Self-Healing Silicones for Outdoor High Voltage Insulation: Mechanism, Applications and Measurements
Abstract
1. Introduction
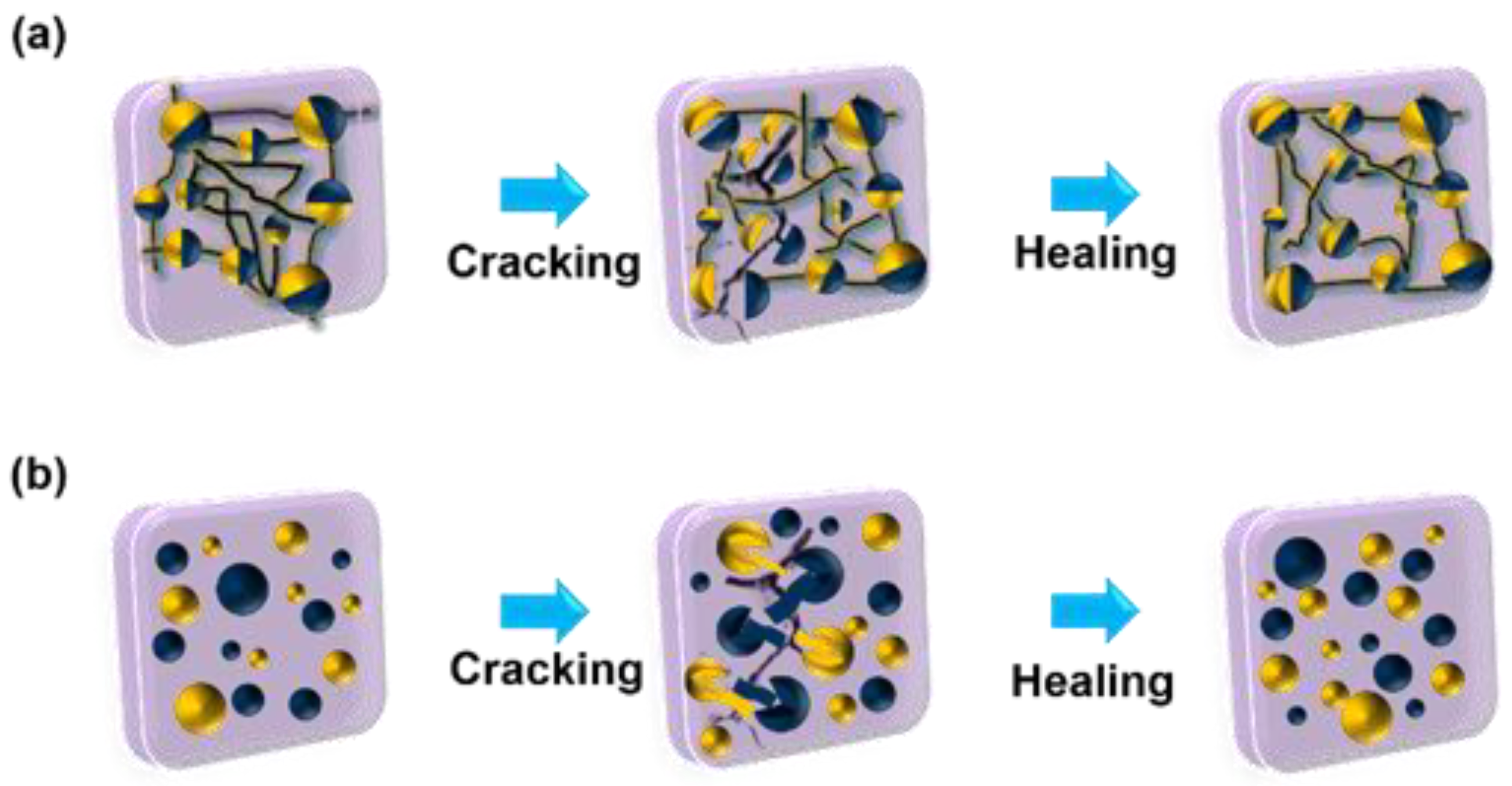
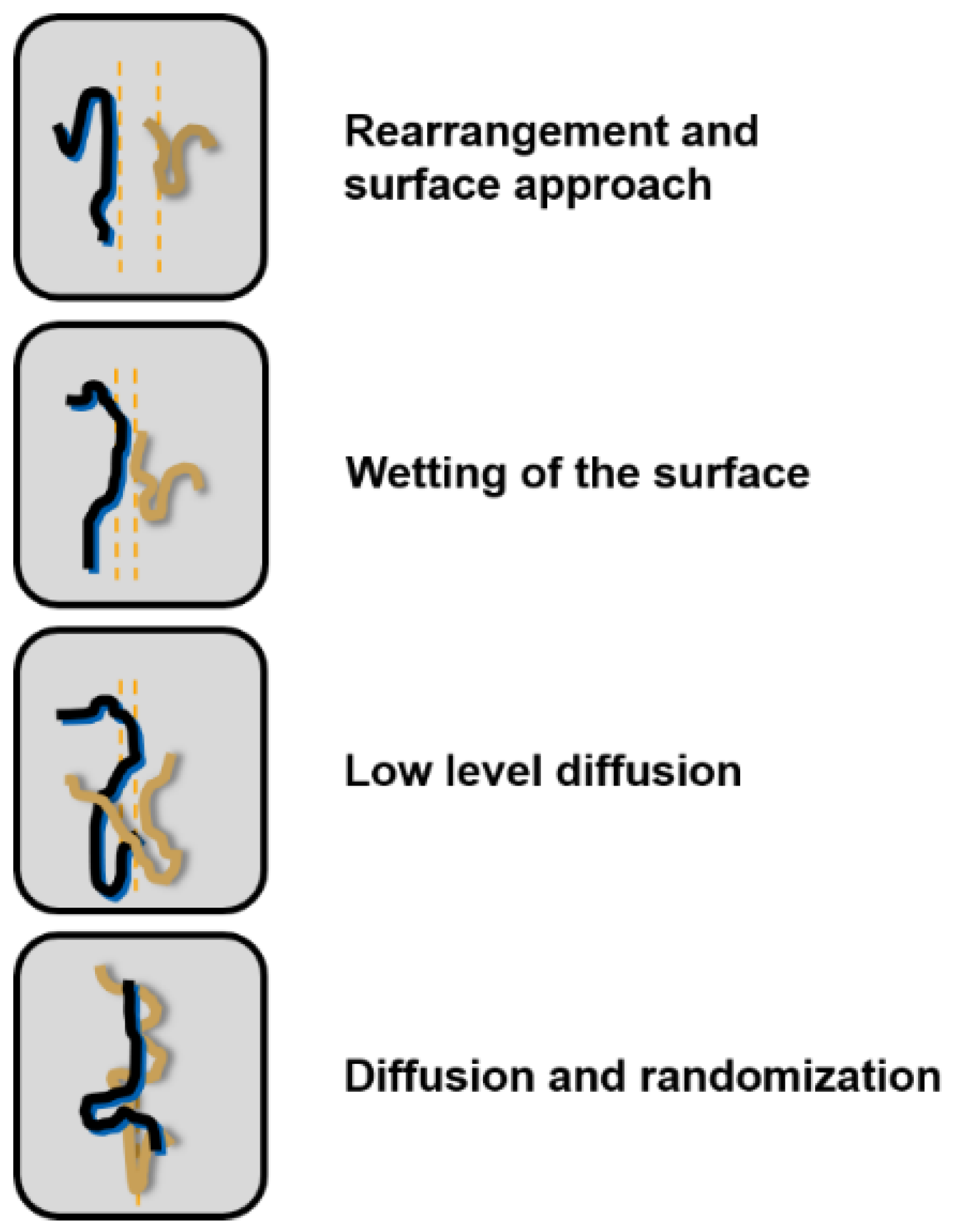
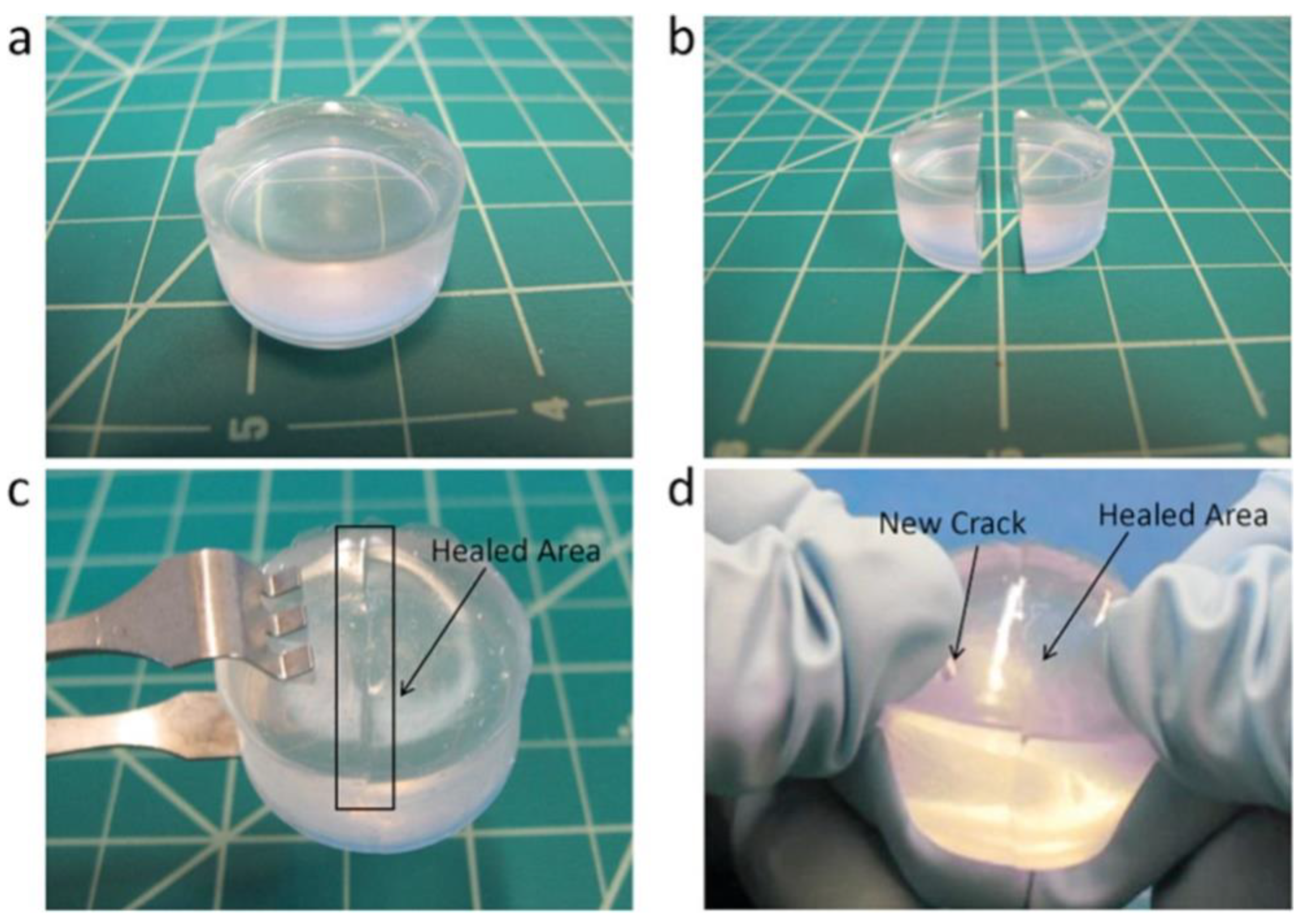
2. Self-Healing Mechanism of Silicone Rubber Insulators
2.1. Effect of Base Material
2.2. Effect of Fillers
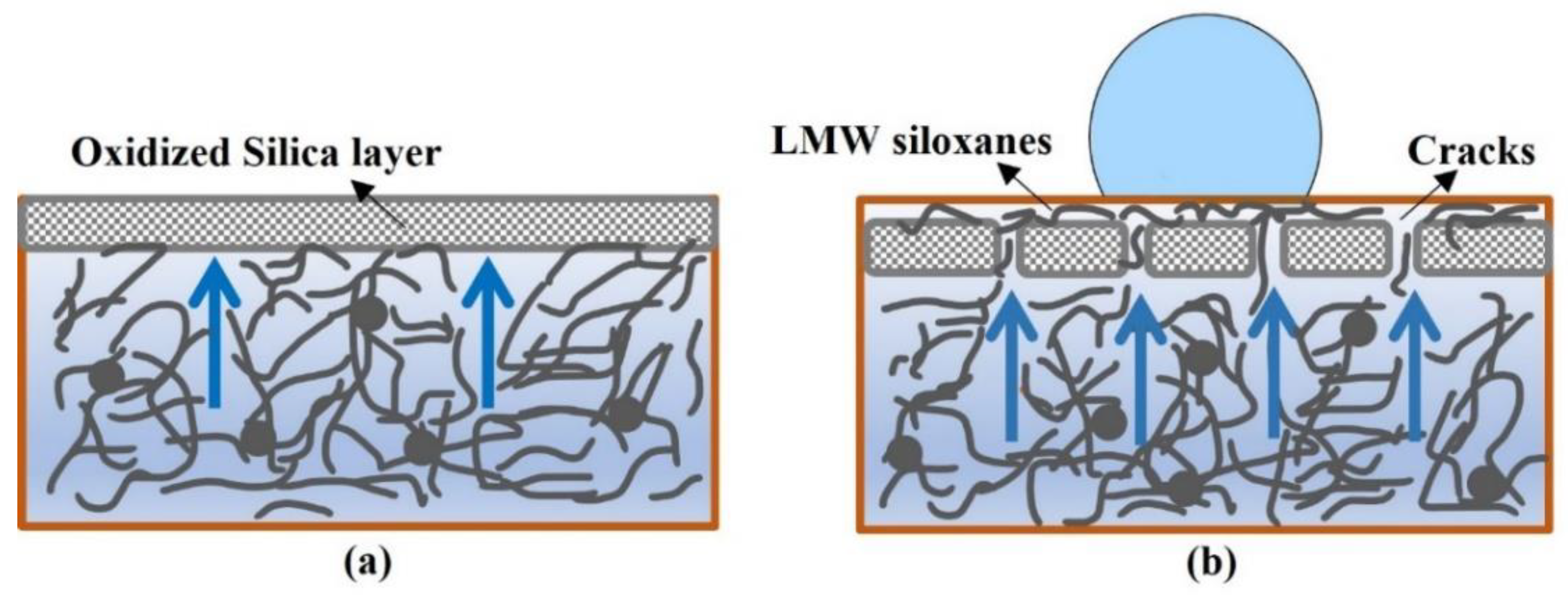
3. Effect of Electrical and Environmental Stresses on Self-Healing Silicone Rubber Insulators
4. Screening Measurements for Outdoor High Voltage Insulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wool, R.P. Self-healing materials: A review. Soft Matter 2008, 4, 400–418. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Dai, S.; Chen, Y.; Ding, J.; Yuan, N. A High Stretchable and Self–Healing Silicone Rubber with Double Reversible Bonds. ChemistrySelect 2019, 4, 10719–10725. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- van der Zwaag, S.; Brinkman, E. Self Healing Materials; IOS Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.A.; Hernández Santana, M. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review. Mater. Horiz. 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Li, C.H.; Zuo, J.L. Self-Healing Polymers Based on Coordination Bonds. Adv. Mater. 2020, 32, 1–29. [Google Scholar] [CrossRef]
- Xiang, H.P.; Rong, M.Z.; Zhang, M.Q. A facile method for imparting sunlight driven catalyst-free self-healability and recyclability to commercial silicone elastomer. Polymer 2017, 108, 339–347. [Google Scholar] [CrossRef]
- Habault, D.; Zhang, H.; Zhao, Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244–7256. [Google Scholar] [CrossRef]
- Chuo, T.W.; Wei, T.C.; Liu, Y.L. Electrically driven self-healing polymers based on reversible guest-host complexation of β-cyclodextrin and ferrocene. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3395–3403. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Lin, B.; Liang, X.; Chen, Y. Design of Self-Healing Supramolecular Rubbers by Introducing Ionic Cross-Links into Natural Rubber via a Controlled Vulcanization. ACS Appl. Mater. Interfaces 2016, 8, 17728–17737. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, D.S. Self-Healing and Rheological Properties of Polyhydroxyurethane Elastomers Based on Glycerol Carbonate Capped Prepolymers. Macromol. Res. 2019, 27, 460–469. [Google Scholar] [CrossRef]
- Kato, Y.; Minakuchi, S.; Ogihara, S.; Takeda, N. Self-healing composites structure using multiple through-thickness microvascular channels. Adv. Compos. Mater. 2021, 30, 1–18. [Google Scholar] [CrossRef]
- Li, G.L.; Zheng, Z.; Möhwald, H.; Shchukin, D.G. Silica/polymer double-walled hybrid nanotubes: Synthesis and application as stimuli-responsive nanocontainers in self-healing coatings. ACS Nano 2013, 7, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cao, K.; Chen, M.; Wu, L. Synthesis of UV-Responsive Self-Healing Microcapsules and Their Potential Application in Aerospace Coatings. ACS Appl. Mater. Interfaces 2019, 11, 33314–33322. [Google Scholar] [CrossRef] [PubMed]
- Blaiszik, B.J.; Sottos, N.R.; White, S.R. Nanocapsules for self-healing materials. Compos. Sci. Technol. 2008, 68, 978–986. [Google Scholar] [CrossRef]
