Analysis of Reject Water Formed in the Mechanical Dewatering Process of Digested Sludge Conditioned by Physical and Chemical Methods
Abstract
:1. Introduction
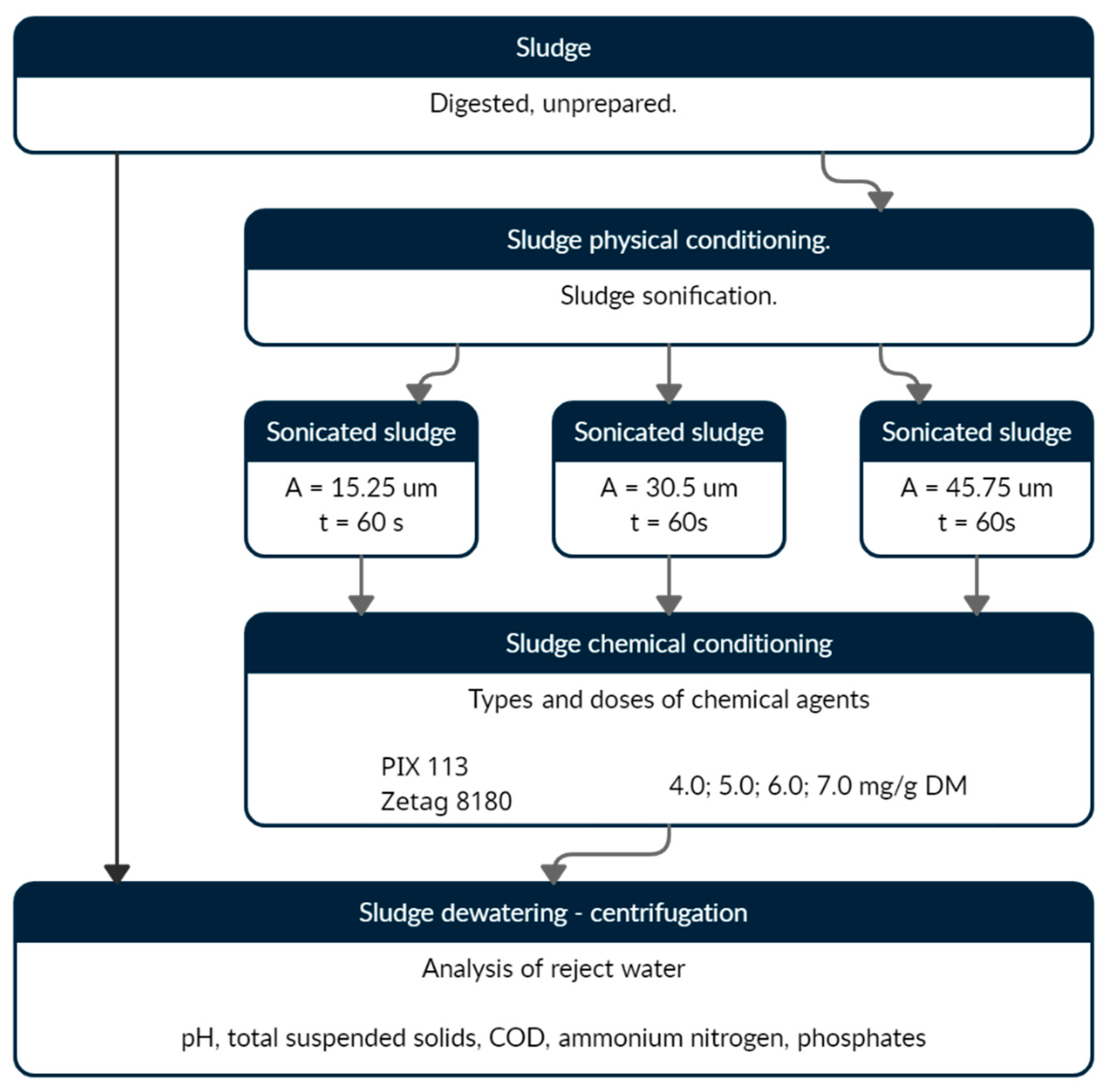
2. Materials and Methods
- -
- the thickening of primary sludge in primary sedimentation tanks,
- -
- the final thickening of primary sludge in gravity thickeners,
- -
- the thickening of excess sludge in a mechanical thickener,
- -
- the single-stage mesophilic anaerobic digestion of the mixed sludge,
- -
- the stabilization and thickening of digested sludge in open digestion chambers (ODC),
- -
- the mechanical dewatering of digested sludge using belt filter presses, and
- -
- thermal drying.
3. Results
4. Discussion
5. Conclusions
- -
- The concentration of COD in the reject water increased with the increase of the amplitude of the sonication wave. The use of chemical agents for sludge conditioning reduced the COD value in the reject water. The COD concentration decreased with the increase of the chemical dose. The best COD reduction effect (to 280 mgO2/dm3) was observed for reject water obtained from non-sonicated sludge prepared with PIX 113 coagulant at a dose of 7.0 mg/g DM.
