Plastic Waste Precursor-Derived Fluorescent Carbon and Construction of Ternary FCs@CuO@TiO2 Hybrid Photocatalyst for Hydrogen Production and Sensing Application
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of FCs and FCs@CuO@TiO2 Composite
2.3. Characterization Techniques
2.4. Fluorescence Sensing of Cu2+ Ions
2.5. Photocatalytic and Photo Electrochemical System
3. Results and Discussion
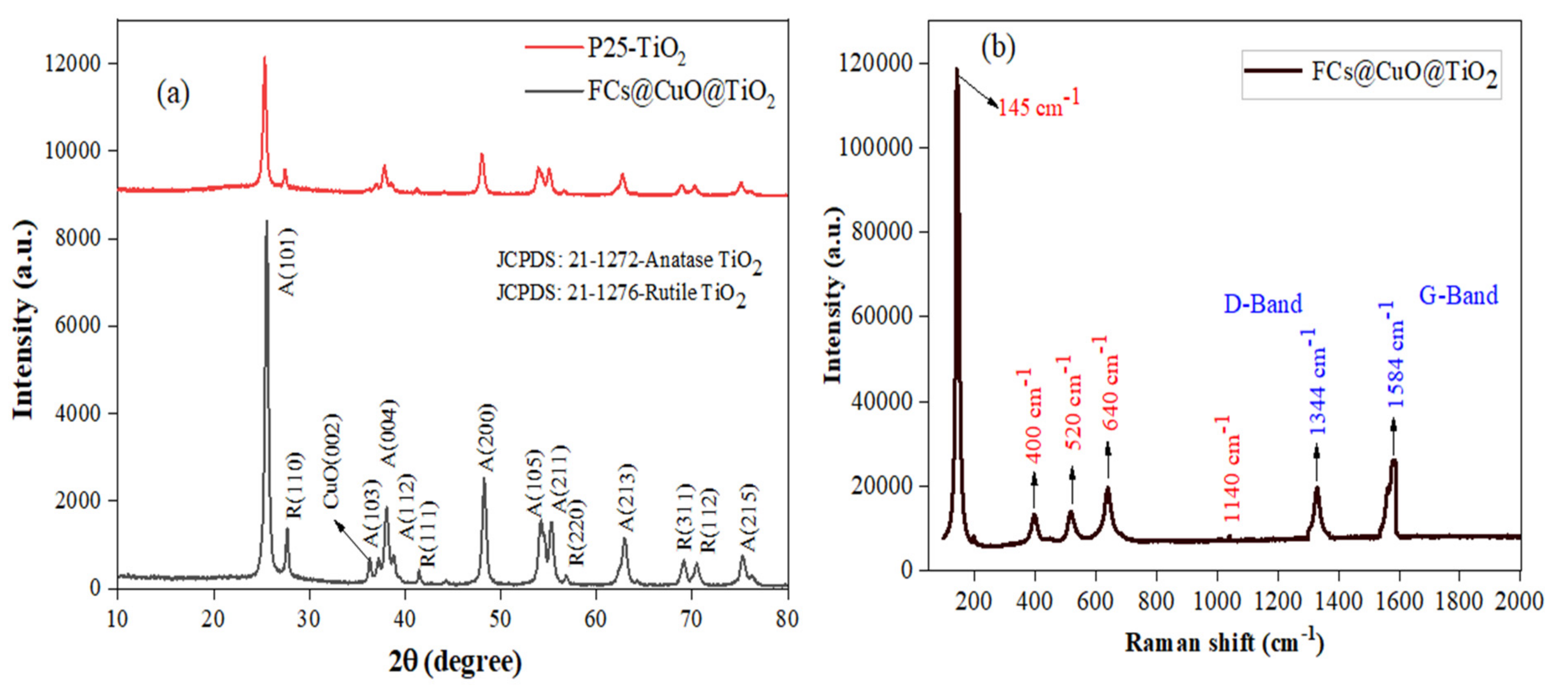
3.1. Characterization of FCs
3.2. Sensing Application of FCs towards Cu2+ Ions
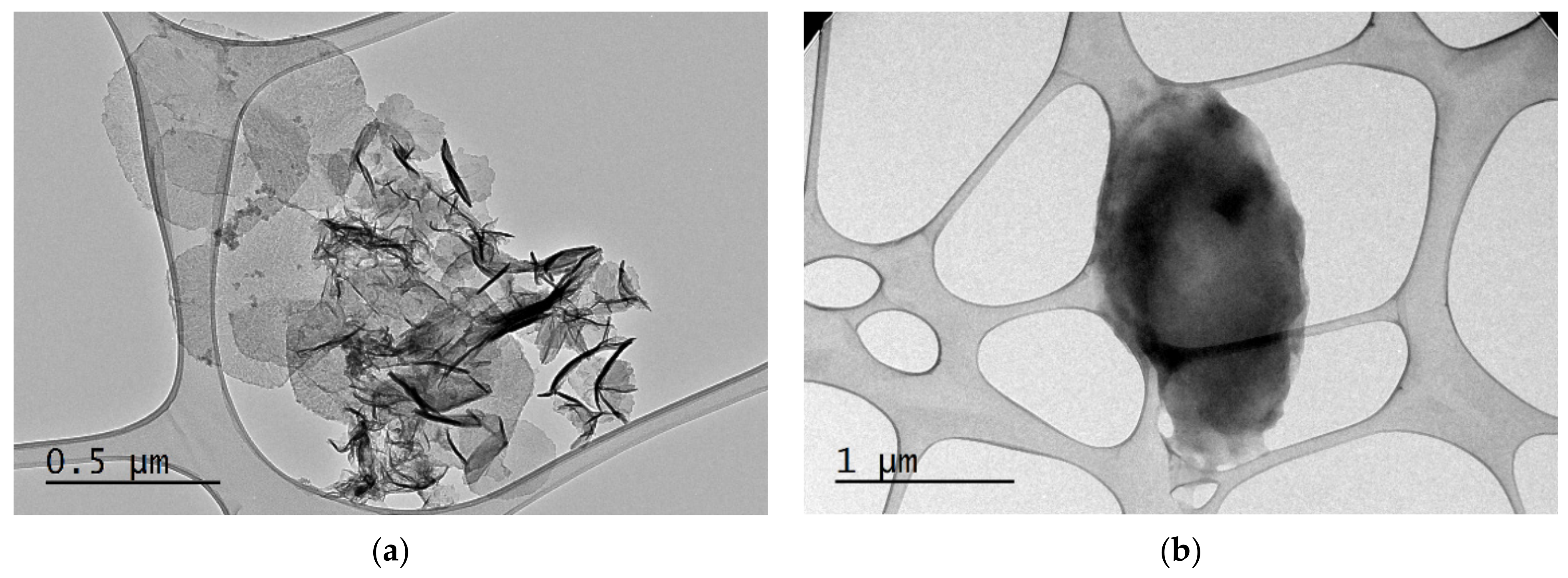
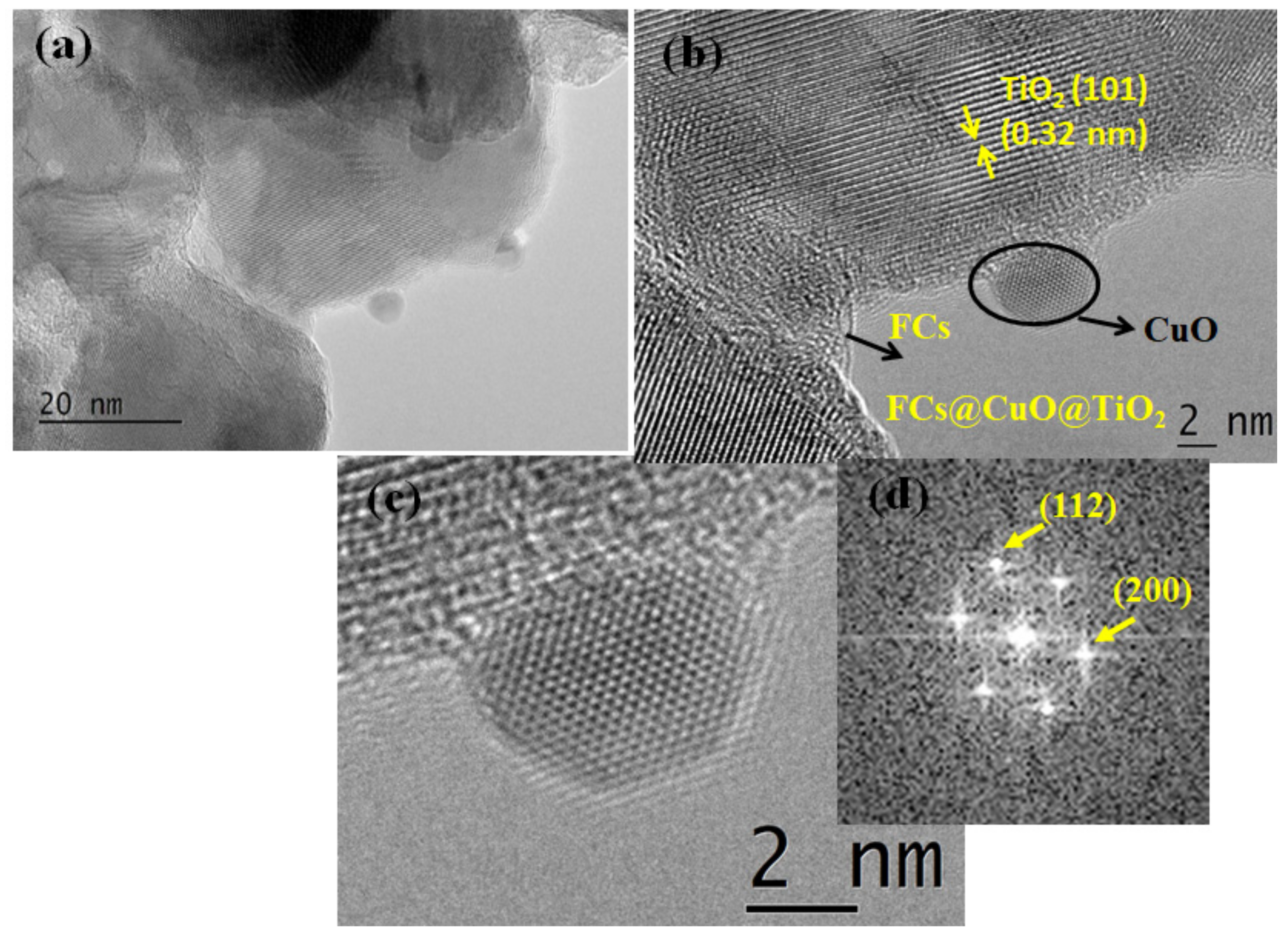
3.3. Morphological Characterization of FCs and FCs@CuO@TiO2 Composite
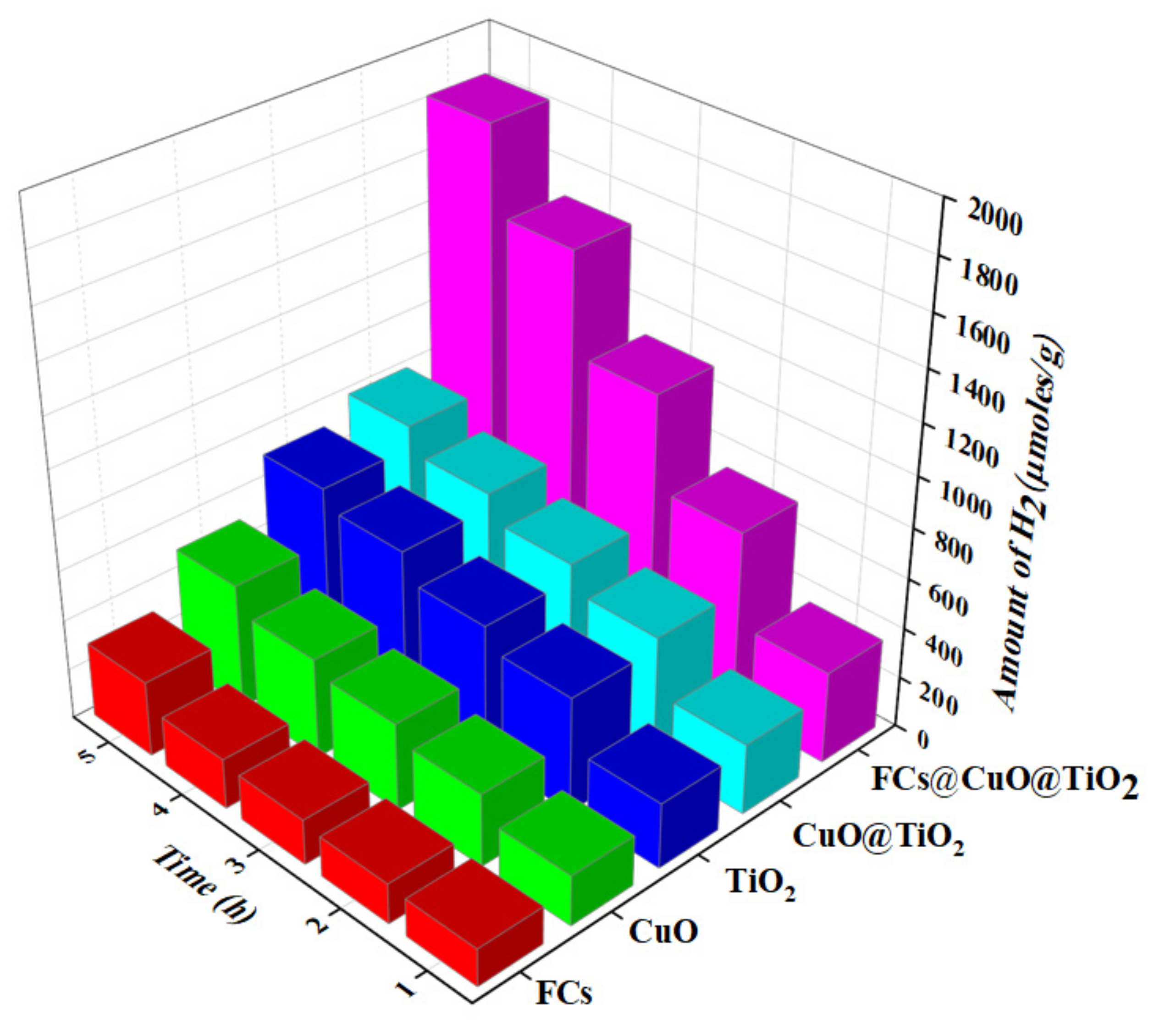
3.4. Photocatalytic Hydrogen Production via Water Splitting
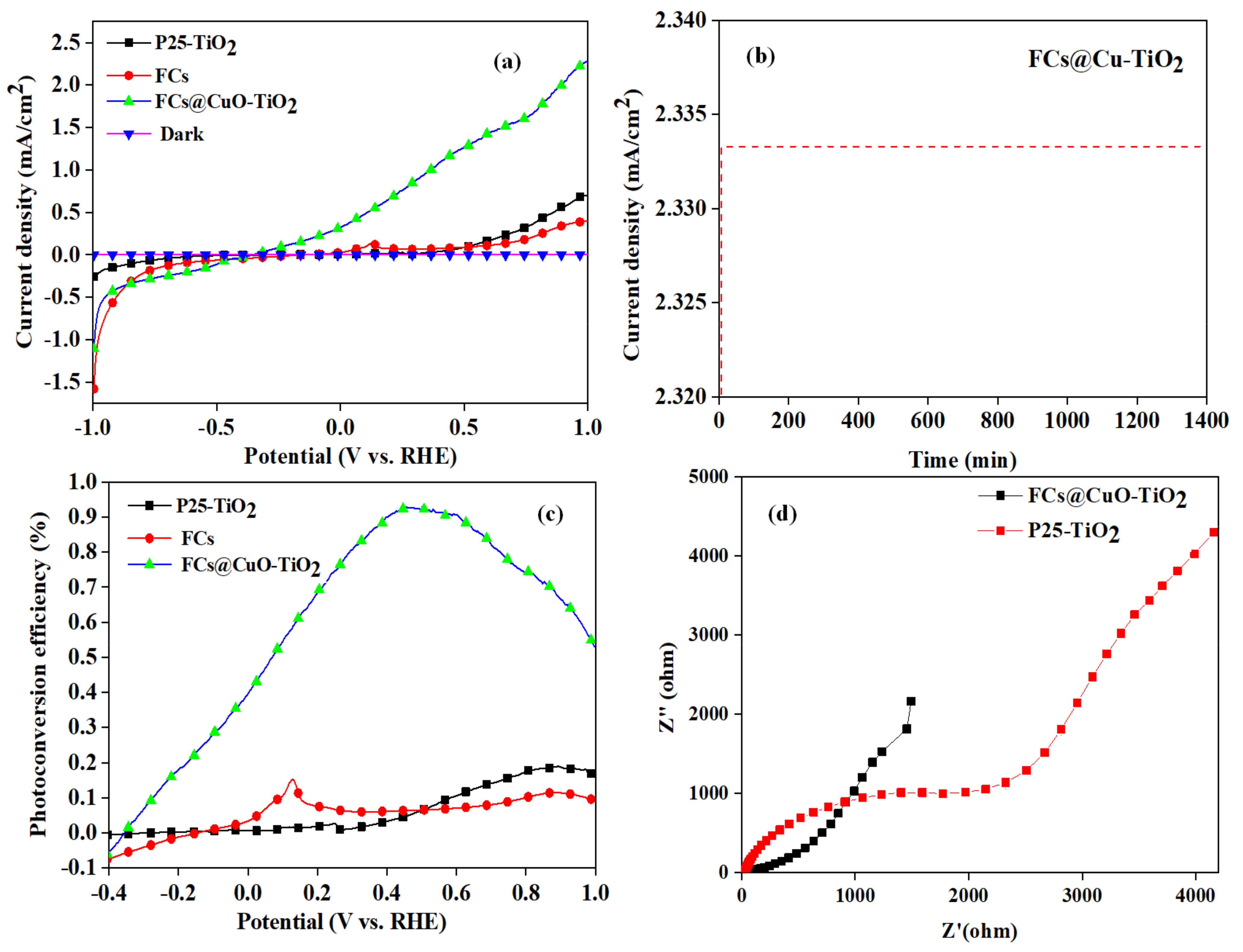
3.5. PEC Water Splitting Performance
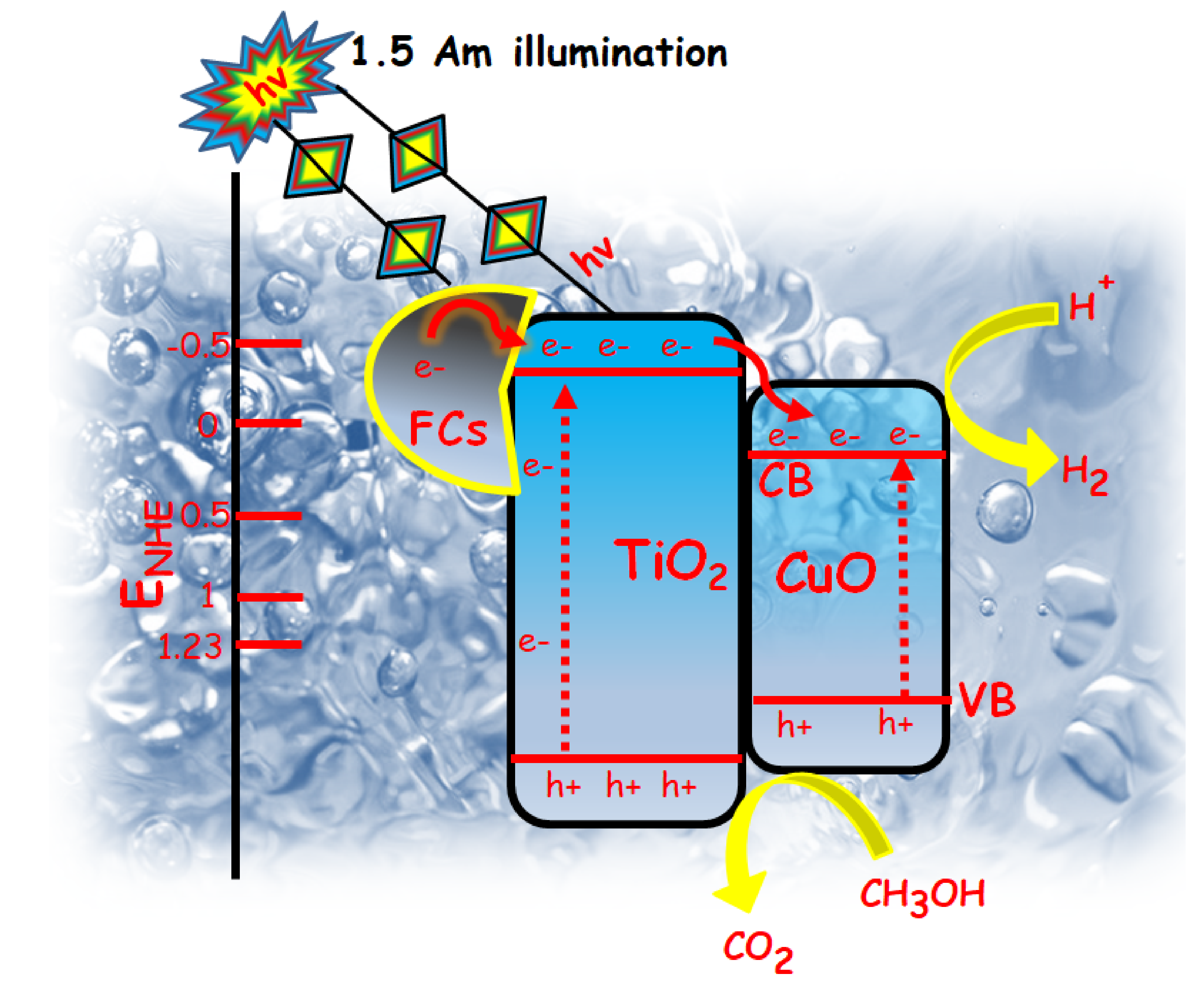
