Study of the NH3-SCR Mechanism on LaMnO3 Surfaces Based on the DFT Method
Abstract
1. Introduction
2. Computational Methods and Models
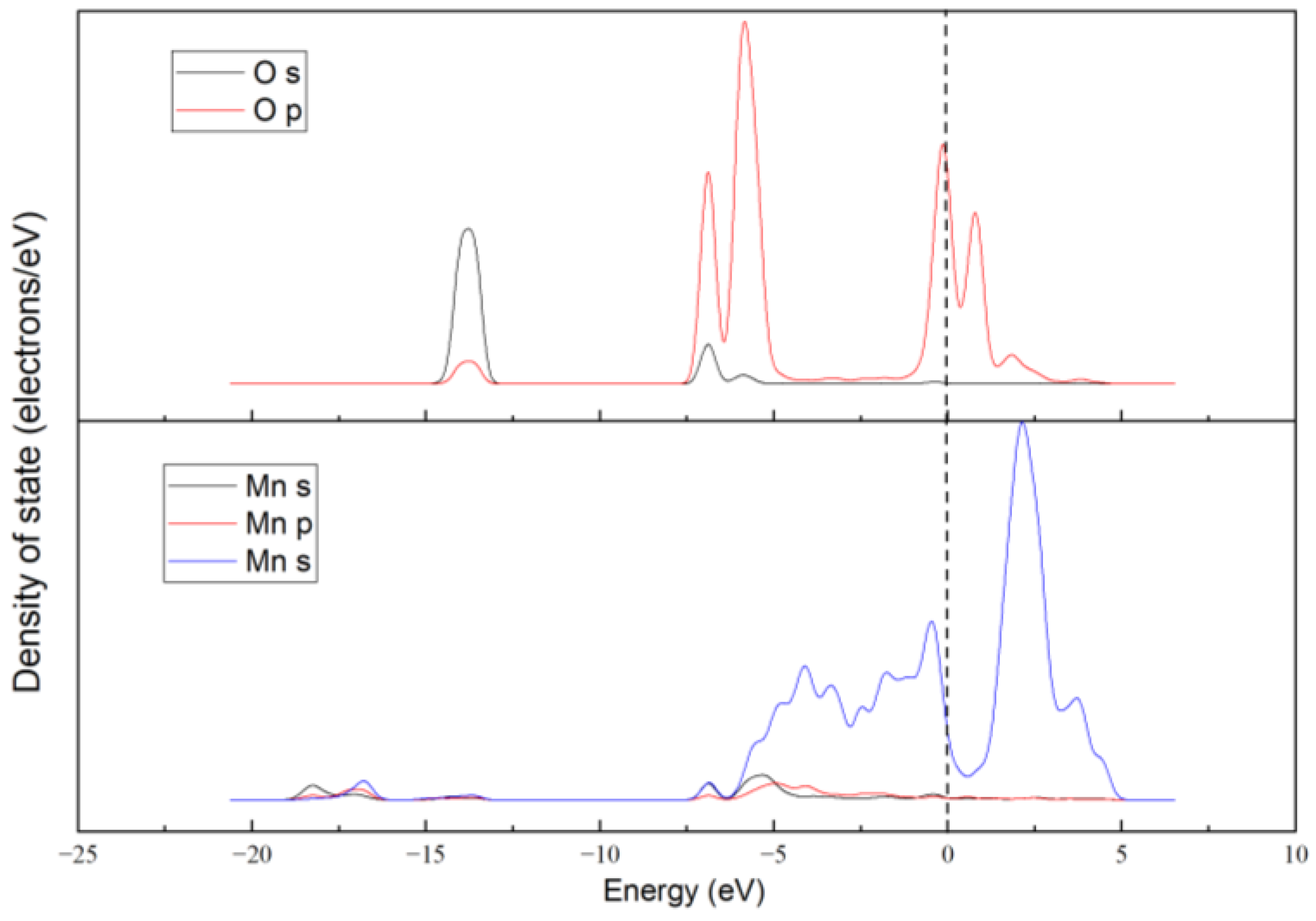
3. Results and Discussion
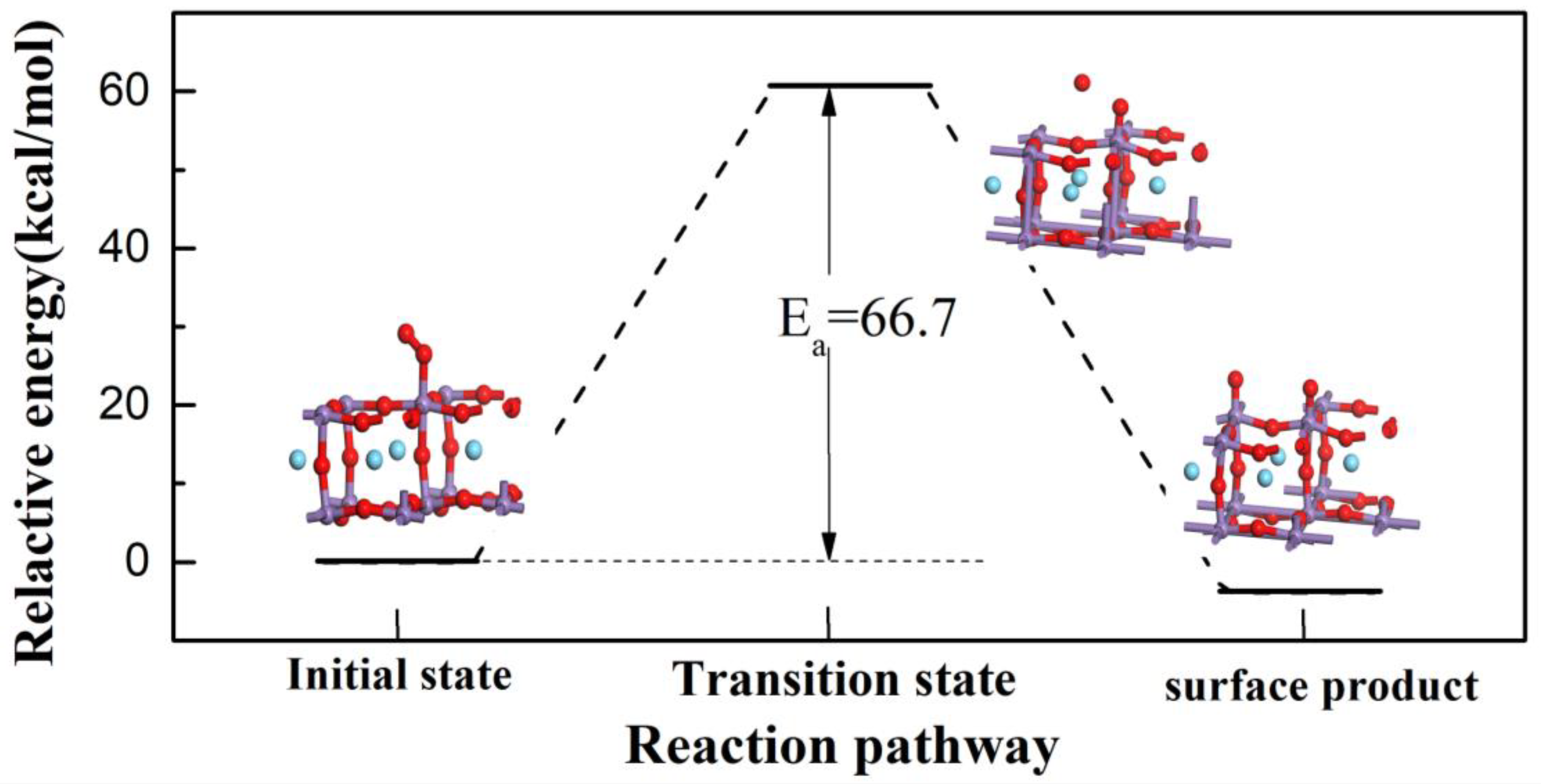
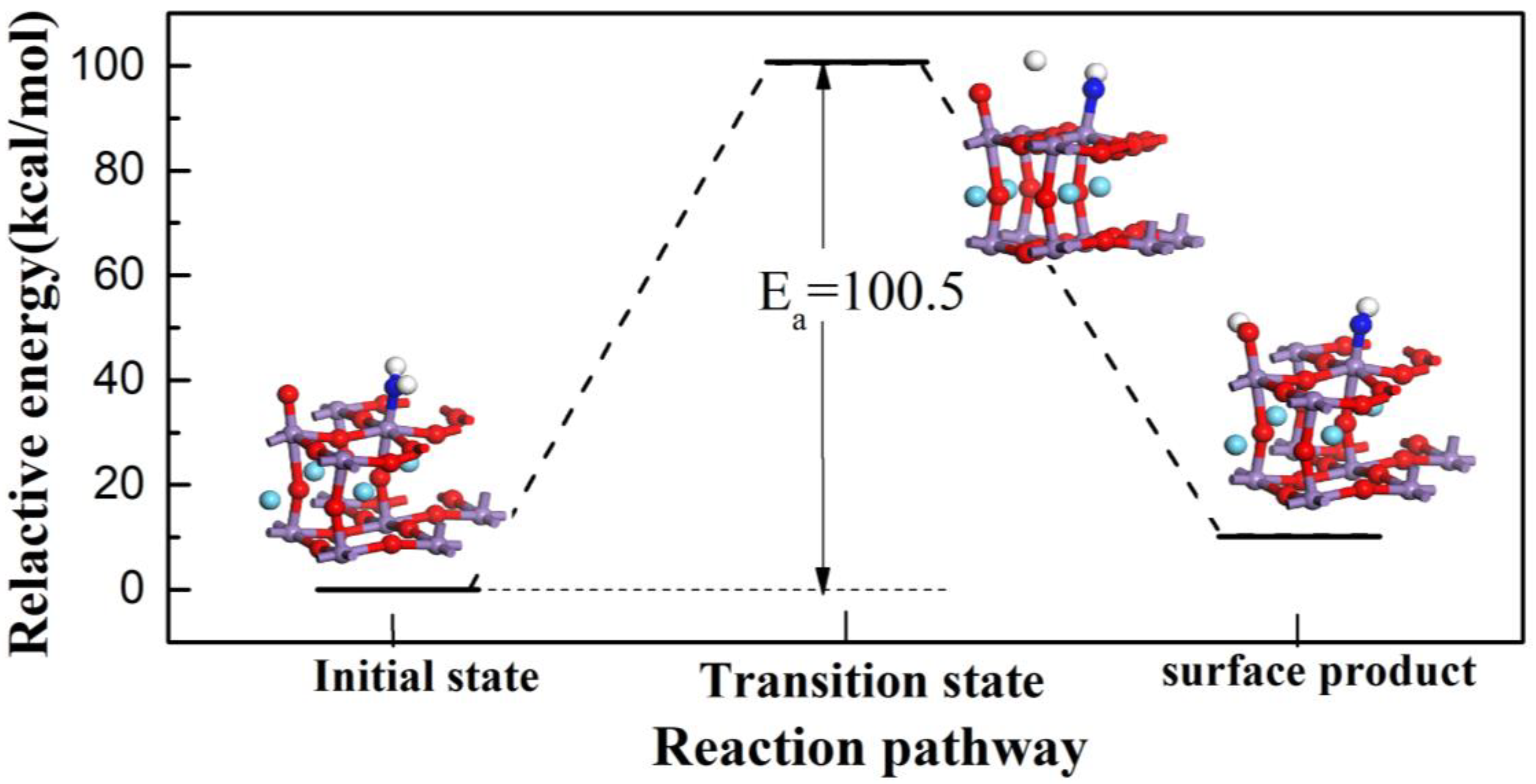
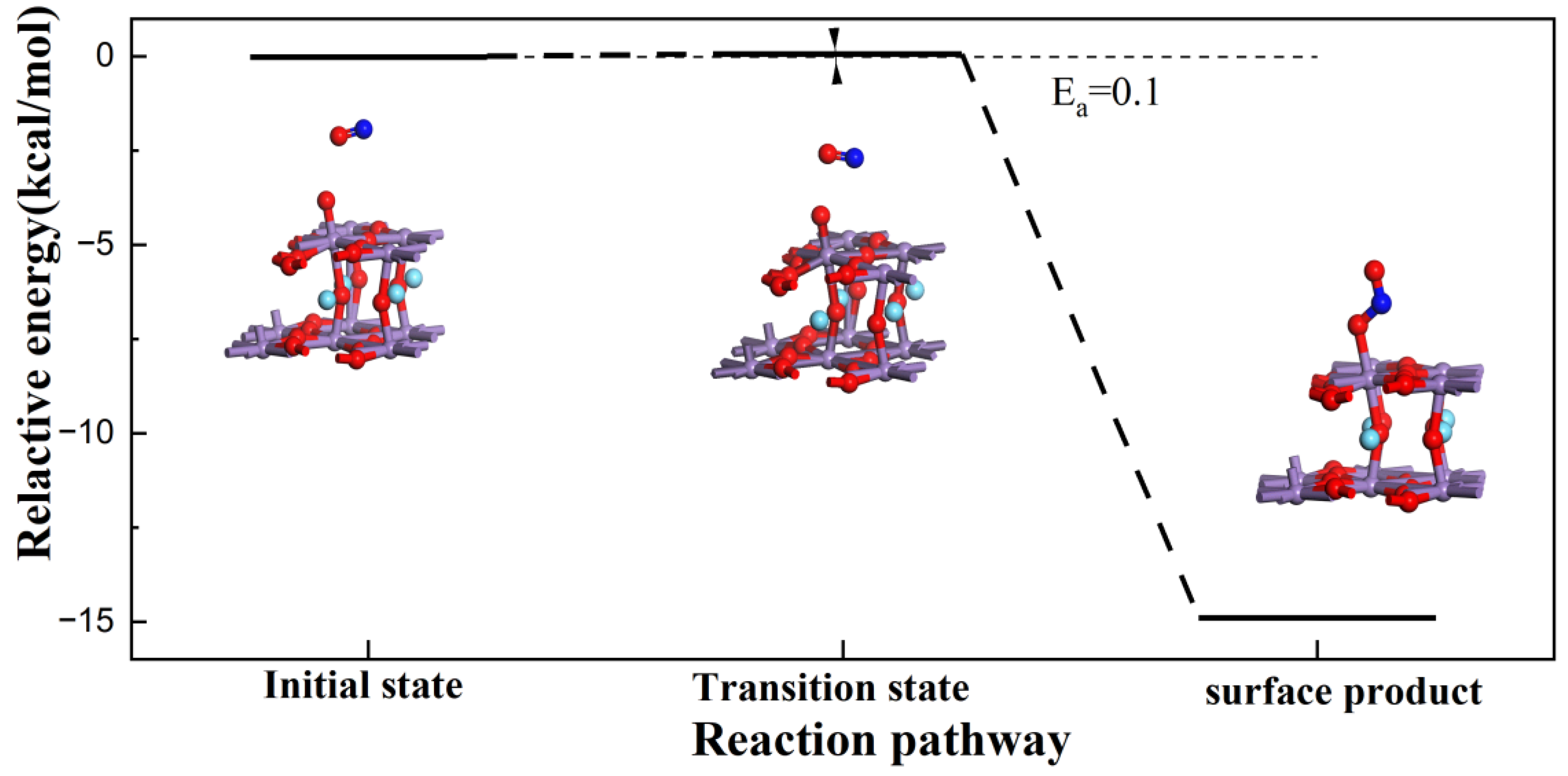
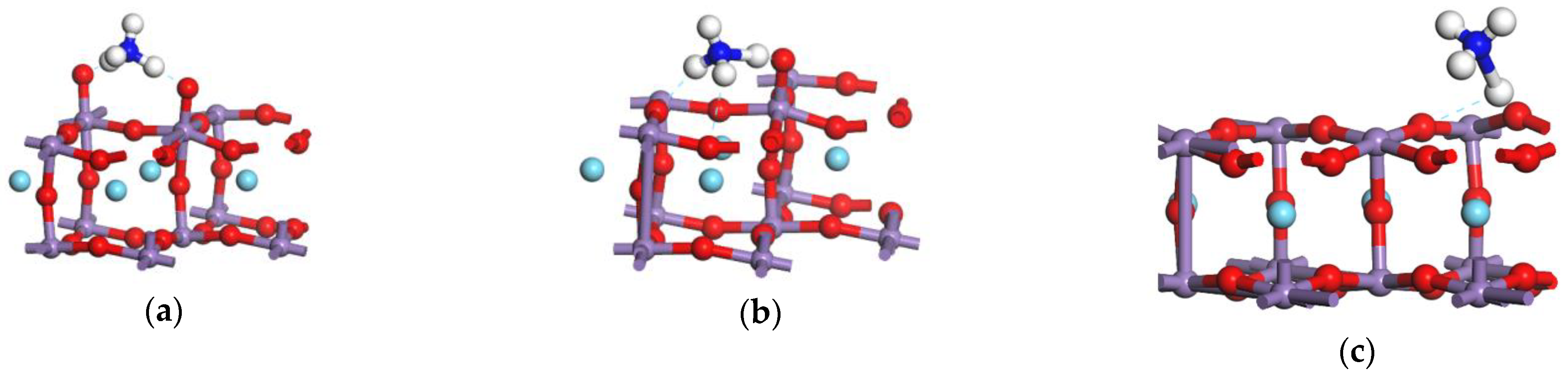
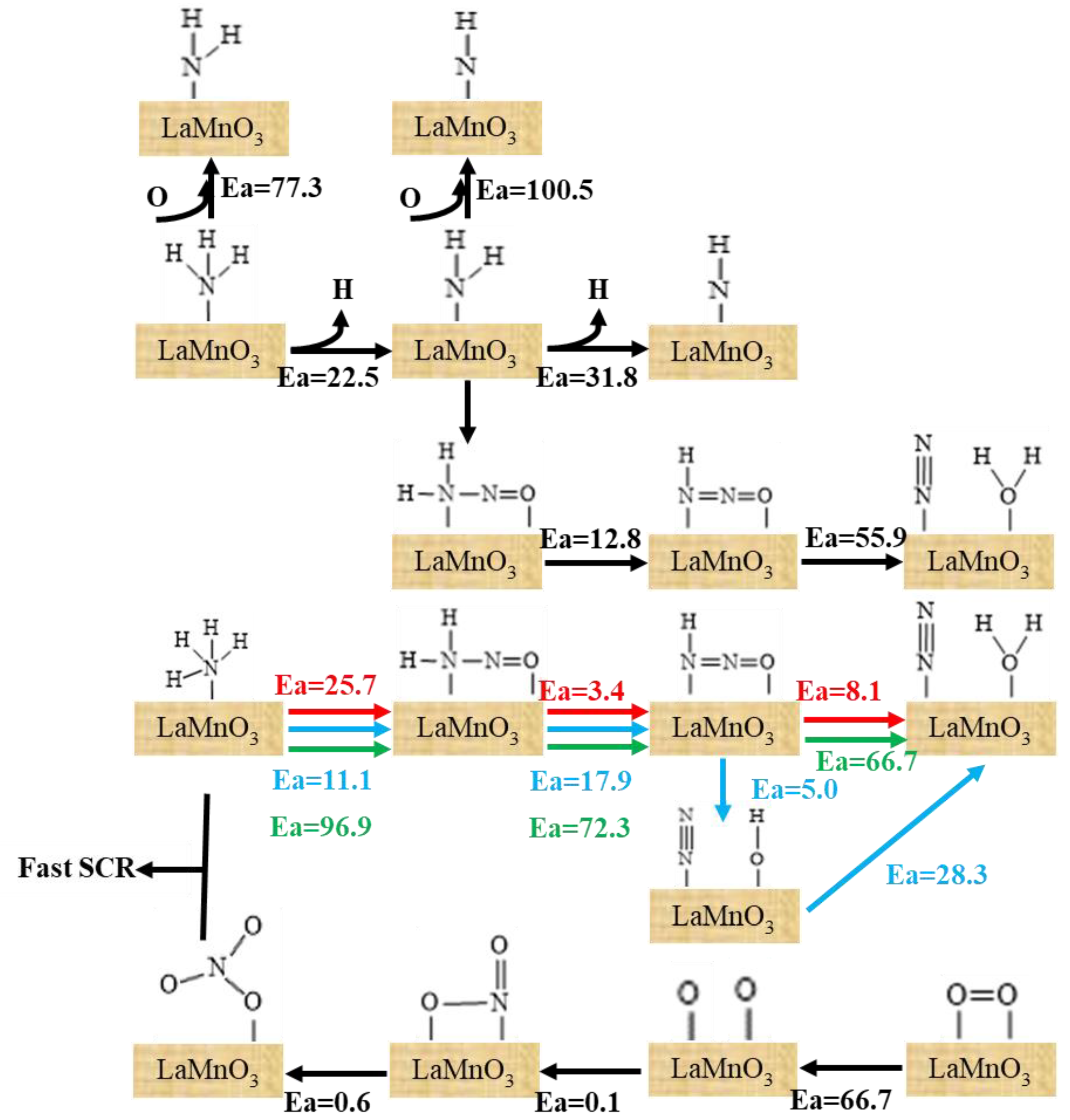
3.1. Reaction of L Acid
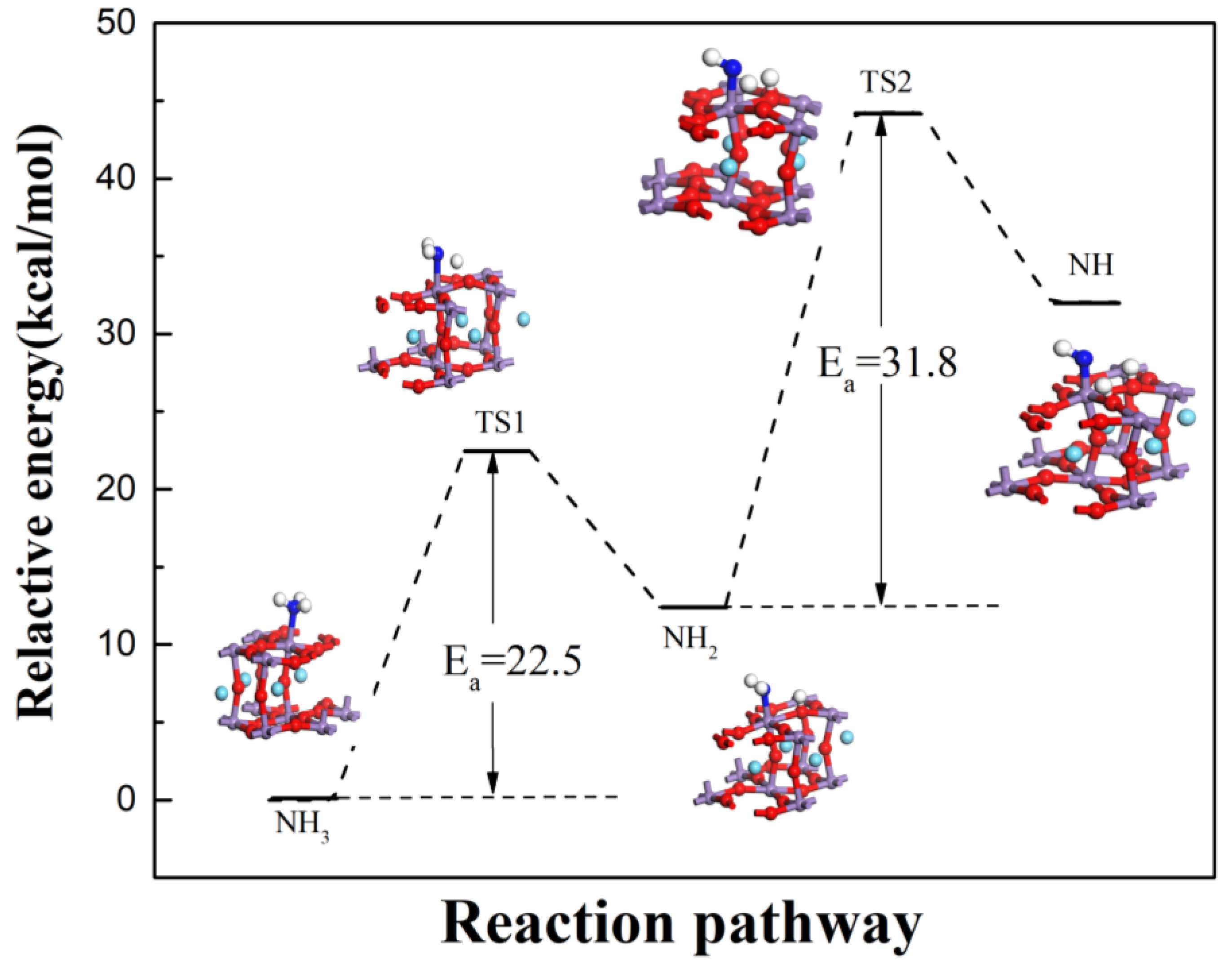
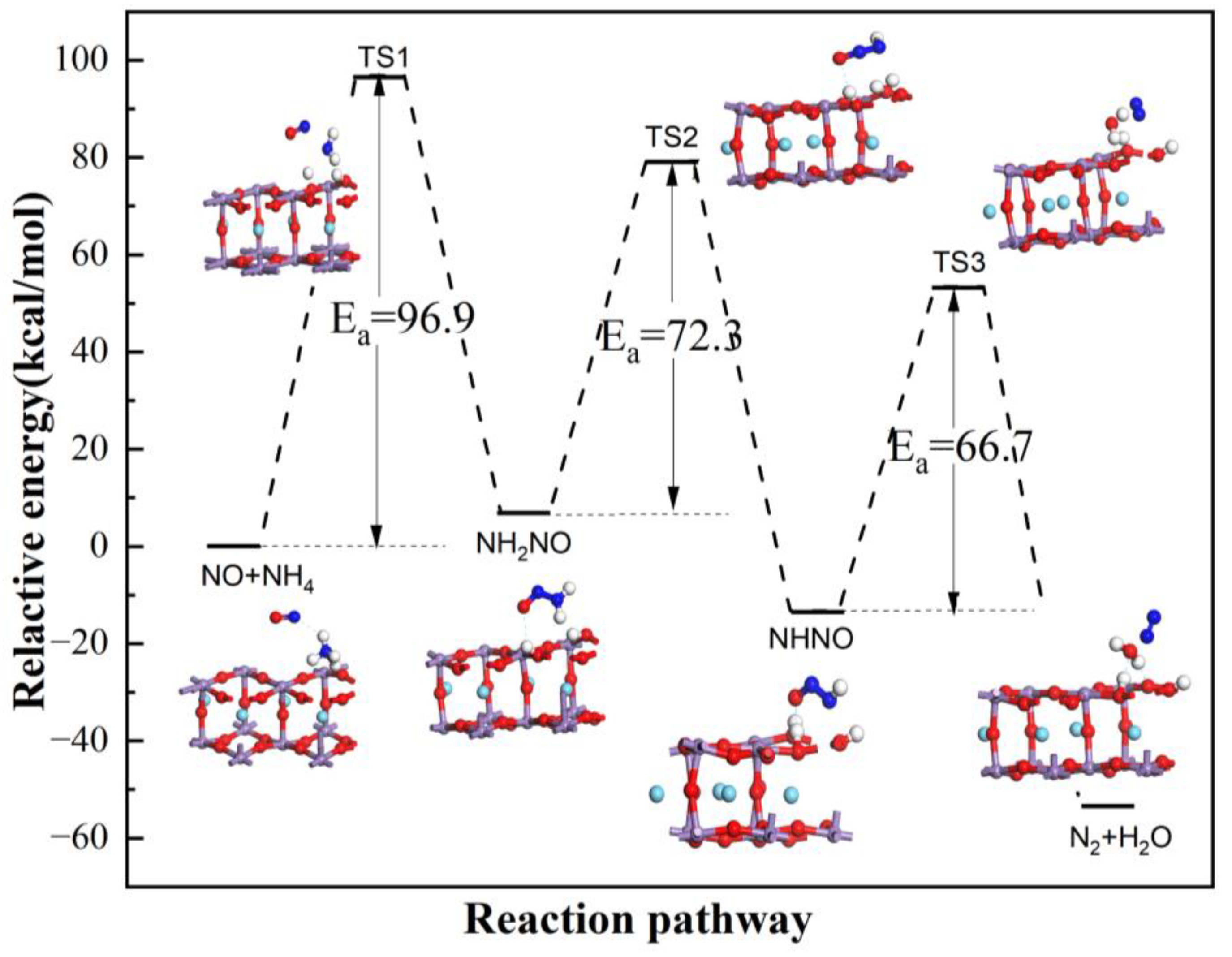
3.2. Reaction Process on B Acid
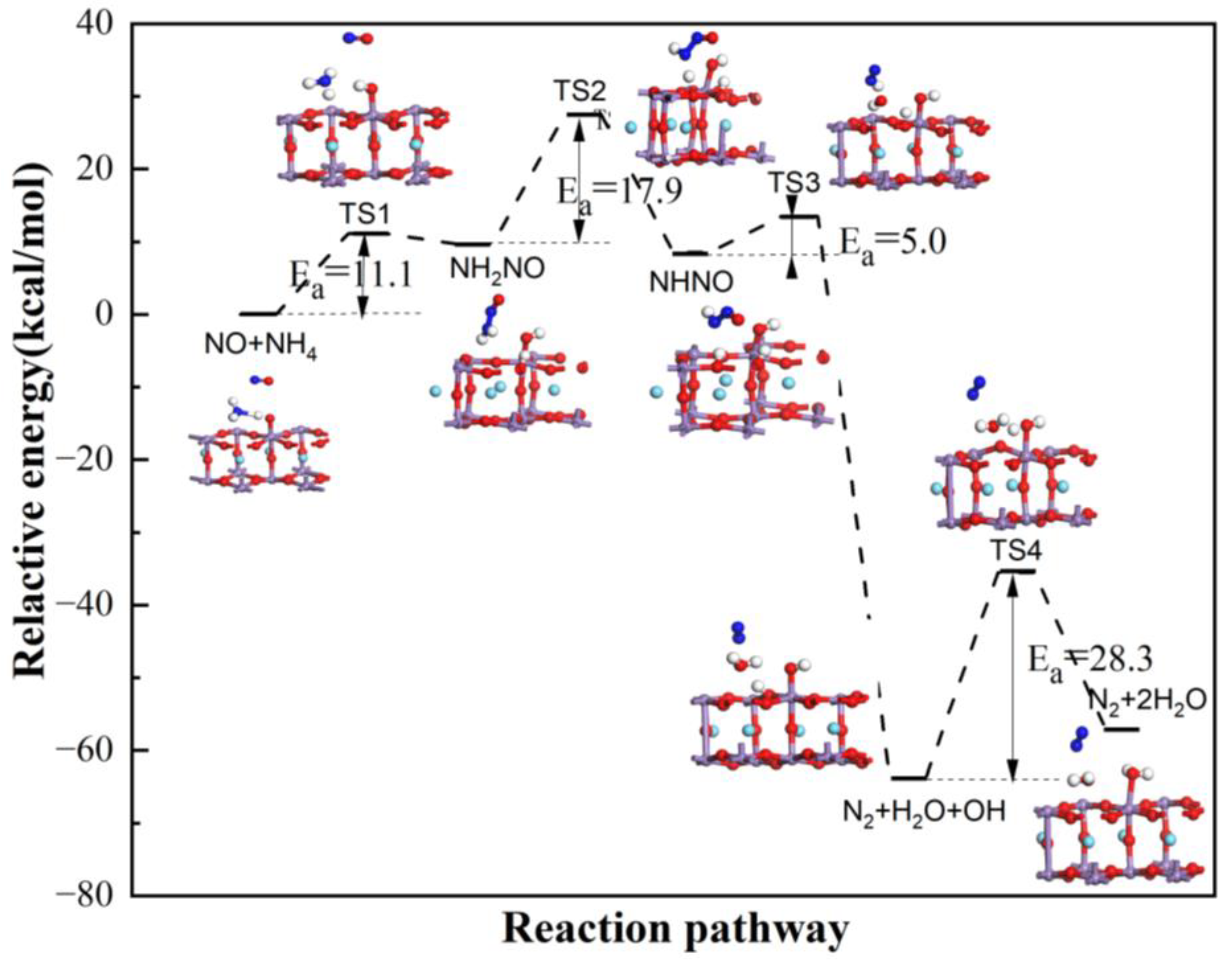
3.3. The Main Reaction Pathway Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heck, R.M. Catalytic abatement of nitrogen oxides–stationary applications. Catal. Today 1999, 4, 519–523. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.; Ledakowicz, S. Trends in NO(x) abatement: A review. Sci Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xia, Y.-F.; Lu, B.-H.; Liu, N.; Zhang, L.; Li, S.-J.; Li, W. Emission inventory and trends of NO x for China, 2000–2020. J. Zhejiang Univ. Sci. A 2014, 15, 454–464. [Google Scholar] [CrossRef]
