Review of the Current Status of Ammonia-Blended Hydrogen Fuel Engine Development
Abstract
:1. Introduction
2. Ammonia Fuel Engine’s Characteristics and Problems
2.1. Physical and Chemical Properties of Ammonia
2.2. Advantages of Ammonia as a Fuel
2.3. Problems with Ammonia-Fueled Engines
- It has also been found in some tests that the combustion of ammonia as a fuel or mixed with other fuels leads to an increase in NOx emissions in the exhaust gas. However, the widely used SCR technology in automobiles treats and cleans up to 90% of the emitted NOx, which can mitigate the possible deterioration of emissions caused by ammonia fuel [13,28,29];
- Ammonia as a fuel often results in the presence of ammonia escape from the engine due to incomplete combustion. However, it was found experimentally that this phenomenon was mitigated to some extent after the installation of ammonia treatment devices;
- For the poor combustion performance of ammonia, ammonia-blending engine research has been widely carried out in the world; it can be blended by using other fuels as combustion aids to effectively improve the ammonia combustion phenomenon [30].
3. Pure Ammonia Fueled Engines
4. Common Ammonia-Blended Fuel
4.1. Spark-Ignition Engines
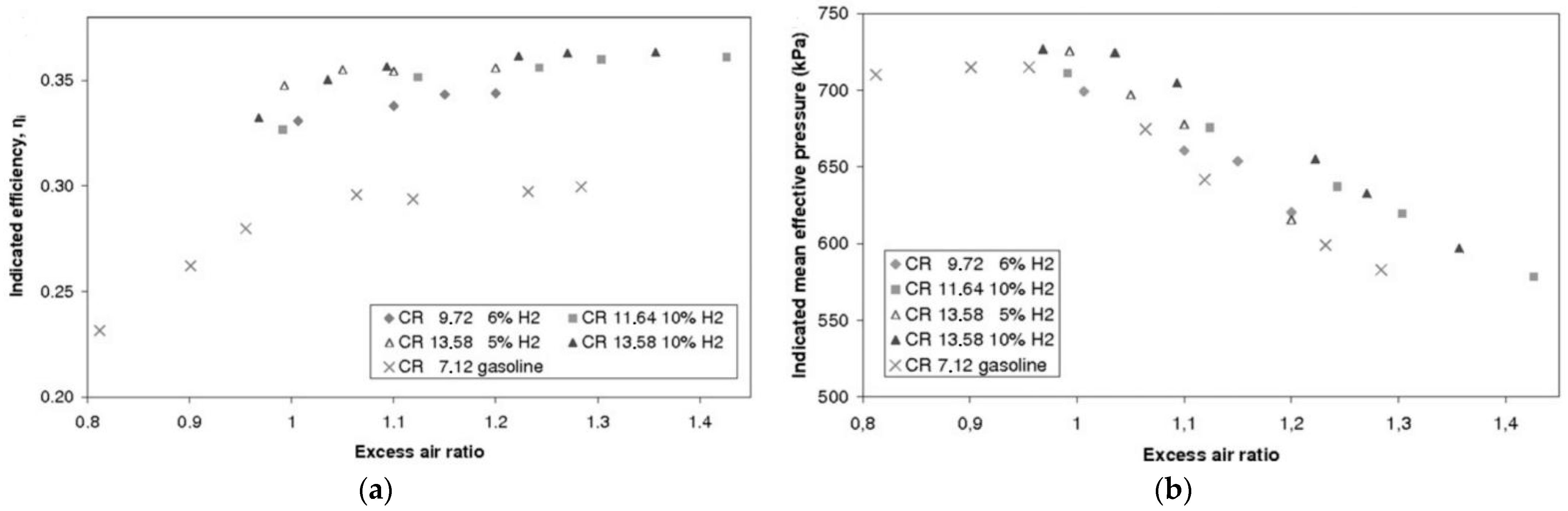
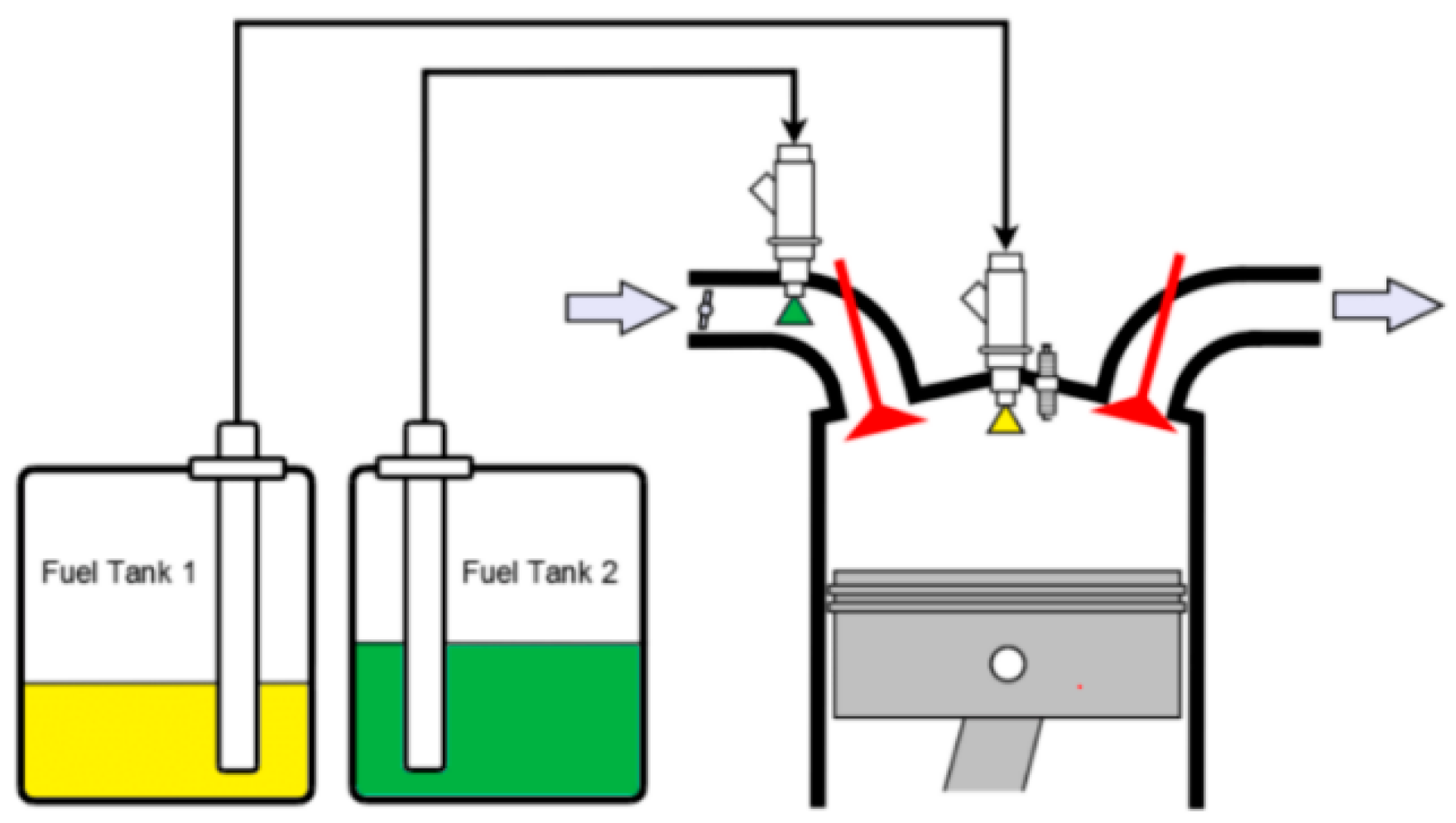
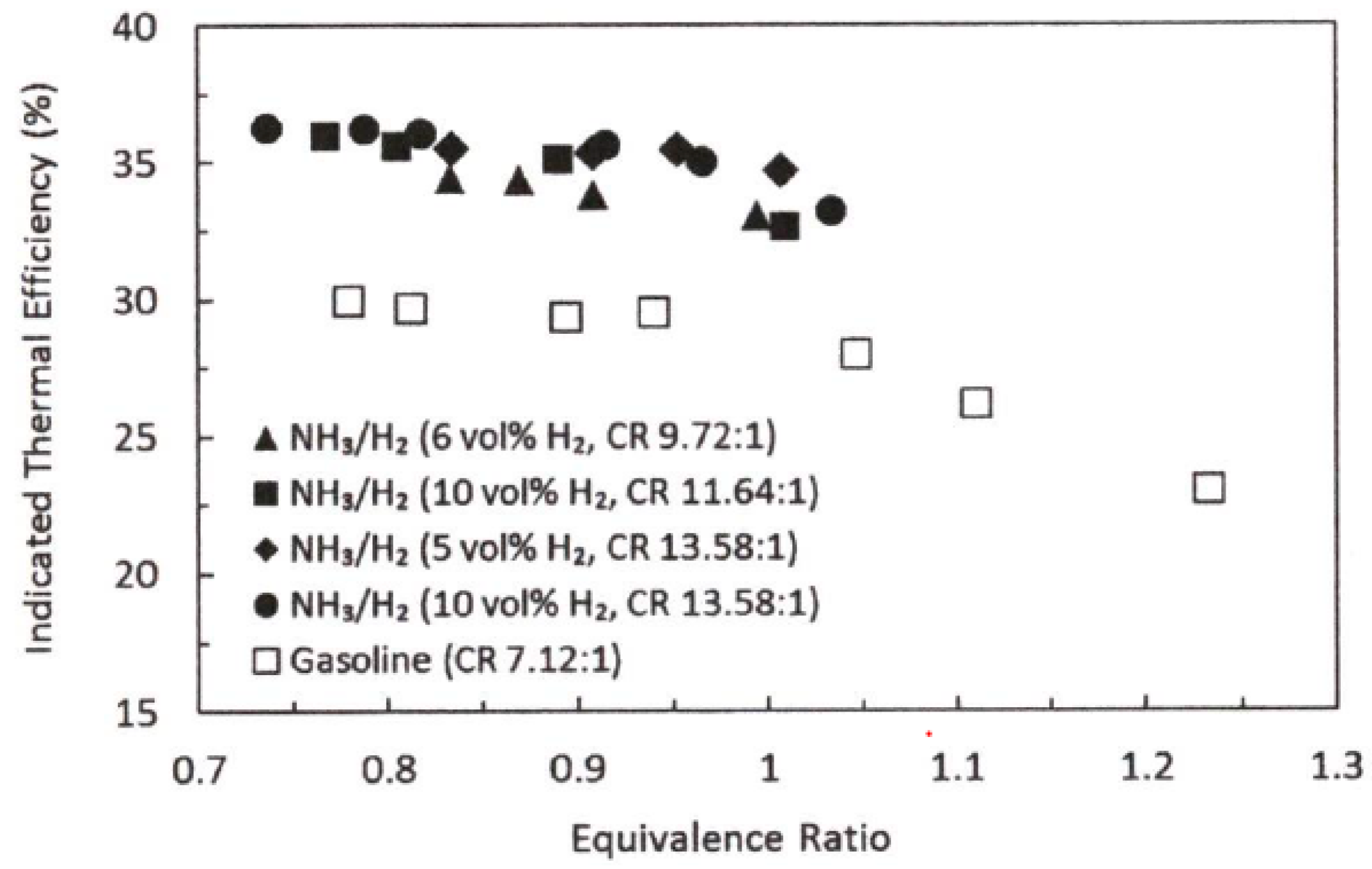
4.1.1. Hydrogen
4.1.2. Natural Gas
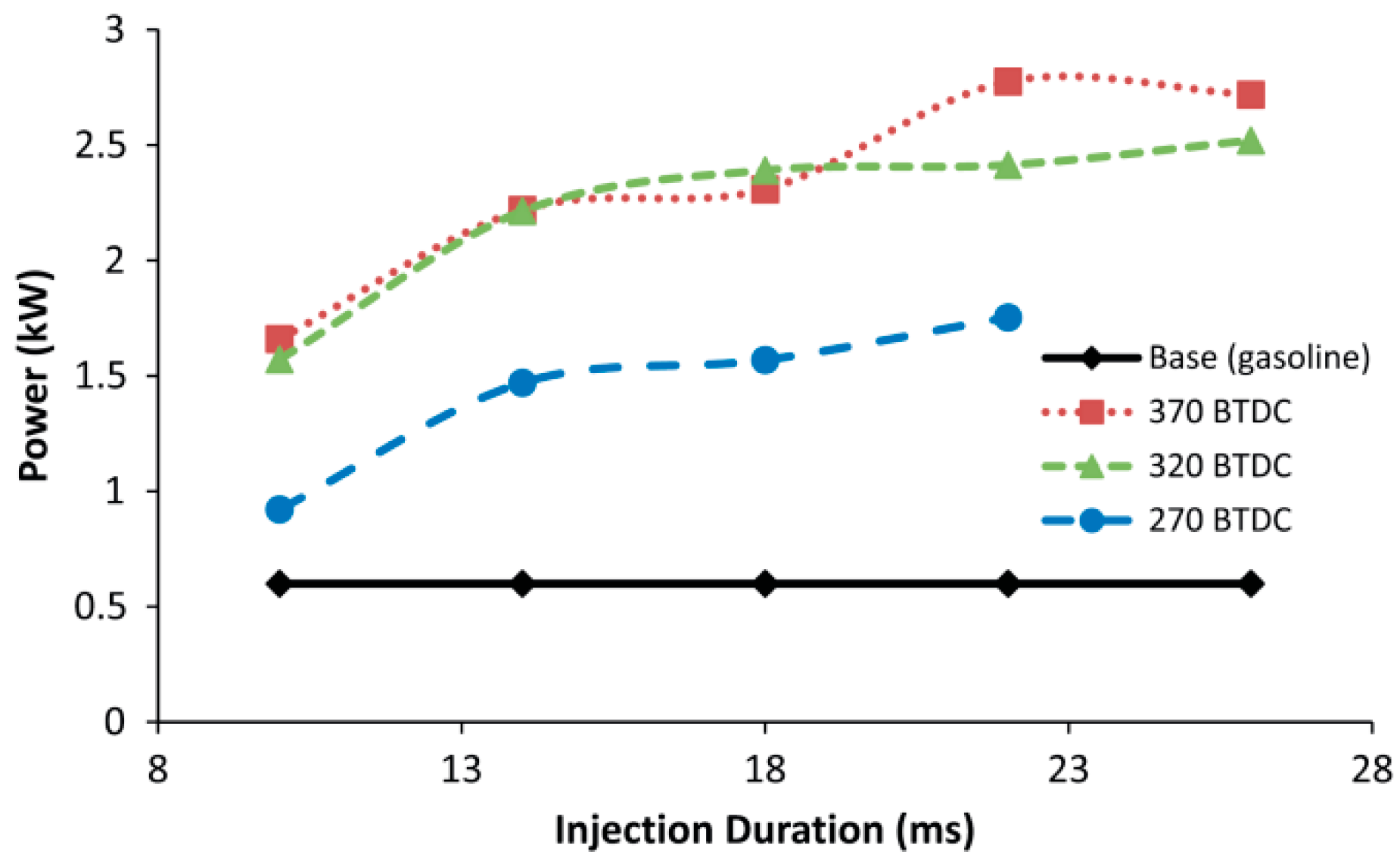
4.1.3. Gasoline
4.2. Compression-Ignition Engine
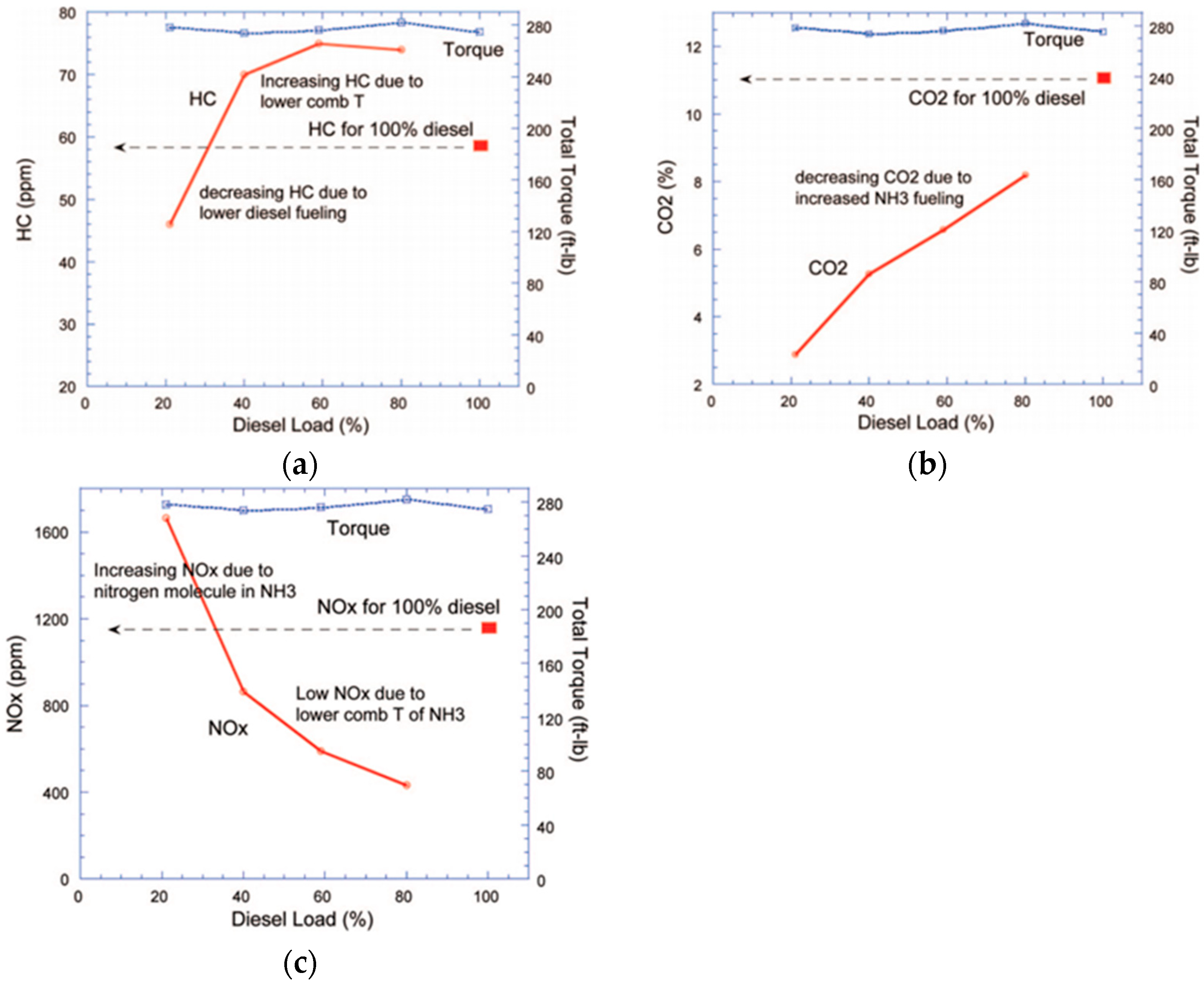
4.2.1. Diesel
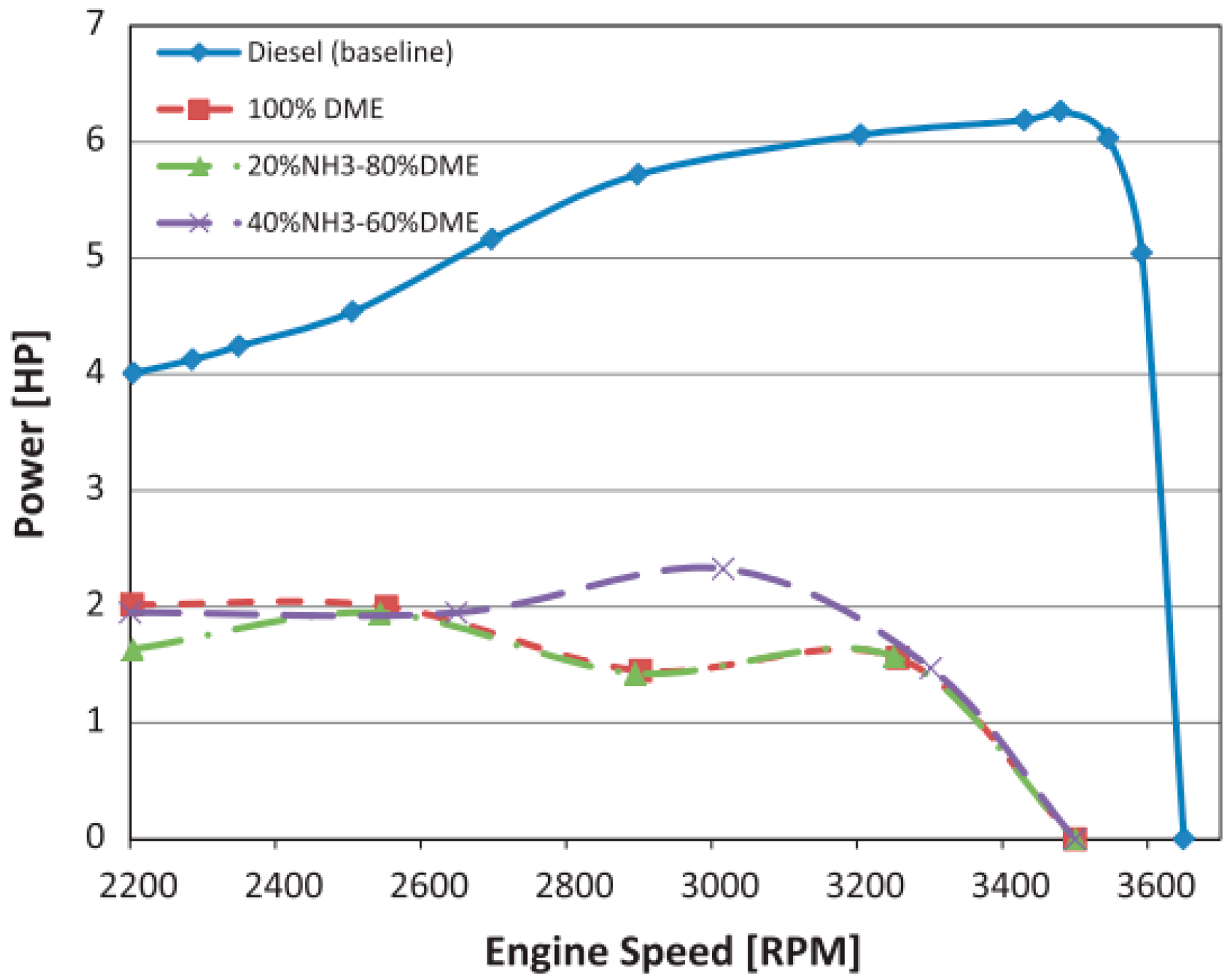
4.2.2. DME
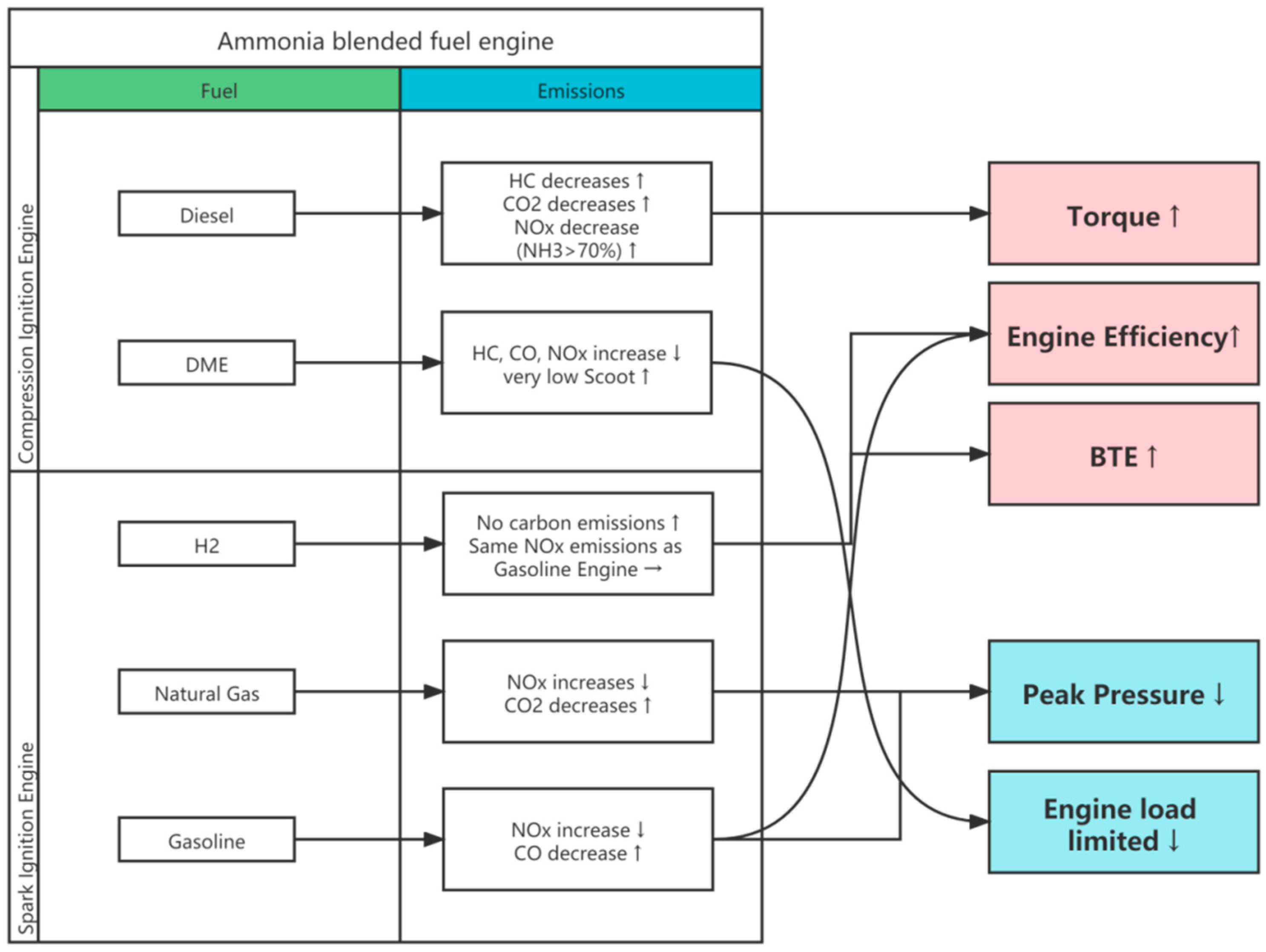
4.3. Comparison Analysis
- The blending of ammonia with hydrogen can improve the combustion properties of ammonia with less negative impact compared to other blended fuels;
- Both ammonia and hydrogen have a higher octane number and can try to burn at a higher compression ratio, thus improving the engine cycle thermal efficiency and output power;
- Theoretically no additional carbon-containing substances are emitted from the emission;
