Abstract
Hybrid donor extractants are a promising class of compounds for the separation of trivalent actinides and lanthanides. In this paper, we present a new ligand from the bipyridyl-dicarboxylic acid diamide family—N,N’-diethyl-N,N’-bis(2,4,5-trimethylphenyl)-[2,2’-bipyridine]-6,6’-dicarboxamide. The synthesis of N-ethyl-2,4,5-trimethylaniline from pseudocumene by selective acetylation is presented. The target ligand was obtained using this aminylene. Chemical synthesis of its complexes with Ln(NO3)3 and their spectroscopic analysis showed that the structure of the complexes is near to the corresponding structures of well-known di-methylated dianilides. A series of studies on the photophysical, complexing, and extraction properties of this ligand and its complexes were carried out. It was shown that the extraction system based on this ligand can selectively isolate americium from the solution of high-level waste imitator.
1. Introduction
The most important problem of nuclear power is the management of high-level radioactive waste (HLW), which is generated during reprocessing of spent nuclear fuel. HLW contains minor actinides such as americium, neptunium, and curium, as well as fission products such as cesium, strontium, iodine, lanthanides, and other elements. Currently, vitrification is a common technology for the immobilization of HLW [1]. However, the half-life of some fission and activation products can reach millions of years, which leads to economic and environmental problems. To minimize the impact of such problems, the concept of fractionation of HLW and transmutation of individual radionuclides in fast-neutron reactors has been proposed. This strategy would reduce the economic and environmental pressure at the HLW disposal stage. However, the choice of chemical and technological procedures for implementing this approach remains an open question. The concept required the separation of trivalent actinides and lanthanides, which is a challenging task because of their similar chemical properties. The process requires selective and efficient ligands in solvent extraction, in contrast to the current process with dozens of stages. A large number of extractants have been studied so far, nevertheless, the search for an extractant that meets all requirements is still an urgent task [2,3,4,5].
Tetradentate ligands containing both hard and soft donor centers are of particular interest for the extraction separation of radioactive waste [6], and the study of the effect of substituents in different ligand positions on the properties of complexes is an important task for the rational design of potential extractants. Besides solvent extraction properties, one of the important characteristics of ligand behavior during complexation is the stability constant of the complexes, which indicates the strength of the formed complexes. The aim of this work was to establish the extraction properties with respect to the components of the HLW of a new representative of N,O-donor tetradentate ligands, N,N’-diethyl-N,N’-bis(2,4,5-trimethylphenyl)-[2,2’-bipyridine]-6,6’-dicarboxamide (1) (Figure 1), and to establish the fundamental laws of complex formation and photophysical properties.
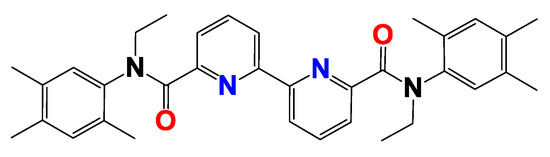
Figure 1.
Structure of N,N’–diethyl–N,N’–bis(2,3,4–trimethylphenyl)–[2,2′–bipyridine]–6,6′–dicarboxamide (1).
2. Materials and Methods
The nuclear magnet resonance (NMR) spectra were measured with a BRUKER AVANCE-600 MHz and AVANCE-400 MHz NMR spectrometers at 22 ± 2 °C in 5 mm probe tubes (with the solvent as internal reference). The 2D NOESY and 2D ROESY experiments were made in CDCl3 or acetonitrile-d3 at about 50 mg/mL concentration of substances. MALDI-TOF Mass spectra were obtained with a MALDI-TOF Reflex 3 instrument (BRUKER) in the positive ion mode (UV laser, 337 nm) with use of cinnamic acid as a matrix. HPLC–CI MS analysis of the final 2,3,4–triMe diamide was made with an Agilent 1100 instrument with Onyx Monolithic C18 (50 × 4.6 mm) column eluted with acetonitrile (HPLC–S Gradient Grade, Biosolve BV). Acetonitrile (99,95%, HPLC-S Gradient Grade, Biosolve BV) was used. Karl Fisher titration indicated that the water content in the acetonitrile was 400 ± 20 ppm (Mettler Toledo, C20, Coulometric KF Titrator). The commercially available lanthanide metals nitrates Ln(NO3)3·nH2O (n = 4–6) (purity > 99%) were used both for titration and chemical synthesis.
2.1. Spectrophotimetric Titration
Ultraviolet-visible (UV-vis) spectra were collected at ambient temperature (24.5 ± 1.0 °C) in the wavelength region 260–500 nm (1 nm interval) on a Hitachi U-1900 spectrophotometer using 10 mm path length quartz cells (Hellma, Müllheim, Germany). The implementation of the Beer–Lambert law was determined for all ligands within the range 0.01–0.1 mM. The binding stoichiometry of one-step complex formation between two different molecules was determined by the method of continuous variation. For the method of continuous variation, solutions of the ligand and metal salt were prepared at a concentration of ≈0.1 mM. For the spectrophotometric titration, a stock solution of the ligand was prepared (ca. 1–4 mM) and then a ligand working solution (ca. 10–40 µM) was prepared from the initial solution. A titrant solution Ln(NO3)3·nH2O (n = 4–6) (ca. 1–5 mM) was prepared by dissolution of a sample of Ln nitrate hydrate in the working ligand solution. A 2 mL working ligand solution was titrated with 1 μL Ln(NO3)3·nH2O solution. Preliminary kinetic experiments showed that the complexation reaction is fast and the absorbance becomes stable within 3–5 s. The stability constants of the lanthanide complexes were calculated by nonlinear least-squares regression analysis using the HypSpec2014 program7, based on Equations (1) and (2) for the complexes:
2,3,4-triMe + Ln(NO3)3 → [2,3,4-triMeLn(NO3)3]
Luminescence spectra were measured on a Hitachi F-7000 luminescence spectrometer at 77 K and 300 K. Luminescence was recorded upon excitation with light λex = 320 nm. The quantum yield of the luminescence of the powders of the complexes was determined using an attachment with an integrating sphere, solutions of the complexes in acetonitrile—by the method of reference dye with rhodamine 6G as a reference.