- Müller, J.-F. Geographical distribution and seasonal variation of surface emissions and deposition velocities of atmospheric trace gases. J. Geophys. Res. Atmos. 1992, 97, 3787–3804. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Woo, Z.; Liu, S.I. Recent Advances in Catalytic DeNOX Science and Technology. Catal. Rev. 2006, 48, 43–89. [Google Scholar]
- Forzatti, P. Environmental catalysis for stationary applications. Catal. Today 2000, 62, 51–65. [Google Scholar] [CrossRef]
- Koh, H.L.; Lee, S.H.; Kim, K.L. The Effect of MoO3 Addition to V2O5/Al2O3 Catalysts for the Selective Catalytic Reduction of NO by NH3. React. Kinet. Catal. Lett. 2000, 71, 239–244. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Zhang, P.F.; Dai, S. Recent advances of Lanthanum based perovskite oxides for catalysis. ACS Catal. 2015, 5, 6370–6385. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as substitutes of noble metals for heterogeneous catalysis: Dream or reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar]
- PENAMA; FIERROJLG. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, Y.; Yao, C.; Zuo, S.; Lu, X.; Luo, S.; Ni, C. La1−xCexMnO3 attapulgite nanocomposites as catalysts for NO reduction with NH3 at low temperature. Particuology 2016, 26, 66–72. [Google Scholar] [CrossRef]
- Zhang, R.D.; Luo, N.; Yang, W.; Liu, N.; Chen, B. Low-temperature selective catalytic reduction of NO with NH3 using perovskite-type oxides as the novel catalysts. J. Mol. Catal. A Chem. 2013, 371, 86–93. [Google Scholar] [CrossRef]