- -
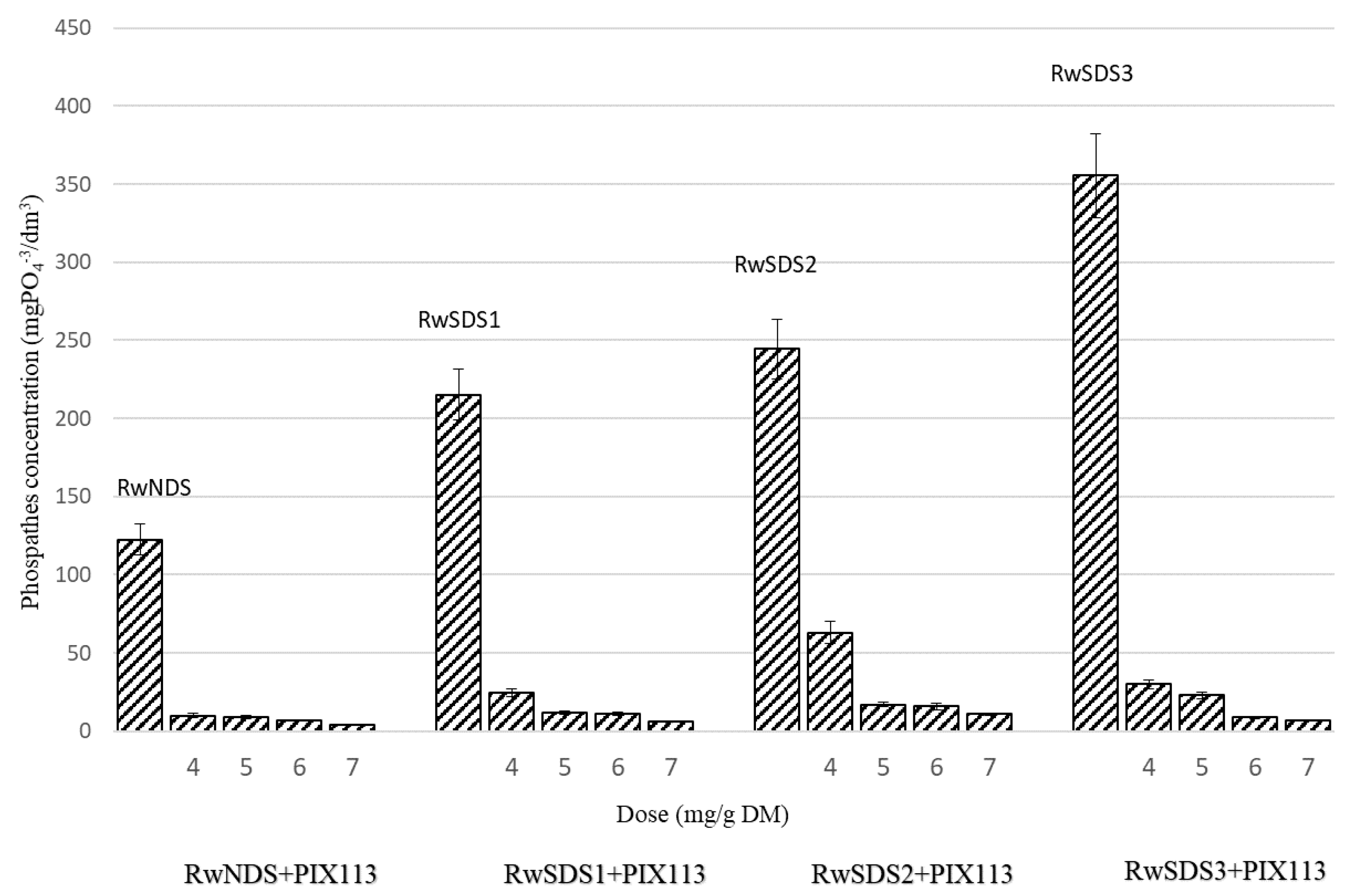
- The phosphate concentration in the reject water obtained from sonicated sludge increased along with the increase of the ultrasonic field’s amplitude. However, it decreased with the increase of the dose of chemicals agents added to the sludge. The lowest value of phosphate concentration (4.3 mgPO4−3/dm3) was observed for the reject water from non-sonicated sludge prepared with PIX 113 at a dose of 7.0 mg/g DM.
