Metagenomic Analysis of the Long-Term Synergistic Effects of Antibiotics on the Anaerobic Digestion of Cattle Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Sampling
2.2. Genomic DNA Isolation
2.3. DNA Sequencing
2.4. Statistical Analysis
3. Results and Discussion
3.1. Long-Term Synergistic Effects of AMO, ENRO and MET on CH4 Production and VFA Concentrations
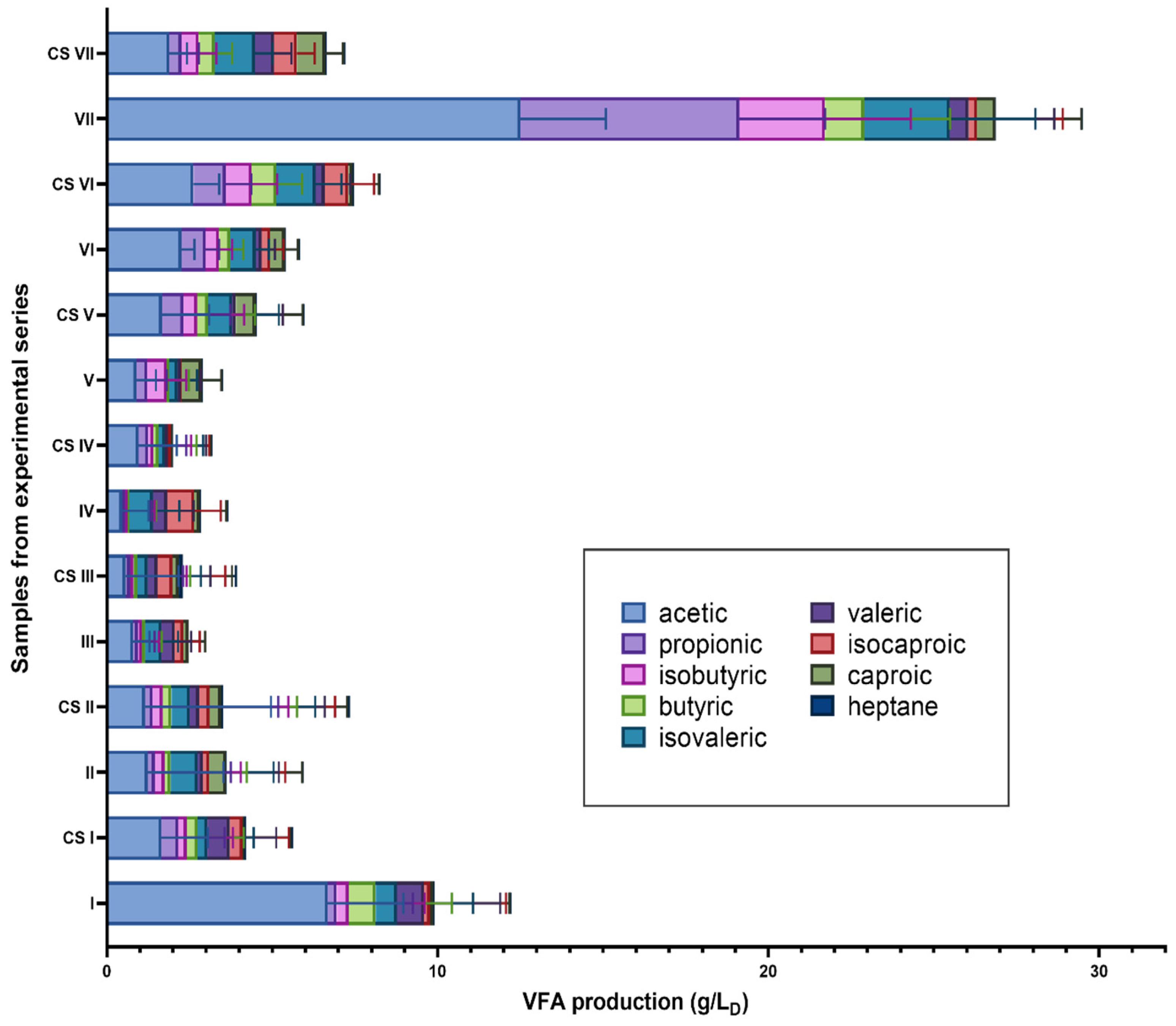
3.2. Concentrations of Volatile Fatty Acids
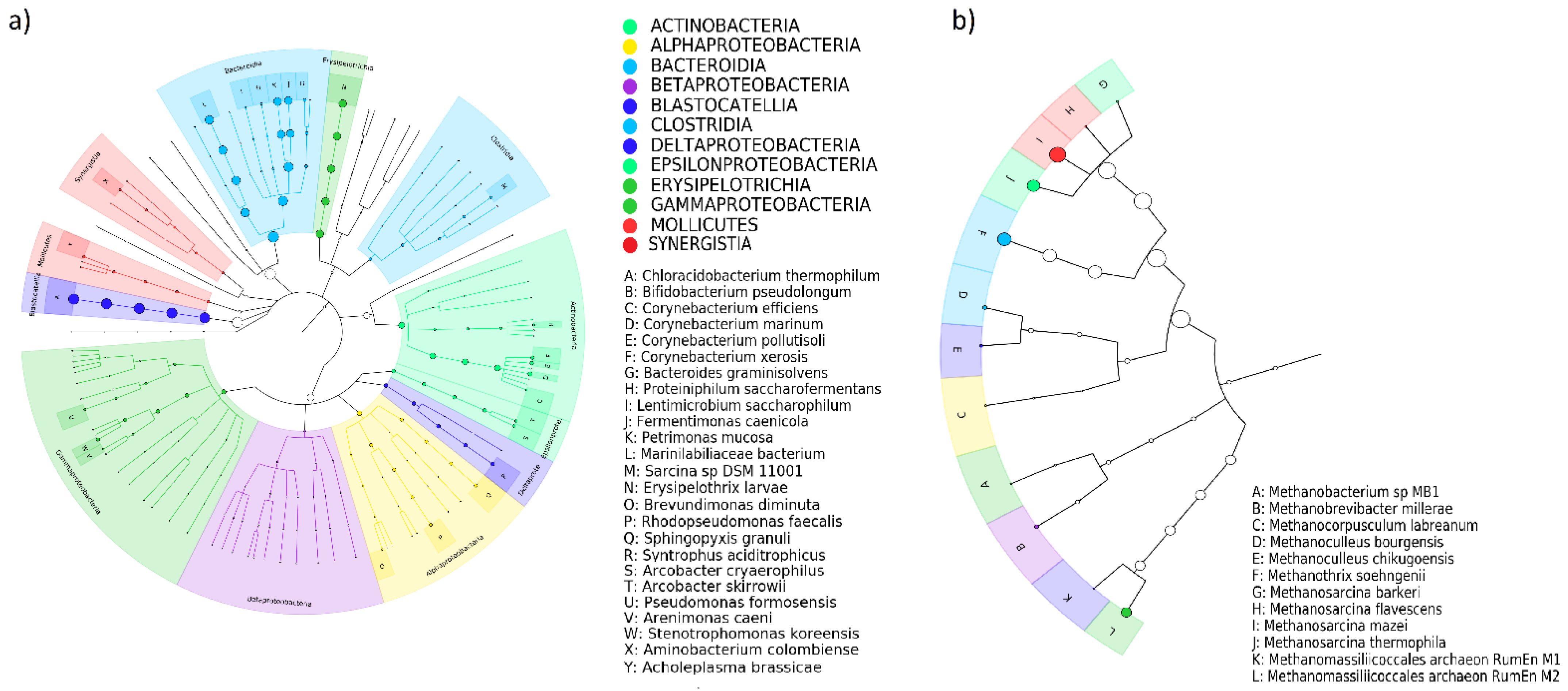
3.3. Long-Term Synergistic Effects of AMO, ENR and MET on the Microbial Biodiversity of Digestates
3.3.1. Bacteria
3.3.2. Archaea
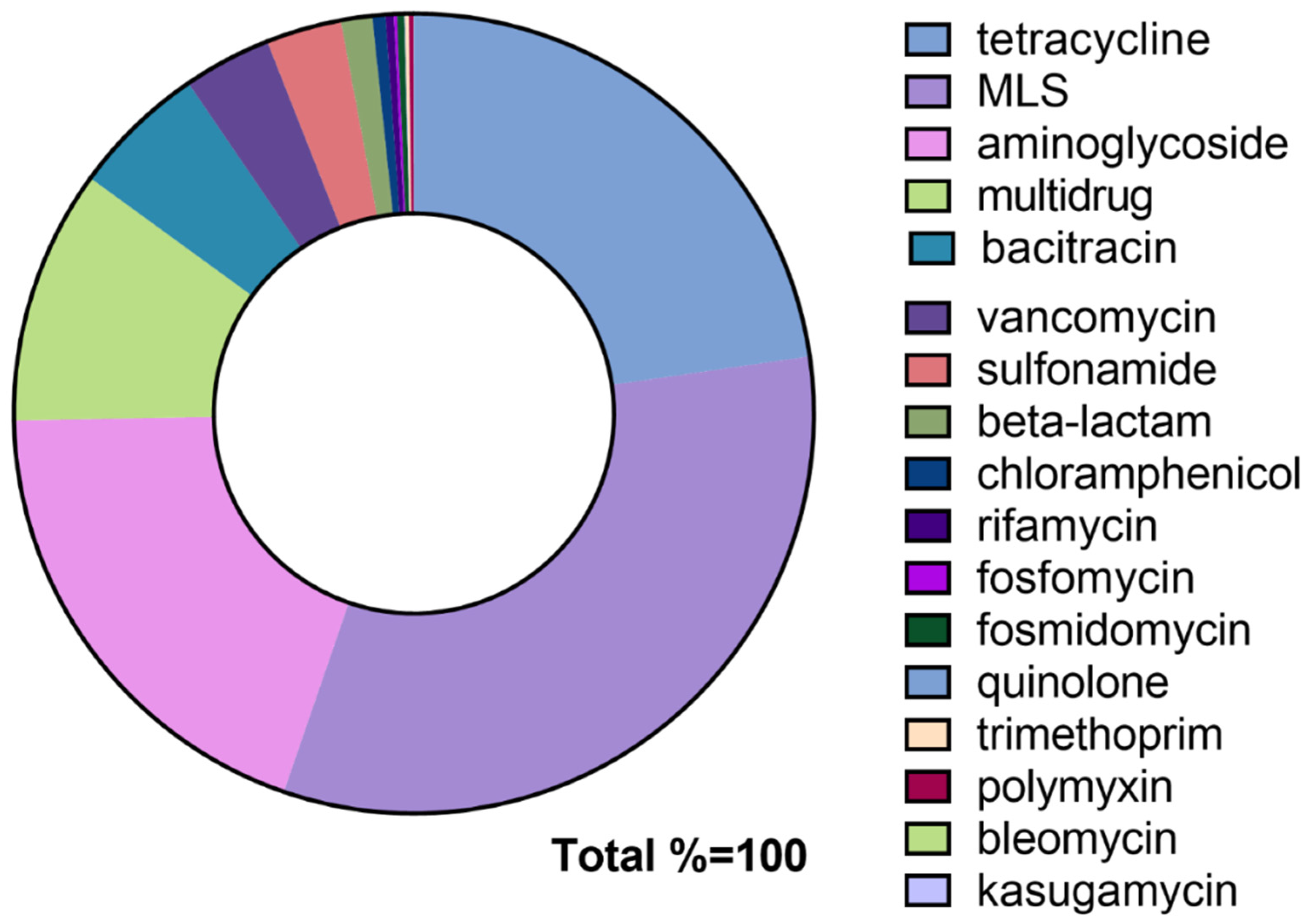
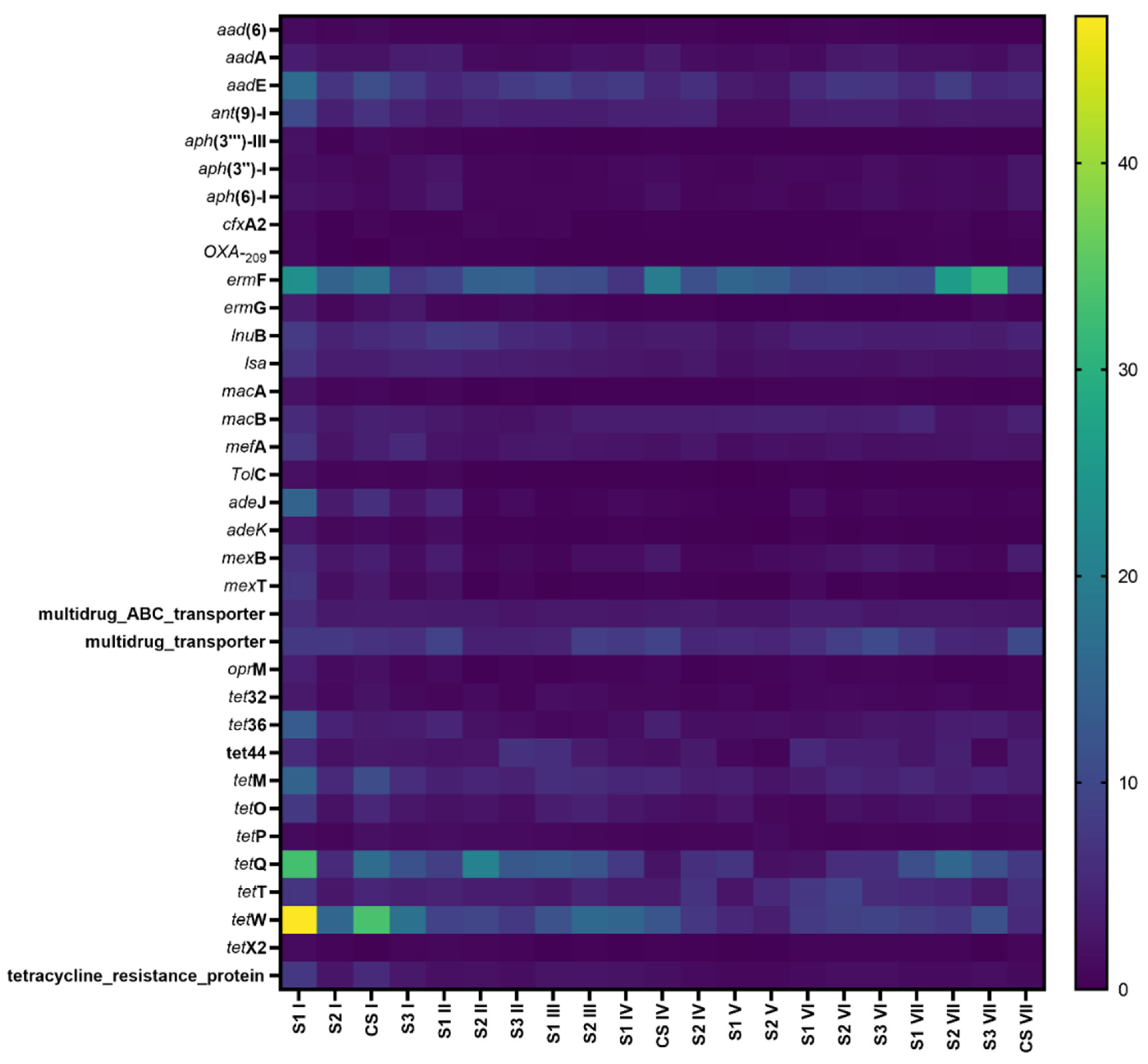
3.4. The Effects of Antibiotics on the Total Abundance of ARGs and Resistant Types
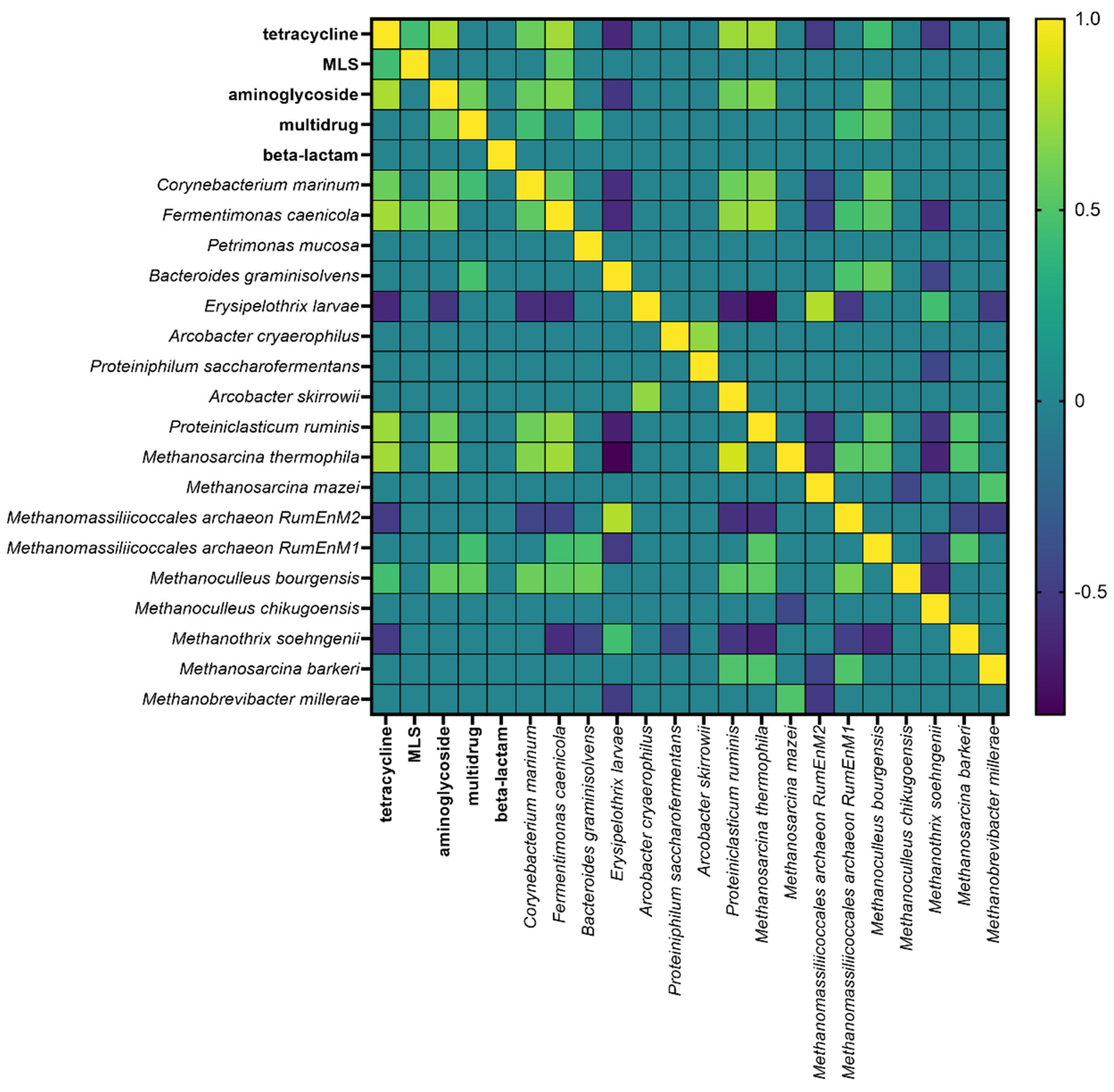
3.5. Results of Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reyes-Contreras, C.; Vidal, G. Methanogenic toxicity evaluation of chlortetracycline hydrochloride. Electron. J. Biotechnol. 2015, 18, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Moulod, M.; Jalali, A.; Asmatulu, R. Biogas derived from municipal solid waste to generate electrical power through solid oxide fuel cells. Int. J. Energy Res. 2016, 45, 2091–2104. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Advances in the pretreatment of brown macroalgae for biogas production. Fuel Process. Technol. 2019, 195, 106151. [Google Scholar] [CrossRef]
- Wu, N.; Qiao, M.; Zhang, B.; Cheng, W.D.; Zhu, Y.G. Abundance and diversity of tetracycline resistance genes in soils adjacent to representative swine feedlots in China. Environ. Sci. Technol. 2010, 44, 6933–6939. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed (Text with EEA relevance). Available online: https://eur-lex.europa.eu/eli/reg/2003/1829/oj (accessed on 3 March 2022).
- Aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment-Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef] [PubMed]
- KIES, F.K.; Boutchebak, S.; Bendaida, N. Soil Contamination by Pharmaceutical Pollutants: Adsorption of an Antibiotic (Amoxicillin) on an Agricultural Land. Proceedings 2020, 30, 60. [Google Scholar] [CrossRef]
- Álvarez-Esmorís, C.; Conde-Cid, M.; Ferreira-Coelho, G.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Adsorption/desorption of sulfamethoxypyridazine and enrofloxacin in agricultural soils. Sci. Total Environ. 2020, 706, 136015. [Google Scholar] [CrossRef]
- Pan, Y.; Yi, J.; Zhou, B.; Xie, S.; Chen, D.; Tao, Y.; Qu, W.; Liu, Z.; Huang, L.; Yuan, Z. Disposition and Residue Depletion of Metronidazole in Pigs and Broilers. Sci. Rep. 2017, 7, 7203. [Google Scholar] [CrossRef] [Green Version]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Koniuszewska, I. Inhibitors of the methane fermentation process with particular emphasis on the microbiological aspect: A review. Energy Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.J.; M-Ridha, M.J.; Abed, K.M.; Elgharbawy, A.A.M. Removal of levofloxacin and ciprofloxacin from aqueous solutions and an economic evaluation using the electrocoagulation process. Int. J. Environ. Anal. Chem. 2021, 1–19. [Google Scholar] [CrossRef]