- Guo, J.; Shi, X.; Fan, A.; Li, J.; Chu, Y.; Yuan, S. Study on the Preparation and Denitration Performance of Ce Modified La–Mn Perovskite Catalyst. Adv. Eng. Sci. 2021, 4, 233–239. [Google Scholar]
- Wang, R.; Gui, K.; Liang, H. Effect of Ce-doped on performance of supported perovskite catalyst LaMnO3/hematite for SCR of NO by NH3. Chem. Ind. Eng. Process 2016, 35, 192–199. [Google Scholar]
- Ramis, G.; Larrubia, M.A.; Busca, G. On the chemistry of ammonia over oxide catalysts: Fourier transform infrared study of ammonia, hydrazine and hydroxylamine adsorption over iron–titania catalyst. Top. Catal. 2000, 11–12, 161–166. [Google Scholar] [CrossRef]
- Gallardo, J.; Escribano, A.; Ramis, V.S. An FT-IR study of ammonia adsorption and oxidation over anatase-supported metal oxides. Appl. Catal. B Environ. 1997, 13, 45–58. [Google Scholar]
- Yang, S.; Wang, C.; Li, J.; Yan, N.; Ma, L.; Chang, H. Low temperature selective catalytic reduction of NO with NH3 over Mn–Fe spinel: Performance, mechanism and kinetic study. Appl. Catal. B Environ. 2011, 110, 71–80. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Wang, D.Q.; Wang, J.; Chen, Q.; Zhang, Z.; Pan, X.; Miao, Z.; Zhang, B.; Wu, Z.; Yang, X. BiMnO3 perovskite catalyst for selective catalytic reduction of NO with NH3 at low temperature. Chin. J. Catal. 