Enhanced Biodiesel Synthesis via a Homogenizer-Assisted Two-Stage Conversion Process Using Waste Edible Oil as Feedstock
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Factors Affecting the Esterification Reaction in the First Stage
3.1.1. Effect of Rotational Speed on the Acid Value
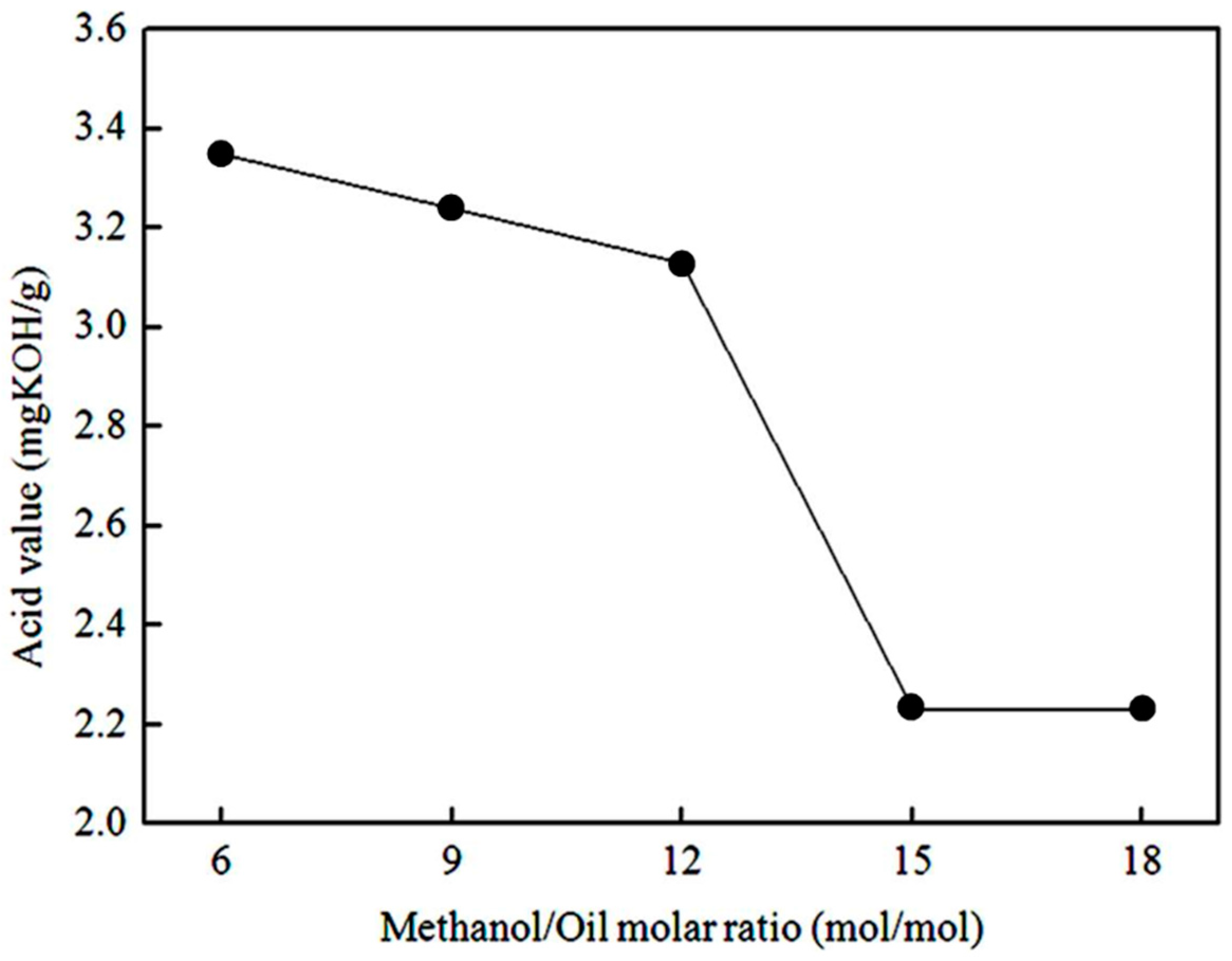
3.1.2. Effect of Methanol/Oil Molar Ratio on the Acid Value
