Abstract
This article reports on the influence of resorcinol (RC) on the kinetics of underpotential deposition of hydrogen (UPD of H) and the oxygen evolution reaction (OER), studied on a polycrystalline Pt electrode in a 0.5 M sulphuric acid supporting solution. It is well known that both PEM fuel cells and water electrolysers’ electrodes often contain significant amounts of nanostructured Pt or other types of noble metal particles. These materials provide the superior catalytic activity of electrochemical reactions such as OER (oxygen evolution reaction), HER (hydrogen evolution reaction) and ORR (oxygen reduction reaction). The trace amounts of phenolic substances contained in air or water could be harmful (when in contact with a fuel cell/water electrolyser’s working environment) to the abovementioned catalytic surfaces. Hence, they could potentially have severe detrimental effects on the kinetics of these processes. The results obtained in this work provided evidence for the detrimental role of Pt surface-adsorbed resorcinol molecules (or their electrodegradation products) on the kinetics of UPD of H and the oxygen evolution reaction. The above was revealed through evaluation of the associated charge-transfer resistance and capacitance parameters, comparatively derived on a platinum electrode, for the initial and the resorcinol-modified H2SO4 electrolyte.
1. Introduction
Hydrogen and oxygen-based reactions on metal-based electrodes are some of the most important electrochemical processes as proton-exchange membrane (PEM) fuel-cell and water electrolysis technologies continue their expansion [1,2,3,4,5,6]. The presence of airborne inorganic (e.g., SO2, NOx and H2S molecules) and numerous organic compounds could have a significant negative impact on the performance of these devices. Resorcinol, for example, is a hydroxy derivative of phenol, which is broadly used in rubber production and numerous speciality chemicals, including dyes, pharmaceuticals and cosmetics, and agricultural fertilizers. As a consequence, large quantities of resorcinol are regularly disposed of into the environment as part of industrially based effluent [7,8,9].
Moreover, numerous works published in the contemporary literature have shown that resorcinol molecules (similar to other phenols) under suitable catalytic conditions are prone to undergoing a sequence of potential-dependent surface-adsorption and electrodegradation steps [10,11,12]. The latter is of special importance for some noble and semi-noble metals, which, due to their superior hydrogen and oxygen evolution characteristics, are frequently used to make electrodes for water electrolysers.
The so-called process of underpotentially deposited hydrogen (UPD H) emerges at positive potentials with reference to the reversible potential of H2, when the Gibbs energy of hydrogen atoms binding with the metal surface is numerically greater than half the Gibbs energy of bonding in the hydrogen molecule. The above phenomenon is characteristic of several noble/semi-noble metals, including platinum, iridium, rhodium and palladium [3,13]. The process of UPD of H could be considered as a prerequisite for a bulk hydrogen evolution reaction (HER) with some of the most active noble metal HER catalysts. Equation (1) represents the process of UPD of H in an acidic solution for Pt:
where:
H3O+ + Pt + e− (in Pt) ⇔ Pt − Hads + H2O
CH+ (1 − θH) E θH
E is the applied potential, CH+ is the proton concentration, θH is the fraction of site occupancy for the adsorbed H and 1 − θH is the fraction of free Pt sites available for adsorption, where 0 ≤ θH ≤ 1.
Continuous desorption of previously electrosorbed H atoms takes place, thus forming hydrogen molecules and initiating the HER (hydrogen evolution reaction) [2].
On the other hand, the oxygen evolution reaction (OER) is a complex electrochemical process involving four electrons and four protons (Equation (2)), which involves several intermediate steps (Equations (3)–(5)). Initially, partial oxidation (deprotonation) of water molecules leads to the formation of surface-adsorbed OHads species (Equation (3)). Next, electron transfer and deprotonation of OHads in the second step result in the formation of Oads (Equation (4)), which is a prerequisite for the chemical recombination of Oads in the next step and the generation of O2 molecules in Equation (5) [14,15].