2012, 33, 1448–1454. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Madia, G. Reaction pathways in the selective catalytic reduction process with NO and NO2 at low temperatures. Ind. Eng. Chem. Res. 2001, 40, 52–59. [Google Scholar] [CrossRef]
- Shi, H.Y.; Li, X.Z.; Xia, J.W.; Lu, X.; Zuo, S.; Luo, S.; Yao, C. Sol-gel synthesis of LaBO3/attapulgite (B=Mn, Fe, Co, Ni) nanocomposite for NH3-SCR of NO at low temperature. J. Inorg. Organomet. Polym. Mater. 2017, 27, 166–172. [Google Scholar] [CrossRef]
- Tuo, K.; Zhang, P.; Wang, L.; Huang, H.Y. Perovskite catalysts for selective catalytic reduction of NOx with NH3. J. Huazhong Agric. Univ. 2020, 5, 26–34. [Google Scholar]
- Soyer, S.; Uzun, A.; Senkan, S.; Onal, I. A quantum chemical study of nitric oxide reduction by ammonia (SCR reaction) on V2O5 catalyst surface. Catal. Today 2006, 118, 268–278. [Google Scholar] [CrossRef]
- Sun, C.; Dong, L.; Yu, W.; Liu, L.; Li, H.; Gao, F.; Chen, Y. Promotion effect of tungsten oxide on SCR of NO with NH3 for the V2O5–WO3/Ti0.5Sn0.5O2 catalyst: Experiments combined with DFT calculations. J. Mol. Catal. A Chem. 2011, 346, 29–38. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Zhong, L. DFT Analysis of the Reaction Mechanism for NH3 -SCR of NOx over Mn/γ-Al2O3 Catalyst. J. Phys. Chem. C 2019, 123, 25185–25196. [Google Scholar] [CrossRef]