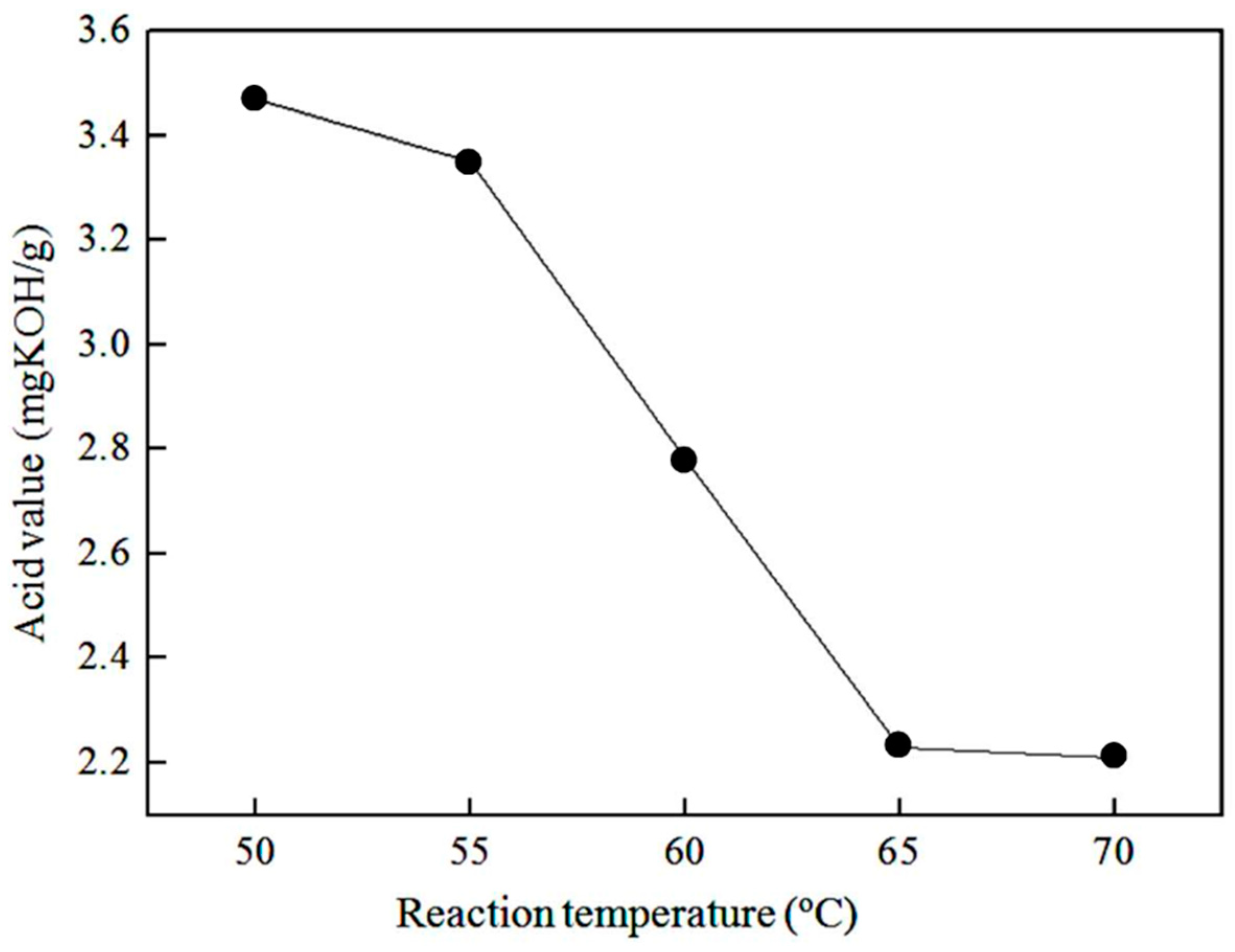
3.1.3. Effect of Reaction Temperature on the Acid Value
3.1.4. Effect of Catalyst Amount on the Acid Value
3.1.5. Effect of Reaction Time on the Acid Value
3.2. Factors Affecting the Transesterification Reaction in the Second Stage
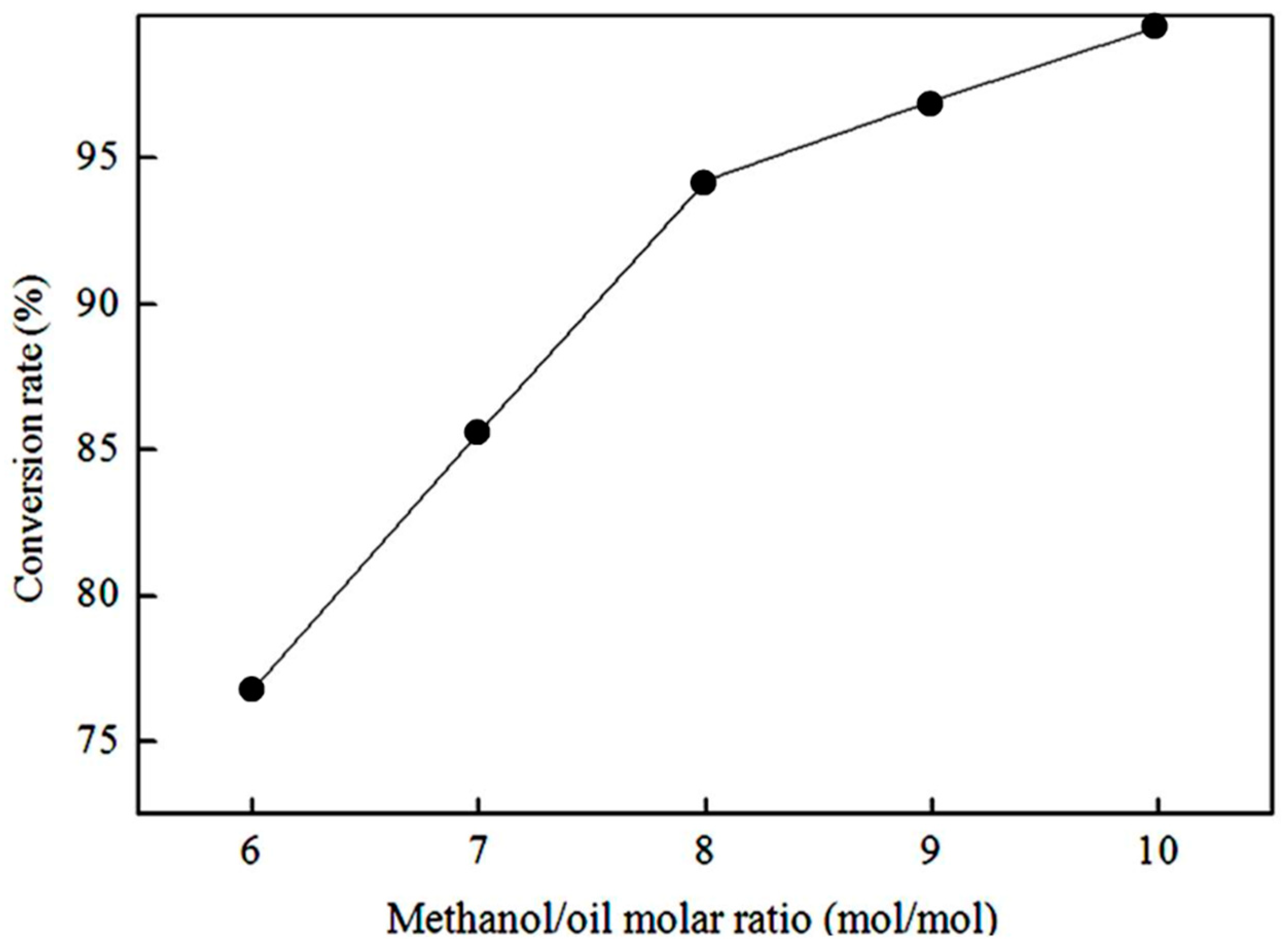
3.2.1. Effect of methanol/oil molar ratio on the biodiesel conversion
3.2.2. Effect of Catalyst Amount on the Biodiesel Conversion
