Numerical Analysis of VPSA Technology Retrofitted to Steam Reforming Hydrogen Plants to Capture CO2 and Produce Blue H2
Abstract
:1. Introduction
2. VPSA Mathematical Model
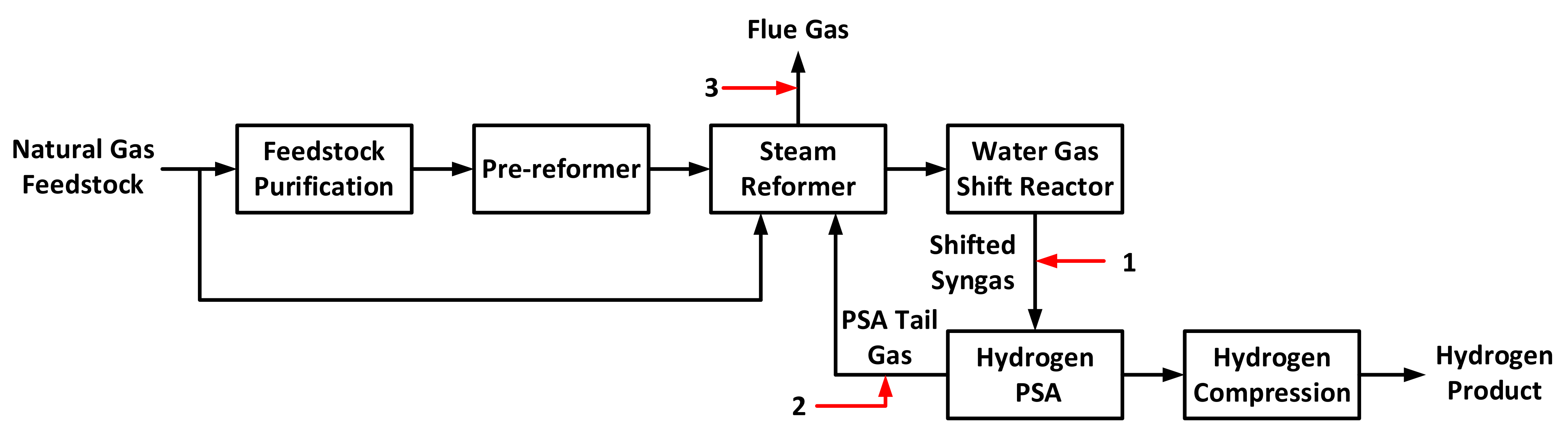
3. Design Basis and Cycle Configurations
4. Results and Discussion
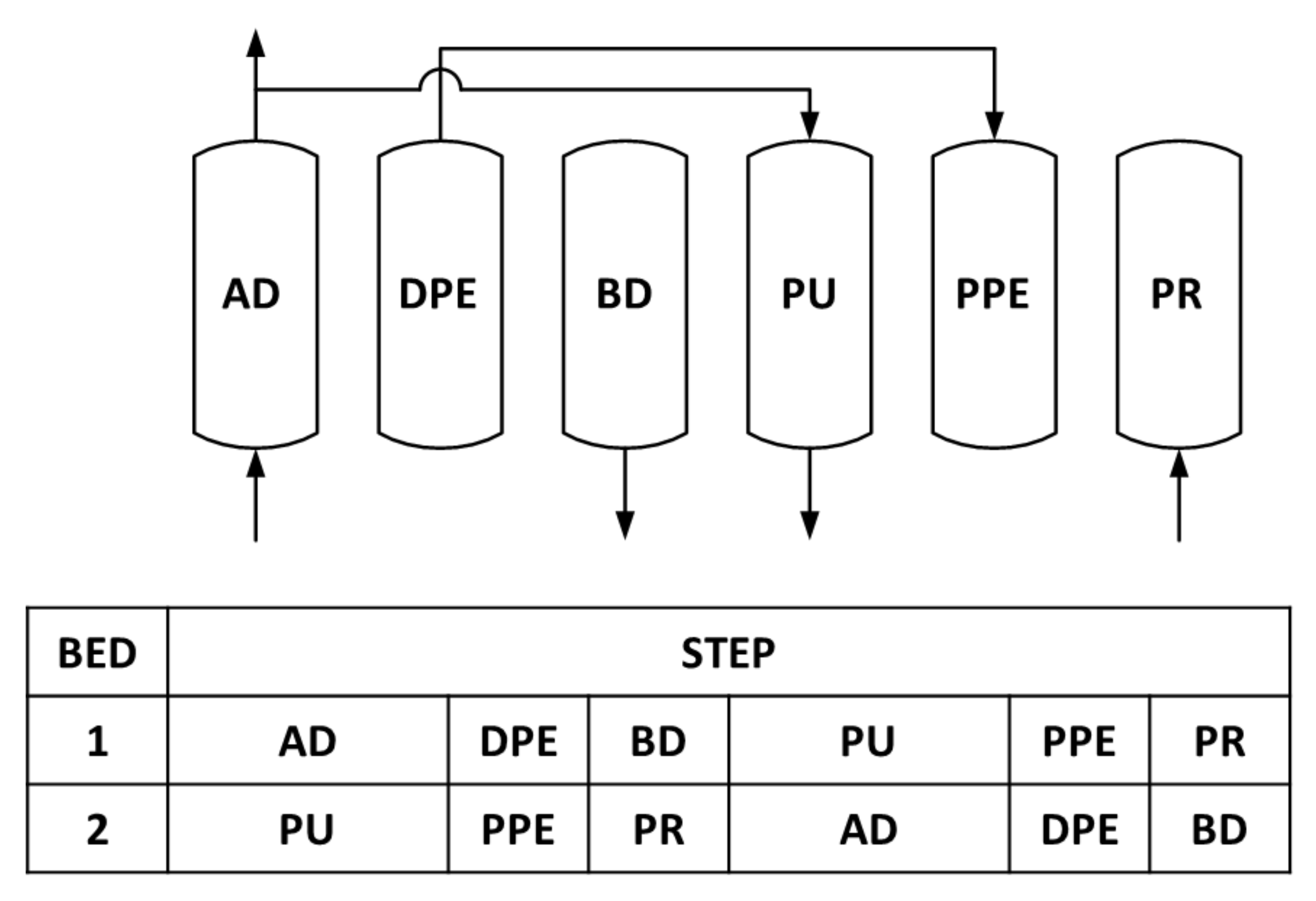
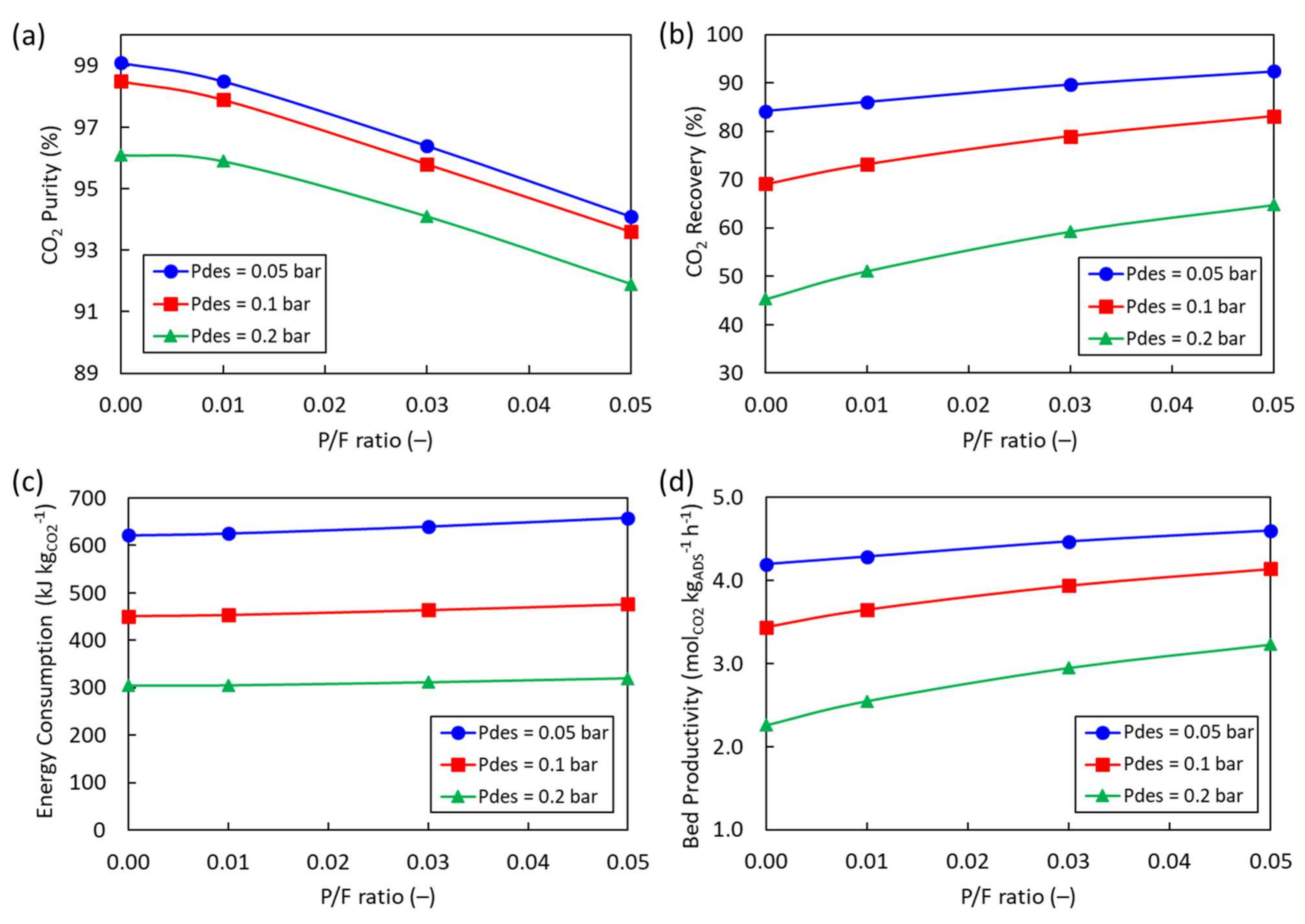
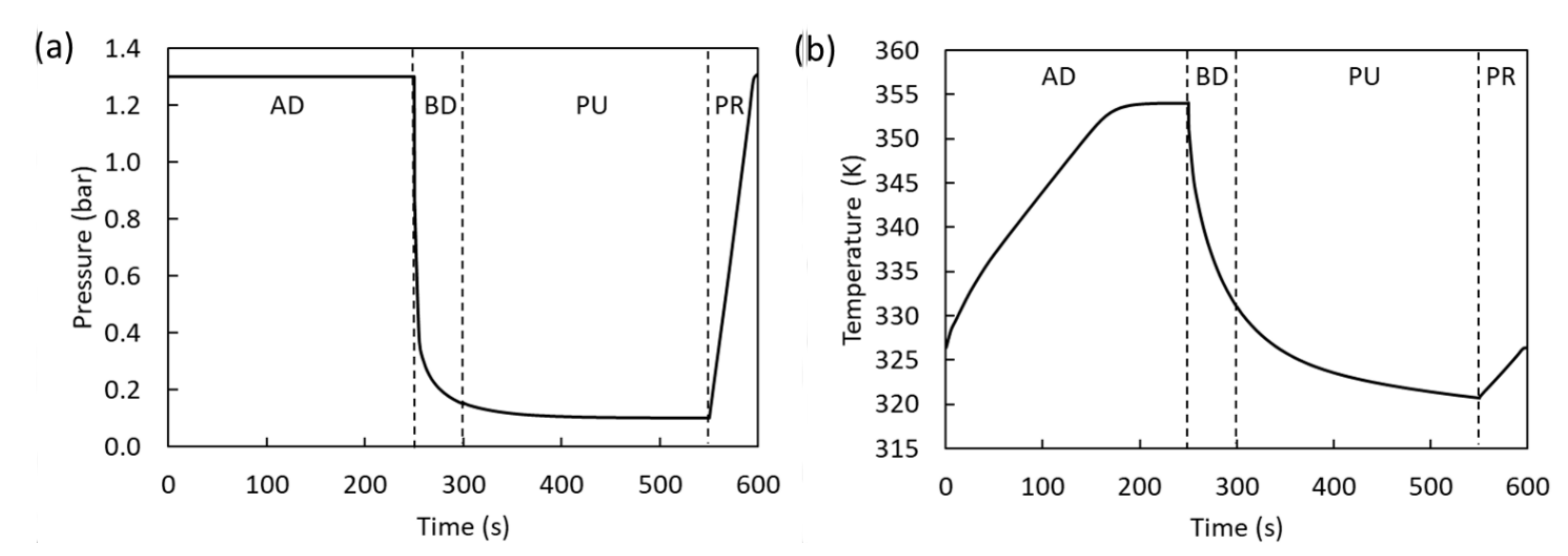
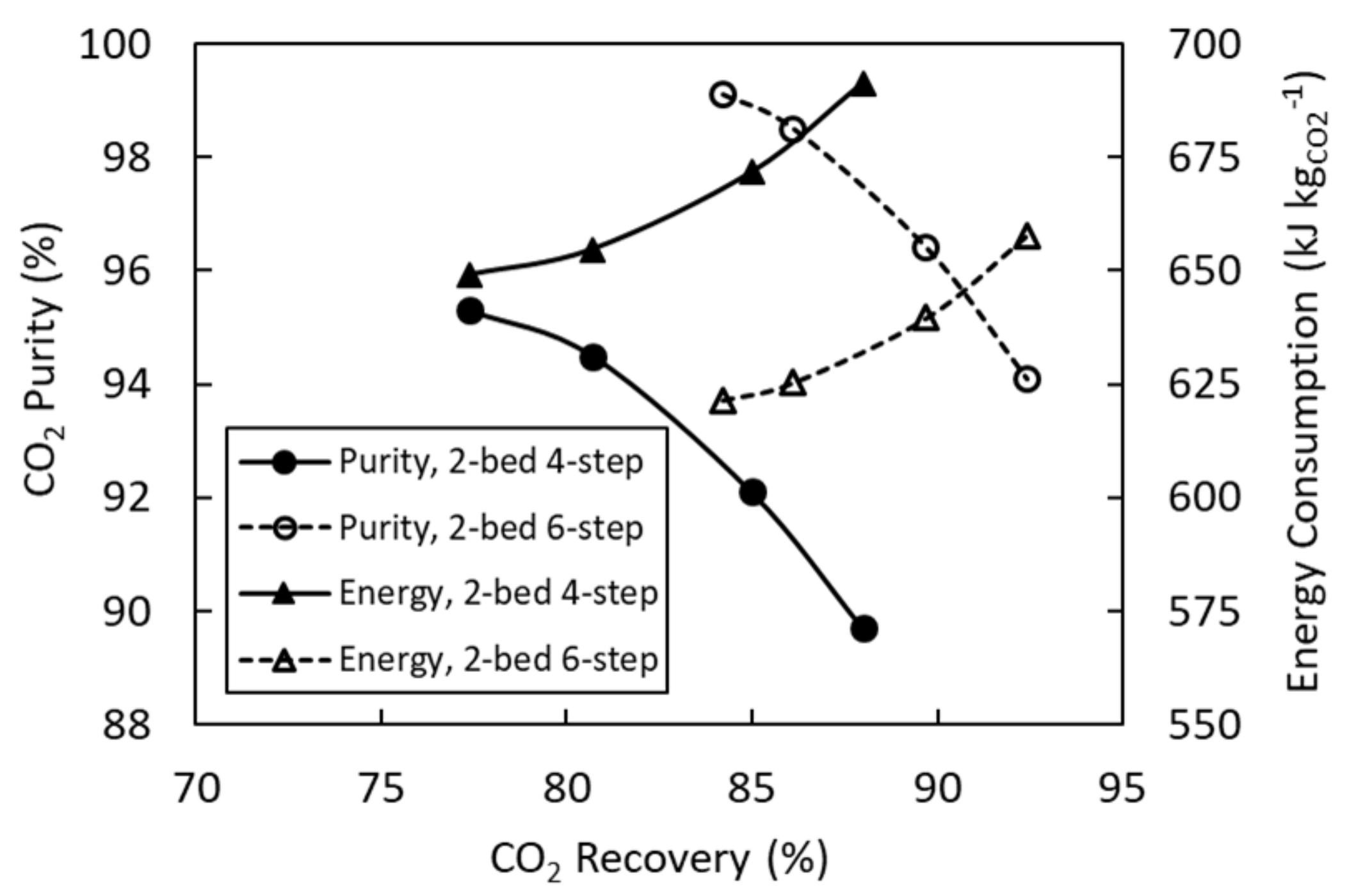
4.1. 2-Bed, 4-Step Simulations
4.2. 2-Bed, 6-Step Simulations
4.3. Cycle Configuration Comparison
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Ab | Bed surface area (m2) |
| b1,i | Equilibrium constant of component i for site 1 (bar−1) |
| b2,i | Equilibrium constant of component i for site 2 (bar−1) |
| b1,i,0 | Pre-exponential equilibrium constant coefficient of component i for site 1 (bar−1) |
| b2,i,0 | Pre-exponential equilibrium constant coefficient of component i for site 2 (bar−1) |
| ci | Gas phase concentration of component i (mol m−3) |
| cp,s | Adsorbent particle specific heat capacity (J kg−1 K−1) |
| cT | Total gas phase concentration (mol m−3) |
| Cv | Valve flow coefficient (kg s−1 Pa−1/2) |
| Db | Bed diameter (m) |
| Dm | Molecular diffusivity (m2 s−1) |