- Anstrom, M.; Topsøe, N.-Y.; Dumesic, J.A. Density functional theory studies of echanistic aspects of the SCR reaction on vanadium oxide catalysts. J. Catal. 2003, 213, 115–125. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Yang, Y.; Wang, Z.; Zheng, Y. A catalytic reaction scheme for NO reduction by CO over Mn-terminated LaMnO3 perovskite: A DFT study. Fuel Process. Technol. 2021, 216, 106798. [Google Scholar] [CrossRef]
- Gavin, A.L.; Watson, G.W. Modelling oxygen defects in orthorhombic LaMnO3 and its low index surfaces. Phys. Chem. Chem. Phys. 2017, 19, 24636–24646. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Yang, Y.; Yu, Y.; Yan, X.; Zhang, Z. Insights into the catalytic behavior of LaMnO3 perovskite for Hg0 oxidation by HCl. J. Hazard. Mater. 2020, 383, 121156. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Cao, H.; Chi, Z.; Chen, C.; Duan, X.; Xie, Y.; Qi, F.; Song, W.; Liu, J.; et al. Role of oxygen vacancies and Mn sites in hierarchical Mn2O3/LaMnO3-δ perovskite composites for aqueous organic pollutants decontamination. Appl. Catal. B Environ. 2019, 245, 546–554. [Google Scholar] [CrossRef]