3.2.3. Effect of Reaction Temperature on the Biodiesel Conversion
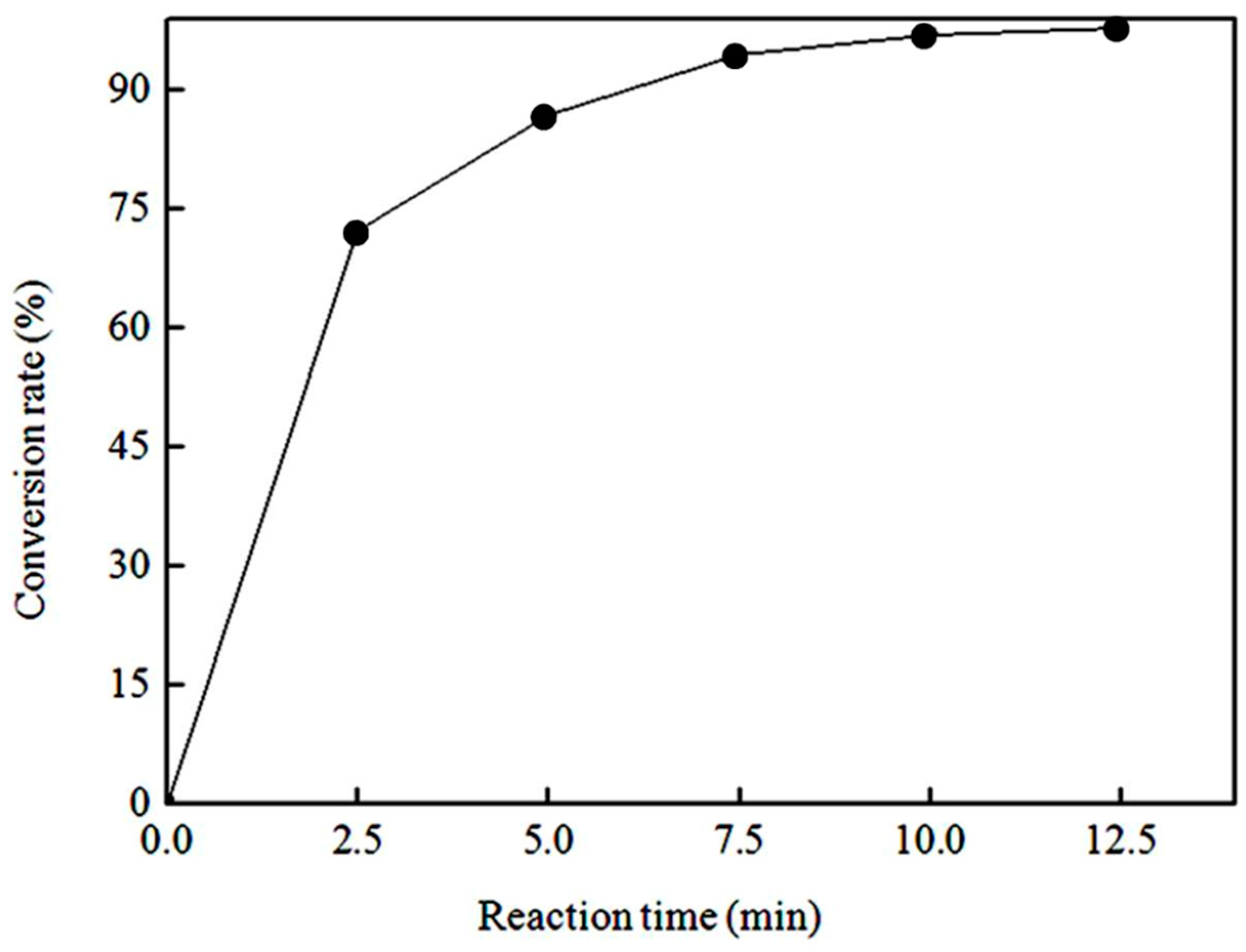
3.2.4. Effect of Reaction Time on the Biodiesel Conversion
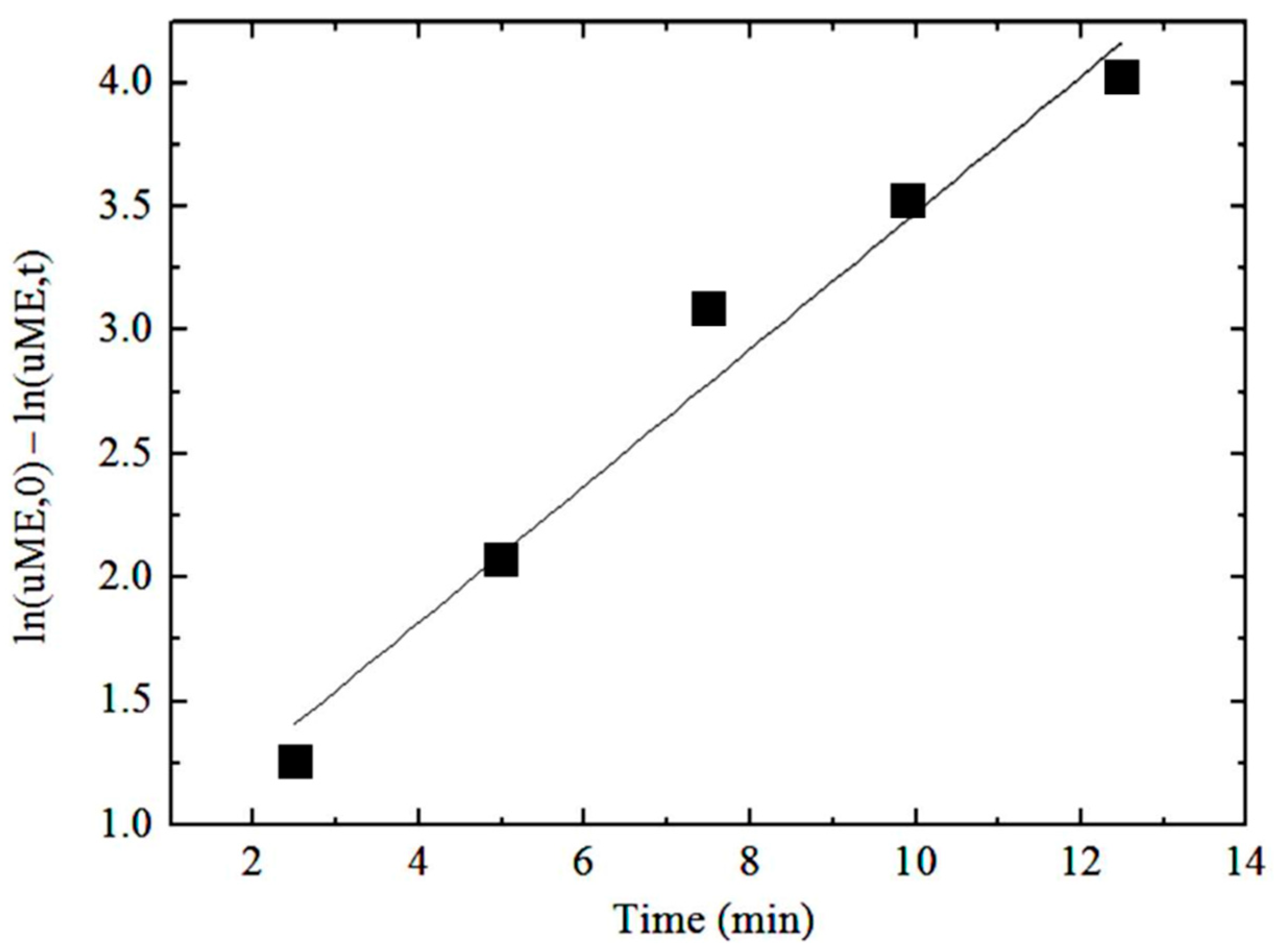
3.2.5. Reaction Rate Constant k
4. Conclusions
- (1)
- When waste edible oil was used to produce biodiesel by a two-stage transesterification process assisted by a homogenizer, in the first step (acid-catalyzed stage) the acid value was effectively decreased lower than 2 mg KOH/g within only 10 min with the following suggested operating conditions: homogenizer speed 8000 rpm, methanol/oil molar ratio 15:1, added catalyst amount 4 wt% (sulfuric acid) and reaction temperature 65 °C.
- (2)
- In the second step (the base-catalyzed stage), the conversion rate of biodiesel reached 97% after 10 min and met the standard value (at least 96.5%) of EN 14214 with the following reaction conditions: homogenizer speed 8000 rpm, alcohol/oil molar ratio 9:1 (mol/mol), sodium hydroxide addition level 1 wt% and reaction temperature at 65 °C.
- (3)
- Additionally, by increasing the reaction temperature from 60 to 65 °C, the reaction rate constant changed significantly from 0.2599 to 0.3515 min−1.