2H2O ⇔ 4H+ + O2 + 4e−
H2O ⇔ OHads + H+ + e−
OHads ⇔ Oads + H+ + e−
2Oads ⇔ O2↑
In this work, the authors would like to give some insight into the dependence of the UPD of H and OER reactions’ kinetics on simultaneous surface adsorption and electrodegradation of resorcinol molecules, examined on a poly Pt electrode surface in a 0.5 M sulphuric acid supporting electrolyte.
2. Materials and Methods
2.1. Materials
A supporting electrolyte of 0.5 M sulphuric acid was prepared from superior quality H2SO4 provided by MERCK and ultra-pure water with 18.2 MΩ cm resistivity, produced by the Millipore Direct-Q3 UV water purification system. The concentration of resorcinol (Sigma-Aldrich, Warsaw, Poland > 99%) in the supporting electrolyte was at the level of 1 × 10−5 M.
A typical three-compartment electrochemical cell made of Pyrex glass was used to carry out all electrochemical measurements. The cell contained three electrodes: a polycrystalline Pt working electrode (WE; SA = 0.94 cm2, comparatively derived through the ac. impedance measurements of the double-layer capacitance and the cyclic voltammetry estimations of the UPD H monolayer charge), a Pd RHE (reversible hydrogen electrode) as a reference and a CE (counter-electrode) made from a Pt wire (1.0 mm diameter, 99.9998% purity, Johnson Matthey, Inc., Audubon, PA, USA).
2.2. Electrochemical Measurements
All electrochemical investigations were conducted at temperature of 293 K by means of the Biologic SP-240 Electrochemical System. Electrochemical impedance spectroscopy and cyclic voltammetry were carried out in this work. For the ac. impedance measurements, the generator provided an output signal of 5 mV and the frequency range was swept between 1.0 × 105 and 0.5 Hz or between 0.5 × 105 and 10 Hz for the OER and the UPD of H processes, respectively. The cyclic voltammetry experiments were carried out at a sweep-rate of 50 mV s−1 over the working electrode potential ranges of 0.05–0.80 V and 0.40–1.80 (or 2.10) V vs. RHE. The instrument was controlled by EC-Lab® software (Biologic, Seyssinet-Pariset, France) for Windows. Three impedance measurements were conducted at each potential value. The reproducibility of the results thus obtained was typically below 10%. Data analysis was performed with the ZView 3.5 (Corrview 3.5) software package for Windows (Scribner Associates, Southern Pines, NC, USA).
3. Results and Discussion
3.1. Cyclic Voltammetry Behaviour of Pt in 0.5 M Sulphuric Acid, in the Absence and Presence of Resorcinol
The cyclic voltammetric behaviour of the poly Pt electrode in contact with the 0.5 M H2SO4 supporting solution, in the absence and presence of resorcinol (at 1 × 10−5 M), is shown in Figure 1 and Figure 2. Hence, the voltammetric features, appearing over the potential span of 0.05–0.40 V and shown as a red curve in Figure 1, could be assigned to the well-known reversible process of UPD of H (hydrogen underpotential deposition) on the polycrystalline Pt electrode (in reference to Equation (1)). The voltammogram is characterised by the presence of two principal reversible adsorption states, where the first, centred at a ~0.13 V/RHE peak, corresponds to adsorption on (110) sites; a second one, positioned at 0.27 V, principally refers to adsorption on (100) sites of geometry [16,17]. The mean H monolayer electric charge for the polycrystalline Pt electrode over the potential range of 0.05–0.80 V (after subtracting the double-layer charge contribution) was typically estimated at 210 μC cm−2 [17]. After introduction of resorcinol (at 1.0 × 10−5 M) into the supporting H2SO4 solution, the voltametric charge (blue curve in Figure 1) became radically depleted (to the mean charge value of about 62 µC cm−2 after the Cdl - double-layer capacitance charge correction), due to simultaneous surface co-adsorption and interaction of the RC molecules with the hydrogen atoms (see the proposed RC-to-H surface interaction model in Scheme 1). It should be stressed that RC concentration as low as 1 × 10−6 M had practically no effect on the hydrogen adsorption sites of the Pt electrode. In addition, for the RC concentration of 1 × 10−4 M, the cyclic voltammetry profile did not change beyond that shown in the blue curve of Figure 1 (see Figure S1 in the Supplementary Materials). The latter effect is also demonstrated through the recorded ac. impedance charge transfer resistance and capacitance parameters in Section 3.2.