- Ren, D.; Gui, K.; Gu, S. Mechanism of Improving the SCR NO Removal Activity of Fe2O3 Catalyst by Doping Mn. J. Alloy. Compd. 2021, 867, 158787. [Google Scholar] [CrossRef]
- Ren, D.; Gui, K.; Gu, S. Quantum chemistry study of SCR-NH3 nitric oxide reduction on Ce-doped γFe2O3 catalyst surface. Mol. Catal. 2021, 502, 111373. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Bergner, A.; Dolg, M.; Küchle, W.; Stoll, H.; Preuß, H. Ab initio energy-adjusted pseudopotentials for elements of groups. Mol. Phys. 1993, 80, 1431–1441. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhang, B.; Liu, F. Mechanistic studies of mercury adsorption and oxidation by oxygen over spinel-type MnFe2O4. Hazard. Mater. 2017, 321, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Gui, K.; Gu, S.; Wei, Y. Study of the nitric oxide reduction of SCR-NH3 on nano-γFe2O3 catalyst surface with quantum chemistry. Appl. Surf. Sci. 2020, 509, 144659. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1997, 49, 225–232. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Lin, Y.S.; Chang, M. Effects of water vapor and trace gas impurities in flue gas on CO2/N2 separation using ZIF-68. Phys. Chem. C 2014, 118, 6744–6751. [Google Scholar] [CrossRef]