- Chen, D.; Wang, D.; Yang, Y.; Huang, Q.; Zhu, S.; Zheng, Z. Self-Healing Materials for Next-GenerationEnergy Harvesting and Storage Devices. Adv. Energy Mater. 2017, 7, 1–23. [Google Scholar] [CrossRef]
- Song, T.; Jiang, B.; Li, Y.; Ji, Z.; Zhou, H.; Jiang, D.; Seok, I.; Murugadoss, V.; Wen, N.; Colorado, H. Self-healing Materials: A Review of Recent Developments. ES Mater. Manuf. 2021, 14, 1–19. [Google Scholar] [CrossRef]
- Gorur, R.; Chenrey, E.; Burnham, T. Outdoor Insulators; Ravi S Gorur Inc.: Phoenix, AZ, USA, 1999. [Google Scholar]
- Cherney, E. 50 years in the development of polymer suspension-type insulators. IEEE Electr. Insul. Mag. 2013, 29, 18–26. [Google Scholar] [CrossRef]
- Mackevich, J.; Simmons, S. Polymer outdoor insulating materials. II. Material considerations. IEEE Electr. Insul. Mag. 1997, 13, 10–16. [Google Scholar] [CrossRef]
- Kim, S.; Cherney, E.A.; Hackam, R. Hydrophobic behavior of insulators coated with RTV silicone rubber. IEEE Trans. Electr. Insul. 1992, 27, 610–622. [Google Scholar] [CrossRef]
- Wu, D.; Meure, S.; Solomon, S. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. [Google Scholar] [CrossRef]
- Kim, Y.H.; Wool, R.P. A Theory of Healing at a Polymer-Polymer Interface. Macromolecules 1983, 16, 1115–1120. [Google Scholar] [CrossRef]
- Prager, S.; Tirrell, M. The healing process at polymer-polymer interfaces. J. Chem. Phys. 1981, 75, 5194–5198. [Google Scholar] [CrossRef]
- Welp, K.A.; Wool, R.P.; Agrawal, G.; Satija, S.K.; Pispas, S.; Mays, J. Direct observation of polymer dynamics: Mobility comparison between central and end section chain segments. Macromolecules 1999, 32, 5127–5138. [Google Scholar] [CrossRef]
- Kantor, S.W.; Grubb, W.T.; Osthoff, R.C. The Mechanism of the Acid- and Base-catalyzed Equilibration of Siloxanes. J. Am. Chem. Soc. 1954, 76, 5190–5197. [Google Scholar] [CrossRef]
- Schmolke, W.; Perner, N.; Seiffert, S. Dynamically Cross-Linked Polydimethylsiloxane Networks with Ambient-Temperature Self-Healing. Macromolecules 2015, 48, 8781–8788. [Google Scholar] [CrossRef]
- Zheng, P.; McCarthy, T.J. A Surprise from 1954: Siloxane Equilibration Is a Simple, Robust, and Obvious Polymer Self-Healing Mechanism. Am. Chem. Soc. 2012, 4, 2024–2027. [Google Scholar] [CrossRef]
- Shemella, P.T.; Laino, T.; Fritz, O.; Curioni, A. Understanding the Self-Healing Hydrophobic Recovery of HighVoltage Insulators. J. Phys. Chem. B 2012, 116, 7351–7356. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Sun, J.; Yuan, J.; Yang, Z.; Gao, C.; Wu, Y. A Type of Hydrogen Bond Cross-Linked Silicone Rubber with the Thermal-Induced Self-Healing Properties Based on the Nonisocyanate Reaction. Ind. Eng. Chem. Res. 2019, 58, 21452–21458. [Google Scholar] [CrossRef]
- Kang, J.; Son, D.; Wang, G.J.N.; Liu, Y.; Lopez, J.; Kim, Y.; Oh, J.Y.; Katsumata, T.; Mun, J.; Lee, Y.; et al. Tough and Water-Insensitive Self-Healing Elastomer for Robust Electronic Skin. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef]
- Strakowska, A.; Kosmalska, A.; Masłowski, M.; Szmechtyk, T.; Strzelec, K.; Zaborski, M. POSS as promoters of self-healing process in silicone composites. Polym. Bull. 2019, 76, 3387–3402. [Google Scholar] [CrossRef]
- Cherney, E.A. Silicone rubber dielectrics modified by inorganic fillers for outdoor high voltage insulation applications. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 1108–1115. [Google Scholar] [CrossRef]