- -
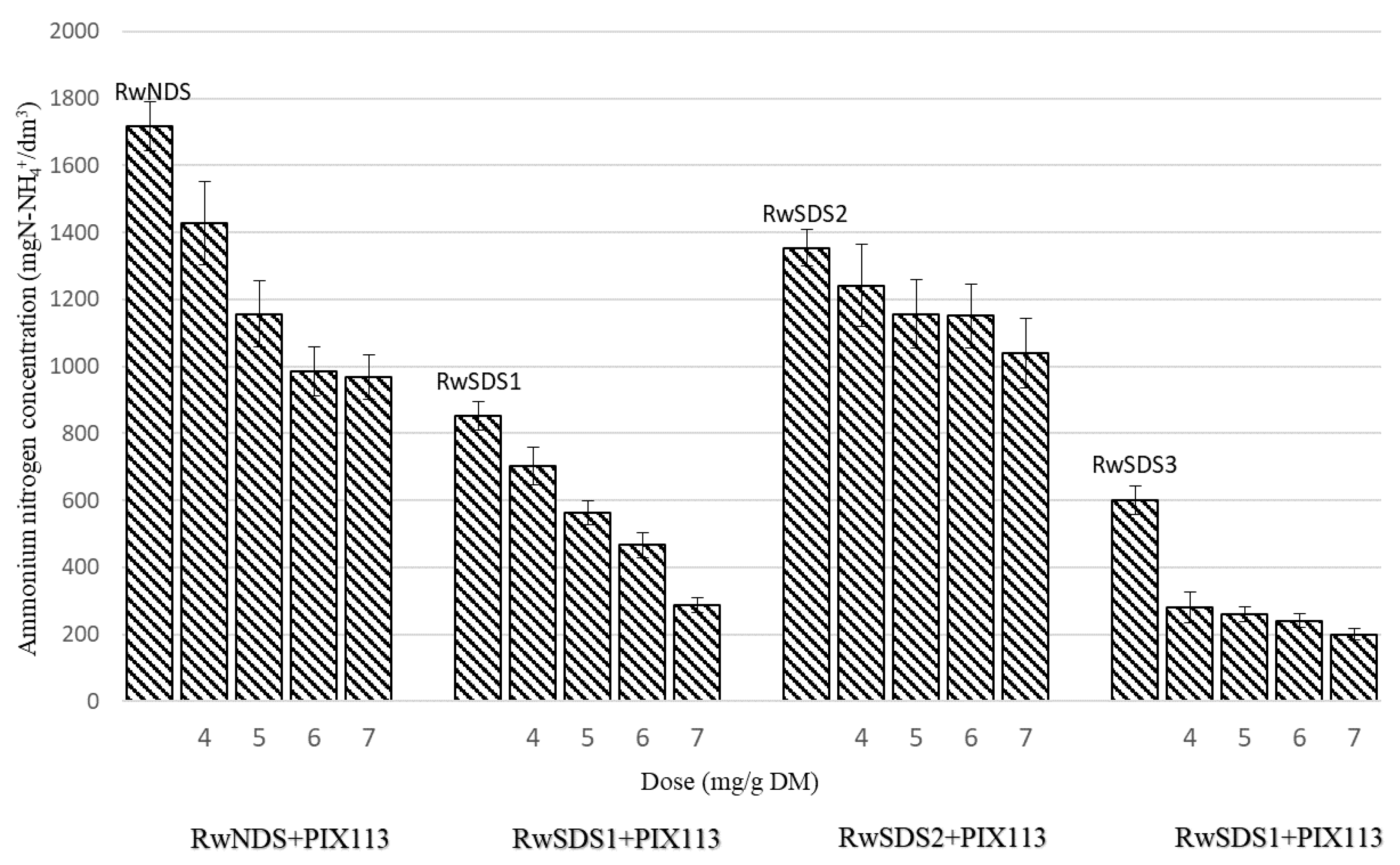
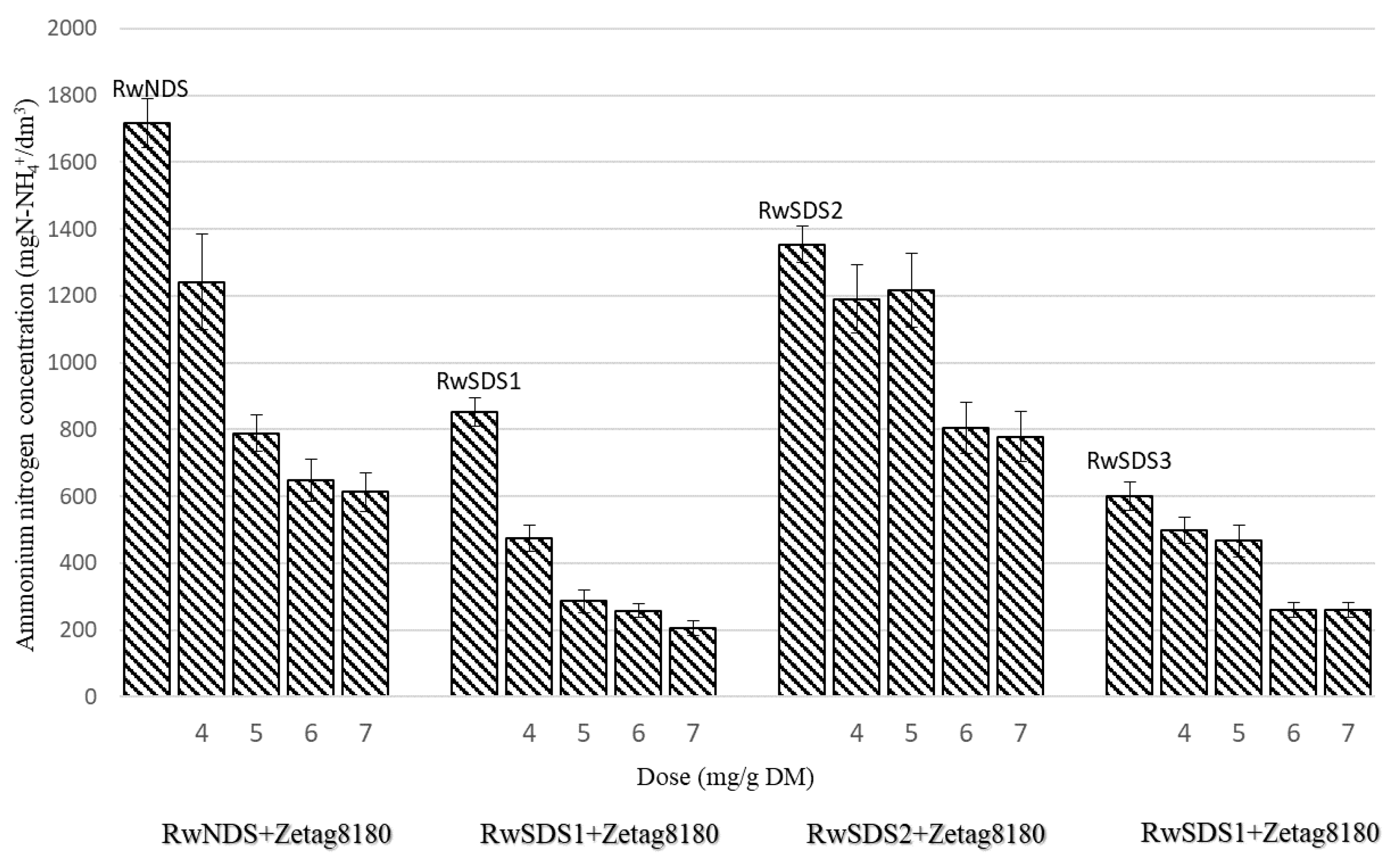
- The concentration of ammonium nitrogen in the reject water decreased with the increase of the amplitude of the ultrasonic field applied for the physical conditioning of the sludge. The addition of chemical agents to the sludge resulted in the further reduction in the concentration of ammonium nitrogen in the reject water. The lowest concentration of ammonium nitrogen (200 mg N-NH4+/dm3) was observed in the reject water obtained after the filtration of the sonicated sludge prepared with the addition of PIX 113 (at a dose of 7.0 mg/g DM).
- -
- The sludge sonication increases the content of impurities (COD, phosphates) in the reject water along with an increase in the amplitude of the ultrasonic wave. The introduction of chemical agents causes a decrease of pollutants with an increase of the chemical dose.
- -
- PIX 113 coagulant gives much better results regarding the reduction of the impurities in the reject water than the polyelectrolyte Zetag 8180.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wójcik, M.; Stachowicz, F. Influence of physical, chemical and dual sewage sludge conditioning methods on the dewatering efficiency. Powder Technol. 2019, 344, 96–102. [Google Scholar] [CrossRef]
- Zawieja, I.; Worwąg, M. Biogas Production from excess sludge oxidized with peracetic acid (PAA). Energies 2021, 14, 3434. [Google Scholar] [CrossRef]
- Chen, Z.; Afzal, M.T.; Salema, A.A. Microwave drying of wastewater sewage sludge. J. Clean Energy Technol. 2014, 2, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Wolny, L.; Wolski, P. Ultrasounds energy as an agent of polyelectrolyte modification prior to sewage sludge conditioning. Energies 2021, 14, 6165. [Google Scholar] [CrossRef]
- Li, H.; Zou, S.; Li, C.; Jin, Y. Alkaline post-treatment for improved sludge anaerobic digestion. Bioresour. Technol. 2013, 140, 187–191. [Google Scholar] [CrossRef]
- Kubacki, P.; Borowski, S. Physico-chemical characteristics and treatment methods of anaerobic supernatant. Przemysl. Chemiczny 2014, 93, 528–530. [Google Scholar]
- Guo, C.H.; Stabnikov, V.; Ivanov, V. The removal of nitrogen and phosphorus from reject water of municipal wastewater treatment plant using ferric and nitrate bioreductions. Bioresour. Technol. 2010, 101, 3992–3999. [Google Scholar] [CrossRef]
- Sperczyńska, E. Phosphates removal from reject water from digestion of sludge. Ecol. Eng. 2016, 48, 196–201. (In Polish) [Google Scholar]
- Wang, S.; Yu, H.; Su, Q.; Zuo, J. Exploring the role of heterotrophs in partial nitritation-anammox process treating thermal hydrolysis process—Anaerobic digestion reject water. Bioresour. Technol. 2021, 341, 125762. [Google Scholar] [CrossRef]
