Occurrence and Migration Mechanisms of Methane in Marine Shale Reservoirs
Abstract
1. Introduction
2. Theory
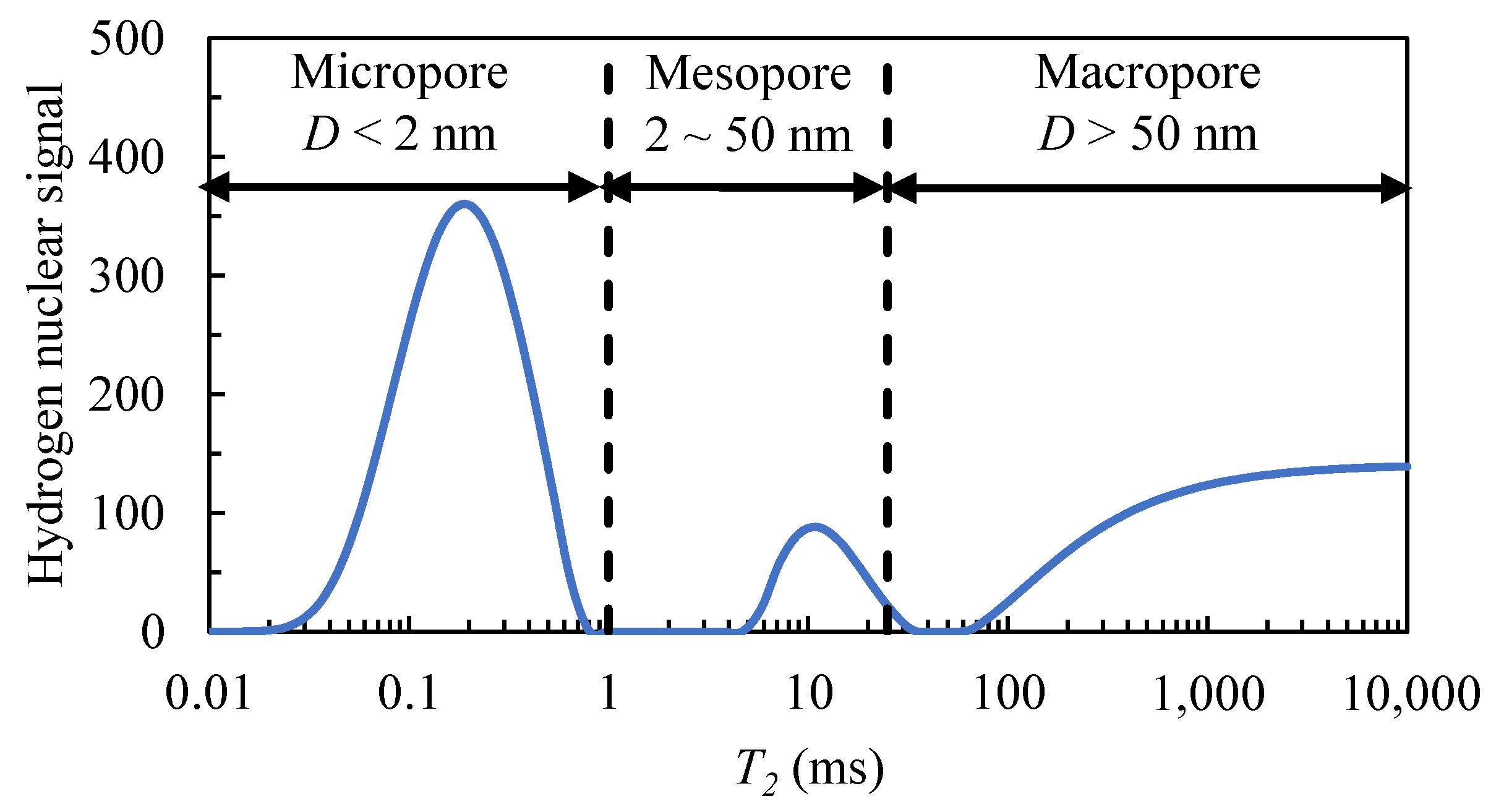
2.1. Nuclear Magnetic Resonance Technology
2.2. Marine Shale Reservoir Characteristics
3. Samples and Methods
4. Results
5. Discussion
5.1. Shale Gas Occurrence Mechanism
5.2. Shale Gas Migration Mechanism
6. Conclusions
- (1)
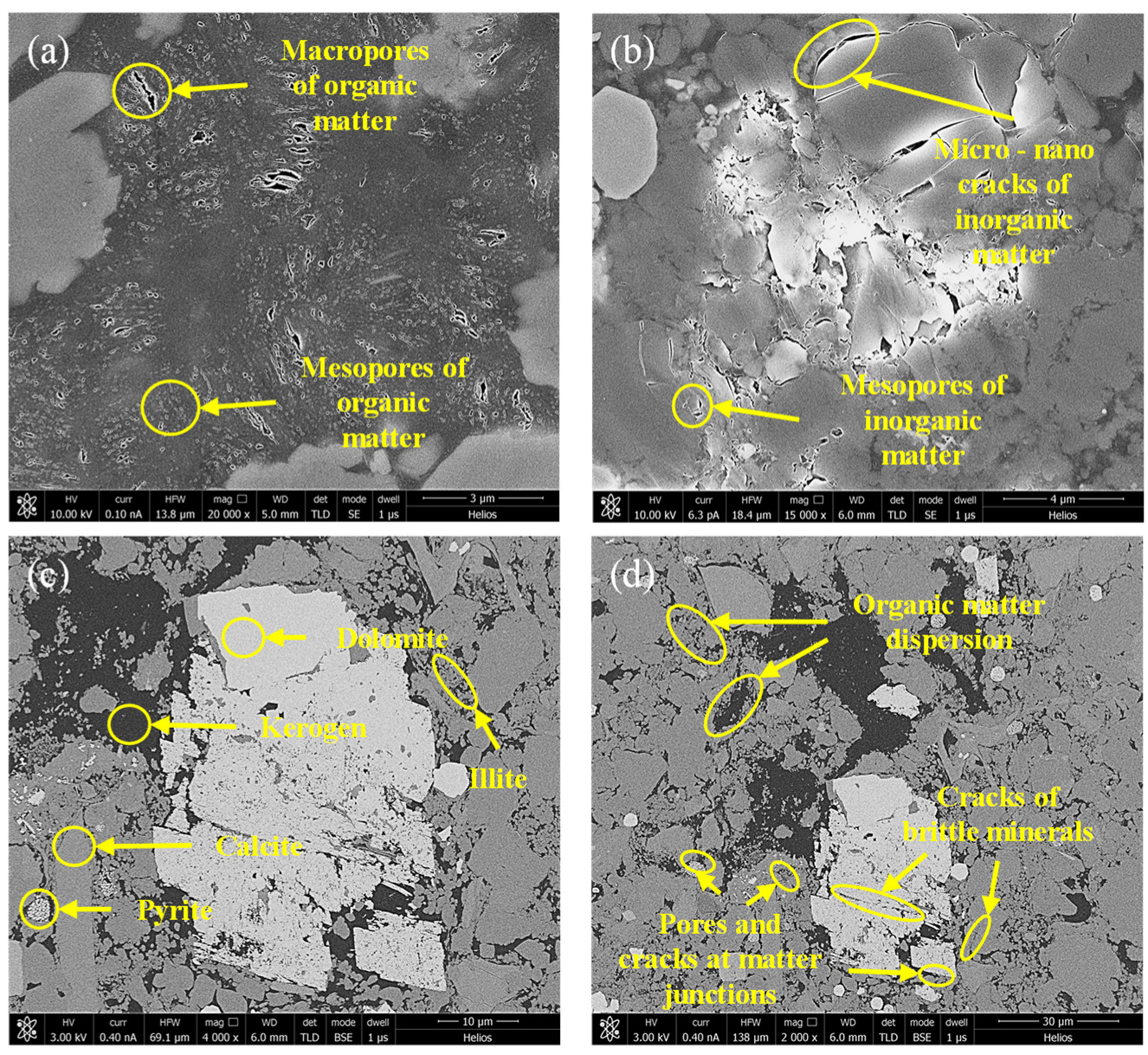
- Marine shale reservoirs have many organic matter pores and poor connectivity. Most organic pores are cylindrical or spherical micropores and mesopores (less than 50 nm in diameter). The pore size of inorganic pores is obviously larger, and the connectivity is better, mostly irregular cylindrical or flat. There are many micro–nano cracks in brittle minerals. In addition, there are many pores and cracks in the mineral cementation. According to the LTNA test results, most of the shale pores are micropores and mesopores, which provide the vast majority of specific surface areas. In addition, the contribution of macropores to pore volume cannot be ignored.