Figure 1.
Cyclic voltammograms for a polycrystalline Pt electrode surface, recorded over the potential range characteristic of UPD of H in a 0.5 M H2SO4 supporting solution (in the second cycle) at a sweep-rate of 50 mV s−1, in the absence and presence of resorcinol (at 1 × 10−5 M).
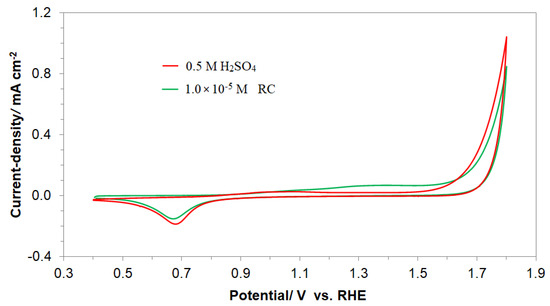
Figure 2.
Cyclic voltammograms for a polycrystalline Pt electrode surface, recorded in a 0.5 M H2SO4 supporting solution (in the second cycle) at a sweep-rate of 50 mV s−1, in the absence and presence of resorcinol (at 1 × 10−5 M), over the potential range characteristic of resorcinol electrooxidation and OER.
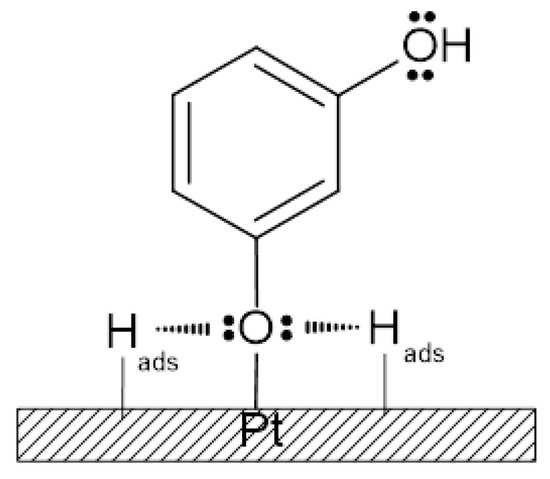
Scheme 1.
The proposed Pt surface interaction of co-adsorbed resorcinol molecules with UPD hydrogen atoms.
On the other hand, Figure 2 presents similar cyclic voltammograms, but reported over the potential range characteristic of the resorcinol (green plot) surface’s electrooxidation/degradation (see the wide anodic feature at 1.2–1.5 V here and in [18] for the details of the process). Additionally, it is worth noting that the oxygen evolution reaction commences just beyond ~1.65 V. Most importantly, it should be observed in Figure 2 that the recorded current densities for the RC-based solution are significantly reduced compared with those derived for the corresponding electrode potentials but in the absence of resorcinol. Furthermore, for the RC concentration of 1 × 10−6 M, the recorded voltammetric current densities for the potential range of OER are practically superimposed with those of the RC-free solution. Moreover, the cyclic voltammograms recorded in the presence of resorcinol (at the concentrations of 1 × 10−5 and 1 × 10−4 M) are also practically identical (see Figure S2 in the Supplementary Materials). As previously reported by Duan et al. [19], Chen et al. [20], and Pierozynski, and Piotrowska [21], electrochemical degradation of resorcinol leads to the destruction of its aromatic ring structure, where maleic, oxalic and formic acids are found to be key intermediate electrooxidation products of resorcinol. Hence, it is strongly believed that such surface-adsorbed intermediates could then impediment the oxygen evolution reaction through the surface repulsive interaction between the RC oxidation intermediate(s) and hydroxyl or oxygen species, as shown as an example in Scheme 2.
