Comparative Study of Methane Production in a One-Stage vs. Two-Stage Anaerobic Digestion Process from Raw Tomato Plant Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Inoculums
2.3. Batch Assays and Experimental Design
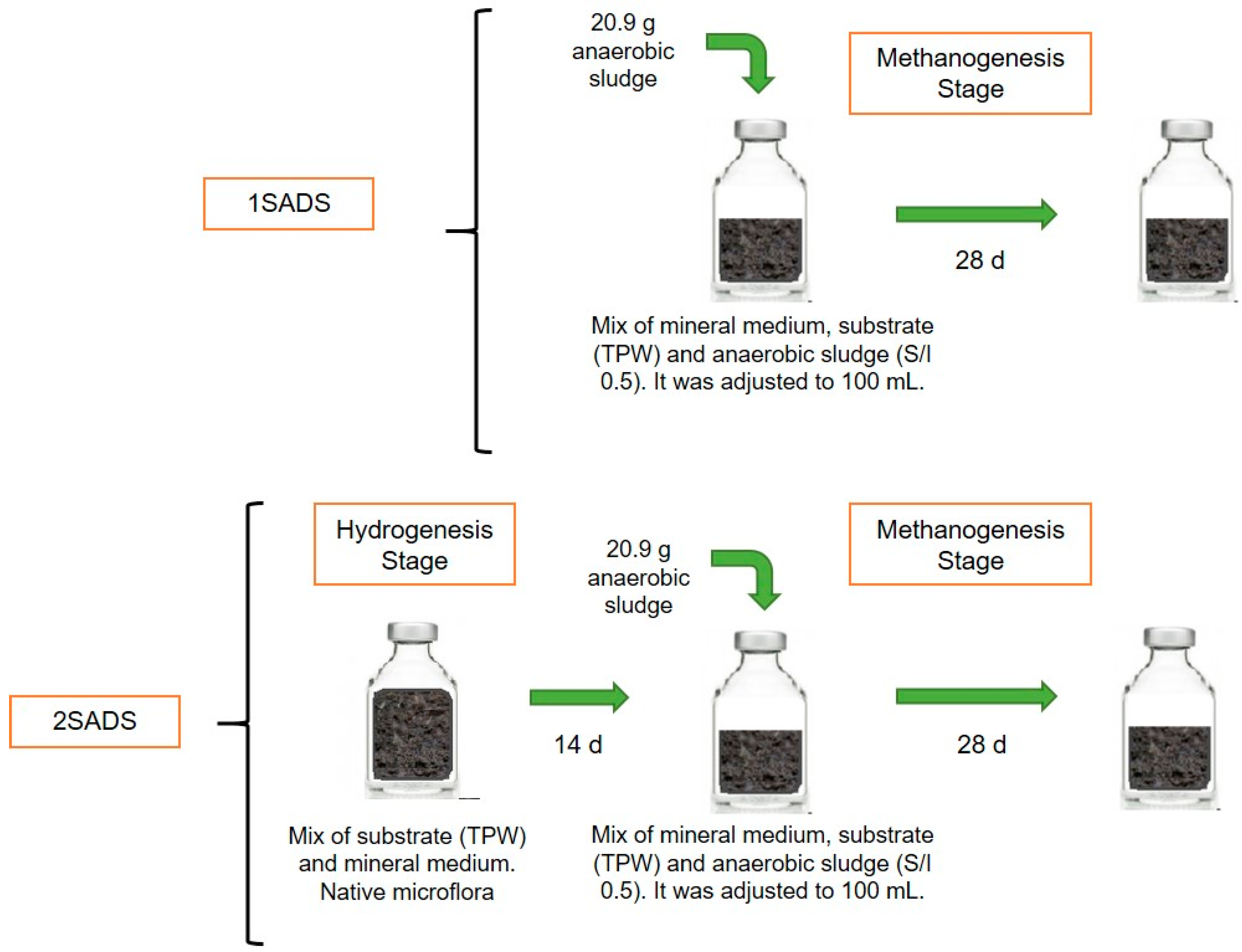