- Ghunem, R.; Ilhan, S.; Uckol, H.; Tuzun, D.; Hadjadj, Y. Viability of Fillers in HTV Silicone Rubber in the AC and DC Inclined Plane Tests, IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 2144–2151. [Google Scholar] [CrossRef]
- Cherney, E.A. RTV Silicone—A High Tech Solution for a Dirty Insulator Problem. IEEE Electr. Insul. Mag. 1995, 11, 8–14. [Google Scholar] [CrossRef]
- Jansen, H.; Herden, A.; Kämer, H.C. The loss and Recovery of Hydrophobicity on Silicone Rubber Surfaces. In Proceedings of the 10th International Symposium on High Voltage Engineering, Montreal, QC, Canada, 25–29 August 1997; pp. 145–148. [Google Scholar]
- Deng, H.; Cherney, E.A.; Hackam, H. Effects of particles size of ATH filler on the performance of RTV rubber coatings. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP’93), Pocono Manor, PA, USA, 17–20 October 1993; pp. 598–604. [Google Scholar] [CrossRef]
- Momen, G.; Farzaneh, M. Survey of micro/nano filler use to improve silicone rubber for outdoor insulators. Rev. Adv. Mater. Sci. 2011, 27, 1–13. [Google Scholar]
- Kim, J.; Chaudhury, M.K.; Owen, M.J. Hydrophobicity loss and recovery of silicone HV insulation. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 695–702. [Google Scholar] [CrossRef]
- Kim, S.H.; Hackam, R.; Cherney, E.A. Suppression mechanism of leakage current on RTV coated porcelain and silicone rubber insulators. IEEE Trans. Power Deliv. 1991, 6, 1549–1556. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, L.; Yang, K.; Li, C.R.; Tu, Y. Study on hydrophobicity recovery characteristics and mechanism of HTV silicone rubber after corona deterioration. In 2007 Annual Report—Conference on Electrical Insulation and Dielectric Phenomena; IEEE: Vancouver, BC, Canada, 2007; pp. 308–311. [Google Scholar] [CrossRef]
- Nazir, M.T.; Phung, B.T.; Yu, S.; Li, S. Resistance against AC corona discharge of micro-ATH/nano-Al2O3 co-filled silicone rubber composites. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 657–667. [Google Scholar] [CrossRef]
- Mehmood, B.; Akbar, M.; Ullah, R. Accelerated aging effect on high temperature vulcanized silicone rubber composites under DC voltage with controlled environmental conditions. Eng. Fail. Anal. 2020, 118, 104870. [Google Scholar] [CrossRef]
- Homma, H.; Kuroyagi, T.; Izumi, K.; Mirley, C.L.; Ronzello, J.; Boggs, S.A. Diffusion of low molecular weight siloxane from bulk to surface. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 370–375. [Google Scholar] [CrossRef]
- Kim, S.H.; Cherney, E.A.; Hackam, R. Effects of Filler Level in RTV Silicone Rubber Coatings Used in HV Insulators. IEEE Trans. Electr. Insul. 1992, 27, 1065–1072. [Google Scholar] [CrossRef]
- Venkatesulu, B.; Thomas, M.J. Corona aging studies on silicone rubber nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 625–634. [Google Scholar] [CrossRef]
- Wang, K.; Jianguo, W.; Feng, Z.; Pengfei, F.; Xiangyang, P.; Zhihai, X. Effect of fumed silica content on the microstructure and hydrophobicity recovery of silicon rubber. Diangong Jishu Xuebao/Trans. China Electrotech. Soc. 2016, 31, 31–39. [Google Scholar]
- Vinod, P.; Desai, B.A.; Sarathi, R.; Kornhuber, S. Investigation on the electrical, thermal and mechanical properties of silicone rubber nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1876–1884. [Google Scholar] [CrossRef]