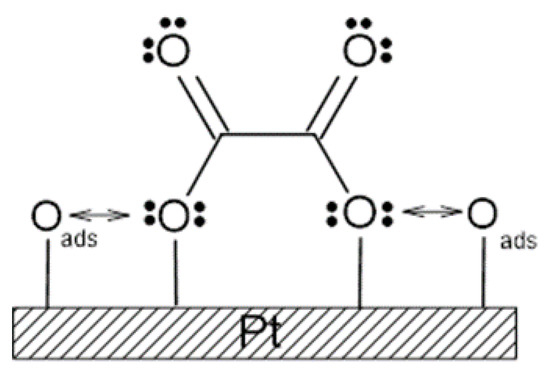
Scheme 2.
The proposed dipole–dipole interaction effect between resorcinol’s electrodegradation intermediates (here shown as oxalic acid) and oxygen atoms on the surface of a polycrystalline Pt electrode.
3.2. Ac. Impedance Behaviour of Pt in 0.5 M H2SO4, in the Absence and Presence of Resorcinol
The electrochemical Ac. impedance behaviour of a polycrystalline Pt electrode in a pure 0.5 M H2SO4 solution and in the presence of 1.0 × 10−5 M RC, examined over the potential range characteristic of the underpotential deposition of hydrogen, is shown in Figure 3A (for a selected electrode potential of 100 mV vs. RHE) and Table 1. The process of UPD of H generates a semicircle over the high and intermediate frequency range in the Nyquist impedance plot, along with a vertical line, which characterizes purely capacitive behaviour (but often deviates from a 90° angle, due to the so-called capacitance dispersion effect [18,22], related to surface roughness and heterogeneity) at reasonably low frequencies. Thus, the results recorded in Table 1 (obtained by fitting the data using the equivalent circuit presented in Figure 3B) for the kinetics of UPD of H in pure 0.5 M H2SO4 principally followed those of other data available in the literature (see, for example, [3,22] for details). Thus, the corresponding charge transfer resistance parameter RH (proportional to the inverse of the exchange rate for the process of UPD of H) considerably increased from 0.47 (at 100 mV) to reach 2.65 Ω cm2 at 400 mV (where H adsorption on the poly Pt surface becomes radically depleted). Moreover, the double-layer capacitance value Cdl for the Pt electrode fluctuated between 21 and 79 μF cm−2 for the corresponding electrode potential range. On the other hand, the H adsorption pseudocapacitance parameter, CpH, fluctuated between 209 (at 100 mV) and 179 μF cm−2 at 400 mV, thus exhibiting similar variation to that of the current density changes in the CV (cyclic voltammetry) profile of Figure 1.
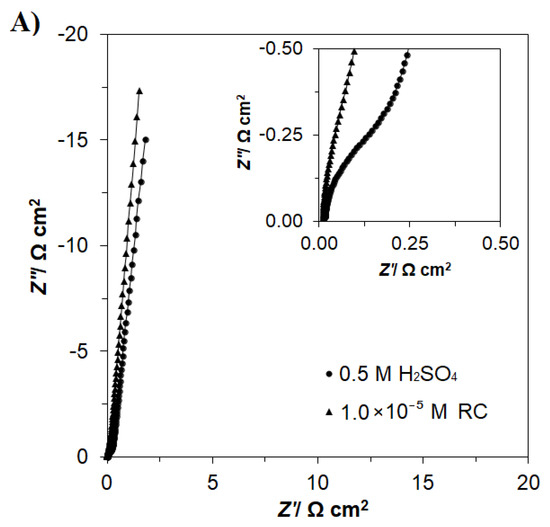
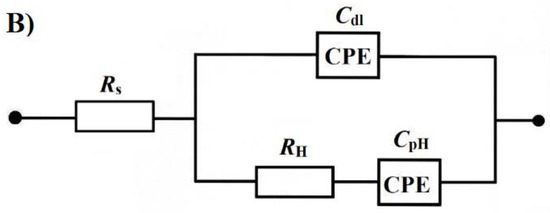
Figure 3.