- The main pollutant in the emissions of blended fuels is NOx, which is emitted at levels similar to those emitted by fossil fuel combustion and can be effectively cleaned by SCR technology or the ternary catalysts now widely used.
5. Problems and Solutions of Ammonia-Blended Hydrogen Engine
5.1. Problems
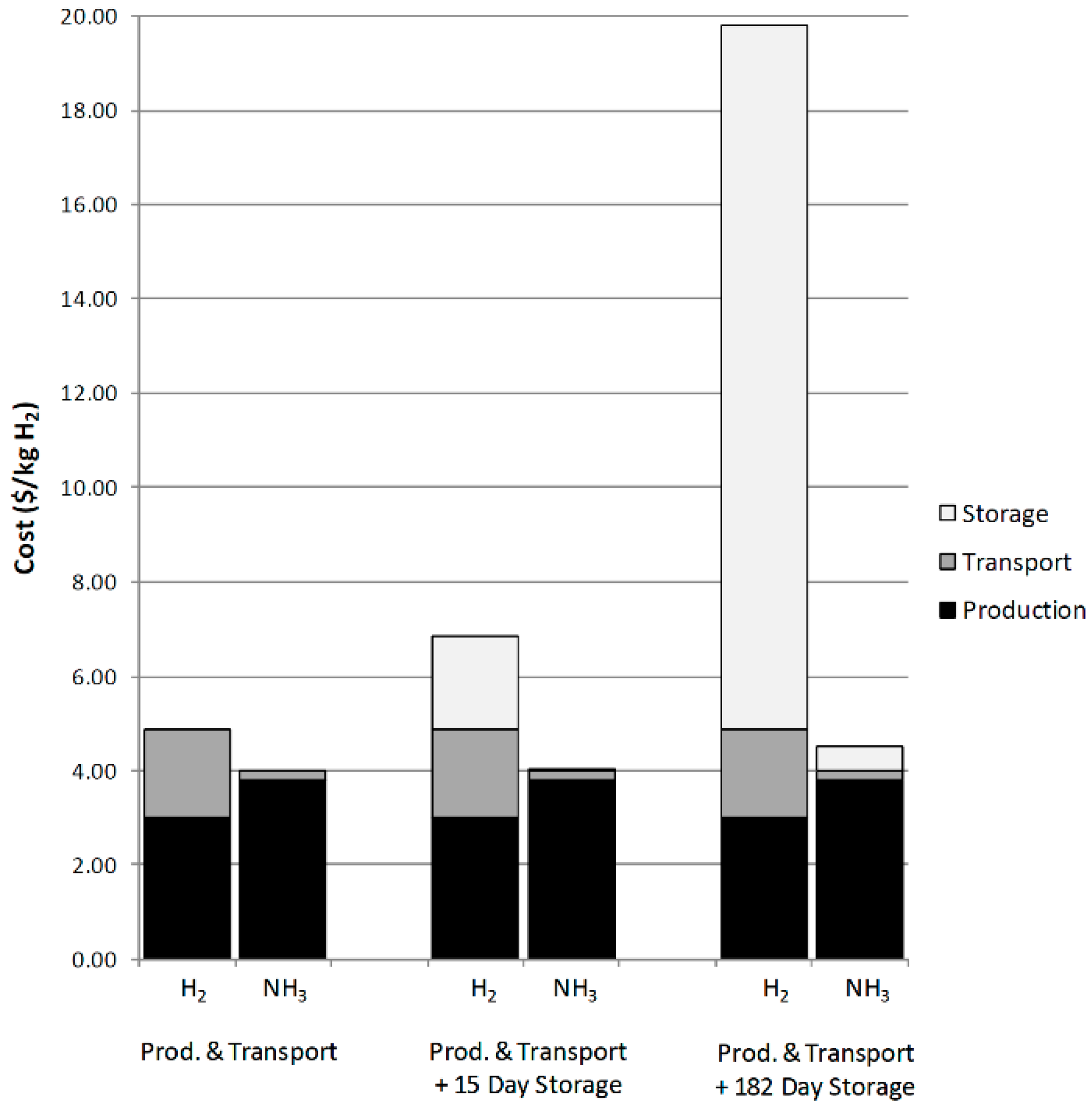
5.2. Hydrogen Production Methods
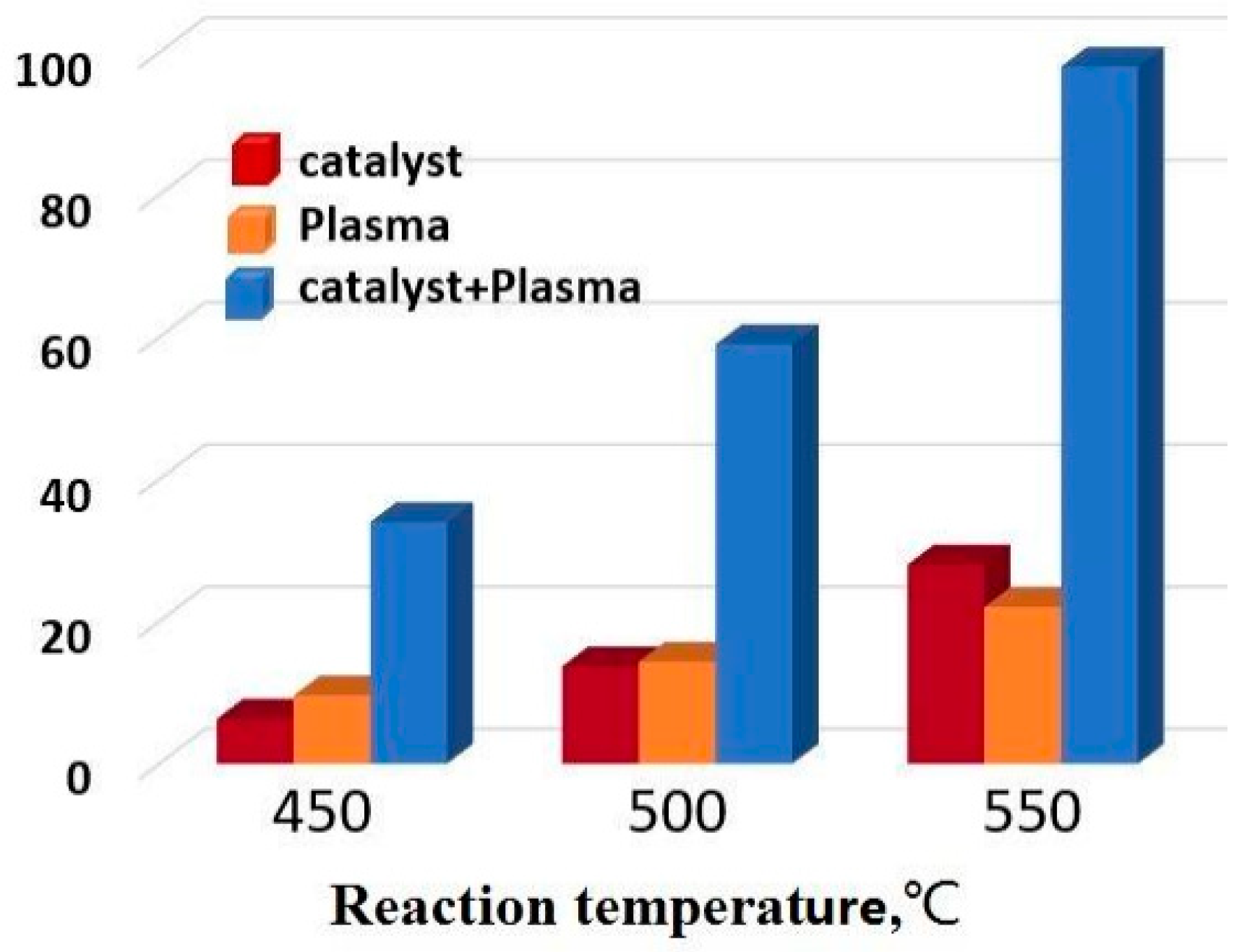
5.3. Ammonia Decomposition
5.4. Summary
6. Discussion
- This paper firstly introduces the physicochemical properties of ammonia fuel, its high calorific value, high octane number, good combustion efficiency, pure combustion products, low cost, easy transportation, etc., which can support ammonia as an excellent fuel; ammonia fuel also has many disadvantages, such as toxicity and corrosiveness, low calorific value, high ignition point, low minimum ignition energy, etc., which limit the development of ammonia as a fuel;