2.3.1. One-Stage Anaerobic System (1SADS)
2.3.2. Two-Stage Anaerobic System (2SADS)
2.4. Statistic Analysis
2.5. Analytical Methods
3. Results
3.1. One-Stage Anaerobic Digestion System (1SADS)
3.2. Two-Stage Anaerobic Digestion System (2SADS)
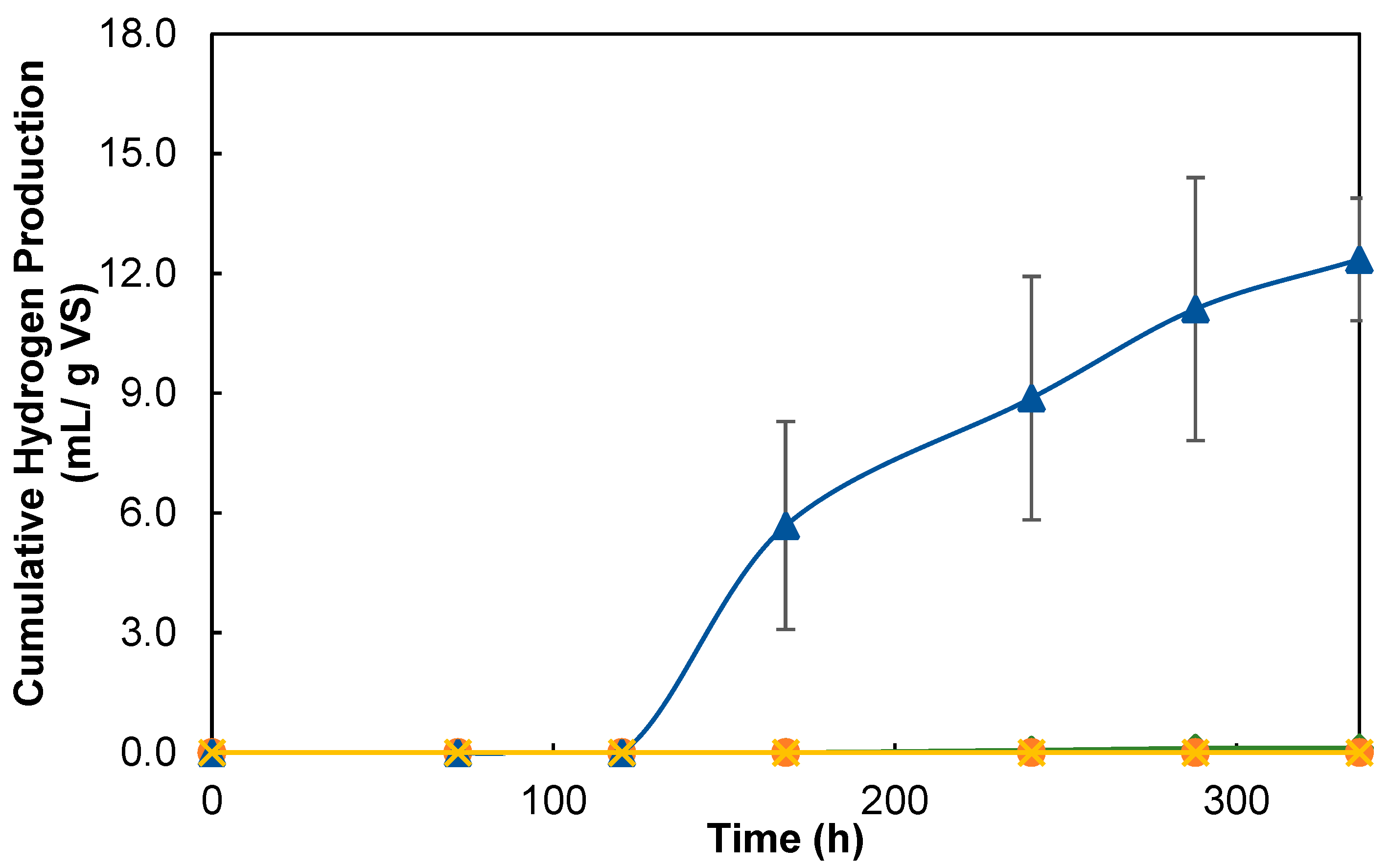
