probe Set. Syst. Appl. Microbiol. 1999, 22, 434–444. [Google Scholar] [CrossRef]
- Eisenthal, R.; Cornish-Bowden, A. The Direct Linear Plot. A New Graphical Procedure for Estimating Enzyme Kinetic Parameters. Biochem. J. 1974, 139, 715–720. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; Van Loosdrecht, M.C.M. Activated Sludge Models ASM, ASM2, ASM2d and ASM3; IWA Scientific and Technical Report 9; IWA Publishing: London, UK, 2000. [Google Scholar]
- Jiménez, E.; Giménez, J.B.; Seco, A.; Ferrer, J.; Serralta, J. Effect of PH, Substrate and Free Nitrous Acid Concentrations on Ammonium Oxidation Rate. Bioresour. Technol. 2012, 124, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Puyol, D.; Carvajal-Arroyo, J.M.; Sierra-Alvarez, R.; Field, J.A. Nitrite (Not Free Nitrous Acid) Is the Main Inhibitor of the Anammox Process at Common PH Conditions. Biotechnol Lett. 2014, 36, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Levenspiel, O. The Monod Equation: A Revisit and a Generalization to Product Inhibition Situations. Biotechnol. Bioeng. 1980, 22, 1671–1687. [Google Scholar] [CrossRef]
- Andrews, J.F. A Mathematical Model for the Continuous Culture of Microorganisms Utilizing Inhibitory Substrates. Biotechnol. Bioeng. 1968, 10, 707–723. [Google Scholar] [CrossRef]
- Prinz, H. Hill Coefficients, Dose–Response Curves and Allosteric Mechanisms. J. Chem. Biol. 2010, 3, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Pijuan, M.; Yuan, Z. Free Nitrous Acid Inhibition on Anoxic Phosphorus Uptake and Denitrification by Poly-Phosphate Accumulating Organisms. Biotechnol. Bioeng. 2007, 98, 903–912. [Google Scholar] [CrossRef]
- Moriasi, D.N.; Arnold, J.G.; van Liew, M.W.; Bingner, R.L.; Harmel, R.D.; Veith, L.T. Model Evaluation Guidelines for Systematic Quantification of Accuracy in Watershed Simulations. Trans. ASABE 2007, 50, 885–900. [Google Scholar] [CrossRef]
- Dai, Y.; Yuan, Z.; Wang, X.; Oehmen, A.; Keller, J. Anaerobic Metabolism of Defluviicoccus Vanus Related Glycogen Accumulating Organisms (GAOs) with Acetate and Propionate as Carbon Sources. Water Res. 2007, 41, 1885–1896. [Google Scholar] [CrossRef]
- Oehmen, A.; Carvalho, G.; Lopez-Vazquez, C.M.; van Loosdrecht, M.C.M.; Reis, M.A.M. Incorporating Microbial Ecology into the Metabolic Modelling of Polyphosphate Accumulating Organisms and Glycogen Accumulating Organisms. Water Res. 2010, 44, 4992–5004. [Google Scholar] [CrossRef]
- Shen, N.; Zhou, Y. Enhanced Biological Phosphorus Removal with Different Carbon Sources. Appl. Microbiol. Biotechnol. 2016, 100, 4735–4745. [Google Scholar] [CrossRef]
- Ye, L.; Pijuan, M.; Yuan, Z. The Effect of Free Nitrous Acid on the Anabolic and Catabolic Processes of Glycogen Accumulating Organisms. Water Res. 2010, 44, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Tayà, C.; Guisasola, A.; Baeza, J.A. Glycerol as a Sole Carbon Source for Enhanced Biological Phosphorus Removal. Water Res. 2012, 46, 2983–2991. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreadakis, D.; Noutsopoulos, C.; Mamais, D.; Charalambous, V.; Koukoura, A.; Malamis, S. The Inhibitory Effect of Free Nitrous Acid and Free Ammonia on the Anoxic Phosphorus Uptake Rate of Polyphosphate-Accumulating Organisms. Energies 2022, 15, 2108. https://doi.org/10.3390/en15062108
Andreadakis D, Noutsopoulos C, Mamais D, Charalambous V, Koukoura A, Malamis S. The Inhibitory Effect of Free Nitrous Acid and Free Ammonia on the Anoxic Phosphorus Uptake Rate of Polyphosphate-Accumulating Organisms. Energies. 2022; 15(6):2108. https://doi.org/10.3390/en15062108
Chicago/Turabian StyleAndreadakis, Dimitris, Constantinos Noutsopoulos, Daniel Mamais, Vera Charalambous, Asimina Koukoura, and Simos Malamis. 2022. "The Inhibitory Effect of Free Nitrous Acid and Free Ammonia on the Anoxic Phosphorus Uptake Rate of Polyphosphate-Accumulating Organisms" Energies 15, no. 6: 2108. https://doi.org/10.3390/en15062108
APA StyleAndreadakis, D., Noutsopoulos, C., Mamais, D., Charalambous, V., Koukoura, A., & Malamis, S. (2022). The Inhibitory Effect of Free Nitrous Acid and Free Ammonia on the Anoxic Phosphorus Uptake Rate of Polyphosphate-Accumulating Organisms. Energies, 15(6), 2108. https://doi.org/10.3390/en15062108