- (2)
- The adsorption of methane is controlled by dispersion force between pore surface molecules and methane molecules. Dispersion force is negatively correlated with distance. The binding effect of the outer methane is weakened, and free gas is gradually separated from the hole wall. Adsorbed gas volume depends on the pore surface area and gas pressure, and the free gas volume depends on the pore volume and gas pressure. Micropores and mesopores with complex structures and large specific surface areas can provide more adsorption sites for methane, which are the major enrichment spaces of adsorbed gas. Macropores with large pore volumes are the ideal space for free gas.
- (3)
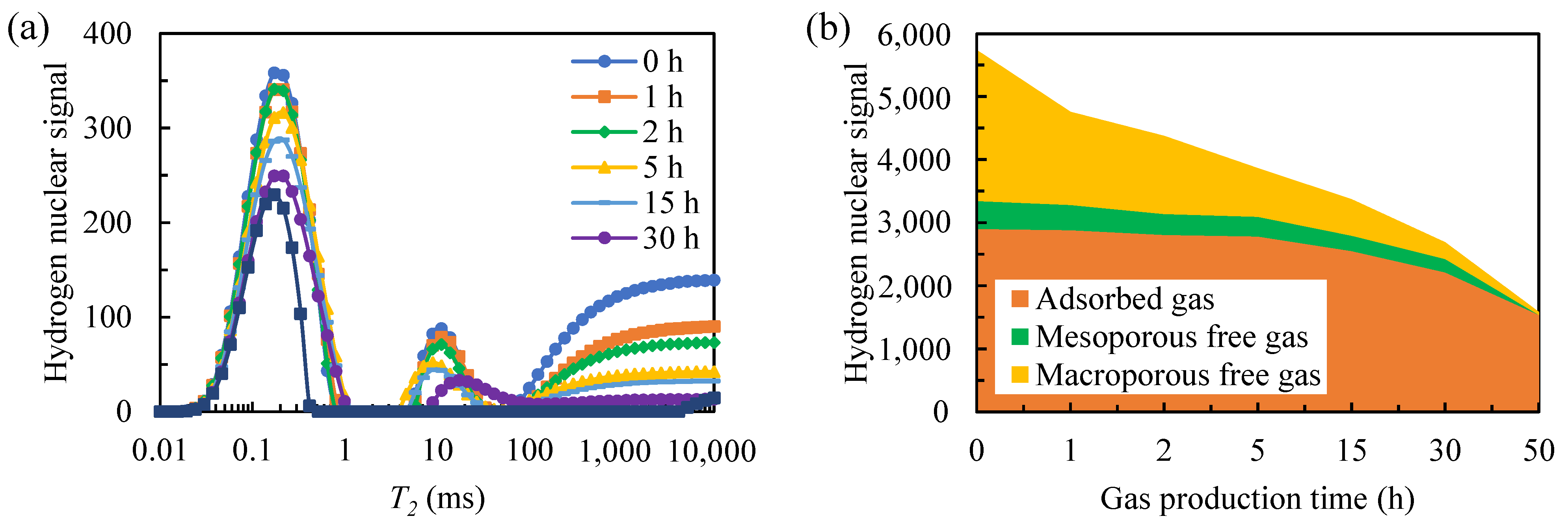
- Under constant temperature and pressure conditions, the adsorption–desorption of methane is in a dynamic equilibrium state. The free gas first enters the low-pressure zone from the high-pressure zone under the action of the pressure difference inside and outside the pore. When the pore pressure is lower than the critical desorption pressure, the adsorption–desorption equilibrium of methane is broken, and the adsorbed gas begins to desorb. Therefore, a large amount of free gas is produced in the early stage of shale gas reservoir development. In the later period, adsorption gas replaces free gas to become the main gas. This makes shale gas wells generally have the characteristics of early high yield, fast decay rate and long production cycle. It should be noted that the desorption process of adsorbed gas is slow and persistent, and improving the recovery rate of shale gas adsorbed gas is the key to future shale gas development research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chandra, D.; Vishal, V. A Critical Review on Pore to Continuum Scale Imaging Techniques for Enhanced Shale Gas Recovery. Earth Sci. Rev. 2021, 217, 103638. [Google Scholar] [CrossRef]
- Dasani, D.; Wang, Y.; Tsotsis, T.T.; Jessen, K. Laboratory-Scale Investigation of Sorption Kinetics of Methane/Ethane Mixtures in Shale. Ind. Eng. Chem. Res. 2017, 56, 9953–9963. [Google Scholar] [CrossRef]
- Dang, W.; Zhang, J.C.; Tang, X.; Wei, X.L.; Li, Z.M.; Wang, C.H.; Chen, Q.; Liu, C. Investigation of Gas Content of Organic-Rich Shale: A Case Study from Lower Permian Shale in Southern North China Basin, Central China. Geosci. Front. 2018, 9, 559–575. [Google Scholar] [CrossRef]
- Colosimo, F.; Thomas, R.; Lloyd, J.R.; Taylor, K.G.; Boothman, C.; Smith, A.D.; Lord, R.; Kalin, R.M. Biogenic Methane in Shale Gas and Coal Bed Methane: A Review of Current Knowledge and Gaps. Int. J. Coal Geol. 2016, 165, 106–120. [Google Scholar] [CrossRef]