4. Plausible Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beg, M.D.H.; Kormin, S.; Bijarimi, M.; Zaman, H.U. Preparation and Characterization of Low-Density Polyethylene/Thermoplastic Starch Composites. Adv. Polym. Technol. 2015, 35, 21521–21529. [Google Scholar] [CrossRef] [Green Version]
- Ugoeze, K.C.; Amogu, E.O.; Oluigbo, K.E.; Nwachukwu, N. Environmental and public health impacts of plastic wastes due to healthcare and food products packages: A Review. J. Environ. Public Health 2021, 5, 1–31. [Google Scholar]
- Siegle, L. Turning the Tide on Plastic: How Humanity (and You) Can Make Our Globe Clean Again; Hachette: New York, NY, USA, 2018. [Google Scholar]
- Zhuo, C.; Levendis, Y.A. Upcycling waste plastics into carbon nanomaterials: A review. J. Appl. Polym. Sci. 2014, 131, 39931. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Kaur, S.; Lee, J.; Mehta, A.; Kumar, S.; Kim, K.-H.; Basu, S.; Rawat, M. Highly fluorescent carbon dots derived from Mangifera indica leaves for selective detection of metal ions. Sci. Total Environ. 2020, 720, 137604. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Mishra, A.; Kainth, S.; Basu, S. Carbon quantum dots/TiO2 nanocomposite for sensing of toxic metals and photodetoxification of dyes with kill waste by waste concept. Mater. Des. 2018, 155, 485–493. [Google Scholar] [CrossRef]
- Ravi, S.; Vadukumpully, S. Sustainable carbon nanomaterials: Recent advances and its applications in energy and environmental remediation. J. Environ. Chem. Eng. 2016, 4, 835–856. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Feng, Z.; Adolfsson, K.H.; Xu, Y.; Fang, H.; Hakkarainen, M.; Wu, M. Carbon dot/polymer nanocomposites: From green synthesis to energy, environmental and biomedical applications. Sustain. Mater. Technol. 2021, 29, e00304. [Google Scholar] [CrossRef]