- Wang, W.G.; Xie, H.C.; Wang, H.; Xue, H.; Wang, J.J.; Zhou, M.; Dai, X.H.; Wang, Y.Y. Organic compounds evolution and sludge properties variation along partial nitritation and subsequent anammox processes treating reject water. Water Res. 2020, 184, 116197. [Google Scholar] [CrossRef]
- Kim, I.-T.; Lee, Y.-E.; Jeong, Y.; Yoo, Y.-S. A novel method to remove nitrogen from reject water in wastewater treatment plants using a methane- and methanol-dependent bacterial consortium. Water Res. 2020, 172, 115512. [Google Scholar] [CrossRef] [PubMed]
- Erdirençelebi, D.; Küçükhemek, M. Diagnosis of the anaerobic reject water effects on WWTP operational characteristics as a precursor of bulking and foaming. Water Sci. Technol. 2015, 71, 572–579. [Google Scholar] [CrossRef]
- Myszograj, S. Quantity and characteristics of sludge liquids formed in wastewater treatment plants. Eng. Prot. Environ. 2008, 11, 219–227. (In Polish) [Google Scholar]
- Morales, N.; Val del Río, A.; Vázquez-Padín, J.R.; Méndez, R.; Mosquera-Corral, A.; Campos, J.L. Integration of the Anammox process to the rejection water and mainstream lines of WWTPs. Chemosphere 2015, 140, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, W.; Karolinczak, B.; Gajewska, M.; Wojciechowska, E. Application of subsurface vertical flow constructed wetlands to reject water treatment in dairy wastewater treatment plant. Environ. Technol. 2017, 38, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Rikmann, E.; Zekker, I.; Tenno, T.; Saluste, A.; Tenno, T. Inoculum-free start-up of biofilm- and sludge-based deammonification systems in pilot scale. J. Environ. Sci. Technol. 2018, 15, 133–148. [Google Scholar] [CrossRef]
- Wett, B.; Podmirseg, S.B.; Gomez-Brandon, M.; Hell, M.; Nyhuis, G.; Bott, C.; Murthy, S. Expanding DEMON sidestream deammonification technology towards mainstream application. Water Environ. Res. 2015, 87, 2084–2089. [Google Scholar] [CrossRef]
- Qian, Y.; Ding, Y.; Ma, H.; Chi, Y.; Yuan, H.-Y.; Li, Y.; Tian, S.; Zhang, B. Startup and performance of a novel single-stage partial nitritation/anammox system for reject water treatment. Bioresour. Technol. 2021, 321, 124432. [Google Scholar] [CrossRef]
- Mehrdad, M.; Park, H.; Ramalingam, K.; Fillos, J.; Beckmann, K.; Deur, A.; Chandran, K. Anammox moving bed biofilm reactor pilot at the 26th Ward wastewater treatment plants in Brooklyn, New York: Start-up, biofilm population diversity and performance optimization. Water Sci. Technol. 2014, 70, 1448–1455. [Google Scholar] [CrossRef]
- Gu, Z.; Li, Y.; Yang, Y.; Xia, S.; Hermanowicz, S.W.; Alvarez-Cohen, L. Inhibition of anammox by sludge thermal hydrolysis and metagenomic insights. Bioresour. Technol. 2018, 270, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Sperczyńska, E. Use of zeolite for removal of ammonium nitrogen from reject water. Eng. Prot. Environ. 2016, 19, 391–399. (In Polish) [Google Scholar] [CrossRef]
