Radio-Oxidation of Electric Cabled Models: Ageing Evaluation at the Atomic Scale
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
- primary antioxidant Irganox 1076 (chemical formula given Figure 1A)
- secondary antioxidant Irganox PS802 (chemical formula given Figure 1B)
- flame retardant ATH (aluminium trihydrate, whose chemical formula is Al(OH)3).
- XLPE M1: XLPE “as received”
- XLPE M2(Irg1076): XLPE + 1 phr of Irganox 1076
- XLPE M3(IrgPS802): XLPE + 1 phr of Irganox PS802
- XLPE M4(Irg1076-IrgPS802): XLPE + 1 phr of Irganox 1076 + 1 phr of Irganox PS802
- XLPE M7(Irg1076-IrgPS802-ATH50): XLPE + 1 phr of Irganox 1076 + 1 phr of Irganox PS802 + 50 phr of ATH
2.2. Irradiation Conditions
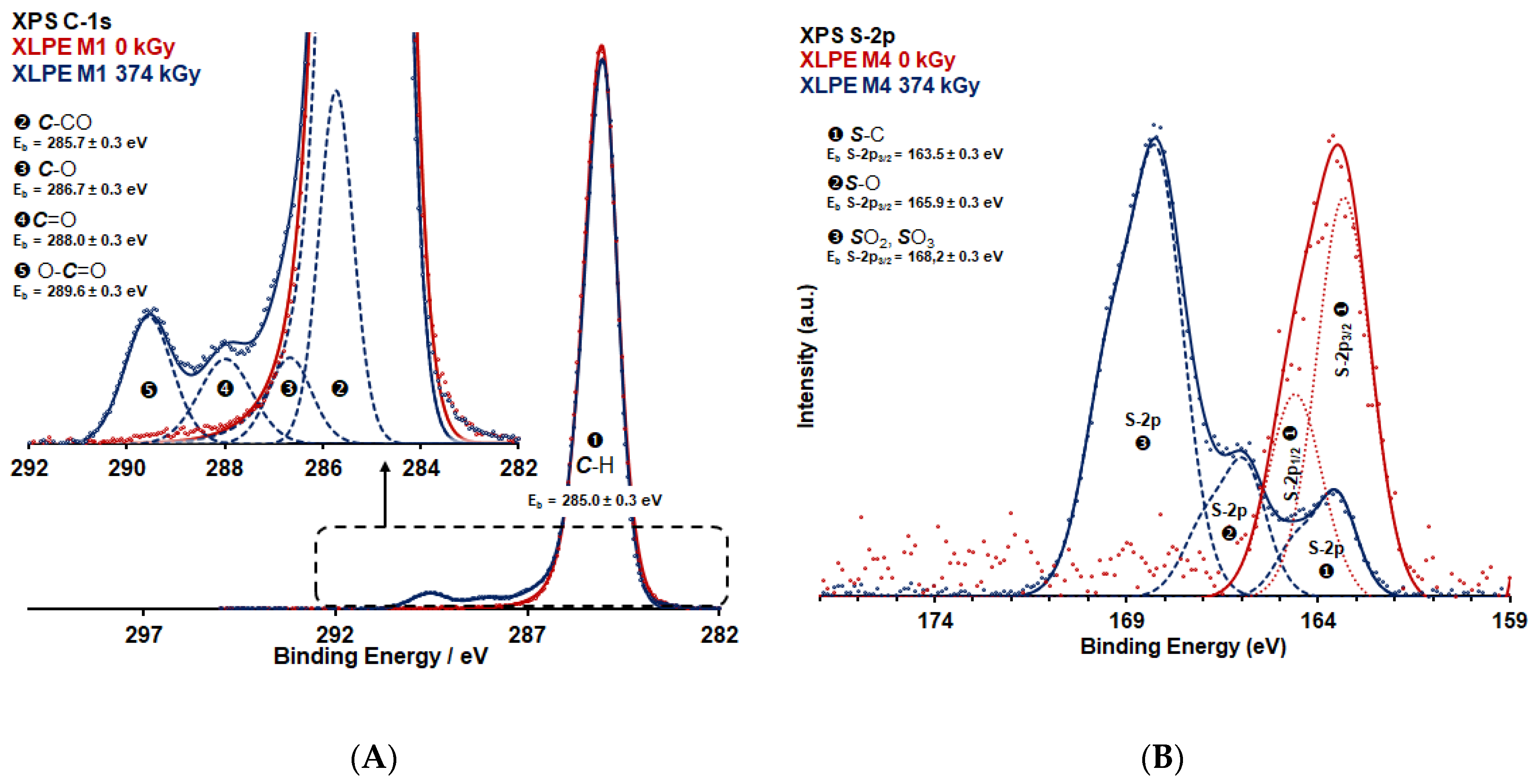
2.3. Characterisation Using XPS
3. Results
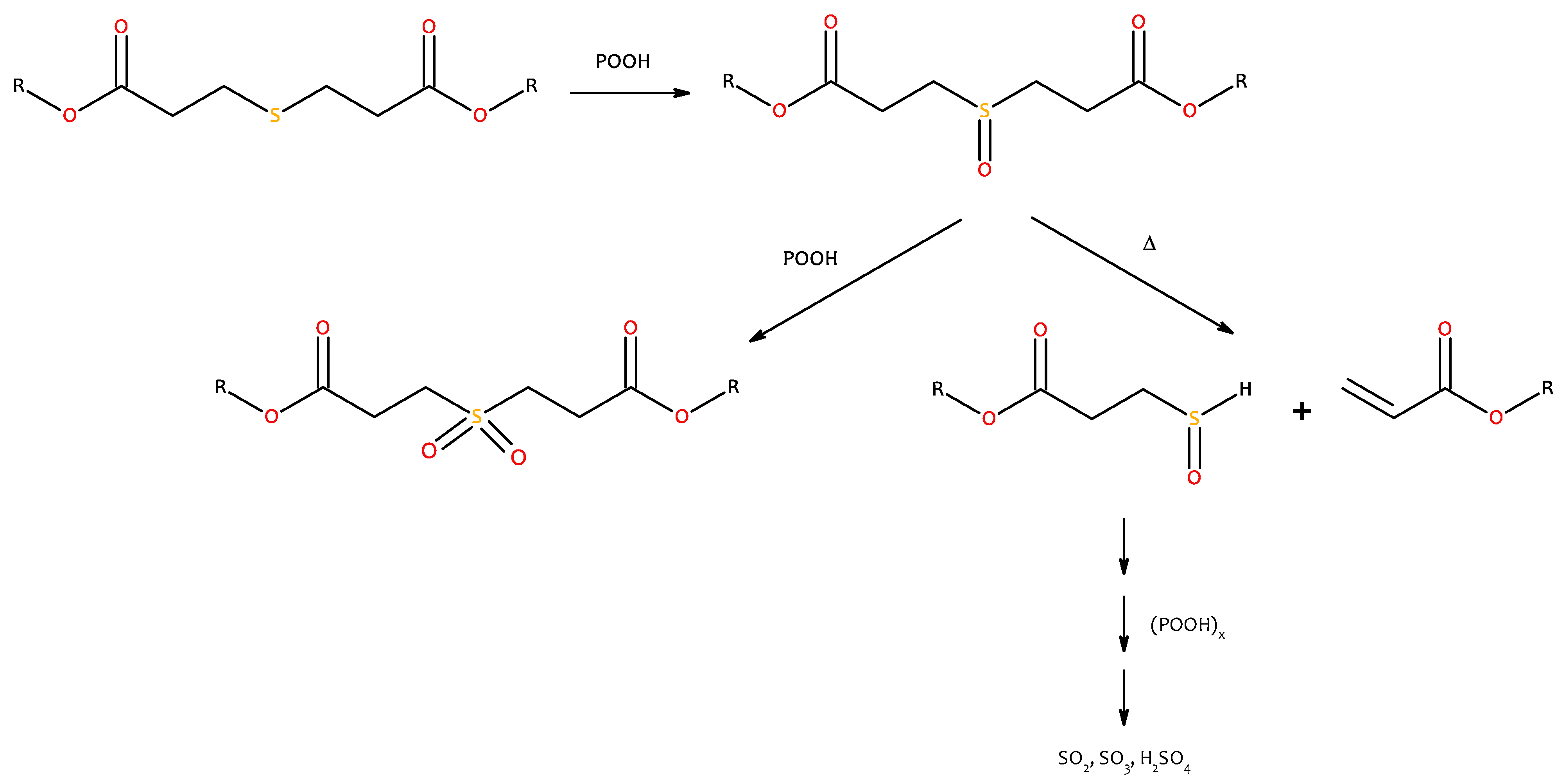
4. Discussion
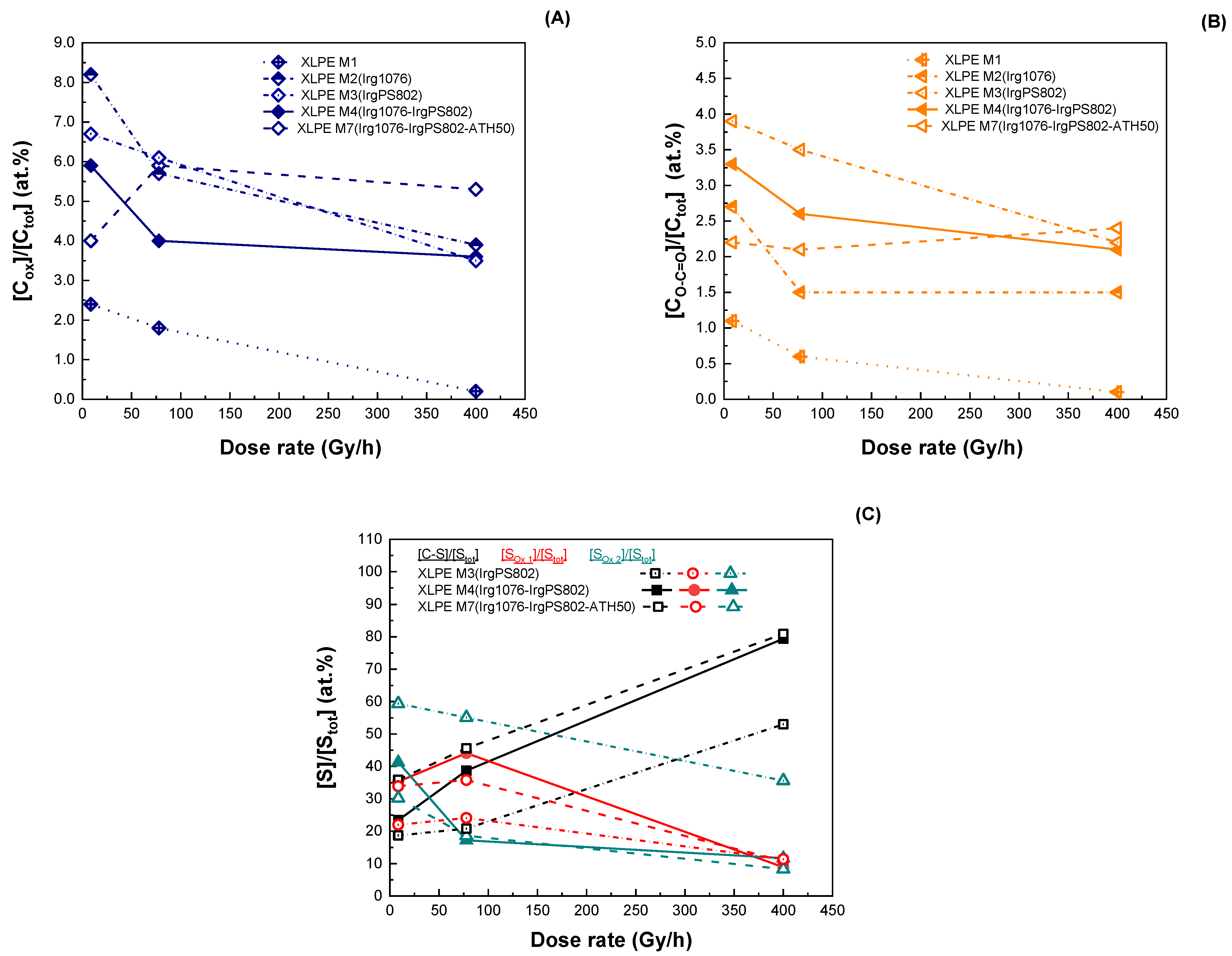
4.1. Dose-Rate Effect
4.2. Dose Effect
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartoníček, B.; Hnát, V.; Plaček, V. Assessment of the insulation degradation of cables used in nuclear power plants. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1999, 151, 423–426. [Google Scholar] [CrossRef]
- Calmet, J.; Carlin, F.; Nguyen, T.; Bousquet, S.; Quinot, P. Irradiation ageing of CSPE/EPR control command electric cables. Correlation between mechanical properties and oxidation. Radiat. Phys. Chem. 2002, 63, 235–239. [Google Scholar] [CrossRef]
- Dole, P.; Chauchard, J. Aging of elastomers in nuclear power plants. Effect of temperature and irradiation. Angew. Makromol. Chem. 1996, 237, 79–98. [Google Scholar] [CrossRef]
- Reynolds, A.; Bell, R.; Bryson, N.; Doyle, T.; Hall, M.; Mason, L.; Quintric, L.; Terwilliger, P. Dose-rate effects on the radiation-induced oxidation of electric cable used in nuclear power plants. Radiat. Phys. Chem. 1995, 45, 103–110. [Google Scholar] [CrossRef]