- The paper then reviews the feasible and adopted solutions proposed by some researchers for the drawbacks of ammonia fuel; based on the special physicochemical properties of ammonia, the paper then introduces the early and recent research on pure ammonia-fueled engines, explaining that there are still many research limitations in the development of pure ammonia-fueled engines, and emphasizing the need for research on ammonia-blended engines;
- Then, this paper compares and analyzes different ammonia-blended engines in terms of their combustion performance and combustion emission products. Among them, ammonia blended with hydrogen is considered as a more ideal blending scheme, although further research and experimental verification are still needed;
- Finally, for hydrogen storage, the feasibility of hydrogen production is explored in this paper. Some of the research on ammonia decomposition for hydrogen production is highlighted. This method can reduce the comprehensive cost and avoid the space problem and safety problem caused by hydrogen storage. However, the current research in this area is mainly at the stage of experimental analysis, which will be affected by factors such as ammonia flow rate and catalytic temperature in the process of practical application, and further structural and performance optimization is needed to obtain a more stable and sufficient supply of hydrogen source.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zemin, J. Reflections on energy issues in China. J. Shanghai Jiaotong Univ. 2008, 42, 345–359. [Google Scholar] [CrossRef]
- Zou, C.; Xiong, B.; Huaqing, X.; Zheng, D. The role of new energy in carbon neutral. Pet. Explor. Dev. 2021, 48, 411–420. [Google Scholar] [CrossRef]
- IPPC. Global Warming of 1.5 °C. Available online: https://www.ipcc.ch/sr15 (accessed on 24 September 2021).
- IEA. Global CO2 Emissions in 2019. Available online: https://www.iea.org/articles/global-co2-emissions-in-2019 (accessed on 24 September 2021).
- IEA. CO2 Emissions from Fuel Combustion Highlights. Available online: https://www.iea.org/data-and-statistics/data-product/co2-emissions-from-fuel-combustion-highlights#highlights (accessed on 24 September 2021).