- Javadpour, F.; Fisher, D.; Unsworth, M. Nanoscale Gas Flow in Shale Gas Sediments. J. Can. Pet. Technol. 2007, 46, 55–61. [Google Scholar] [CrossRef]
- Yin, Y.; Qu, Z.G.; Zhang, J.F. An Analytical Model for Shale Gas Transport in Kerogen Nanopores Coupled with Real Gas Effect and Surface Diffusion. Fuel 2017, 210, 569–577. [Google Scholar] [CrossRef]
- Guo, C.; Xu, J.; Wu, K.; Wei, M.; Liu, S. Study on Gas Flow through Nano Pores of Shale Gas Reservoirs. Fuel 2015, 143, 107–117. [Google Scholar] [CrossRef]
- Zhang, R.H.; Zhang, L.H.; Wang, R.H.; Zhao, Y.L.; Zhang, D. liang Research on Transient Flow Theory of a Multiple Fractured Horizontal Well in a Composite Shale Gas Reservoir Based on the Finite-Element Method. J. Nat. Gas Sci. Eng. 2016, 33, 587–598. [Google Scholar] [CrossRef]
- Kaiser, M.J. Profitability Assessment of Haynesville Shale Gas Wells. Energy 2012, 38, 315–330. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. Shale Gas Potential of the Lower Jurassic Gordondale Member, Northeastern British Columbia, Canada. Bull. Can. Pet. Geol. 2007, 55, 51–75. [Google Scholar] [CrossRef]
- Chalmers, G.R.L.; Bustin, R.M. Lower Cretaceous Gas Shales in Northeastern British Columbia, Part II: Evaluation of Regional Potential Gas Resources. Bull. Can. Pet. Geol. 2008, 56, 22–61. [Google Scholar] [CrossRef]
- Liu, S.; Sang, S.; Wang, G.; Ma, J.; Wang, X.; Wang, W.; Du, Y.; Wang, T. FIB-SEM and X-Ray CT Characterization of Interconnected Pores in High-Rank Coal Formed from Regional Metamorphism. J. Pet. Sci. Eng. 2017, 148, 21–31. [Google Scholar] [CrossRef]
- Raut, U.; Famá, M.; Teolis, B.D.; Baragiola, R.A. Characterization of Porosity in Vapor-Deposited Amorphous Solid Water from Methane Adsorption. J. Chem. Phys. 2007, 127, 204713. [Google Scholar] [CrossRef] [PubMed]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, Genesis, and Distribution of Nanometer-Scale Pores in Siliceous Mudstones of the Mississippian Barnett Shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Martini, A.M.; Walter, L.M.; Ku, T.C.W.; Budai, J.M.; McIntosh, J.C.; Schoell, M. Microbial Production and Modification of Gases in Sedimentary Basins: A Geochemical Case Study from a Devonian Shale Gas Play, Michigan Basin. Am. Assoc. Pet. Geol. Bull. 2003, 87, 1355–1375. [Google Scholar] [CrossRef]
- Montgomery, S.L.; Jarvie, D.M.; Bowker, K.A.; Pollastro, R.M. Mississippian Barnett Shale, Fort Worth Basin, North-Central Texas: Gas-Shale Play with Multi–Trillion Cubic Foot Potential. Am. Assoc. Pet. Geol. Bull. 2005, 89, 155–175. [Google Scholar] [CrossRef]