- Eitel, Z.; Sóki, J.; Urbán, E.; Nagy, E. The prevalence of antibiotic resistance genes in Bacteroides fragilis group strains isolated in different European countries. Anaerobe 2013, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ingerslev, F.; Halling-Sørensen, B. Biodegradability of metronidazole, olaquindox, and tylosin and formation of tylosin degradation products in aerobic soil-manure slurries. Ecotoxicol. Environ. Saf. 2001, 48, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Buta, M.; Korzeniewska, E.; Harnisz, M.; Hubeny, J.; Zieliński, W.; Rolbiecki, D.; Bajkacz, S.; Felis, E.; Kokoszka, K. Microbial and chemical pollutants on the manure-crops pathway in the perspective of “One Health” holistic approach. Sci. Total Environ. 2021, 785, 147411. [Google Scholar] [CrossRef]
- Korzeniewska, E.; Harnisz, M. Sources, Occurrence, and Environmental Risk Assessment of Antibiotics and Antimicrobial-Resistant Bacteria in Aquatic Environments of Poland. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; Volume 87, pp. 179–193. [Google Scholar]
- Amador, P.P.; Fernandes, R.M.; Prudêncio, M.C.; Barreto, M.P.; Duarte, I.M. Antibiotic resistance in wastewater: Occurrence and fate of Enterobacteriaceae producers of Class A and Class C β-lactamases. J. Environ. Sci. Health–Part A Toxic/Hazardous Subst. Environ. Eng. 2015, 50, 26–39. [Google Scholar] [CrossRef]
- Flores-Orozco, D.; Patidar, R.; Levin, D.B.; Sparling, R.; Kumar, A.; Çiçek, N. Effect of ceftiofur on mesophilic anaerobic digestion of dairy manure and the reduction of the cephalosporin-resistance gene cmy-2. Bioresour. Technol. 2020, 301, 122729. [Google Scholar] [CrossRef]
- Gurmessa, B.; Pedretti, E.F.; Cocco, S.; Cardelli, V.; Corti, G. Manure anaerobic digestion effects and the role of pre- and post-treatments on veterinary antibiotics and antibiotic resistance genes removal efficiency. Sci. Total Environ. 2020, 721, 137532. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, J.; Tian, S.; Liu, C.; Liu, L.; Wei, L.; Fan, H.; Zhang, T.; Wang, L.; Zhu, G.; et al. Higher temperatures do not always achieve better antibiotic resistance gene removal in anaerobic digestion of swine manure. Appl. Environ. Microbiol. 2019, 85, e02878-18. [Google Scholar] [CrossRef] [Green Version]
- Larrañaga, O.; Brown-Jaque, M.; Quirós, P.; Gómez-Gómez, C.; Blanch, A.R.; Rodríguez-Rubio, L.; Muniesa, M. Phage particles harboring antibiotic resistance genes in fresh-cut vegetables and agricultural soil. Environ. Int. 2018, 115, 133–141. [Google Scholar] [CrossRef]
- Bai, Y.; Ruan, X.; Xie, X.; Yan, Z. Antibiotic resistome profile based on metagenomics in raw surface drinking water source and the influence of environmental factor: A case study in Huaihe River Basin, China. Environ. Pollut. 2019, 248, 438–447. [Google Scholar] [CrossRef]
- Koniuszewska, I.; Harnisz, M.; Korzeniewska, E.; Czatzkowska, M.; Jastrzębski, J.P.; Paukszto, Ł.; Bajkacz, S.; Felis, E.; Rusanowska, P. The Effect of Antibiotics on Mesophilic Anaerobic Digestion Process of Cattle Manure. Energies 2021, 14, 1125. [Google Scholar] [CrossRef]
- Ozbayram, E.G.; Arikan, O.; Ince, B.; Cetecioglu, Z.; Aydin, S.; Ince, O. Acute effects of various antibiotic combinations on acetoclastic methanogenic activity. Environ. Sci. Pollut. Res. 2015, 22, 6230–6235. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Cetecioglu, Z.; Arikan, O.; Ince, B.; Ozbayram, E.G.; Ince, O. Inhibitory effects of antibiotic combinations on syntrophic bacteria, homoacetogens and methanogens. Chemosphere 2015, 120, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ince, B.; Ince, O. Application of real-time PCR to determination of combined effect of antibiotics on Bacteria, Methanogenic Archaea, Archaea in anaerobic sequencing batch reactors. Water Res. 2015, 76, 88–98. [Google Scholar] [CrossRef]