- (4)
- Synthesizing biodiesel from waste edible oil took at least 4 h via the conventional acid-catalyzed approach [47], while the whole process of the improved method in this study only took 20 min in total, which is a very considerable time reduction. Note that the optimal reaction time (20 min) herein is also much shorter compared to the 140 min reported by Hsiao et al. [16], who also utilized the two-stage catalytic process, but instead used a conventional mechanical stirring approach.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez, D.E.; Goodwin, J.G.; Bruce, D.A. Transesterification of triacetin with methanol on solid acid and base catalysts. Appl. Catal. A Gen. 2005, 295, 97–105. [Google Scholar] [CrossRef]
- Vicente, G.; Martınez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Rout, A.K.; Samanta, S. A review on the effect of engine performance and emission characteristics of CI engine using diesel-biodiesel-additives fuel blend. Mater. Today Proc. 2022, 51, 2224–2232. [Google Scholar] [CrossRef]
- Gaur, A.; Dwivedi, G.; Baredar, P.; Jain, S. Influence of blending additives in biodiesel on physiochemical properties, engine performance, and emission characteristics. Fuel 2022, 321, 124072. [Google Scholar] [CrossRef]
- Hanif, M.; Bhatti, H.N.; Hanif, M.A.; Rashid, U.; Hanif, A.; Moser, B.R.; Alsalme, A. A novel heterogeneous superoxide support-coated catalyst for production of biodiesel from roasted and unroasted sinapis arvensis seed oil. Catalysts 2021, 11, 1421. [Google Scholar] [CrossRef]
- Mustayen, A.G.M.B.; Rasul, M.G.; Wang, X.; Bhuiya, M.M.K.; Negnevitsky, M.; Hamilton, J. Theoretical and experimental analysis of engine performance and emissions fueled with Jojoba Biodiesel. Energies 2022, 15, 6282. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of physicochemical properties, production process, performance and emissions characteristics of 2nd generation biodiesel feedstock: Jatropha curcas. Fuel 2021, 285, 119110. [Google Scholar] [CrossRef]
- Ghadge, S.V.; Raheman, H. Process optimization for biodiesel production from mahua (Madhuca indica) oil using response surface methodology. Bioresour. Technol. 2006, 97, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ou, S.; Liu, P.; Xue, F.; Tang, S. Comparison of two different processes to synthesize biodiesel by waste cooking oil. J. Mol. Catal. A Chem. 2006, 252, 107–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, M.A.; McLean, D.D.L.; Kates, M. Biodiesel production from waste cooking oil: 1. process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Bournay, L.; Casanave, D.; Delfort, B.; Hillion, G.; Chodorge, J.A. New heterogeneous process for biodiesel production: A way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal. Today 2005, 106, 190–192. [Google Scholar] [CrossRef]
- Singh, A.K.; Fernando, S.D.; Hernandez, R. Base-catalyzed fast transesterification of soybean oil using ultrasonication. Energy Fuels 2007, 21, 1161–1164. [Google Scholar] [CrossRef]
- dos Reis, S.C.M.; Lachter, E.R.; Nascimento, R.S.; Rodrigues, J.A.; Reid, M.G. Transesterification of Brazilian vegetable oils with methanol over ion-exchange resins. J. Am. Oil Chem. Soc. 2005, 82, 661–665. [Google Scholar] [CrossRef]
- Nayab, R.; Imran, M.; Ramzan, M.; Tariq, M.; Taj, M.B.; Akhtar, M.N.; Iqbal, H.M. Sustainable biodiesel production via catalytic and non-catalytic transesterification of feedstock materials—A review. Fuel 2022, 328, 125254. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Lin, W.T.; Chiu, W.C.; Hou, S.S. Two-Stage Biodiesel synthesis from used cooking oil with a high acid value via an ultrasound-assisted method. Energies 2021, 14, 3703. [Google Scholar] [CrossRef]
- He, C.; Mei, Y.; Zhang, Y.; Liu, L.; Li, P.; Zhang, Z.; Jing, Y.; Li, G.; Jiao, Y. Enhanced biodiesel production from diseased swine fat by ultrasound-assisted two-step catalyzed process. Bioresour. Technol. 2020, 304, 123017. [Google Scholar] [CrossRef]