- Zulfajri, M.; Sudewi, S.; Ismulyati, S.; Rasool, A.; Adlim, M.; Huang, G.G. Carbon Dot/Polymer Composites with Various Precursors and Their Sensing Applications: A Review. Coatings 2021, 11, 1100. [Google Scholar] [CrossRef]
- Meseldžija, S.; Petrovic, J.; Onjia, A.; Volkov-Husovic, T.; Nesic, A.; Vukelic, N. Utilization of agro-industrial waste for removal of copper ions from aqueous solutions and mining-wastewater. J. Ind. Eng. Chem. 2019, 75, 246–252. [Google Scholar] [CrossRef]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Kaur, N.; Mehta, A.; Mishra, A.; Chaudhary, S.; Rawat, M.; Basu, S. Amphiphilic carbon dots derived by cationic surfactant for selective and sensitive detection of metal ions. Mater. Sci. Eng. C 2019, 95, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, A.; Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Optical bio (sensing) using nitrogen doped graphene quantum dots: Recent advances and future challenges. Trends Anal. Chem. 2018, 108, 110–121. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [Green Version]
- Tofighy, M.A.; Mohammadi, T. Divalent heavy metal ions removal from contaminated water using positively charged membrane prepared from a new carbon nanomaterial and HPEI. Chem. Eng. J. 2020, 388, 124192. [Google Scholar] [CrossRef]
- Stern, B.R. Essentiality and toxicity in copper health risk assessment: Overview, update and regulatory considerations. J. Toxicol. Environ. Health Part A 2010, 73, 114–127. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Arbabi, M.; Golshani, N. Removal of copper ions Cu(II) from industrial wastewater: A review of removal methods. Int. J. Epidemiol. Res. 2016, 3, 283–293. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Basabe-Desmonts, L.; Reinhoudt, D.N.; Crego-Calama, M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev. 2007, 36, 993–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Raj, S.K.; Yadav, V.; Bhadu, G.R.; Patidar, R.; Kumar, M.; Kulshrestha, V. Synthesis of highly fluorescent and water soluble graphene quantum dots for detection of heavy metal ions in aqueous media. Environ. Sci. Pollut. Res. 2021, 28, 46336–46342. [Google Scholar] [CrossRef]
- Peveler, W.J.; Yazdani, M.; Rotello, V.M. Selectivity and specificity: Pros and cons in sensing. ACS Sens. 2016, 1, 1282–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Adverse Health Effects of Heavy Metals in Children (No. WHO/HSE/PHE/EPE/11.01.07); World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/bitstream/handle/10665/336875/WHO-HSE-PHE-EPE-11.01.07-eng.pdf (accessed on 20 December 2021).