- Ramis, G.; Angeles Larrubia, M. An FT-IR study of the adsorption and oxidation of N-containing compounds over Fe2O3/Al2O3 SCR catalysts. J. Mol. Catal. A Chem. 2004, 215, 161–167. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, Z.; Liu, F. A skeletal reaction scheme for selective catalytic reduction of NOx with NH3 over CeO2/TiO2 catalyst. Fuel Process. Technol. 2018, 174, 17–25. [Google Scholar] [CrossRef]
- Yao, H. Periodic DFT study on mechanism of selective catalytic reduction of NO via NH3 and O2 over the V2O5 (001) surface: Competitive sites and pathways. J. Catal. 2013, 305, 67–75. [Google Scholar] [CrossRef]
- Gu, S.; Gui, K.; Ren, D.; Wei, Y. The effects of manganese precursors on NO catalytic removal with MnOxSiO2 catalyst at low temperature. React. Kinet. Mech. Catal. 2020, 130, 195–215. [Google Scholar] [CrossRef]
- Gu, S.; Gui, K.; Ren, D.; Wei, Y. Understanding the adsorption of NH3, NO and O2 on the MnOxSiO2 β-cristobalite (101) surface with density functional theory. React. Kinet. Mech. Catal. 2020, 130, 741–751. [Google Scholar] [CrossRef]
- Savara, A.; Li, M.-J.; Sachtler, W.M.; Weitz, E. Catalytic reduction of NH4NO3 by NO: Effects of solid acids and implications for low temperature De-NOx processes. Appl. Catal. B Environ. 2008, 81, 251–257. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Kamasamudram, K.; Epling, W.S. In Situ-DRIFTS Study of Selective Catalytic Reduction of NOx by NH3 over Cu-Exchanged SAPO-34. ACS Catal. 2013, 3, 871–881. [Google Scholar] [CrossRef]