| dP | Adsorbent particle diameter (m) |
| Dz | Axial mass dispersion coefficient (m2 s−1) |
| F | Mass flowrate (kg s−1) |
| Fi | Assigned mass flowrate (kg s−1) |
| (–∆H1,i) | Heat of adsorption of component i for site 1 (J mol−1) |
| (–∆H2,i) | Heat of adsorption of component i for site 2 (J mol−1) |
| () | Averaged heat of adsorption of component i (J mol−1) |
| Hg | Gas phase enthalpy per unit volume (J m−3) |
| hw | Heat transfer coefficient between the gas phase and the bed wall (W m−2 K−1) |
| Ji | Diffusive flux of component i (mol m−2 s−1) |
| JT | Thermal diffusive flux (W m−2) |
| kg | Gas thermal conductivity (W m−1 K−1) |
| kLDF,i | LDF coefficient of component i (s−1) |
| k1,i | First parameter of LDF coefficient linear equation of component i (s−1 K−1) |
| k2,i | Second parameter of LDF coefficient linear equation of component i (s−1) |
| kz | Axial thermal dispersion coefficient (W m−1 K−1) |
| Lb | Bed length (m) |
| mads | Adsorbent mass (kg) |
| MWi | Molecular weight of component i (kg mol−1) |
| P | Pressure (bar) |
| ΔP | Pressure difference (bar) |
| Pads | Adsorption pressure (bar) |
| Patm | Atmospheric pressure (bar) |
| Pdes | Desorption pressure (bar) |
| Pi | Partial pressure of component i (bar) |