- Simmons, K.L.; Fifield, L.S.; Westman, M.P.; Ramuhalli, P.; Pardini, A.F.; Tedeschi, J.R.; Jones, A.M. Determining Remaining Useful Life of Aging Cables in Nuclear Power Plants?; Interim Study FY13; Pacific Northwest National Laboratory: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Yamamoto, T.; Minakawa, T. JNES-SS-0903 Final Report of the Project of “Assessment of Cable Aging for Nuclear Power Plants”; Japan Nuclear Energy Safety Organization: Tokyo, Japan, 2009. [Google Scholar]
- Celina, M.; George, G. Characterisation and degradation studies of peroxide and silane crosslinked polyethylene. Polym. Degrad. Stab. 1995, 48, 297–312. [Google Scholar] [CrossRef]
- Mopsik, F.I. Radiation-induced dielectric loss in hydrocarbon polymers. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1989–1993. [Google Scholar] [CrossRef]
- Tamblyn, J.W.; Newland, G.C. Induction period in the aging of polypropylene. J. Appl. Polym. Sci. 1965, 9, 2251–2260. [Google Scholar] [CrossRef]
- Cracco, F.; Arvia, A.J.; Dole, M. ESR Studies of Free Radical Decay in Irradiated Polyethylene. J. Chem. Phys. 1962, 37, 2449–2457. [Google Scholar] [CrossRef]
- Faucitano, A.; Buttafava, A.; Martinotti, F.; Ferloni, P.; Magistris, A. The mechanism of gamma-radiolysis of polymethylene, polypropylene and poly-n-butylene oxides: An ESR investigation. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1992, 40, 347–355. [Google Scholar] [CrossRef]
- Nunome, K.; Muto, H.; Toriyama, K.; Iwasaki, M. ESR studies of local concentrations of radicals in polyethylene irradiated at 1.5, 4.2 and 77 K. Chem. Phys. Lett. 1976, 39, 542–546. [Google Scholar] [CrossRef]
- Yamazaki, T.; Seguchi, T. ESR study on chemical crosslinking reaction mechanisms of polyethylene using a chemical agent—II. The effect of phenolic antioxidants. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 2431–2439. [Google Scholar] [CrossRef]