2.2. Synthesis of the 2,3,4–triMe Diamide and their Complexes
2,4,5-Trimethylacetophenone. To an ice-cooled suspension of 15 g (0.11 mol) of AlCl3 in DCM, the solution of acetylchloride (8.35 mL, 0.12 mol) in 15 mL of DCM was dropwise added under vigorous stirring. After addition was completed, the mixture was stirred for 15 min and then the solution of pseudocumol (13.8 mL, 0.1 mol) in 15 mL of DCM was added. The reaction mixture was stirred for 1.5 h at room temperature and then placed into crushed ice (200 g) with 50 mL of conc. HCl. The organic layer was separated and successively washed with 50 mL of 10% HCl, 2 × 100 mL of water, 100 mL of 5% NaHCO3, and 2 × 100 mL of water and after that the organic layer dried over CaCl2. The solvent was removed and organic residue distillated in a vacuum yielded 14 g (86%) of colorless liquid. bp 115–116 °C/7 torr (107–108 °C/7 torr [7]). Then, 1H NMR (600 MHz, CHLOROFORM-d) δ ppm, 2.27 (s, 3 H), 2.28 (s, 3 H), 2.49 (s, 3 H), 2.56 (s, 3 H), 7.01 (s, 1 H), 7.50 (s, 1 H); p13C NMR (151 MHz, CHLOROFORM-d) δ ppm 19.16, 19.57, 21.16, 29.27, 131.03, 133.41, 133.59, 134.87, 136.10, 140.73, 201.11.
2,4,5–Trimethylacetanilide. First, 32.4 g (0.2 mol) of 2,4,5–trimethylacetophenone was dissolved at 60–70 °C in 200 mL of acetic acid containing 50 mL H2SO4. After that, the 19.5 g (0.3 mol) NaN3 was added portionwise under vigorous. The reaction was stirred under the titled conditions for 3 h after the reaction mixture was placed into 2 l of crushed ice and maintained until warming to the room temperature. The precipitate obtained was filtered off, washed with water until neutral, and air dried. Yielded 29.38 g (83%) of titled compound as white powder. mp = 96–97 °C [8]. Then, 1H NMR (600 MHz, CDCl3) δ ppm 7.41 (s, 1H, NH); 7.02, 6.96 (2s, 2H, CHarom), 2,49 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.22 (s, 3H, CH3), 2.19 (s, 3H, CH3).
N–Ethyl–2,4,5–trimethylaniline. To a stirring suspension of LiAlH4 (3 g, 79 mmol) in 50 mL of dry THF, a solution of 17.7 g (0.1 mol) of 2,4,5-trimethylacetanilide in 100 mL of dry THF was added with cooling in a water bath. After the end of hydrogen evolution, the reaction mixture was refluxed with stirring for 6 h and left overnight. The reaction mixture was decomposed by sequentially adding 3 mL of water, 3 mL of 15% NaOH solution, and 9 mL of water while stirring and cooling in a water bath. The precipitate was filtered off and washed with THF (3 × 20 mL). The solution was dried over anhydrous CaCl2. The solution was evaporated, isolating 11.9 g (73%) of the solid target amine. Mp 47–48 °C. Then, 1H NMR (600 MHz, CDCl3) δ ppm 7.02 (s, 1H, CHAr), 6.96 (s, 1H, CHAr); 2.99 (q, 2H, CH2), 2.52 (s, 1H, NH); 2.23 (s, 3H, CH3), 2.18 (s, 3H, CH3), 2.14 (s, 3H, CH3); 1.07 (t, 3H, CH3). 13C NMR (151 MHz, CHLOROFORM-d) δ ppm 15.06, 16.78, 18.47, 19.75, 38.72, 111.77, 119.17, 124.32, 131.44, 134.64, 144.33.