| Pi0 | Surface pressure of component i (bar) |
| Pr | Prandtl number (–) |
| P/F | Purge-to-feed ratio (–) |
| qi* | Adsorbed phase concentration of component i at equilibrium (mol kg−1) |
| Averaged adsorbed phase concentration of component i (mol kg−1) | |
| qs1,i | Saturation capacity of component i for site 1 (mol kg−1) |
| qs2,i | Saturation capacity of component i for site 2 (mol kg−1) |
| qT | Total adsorbed phase concentration (mol kg−1) |
| R | Ideal gas constant (J mol−1 K−1) |
| Re | Reynolds number (–) |
| Sc | Schimdt number (–) |
| t | Time (s) |
| tAD | Adsorption time (s) |
| tBD | Blowdown time (s) |
| tcycle | Cycle time (s) |
| tDPE | Depressurizing pressure equalization time (s) |
| tPPE | Pressurizing pressure equalization time (s) |
| tPR | Feed pressurization time (s) |
| tPU | Purge time (s) |
| T | Temperature (K) |
| Tw | Bed wall temperature (K) |
| u | Interstitial gas velocity (m s−1) |
| Ug | Gas phase internal energy per unit volume (J m−3) |
| Us | Adsorbent particle internal energy per unit volume (J m−3) |
| VSP | Valve stem position (–) |
| xi | Adsorbed phase molar fraction of component i (–) |
| yi | Gas phase molar fraction of component i (–) |