- Bowker, K.A. Barnett Shale Gas Production, Fort Worth Basin: Issues and Discussion. Am. Assoc. Pet. Geol. Bull. 2007, 91, 523–533. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Alcañiz-Monge, J.; de La Casa-Lillo, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Advances in the Study of Methane Storage in Porous Carbonaceous Materials. Fuel 2002, 81, 1777–1803. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Chen, S.; Li, W. Characteristics of the Nanoscale Pore Structure in Northwestern Hunan Shale Gas Reservoirs Using Field Emission Scanning Electron Microscopy, High-Pressure Mercury Intrusion, and Gas Adsorption. Energy Fuels 2014, 28, 945–955. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Sun, Z.; Lu, Z.; Du, Z.; Liang, H.; Lu, D. A New Method for Analysis of Dual Pore Size Distributions in Shale Using Nitrogen Adsorption Measurements. Fuel 2017, 210, 446–454. [Google Scholar] [CrossRef]
- Moridis, G.J.; Blasingame, T.A.; Freeman, C.M. Analysis of Mechanisms of Flow in Fractured Tight-Gas and Shale-Gas Reservoirs. SPE Lat. Am. Caribb. Pet. Eng. Conf. Proc. 2010, 2, 1310–1331. [Google Scholar] [CrossRef]
- Lin, D.; Wang, J.; Yuan, B.; Shen, Y. Review on Gas Flow and Recovery in Unconventional Porous Rocks. Adv. Geo-Energy Res. 2017, 1, 39–53. [Google Scholar] [CrossRef]
- Roy, S.; Raju, R.; Chuang, H.F.; Cruden, B.A.; Meyyappan, M. Modeling Gas Flow through Microchannels and Nanopores. J. Appl. Phys. 2003, 93, 4870. [Google Scholar] [CrossRef]
- Freeman, C.M.; Moridis, G.J.; Blasingame, T.A. A Numerical Study of Microscale Flow Behavior in Tight Gas and Shale Gas Reservoir Systems. Transp. Porous Media 2011, 90, 253–268. [Google Scholar] [CrossRef]
- Sheng, M.; Li, G.; Huang, Z.; Tian, S. Shale Gas Transient Flow Model with Effects of Surface Diffusion. Acta Pet. Sin. 2014, 35, 347. [Google Scholar] [CrossRef]
- Wu, K.; Li, X.; Wang, C.; Yu, W.; Chen, Z. A Model for Surface Diffusion of Adsorbed Gas in Nanopores of Shale Gas Reservoirs. Proc. Annu. Offshore Technol. Conf. 2015, 1, 199–221. [Google Scholar] [CrossRef]
- Mitchell, J.; Fordham, E.J. Contributed Review: Nuclear Magnetic Resonance Core Analysis at 0.3 T. Rev. Sci. Instrum. 2014, 85, 111502. [Google Scholar] [CrossRef]
- Mu, Y.; Hu, Z.; Chang, J.; Duan, X.; Li, Y.; Li, Y.; Niu, W. Effect of Water Occurrence on Shale Seepage Ability. J. Pet. Sci. Eng. 2021, 204, 108725. [Google Scholar] [CrossRef]
- Mu, Y.; Hu, Z.; Shen, R.; Chang, J.; Duan, X.; Li, Y.; Guo, Q. Water Occurrence Characteristics of Gas Shale Based on 2D NMR Technology. Energy Fuels 2022, 36, 910–921. [Google Scholar] [CrossRef]