(A) Nyquist impedance plots for the process of underpotential deposition of hydrogen (UPD of H) on a polycrystalline Pt electrode in contact with 0.5 M H2SO4 and in the presence of RC for the concentration indicated, recorded at the potential of 100 mV vs. RHE. Additionally, the related Bode plot is presented in Figure S3 in the Supplementary Materials. (B) Equivalent circuit used to fit the above process, where CpH is the Faradaic pseudocapacitance, RH is the Faradaic resistance and Cdl is the double-layer capacitance (both capacitance parameters are CPE: constant phase element−modified), jointly in series with an uncompensated solution resistance, RS. The data derived from the equivalent circuit are represented by the solid lines.

Table 1.
Spectroscopy-derived Ac. impedance parameters for the UPD of H and OER processes on a polycrystalline Pt electrode surface in contact with 0.5 M H2SO4 and in the presence of RC (1.0 × 10−5 M and 293 K), obtained by fitting the equivalent circuits shown in Figure 3B and Figure 4B to the recorded impedance data (the values of the dimensionless φ parameters for the CPE circuit’s components oscillated between 0.89 and 1.00 and between 0.94 and 0.99 for UPD of H and OER, correspondingly; the reproducibility of impedance was usually below 10%; χ2 = 6 × 10−5 to 3 × 10−4).
Next, the introduction of resorcinol (at a concentration of 1.0 × 10−5 M) into the supporting electrolyte caused the RH parameter to radically increase, i.e., to 1.16 Ω cm2 at 100 mV (2.5 times) and to 5.08 Ω cm2 at 400 mV (1.9 times), compared with the RC-free electrolyte. Moreover, the recorded CpH parameter values significantly decreased to reach 73 μF cm−2 (qualitatively comparable with that of Cdl) at the electrode potential of 350 mV (Table 1). It should be noted that these observations are not only completely in line with the findings for cyclic voltammetry and ac. impedance of Figure 1 and the inset in Figure 3A, respectively, but are also in general agreement with the proposed Pt surface interaction of co-adsorbed RC molecules with the UPD hydrogen atoms in Scheme 1.
On the other hand, the characteristics of the ac. impedance of the OER for a polycrystalline Pt electrode, experimentally recorded in 0.5 M H2SO4 and comparatively in RC-modified (at 1.0 × 10−5 M) sulphuric acid, are shown in Figure 4A,B and Table 1. For all the examined potentials, the impedance behaviour exhibited single, somewhat “depressed” semicircles (a single-step charge transfer reaction) in the explored frequency range (see examples of the Nyquist impedance plots for the potential of 1800 mV in Figure 4A) along with a low-frequency noisy feature (observed below 1 Hz), which is typically assigned to the so-called O2 bubbling effect (see, for example, [23] for details). This part of the spectrum was not included in the impedance analysis and fitting. The parameters of Faradaic reaction resistance, Rct, and double-layer capacitance, Cdl, were obtained by means of a constant phase element (CPE)-modified Randles equivalent circuit model (see Figure 4B), and have been presented in Table 1.