| z | Axial distance along the bed (m) |
| Greek letters | |
| γ | Ratio of specific heat capacities cp/cv (–) |
| ε | External bed void fraction (–) |
| εp | Adsorbent particle void fraction (–) |
| η | Vacuum pump efficiency (–) |
| μ | Gas viscosity (Pa s) |
| ρg | Gas density (kg m−3) |
| ρs | Adsorbent particle density (kg m−3) |
| ψeq | Reduced grand potential at equilibrium (mol kg−1) |
| ψi | Reduced grand potential of component i (mol kg−1) |
References
- IEA. Global Energy Review: CO2 Emissions in 2020. 2021. Available online: https://www.iea.org/articles/global-energy-review-co2-emissions-in-2020 (accessed on 28 December 2021).
- IEA. The Future of Hydrogen. 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 28 December 2021).
- BEIS. UK Hydrogen Strategy. 2021. Available online: https://www.gov.uk/government/publications/uk-hydrogen-strategy (accessed on 28 December 2021).
- Meyers, R.A. Handbook of Petroleum Refining Processes, 3rd ed.; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Collodi, G. Hydrogen production via steam reforming with CO2 capture. Chem. Eng. Trans. 2010, 19, 37–42. [Google Scholar]
- Meerman, J.C.; Hamborg, E.S.; van Keulen, T.; Ramirez, A.; Turkenburg, W.C.; Faaij, A.P.C. Techno-economic assessment of CO2 capture at steam methane reforming facilities using commercially available technology. Int. J. Greenh. Gas Control 2012, 9, 160–171. [Google Scholar] [CrossRef]
- Voss, C. CO2 removal by PSA: An industrial view on opportunities and challenges. Adsorption 2014, 20, 295–299. [Google Scholar] [CrossRef]
- IEA. The Shell Quest Carbon Capture and Storage Project. 2019. Available online: https://ieaghg.org/publications/technical-reports/reports-list/9-technical-reports/949-2019-04-the-shell-quest-carbon-capture-and-storage-project (accessed on 28 December 2021).
- IEA. The Carbon Capture Project at Air Products’ Port Arthur Hydrogen Production Facility. 2018. Available online: https://ieaghg.org/publications/technical-reports/reports-list/9-technical-reports/956-2018-05-the-ccs-project-at-air-products-port-arthur-hydrogen-production-facility (accessed on 28 December 2021).
- IEA. Reference Data and Supporting Literature Review for SMR Based Hydrogen Production with CCS. 2017. Available online: https://ieaghg.org/publications/technical-reports/reports-list/10-technical-reviews/778-2017-tr3-reference-data-supporting-literature-reviews-for-smr-based-hydrogen (accessed on 28 December 2021).