- Bień, B.; Bień, J. Conditioning of sewage sludge with physical, chemical and dual methods to improve Sewage sludge dewatering. Energies 2021, 14, 5079. [Google Scholar] [CrossRef]
- Wu, X.; Modin, O. Ammonium recovery from reject water combined with hydrogen production in a bioelectrochemical reactor. Bioresour. Technol. 2013, 146, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piaskowski, K.; Ćwikałowska, M. Profile of changes of orthophosphate concentrations during sewage and sewage sludge treatment. Rocz. Ochr. Sr. 2007, 9, 183–197. [Google Scholar]
- Wang, R.; Li, Y.; Wang, W.; Chen, Y.; Vanrolleghem, P.A. Effect of high orthophosphate concentration on mesophilic anaerobic sludge digestion and its modeling. Chem. Eng. J. 2015, 260, 791–800. [Google Scholar] [CrossRef]
- Battistoni, P.; Pavan, P.; Prisciandaro, M.; Cecchi, F. Struvite crystallization: A feasible and reliable way to fix phosphorus in anaerobic supernatants. Water Res. 2000, 34, 3033–3041. [Google Scholar] [CrossRef]
- Claudia Santiviago, C.; Peralta, J.; López, I. Phosphorus removal from wastewater through struvite crystallization in a continuous fluidized-bed reactor: An improved comprehensive model. Chem. Eng. J. 2021, 430, 132903. [Google Scholar] [CrossRef]
- Hong, T.; Wei, L.; Cui, K.; Chen, T.; Luo, L.; Fu, M.; Zhang, Q. A constant composition technique for quantifying the effect of As(V) on struvite crystallization under various operational conditions. J. Cryst. Growth 2020, 552, 125925. [Google Scholar] [CrossRef]
- Mudragada, R.; Kundral, S.; Coro, E.; Moncholi, M.E.; Laha, S.; Tansel, B. Phosphorous removal during sludge dewatering to prevent struvite formation in sludge digesters by full scale evaluation. J. Water Process Eng. 2014, 2, 37–42. [Google Scholar] [CrossRef]
- Ren, W.; Zhou, Z.; Jiang, L.M.; Hu, D.; Qiu, Z.; Wei, H.; Wang, L. A cost-effective method for the treatment of reject water from sludgedewatering process using supernatant from sludge lime stabilization. Sep. Purif. Technol. 2015, 142, 123–128. [Google Scholar] [CrossRef]
- Podstawczyk, D.; Witek-Krowiak, A.; Dawiec-Liśniewska, A.; Chrobot, P.; Skrzypczak, D. Removal of ammonium and orthophosphates from reject water generated during dewatering of digested sewage sludge in municipal wastewater treatment plant using adsorption and membrane contactor system. J. Clean. Prod. 2017, 161, 277–287. [Google Scholar] [CrossRef]
- Bradford-Hartke, Z.; Lane, J.; Lant, P.; Leslie, G. Environmental benefits and burdens of phosphorus recovery from municipal wastewater. Environ. Sci. Technol. 2015, 49, 8611–8622. [Google Scholar] [CrossRef] [PubMed]