- Sarve, A.N.; Varma, M.N.; Sonawane, S.S. Ultrasound assisted two-stage biodiesel synthesis from non-edible Schleichera triguga oil using heterogeneous catalyst: Kinetics and thermodynamic analysis. Ultrason. Sonochem. 2016, 29, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Hanh, H.D.; Dong, N.T.; Okitsu, K.; Nishimura, R.; Maeda, Y. Biodiesel production through transesterification of triolein with various alcohols in an ultrasonic field. Renew. Energy 2009, 34, 766–768. [Google Scholar] [CrossRef]
- Hanh, H.D.; Dong, N.T.; Starvarache, C.; Okitsu, K.; Maeda, Y.; Nishimura, R. Methanolysis of triolein by low frequency ultrasonic irradiation. Energy Convers. Manag. 2008, 49, 276–280. [Google Scholar] [CrossRef]
- Barnard, T.M.; Leadbeater, N.E.; Boucher, M.B.; Stencel, L.M.; Wilhite, B.A. Continuous-flow preparation of biodiesel using microwave heating. Energy Fuels 2007, 21, 1777–1781. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Production of FAME by palm oil transesterification via supercritical methanol technology. Biomass Bioenergy 2009, 33, 1096–1099. [Google Scholar] [CrossRef]
- Yatish, K.V.; Omkaresh, B.R.; Kattimani, V.R.; Lalithamba, H.S.; Sakar, M.; Balakrishna, R.G. Solar energy-assisted reactor for the sustainable biodiesel production from Butea monosperma oil: Optimization, kinetic, thermodynamic and assessment studies. Energy 2023, 263, 125768. [Google Scholar] [CrossRef]
- Koc, A.B. Ultrasonic monitoring of glycerol settling during transesterification of soybean oil. Bioresour. Technol. 2009, 100, 19–24. [Google Scholar] [PubMed]
- Hsiao, M.C.; Lin, C.C.; Chang, Y.H.; Chen, L.C. Ultrasonic mixing and closed microwave irradiation-assisted transesterification of soybean oil. Fuel 2010, 89, 3618–3622. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Lin, C.C.; Chang, Y.H. Microwave irradiation-assisted transesterification of soybean oil to biodiesel catalyzed by nanopowder calcium oxide. Fuel 2011, 90, 1963–1967. [Google Scholar] [CrossRef]
- Haghighi, S.F.M.; Parvasi, P.; Jokar, S.M.; Basile, A. Investigating the effects of ultrasonic frequency and membrane technology on biodiesel production from chicken waste. Energies 2021, 14, 2133. [Google Scholar] [CrossRef]
- Sundaramahalingam, M.A.; Karthikumar, S.; Kumar, R.S.; Samuel, K.J.; Shajahan, S.; Sivasubramanian, V.; Sivashanmugam, P.; Varalakshmi, P.; Syed, A.; Marraiki, N.; et al. An intensified approach for transesterification of biodiesel from Annona squamosa seed oil using ultrasound-assisted homogeneous catalysis reaction and its process optimization. Fuel 2021, 291, 120195. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Kuo, J.Y.; Hsieh, S.A.; Hsieh, P.H.; Hou, S.S. Optimized conversion of waste cooking oil to biodiesel using modified calcium oxide as catalyst via a microwave heating system. Fuel 2020, 266, 117114. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Liao, P.H.; Lan, N.V.; Hou, S.S. Enhancement of biodiesel production from high-acid-value waste cooking oil via a microwave reactor using a homogeneous alkaline catalyst. Energies 2021, 14, 437. [Google Scholar] [CrossRef]
- Amesho, K.T.; Lin, Y.C.; Chen, C.E.; Cheng, P.C.; Shangdiar, S. Kinetics studies of sustainable biodiesel synthesis from Jatropha curcas oil by exploiting bio-waste derived CaO-based heterogeneous catalyst via microwave heating system as a green chemistry technique. Fuel 2022, 323, 123876. [Google Scholar] [CrossRef]
- Gouda, S.P.; Ngaosuwan, K.; Assabumrungrat, S.; Selvaraj, M.; Halder, G.; Rokhum, S.L. Microwave assisted biodiesel production using sulfonic acid-functionalized metal-organic frameworks UiO-66 as a heterogeneous catalyst. Renew. Energy 2022, 197, 161–169. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Kuo, J.Y.; Hsieh, P.H.; Hou, S.S. Improving biodiesel conversions from blends of high-and low-acid-value waste cooking oils using sodium methoxide as a catalyst based on a high speed homogenizer. Energies 2018, 11, 2298. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Hou, S.S.; Kuo, J.Y.; Hsieh, P.H. Optimized conversion of waste cooking oil to biodiesel using calcium methoxide as catalyst under homogenizer system conditions. Energies 2018, 11, 2622. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Chang, L.W.; Hou, S.S. Study of solid calcium diglyceroxide for biodiesel production from waste cooking oil using a high speed homogenizer. Energies 2019, 12, 3205. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.H.; Ramavandi, B. Generation of biodiesel from edible waste oil using ZIF-67-KOH modified Luffa cylindrica biomass catalyst. Fuel 2022, 322, 124181. [Google Scholar] [CrossRef]