- Sonachalam, M.; Paulpandian, P.; Manieniyan, V. Emission reduction in diesel engine with acetylene gas and biodiesel using inlet manifold injection. Clean Technol. Environ. Policy 2020, 22, 2177–2191. [Google Scholar]
- Sonachalam, M.; Manieniyan, V. Impact of secondary fuel injector in various distance on direct injection diesel engine using acetylene-bio diesel in reactivity controlled compression ignition mode. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 22, 2177–2191. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges–ScienceDirect. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Su, H.; Linkou, V.; Bladergroen, B.J. Membrane electrode assemblies with low noble metal loadings for hydrogen production from solid polymer electrolyte water electrolysis. Int. J. Hydrog. Energy 2013, 38, 9601–9608. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z. Hydrogen Fuel Engine Combustion and Optimal Control; Zhejiang University: Zhejiang, China, 2001. [Google Scholar]
- Yang, Z.; Wang, L.; Wei, L. Prospect and Development on Hydrogen fueled Engines. In Proceedings of the China International Hydrogen Energy Conference, Shanghai, China, September 2007; China Industrial Gases Industry Association: Beijing, China, 2007. [Google Scholar]
- Agustin, V.-M.; Rene, B.-A. Ammonia Production Technologies—ScienceDirect. In Techno-Economic Challenges of Green Ammonia as an Energy Vector; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–83. [Google Scholar]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Jianquan, H.; Shan, X.; Yichun, W.; Jianxiang, Z.; Wenzhong, M. Study on low carbon ammonia engine. Renew. Energy Resour. 2014, 32, 1505–1509. [Google Scholar]
- Danan, C.; Jun, L.; Hongyu, H.; Ying, C.; Zhaohong, H.; Lisheng, D. Progress in Ammonia Combustion and Reaction Mechanism. Chemistry 2020, 6, 508–515. [Google Scholar]
- The Royal Society. Ammonia: Fuel and Energy Store; The Royal Society: London, UK, 2020. [Google Scholar]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.; Hodgetts, R.Y.; Bakker, J.M.; Vallana, F.M.F.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Lloyd’s Register. Decarbonising Shipping-Could Ammonia Be the Fuel of the Future; Lloyd’s Register: London, UK, 2021. [Google Scholar]
- Ma, J.; Liu, X.D.; Chen, Y.S. Current Status and Countermeasures for China’s New Energy Automobile Industry and Technology Development. China J. Highw. Transp. 2018, 31, 1–19. [Google Scholar] [CrossRef]
- Pengyan, G.; Fang, S.; Lijun, W.; Fangge, S.; Peng, Z. Research Status and Development Trend for Ammonia-fueled Engines. Veh. Engine 2016, 1–5, 13. [Google Scholar] [CrossRef]
- Zacharakisjutz, G.E. Performance Characteristics of Ammonia Engines Using Direct Injection Strategies. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2013. [Google Scholar]
- Liu, R.; Ting, D.S.K.; Checkel, M.D. Ammonia as a Fuel for SI Engine; SAE Technical Paper; SAE: Warrendale, PA, USA, 2003. [Google Scholar]
- Zeng, S.J.; Shang, D.W.; Yu, M.; Chen, H.; Dong, H.; Zhang, X. Applications and perspectives of NH3 separation and recovery with ionic liquids. CIESC J. 2019, 70, 10. [Google Scholar]
- Xu, X.; Xu, Q.; Huang, G.; Wang, L.; Huang, L. Removal of ammonia by absorption combined with electrochemical oxidation on RuO2/Ti anode. CIESC J. 2016, 67, 7. [Google Scholar]
- Giddey, S.; Badwal, S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrog. Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Jha, P.; Ramgir, N.S.; Sharma, P.K.; Datta, N.; Kailasaganapathi, S.; Kaur, M.; Koiry, S.P.; Saxena, V.; Chauhan, A.K.; Debnath, A.K.; et al. Charge transport and ammonia sensing properties of flexible polypyrrole nanosheets grown at air–liquid interface. Mater. Chem. Phys. 2013, 140, 300–306. [Google Scholar] [CrossRef]
- Feng, X. Methanol-Ammonia and the New Energy Economy; Chemical Industry Press: Beijing, China, 2010; pp. 60–62. Available online: https://baike.baidu.com/item/%E7%94%B2%E9%86%87%C2%B7%E6%B0%A8%E5%92%8C%E6%96%B0%E8%83%BD%E6%BA%90%E7%BB%8F%E6%B5%8E/3274627?fr=aladdin (accessed on 20 January 2022).
- Reiter, A.J.; Kong, S.C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Toyota Motor Corporation. Exhaust Cleaning Device for Engines. CN200980159001.8. 11 April 2004. Available online: https://wenku.baidu.com/view/a0ce8cf769d97f192279168884868762cbaebbe5?fr=xueshu (accessed on 20 January 2020).