- Gillen, K.; Bernstein, R.; Clough, R.; Celina, M. Lifetime predictions for semi-crystalline cable insulation materials: I. Mechanical properties and oxygen consumption measurements on EPR materials. Polym. Degrad. Stab. 2006, 91, 2146–2156. [Google Scholar] [CrossRef]
- Seguchi, T.; Tamura, K.; Ohshima, T.; Shimada, A.; Kudoh, H. Degradation mechanisms of cable insulation materials during radiation–thermal ageing in radiation environment. Radiat. Phys. Chem. 2010, 80, 268–273. [Google Scholar] [CrossRef]
- Fayolle, B.; Audouin, L.; Verdu, J. A critical molar mass separating the ductile and brittle regimes as revealed by thermal oxidation in polypropylene. Polymer 2004, 45, 4323–4330. [Google Scholar] [CrossRef]
- Celina, M.; Gillen, K.; Wise, J.; Clough, R. Anomalous aging phenomena in a crosslinked polyolefin cable insulation. Radiat. Phys. Chem. 1996, 48, 613–626. [Google Scholar] [CrossRef]
- Ekelund, M.; Fantoni, P.; Gedde, U. Thermal ageing assessment of EPDM-chlorosulfonated polyethylene insulated cables using line resonance analysis (LIRA). Polym. Test. 2011, 30, 86–93. [Google Scholar] [CrossRef]
- Clough, R.L.; Gillen, K.T. Investigation of Cable Deterioration Inside Reactor Containment. Nucl. Technol. 1982, 59, 344–354. [Google Scholar] [CrossRef]
- Papet, G.; Audouin-Jirackova, L.; Verdu, J. Diffusion controlled radiochemical oxidation of low density polyethylene—II. Kinetic modelling. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1989, 33, 329–335. [Google Scholar] [CrossRef]
- Ferry, M.; Carpentier, F.; Cornaton, M. Radio-Oxidation Ageing of XLPE Containing Different Additives and Filler: Effect on the Gases Emission and Consumption. Polymers 2021, 13, 2845. [Google Scholar] [CrossRef]
- Hettal, S.; Roland, S.; Sipila, K.; Joki, H.; Colin, X. A new analytical model for predicting the radio-thermal oxidation kinetics and the lifetime of electric cable insulation in nuclear power plants. application to silane cross-linked polyethylene. Polym. Degrad. Stab. 2021, 185, 109492. [Google Scholar] [CrossRef]