| Mn–N Bond Length (Å) | Angle of N–H (°) | Mulliken Charge of NH3 (a.u) | Adsorption Energy (kJ/mol) |
|---|---|---|---|
| 2.107 | 109.782 | 0.327 | −97.9 |
| Mn–O Bond Length (Å) | O–O Bond Length (Å) | Mulliken Charge of O2 (a.u) | Adsorption Energy (kJ/mol) |
|---|---|---|---|
| 2.015 | 1.268 | -0.129 | −47.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, D.; Wu, K.; Luo, S.; Li, Y.; Gui, K.; Zuo, Z.; Guo, X. Study of the NH3-SCR Mechanism on LaMnO3 Surfaces Based on the DFT Method. Energies 2022, 15, 9099. https://doi.org/10.3390/en15239099
Ren D, Wu K, Luo S, Li Y, Gui K, Zuo Z, Guo X. Study of the NH3-SCR Mechanism on LaMnO3 Surfaces Based on the DFT Method. Energies. 2022; 15(23):9099. https://doi.org/10.3390/en15239099
Chicago/Turabian StyleRen, Dongdong, Kai Wu, Siyi Luo, Yongjie Li, Keting Gui, Zongliang Zuo, and Xianjun Guo. 2022. "Study of the NH3-SCR Mechanism on LaMnO3 Surfaces Based on the DFT Method" Energies 15, no. 23: 9099. https://doi.org/10.3390/en15239099
APA StyleRen, D., Wu, K., Luo, S., Li, Y., Gui, K., Zuo, Z., & Guo, X. (2022). Study of the NH3-SCR Mechanism on LaMnO3 Surfaces Based on the DFT Method. Energies, 15(23), 9099. https://doi.org/10.3390/en15239099