- Aydin, S.; Ince, B.; Cetecioglu, Z.; Ozbayram, E.G.; Shahi, A.; Okay, O.; Arikan, O.; Ince, O. Performance of anaerobic sequencing batch reactor in the treatment of pharmaceutical wastewater containing erythromycin and sulfamethoxazole mixture. Water Sci. Technol. 2014, 70, 1625–1632. [Google Scholar] [CrossRef]
- Rusanowska, P.; Harnisz, M.; Zieliński, M.; Dębowski, M.; Korzeniewska, E.; Kisielewska, M.; Amenda, E. Individual and Synergistic Effects of Metronidazole, Amoxicillin, and Ciprofloxacin on Methane Fermentation with Sewage Sludge. Clean—Soil Air Water 2020, 48, 1900281. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M. Improvement of biohydrogen production using a reduced pressure fermentation. Bioprocess Biosyst. Eng. 2015, 38, 1925–1933. [Google Scholar] [CrossRef]
- Federation, W.E. APHA, AWWA, WEF. “Standard Methods for examination of water and wastewater”. An. Hidrol. Médica 2012, 5, 185–186. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Waldron, L.; Ballarini, A.; Narasimhan, V.; Jousson, O.; Huttenhower, C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods 2012, 9, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Jiang, X.T.; Chai, B.; Li, L.; Yang, Y.; Cole, J.R.; Tiedje, J.M.; Zhang, T. ARGs-OAP v2.0 with an expanded SARG database and Hidden Markov Models for enhancement characterization and quantification of antibiotic resistance genes in environmental metagenomes. Bioinformatics 2018, 34, 2263–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, L.; Yang, Y.; Littier, H.; Ray, P.; Zhang, T.; Pruden, A.; Strickland, M.; Knowlton, K. Metagenomic analysis of antibiotic resistance genes in dairy cow feces following therapeutic administration of third generation cephalosporin. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Wang, Y.; Lichtfouse, E.; Li, Z.; Kumar, P.S.; Liu, J.; Feng, D.; Yang, Q.; Liu, F. Effect of Antibiotics on the Microbial Efficiency of Anaerobic Digestion of Wastewater: A Review. Front. Microbiol. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Sun, C.; Li, W.; Chen, Z.; Qin, W.; Wen, X. Responses of antibiotics, antibiotic resistance genes, and mobile genetic elements in sewage sludge to thermal hydrolysis pre-treatment and various anaerobic digestion conditions. Environ. Int. 2019, 133, 105156. [Google Scholar] [CrossRef]
- Koniuszewska, I.; Korzeniewska, E.; Harnisz, M.; Czatzkowska, M. Intensification of biogas production using various technologies: A review. Int. J. Energy Res. 2020, 44, 6240–6258. [Google Scholar] [CrossRef]
- Schulz, F.; Wagner, K.; Brinkmann, J.; March, S.; Hinterstoißer, P.; Schüler, M.; Warnecke, S.; Paulsen, H.M. Welfare of dairy cattle in summer and winter—A comparison of organic and conventional herds in a farm network in Germany. Landbauforschung 2020, 70, 83–96. [Google Scholar] [CrossRef]
- Tufan, T.; Arslan, C. Beef Cattle Feeding Principles of Kars Province Enterprises During the Winter Period. Bahri Dağdaş Hayvancılık Araştırma Derg. 2021, 10, 1–9. [Google Scholar]
- Pan, X.; Qiang, Z.; Ben, W.; Chen, M. Residual veterinary antibiotics in swine manure from concentrated animal feeding operations in Shandong Province, China. Chemosphere 2011, 84, 695–700. [Google Scholar] [CrossRef]