- Maléchaux, A.; Colombani, J.; Amat, S.; Marque, S.; Dupuy, N. Influence of Gamma Irradiation on Electric Cables Models: Study of Additive Effects by Mid-Infrared Spectroscopy. Polymers 2021, 13, 1451. [Google Scholar] [CrossRef] [PubMed]
- Przybytniak, G.; Sadło, J.; Walo, M. EPR studies on the interaction between additives and polyethylene matrix initiated by gamma radiation. Express Polym. Lett. 2020, 14, 556–565. [Google Scholar] [CrossRef]
- Przybytniak, G.; Sadło, J.; Walo, M.; Wróbel, N.; Žák, P. Comparison of radical processes in non-aged and radiation-aged polyethylene unprotected or protected by antioxidants. Mater. Today Commun. 2020, 25, 101521. [Google Scholar] [CrossRef]
- Suraci, S.V.; Fabiani, D. Aging Modeling of Low-Voltage Cables Subjected to Radio-Chemical Aging. IEEE Access 2021, 9, 83569–83578. [Google Scholar] [CrossRef]
- Suraci, S.V.; Fabiani, D.; Roland, S.; Colin, X. Multi scale aging assessment of low-voltage cables subjected to radio-chemical aging: Towards an electrical diagnostic technique. Polym. Test. 2021, 103, 107352. [Google Scholar] [CrossRef]
- Xu, A.; Roland, S.; Colin, X. Physico-chemical characterization of the blooming of Irganox 1076® antioxidant onto the surface of a silane-crosslinked polyethylene. Polym. Degrad. Stab. 2019, 171, 109046. [Google Scholar] [CrossRef]
- Beamson, G.; Clark, D.; Kendrick, J.; Briggs, D. Observation of vibrational asymmetry in the high resolution monochromatized XPS of hydrocarbon polymers. J. Electron. Spectrosc. Relat. Phenom. 1991, 57, 79–90. [Google Scholar] [CrossRef]
- Briggs, D.; Fairley, N. XPS of chemically modified low-density polyethylene surfaces: Observations on curve-fitting the C 1s spectrum. Surf. Interface Anal. 2002, 33, 283–290. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta Esca 300 Database; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Buncick, M.; Thomas, D.; McKinny, K.; Jahan, M. Structural changes of ultra-high molecular weight polyethylene exposed to X-ray flux in X-ray photoelectron spectroscopy detected by valence band and electron spin resonance spectroscopy. Appl. Surf. Sci. 2000, 156, 97–109. [Google Scholar] [CrossRef]
- Delhalle, J.; André, J.M.; Pireaux, J.J.; Caudano, R.; Verbist, J.J. Electronic structure of polyethylene: Theory and ESCA measurements. J. Chem. Phys. 1974, 60, 595–600. [Google Scholar] [CrossRef]
- Briggs, D.; Brewis, D.M.; Dahm, R.H.; Fletcher, I.W. Analysis of the surface chemistry of oxidized polyethylene: Comparison of XPS and ToF-SIMS. Surf. Interface Anal. 2003, 35, 156–167. [Google Scholar] [CrossRef]
- Holländer, A.; Klemberg-Sapieha, J.E.; Wertheimer, M.R. Vacuum-ultraviolet induced oxidation of the polymers polyethylene and polypropylene. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 2013–2025. [Google Scholar] [CrossRef]
- Rivaton, A.; Cambon, S.; Gardette, J.L. Radiochemical ageing of EPDM elastomers. 3. Mechanism of radiooxidation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 227, 357–368. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. XPS Study of Sulfur and Phosphorus Compounds with Different Oxidation States. Sains Malays. 2018, 47, 1913–1922. [Google Scholar]
- Siow, K.S. Plasma Based Methods for Producing Controlled Polymer Surfaces with Sulfur and Phosphorus Containing Chemical Groups and Interactions Between Such Surfaces and Proteins; Ian Wark Research Institute: Adelaide, SA, Australia, 2007; unpublished. [Google Scholar]
- Decker, C.; Mayo, F.R. Aging and degradation of polyolefins. II. g-initiated oxidations of atactic polypropylene. J. Polym. Sci. 1973, 11, 2847. [Google Scholar] [CrossRef]
- Pospíšil, J. The key role of antioxidant transformation products in the stabilization mechanisms—A critical analysis. Polym. Degrad. Stab. 1991, 34, 85–109. [Google Scholar] [CrossRef]