2,3,4–triMe Diamide. 2.44 g (10 mmol) of 2,2’-bipyridyl-6,6’-dicarboxylic acid in 25 mL of absolute THF was boiled with 20 mL of thionyl chloride and 0.3 mL of DMF for 2.5 h. The resulting solution was evaporated to dryness in vacuum and redissolved in 20 mL of dry THF. The solution of dichloranhydride was added portionwise with stirring to a mixture of 3.58 g (22 mmol) of N-ethyl-2,4,5-trimethylaniline, 4 mL of triethylamine, and 35 mL of dry THF. After completion of the addition, the mixture was stirred for 5 h at 40–50 °C and left overnight. An equal volume of water was added to the reaction mixture, and the organic layer was separated. The aqueous fraction was extracted with diethyl ether (3 × 20 mL). The combined organic layers were washed with water and dried over anhydrous Na2SO4. The solvent was removed in vacuo. The residue was treated with 2 mL of ethyl acetate. The precipitate formed, was filtered off, washed with cold ethyl acetate, hexane, and dried in air. Yield 3.42 g (64%) as a white powder. 1H NMR (400 MHz, CHLOROFORM-d) δ ppm 1.25 (t, J = 6.11 Hz, 3 H), 1.96–2.09 (m, 6 H), 2.11 (br. s., 5 H), 3.65 (dd, J = 10.27, 6.11 Hz, 1 H), 4.12 (dd, J = 11.98, 6.11 Hz, 1 H), 6.76 (br. s., 1 H), 6.92 (d, J = 6.11 Hz, 1 H), 7.53 (d, J = 6.97 Hz, 1 H), 7.59 (t, J = 6.97 Hz, 1 H), 7.65 (d, J = 6.36 Hz, 1 H); MALDI-TOF MS: 535 [M+H]+. 13C NMR (101 MHz, CHLOROFORM-d) δ ppm 12.45 (CH2CH3) 17.36, 17.40 (2-CH3) 19.06 (4-CH3, 5-CH3) 44.96, 45.01 (CH2CH3) 121.40 (5PyCH) 123.64 (3PyCH) 129.84, 129.90 (6PhCH) 131.87, 131.91 (3PhCH) 131.97, 132.06 (3PhC) 134.33, 135.55 (2PhC) 136.51, 136.55 (4PyCH) 139.30, 139.35 (3PhC) 153.16, 153.24 (6PyC) 153.49, 153.52 (2PyC) 167.52, 167.60 (C=O). IR (KBr) cm−1: 2972, 2956, 2929, 2870, 1637, 1576, 1512, 1450, 1400, 1319, 1124. Mass HRMS ESI Found: [M+H] 535.30585; molecular formula C34H39N4O2 requires [M+H] 535.30675.
2.3. Complexes of 2,3,4–triMe Diamide with La, Sm, Eu, Gd and Tb
A solution of 100 mg of the corresponding lanthanide nitrate hydrate was added to a solution of 100 mg of the ligand in 1.5 mL of acetonitrile. The resulting mixture was heated to a boil, cooled to room temperature, and left for 24 h. The precipitate was filtered, washed with absolute acetonitrile (2 × 0.5 mL), and dried in air to a constant mass.
2,3,4–triMe[La(NO3)3] 108 mg (67%). Anal Calc. for C34H38LaN7O11: C 47.51, H 4.46, N 11.41; Found C 47.38; H 4.71; N 11.44; 1H NMR (600 MHz, ACETONITRILE-d3) δ ppm 1.29 (t, J = 7.06 Hz, 12 H), 2.19 (s) 2.20 (s) 3.74 (dq, J = 13.50, 6.42 Hz, 1 H), 3.79 (dq, J = 13.30, 6.97 Hz, 1 H), 4.16 (dq, J = 13.20, 7.15 Hz, 2 H), 4.21 (dq, J = 13.20, 6.90 Hz, 1 H), 6.94 (dd, J = 7.70, 5.78 Hz, 2 H), 7.08 (s, 1 H), 7.12 (br. s., 1 H), 7.13 (br. s., 1 H), 7.15 (s, 1 H), 7.82 (t, J = 7.98 Hz, 2 H), 8.22 (d, J = 7.98 Hz, 2 H); MALDI-TOF MS m/z (Irel (%)): 797 ([M–NO2]+). IR (KBr) cm−1: 3089, 2978, 2937, 2924, 2875, 1610, 1585, 1572, 1506, 1489, 1473, 1460, 1429, 1286, 1028, 1007.
2,3,4–triMe[Sm(NO3)3] 124 mg (76%). Anal Calc. for C34H38N7O11Sm: C 46.88, H 4.40, N 11.26; Found C 46.69; H 4.32; N 11.19; MALDI-TOF MS m/z (Irel (%)): 810 ([M–NO2]+) 100%, 781 ([M–NO2–C2H6]+) 15%. IR (KBr) cm−1: 3091, 2976, 2922, 2873, 1606, 1587, 1574, 1491, 1464, 1431, 1294, 1028, 1009.
2,3,4–triMe[Eu(NO3)3] 131 mg (80%). Anal Calc. for C34H38EuN7O11: C 46.80, H 4.39, N 11.24; Found C 46.71; H 4.26; N 11.14; MALDI-TOF MS m/z (Irel (%)):811 ([M–NO2]+). IR (KBr) cm−1: 3091, 2972, 2924, 2877, 1606, 1589, 1574, 1493, 1470, 1431, 1296, 1030, 1009.
2,3,4–triMe[Gd(NO3)3] 127 mg (77%). Anal Calc. for C34H38GdN7O11: C 46.51, H 4.36, N 11.17; Found C 46.68; H 4.42; N 11.22; MALDI-TOF MS m/z (Irel (%)): 816 ([M–NO2]+). IR (KBr) cm−1: 3091, 2978, 2937, 2922, 2875, 1608, 1589, 1574, 1475, 1431, 1298, 1030, 1011.
2,3,4–triMe[Tb(NO3)3] 135 mg (82%). %). Anal Calc. for C34H38N7O11Tb: C 46.43, H 4.35, N 11.15; Found C 46.35; H 4.48; N 11.09; MALDI-TOF MS m/z (Irel (%)):817 ([M–NO2]+). IR (KBr) cm−1: 3091, 2978, 2939, 2924, 2877, 1608, 1589, 1576, 1506, 1487, 1475, 1431, 1300, 1030, 1011.
2,3,4–triMe[Dy(NO3)3] 118 mg 68%. Anal Calc. for C34H38DyN7O11: C 46.24, H 4.34, N 11.10; Found C 46.11; H 4.19; N 11.02; MALDI-TOF MS m/z (Irel (%)):822 ([M–NO2]+). IR (KBr) cm−1: 3089, 2976, 2935, 2924, 2870, 1608, 1589, 1576, 1477, 1431, 1300, 1030, 1011.