- Park, J.H.; Beum, H.T.; Kim, J.N.; Cho, S.H. Numerical analysis on the power consumption of the PSA process for recovering CO2 from flue gas. Ind. Eng. Chem. Res. 2002, 41, 4122–4131. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Webley, P.; Li, G.; Singh, R.; Todd, R. Capture of CO2 from flue gas streams with zeolite 13X by vacuum-pressure swing adsorption. Adsorption 2008, 14, 575–582. [Google Scholar] [CrossRef]
- Luberti, M.; Oreggioni, G.D.; Ahn, H. Design of a rapid vacuum pressure swing adsorption process for post-combustion CO2 capture from a biomass-fuelled CHP plant. J. Environ. Chem. Eng. 2017, 5, 3973–3982. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Farooq, S.; Knaebel, K.S. Pressure Swing Adsorption; VCH Publishers: New York, NY, USA, 1994. [Google Scholar]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Capocelli, M.; Luberti, M.; Inno, S.; D’Antonio, F.; Di Natale, F.; Lancia, A. Post-combustion CO2 capture by RVPSA in a large-scale steam reforming plant. J. CO2 Util. 2019, 32, 53–65. [Google Scholar] [CrossRef]
- Oreggioni, G.D.; Brandani, S.; Luberti, M.; Baykan, Y.; Friedrich, D.; Ahn, H. CO2 capture from syngas by an adsorption process at a biomass gasification CHP plant: Its comparison with amine-based CO2 capture. Int. J. Greenh. Gas Control 2015, 35, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Yu, X.; Shi, W.; Shen, Y.; Zhang, D.; Tang, Z. Two-stage VSA/PSA for capturing carbon dioxide (CO2) and producing hydrogen (H2) from steam methane reforming gas. Int. J. Hydrogen Energy 2020, 45, 24870–24882. [Google Scholar] [CrossRef]
- Sircar, S.; Kratz, W.C. Simultaneous production of hydrogen and carbon dioxide from steam reformer off-gas by pressure swing adsorption. Sep. Sci. Technol. 1988, 23, 2397–2415. [Google Scholar] [CrossRef]
- Streb, A.; Hefti, M.; Gazzani, M.; Mazzotti, M. Novel adsorption process for co-production of hydrogen and CO2 from a multicomponent stream. Ind. Eng. Chem. Res. 2019, 58, 17489–17506. [Google Scholar] [CrossRef] [Green Version]
- Streb, A.; Mazzotti, M. Novel adsorption process for co-production of hydrogen and CO2 from a multicomponent stream—Part 2: Application to steam methane reforming and autothermal reforming gases. Ind. Eng. Chem. Res. 2020, 59, 10093–10109. [Google Scholar] [CrossRef]
- Golmakani, A.; Fatemi, S.; Tamnanloo, J. CO2 capture from the tail gas of hydrogen purification unit by vacuum swing adsorption process, using SAPO-34. Ind. Eng. Chem. Res. 2016, 55, 334–350. [Google Scholar] [CrossRef]
- Shi, W.; Yang, H.; Shen, Y.; Fu, Q.; Zhang, D.; Fu, B. Two-stage PSA/VSA to produce H2 with CO2 capture via steam methane reforming (SMR). Int. J. Hydrogen Energy 2018, 43, 19057–19074. [Google Scholar] [CrossRef]
- Mhaskar, P.R.; Moharir, A.S. Heuristics for synthesis and design of pressure-swing adsorption processes. Adsorption 2012, 18, 275–295. [Google Scholar] [CrossRef]
- Brea, P.; Delgado, J.A.; Agueda, V.I.; Gutierrez, P.; Uguina, M.A. Multicomponent adsorption of H2, CH4, CO and CO2 in zeolites NaX, CaX and MgX. Evaluation of performance in PSA cycles for hydrogen purification. Microporous Mesoporous Mater. 2019, 286, 187–198. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Li, P.; Wang, J.; Yu, J. CO2 capture from flue gas by two successive VPSA units using 13XAPG. Adsorption 2012, 18, 445–459. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Rao, V.R.; Guntuka, S.; Sharratt, P.; Haghpanah, R.; Rajendran, A.; Amanullah, M.; Karimi, I.A.; Farooq, S. CO2 capture from dry flue gas by vacuum swing adsorption: A pilot plant study. AIChE J. 2014, 60, 1830–1842. [Google Scholar] [CrossRef]
- Luberti, M.; Friedrich, D.; Brandani, S.; Ahn, H. Design of a H2 PSA for cogeneration of ultrapure hydrogen and power at an advanced integrated gasification combined cycle with pre-combustion capture. Adsorption 2014, 20, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Luberti, M.; Ahn, H. Design of an industrial multi-bed (V)PSA unit for argon concentration. Sep. Purif. Technol. 2021, 261, 118254. [Google Scholar] [CrossRef]
- Luberti, M.; Kim, Y.-H.; Lee, C.-H.; Ferrari, M.-C.; Ahn, H. New momentum and energy balance equations considering kinetic energy effect for mathematical modelling of a fixed bed adsorption column. Adsorption 2015, 21, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Ergun, S. Fluid flow through packed columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Santori, G.; Luberti, M. Thermodynamics of thermally-driven adsorption compression. Sustain. Mater. Technol. 2016, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- OriginLab. 2019. Available online: https://www.originlab.com/ (accessed on 28 December 2021).