- Ferry, M.; Bessy, E.; Harris, H.; Lutz, P.J.; Ramillon, J.-M.; Ngono-Ravache, Y.; Balanzat, E. Aliphatic/Aromatic Systems under Irradiation: Influence of the Irradiation Temperature and of the Molecular Organization. J. Phys. Chem. B 2013, 117, 14497–14508. [Google Scholar] [CrossRef]
- Ferry, M.; Ramillon, J.; Been, T.; Lutz, P.; Ngono-Ravache, Y.; Balanzat, E. Energy migration effect on the formation mechanism of different unsaturations in ethylene/styrene random copolymers. Polym. Degrad. Stab. 2018, 160, 210–217. [Google Scholar] [CrossRef]
- Ferry, M.; Pellizzi, E.; Boughattas, I.; Fromentin, E.; Dauvois, V.; de Combarieu, G.; Coignet, P.; Cochin, F.; Ngono-Ravache, Y.; Balanzat, E.; et al. Effect of cumulated dose on hydrogen emission from polyethylene irradiated under oxidative atmosphere using gamma rays and ion beams. Radiat. Phys. Chem. 2016, 118, 124–127. [Google Scholar] [CrossRef]
- Kikkawa, K.; Nakahara, Y.; Ohkatsu, Y. Antagonism between hindered amine light stabilizers and sulfur-containing compounds. Polym. Degrad. Stab. 1987, 18, 237–245. [Google Scholar] [CrossRef]
- Xu, A.; Roland, S.; Colin, X. Thermal ageing of a silane-crosslinked polyethylene stabilised with a thiodipropionate antioxidant. Polym. Degrad. Stab. 2020, 181, 109276. [Google Scholar] [CrossRef]
- Richaud, E.; Monchy-Leroy, C.; Colin, X.; Audouin, L.; Verdu, J. Kinetic modelling of stabilization coupled with stabilizer loss by evaporation. Case of dithioester stabilized polyethylene. Polym. Degrad. Stab. 2009, 94, 2004–2014. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M. Perspectives on additives for polymers. Part 2. Aspects of photostabilization and role of fillers and pigments. J. Vinyl Addit. Technol. 2020, 27, 211–239. [Google Scholar] [CrossRef]
- Allen, S.N.; Edge, M. Perspectives on additives for polymers. 1. Aspects of stabilization. J. Vinyl Addit. Technol. 2020, 27, 5–27. [Google Scholar] [CrossRef]