- Koskue, V.; Rinta-Kanto, J.M.; Freguia, S.; Ledezma, P.; Kokko, M. Optimising nitrogen recovery from reject water in a 3-chamber bioelectroconcentration cell. Sep. Purif. Technol. 2021, 264, 118428. [Google Scholar] [CrossRef]
- Hao, X.; Wang, C.; Loosdrecht, M.C.M.; Hu, Y. Looking beyond struvite for P-recovery. Environ. Sci. Technol. 2013, 47, 4965–4966. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.T.; Li, B.; Boiarkina, I.; Baroutian, S.; Yu, W.; Young, B.R. Phosphate recovery from hydrothermally treated sewage sludge using struvite precipitation. Bioresour. Technol. 2017, 239, 171–179. [Google Scholar] [CrossRef]
- Desloover, J.; Woldeyohannis, A.A.; Verstraete, W.; Boon, N.; Rabaey, K. Electrochemical resource recovery from digestate to prevent ammonia toxicity during anaerobic digestion. Environ. Sci. Technol. 2012, 46, 12209–12216. [Google Scholar] [CrossRef]
- Koskue, V.; Freguia, S.; Ledezma, P.; Kokko, M. Efficient nitrogen removal and recovery from real digested sewage sludge reject water through electroconcentration. J. Environ. Chem. Eng. 2021, 9, 106286. [Google Scholar] [CrossRef]
- Jermakka, J.; Thompson, E.; Ledezma, P.; Freguia, S. Electro-concentration for chemical-free nitrogen capture as solid ammonium bicarbonate. Sep. Purif. Technol. 2018, 203, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Ward, A.J.; Arola, K.; Brewster, E.T.; Mehta, C.M.; Batstone, D.J. Nutrient recovery from wastewater through pilot scale electrodialysis. Water Res. 2018, 135, 57–65. [Google Scholar] [CrossRef]
- Bień, B.; Bień, J. Dewatering of sewage sludge treated by the combination of ultrasonic field and chemical methods. Desalin. Water Treat. 2020, 199, 72–78. [Google Scholar] [CrossRef]
- Kamizela, T.; Kowalczyk, M. Impact of conditioning substances and filtration pressure on dewatering efficiency of sewage. Energies 2021, 14, 361. [Google Scholar] [CrossRef]
- Hu, D.; Zhou, Z.; Niu, T.; Wei, H.; Dou, W.; Jiang, L.M.; Lv, Y. Co-treatment of reject water from sludge dewatering and supernatant from sludge lime stabilization process for nutrient removal: A cost-effective approach. Sep. Pur. Technol. 2017, 172, 357–365. [Google Scholar] [CrossRef]
- Technologie Sanitarne. Available online: http://www.technologie-sanitarne.com/Koagulant_zelazowy_Pix_113_-3-205541-66_60_73.html (accessed on 22 January 2022).
- Kemipol. Available online: http://www.old.kemipol.com.pl/img/pdf/karty_2009/20-1-K-PIX_113-SIARCZAN_VI_ZELAZA_III_Xn.pdf (accessed on 22 January 2022).
- Aniq. Available online: https://aniq.org.mx/pqta/pdf/Zetag_8180(HT).pdf (accessed on 22 January 2022).
- PN-ISO 6060: 2006. Available online: http://sklep.pkn.pl/pn-iso-6060-2006p.html (accessed on 22 January 2022).