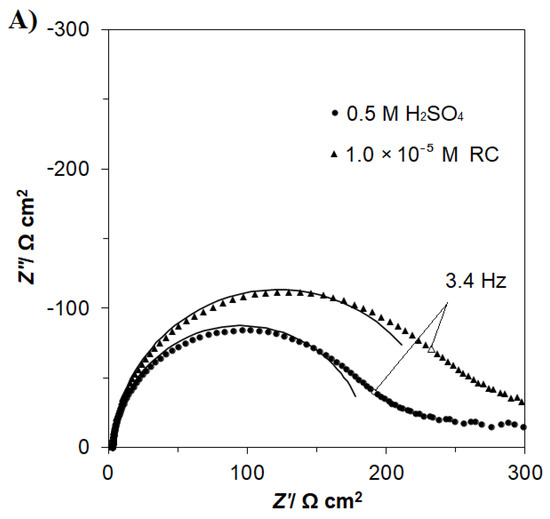
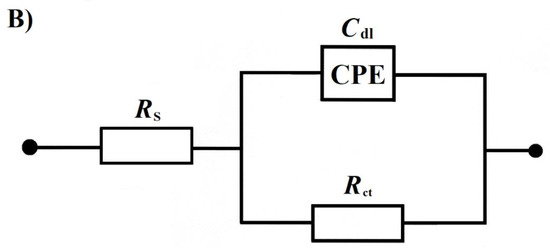
Figure 4.
(A) Nyquist impedance plots for the process of the oxygen evolution reaction (OER), recorded at the potential of 1800 mV vs. RHE. (B) Equivalent circuit for the OER. The circuit includes a constant phase element (CPE) for distributed capacitance; Rct corresponds to the OER charge-transfer resistance, whereas the elements Cdl and RS are the same as those in Figure 3B. The data derived from the equivalent circuit are represented by the solid lines.
Hence, in the absence of resorcinol, the recorded Rct radically decreased from 874.2 Ω cm2 at 1700 mV to reach 2.8 Ω cm2 at the electrode potential of 2100 mV/RHE. Simultaneously, the recorded Cdl values increased from 54 to 90 μF cm−2 upon potential augmentation from 1700 to 2100 mV. Again, as in case of the UPD of H process, the introduction of resorcinol into the supporting solution resulted in a significant increase in the impedance-derived charge transfer resistance parameter, which came to 1097.0 and 3.3 Ω cm2 for the electrode potentials of 1700 and 2100 mV vs. RHE, correspondingly. This could be translated to a rise in the Rct parameter by ~1.2 times and is in good agreement with the findings of Figure 2 and Figure 4A, as well as the dipole–dipole repulsive interaction model suggested for the RC electrodegradation products (e.g., oxalic acid) and oxygen atoms on the surface of the poly Pt electrode in Scheme 2.
4. Conclusions
Application of cyclic voltammetry and electrochemical ac. impedance spectroscopy techniques to study the influence of resorcinol (an important industrial chemical and a possible contaminant of electrolysers/fuel cell systems) on the kinetics of the UPD of H and OER processes, examined on a polycrystalline Pt electrode under acidic conditions, provided confirmation of the considerable damaging role of platinum-electrosorbed resorcinol molecules (or its electrodegradation intermediates) on the rates of the underpotential deposition of hydrogen (frequently an antecedent process to hydrogen evolution in platinum series metals) and the oxygen evolution reaction.
Further laboratory work is necessary in order to understand the details of the process and the extent to which RC competes with UPD of H electrosorption on platinum, as well as that of the surface interactions of electrosorbed RC electrodegradation products and OER’s intermediate species (OHads and Oads).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en15031092/s1, Figure S1: Cyclic voltammograms for polycrystalline Pt electrode surface, recorded over the potential range characteristic to UPD of H in 0.5 M H2SO4 supporting solution (on the 2nd cycle), at a sweep-rate of 50 mV s−1, in the absence and presence of resorcinol (at the concentration of 1 × 10−6, 1 × 10−5 and 1 × 10−4 M); Figure S2: Cyclic voltammograms for polycrystalline Pt electrode surface, recorded in 0.5 M H2SO4 supporting solution (on the 2nd cycle) at a sweep-rate of 50 mV s−1, in the absence and presence of resorcinol (at the concentration of 1 × 10−6, 1 × 10−5 and 1 × 10−4 M), over the potential range characteristic to resorcinol electrooxidation and OER; Figure S3: Complex-plane Bode impedance plot for the process of underpotential deposition of hydrogen (UPD of H) on polycrystalline Pt electrode in contact with 0.5 M H2SO4 and in the presence of RC for the concentration 1 × 10−5 M, recorded for the potential of 100 mV vs. RHE.