- How to Sustain a World Population of 10 Billion People? Available online: https://www.theworldcounts.com (accessed on 20 December 2021).
- Qadri, R.; Faiq, M.A. Freshwater pollution: Effects on aquatic life and human health. In Fresh Water Pollution Dynamics and Remediation; Springer: Singapore, 2020; pp. 15–26. [Google Scholar]
- Gujre, N.; Mitra, S.; Soni, A.; Agnihotri, R.; Rangan, L.; Rene, E.R.; Sharma, M.P. Speciation, contamination, ecological and human health risks assessment of heavy metals in soils dumped with municipal solid wastes. Chemosphere 2021, 262, 128013. [Google Scholar] [CrossRef]
- Fatima, B. Characterization and Treatment of Real Wastewater from an Electroplating Company by Raw Chitin. In Recent Advancements in the Metallurgical Engineering and Electrodeposition; IntechOpen: London, UK, 2020; p. 107. Available online: https://www.intechopen.com/books/recent-advancements-in-the-metallurgical-engineering-and-electrodeposition/characterization-and-treatment-of-real-wastewater-from-an-electroplating-company-by-raw-chitin (accessed on 20 December 2021).
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol–gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.; Wang, W.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Song, B.; Xue, W.; Li, X.; et al. Recent advances in application of transition metal phosphides for photocatalytic hydrogen production. Chem. Eng. J. 2021, 405, 126547. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X.; et al. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 18, 116200. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, G.; Huang, D.; Zhang, C.; He, D.; Zhou, C.; Wang, W.; Xiong, W.; Song, B.; Yi, H.; et al. In Situ Grown Single-Atom Cobalt on Polymeric Carbon Nitride with Bidentate Ligand for Efficient Photocatalytic Degradation of Refractory Antibiotics. Small 2020, 16, e2001634. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wang, Y.; Yu, H.; Zhang, R.; Li, J.; Zang, Z.; Li, X. MXene Ti3C2Tx-Derived Nitrogen-Functionalized Heterophase TiO2 Homojunctions for Room-Temperature Trace Ammonia Gas Sensing. ACS Appl. Mater. Interfaces 2021, 13, 56485–56497. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, Y.; Zhang, R.; Li, J.; Li, X.; Zang, Z. Conductometric room temperature ammonia sensors based on titanium dioxide nanoparticles decorated thin black phosphorus nanosheets. Sens. Actuators B Chem. 2021, 349, 130770. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Wang, Y.; Tai, H.; Guo, Y. UV Illumination-enhanced molecular ammonia detection based on a ternary-reduced graphene oxide–titanium dioxide–Au composite film at room temperature. Anal. Chem. 2018, 91, 3311–3318. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kumar, A.; Sahu, S.K.; Kumar, S. Synthesis of green fluorescent carbon quantum dots using waste polyolefins residue for Cu2+ ion sensing and live cell imaging. Sens. Actuators B Chem. 2018, 254, 197–205. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Gholizadeh, M.; Hu, X. Different reaction behaviours of light or heavy density polyethylene during the pyrolysis with biochar as the catalyst. J. Hazard. Mater. 2020, 399, 123075. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berge, N.; et al. Recent advances in hydrothermal carbonisation: From tailored carbon materials and biochemicals to applications and bioenergy. Green Chem. 2020, 22, 4747–4800. [Google Scholar] [CrossRef]
- Li, Z.; Tian, B.; Zhen, W.; Wu, Y.; Lu, G. Inhibition of hydrogen and oxygen recombination using oxygen transfer reagent hemin chloride in Pt/TiO2 dispersion for photocatalytic hydrogen generation. Appl. Catal. B Environ. 2017, 203, 408–415. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Z.; Wang, H.; Cheng, W.; Chen, M.; Shangguan, W.; Ding, G. TiO2–graphene nanocomposites for photocatalytic hydrogen production from splitting water. Int. J. Hydrogen Energy 2012, 37, 2224–2230. [Google Scholar] [CrossRef]