3.2.1. Hydrogenesis Stage
3.2.2. Methanogenesis Stage
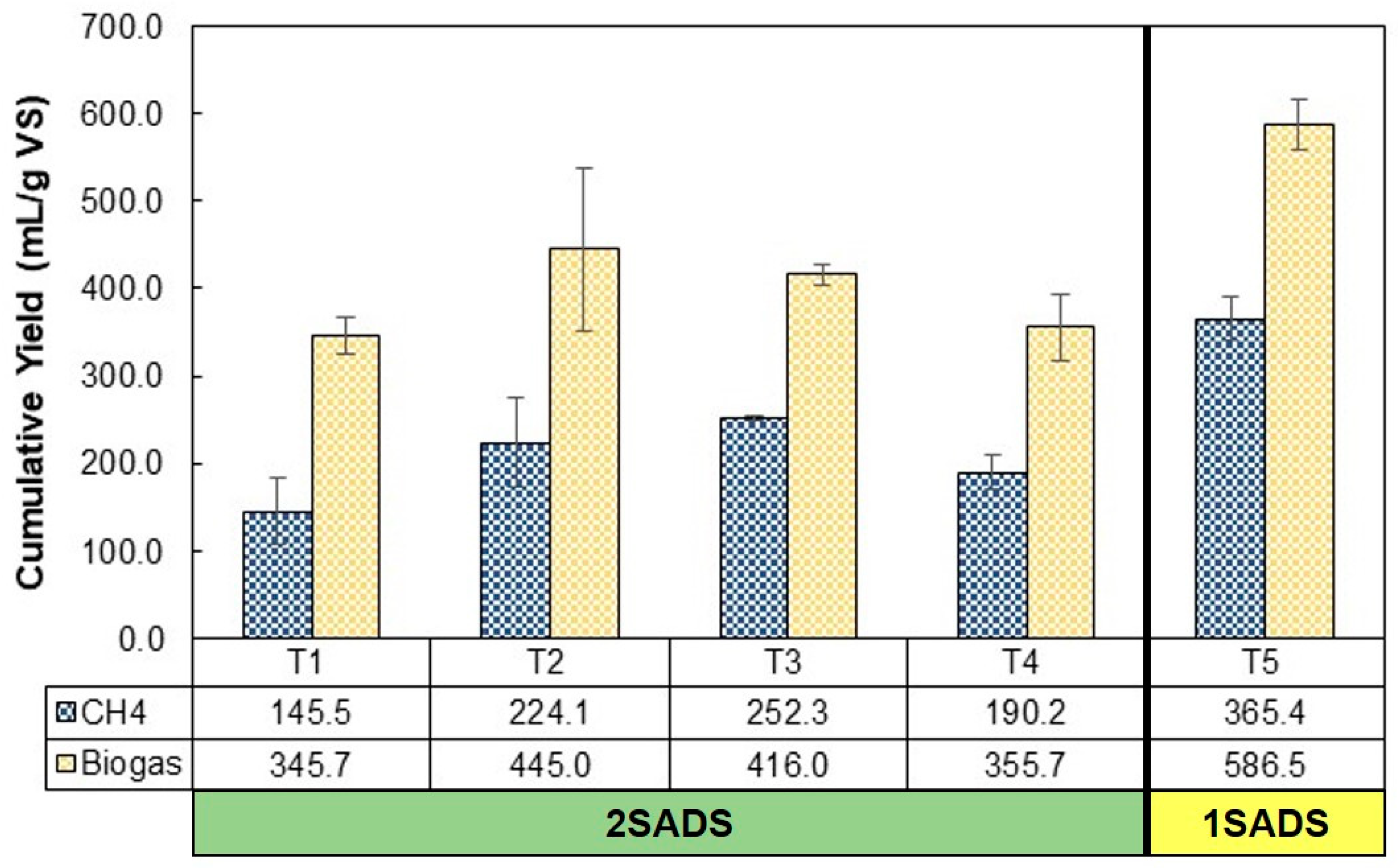
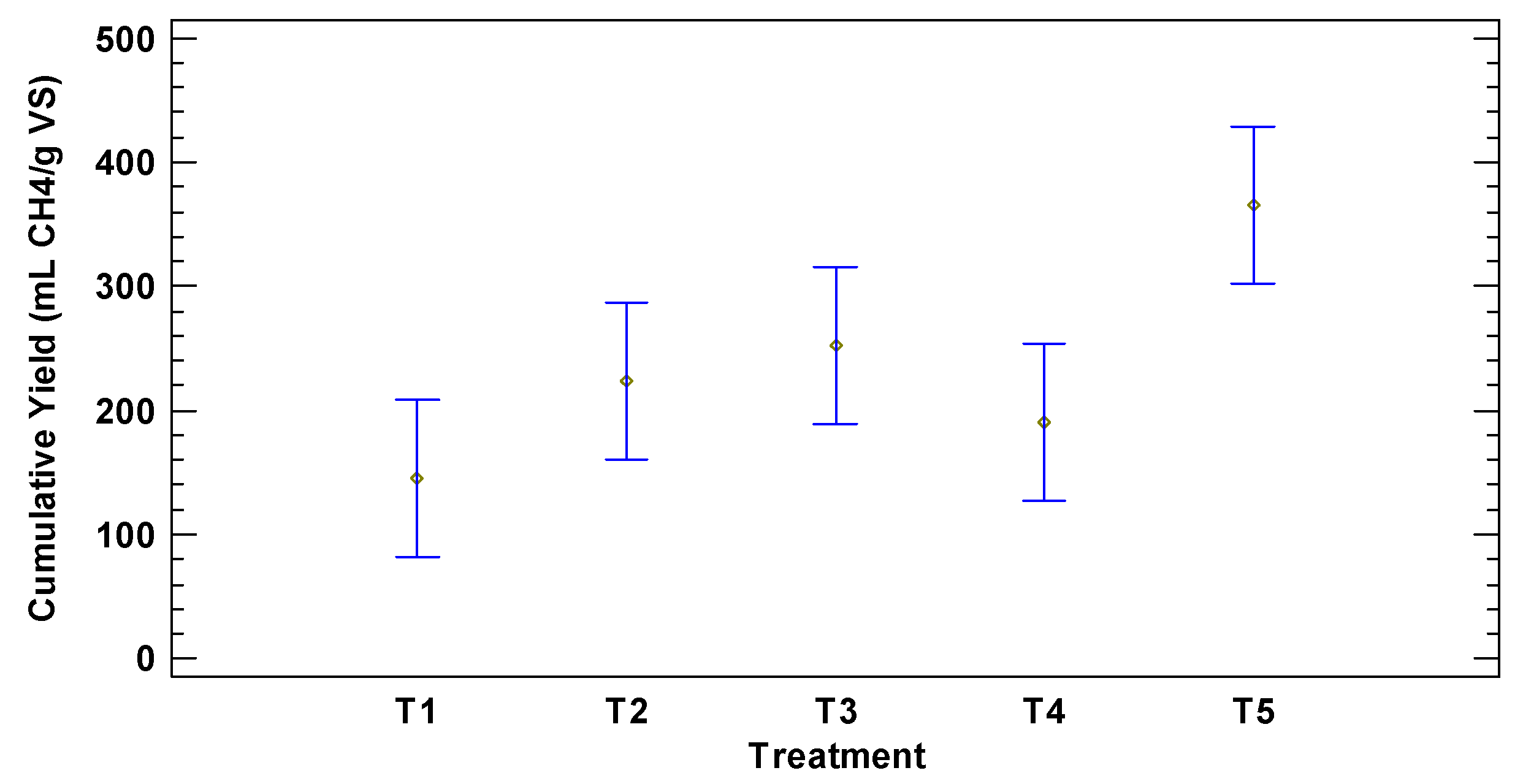
3.3. Comparison of Methane Production between 1SADS VS 2SADS
3.4. Comparison with Other Works
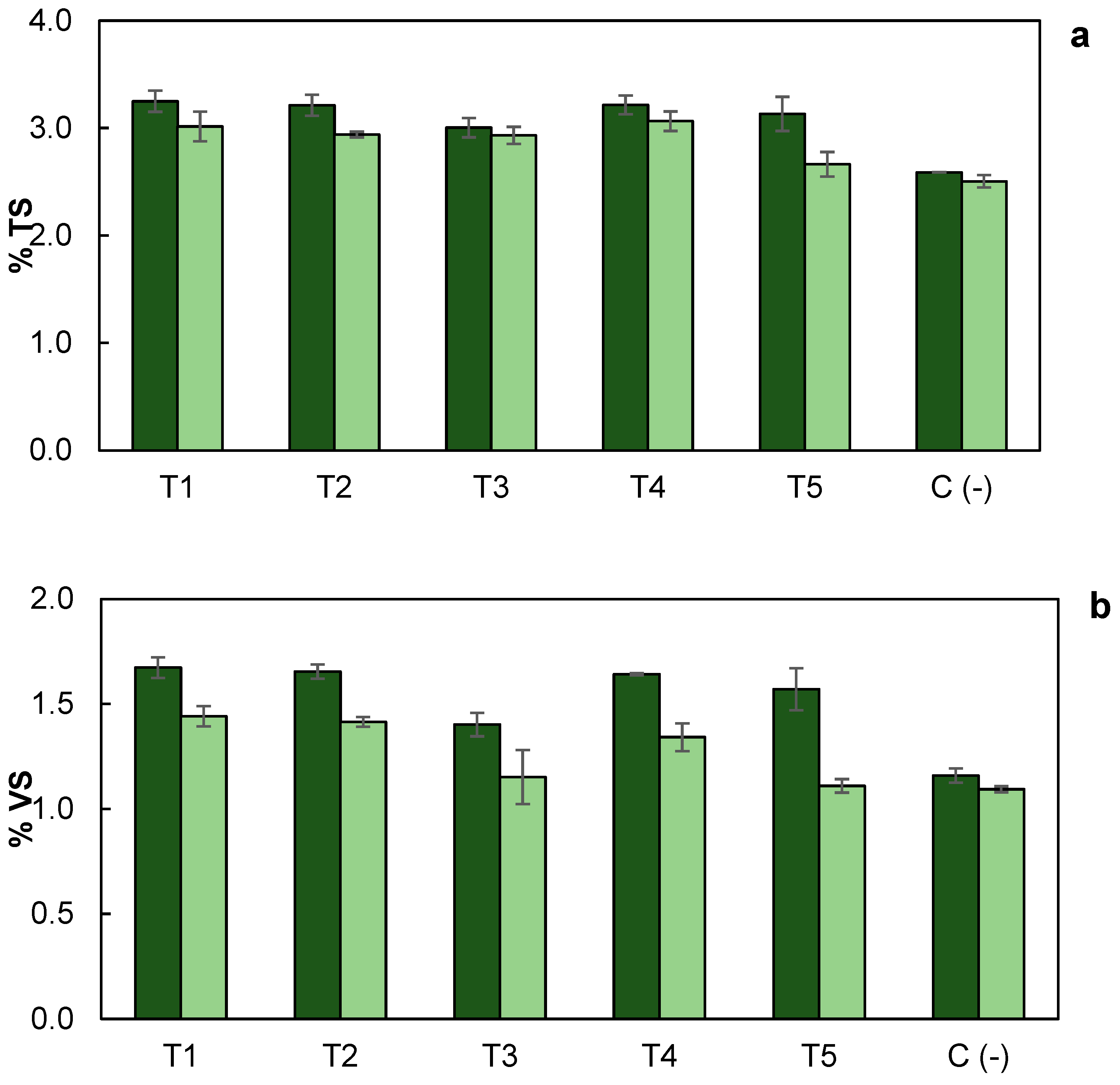
3.5. Total Solids and Total Volatile Solids Removal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rena; Bin Zacharia, K.M.; Yadav, S.; Machhirake, N.P.; Kim, S.-H.; Lee, B.-D.; Jeong, H.; Singh, L.; Kumar, S.; Kumar, R. Bio-hydrogen and bio-methane potential analysis for production of bio-hythane using various agricultural residues. Bioresour. Technol. 2020, 309, 123297. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of United Nations (FAO). 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 21 September 2022).