- Zawieja, I.; Wolny, L. Effect of Sonicator Power on the Biodegradability of sewage sludge. Rocz. Ochr. Sr. 2011, 13, 1719–1730. (In Polish) [Google Scholar]
- Molaey, R.; Yesil, H.; Calli, B.; Tugtas, A.E. Enhanced heavy metal leaching from sewage sludge through anaerobic fermentation and air-assisted ultrasonication. Chemosphere 2021, 279, 130548. [Google Scholar] [CrossRef] [PubMed]
- Kupczyk, K.; Zieliński, M.; Dębowski, M.; Szwarc, D.; Rokicka, M.; Hajduk, A. Utrasonic disintegration as a method of conditioning the biomass of microalgae Chlorella vulgaris before the process of methane fermentation. Logistyka 2015, 4, 9373–9378. (In Polish) [Google Scholar]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Comparison of ultrasound and thermal pretreatment of Scenedesmus biomass on methane production. Bioresour. Technol. 2012, 110, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Tiehm, A.; Nickel, K.; Neis, U. The use of ultrasound to accelerate the anaerobic digestion of sewage sludge. Water Sci. Technol. 1997, 36, 121–128. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Zhang, G.; Ren, Z.; Zhu, Y. Critical review on ultrasound lysis-cryptic growth for sludge reduction. J. Environ. Chem. Eng. 2021, 9, 106263. [Google Scholar] [CrossRef]
- Hajduk, A.; Dębowski, M.; Zieliński, M.; Kłodowska, I.; Rozpondek, P.; Rokicka, M.; Kupczyk, K.; Szwarc, D.; Łączyńska, B. Disintegration sludge in a loop reactor. Eko-Doc. Proc. 2015, 5, 150–157. [Google Scholar]
- Rai, C.L.; Struenkmann, G.; Mueller, J.; Rao, P.G. Influence of ultrasonic disintegration on sludge growth reduction and its estimation by respirometry. Environ. Sci. Technol. 2004, 38, 5779–5785. [Google Scholar] [CrossRef]
- Tiehm, A.; Nickel, K.; Zellhorn, M.; Neis, U. Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Res. 2001, 35, 2003–2009. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, G.; Wang, W. Ultrasonic treatment of biological sludge: Floc disintegration, cell lysis and inactivation. Bioresour. Technol. 2007, 98, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Negral, L.; Marañón, E.; Fernández-Nava, Y.; Castrillón, L. Short term evolution of soluble COD and ammonium in pre-treated sewage sludge by ultrasound and inverted phase fermentation. Chem. Eng. Process. 2013, 69, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Battistoni, P.; Paci, B.; Fatone, F.; Pavan, P. Phosphorus removal from anaerobic supernatants: Start-up and steady-state conditions of a fluidized bed reactor full-scale plant. Ind. Eng. Chem. Res. 2006, 45, 663–669. [Google Scholar] [CrossRef]
- Ren, W.; Zhou, Z.; Wan, L.; Hu, D.; Jiang, L.M.; Wang, L. Optimization of phosphorus removal from reject water of sludge thickening and dewatering process through struvite precipitation. Desal. Water Treat. 2016, 57, 15515–15523. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.Q.; Babatunde, A.O.; Kearney, P. Two strategies for phosphorus removal from reject water of municipal wastewater treatment plant using alum sludge. Water Sci. Technol. 2009, 60, 3181–3188. [Google Scholar] [CrossRef]



| Chemical Reagent | Description |
|---|---|
| PIX 113 | A ferric coagulant, a dark brown water solution of ferric sulphate, with total iron (Fe) content of 11.4 ± 12.2%, and ironions Fe2+ content of 0.4 ± 0.3% [43,44] |
| Zetag 8180 | Copolymer of acrylamide and quaternized cationic monomer, highly effective, supplied as off-white granular solid powder, manufactured by BASF Corporation [45] |
| Lp | Symbol | Description |
|---|---|---|
| 1. | RwNDS | Reject water from non-sonicated digested sludge |
| 2. | RwSDS1 RwSDS2 RwSDS3 | Reject water from sonicated digested sludge. The time of sonication 60s, the amplitude: A1 = 15.25 µm (RwSDS1); A2 = 30.5 µm (RwSDS2); A3 = 45.75 µm (RwSDS3) respectively. |
| 3. | RwNDS + PIX113 RwNDS + Zetag 8180 | Reject water from non-sonicated sludge prepared with chemical reagents: PIX 113, Zetag 8180 |
| 4. | RwSDS1 + PIX113 RwSDS1 + Zetag 8180 | Reject water from sonicated digested sludge (t = 60 s, A1 = 15.25 µm) and chemically prepared with: PIX 113, Zetag 8180 |