- Nascimento, A.L.; Souza, A.J.; Andrade, P.A.M.; Andreote, F.D.; Coscione, A.R.; Oliveira, F.C.; Regitano, J.B. Sewage sludge microbial structures and relations to their sources, treatments, and chemical attributes. Front. Microbiol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J.L.; Van Eerde, A. Pollution by antibiotics and antimicrobial resistance in live stock and poultry manure in china, and countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, X.; Qin, J.; Lu, N.; Cheng, G.; Wu, N.; Pan, Y.; Li, J.; Zhu, L.; Wang, X.; et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat. Commun. 2013, 4, 2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hu, Y.; Cao, J.; Bi, Y.; Lv, N.; Liu, F.; Liang, S.; Shi, Y.; Jiao, X.; Gao, G.F.; et al. Antibiotic resistance gene reservoir in live poultry markets. J. Infect. 2019, 78, 445–453. [Google Scholar] [CrossRef]
- Smillie, C.S.; Smith, M.B.; Friedman, J.; Cordero, O.X.; David, L.A.; Alm, E.J. Ecology drives a global network of gene exchange connecting the human microbiome. Nature 2011, 480, 241–244. [Google Scholar] [CrossRef]
- Koniuszewska, I.; Czatzkowska, M.; Harnisz, M.; Korzeniewska, E. The impact of antimicrobial substances on the methanogenic community during methane fermentation of sewage sludge and cattle slurry. Appl. Sci. 2021, 11, 369. [Google Scholar] [CrossRef]
- Cheng, D.; Feng, Y.; Liu, Y.; Xue, J.; Li, Z. Dynamics of oxytetracycline, sulfamerazine, and cipro fl oxacin and related antibiotic resistance genes during swine manure composting. J. Environ. Manage. 2019, 230, 102–109. [Google Scholar] [CrossRef]
- Nurk, L.; Knörzer, S.; Jacobi, H.F.; Spielmeyer, A. Elimination of sulfonamides and tetracyclines during anaerobic fermentation—A “ Cheshire Cat ” phenomenon. Sustain. Chem. Pharm. 2019, 13, 100157. [Google Scholar] [CrossRef]
- Wen, Q.; Yang, S.; Chen, Z. Mesophilic and thermophilic anaerobic digestion of swine manure with sulfamethoxazole and norfloxacin: Dynamics of microbial communities and evolution of resistance genes. Front. Environ. Sci. Eng. 2021, 15, 94. [Google Scholar] [CrossRef]
- Yin, F.; Dong, H.; Ji, C.; Tao, X.; Chen, Y. Effects of anaerobic digestion on chlortetracycline and oxytetracycline degradation efficiency for swine manure. Waste Manag. 2016, 56, 540–546. [Google Scholar] [CrossRef]
- García-Ruíz, M.J.; Castellano-Hinojosa, A.; Armato, C.; González-Martínez, A.; González-López, J.; Osorio, F. Biogas production and microbial community structure in a stable-stage of a two-stage anaerobic digester. AIChE J. 2020, 66, e16807. [Google Scholar] [CrossRef]
- Wang, S.; Du, K.; Yuan, R.; Chen, H.; Wang, F.; Zhou, B. Effects of sulfonamide antibiotics on digestion performance and microbial community during swine manure anaerobic digestion. Environ. Eng. Res. 2020, 26, 190471. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Jo, J.H.; Park, D.; Lee, D.S.; Park, J.M. Comprehensive study on a two-stage anaerobic digestion process for the sequential production of hydrogen and methane from cost-effective molasses. Renew. Energy 2010, 35, 6194–6202. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J.; Frear, C. Bioresource Technology The effects of the antibiotics ampicillin, florfenicol, sulfamethazine, and tylosin on biogas production and their degradation efficiency during anaerobic digestion. Bioresour. Technol. 2013, 149, 244–252. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, H.; Wyckoff, K.N.; He, Q. Linkages of Firmicutes and Bacteroidetes populations to methanogenic process performance. J. Ind. Microbiol. Biotechnol. 2016, 43, 771–781. [Google Scholar] [CrossRef]