2.4. Solvent Extraction Studies
Solvent extraction experiments were carried out as follows. First, 0.5 mL of organic solution of the extractant in 3-nitrobenzotrifluoride (“F-3”) and 0.5 mL of aqueous phase containing nitric acid and radionuclides were mixed in 1.5 mL polypropylene vial. Three sets of aqueous solutions were prepared for extraction experiments. The first one contained trace concentrations of 241Am (≈1500 Bq mL−1) and 152Eu (≈2500 Bq mL−1). The second set contained lanthanum and all lanthanides (except promethium) in total concentration, 10−4 mol L−1. The third was an imitator of High-level liquid waste with or without 241Am. The phases were stirred for 15 min on a vortex shaker at 25 ± 1 °C in an air thermostat. Then, samples were centrifuged (5 min, 6000 rpm) and aliquots of each phase were taken for determination of radionuclide or lanthanide concentration. Content of 241Am (Eγ = 59.5 keV) and 152Eu (Eγ = 121.8 keV) was determined by gamma-spectrometry using a high-pure germanium detector GR 3818 (Canberra Ind.) in the first set of experiments. Initial aqueous phase and equilibrium aqueous phases of the third set of samples were analyzed by ICP-OES (Agilent ICP-OES 720). The distribution ratios (D) of metals were calculated for the first set of experiments as the ratio of the counting rate in the organic and aqueous phase. For the second and the third sets of samples, activity/concentration in organic phase was calculated as difference between concentrations in initial and equilibrium
3. Results and Discussion
3.1. Synthesis
To introduce three electron-donating methyl groups into different positions of the aromatic ring of aniline, either direct nitration of pseudocumene or a multistage scheme including acylation followed by Schmidt rearrangement is possible. Since the nitration of pseudocumene proceeds ambiguously, we used selective acetylation, followed by rearrangement and reduction of acetanilide, which according to our early studies [9], give the corresponding donor-substituted N-ethylanilines in high overall yields (Scheme 1). As a result, N-ethyl-2,4,5-trimethylaniline was obtained with a yield of 52% based on the initial pseudocumene.
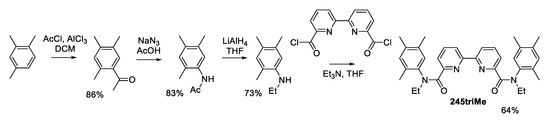
Scheme 1.
General scheme of 2,3,5Me synthesis.
Acylation of the resulting N-ethyl-2,4,5-trimethylaniline with 2,2-bipyridyldicarboxylic acid chloride leads to the target 2,3,4-triMe diamide in moderate yield. Further incertion of methyl groups into the phenyl ring of the amide group leads to a significant steric loading of the amide fragments of the molecule, and, as a consequence, the appearance of a significant asymmetry in the most stable conformation in solution. This asymmetry complicates the NMR spectra for both 1H and 13C nuclei. The 1H NMR spectra show separate signals for the methylene groups of the protons of the N-ethyl group of two amide fragments. In the 13C spectra, signals from all carbon atoms of the molecule are observed, which indicates the absence of a symmetry plane in the most stable conformers in solution. A similar effect was observed in sterically loaded 2-methylcarboxyanilide [9]. The chemical synthesis of complexes of lanthanides with diamide 2,3,4-triMe leads to the formation of complexes only with a composition of 1 to 1 metal:ligand for ions of the beginning, middle, and end of the lanthanide series (Scheme 2).
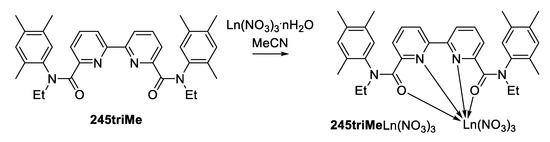
Scheme 2.
Sythesis of 2,4,5triMeLn(NO3)3 complexes.
The mass spectra obtained by the MALDI-TOF method in the laser desorption mode show that for all the complexes obtained, the only peak in the spectra is the peak of the fragment ion, which corresponds to the loss of one nitrate anion by the molecular ion. This behavior is typical for complexes of bipyridyl amides [10,11,12]. The IR spectra of the complexes confirm the coordination of the metal ion with the amide oxygen atoms and nitrogen atoms of the bipyridyl fragment. Since there is a decrease in the frequency of the stretching vibrations of the amide group from 1637 cm−1 in free diamide to 1606–1610 cm−1 in its complexes, with a simultaneous decrease in frequency ν(C=N) vibrations of the pyridine ring from 1576 cm−1 in the free ligand to 1572–1574 cm−1 in the complexes. The bidentate-chelate nature of the coordination of the three nitrate anions is also confirmed by IR spectroscopy data. The IR spectra show bands of asymmetric (νa(NO2)) and symmetric (νs(NO2)) vibrations of the nitrate anion, as well as a band of stretching vibrations ν(N=O) in the ranges characteristic of bidentate-chelate coordination [10]. So, we can assume the structure of the complexes are near to the corresponding structures of well-known dimethylated dianilides [12].
3.2. Spectrophotometric Study
Solutions of europium complexes absorb in the UV range (<350 nm). The absorption spectrum maximum falls at a wavelength of 325 nm (Figure 2). The shape of the absorption spectrum of a solution of the europium complex repeats the spectra of complexes of lanthanides with ligands based on 2,2’-bipyridyl-dicarboxamides [10,12,13,14]. This suggests that the introduction of any number of methyl substituents at any position of the phenyl ring does not change the shape of the absorption spectrum of the complex solution.