- Nazir, M.T.; Phung, B.T.; Hoffman, M. Performance of silicone rubber composites with SiO2 micro/nano-filler under AC corona discharge. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2804–2815. [Google Scholar] [CrossRef]
- Zakaria, I.H.; Kamaruddin, M.T.; Arie, Y.Z.; Ahmad, M.H.; Ahmad, A.; Muhamad, N.A.; Adzis, Z. Self-healing properties of silicone rubber against relative humidity and nanofiller. Indones. J. Electr. Eng. Comput. Sci. 2017, 6, 166–171. [Google Scholar] [CrossRef][Green Version]
- Amin, M.; Akbar, M.; Amin, S. Hydrophobicity of silicone rubber used for outdoor insulation (An overview). Rev. Adv. Mater. Sci. 2007, 16, 10–26. [Google Scholar]
- Li, Z.; Cui, J.; Zhou, Y.; Wang, L.; Liang, X.; Liu, Y.; Tang, J.; Zhou, G. Study on the hydrophobicity of composite insulator in service. Gaodianya Jishu/High Volt. Eng. 2006, 32, 24–26. [Google Scholar]
- Long, C.; Zheng, W.; Yi, L.; Jingjing, L. Study on Surface Hydrophobic Properties and Microstructure of Serviced Composite Insulator. Insul. Mater. 2020, 53, 19–26. [Google Scholar]
- Nazir, M.T.; Xingliang, J.; Akram, S. Laboratory investigation on hydrophobicity of new silicon rubber insulator under different environmental conditions. Dimensions 2012, 60, 7. [Google Scholar]
- Janssen, H.; Herden, A.; Karner, H.C. LMW components in silicone rubbers and epoxy resins. In Proceedings of the 1999 Eleventh International Symposium on High Voltage Engineering, London, UK, 23–27 August 1999; Volume 4, pp. 18–21. [Google Scholar] [CrossRef]
- Gubanski, S.M. Modern outdoor insulation-concerns and challenges. IEEE Electr. Insul. Mag. 2005, 21, 5–11. [Google Scholar] [CrossRef]
- Jia, Z.; Gao, H.; Guan, Z.; Wang, L.; Yang, J. Study on hydrophobicity transfer of RTV coatings based on a modification of absorption and cohesion theory. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 317–1324. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Wang, S.; Fu, Y. Plasma treatment to improve the hydrophobicity of contaminated silicone rubber—The role of LMW siloxanes. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 416–422. [Google Scholar] [CrossRef]
- Hackam, R. Low-molecular weight silicone fluid in RTV silicone rubber coatings. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 461–462. [Google Scholar] [CrossRef]
- Swift, D.A.; Spellman, C.; Haddad, A. Hydrophobicity transfer from silicone rubber to adhering pollutants and its effect on insulator performance. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 820–829. [Google Scholar] [CrossRef]
- Kumagai, S.; Yoshimura, N. Hydrophobic Transfer of RTV Silicone Rubber Aged in Single and Multiple Environmental Stresses and the Behavior of LMW Silicone Fluid. IEEE Power Eng. Rev. 2002, 22, 54. [Google Scholar] [CrossRef]
- Vas, J.V.; Thomas, M.J. Surface degradation of silicone rubber nanocomposites due to DC corona discharge. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1175–1182. [Google Scholar] [CrossRef]
- Kim, S.H.; Cherney, E.A.; Hackam, R.; Rutherford, K.G. Chemical Changes at the Surface of Rtv Silicone Rubber Coatings on Insulators During Dry-Band Arcing. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 106–123. [Google Scholar] [CrossRef]