- Wakao, N.; Funazkri, T. Effect of fluid dispersion coefficients on particle-to-fluid mass transfer coefficients in packed beds. Chem. Eng. Sci. 1978, 33, 1375–1384. [Google Scholar] [CrossRef]
- Specchia, V.; Baldi, G.; Sicardi, S. Heat transfer in packed bed reactors with one phase flow. Chem. Eng. Commun. 1980, 4, 361–380. [Google Scholar] [CrossRef]
- Process System Enterprise Ltd. 2020. Available online: https://www.psenterprise.com/ (accessed on 28 December 2021).
- Liu, Z.; Grande, C.A.; Li, P.; Yu, J.; Rodrigues, A.E. Multi-bed vacuum pressure swing adsorption for carbon dioxide capture from flue gas. Sep. Purif. Technol. 2011, 81, 307–317. [Google Scholar] [CrossRef]
- IEA. Techno-Economic Evaluation of SMR Based Standalone (Merchant) Hydrogen Plant with CSS. 2017. Available online: https://ieaghg.org/component/content/article/49-publications/technical-reports/784-2017-02-smr-based-h2-plant-with-ccs (accessed on 28 December 2021).
- Yang, R.T. Gas Separation by Adsorption Processes; Butterworths: Boston, MA, USA, 1987. [Google Scholar]




| Equilibrium Parameter (Dual-Site Langmuir Isotherm) | qs1/qs2 (mol kg−1) | b01/b02 (bar−1) | (–ΔH1)/(–ΔH2) (J mol−1) | |
| H2 | 4.529/0 | 2.431 × 10−4/0 | 7018/0 | |
| CH4 | 4.529/0 | 8.313 × 10−5/0 | 16,294/0 | |
| CO | 4.529/0 | 9.411 × 10−5/0 | 18,182/0 | |
| CO2 | 2.500/2.029 | 3.521 × 10−5/6.318 × 10−6 | 32,244/30,937 | |
| Kinetic Parameter (kLDF = k1 × T + k2) | k1 (s−1 K−1) | k2 (s−1) | ||
| H2 | 0 | 100 | ||
| CH4 | 0.0115 | −2.8561 | ||
| CO | 0.0137 | –3.6086 | ||
| CO2 | 0.0099 | –2.8631 | ||
| Adsorbent Parameters | |
| ρs (kg m−3) | 1256 [25] |
| εp (–) | 0.404 [25] |
| cp,s (J kg−1 K−1) | 920 [13] |
| dp (m) | 2.5 × 10−3 [13] |
| Bed Parameters | |
| Lb (m) | 4.5 (2-bed, 4-step); 6.0 (2-bed, 6-step) |
| Db (m) | 3.0 |
| ε (–) | 0.53 [25] |
| Dz (m2 s−1) | 1.5 × 10−3 |
| kz (W m−1 K−1) | 1.4 |
| hw (W m−2 K−1) | 55.1 |
| Valve Parameters | |
| Cv (kg s−1 Pa−1/2) | 1.0 × 10−3 (feed) |
| 1.0 × 10−3 (product) | |
| 5.0 × 10−3 (blowdown) | |
| 1.5 × 10−3 (purge top) | |
| 5.0 × 10−3 (purge bottom) | |
| 3.0 × 10−4 (equalization) | |
| Operating Conditions | |
| Feed composition (mol%) | 23.87% H2; 10.15% CH4; 14.65% CO; 51.34% CO2 |
| Adsorption pressure (bar) | 1.3 |
| Desorption pressure (bar) | 0.05–0.2 |
| Purge-to-feed ratio (–) | 0–0.05 |
| Feed temperature (K) | 301.15 |
| Molar feed flowrate (mol s−1) | 146.27 (per PSA train) |
| Volumetric feed flowrate (m3 s−1) | 2.82 (per PSA train) |
| Run # | Pdes (bar) | P/F Ratio (–) | CO2 Purity (%) | CO2 Recovery (%) | Specific Energy Consumption (kJ kgCO2−1) | Bed Productivity (molCO2 h−1 kgads−1) |
|---|---|---|---|---|---|---|
| Run 1 | 0.05 | 0 | 95.3 | 77.4 | 649.1 | 5.57 |
| Run 2 | 0.01 | 94.5 | 80.7 | 654.7 | 5.81 | |
| Run 3 | 0.03 | 92.1 | 85.0 | 671.7 | 6.12 | |
| Run 4 | 0.05 | 89.7 | 88.0 | 691.3 | 6.33 | |