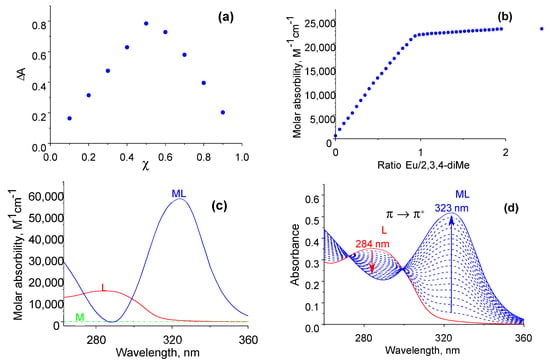
Figure 2.
Spectrophotometric titration of 2·10−5 M 2,3,4-triMe anilide with a solution of 2·10−3 M Eu(NO3)3•6H2O in acetonitrile (with water content ppm 400ppm): UV titration at 25 °C (a), titration curve at 323 nm (b), isomolar series for the europium complex with 2,3,4-triMe anilide (c), factorial analysis (d).
The introduction of three methyl groups into the anilide fragment of the diamide had no effect on the maximum absorption spectrum of the ligand (at 284 nm) when compared with dimethyl-substituted diamides (Figure 2a) [12]. The composition of the equilibrium solution was studied using titration curves (Figure 2b), isomolar series method (Figure 2c), and factorial analyzes (Figure 2d). Based on the data obtained, it was found that only one complex particle with a lanthanide–diamide ratio of 1:1 is formed in the solution. The stability constants of complexes of trivalent lanthanide ions with 2,3,4-triMe diamide were determined using spectrophotometric titration. When the metal solution was added, the π–π* absorption band of diamide shifted by ~40 nm (Figure 2b). The results were processed using the HypSpec2014 program. Factor analysis showed the presence of three components in the solution: a ligand, lanthanide nitrate crystal hydrate, and a complex particle.
Solutions of lanthanide complexes absorb in the UV range (<350 nm). The absorption spectrum maxima of the complexes were in the wavelength range from 322 nm to 325 nm (Figure 2a). The shape of the absorption spectrum of a solution of the europium complex repeats the spectra of complexes of lanthanides with ligands based on 2,2’-bipyridyl-dicarboxamides [10,12]. This suggests that the introduction of any amount of methyl substituents at any position of the phenyl ring of the unsubstituted ligand does not affect the change in the absorption spectra of solutions of the complexes.
As can be seen from Figure 3 (Table 1), the values of the stability constants of the 2,4,5–triMe complexes of diamide with lanthanides decrease almost linearly with a decrease in the ionic radius of the metal. Although, the opposite dependence was observed for the stability constants of lanthanide complexes with carboxylic acids, such as NTA, CDTA, and EDTA [13]. It can be assumed that an increase in the number of methyl substituents on the remote phenyl fragment of the ligand affects the cavity of the ligand upon coordination with the lanthanide; the greater the number of methyl substituents on the phenyl ring, the higher the stability of the lanthanide complexes. However, the stability values of complexes for trimethyl-substituted diamide with the second half of the lanthanide series (from dysprosium to lutetium) are lower than those for the unsubstituted ligand [10]. It was also found that the stability constants of complexes of cerium and europium nitrates with 2,4,5-triMe diamide are higher than those of unsubstituted anilide.
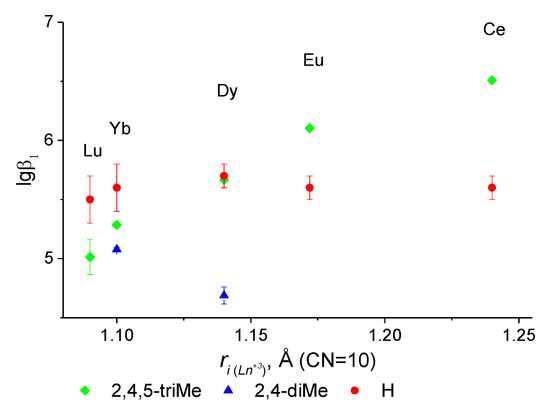
Figure 3.
Dependence of the stability of (logβ1) lanthanide complexes on the ionic radius for the 2,3,4-triMe ligand when compared with unsubstituted diamide (H) [10] and 2,4-diMe diamide [12].

Table 1.
Stability values of complexes (logβ1) of trivalent lanthanide ions with 2,3,4-triMe diamide in comparison with 2,4-diMe and unsubstituted diamide in acetonitrile and N,PO tetradentate ligands.
When comparing the stability constants of complexes for dysprosium nitrate with trimethyl-substituted, unsubstituted, and 2,4-dimethyl-substituted anilides. It was established that the stability values of complexes with unsubstituted and 2,4,5-triMe diamides are within the confidence interval and are an order of magnitude higher than the stability constant of the complex with 2,5-diMe diamide. Additionally, comparing the constants for the diphosphate tetradentate ligands and 2,4,5–triMe, we note that under identical conditions, the binding constants for Eu(III) are close [15].
Figure 2 shows that the value of the stability constant of the complex of the unsubstituted ligand with ytterbium and lutetium is higher regardless of the number of methyl substituents introduced into the structure of the unsubstituted ligand. At the same time, with an increase in the number of methyl substituents, an increase in the value of the stability of complexes with ytterbium was found.