- Yuan, X.; Lu, H.; Lan, L.; Wang, H.; Wen, X.; Liao, Y.; Zhang, F. Study on the effect of corona on hydrophobicity recovery performance of RTV silicone rubber and its failure criterion. In Proceedings of the 2016 Electrical Insulation Conference (EIC), Montreal, QC, Canada, 19–22 June 2016; pp. 215–218. [Google Scholar] [CrossRef]
- Dai, R.; Wang, S.; Lu, F. RTV silicone rubber hydrophobicity loss and recovery character under AC corona. In Proceedings of the 2011 International Conference on Electrical and Control Engineering, Yichang, China, 16–18 September 2011; pp. 807–810. [Google Scholar] [CrossRef]
- Kumagai, S.; Yoshimura, N. Hydrophobic transfer of RTV silicone rubber aged in single and multiple environmental stresses and the behavior of LMW silicone fluid. IEEE Trans. Power Deliv. 2003, 18, 506–516. [Google Scholar] [CrossRef]
- Hillborg, H.; Gedde, U.W. Hydrophobicity recovery of polydimethylsiloxane after exposure to corona discharges. Polymer 1998, 39, 1991–1998. [Google Scholar] [CrossRef]
- Hillborg, H.; Gedde, U.W. Hydrophobicity changes in silicone rubbers. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 703–717. [Google Scholar] [CrossRef]
- Hillborg, H.; Gedde, U.W. Hydrophobicity recovery of polydimethylsiloxane after repeated exposure to corona discharges. Influence of crosslink density. In Proceedings of the 1999 Annual Report Conference on Electrical Insulation and Dielectric Phenomenon (Cat. No. 99CH36319), Austin, TX, USA, 17–20 October 1999; Volume 2, pp. 751–755. [Google Scholar] [CrossRef]
- Fritz, J.L.; Owen, M.J. Hydrophobic recovery of plasma-treated polydimethylsiloxane. J. Adhes. 1995, 54, 33–45. [Google Scholar] [CrossRef]
- Yoshimura, N.; Kumagai, S.; Nishimura, S. Electrical and environmental aging of silicone rubber used in outdoor insulation. IEEE Trans. Dielectr. Electr. Insul. 2020, 6, 632–650. [Google Scholar] [CrossRef]
- Zhu, Y. Influence of corona discharge on hydrophobicity of silicone rubber used for outdoor insulation. Polym. Test. 2019, 74, 14–20. [Google Scholar] [CrossRef]
- Dai, H.Q.; Mei, H.W.; Wang, L.M.; Zhao, C.L.; Jia, Z.D.; Guan, Z.C. Volatility of hydrophobicity on the polluted surface during hydrophobicity transfer. Zhongguo Dianji Gongcheng Xuebao/Proc. Chin. Soc. Electr. Eng. 2014, 34, 971–981. [Google Scholar] [CrossRef]
- Du, B.X.; Xu, H.; Liu, Y. Effects of wind condition on hydrophobicity behavior of silicone rubber in corona discharge environment. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 385–393. [Google Scholar] [CrossRef]
- Deng, H.; Hackam, R.; Cherney, E.A. Effects of applied heat on LMW silicone fluid in RTV silicone rubber coatings used in outdoor HV insulation. In Proceedings of the 1995 Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Virginia Beach, VA, USA, 22–25 October 1995; pp. 459–462. [Google Scholar] [CrossRef]
- Chang, J.W.; Gorur, R.G. Surface recovery of silicone rubber used for HV outdoor insulation. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 1039–1046. [Google Scholar] [CrossRef]
- Chakraborty, R.; Reddy, B.S. Studies on high temperature vulcanized silicone rubber insulators under arid climatic aging. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1751–1760. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Fang, J. Influence of environmental factor on hydrophobicity transfer of silicone rubber used for outdoor insulation. In Proceedings of the 2017 International Symposium on Electrical Insulating Materials (ISEIM), Toyohashi, Japan, 11–15 September 2017; Volume 1, pp. 32–35. [Google Scholar] [CrossRef]