- Xin, L.; Guo, Z.; Xiao, X.; Peng, C.; Zeng, P.; Feng, W.; Xu, W. Feasibility of anaerobic digestion on the release of biogas and heavy metals from rice straw pretreated with sodium hydroxide. Environ. Sci. Pollut. Res. 2019, 26, 19434–19444. [Google Scholar] [CrossRef]
- She, Y.; Hong, J.; Zhang, Q.; Chen, B.Y.; Wei, W.; Xin, X. Revealing microbial mechanism associated with volatile fatty acids production in anaerobic acidogenesis of waste activated sludge enhanced by freezing/thawing pretreatment. Bioresour. Technol. 2020, 302, 122869. [Google Scholar] [CrossRef]
- Krakat, N.; Schmidt, S.; Scherer, P. Potential impact of process parameters upon the bacterial diversity in the mesophilic anaerobic digestion of beet silage. Bioresour. Technol. 2011, 102, 5692–5701. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, J.; Wang, X.; Zhang, K.; Yin, Y.; Zhang, R.; Zhang, S. Effects of tylosin, ciprofloxacin, and sulfadimidine on mcrA gene abundance and the methanogen community during anaerobic digestion of cattle manure. Chemosphere 2019, 221, 81–88. [Google Scholar] [CrossRef]
- Niestępski, S.; Harnisz, M.; Ciesielski, S.; Korzeniewska, E.; Osińska, A. Environmental fate of Bacteroidetes, with particular emphasis on Bacteroides fragilis group bacteria and their specific antibiotic resistance genes, in activated sludge wastewater treatment plants. J. Hazard. Mater. 2020, 394, 122544. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Hu, H.W.; Zhang, Y.J.; Wang, J.T.; Hayden, H.; Tang, Y.Q.; He, J.Z. Aerobic composting reduces antibiotic resistance genes in cattle manure and the resistome dissemination in agricultural soils. Sci. Total Environ. 2018, 612, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gu, J.; Wang, X.; Li, Y.; Liu, J.; Lu, C.; Qiu, L. Response of antibiotic resistance genes abundance by graphene oxide during the anaerobic digestion of swine manure with copper pollution. Sci. Total Environ. 2019, 654, 292–299. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, M.; Ma, X.; Gao, Q.; Wang, T.; Shi, X. High variations of methanogenic microorganisms drive full-scale anaerobic digestion process. Environ. Int. 2019, 126, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, W.; Lee, C. Absolute dominance of hydrogenotrophic methanogens in full-scale anaerobic sewage sludge digesters. J. Environ. Sci. 2013, 25, 2272–2280. [Google Scholar] [CrossRef]
- Huerta, B.; Marti, E.; Gros, M.; López, P.; Pompêo, M.; Armengol, J.; Barceló, D.; Balcázar, J.L.; Rodríguez-Mozaz, S.; Marcé, R. Exploring the links between antibiotic occurrence, antibiotic resistance, and bacterial communities in water supply reservoirs. Sci. Total Environ. 2013, 456–457, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, Y.; Li, J.; Zhang, H.; Shi, R.; Chen, J.; Li, H. Distribution pattern of antibiotic resistance genes and bacterial community in agricultural soil samples of Wuliangsuhai watershed. China. Agric. Ecosyst. Environ. 2020, 295, 106884. [Google Scholar] [CrossRef]