3.3. Luminescent Study
Phosphorescence spectrum of the gadolinium complex with the studied ligand has the maximum at the wavelength ~530 nm (Figure 4). We used the highest energy component of this spectrum to determine the ligand triplet level energy [13]. Its energy was 18,170 cm−1. Thus, the energy difference between the ligand triplet level and the europium ion resonance level, 5D0 is ~990 cm−1. This is significantly lower compared to complexes with two methyl substituents in the ligand [12]. According to the literature data [16], for efficient energy transfer from the ligand to the europium ion, it is necessary that the energy difference between the ligand triplet level and the europium ion resonance level 5D0 should be in the range of 2000–3500 cm−1. This means that in the studied europium complex, the back energy transfer from the europium ion to the ligand occurs. This may be the reason for the low luminescence quantum yield.
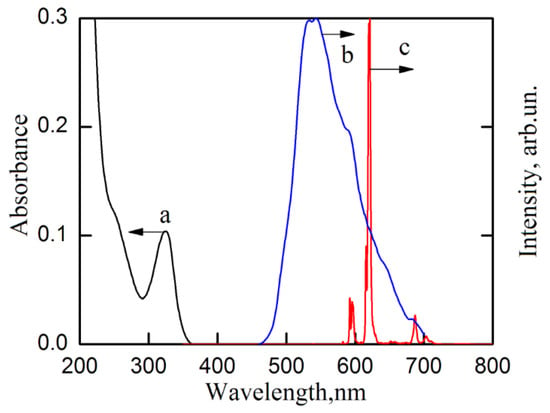
Figure 4.
Absorbance spectra of europium complex acetonitrile solution, concentration C = 1·10−5 mol/l (a); phosphorescence emission spectrum of gadolinium complex in solid state at 77 K, λex = 395 nm (b); luminescence emission spectrum of europium complex in solid state at 77 K, λex = 320 nm (c).
The luminescence emission spectrum of the europium complex shows a typical luminescent signal in the red region of the spectrum, which is typical for the europium ion light emission, corresponding to energy transitions 5D0→7FJ, where J = 0–4 (Figure 4). The most intense peak in the luminescence emission spectrum of the europium complex is the spectral band corresponding to “hypersensitive” transition 5D0→7F2 (in the spectral region 610–630 nm), the relative intensity of which is 75% of the total luminescence emission of this complex. Europium complex asymmetry ratio (R), which shows the deviation of the europium ion position from the inverse center, was calculated as the ratio of the integral peak luminescence intensities that correspond to energy transitions 5D0→7F2 and 5D0→7F1 (in spectral region 585–605 nm). When studying a sample of the complex in solid state, the asymmetry coefficient was 6.3–6.4 and did not depend on temperature. When measuring the europium complex in acetonitrile solution, the value of the asymmetry coefficient increased to 8.1, i.e., the deviation of the position of the europium ion from the inverse center increased in the presence of solvent molecules.
Europium ion luminescence lifetime of the excited state 5D0 was determined from the luminescence decay kinetics. It practically does not depend on the conditions of the experiment. Further, (solution\solid state, 300 K/77 K) and equaled 1.35 ms (Table 2). This is an underestimated value compared to the luminescence lifetime of other complexes of this type [12].

Table 2.
Luminescent characteristics of the europium complex.
The luminescence radiative lifetime of the europium complex was calculated using formula [14]:
where A0→1 = AMD,0n3—Einstein coefficient of transition 5D0→7F1, n—refractive index of solvent, Itot—integral luminescence intensity of europium complex, I0→1—integral intensity of luminescence band correspond to transition 5D0→7F1. The luminescence radiative lifetime of the europium complex in solid state was slightly lower than the luminescence radiative lifetime of europium complex in acetonitrile solution.
The sensibilization efficiency (ηsens, %) of europium was calculated using formula:
where QEu—luminescence quantum yield, QL—internal luminescence quantum yield.
The luminescence quantum yield of europium complex in solid state was measured using an integrating sphere and was equal to 2.7%. The luminescence quantum yield of a europium complex in acetonitrile solution was very low compared to other europium complexes with ligands of similar structure [12,13,14]. This is explained by the too low energy of the ligand triplet level and the back energy transfer from the europium ion to the ligand. The sensitization efficiency of the europium complex solution is also quite low and equals 0.41% for the same reason.
Terbium, samarium, and dysprosium complexes with this ligand have an extremely low luminescence intensity, which makes it impossible to analyze their luminescent properties.
3.4. Solvent Extraction Studies
After studying the complexation and properties of the complexes, we established the extraction properties of the ligand (1) with respect to the main components of the highly active waste. F-3 was used as a solvent due to its high radiolytic resistance and fire-explosive stability.
3.4.1. Extraction of Am(III), Eu(III) and Lanthanides from Nitric Acid Solutions
For the initial evaluation of the extraction properties in relation to the three-valent f-elements, we carried out extraction of indicator amounts of americium and europium depending on the content of nitric acid. Obtained distribution ratios of americium(III) and europium(III) and their separations factors are shown in Figure 5. This dependence takes a typical form for bipyridyl-dicarboxylic acid diamides [17]. It reaches saturation at a nitric acid content of 3 mol/L. This phenomenon is due to competitive protonation of the ligand with respect to extraction of metal cations [16].