- Raza, M.H.; Khattak, A.; Ali, A.; Butt, S.U.; Iqbal, B.; Ulasyar, A.; Alahmadi, A.A.; Ullah, N.; Khan, A. Surface recovery investigation of silicone rubber composites for outdoor electrical insulation under accelerated temperature and humidity. Polymers 2021, 13, 3024. [Google Scholar] [CrossRef]
- Venkatesulu, B.; Thomas, M.J. Long-term accelerated weathering of outdoor silicone rubber insulators. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 418–424. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Zhou, Y.; Tang, J.; Cui, J.; Liu, Y. Influence of temperature on the hydrophobicity of silicone rubber surfaces. In Proceedings of the 2004 Annual Report Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Boulder, CO, USA, 20 October 2004; pp. 679–682. [Google Scholar] [CrossRef]
- Hackam, R. Outdoor HV composite polymeric insulators. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 557–585. [Google Scholar] [CrossRef]
- Reynders, J.P.; Jandrell, I.R.; Reynders, S.M. Review of aging and recovery of silicone rubber insulation for outdoor use. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 620–631. [Google Scholar] [CrossRef]
- Tokoro, T.; Hackam, R. Loss and recovery of hydrophobicity and surface energy of HTV silicone rubber. IEEE Trans. Dielectr. Electr. Insul. 2001, 8, 1088–1097. [Google Scholar] [CrossRef]
- Ali, M.; Hackam, R. Recovery of hydrophobicity of htv silicone rubber after accelerated aging in saline solutions. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 842–852. [Google Scholar] [CrossRef]
- Ullah, I.; Akbar, M.; Khan, H.A. Degradation analysis of RTV-SiR based composites under both polarities DC voltage for insulators coating. Mater. Today Commun. 2021, 29, 102890. [Google Scholar] [CrossRef]
- Ullah, I.; Akbar, M. Anti-aging characteristics of RTV-SiR aided HV insulator coatings: Impact of DC polarity and fillers. Mater. Chem. Phys. 2022, 278, 125634. [Google Scholar] [CrossRef]
- Kaaiye, S.F.; Nyamupangedengu, C. Comparative study of AC and DC inclined plane tests on silicone rubber (SiR) insulation. High Volt. 2017, 2, 119–128. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, L.; Li, C.R.; Tu, Y.; Yang, K. Effect of corona on the hydrophobicity recovery characteristics of HTV silicone rubber. In Proceedings of the 2007 IEEE International Conference on Solid Dielectrics, Winchester, UK, 8–13 July 2007; pp. 118–121. [Google Scholar] [CrossRef]
- Yan, Z.; Liang, X.; Wu, C.; Bao, W.; Li, S.; Liu, Y. Aging and recovery of superhydrophobic silicone rubber under electrical and non-electrical stresses. In Proceedings of the 2015 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Ann Arbor, MI, USA, 18–21 October 2015; pp. 189–192. [Google Scholar] [CrossRef]
- CIGRE TB 255: Material properties for non-ceramic outdoor Insulation. In State of the Art Report from CIGRE WG D1.14; CIGRE: Paris, France, 2004.
- CIGRE WG D1.14: Evaluation of Dynamic Hydrophobicity Properties of Polymeric Materials for Non-Ceramic Outdoor Insulation Retention and Transfer of Hydrophobicity; TB no. 442; CIGRE: Paris, France, 2010.