- Servicio de Información Agroalimentaria y Pesquera (SIAP). 2022. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 21 September 2022).
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014, 32, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Zaccardelli, M.; Lovelli, S.; Perrone, D.; Francia, E.; Milc, J.; Ulrici, A.; Pecchioni, N. Biomass production and dry matter partitioning of processing tomato under organic vs. conventional cropping systems in a Mediterranean environment. Sci. Hortic. 2017, 224, 163–170. [Google Scholar] [CrossRef]
- Pietzsch, M.; Meyer, J. Use of reject heat from biogas power plants for greenhouse heating. Acta Hort. 2008, 801, 719–724. [Google Scholar] [CrossRef]
- Oleszek, M.; Tys, J.; Wiącek, D.; Król, A.; Kuna, J. The possibility of meeting greenhouse energy and CO2 demands through utilization of cucumber and tomato residues. BioEnergy Res. 2016, 9, 624–632. [Google Scholar] [CrossRef]
- Iwaszczuk, N.; Szyba, M.; Iwaszczuk, A.; Yakubiv, V. Production of agricultural biogas from waste—An element of socially responsible actions in the food sector. Acta Innov. 2019, 33, 52–62. [Google Scholar] [CrossRef]
- Janas, M.; Zawadzka, A.A. Assessment of environmental impact of agricultural biogas plant. Acta Innov. 2018, 27, 24–31. [Google Scholar] [CrossRef]
- Camarena-Martínez, S.; Martínez-Martínez, J.H.; Saldaña-Robles, A.; Nuñez-Palenius, H.G.; Costilla-Salazar, R.; Valdez-Vazquez, I.; Lovanh, N.; Ruiz-Aguilar, G.M. Effects of experimental parameters on methane production and volatile solids removal from tomato and pepper plant wastes. BioResources 2020, 15, 4763–4780. [Google Scholar] [CrossRef]
- Cremonez, P.A.; Teleken, J.G.; Weiser Meier, T.R.; Alves, H.J. Two-Stage anaerobic digestion in agroindustrial waste treatment: A review. J. Environ. Manag. 2021, 281, 111854. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Salakkan, A.; Plamgklang, P.; Sittijunda, S.; Kongkeitkajorn, M.B.; Lunprom, S.; Reungsang, A. Bio-Hydrogen and Methane Production from Lignocelluulosic Materials in Biomass for Bioenergy: Recent Trends and Future Challenges; Abd El-Fatah, A., Ed.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/biomass-for-bioenergy-recent-trends-and-future-challenges/bio-hydrogen-and-methane-production-from-lignocellulosic-materials (accessed on 1 October 2022).