- Beißmann, S.; Reisinger, M.; Grabmayer, K.; Wallner, G.; Nitsche, D.; Buchberger, W. Analytical evaluation of the performance of stabilization systems for polyolefinic materials. Part I: Interactions between hindered amine light stabilizers and phenolic antioxidants. Polym. Degrad. Stab. 2014, 110, 498–508. [Google Scholar] [CrossRef]




| Facility | Panoza | Panoza | Roza |
|---|---|---|---|
| Irradiation temperature (°C) | 45 | 45 | 21 |
| Dose rate (Gy·h−1) | 8.5 | 78 | 400 |
| Dose (kGy) | 67 | 67, 220, 374 | 67 |
| XLPE M1 | XLPE M2(Irg1076) | XLPE M3(IrgPS802) | XLPE M4(Irg1076-IrgPS802) | XLPE M7(Irg1076-IrgPS802-ATH50) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose rate (Gy·h−1) | - | 8.5 | 78 | 400 | - | 8.5 | 78 | 400 | - | 8.5 | 78 | 400 | - | 8.5 | 78 | 400 | - | 8.5 | 78 | 400 |
| [Otot]/[Otot+Ctot] | 3.1 | 5.5 | 5.2 | 2.7 | 9.9 | 10.3 | 9.7 | 9.7 | 5.1 | 13.5 | 12.9 | 9.1 | 9.1 | 11.9 | 8.6 | 8.2 | 9.4 | 9.1 | 8.7 | 7.9 |
| [Stot]/[Stot+Ctot] | - | - | 0.4 | 2.7 | 2.9 | 1.8 | <1 | 2.3 | 1.9 | 1.5 | 1.1 | 1.6 | 1.5 | 2.6 | ||||||
| [Cox]/[Ctot] | 0 | 2.4 | 1.8 | 0.2 | 6.4 | 8.2 | 5.7 | 3.9 | 0.4 | 6.7 | 6.1 | 3.5 | 5.0 | 5.9 | 4.0 | 3.6 | 4.5 | 4.0 | 5.9 | 5.3 |
| [O-C=O]/[Ctot] | 0 | 1.1 | 0.6 | 0.1 | 2.1 | 2.7 | 1.5 | 1.5 | 0.4 | 3.9 | 3.5 | 2.2 | 2.1 | 3.3 | 2.6 | 2.1 | 2.1 | 2.2 | 2.1 | 2.4 |
| [C-O, C=O]/[Ctot] | 0 | 1.3 | 1.2 | 0.1 | 4.3 | 5.5 | 4.2 | 2.4 | 0 | 2.8 | 2.6 | 1.3 | 2.9 | 2.6 | 1.4 | 1.5 | 2.4 | 1.8 | 3.8 | 2.9 |
| [C-S]/[Stot] | 100 | 18.7 | 20.8 | 53.0 | 100 | 23.4 | 38.7 | 79.4 | 100 | 35.9 | 45.6 | 80.9 | ||||||||
| [S=O]/[Stot] | - | - | 0 | 22.0 | 24.1 | 11.4 | 0 | 35.3 | 44.1 | 8.9 | 0 | 33.9 | 35.8 | 10.8 | ||||||
| [SO2, SO3]/[Stot] | 0 | 59.4 | 55.1 | 35.6 | 0 | 41.3 | 17.2 | 11.7 | 0 | 30.2 | 18.6 | 8.3 | ||||||||