- Kornhuber, S.; Weber, J. Evaluation of the influence of low molecular weight components to the retention of the hydrophobicity of silicones by using the dynamic drop test. In Proceedings of the 2017 International Symposium on Electrical Insulating Materials (ISEIM), Toyohashi, Japan, 11–15 September 2017; Volume 1, pp. 25–28. [Google Scholar] [CrossRef]
- Bar, C.; Barsch, R.; Hergert, A.; Kindersberger, J. Evaluation of the retention and recovery of hydrophobicity of insulating materials in high voltage outdoor applications under AC and DC stresses with the Dynamic Drop Test. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 294–303. [Google Scholar] [CrossRef]
- Hergert, A.; Kindersberger, J.; Bär, C.; Bärsch, R. Test Methods for the Evaluation of Dynamic Hydrophobicity Properties of Polymeric Insulating Materials for High-Voltage Applications. In Proceedings of the Conference on Silicone Insulation, Burghausen, Germany, 6–7 June 2013. [Google Scholar]
- Zhidong, J.; Haifeng, G.; Liming, W.; Zhicheng, G.; Jie, Y. Study on the hydrophobic transfer based on the theory of absorption and cohesion. In Proceedings of the 7th International Conference on Properties and Applications of Dielectric Materials, Nagoya, Japan, 1–5 June 2003; Volume 1, pp. 162–166. [Google Scholar] [CrossRef]
- Hergert, A.; Kindersberger, J.; Bär, C.; Bärsch, R. Transfer of hydrophobicity of polymeric insulating materials for high voltage outdoor application. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1057–1067. [Google Scholar] [CrossRef]
- IEC 60815; Selection and Dimensioning of High-Voltage Insulators for Polluted Conditions-Part 3: Polymer Insulators for a.c. Systems. International Electrotechical Committee: Geneva, Switzerland, 2008.
- CIGRE Report: Artificial Pollution Test for Polymer Insulators-Results of Round Robin Test; CIGRE Working Group C4.303: Paris, France, 2013.
- Chakraborty, R.; Reddy, S.B. Performance of Silicone Rubber Insulators under Thermal and Electrical Stress. IEEE Trans. Ind. Appl. 2017, 53, 2446–2454. [Google Scholar] [CrossRef]
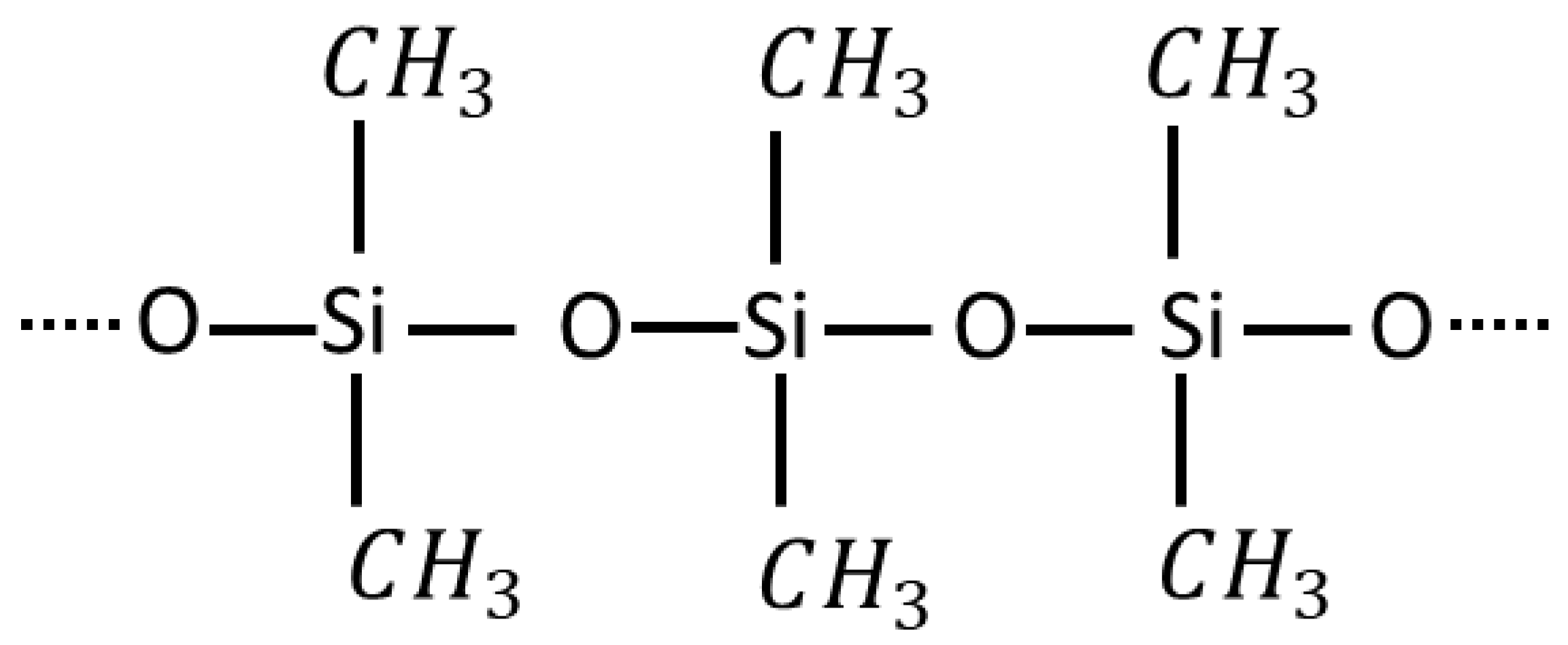



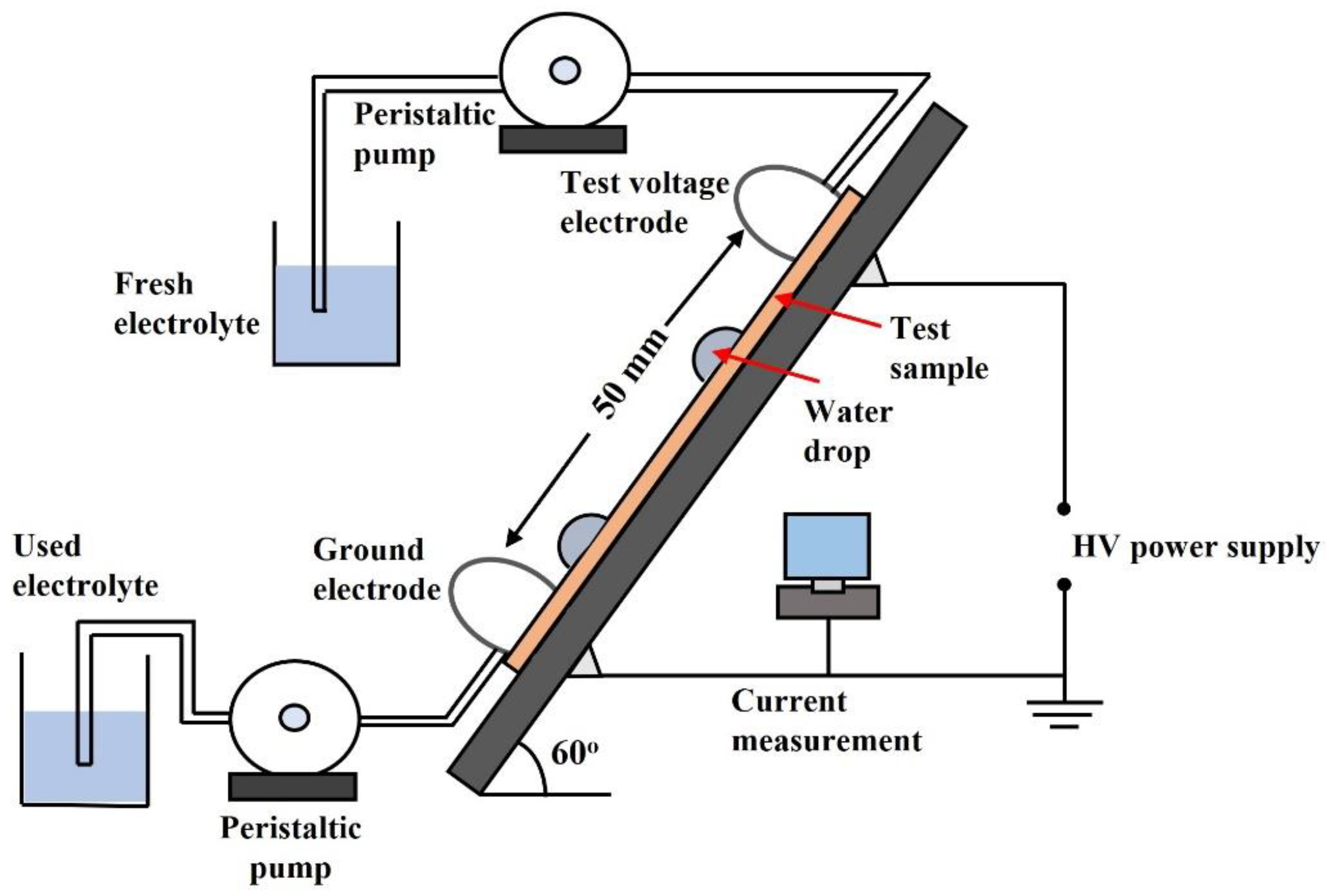

| Parameters | Static Contact Angle [42,73,90] | Dynamic Drop Test (DDT) [91,92,93,94] | Hydrophobicity Transfer Test [89,95,96,97] |
|---|---|---|---|
| Purpose of the test | To quantify the recovery of hydrophobicity after aging test | To evaluate the hydrophobicity retention and recovery in short time | To evaluate the polymer’s ability to transfer intrinsic LMW species onto pollution particles |