- Washburn, K.E.; Birdwell, J.E. Updated Methodology for Nuclear Magnetic Resonance Characterization of Shales. J. Magn. Reson. 2013, 233, 17–28. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, S.; Li, J.; Chang, X. 1D and 2D Nuclear Magnetic Resonance (NMR) Relaxation Behaviors of Protons in Clay, Kerogen and Oil-Bearing Shale Rocks. Mar. Pet. Geol. 2020, 114, 104210. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.; Cai, Y.; Yao, Y.; Pan, Z.; Zhou, Y. Application of Nuclear Magnetic Resonance (NMR) in Coalbed Methane and Shale Reservoirs: A Review. Int. J. Coal Geol. 2020, 218, 103261. [Google Scholar] [CrossRef]
- Fleury, M.; Romero-Sarmiento, M. Characterization of Shales Using T1–T2 NMR Maps. J. Pet. Sci. Eng. 2016, 137, 55–62. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, Y.; Zhuang, X.; Li, G.; Wang, X. Poroperm Characteristics of High-Rank Coals from Southern Qinshui Basin by Mercury Intrusion, SEM-EDS, Nuclear Magnetic Resonance and Relative Permeability Analysis. J. Nat. Gas Sci. Eng. 2018, 51, 116–128. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, Y.; Liu, D.; Cai, Y.; Liu, Y. Characterizations of Full-Scale Pore Size Distribution, Porosity and Permeability of Coals: A Novel Methodology by Nuclear Magnetic Resonance and Fractal Analysis Theory. Int. J. Coal Geol. 2018, 196, 148–158. [Google Scholar] [CrossRef]
- Zou, C.; Dong, D.; Wang, S.; Li, J.; Li, X.; Wang, Y.; Li, D.; Cheng, K. Geological Characteristics and Resource Potential of Shale Gas in China. Pet. Explor. Dev. 2010, 37, 641–653. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Yang, T.; Wang, S.; Huang, J. Geological Difference and Its Significance of Marine Shale Gases in South China. Pet. Explor. Dev. 2016, 43, 547–559. [Google Scholar] [CrossRef]
- Ji, W.; Song, Y.; Rui, Z.; Meng, M.; Huang, H. Pore Characterization of Isolated Organic Matter from High Matured Gas Shale Reservoir. Int. J. Coal Geol. 2017, 174, 31–40. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Fu, C.; Zhang, H.; Zhu, Y.; Zuo, Z. Micro and Nano-Size Pores of Clay Minerals in Shale Reservoirs: Implication for the Accumulation of Shale Gas. Sediment. Geol. 2016, 342, 180–190. [Google Scholar] [CrossRef]
- Wilson, M.J.; Shaldybin, M.V.; Wilson, L. Clay Mineralogy and Unconventional Hydrocarbon Shale Reservoirs in the USA. I. Occurrence and Interpretation of Mixed-Layer R3 Ordered Illite/Smectite. Earth Sci. Rev. 2016, 158, 31–50. [Google Scholar] [CrossRef]
- Feng, D.; Li, X.; Wang, X.; Li, J.; Sun, F.; Sun, Z.; Zhang, T.; Li, P.; Chen, Y.; Zhang, X. Water Adsorption and Its Impact on the Pore Structure Characteristics of Shale Clay. Appl. Clay Sci. 2018, 155, 126–138. [Google Scholar] [CrossRef]