- Gebreyes, W.A.; Thakur, S.; Morrow, W.E.M. Comparison of prevalence, antimicrobial resistance, and occurrence of multidrug-resistant Salmonella in antimicrobial-free and conventional pig production. J. Food Prot. 2006, 69, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Marano, R.B.M.; Gupta, C.L.; Cozer, T.; Jurkevitch, E.; Cytryn, E. Hidden Resistome: Enrichment Reveals the Presence of Clinically Relevant Antibiotic Resistance Determinants in Treated Wastewater-Irrigated Soils. Environ. Sci. Technol. 2021, 55, 6814–6827. [Google Scholar] [CrossRef]
- Zhou, Z.; Yao, H. Effects of composting different types of organic fertilizer on the microbial community structure and antibiotic resistance genes. Microorganisms 2020, 8, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerqueira, F.; Matamoros, V.; Bayona, J.M.; Berendonk, T.U.; Elsinga, G.; Hornstra, L.M.; Piña, B. Antibiotic resistance gene distribution in agricultural fields and crops. A soil-to-food analysis. Environ. Res. 2019, 177, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wu, N.; Tian, J.; Liu, X.; Wu, S.; Mo, Q.; Lu, S. Review on the removal of antibiotic resistance genes from livestock manure by composting. IOP Conf. Ser. Earth Environ. Sci. 2019, 237, 052010. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, M.; Wang, W.; Zhang, L.; Ma, T.; Liu, G.; Zhang, Y.; Shang, Z.; Chen, X.; Shi, Y.; et al. Exploring the Prevalence and Distribution Patterns of Antibiotic Resistance Genes in Bovine Gut Microbiota Using a Metagenomic Approach. Microb. Drug Resist. 2020, 00, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Lins, P.; Reitschuler, C.; Illmer, P. Methanosarcina spp., the key to relieve the start-up of a thermophilic anaerobic digestion suffering from high acetic acid loads. Bioresour. Technol. 2014, 152, 347–354. [Google Scholar] [CrossRef]
- Smith, K.S.; Ingram-Smith, C. Methanosaeta, the forgotten methanogen? Trends Microbiol. 2007, 15, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, Z.; Michel, F.C.; Wittum, T.; Morrison, M. Development and application of real-time PCR assays for quantification of erm genes conferring resistance to macrolides-lincosamides-streptogramin B in livestock manure and manure management systems. Appl. Environ. Microbiol. 2007, 73, 4407–4416. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Fluharty, F.L.; Morrison, M.; Yu, Z. Technical note: Occurrence in fecal microbiota of genes conferring resistance to both macrolide-lincosamide-streptogramin B and tetracyclines concomitant with feeding of beef cattle with tylosin 1. J. Anim. Sci. 2004, 86, 2385–2391. [Google Scholar] [CrossRef]
- Sóki, J. Extended role for insertion sequence elements in the antibiotic resistance of Bacteroides. World J. Clin. Infect. Dis. 2013, 3, 1–12. [Google Scholar] [CrossRef]
- Whittle, G.; Hund, B.D.; Shoemaker, N.B.; Salyers, A.A. Characterization of the 13-Kilobase ermF Region of the Bacteroides Conjugative Transposon CTnDOT. Appl. Environ. Microbiol. 2001, 67, 3488–3495. [Google Scholar] [CrossRef] [Green Version]
- Zhanel, G.G.; Cheung, D.; Adam, H.; Zelenitsky, S.; Golden, A.; Schweizer, F.; Gorityala, B.; Lagacé-Wiens, P.R.S.; Walkty, A.; Gin, A.S.; et al. Review of Eravacycline, a Novel Fluorocycline Antibacterial Agent. Drugs 2016, 76, 567–588. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, J.; López, L.; Soto, C.Y. Detection and diversity evaluation of tetracycline resistance genes in grassland-based production systems in Colombia, South America. Front. Microbiol. 2011, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; An, X.; Li, H.; Su, J.; Ma, Y.; Zhu, Y.G. Long-term field application of sewage sludge increases the abundance of antibiotic resistance genes in soil. Environ. Int. 2016, 92–93, 1–10. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, W.; Xie, S.; Zeng, M.; Liu, H.; Yang, J.; Liu, X.; Yang, F. Increasing prevalence of antibiotic resistance genes in manured agricultural soils in northern China. Front. Environ. Sci. Eng. 2020, 14, 1. [Google Scholar] [CrossRef]