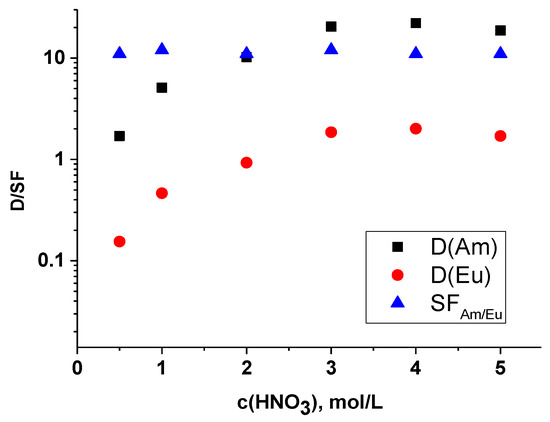
Figure 5.
Dependence of americium and europium distribution ratios and selectivity factor on nitric acid content. Organic phase-0.05 mol/L ligand in F-3 solvent.
Compared with earlier studies of extraction systems under identical conditions, the tri-substituted ligand has a higher extraction efficiency than the mono- and di-substituted ones [12,17].
The selectivity factor takes a value slightly greater than 10, indicating a moderate but sufficient selectivity to americium(III) in the presence of europium(III).
Additionally, to establish the selectivity in more detail, we performed extraction experiments with a series of lanthanides (except for promethium). The obtained distribution ratios are shown in Figure 6. The course of the dependence on the Ln-number is nonlinear in nature, similar to that for dimethylaryl-substituted ligands. It is interesting to note that the nature of this relationship indicates that this ligand is more similar to 2,5- than to 3,4- or even to 2,4-dimethyl-substituted aryls of bipyridn-di-carboxylic acid [12]. This indicates that positions 2 and 5 play a greater role than positions 3 and 4, which is most likely due to the steric factor [9].The overall selectivity Am(III)/Ln(III), where Ln is those lanthanides that are in HLW (La-Gd), takes the value 7.
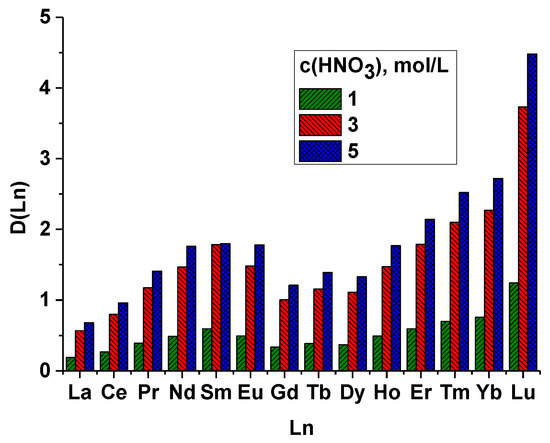
Figure 6.
Dependence of the distribution ratio on the order number Ln at different contents of nitric acid.
3.4.2. Solvent Extraction of Am(III) from HLW Imitator
To check the possibility of using diamide (1) for the extraction of Am(III) from the HLW simulant solution, the distribution ratios of the main components of the HLW simulant solution whose composition is shown in Table 3. Oxalic acid and polyaminocarboxylate HEDTA (N-(2-hydroxyethyl)ethylenediamine-N,N′,N′-triacetic acid) were used to suppress the extraction of Zr, Pd, and Mo. The obtained distribution coefficients are presented in Table 4.

Table 3.
Composition of the HLW imitator solution used in this work [18].

Table 4.
Distribution ratios of the HLW imitator solution used in this work.
The obtained distribution coefficients indicate that this system proves promising in terms of selectivity when using the above-mentioned simulant solution in the presence of masking water-soluble complexons.
4. Conclusions
We synthesized a new ligand, N,N′-diethyl-N,N′-bis(2,4,5-trimethylphenyl)-[2,2′-bipyridine]-6,6′-dicarboxamide for the selective isolation of americium(III) from highly reactive wastes and showed the effect of the third substituent on photophysical properties, cation binding constants, and extraction properties. Chemical synthesis of its complex compounds and their analysis by infrared spectroscopy showed that the structure of complex compounds of this ligand with lanthanide nitrates is close to the corresponding structures of well-known dimethylated dianilides. In terms of photophysical properties, it was shown that the introduction of a third substituent in the aryl fragment of the amide group leads to a decrease in the quantum yields of luminescence. Extraction tests on lanthanides and established binding constants indicate that the introduction of the additional substituent causes it to be more similar to 2,5- than to 3,4- or even to 2,4-dimethyl-substituted aryls of bipyr-idn-di-carboxylic acid. Tests on a solution of simulant HLLW with maintenance of water-soluble complexes for palladium and zirconium show the possibility of selectively isolating americium using a solution of this ligand in the solvent meta-nitrobenzotrifluoride.