- Newhall, H.K.; Starkman, E.S. Theoretical Performance of Ammonia as a Gas Turbine Fuel; SAE Technical Paper; SAE International: Warrendale, PA, USA, 1966. [Google Scholar]
- Thurston, R.H. A History of the Growth of the Steam-Engine. Nature 1972, 19, 381–382. [Google Scholar]
- Grimes, P.G. Energy Depot Fuel Production and Utilization. In Proceedings of the International Automotive Engineering Congress & Exposition. 1965. Available online: https://www.researchgate.net/publication/296647846_Energy_Depot_Fuel_Production_and_Utilization (accessed on 20 January 2022).
- Gray, J.T., Jr.; Dimitroff, E.; Meckel, N.T.; Quillian, R.D., Jr. Ammonia Fuel-Engine Compatibility and Combustion. In Proceedings of the Automotive Engineering Congress & Exposition. 1966. Available online: https://www.researchgate.net/publication/296646977_Ammonia_Fuel_-_Engine_Compatibility_and_Combustion (accessed on 20 January 2022).
- Pearsall, T.J.; Garabedian, C.G. Combustion of Anhydrous Ammonia in Diesel Engines; SAE Technical Paper 670947; SAE International: Warrendale, PA, USA, 1967. [Google Scholar]
- Pearsall, T.J. Ammonia Application to Reciprocating Engines. Volume 1. 1967. Available online: https://www.researchgate.net/publication/235199347_AMMONIA_APPLICATION_TO_RECIPROCATING_ENGINES_VOLUME_1 (accessed on 20 January 2022).
- Li, J.; Huang, H.; Kobayashi, N.; He, Z.; Osaka, Y.; Zeng, T. Numerical study on effect of oxygen content in combustion air on ammonia combustion. Energy 2015, 93, 2053–2068. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Jeanmart, H.; Vandooren, J. Ammonia Combustion in spark ignition engine conditions. In Proceedings of the 7th Annual NH3 Fuel Conference, Romulus, MI, USA, 26–28 September 2010. [Google Scholar]
- Mørch, C.S.; Bjerre, A.; Gøttrup, M.P.; Sorenson, S.C.; Schramm, J. Ammonia/hydrogen mixtures in an Si-engine: Engine performance and analysis of a proposed fuel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Westlye, F.R.; Ivarsson, A.; Schramm, J. Experimental investigation of nitrogen based emissions from an ammonia fueled SI-engine. Fuel 2013, 111, 239–247. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.H.; Park, J.H.; Kwon, O.C. Studies on properties of laminar premixed hydrogen-added ammonia/air flames for hydrogen production. Int. J. Hydrog. Energy 2010, 35, 1054–1064. [Google Scholar] [CrossRef]
- Kumar, P.; Meyer, T.R. Experimental and modeling study of chemical-kinetics mechanisms for H2–NH3–air mixtures in laminar premixed jet flames. Fuel 2013, 108, 166–176. [Google Scholar] [CrossRef]
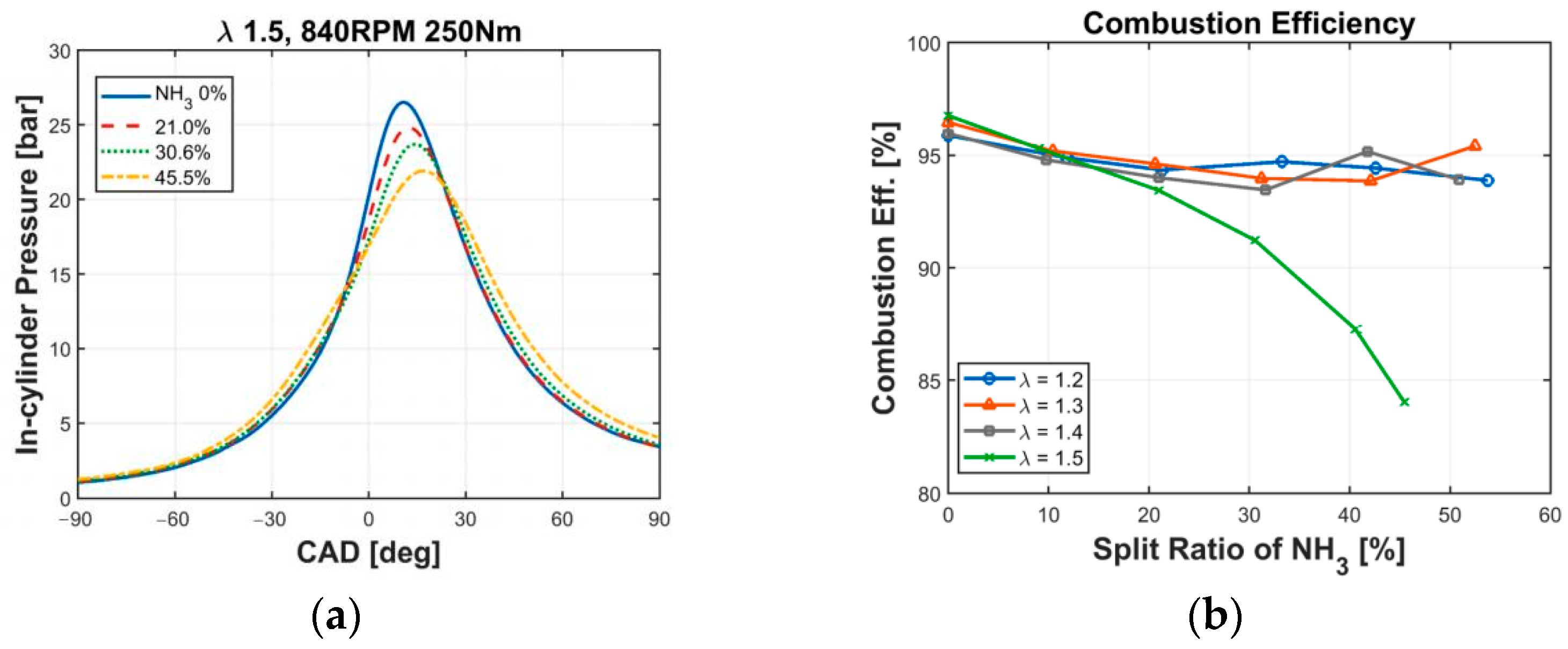
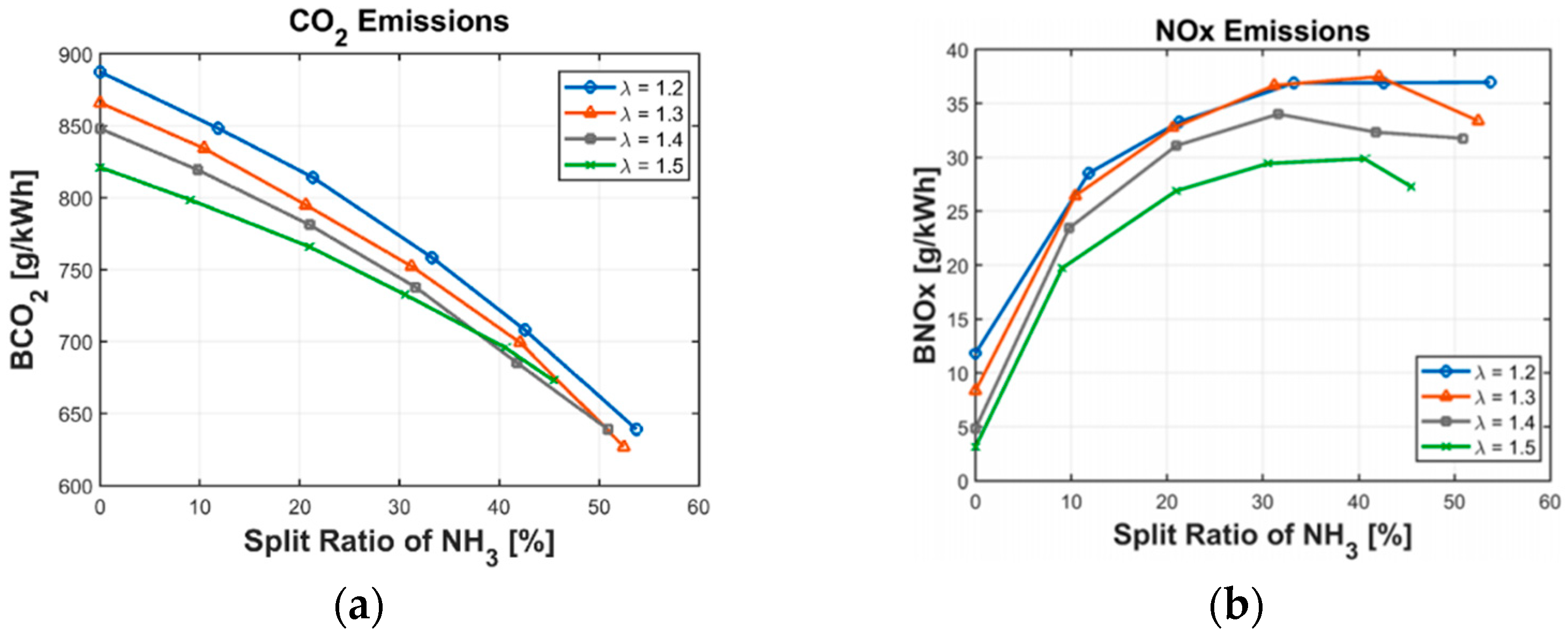
- Oh, S.; Park, C.; Kim, S.; Kim, Y.; Choi, Y.; Kim, C. Natural gas–ammonia dual-fuel combustion in spark-ignited engine with various air–fuel ratios and split ratios of ammonia under part load condition. Fuel 2021, 290, 120095. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.C. Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Appl. Energy 2014, 116, 206–215. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Demonstration of Compression-Ignition Engine Combustion Using Ammonia in Reducing Greenhouse Gas Emissions. Energy Fuels 2008, 22, 2963–2971. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Diesel Engine Operation Using Ammonia as a Carbon-Free Fuel. In Proceedings of the Internal Combustion Engine Division Fall Technical Conference, Online, 4–6 November 2010; Volume 49446, pp. 111–117. [Google Scholar]
- Gross, C.W.; Kong, S.C. Performance characteristics of a compression-ignition engine using direct-injection ammonia–DME mixtures. Fuel 2013, 103, 1069–1079. [Google Scholar] [CrossRef]
- Lesmana, H.; Zhang, Z.; Li, X.; Zhu, M.; Xu, W.; Zhang, D. NH3 as a Transport Fuel in Internal Combustion Engines: A Technical Review. J. Energy Resour. Technol. 2019, 141, 070703. [Google Scholar] [CrossRef]
- Bartels, J.R. A Feasibility Study of Implementing an Ammonia Economy; Iowa State University: Ames, IA, USA, 2008. [Google Scholar]
- Yeh, T.F.; Syu, J.M.; Cheng, C.; Chang, T.H.; Teng, H. Graphite Oxide as a Photocatalyst for Hydrogen Production from Water. Adv. Funct. Mater. 2010, 20, 2255–2262. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Liu, C.; Yang, Y.; Li, F.; Gong, Y.; Liu, M. Progress in Hydrogen Preparation Technology. Plat. Finish. 2019, 41, 6. Available online: https://wenku.baidu.com/view/fcc477617ed184254b35eefdc8d376eeafaa1713?fr=xueshu (accessed on 20 January 2022).
- Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrog. Energy 2013, 38, 2039–2061. [Google Scholar] [CrossRef]
- Laube, A.; Hofer, A.; Ressel, S.; Chica, A.; Bachmann, J.; Struckmann, T. PEM water electrolysis cells with catalyst coating by atomic layer deposition. Int. J. Hydrog. Energy 2021, 46, 38972–38982. [Google Scholar] [CrossRef]
- Ayers, K. High efficiency PEM water electrolysis: Enabled by advanced catalysts, membranes, and processes. Curr. Opin. Chem. Eng. 2021, 33, 100719. [Google Scholar] [CrossRef]
- Brisse, A.; Schefold, J.; Zahid, M. High temperature water electrolysis in solid oxide cells. Int. J. Hydrog. Energy 2008, 33, 5375–5382. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient water oxidation using nanostructured α-nickel-hydroxide as an electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7784. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Song, T.; Wu, J.; Shen, L.; Xiao, J. Experimental investigation on hydrogen production from biomass gasification in interconnected fluidized beds. Biomass Bioenergy 2012, 36, 258–267. [Google Scholar] [CrossRef]
- Levin, D.B.; Chahine, R. Challenges for renewable hydrogen production from biomass—ScienceDirect. Int. J. Hydrog. Energy 2010, 35, 4962–4969. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Wang, D.; He, C.; Jiang, W. Research of industrial hydrogen production at home and abroad. Ind. Catal. 2018, 26, 26–30. [Google Scholar] [CrossRef]
- Roel, V.; Liang, Y.; Schoonman, J. Solar hydrogen production with nanostructured metal oxides. J. Mater. Chem. 2008, 18, 2311. [Google Scholar]
- Joshi, A.S.; Dincer, I.; Reddy, B.V. Solar hydrogen production: A comparative performance assessment. Int. J. Hydrog. Energy 2011, 36, 11246–11257. [Google Scholar] [CrossRef]
- Wu, H. Research Progress in Several Methods of Hydrogen Producing Technology. Guangdong Chem. Ind. 2013, 40, 111–112. [Google Scholar] [CrossRef]
- Kogan, A. Direct solar thermal splitting of water and on site separation of the products I. Theoretical evaluation of hydrogen yield. Int. J. Hydrog. Energy 1997, 22, 481–486. [Google Scholar] [CrossRef]
- Kogan, A. Direct solar thermal on-site separation of the products-II. experimental feasibility study. Int. J. Hydrog. Energy 1998, 23, 89–98. [Google Scholar] [CrossRef]
- Kogan, A. Direct solar thermal splitting of water and on-site separation of the products. III. Improvement of reactor efficiency by steam entrainment. Int. J. Hydrog. Energy 1998, 25, 739–745. [Google Scholar] [CrossRef]
- Kogan, A. Direct solar thermal splitting of water and on-site separation of the products-IV. Development of porous ceramic membranes for a solar thermal water-splitting reactor. Int. J. Hydrog. Energy 2000, 25, 1043–1050. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.F.; Wang, T.; Li, H.; Li, C. Photonic, and photocatalytic behavior of TiO2 mediated by Fe, CO, Ni, N doping and co-doping. Phys. B Condens. Matter 2015, 478, 6–11. [Google Scholar] [CrossRef]
- Hwang, K.S.; Kim, S.D.; Hwangbo, S.; Kim, J.T. Effect of Metal (Ni2+, Fe3+, and Mo3+) Doping in TiO2 Thin Films on Antimicrobial Activity. J. Nanoelectron. Optoelectron. 2017, 12, 413–417. [Google Scholar] [CrossRef]
- Thomas, G.; Parks, G. Potential Roles of Ammonia in a Hydrogen Economy. 2006. Available online: https://www.researchgate.net/publication/331595915_Potential_Roles_of_Ammonia_in_a_Hydrogen_Economy (accessed on 20 January 2022).
- Li, Y.; Yao, L.; Song, Y.; Liu, S.; Zhao, J.; Ji, W.; Au, C.T. Core–shell structured microcapsular-like Ru@SiO2reactor for efficient generation of COx-free hydrogen through ammonia decomposition Electronic supplementary information (ESI) available: N2adsorption–desorption data, H2-TPD and H2-TPR. Chem. Commun. 2010, 46, 5298–5300. [Google Scholar] [CrossRef] [PubMed]
- Avery, W.H. A role for ammonia in the hydrogen economy. Int. J. Hydrog. Energy 1988, 13, 761–773. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrog. Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannessen, T.; Sørensen, R.Z.; Nørskov, J.K. Towards an ammonia-mediated hydrogen economy? Catal. Today 2006, 111, 140–144. [Google Scholar] [CrossRef]
- Zhu, C. Study of Nickel-Based Low-Temperature Ammonia Decomposition Catalysts and Their Carriers; Tianjin University: Tianjin, China, 2007. [Google Scholar] [CrossRef]
- Yan, S.; Li, X.; Zhong, L. Gliding Arc Discharge Plasma Assisted Decomposition of Ammonia into Hydrogen. J. Combust. Sci. Technol. 2011, 17, 186–190. [Google Scholar]
- Wang, L. Study on the Synergistic Effect of Plasma-Catalyzed Ammonia Decomposition for Hydrogen Production; University of Technology: Dalian, China, 2013. [Google Scholar]
- Sun, S. Loaded Bimetallic Catalyst for Hydrogen Production by Ammonia Decomposition; Dalian University of Technology: Dalian, China, 2017. [Google Scholar]
- Frigo, S.; Gentili, R. Analysis of the behaviour of a 4-stroke Si engine fuelled with ammonia and hydrogen. Int. J. Hydrog. Energy 2013, 38, 1607–1615. [Google Scholar] [CrossRef]
- Comotti, M.; Frigo, S. Hydrogen generation system for ammonia–hydrogen fuelled internal combustion engines. Int. J. Hydrog. Energy 2015, 40, 10673–10686. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.C. Performance enhancement of ammonia-fueled engine by using dissociation catalyst for hydrogen generation. Int. J. Hydrog. Energy 2014, 39, 2390–2398. [Google Scholar] [CrossRef]





| Properties | Ammonia | Hydrogen | Gasoline | Diesel | Natural Gas |
|---|---|---|---|---|---|
| Density, g/cm3 | 0.77 (liq.) | 0.071 | 0.73 | 0.84 | 0.187 |
| Ignition point, °C | 800 | 400 | 530 | 220 | 650 |
| Latent heat of vaporization | 1370 | 275 | 232.4 | ||
| Low calorific value, kJ/kg | 18,610 | 120,000 | 43,500 | 42,700 | 38,100 |
| Octane number | 110 | 130 | 90~98 | 107 | |
| Explosion limit (volume ratio)/% | 16~28 | 4.5~75 | 1.4~7.6 | 0.7~5.0 | 5~15 |
| Energy Density, MJ/m3 | 11.3 | 3.75 | 31.54 | 35.69 | 7.134 |
| Minimum Ignition Energy, mJ | 680 | 0.02 | 0.20 | 0.63 | 0.32 |
| Type | Combustion Effects | Emission Effects |
|---|---|---|
| Hydrogen | Increased cycle thermal efficiency and engine power | No carbon emissions, NOx emissions are the same as fossil fuels |
| Natural gas | Peak pressure decrease | NOx, NH3 increase, CO2 decrease |
| Gasoline | engine power increase, Peak pressure decrease | NOx, NH3 increase, CO decrease |
| Diesel | Torque increases significantly | HC, CO2 decrease, NOx decrease (ammonia doping ratio below 70%) |
| DME | Maximum engine load limited | HC, CO, NOx increase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Liu, E.; Zhu, N.; Liu, F.; Qian, F. Review of the Current Status of Ammonia-Blended Hydrogen Fuel Engine Development. Energies 2022, 15, 1023. https://doi.org/10.3390/en15031023
Xu X, Liu E, Zhu N, Liu F, Qian F. Review of the Current Status of Ammonia-Blended Hydrogen Fuel Engine Development. Energies. 2022; 15(3):1023. https://doi.org/10.3390/en15031023
Chicago/Turabian StyleXu, Xiaowei, Enlong Liu, Neng Zhu, Fanfu Liu, and Feng Qian. 2022. "Review of the Current Status of Ammonia-Blended Hydrogen Fuel Engine Development" Energies 15, no. 3: 1023. https://doi.org/10.3390/en15031023
APA StyleXu, X., Liu, E., Zhu, N., Liu, F., & Qian, F. (2022). Review of the Current Status of Ammonia-Blended Hydrogen Fuel Engine Development. Energies, 15(3), 1023. https://doi.org/10.3390/en15031023