- Khurshid, M.; Rasool, M.H.; Ashfaq, U.A.; Aslam, B.; Waseem, M.; Ali, M.A.; Almatroudi, A.; Rasheed, F.; Saeed, M.; Guo, Q.; et al. Acinetobacter baumannii sequence types harboring genes encoding aminoglycoside modifying enzymes and 16srRNA methylase; a multicenter study from Pakistan. Infect. Drug Resist. 2020, 13, 2855–2862. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010, 13, 151–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.K.; Shin, H.; Han, D.; Hur, H.G.; Unno, T. Metagenomic analysis reveals the prevalence and persistence of antibiotic- and heavy metal-resistance genes in wastewater treatment plant. J. Microbiol. 2018, 56, 408–415. [Google Scholar] [CrossRef]
- He, P.; Wu, Y.; Huang, W.; Wu, X.; Lv, J.; Liu, P.; Bu, L.; Bai, Z.; Chen, S.; Feng, W.; et al. Characteristics of and variation in airborne ARGs among urban hospitals and adjacent urban and suburban communities: A metagenomic approach. Environ. Int. 2020, 139, 105625. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, Y.; Qi, Z.; Yue, Y.; Min, M.; Peng, S.; Shi, Z.; Gao, Y. Diverse and abundant antibiotic resistance genes from mariculture sites of China’s coastline. Sci. Total Environ. 2018, 630, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hirte, K.; Seiwert, B.; Schüürmann, G.; Reemtsma, T. New hydrolysis products of the beta-lactam antibiotic amoxicillin, their pH-dependent formation and search in municipal wastewater. Water Res. 2016, 88, 880–888. [Google Scholar] [CrossRef]
- Wallace, J.S.; Aga, D.S. Enhancing extraction and detection of veterinary antibiotics in solid and liquid fractions of manure. J. Environ. Qual. 2016, 45, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gómez, B.; Richter, M.; Schüler, M.; Pinhassi, J.; Acinas, S.G.; González, J.M.; Pedrós-Alió, C. Ecology of marine bacteroidetes: A comparative genomics approach. ISME J. 2013, 7, 1026–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S. A critical review on biochar for enhancing biogas production from anaerobic digestion of food waste and sludge. J. Clean. Prod. 2021, 305, 127143. [Google Scholar] [CrossRef]

| Kind of Substrate | Antibiotics Used in Investigate | Antibiotics Combinations Concentration | Effect of Antibiotics | References |
|---|---|---|---|---|
| Seed sludge | Short-term effect of sulfamethoxazole and tetracycline, erythromycin and sulfamethoxazole, erythromycin and tetracycline | 0, 2, 20, 50, 100, 250, and 500 mg/L | Inhibition of CH4 production in reactors fed with erythromycin-sulfamethoxazole and sulfamethoxazole-tetracycline and weak inhibition of CH4 production in reactors fed with the mixture of erythromycin-tetracycline | [24] |
| Synthetic wastewater | Short-term effect of three antibiotics with four combinations; sulfamethoxazole-tetracycline; erythromycin-sulfamethoxazole; erythromycin-tetracycline and erythromycin-tetracycline-sulfamethoxazole | 0, 1, 10, 25, 50, 100 and 250 mg/L | Inhibition of biogas production; microorganisms’ development of resistance to antibiotics | [25] |