Author Contributions
Conceptualization, N.E.B., investigation, A.V.I., T.B.S., A.V.K. and P.I.M.; writing—original draft preparation, N.E.B., T.B.S., A.V.K. and P.I.M.; writing—review and editing, N.E.B. and P.I.M.; supervision, S.V.P.; funding acquisition, V.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by RSF grant no. 21-73-20138.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baisden, P.A.; Choppin, G.R. Nuclear waste management and the nuclear fuel cycle. Radiochem. Nucl. Chem. 2007, 2, 1–63. [Google Scholar]
- Evsiunina, M.V.; Matveev, P.I.; Kalmykov, S.N.; Petrov, V.G. Solvent Extraction Systems for Separation of An(III) and Ln(III): Overview of Static and Dynamic Tests. Moscow Univ. Chem. Bull. 2021, 76, 287–315. [Google Scholar] [CrossRef]
- Matveev, P.; Mohapatra, P.K.; Kalmykov, S.N.; Petrov, V. Solvent extraction systems for mutual separation of Am(III) and Cm(III) from nitric acid solutions. A review of recent state-of-the-art. Solvent Extr. Ion Exch. 2021, 39. [Google Scholar] [CrossRef]
- Leoncini, A.; Huskens, J.; Verboom, W. Ligands for f-element extraction used in the nuclear fuel cycle. Chem. Soc. Rev. 2017, 46, 7229–7273. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Mohapatra, P.K. Separation of trivalent actinides and lanthanides using various ‘N’, ‘S’ and mixed ‘N, O’ donor ligands: A review. Radiochim. Acta 2019, 107, 931–949. [Google Scholar] [CrossRef]
- Alyapyshev, M.; Babain, V.; Kirsanov, D. Isolation and Purification of Actinides Using N, O-Hybrid Donor Ligands for Closing the Nuclear Fuel Cycle. Energies 2022, 15, 7380. [Google Scholar] [CrossRef]
- Mukherjee-Müller, G.; Winkler, T.; Zsindely, J.; Schmid, H. Umwandlung von 6-Methyliden-tricyclo[3.2.1.02,7]oct-3-en-8-onen in Norcaradien/Cycloheptatrien-Derivate. Helv. Chim. Acta 1976, 59, 1763–1796. [Google Scholar] [CrossRef]
- Carlin, R.B.; Moores, M.S. The Fischer Reaction of Cyclohexanone Mesitylhydrazone. Evidence of a 1,4-Methyl Migration. J. Am. Chem. Soc. 1962, 84, 4107–4112. [Google Scholar] [CrossRef]
- Borisova, N.E.; Ivanov, A.V.; Matveev, P.I.; Smirnova, A.A.; Belova, E.V.; Kalmykov, S.N.; Myasoedov, B.F. Screening of the Structure of Americium Extractants Based on a 2,2’-Bipyridyl Scaffold: A Simple Way to a N2,O2-Tetradentate Ligands Library for Rational Design of An/Ln Extractants. ChemistrySelect 2018, 3, 1983–1989. [Google Scholar] [CrossRef]
- Borisova, N.E.; Kostin, A.A.; Eroshkina, E.A.; Reshetova, M.D.; Lyssenko, K.A.; Spodine, E.N.; Puntus, L.N. Lanthanide Complexes with Tetradentate N,N′,O,O′—Dipyridyl-Based Ligands: Structure, Stability, and Photophysical Properties. Eur. J. Inorg. Chem. 2014, 2219–2229. [Google Scholar] [CrossRef]
- Borisova, N.E.; Ivanov, A.V.; Kharcheva, A.V.; Sumyanova, T.B.; Surkova, U.V.; Matveev, P.I.; Patsaeva, S.V. Effect of heterocyclic ring on LnIII coordination, luminescence and extraction of diamides of 2,2′-Bipyridyl-6,6′-dicarboxylic acid. Molecules 2020, 25, 62. [Google Scholar] [CrossRef] [PubMed]
- Borisova, N.E.; Sumyanova, T.B.; Kharcheva, A.V.; Matveev, P.I.; Ivanov, A.V.; Razumova, E.A.; Patsaeva, S.V. The lanthanide complexes of 2,2′-bipyridyl-6,6′-dicarboxylic dimethylanilides: The influence of a secondary coordination sphere on the stability, structure, luminescence and f-element extraction. Dalt. Trans. 2018, 47, 16755–16765. [Google Scholar] [CrossRef] [PubMed]
- Kharcheva, A.V.; Ivanov, A.V.; Borisova, N.E.; Tatiana, P.; Patsaeva, S.V.; Popov, V.V.; Yuzhakov, V.I. Luminescent solutions and films of new europium complexes with chelating ligands. In Proceedings of the Saratov Fall Meeting 2014, Saratov, Russia, 23–26 September 2014; Volume 9448, p. 944813. [Google Scholar]
- Kharcheva, A.V.; Borisova, N.E.; Ivanov, A.V.; Reshetova, M.D.; Kaminskaya, T.P.; Popov, V.V.; Yuzhakov, V.I.; Patsaeva, S.V. Effect of Aliphatic Chain Length in the Ligand on Photophysical Properties and Thin Films Morphology of the Europium Complexes. Russ. J. Inorg. Chem. 2018, 63, 219–228. [Google Scholar] [CrossRef]
- Matveev, P.I.; Huang, P.W.; Kirsanova, A.A.; Ananyev, I.V.; Sumyanova, T.B.; Kharcheva, A.V.; Khvorostinin, E.Y.; Petrov, V.G.; Shi, W.Q.; Kalmykov, S.N.; et al. Way to Enforce Selectivity via Steric Hindrance: Improvement of Am(III)/Eu(III) Solvent Extraction by Loaded Diphosphonic Acid Esters. Inorg. Chem. 2021, 60, 14563–14581. [Google Scholar] [CrossRef] [PubMed]
- Latva, M.; Takalob, H.; Mukkala, V.M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Tkachenko, L.I.; Paulenova, A.; Popova, A.A.; Borisova, N.E. New Diamides of 2,2′-dipyridyl-6,6′-dicarboxylic Acid for Actinide-Lanthanide Separation. Solvent Extr. Ion Exch. 2014, 32, 138–152. [Google Scholar] [CrossRef]
- Alyapyshev, M.; Babain, V.; Tkachenko, L. Various flowsheets of actinides recovery with diamides of heterocyclic dicarboxylic acids. J. Radioanal. Nucl. Chem. 2017, 312, 47–58. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).