- Bouallagui, H.; Touhami, Y.; Ben Cheik, H.R.; Hamdi, M. Bioreactor performance in anaerobic digestión of fruit and vegetable wastes. Process Biochem. 2005, 40, 989–995. [Google Scholar] [CrossRef]
- Shin, H.S.; Han, S.K.; Song, Y.C.; Lee, C.Y. Performance of UASB reactor treating leachate from acidogenic fermenter in the two phase anaerobic digestion of food waste. Water Res. 2001, 35, 3441–3447. [Google Scholar] [CrossRef] [PubMed]
- Salomoni, C.; Caputo, A.; Bonoli, M.; Francioso, O.; Rodriguez-Estrada, M.T.; Palenzona, D. Enhanced methane production in a two-phase anaerobic digestion plant, after CO2 capture and addition to organic wastes. Bioresour. Technol. 2011, 102, 6443–6448. [Google Scholar] [CrossRef] [PubMed]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Schievano, A.; Tenca, A.; Lonati, S.; Manzini, E.; Adani, F. Can two-stage instead of one-stage anaerobic digestion really increase energy recovery from biomass? Appl. Energy 2014, 124, 335–342. [Google Scholar] [CrossRef]
- Pakarinen, O.M.; Tähti, H.P.; Rintala, J.A. One-stage H2 and CH4 and two-stage H2 + CH4 production from grass silage and from solid and liquid fractions of NaOH pre-treated grass silage. Biomass Bioenergy 2009, 33, 1419–1427. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Gilroyed, B.; Yanke, J.; Gruninger, R.; Vedres, D.; McAllister, T.; Hao, X. Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour. Technol. 2015, 185, 79–88. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Flores-Larios, A.; González-Álvarez, V.; Corona-González, R.I.; Méndez-Acosta, H.O. Single and two-stage anaerobic digestion for hydrogen and methane production from acid and enzymatic hydrolysates of Agave tequilana bagasse. Int. J. Hydrogen Energy 2016, 41, 897–904. [Google Scholar] [CrossRef]
- Lobo, B.E.; Sousa, D.R.; Balena, J.G.; Herrera, O.F.; Alves, L.V.; de Aquino, S.F. Evaluation of hydrogen and methane production from sugarcane bagasse hemicellulose hydrolysates by two-stage anaerobic digestion process. Bioresour. Technol. 2016, 218, 436–446. [Google Scholar] [CrossRef]
- dos Santos, L.C.; Adarme, O.F.H.; Baeta, B.; Gurgel, L.; de Aquino, S.F. Production of biogas (methane and hydrogen) from anaerobic digestion of hemicellulosic hydrolysate generated in the oxidative pretreatment of coffee husks. Bioresour. Technol. 2018, 263, 601–612. [Google Scholar] [CrossRef]
- Valdez-Vazquez, I.; Morales, A.L.; Escalante, A.E. History of adaptation determines short-term shifts in performance and community structure of hydrogen-producing microbial communities degrading wheat straw. Microb. Biotechnol. 2017, 10, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rangel, M.; Barboza-Corona, J.E.; Buitrón, G.J.; Valdez-Vazquez, I. Essential nutrients for improving the direct processing of raw lignocellulosic substrates through the dark fermentation process. BioEnergy Res. 2020, 13, 349–357. [Google Scholar] [CrossRef]
- Lara, A.R.; Sánchez, A.; Vázquez, I. Hydration treatments increase the biodegradability of native wheat straw for hydrogen production by a microbial consortium. Int. J. Hydrogen Energy 2014, 39, 19899–19904. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Kang, J.; Kim, K.; Oh, G.; Rhee, S. Analysis on biochemical methane potential of agricultural byproducts with different types of silage storage. J. Mater. Cycles Waste Manag. 2013, 16, 468–474. [Google Scholar] [CrossRef]
- Miftah, A.K.; Sittijunda, S.; Imai, T.; Salakkam, A.; Reungsang, A. Biohydrogen and Methane Production from Sugarcane Leaves Pretreated by Deep Eutectic Solvents and Enzymatic Hydrolysis by Cellulolytic Consortia. Fermentation 2022, 8, 396. [Google Scholar] [CrossRef]