- Davudov, D.; Moghanloo, R.G. Impact of Pore Compressibility and Connectivity Loss on Shale Permeability. Int. J. Coal Geol. 2018, 187, 98–113. [Google Scholar] [CrossRef]
- Zou, C.; Dong, D.; Wang, Y.; Li, X.; Huang, J.; Wang, S.; Guan, Q.; Zhang, C.; Wang, H.; Liu, H.; et al. Shale Gas in China: Characteristics, Challenges and Prospects (I). Pet. Explor. Dev. 2015, 42, 753–767. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Yin, C.; Zhang, Y.; Lin, C. Propagation and Characterization of the Micro Cracks Induced by Hydraulic Fracturing in Shale. Energy 2020, 191, 116449. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, J.; Liang, L. Investigation of Pore Structure and Fractal Characteristics of Organic-Rich Yanchang Formation Shale in Central China by Nitrogen Adsorption/Desorption Analysis. J. Nat. Gas Sci. Eng. 2015, 22, 62–72. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Liu, H. Fractal Characteristics of Shales from a Shale Gas Reservoir in the Sichuan Basin, China. Fuel 2014, 115, 378–384. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, C.; Leung, J.Y.; Wojtanowicz, A.K.; Zhang, D. Multiscale Characterization of Shale Pore-Fracture System: Geological Controls on Gas Transport and Pore Size Classification in Shale Reservoirs. J. Pet. Sci. Eng. 2021, 202, 108442. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; He, D.; Sun, P.; Zou, X. Full-Scale Nanopore System and Fractal Characteristics of Clay-Rich Lacustrine Shale Combining FE-SEM, Nano-CT, Gas Adsorption and Mercury Intrusion Porosimetry. Appl. Clay Sci. 2020, 196, 105758. [Google Scholar] [CrossRef]
- Gou, Q.; Xu, S.; Hao, F.; Yang, F.; Zhang, B.; Shu, Z.; Zhang, A.; Wang, Y.; Lu, Y.; Cheng, X.; et al. Full-Scale Pores and Micro-Fractures Characterization Using FE-SEM, Gas Adsorption, Nano-CT and Micro-CT: A Case Study of the Silurian Longmaxi Formation Shale in the Fuling Area, Sichuan Basin, China. Fuel 2019, 253, 167–179. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Y.; Liu, D.; Zheng, S.; Sun, G.; Chang, Y. Shale Pore Size Classification: An NMR Fluid Typing Method. Mar. Pet. Geol. 2018, 96, 591–601. [Google Scholar] [CrossRef]
- Sigal, R.F. Pore-Size Distributions for Organic-Shale-Reservoir Rocks From Nuclear-Magnetic-Resonance Spectra Combined With Adsorption Measurements. SPE J. 2015, 20, 824–830. [Google Scholar] [CrossRef]
- Hun, L.; Bing, Y.; Xixiang, S.; Xinyi, S.; Lifei, D. Fracturing Fluid Retention in Shale Gas Reservoir from the Perspective of Pore Size Based on Nuclear Magnetic Resonance. J. Hydrol. 2021, 601, 126590. [Google Scholar] [CrossRef]