| Run 5 | 0.1 | 0 | 93.8 | 60.1 | 475.2 | 4.33 |
| Run 6 | 0.01 | 93.4 | 66.5 | 477.0 | 4.79 | |
| Run 7 | 0.03 | 91.3 | 72.8 | 488.7 | 5.24 | |
| Run 8 | 0.05 | 89.0 | 77.0 | 501.7 | 5.54 | |
| Run 9 | 0.2 | 0 | 89.9 | 37.5 | 327.3 | 2.70 |
| Run 10 | 0.01 | 90.2 | 45.9 | 324.1 | 3.30 | |
| Run 11 | 0.03 | 89.1 | 53.7 | 330.1 | 3.86 | |
| Run 12 | 0.05 | 87.0 | 58.8 | 338.1 | 4.23 |
| Run # | Pdes (bar) | P/F Ratio (–) | CO2 Purity (%) | CO2 Recovery (%) | Specific Energy Consumption (kJ kgCO2−1) | Bed Productivity (molCO2 h−1 kgads−1) |
|---|---|---|---|---|---|---|
| Run 13 | 0.05 | 0 | 99.1 | 84.2 | 621.5 | 4.20 |
| Run 14 | 0.01 | 98.5 | 86.1 | 625.4 | 4.29 | |
| Run 15 | 0.03 | 96.4 | 89.7 | 639.7 | 4.47 | |
| Run 16 | 0.05 | 94.1 | 92.4 | 657.8 | 4.60 | |
| Run 17 | 0.1 | 0 | 98.5 | 69.1 | 450.9 | 3.44 |
| Run 18 | 0.01 | 97.9 | 73.2 | 453.5 | 3.65 | |
| Run 19 | 0.03 | 95.8 | 79.0 | 463.9 | 3.94 | |
| Run 20 | 0.05 | 93.6 | 83.1 | 476.4 | 4.14 | |
| Run 21 | 0.2 | 0 | 96.1 | 45.3 | 305.1 | 2.26 |
| Run 22 | 0.01 | 95.9 | 51.1 | 305.7 | 2.55 | |
| Run 23 | 0.03 | 94.1 | 59.3 | 311.9 | 2.95 | |
| Run 24 | 0.05 | 91.9 | 64.8 | 319.9 | 3.23 |
| Cycle Configuration | Adsorbent | Pads (bar) | Pdes (bar) | P/F Ratio (–) | CO2 Purity (%) | CO2 Recovery (%) | Specific Energy Consumption (kJ kgCO2−1) 1 | Bed Productivity (molCO2 h−1 kgads−1) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 2-bed, 4-step | NaX zeolite | 1.3 | 0.05 | 0 | 95.3 | 77.4 | 259.6 | 5.57 | This study |
| 2-bed, 6-step | 0.03 | 96.4 | 89.7 | 255.9 | 4.47 | ||||
| 4-bed, 7-step | SAPO-34 zeolite | 4.0 | 0.05 | 0.03 | 90.5 | 73.5 | 305.0 | 3.34 | [22] |
| Activated carbon | 86.2 | 56.8 | 369.0 | 3.14 | |||||
| 5A zeolite | 88.1 | 75.9 | 284.0 | 3.61 | |||||
| 3-bed, 5-step | Silica gel | 2.0 | 0.2 | N/A | 97.1 | 82.6 | 133.3 | N/A | [23] |
| 88.6 | 97.8 | 133.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luberti, M.; Brown, A.; Balsamo, M.; Capocelli, M. Numerical Analysis of VPSA Technology Retrofitted to Steam Reforming Hydrogen Plants to Capture CO2 and Produce Blue H2. Energies 2022, 15, 1091. https://doi.org/10.3390/en15031091
Luberti M, Brown A, Balsamo M, Capocelli M. Numerical Analysis of VPSA Technology Retrofitted to Steam Reforming Hydrogen Plants to Capture CO2 and Produce Blue H2. Energies. 2022; 15(3):1091. https://doi.org/10.3390/en15031091
Chicago/Turabian StyleLuberti, Mauro, Alexander Brown, Marco Balsamo, and Mauro Capocelli. 2022. "Numerical Analysis of VPSA Technology Retrofitted to Steam Reforming Hydrogen Plants to Capture CO2 and Produce Blue H2" Energies 15, no. 3: 1091. https://doi.org/10.3390/en15031091
APA StyleLuberti, M., Brown, A., Balsamo, M., & Capocelli, M. (2022). Numerical Analysis of VPSA Technology Retrofitted to Steam Reforming Hydrogen Plants to Capture CO2 and Produce Blue H2. Energies, 15(3), 1091. https://doi.org/10.3390/en15031091