- Cai, Y. Identification and characterization of Enterococcus species isolated from forage crops and their influence on silage fermentation. J. Dairy Sci. 1999, 82, 2466–2471. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Pérez-Rangel, M.; Quiroz-Figueroa, F.R.; González-Castañeda, J.; Valdez-Vazquez, I. Microscopic analysis of wheat straw cell wall degradation by microbial consortia for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 151–160. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Kornaros, M. Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies 2021, 14, 5423. [Google Scholar] [CrossRef]


| Treatment | Hydrogenesis Stage | |||
|---|---|---|---|---|
| Initial pH | X1 | Temperature (°C) | X2 | |
| 1 | 5.5 | −1 | 37 | −1 |
| 2 | 5.5 | −1 | 55 | +1 |
| 3 | 6.5 | +1 | 37 | −1 |
| 4 | 6.5 | +1 | 55 | +1 |
| Substrate | Pretreatment | 2SADS | 1SADS | IMY a | Ref. | |
|---|---|---|---|---|---|---|
| Hydrogenesis Stage | Methanogesis Stage | |||||
| Corn silage | Silage and Bioaugmentation with Pyromyces rhizinflata YM600 | 59 mL H2/g VS 120 mL CH4/g VS Uncontrolled pH HRT 4.3 d OLR 5.0 g COD/L-d Semicontinuous | 175 mL CH4/g VS Uncontrolled pH Batch | 358 mL CH4/g VS Uncontrolled pH RT 60 d, 37 °C S/I 1.0 Batch | −51% | [20] |
| Hydrolyzate of Agave tequilana bagasse | Enzimatic (Celluclast 1.5 L 45 °C/10 h) | 3.4 mol H2/mol hexose Initial pH 7.0, RT not mentioned, 37 °C 20% (v/v) hydrolyzate Batch | 240 mL CH4/g COD Initial pH 8.0 RT not mentioned, 37 °C Batch | 90 mL CH4/g COD pH 8.0, RT not mentioned, 37 °C 20% (v/v) hydrolyzate, Batch | 267% | [21] |
| Sugarcane bagasse | Autohydrolysis (182.9 °C, 40.7 min) | 3.7 mmol H2 pH 5.5 RT 19 d, 35 °C S/I 1.85 (g TOC/g VSS) Batch | 1.87 mmol CH4 pH not mentioned RT 20 d, 35 °C S/I 0.4 (g TOC/g VSS) Batch | N.A. | 400% b | [22] |
| Coffee husks | Ozonolysis (liquid-to-solid ratio 10 mL /g, pH 11, 18.5 mg O3/g substrate) | 48.5 mL H2/g COD pH not mentioned RT 700 h, 35 °C S/I 1.8 (g COD/g VSS) Batch | 284.6 mL CH4/g COD pH not mentioned RT 900 h, 35 °C S/I 0.7 (g COD/g VSS) Batch | 91.1 mL CH4/g COD pH not mentioned RT 900 h, 35 °C S/I 0.7 (g COD/g VSS) Batch | 312% | [23] |
| Tomato plant residues | None | 11.6 mL/g VS Initial pH 6.5, RT 14 d, 37 °C Batch | 252.3 mL/g VS Uncontrolled pH RT 28 d, 37 °C S/I 0.5 (g VS/g VS) Batch | 365.4 mL/g VS Uncontrolled pH RT 28 d, 37 °C S/I 0.5 (g VS/g VS)Batch | −31% c | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Aguilar, G.M.L.; Nuñez-Palenius, H.G.; Lovanh, N.; Camarena-Martínez, S. Comparative Study of Methane Production in a One-Stage vs. Two-Stage Anaerobic Digestion Process from Raw Tomato Plant Waste. Energies 2022, 15, 9137. https://doi.org/10.3390/en15239137
Ruiz-Aguilar GML, Nuñez-Palenius HG, Lovanh N, Camarena-Martínez S. Comparative Study of Methane Production in a One-Stage vs. Two-Stage Anaerobic Digestion Process from Raw Tomato Plant Waste. Energies. 2022; 15(23):9137. https://doi.org/10.3390/en15239137
Chicago/Turabian StyleRuiz-Aguilar, Graciela M. L., Hector G. Nuñez-Palenius, Nanh Lovanh, and Sarai Camarena-Martínez. 2022. "Comparative Study of Methane Production in a One-Stage vs. Two-Stage Anaerobic Digestion Process from Raw Tomato Plant Waste" Energies 15, no. 23: 9137. https://doi.org/10.3390/en15239137
APA StyleRuiz-Aguilar, G. M. L., Nuñez-Palenius, H. G., Lovanh, N., & Camarena-Martínez, S. (2022). Comparative Study of Methane Production in a One-Stage vs. Two-Stage Anaerobic Digestion Process from Raw Tomato Plant Waste. Energies, 15(23), 9137. https://doi.org/10.3390/en15239137









