Prospect of Post-Combustion Carbon Capture Technology and Its Impact on the Circular Economy
Abstract
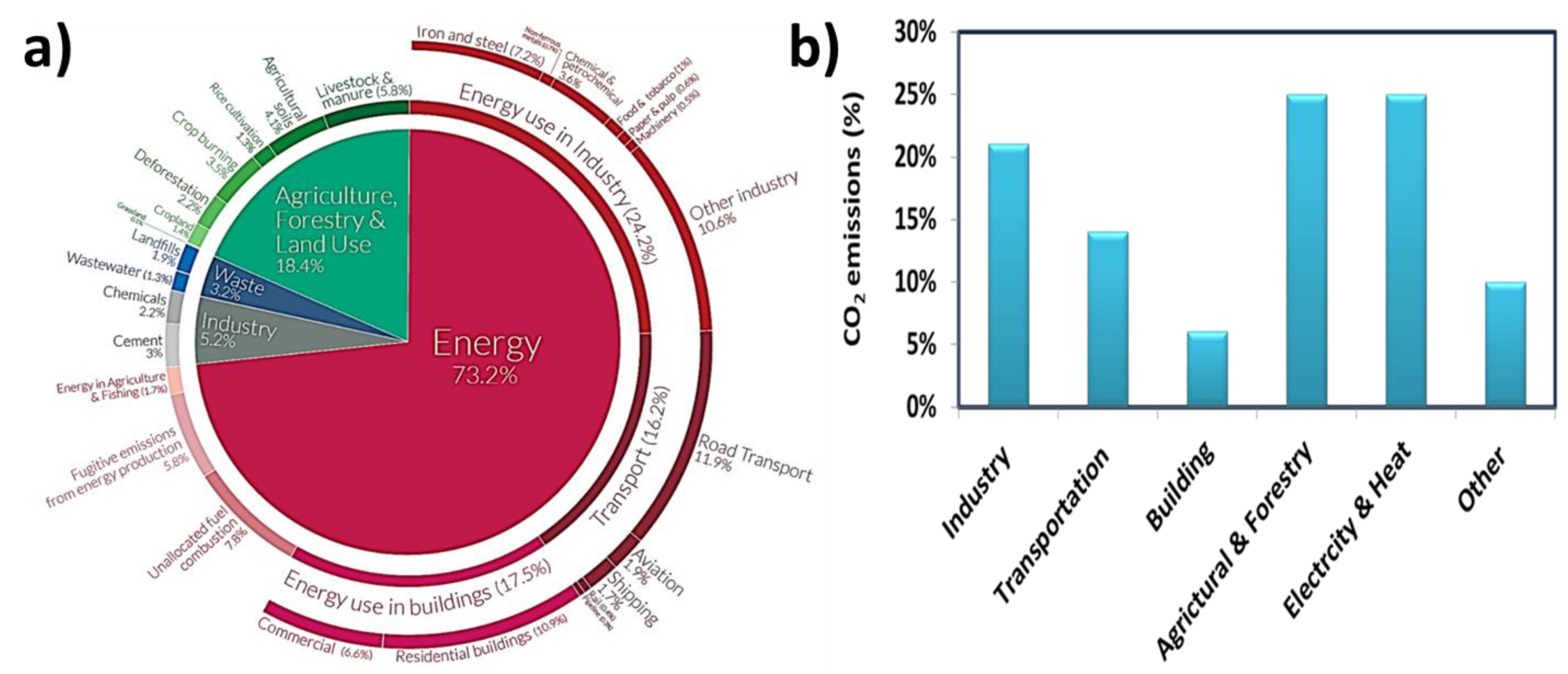
1. Introduction
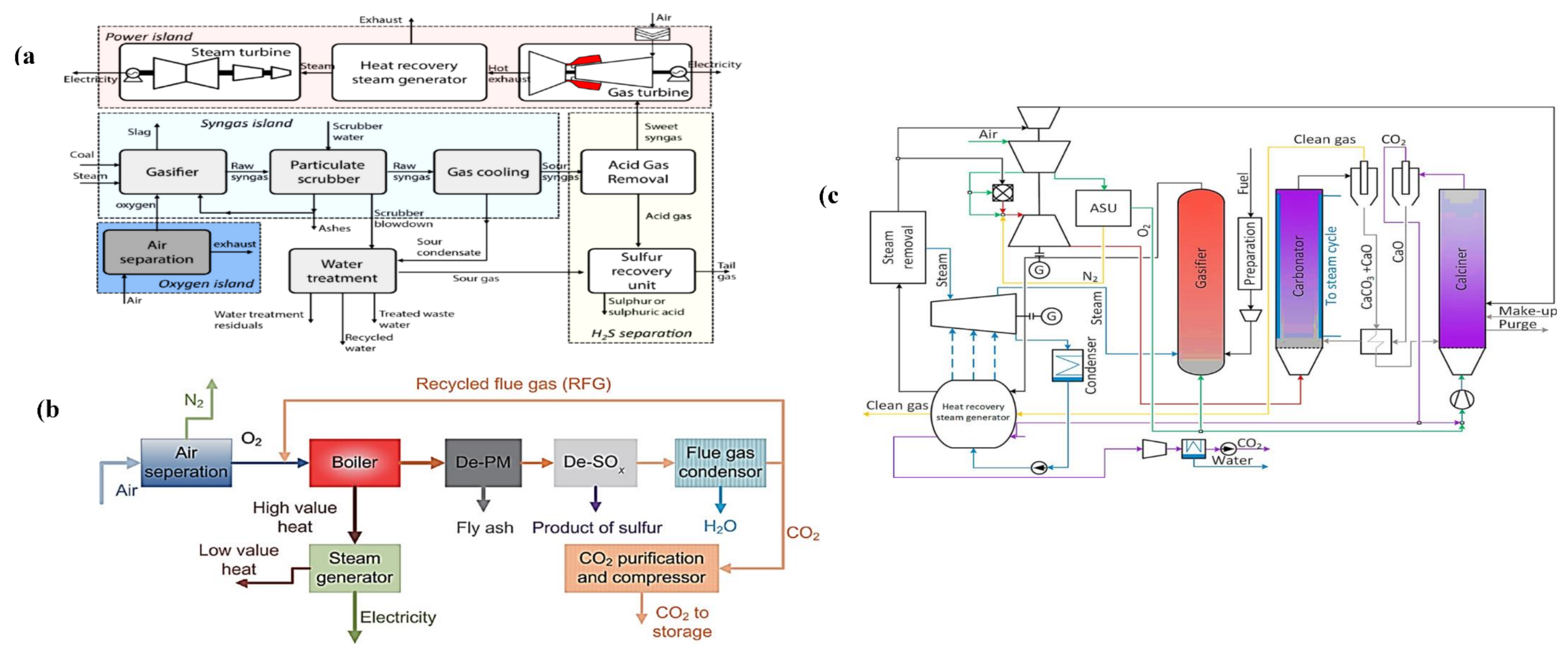
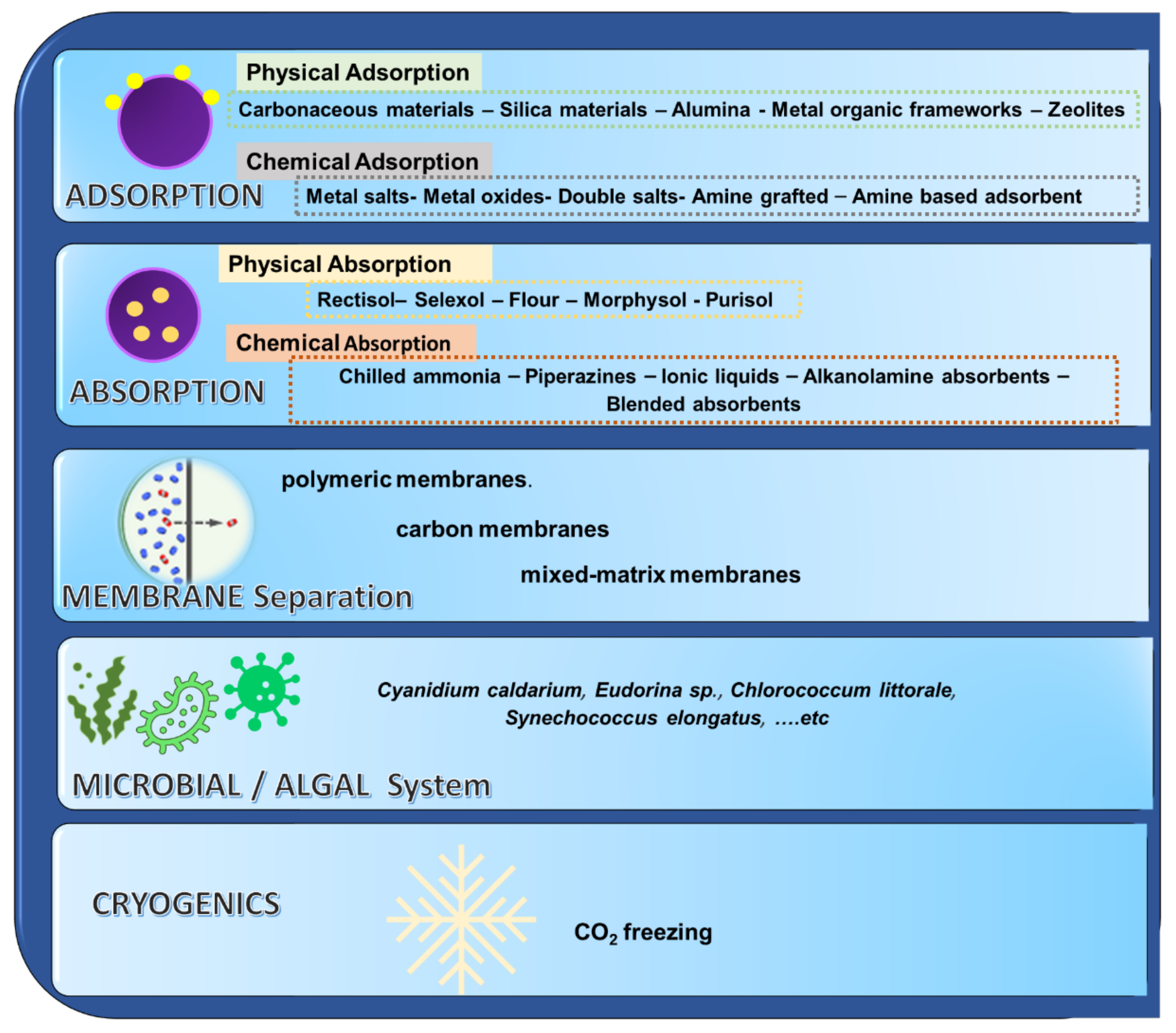
2. Existing Technologies for Capturing CO2
| Technologies | Merits | Demerits | Ref. |
|---|---|---|---|
| Pre combustion (PRC) |
|
| [52] |
| Oxy fuel combustion (OC) |
|
| [53,54] |
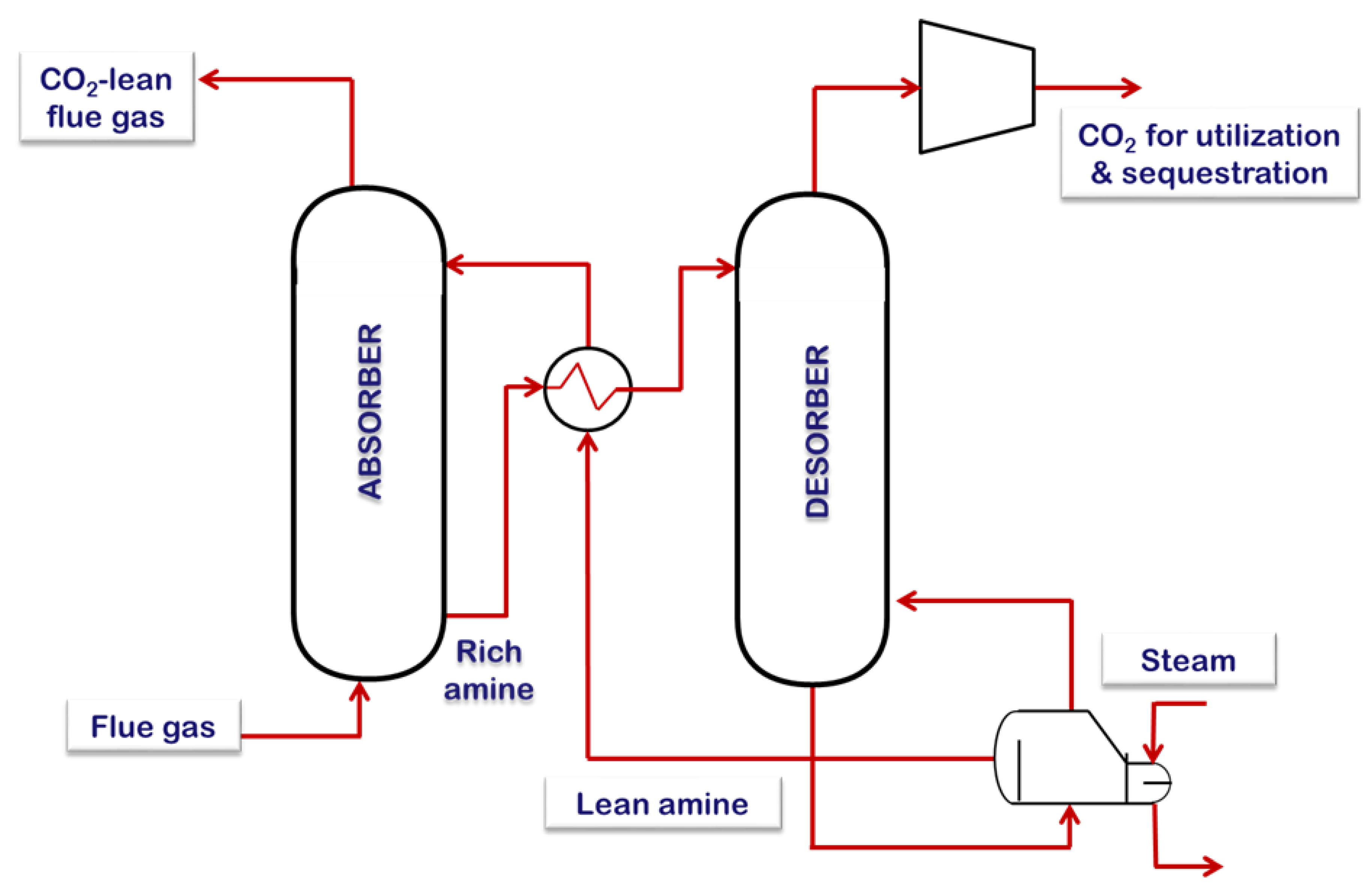
3. Capturing CO2 Using the PC Approach
| Post Combustion CO2 Capture | Strategies for Post Combustion Carbon Dioxide Capturing | |||||
|---|---|---|---|---|---|---|