| 5. | RwSDS2 + PIX113 RwSDS2 + Zetag 8180 | Reject water from sonicated digested sludge (t = 60 s, A1 = 30.5 µm) and chemically prepared with: PIX 113, Zetag 8180 |
| 6. | RwSDS3 + PIX113 RwSDS3 + Zetag 8180 | Reject water from sonicated digested sludge (t = 60 s, A1 = 45.75 µm) and chemically prepared with: PIX 113, Zetag 8180 |
| No | Indicator | Unit | Value |
|---|---|---|---|
| 1 | pH | - | 7.32 |
| 2 | Total suspended solids (TSS) | mg/dm3 | 1142 |
| 3 | Chemical oxygen demand (COD) | mgO2/dm3 | 2240 |
| 4 | Ammonium nitrogen | mgN-NH4+/dm3 | 1718 |
| 5 | Phosphates | mgPO4−3/dm3 | 122.4 |
| 6 | Phosphorus | mgP-PO4−3/dm3 | 40 |
| Parameter | Dose | pH | Phosphates | Phosphorus | Ammonium Nitrogen | COD | TSS |
|---|---|---|---|---|---|---|---|
| Unit | mg/g DM | - | mgPO4−3/dm3 | mgP-PO4−3/dm3 | N-NH4+/dm3 | mg O2/dm3 | mg/dm3 |
| Reject water separated from non-sonicated digested sludge prepared with: | |||||||
| PIX 113 | 4.0 | 6.27 | 10.1 | 3.3 | 1427 | 770 | 350 |
| 5.0 | 5.95 | 8.9 | 2.9 | 1155.7 | 500 | 300 | |
| 6.0 | 5.56 | 7.0 | 2.3 | 984.8 | 320 | 260 | |
| 7.0 | 5.02 | 4.3 | 1.4 | 969 | 280 | 110 | |
| Zetag 8180 | 4.0 | 7.43 | 50.5 | 16.5 | 1241.1 | 2230 | 540 |
| 5.0 | 7.44 | 41.6 | 13.6 | 788.4 | 1930 | 340 | |
| 6.0 | 7.45 | 38.7 | 12.7 | 648.2 | 1850 | 240 | |
| 7.0 | 7.47 | 26.6 | 8.7 | 612.7 | 1670 | 140 | |
| Reject water separated from sonicated (A = 15.25 μm; t = 60 s) sludge | |||||||
| - | 7.8 | 215.2 | 70.3 | 853 | 3679.2 | 1083 | |
| and sludge prepared by: | |||||||
| PIX 113 | 4.0 | 5.9 | 24.9 | 8.1 | 703 | 1394.4 | 783.3 |
| 5.0 | 5.7 | 11.8 | 3.8 | 562 | 604.8 | 683.2 | |
| 6.0 | 5.4 | 11.1 | 3.6 | 466 | 474 | 583.1 | |
| 7.0 | 5.0 | 6.2 | 2.0 | 288 | 386.4 | 333.3 | |
| Zetag 8180 | 4.0 | 7.8 | 98.4 | 32.1 | 474 | 3354 | 500 |
| 5.0 | 7.8 | 95.3 | 31.1 | 286 | 2734.8 | 466.7 | |
| 6.0 | 7.8 | 91.4 | 29.9 | 258 | 2614.4 | 383.3 | |
| 7.0 | 7.9 | 68.4 | 22.3 | 206 | 2390.8 | 266.7 | |
| Reject water separated from sonicated (A = 30.5 μm; t = 60 s) sludge | |||||||
| - | 7.86 | 244.5 | 79.9 | 1354 | 3960 | 1230 | |
| and sludge prepared by: | |||||||
| PIX 113 | 4.0 | 6.4 | 63.0 | 20.6 | 1241.1 | 900 | 580 |
| 5.0 | 6.2 | 17.1 | 5.6 | 1155.7 | 590 | 470 | |
| 6.0 | 5.8 | 15.9 | 5.2 | 1150.6 | 570 | 370 | |
| 7.0 | 5.52 | 11.0 | 3.6 | 1040.1 | 400 | 310 | |
| Zetag 8180 | 4.0 | 7.88 | 156.7 | 51.2 | 1190.8 | 2880 | 770 |
| 5.0 | 7.89 | 134.9 | 44.1 | 1215.9 | 2800 | 560 | |
| 6.0 | 7.90 | 131.9 | 43.1 | 803.9 | 2640 | 480 | |
| 7.0 | 7.95 | 111.7 | 36.5 | 778.8 | 2400 | 440 | |
| Reject water separated from sonicated (A = 47.75 μm; t = 60 s) sludge | |||||||
| - | 7.88 | 355.5 | 116.2 | 600 | 5260 | 2100 | |
| and sludge prepared by: | |||||||
| PIX 113 | 4.0 | 6.1 | 30 | 9.8 | 280 | 1172 | 766.7 |
| 5.0 | 5.8 | 23 | 7.5 | 260 | 862 | 533.3 | |
| 6.0 | 5.4 | 8.8 | 2.9 | 240 | 689.6 | 483.3 | |
| 7.0 | 5.1 | 7 | 2.3 | 200 | 586 | 466.6 | |
| Zetag 8180 | 4.0 | 7.9 | 332.5 | 108.7 | 498 | 5124 | 616.7 |
| 5.0 | 7.92 | 263 | 85.9 | 466 | 4987.4 | 516.7 | |
| 6.0 | 7.93 | 230.5 | 75.3 | 261 | 4577.4 | 433.3 | |
| 7.0 | 7.95 | 213 | 69.6 | 260 | 4440.8 | 400 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bień, B.; Bień, J.D. Analysis of Reject Water Formed in the Mechanical Dewatering Process of Digested Sludge Conditioned by Physical and Chemical Methods. Energies 2022, 15, 1678. https://doi.org/10.3390/en15051678
Bień B, Bień JD. Analysis of Reject Water Formed in the Mechanical Dewatering Process of Digested Sludge Conditioned by Physical and Chemical Methods. Energies. 2022; 15(5):1678. https://doi.org/10.3390/en15051678
Chicago/Turabian StyleBień, Beata, and Jurand D. Bień. 2022. "Analysis of Reject Water Formed in the Mechanical Dewatering Process of Digested Sludge Conditioned by Physical and Chemical Methods" Energies 15, no. 5: 1678. https://doi.org/10.3390/en15051678
APA StyleBień, B., & Bień, J. D. (2022). Analysis of Reject Water Formed in the Mechanical Dewatering Process of Digested Sludge Conditioned by Physical and Chemical Methods. Energies, 15(5), 1678. https://doi.org/10.3390/en15051678