- Charanpahari, A.; Umare, S.; Sasikala, R. Effect of Ce, N and S multi-doping on the photocatalytic activity of TiO2. Appl. Surf. Sci. 2013, 282, 408–414. [Google Scholar] [CrossRef]
- Hou, H.; Shang, M.; Gao, F.; Wang, L.; Liu, Q.; Zheng, J.; Yang, Z.; Yang, W. Highly efficient photocatalytic hydrogen evolution in ternary hybrid TiO2/CuO/Cu thoroughly mesoporous nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 20128–20137. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, A.; Pooja, D.; Thakur, A.; Basu, S. Enhanced photocatalytic water splitting by gold carbon dot core shell nanocatalyst under visible/sunlight. N. J. Chem. 2017, 41, 4573–4581. [Google Scholar] [CrossRef]
- Khemthong, P.; Photai, P.; Grisdanurak, N. Structural properties of CuO/TiO2 nanorod in relation to their catalytic activity for simultaneous hydrogen production under solar light. Int. J. Hydrogen Energy 2013, 38, 15992–16001. [Google Scholar] [CrossRef]
- Gombac, V.; Sordelli, L.; Montini, T.; Delgado, J.J.; Adamski, A.; Adami, G.; Cargnello, M.; Bernal, S.; Fornasiero, P. CuOx−TiO2 Photocatalysts for H2 Production from Ethanol and Glycerol Solutions. J. Phys. Chem. A 2010, 11, 3916–3925. [Google Scholar] [CrossRef]
- Zhu, L.; Hong, M.; Ho, G.W. Fabrication of wheat grain textured TiO2/CuO composite nanofibers for enhanced solar H2 generation and degradation performance. Nano Energy 2015, 11, 28–37. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, M.; He, Y.; Zhou, Z.; Sun, Z. In situ loading CuO quantum dots on TiO2 nanosheets as cocatalyst for improved photocatalytic water splitting. J. Alloys Compd. 2020, 813, 152184. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, S.; Lin, M.; Lin, L.; Cai, Z.; Xu, W. Controlling interface properties for enhanced photocatalytic performance: A case-study of CuO/TiO2 nanobelts. Mater. Adv. 2020, 1, 767–773. [Google Scholar] [CrossRef]


| Catalyst | λEm (nm) | τ1 | τ2 | B1 | B2 | R1 | R2 | A | χ2 | τ1avg (ns) | τ2avg (ns) | τaverage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCs | 486 | 1.18 | 8 | 0.17 | 0.15 | 1.15 | 1.032353 | 525.2 | 22.31 | 1.357 | 8.258823529 | 9.61582352 |
| FCs@CuO@TiO2 | 486 | 1.38 | 8.21 | 0.17 | 0.16 | 1.16 | 1.101176 | 1690 | 60.73 | 1.6008 | 9.040658824 | 10.64145882 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, A.; Rather, R.A.; Belec, B.; Gardonio, S.; Fang, M.; Valant, M. Plastic Waste Precursor-Derived Fluorescent Carbon and Construction of Ternary FCs@CuO@TiO2 Hybrid Photocatalyst for Hydrogen Production and Sensing Application. Energies 2022, 15, 1734. https://doi.org/10.3390/en15051734
Mehta A, Rather RA, Belec B, Gardonio S, Fang M, Valant M. Plastic Waste Precursor-Derived Fluorescent Carbon and Construction of Ternary FCs@CuO@TiO2 Hybrid Photocatalyst for Hydrogen Production and Sensing Application. Energies. 2022; 15(5):1734. https://doi.org/10.3390/en15051734
Chicago/Turabian StyleMehta, Akansha, Rayees Ahmad Rather, Blaz Belec, Sandra Gardonio, Ming Fang, and Matjaz Valant. 2022. "Plastic Waste Precursor-Derived Fluorescent Carbon and Construction of Ternary FCs@CuO@TiO2 Hybrid Photocatalyst for Hydrogen Production and Sensing Application" Energies 15, no. 5: 1734. https://doi.org/10.3390/en15051734
APA StyleMehta, A., Rather, R. A., Belec, B., Gardonio, S., Fang, M., & Valant, M. (2022). Plastic Waste Precursor-Derived Fluorescent Carbon and Construction of Ternary FCs@CuO@TiO2 Hybrid Photocatalyst for Hydrogen Production and Sensing Application. Energies, 15(5), 1734. https://doi.org/10.3390/en15051734