- Mohadesi, M.; Gouran, A.; Dehnavi, A.D. Biodiesel production using low cost material as high effective catalyst in a microreactor. Energy 2021, 219, 119671. [Google Scholar] [CrossRef]
- Aghel, B.; Gouran, A.; Nasirmanesh, F. Transesterification of waste cooking oil using clinoptilolite/industrial phosphoric waste as green and environmental catalysts. Energy 2022, 244, 123138. [Google Scholar] [CrossRef]
- Kiss, A.A.; Omota, F.; Dimian, A.C.; Rothenberg, G. The heterogeneous advantage: Biodiesel by catalytic reactive distillation. Top. Catal. 2006, 40, 141–150. [Google Scholar] [CrossRef]
- Lingfeng, C.; Guomin, X.; Bo, X.; Guangyuan, T. Transesterification of cottonseed oil to biodiesel by using heterogeneous solid basic catalysts. Energy Fuels 2007, 21, 3740–3743. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Heterogeneous esterification of oil with high amount of free fatty acids. Fuel 2007, 86, 906–910. [Google Scholar] [CrossRef]
- Xie, W.; Peng, H.; Chen, L. Transesterification of soybean oil catalyzed by potassium loaded on alumina as a solid-base catalyst. Appl. Catal. A Gen. 2006, 300, 67–74. [Google Scholar] [CrossRef]
- Di Serio, M.; Ledda, M.; Cozzolino, M.; Minutillo, G.; Tesser, R.; Santacesaria, E. Transesterification of soybean oil to biodiesel by using heterogeneous basic catalysts. Ind. Eng. Chem. Res. 2006, 45, 3009–3014. [Google Scholar] [CrossRef]
- Stavarache, C.; Vinatoru, M.; Nishimura, R.; Maeda, Y. Fatty acids methyl esters from vegetable oil by means of ultrasonic energy. Ultrason. Sonochem. 2005, 12, 367–372. [Google Scholar] [CrossRef]
- Stavarache, C.; Vinatoru, M.; Maeda, Y. Aspects of ultrasonically assisted transesterification of various vegetable oils with methanol. Ultrason. Sonochem. 2007, 14, 380–386. [Google Scholar] [CrossRef]
- Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar]
- Zheng, S.; Kates, M.; Dube, M.A.; McLean, D.D. Acid-catalyzed production of biodiesel from waste frying oil. Biomass Bioenergy 2006, 30, 267–272. [Google Scholar] [CrossRef]
- He, H.; Sun, S.; Wang, T.; Zhu, S. Transesterification kinetics of soybean oil for production of biodiesel in supercritical methanol. J. Am. Oil Chem. Soc. 2007, 84, 399–404. [Google Scholar] [CrossRef]



| Revolution (rpm) | Acid Value (mg KOH/g) |
|---|---|
| 3000 | 4.12 |
| 5000 | 3.59 |
| 7000 | 3.57 |
| 8000 | 3.46 |
| 9000 | 3.46 |
| Catalyst Amount (wt%) | Conversion (%) |
|---|---|
| 0.5 | 78.02 |
| 0.8 | 90.34 |
| 1.0 | 97.00 |
| 1.2 | 88.45 |
| 1.5 | 80.25 |
| Temperature (°C) | Rate Constants k (min−1) |
|---|---|
| 50 | 0.2116 |
| 55 | 0.2324 |
| 60 | 0.2599 |
| 65 | 0.3515 |
| 70 | 0.3437 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, M.-C.; Liao, P.-H.; Yang, K.-C.; Lan, N.V.; Hou, S.-S. Enhanced Biodiesel Synthesis via a Homogenizer-Assisted Two-Stage Conversion Process Using Waste Edible Oil as Feedstock. Energies 2022, 15, 9036. https://doi.org/10.3390/en15239036
Hsiao M-C, Liao P-H, Yang K-C, Lan NV, Hou S-S. Enhanced Biodiesel Synthesis via a Homogenizer-Assisted Two-Stage Conversion Process Using Waste Edible Oil as Feedstock. Energies. 2022; 15(23):9036. https://doi.org/10.3390/en15239036
Chicago/Turabian StyleHsiao, Ming-Chien, Peir-Horng Liao, Kuo-Chou Yang, Nguyen Vu Lan, and Shuhn-Shyurng Hou. 2022. "Enhanced Biodiesel Synthesis via a Homogenizer-Assisted Two-Stage Conversion Process Using Waste Edible Oil as Feedstock" Energies 15, no. 23: 9036. https://doi.org/10.3390/en15239036
APA StyleHsiao, M.-C., Liao, P.-H., Yang, K.-C., Lan, N. V., & Hou, S.-S. (2022). Enhanced Biodiesel Synthesis via a Homogenizer-Assisted Two-Stage Conversion Process Using Waste Edible Oil as Feedstock. Energies, 15(23), 9036. https://doi.org/10.3390/en15239036