Author Contributions
Conceptualization, B.P.; methodology, B.P.; investigation, M.Ł., M.K. and T.M.; data curation, T.M., M.Ł. and M.K.; writing—original draft preparation, B.P.; editing, B.P. and T.M.; supervision, B.P. All authors have read and agreed to the published version of the manuscript.
Funding
Project financially supported by the Minister of Education and Science under the program entitled “Regional Initiative of Excellence” for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN. The results in this article were obtained as part of comprehensive study financed by the University of Warmia and Mazury in Olsztyn, Faculty of Agriculture and Forestry, Department of Chemistry (Grant No. 30.610.001-110).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Mateusz Luba would also like to acknowledge a scholarship from the Interdisciplinary Doctoral Studies in Bioeconomy program (POWR.03.02.00-00-1034/16-00), funded by the European Social Fund.
Conflicts of Interest
The authors of this work declare no conflict of interest.
References
- Conway, B.E.; Tilak, B.V. Behavior and characterization of kinetically involved chemisorbed intermediates in electrocatalysis of gas evolution reactions. Adv. Catal. 1992, 38, 1–147. [Google Scholar]
- Conway, B.E.; Bai, L. Determination of adsorption of OPD H species in the cathodic hydrogen evolution reaction at Pt in relation to electrocatalysis. J. Electroanal. Chem. Interfac. Electrochem. 1986, 198, 149–175. [Google Scholar] [CrossRef]
- Morin, S.; Dumont, H.; Conway, B.E. Evaluation of the effect of two-dimensional geometry of Pt single-crystal faces on the kinetics of upd of H using impedance spectroscopy. J. Electroanal. Chem. 1996, 412, 39–52. [Google Scholar] [CrossRef]
- Pierozynski, B. Hydrogen evolution reaction at Pd-modified carbon fibre and nickel-coated carbon fibre materials. Int. J. Hydrogen Energy 2013, 38, 7733–7740. [Google Scholar] [CrossRef]
- Doyle, R.L.; Lyons, M.E.G. An electrochemical impedance study of the oxygen evolution reaction at hydrous iron oxide in base. Phys. Chem. Chem. Phys. 2013, 15, 5224–5237. [Google Scholar] [CrossRef]
- Pierozynski, B.; Mikolajczyk, T.; Luba, M.; Zolfaghari, A. Kinetics of oxygen evolution reaction on nickel foam and platinum-modified nickel foam materials in alkaline solution. J. Electroanal. Chem. 2019, 847, 113194. [Google Scholar] [CrossRef]
- Porras, S.P.; Hartonen, M.; Ylinen, K.; Tornaeus, J.; Tuomi, T.; Santonen, T. Environmental and occupational exposure to resorcinol in Finland. Toxicol. Lett. 2018, 298, 125–133. [Google Scholar] [CrossRef]
- Korbahti, B.K.; Demirbuken, P. Electrochemical oxidation of resorcinol in aqueous medium using boron-doped diamond anode: Reaction kinetics and process optimization with response surface methodology. Front. Chem. 2017, 5, 75. [Google Scholar] [CrossRef] [Green Version]
- Lynch, B.S.; Delzell, E.S.; Bechtel, D.H. Toxicology review and risk assessment of resorcinol: Thyroid effects. Regul. Toxicol. Pharm. 2002, 36, 198–210. [Google Scholar] [CrossRef]
- Rajkumar, D.; Palanivelu, K.; Mohan, N. Electrochemical oxidation of resorcinol for wastewater treatment—A kinetic study. Indian J. Chem. Technol. 2003, 10, 396–401. [Google Scholar]