| XLPE M1 | XLPE M2(Irg1076) | XLPE M3(IrgPS802) | XLPE M4(Irg1076-IrgPS802) | XLPE M7(Irg1076-IrgPS802-ATH50) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (kGy) | 0 | 67 | 220 | 374 | 0 | 67 | 220 | 374 | 0 | 67 | 220 | 374 | 0 | 67 | 220 | 374 | 0 | 67 | 220 | 374 |
| [Otot]/[Ctot] | 3.1 | 5.2 | 8.2 | 10.0 | 9.9 | 9.7 | 4.9 | 10.3 | 5.1 | 12.9 | 12.2 | 13.9 | 9.1 | 8.6 | 12.4 | 14.7 | 9.4 | 8.7 | 12.9 | 14.9 |
| [Stot]/[Ctot] | - | - | 0.4 | 2.9 | 2.4 | 2.6 | <1 | 1.9 | 2.5 | 2.8 | 1.1 | 1.5 | 1.9 | 2.6 | ||||||
| [Cox]/[Ctot] | 0 | 1.8 | 4.6 | 6.3 | 6.4 | 5.7 | 3.2 | 4.0 | 0.4 | 6.1 | 6.6 | 7.5 | 5.0 | 4.0 | 7.3 | 9.8 | 4.5 | 5.9 | 6.4 | 9.8 |
| [O-C=O]/[Ctot] | 0 | 0.6 | 1.9 | 2.7 | 2.1 | 1.5 | 0.7 | 1.0 | 0.4 | 3.5 | 3.6 | 4.2 | 2.1 | 2.6 | 3.3 | 5.1 | 2.1 | 2.1 | 2.8 | 4.5 |
| [C-O, C=O]/[Ctot] | 0 | 1.2 | 2.7 | 3.6 | 4.3 | 4.2 | 2.5 | 3.0 | 0 | 2.6 | 3.0 | 3.3 | 2.9 | 1.4 | 4.0 | 4.4 | 2.4 | 3.8 | 3.6 | 5.3 |
| [C-S]/[Stot] | 100 | 20.8 | 15.6 | 12.5 | 100 | 38.7 | 23.6 | 13.1 | 100 | 45.6 | 27.2 | 13.1 | ||||||||
| [S=O]/[Stot] | - | - | 0 | 24.1 | 16.3 | 9.7 | 0 | 44.1 | 29.0 | 18.0 | 0 | 35.8 | 27.4 | 10.8 | ||||||
| [SO2, SO3]/[Stot] | 0 | 55.1 | 67.8 | 77.8 | 0 | 17.2 | 47.4 | 68.9 | 0 | 18.6 | 45.4 | 76.1 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferry, M.; Miserque, F. Radio-Oxidation of Electric Cabled Models: Ageing Evaluation at the Atomic Scale. Energies 2022, 15, 1631. https://doi.org/10.3390/en15051631
Ferry M, Miserque F. Radio-Oxidation of Electric Cabled Models: Ageing Evaluation at the Atomic Scale. Energies. 2022; 15(5):1631. https://doi.org/10.3390/en15051631
Chicago/Turabian StyleFerry, Muriel, and Frederic Miserque. 2022. "Radio-Oxidation of Electric Cabled Models: Ageing Evaluation at the Atomic Scale" Energies 15, no. 5: 1631. https://doi.org/10.3390/en15051631
APA StyleFerry, M., & Miserque, F. (2022). Radio-Oxidation of Electric Cabled Models: Ageing Evaluation at the Atomic Scale. Energies, 15(5), 1631. https://doi.org/10.3390/en15051631