- Tan, M.; Mao, K.; Song, X.; Yang, X.; Xu, J. NMR Petrophysical Interpretation Method of Gas Shale Based on Core NMR Experiment. J. Pet. Sci. Eng. 2015, 136, 100–111. [Google Scholar] [CrossRef]
- Sondergeld, C.; Tinni, A.; Rai, C.; Besov, A. NMR Considerations in Shale Evaluation. In Proceedings of the SPWLA 57th Annual Logging Symposium, Reykjavik, Iceland, 25–29 June 2016. [Google Scholar]
- Zou, C.; Dong, D.; Wang, Y.; Li, X.; Huang, J.; Wang, S.; Guan, Q.; Zhang, C.; Wang, H.; Liu, H.; et al. Shale Gas in China: Characteristics, Challenges and Prospects (II). Pet. Explor. Dev. 2016, 43, 182–196. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Z.; Liu, X.; Duan, X.; Gao, S.; Wang, W.; Chang, J. Pressure-Dependent Equilibrium Molecular Simulation of Shale Gas and Its Distribution and Motion Characteristics in Organic-Rich Nano-Slit. Fuel 2019, 237, 1040–1049. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Z.; Liu, Q.; Jiang, S.; Liu, K.; Tan, J.; Gao, F. Mechanism of Shale Gas Occurrence: Insights from Comparative Study on Pore Structures of Marine and Lacustrine Shales. Mar. Pet. Geol. 2019, 104, 200–216. [Google Scholar] [CrossRef]
- Chen, L.; Zuo, L.; Jiang, Z.; Jiang, S.; Liu, K.; Tan, J.; Zhang, L. Mechanisms of Shale Gas Adsorption: Evidence from Thermodynamics and Kinetics Study of Methane Adsorption on Shale. Chem. Eng. J. 2019, 361, 559–570. [Google Scholar] [CrossRef]
- Duan, X.; Hu, Z.; Gao, S.; Shen, R.; Liu, H.; Chang, J.; Wang, L. Shale High Pressure Isothermal Adsorption Curve and the Production Dynamic Experiments of Gas Well. Pet. Explor. Dev. 2018, 45, 127–135. [Google Scholar] [CrossRef]






| Length (mm) | Diameter (mm) | Mass (g) | Posity (Helium) (%) | Pulse-Decay Permeability (mD) |
|---|---|---|---|---|
| 57.79 | 25.07 | 73.14 | 3.10 | 3.54 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Mu, Y.; Guo, Q.; Niu, W.; Duan, X.; Chang, J.; Wu, Z. Occurrence and Migration Mechanisms of Methane in Marine Shale Reservoirs. Energies 2022, 15, 9043. https://doi.org/10.3390/en15239043
Hu Z, Mu Y, Guo Q, Niu W, Duan X, Chang J, Wu Z. Occurrence and Migration Mechanisms of Methane in Marine Shale Reservoirs. Energies. 2022; 15(23):9043. https://doi.org/10.3390/en15239043
Chicago/Turabian StyleHu, Zhiming, Ying Mu, Qiulei Guo, Wente Niu, Xianggang Duan, Jin Chang, and Zhenkai Wu. 2022. "Occurrence and Migration Mechanisms of Methane in Marine Shale Reservoirs" Energies 15, no. 23: 9043. https://doi.org/10.3390/en15239043
APA StyleHu, Z., Mu, Y., Guo, Q., Niu, W., Duan, X., Chang, J., & Wu, Z. (2022). Occurrence and Migration Mechanisms of Methane in Marine Shale Reservoirs. Energies, 15(23), 9043. https://doi.org/10.3390/en15239043