- Nady, H.; El-Rabieci, M.M.; Abd El-Hafez, G.M. Electrochemical oxidation behavior of some hazardous phenolic compounds in acidic solution. Egypt. J. Pet. 2017, 26, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Mikolajczyk, T.; Pierozynski, B.; Smoczynski, L.; Wiczkowski, W. Electrodegradation of resorcinol on pure and catalyst-modified Ni foam anodes, studied under alkaline and neutral pH conditions. Molecules 2018, 23, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierozynski, B.; Morin, S.; Conway, B.E. Influence of adsorption of guanidonium cations on H UPD at Pt(hkl) surfaces: Lattice-specific anion-mimetic effects. J. Electroanal. Chem. 1999, 467, 30–42. [Google Scholar] [CrossRef]
- Zeradjanin, A.R.; Masa, J.; Spanos, I.; Schlogl, R. Activity and stability of oxides during oxygen evolution reaction-from mechanistic controversies toward relevant electrocatalytic descriptors. Front. Energy Res. 2021, 8, 613092. [Google Scholar] [CrossRef]
- Favaro, M.; Valero-Vidal, C.; Eichhorn, J.; Toma, F.M.; Ross, P.N.; Yano, J.; Liu, Z.; Crumlin, E.J. Elucidating the alkaline oxygen evolution reaction mechanism on platinum. J. Mater. Chem. A. 2017, 5, 11634–11643. [Google Scholar] [CrossRef] [Green Version]
- Clavilier, J.; El Achi, K.; Petit, M.; Rodes, A.; Zamakhchari, M.A. Electrochemical monitoring of the thermal reordering of platinum single-crystal surfaces after metallographic polishing from the early stage to the equilibrium surfaces. J. Electroanal. Chem. 1990, 295, 33–356. [Google Scholar] [CrossRef]
- Clavilier, J.; Rodes, A.; El Achi, K.; Zamakhchari, M.A. Electrochemistry at platinum single crystal surfaces in acidic media: Hydrogen and oxygen adsorption. J. Chim. Phys. 1991, 88, 1291–1337. [Google Scholar] [CrossRef]
- Mikolajczyk, T.; Luba, M.; Pierozynski, B.; Kowalski, I.M.; Wiczkowski, W. The influence of solution pH on the kinetics of resorcinol electrooxidation (degradation) on polycrystalline platinum. Molecules 2019, 24, 2309. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Zhao, Y.; Liu, W.; Chang, L. Investigation on electro-catalytic oxidation properties of carbon nanotube-Ce-modified PbO2 electrode and its application for degradation of m-nitrophenol. Arab. J. Chem. 2019, 12, 709–717. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Liu, W.; Tu, Y.; Zhang, Y.; Han, W.; Wang, L. Electrochemical degradation of nitrobenzene by anodic oxidation on the constructed TiO2-NTs/SnO2-Sb/PbO2 electrode. Chemosphere 2014, 113, 48–55. [Google Scholar] [CrossRef]
- Pierozynski, B.; Piotrowska, G. Electrochemical degradation of phenol and resorcinol molecules through the dissolution of sacrificial anodes of macro-corrosion galvanic cells. Water 2018, 10, 770. [Google Scholar] [CrossRef] [Green Version]
- Conway, B.E.; Pierozynski, B.A.c. impedance behaviour of processes involving adsorption and reactivity of guanidonium-type cations at Pt(100) surface. J. Electroanal. Chem. 2008, 622, 10–14. [Google Scholar] [CrossRef]
- Audichon, T.; Napporn, T.W.; Kokoh, K.B.; Canaff, C.; Morais, C.; Comminges, C. IrO2 coated on RuO2 as efficient and stable electroactive nanocatalysts for electrochemical water splitting. J. Phys. Chem. C 2016, 120, 2562–2573. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).