| Measurements | Assessment of recovery based on static drop contact angle measurement with respect to resting time | Evaluation via estimating the sample’s failure time (min), i.e., the time when leakage current exceeds a defined level (2 mA for 1 s) | Evaluation through static and dynamic contact-angle measurements at defined transfer time intervals after building silica layer |
| Measuring setup | A goniometer is used for measurement of water contact angle on a flat horizontal polymer surface | Test setup consists of a pair of electrodes, a tilted silicone rubber sample, and a current sensor for detection of leakage current | A mask of adhesive foil is used for applying a uniform silica layer. A mask of 0.36 mm thickness provides good results. A goniometer is used to measure contact angles |
| Voltage | Measurements are conducted without exposing polymer to electric stress | Test voltage (3–6 kV AC rms or DC) is applied until the sample fails | This test can be performed without applying test voltage to silicone rubber |
| Accuracy | It has the lowest accuracy and yields a high scattering of test results. It is not applicable to polluted surface | It is an accurate and reliable aging test for reducing scattering in test results (Interval-based DDT) | It has high accuracy and provides reproducible results with reduced scattering. A too-thick and uneven silica layer may affect its accuracy |
| Reproducibility, standardization | It is not a standardized method for recovery evaluation and provides unreproducible test results | It has the potential to standardize and achieve reproducible results for evaluating hydrophobicity retention and recovery properties | It is an appropriate and reproducible test method for evaluation of dynamic hydrophobic transfer and recovery properties |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamand, F.Z.; Mehmood, B.; Ghunem, R.; Hassan, M.K.; El-Hag, A.; Al-Sulaiti, L.; Abdala, A. Self-Healing Silicones for Outdoor High Voltage Insulation: Mechanism, Applications and Measurements. Energies 2022, 15, 1677. https://doi.org/10.3390/en15051677
Kamand FZ, Mehmood B, Ghunem R, Hassan MK, El-Hag A, Al-Sulaiti L, Abdala A. Self-Healing Silicones for Outdoor High Voltage Insulation: Mechanism, Applications and Measurements. Energies. 2022; 15(5):1677. https://doi.org/10.3390/en15051677
Chicago/Turabian StyleKamand, Fadi Z., Basharat Mehmood, Refat Ghunem, Mohammad K. Hassan, Ayman El-Hag, Leena Al-Sulaiti, and Ahmed Abdala. 2022. "Self-Healing Silicones for Outdoor High Voltage Insulation: Mechanism, Applications and Measurements" Energies 15, no. 5: 1677. https://doi.org/10.3390/en15051677
APA StyleKamand, F. Z., Mehmood, B., Ghunem, R., Hassan, M. K., El-Hag, A., Al-Sulaiti, L., & Abdala, A. (2022). Self-Healing Silicones for Outdoor High Voltage Insulation: Mechanism, Applications and Measurements. Energies, 15(5), 1677. https://doi.org/10.3390/en15051677