| Synthetic wastewater | Short-term effect of erythromycin and sulfamethoxazole mixture | In 10 stages, respectively (mg/L): 0.1 and 0.5; 0.2 and 5; 0.5 and 5; 0.5 and 10; 1 and 10, 1 and 15; 1.5 and 15; 1.5 and 20; 2 and 20; 2.5 and 25; | Inhibition of biogas production; microorganisms’ development of resistance to antibiotics | [26] |
| Synthetic wastewater | Long-term effect of erythromycin-tetracycline-sulfamethoxazole and sulfamethoxazole-tetracycline | 0.5; 5; 10; 15; 20; 25; 40 mg/L for SMX; 0.1; 0.2; 0.5; 1; 1.5; 2; 2.5; 3 mg/L for erythromycin-tetracycline | Increasing antibiotic concentrations has a negative impact on the microbial community structure and function in anaerobic wastewater treatment; increase of AR | [27] |
| Sewage sludge from a municipal WWTP | Amoxicillin, metronidazole, and ciprofloxacin | Static conditions—In first variant, respectively [mg/kg]; 2, 16 and 1024, in second variant: 1, 8, and 512; and last variant: 0.25, 2, and 512, semi-continuous conditions, respectively: 16, 8, and 2 mg/kg | Synergistic effect of antibiotics causes inhibition of CH4 production and accumulation of VFAs | [28] |
| Experimental Series | Antibiotic Concentrations (µg/mL D) | Collected Samples | Sample ID | ||
|---|---|---|---|---|---|
| AMO | ENRO | MET | |||
| Series I | 1 | 0.25 | 0.25 | Sample 1 | S1 I |
| Sample 2 | S2 I | ||||
| Sample 3 | S3 I | ||||
| Control sample | CS I | ||||
| Series II | 2 | 0.5 | 0.5 | Sample 1 | S1 II |
| Sample 2 | S2 II | ||||
| Sample 3 | S3 II | ||||
| Series III | 2.5 | 0.75 | 0.75 | Sample 1 | S1 III |
| Sample 2 | S2 III | ||||
| Series IV | 5 | 1.5 | 1.5 | Sample 1 | S1 IV |
| Sample 2 | S2 IV | ||||
| Control sample | CS IV | ||||
| Series V | 10 | 3 | 3 | Sample 1 | S1 V |
| Sample 2 | S2 V | ||||
| Series VI | 16 | 4 | 4 | Sample 1 | S1 VI |
| Sample 2 | S2 VI | ||||
| Sample 3 | S3 VI | ||||
| Series VII | 32 | 8 | 8 | Sample 1 | S1 VII |
| Sample 2 | S2 VII | ||||
| Sample 3 | S3 VII | ||||
| Control sample | CS VII | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolak, I.; Czatzkowska, M.; Harnisz, M.; Jastrzębski, J.P.; Paukszto, Ł.; Rusanowska, P.; Felis, E.; Korzeniewska, E. Metagenomic Analysis of the Long-Term Synergistic Effects of Antibiotics on the Anaerobic Digestion of Cattle Manure. Energies 2022, 15, 1920. https://doi.org/10.3390/en15051920
Wolak I, Czatzkowska M, Harnisz M, Jastrzębski JP, Paukszto Ł, Rusanowska P, Felis E, Korzeniewska E. Metagenomic Analysis of the Long-Term Synergistic Effects of Antibiotics on the Anaerobic Digestion of Cattle Manure. Energies. 2022; 15(5):1920. https://doi.org/10.3390/en15051920
Chicago/Turabian StyleWolak, Izabela, Małgorzata Czatzkowska, Monika Harnisz, Jan Paweł Jastrzębski, Łukasz Paukszto, Paulina Rusanowska, Ewa Felis, and Ewa Korzeniewska. 2022. "Metagenomic Analysis of the Long-Term Synergistic Effects of Antibiotics on the Anaerobic Digestion of Cattle Manure" Energies 15, no. 5: 1920. https://doi.org/10.3390/en15051920
APA StyleWolak, I., Czatzkowska, M., Harnisz, M., Jastrzębski, J. P., Paukszto, Ł., Rusanowska, P., Felis, E., & Korzeniewska, E. (2022). Metagenomic Analysis of the Long-Term Synergistic Effects of Antibiotics on the Anaerobic Digestion of Cattle Manure. Energies, 15(5), 1920. https://doi.org/10.3390/en15051920