| Merits | Demerits | Ref. | Strategy (Efficiency) | Merits | Demerits | Ref. |
|
| [59,60,61] | Chemical solvent scrubbing (90%) |
|
| [62] |
|
| [63,64,65,66,67] | Physical adsorption (55–92%) |
|
| [68,69] |
| [70,71,72] | Calcium looping (>75%) |
|
| [73,74] | |
|
| [41,75,76,77,78,79] | Membrane separation (Up to 90%) |
|
| [8] |
|
| [75,76,77] | Captured using Algae and other living species |
|
| [80,81] |
3.1. Research Activities in Post-Combustion Capture Technologies
3.1.1. Adsorption Mechanisms for CO2 Capture Using Activated Carbons
Physical-Activated Carbons
| Precursors | Carbonization Approach | Activation Stage | Total Area (m2/g) | Adsorption (mmol/g) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flow Rate (mL/min) | Temp. (°C) | Retention Time (min) | Oxidant | Flow Rate (mL/min) | Temperature (°C) | Retention Time (min) | 0 °C | 25 °C | 100 °C | |||
| Tobacco stem | - | 180 | 600 | KOH | - | 500 600 700 800 | 240 | 786 1086 1922 2399 | 4.76–7.98 | 3.31–4.84 | - | [107] |
| Bamboo | - | 500 | 90 | KOH | - | 603 | 90 | 528 | 4.5 | - | [108] | |
| Shell of almond | - | - | - | Carbon dioxide | 100 | 750 | 240 | 862 | 2.7 | 0.9 | [109] | |
| - | 600 | - | Carbon dioxide | 50 | 400 600 800 900 | 120 | 8 91 326 350 | 0.16 0.15 0.20 0.08 | [110] | |||
| Olive stone | - | - | - | 100 | 800 | 360 | 1215 | 3.1 | 0.8 | [109] | ||
| Shell of coconut | - | - | - | 140 | 800 | 210 | 1327 | 3.9 | - | [111] | ||
| Coffee | 50 | 600 | - | 15 | 800 | - | 590 | 2.35 | - | [112] | ||
| Nut | 500 | 600 | 60 | 500 | 900 | 61 | 570 | 3.49 | - | [49] | ||
| Cotton | - | 600 | - | - | 900 | - | 610 | 2.30 | 0.5 | [113] | ||
| Tobacco stem | - | 180 | 600 | Zn(NO3)2.6H2O | - | 910 | 120 | 340–998 | 92–209 mg/g | 66–145 mg/g | - | [114] |
Chemically Activated Carbons
| Precursors | Stages | Activation Stages | Area | Adsorption Capacity (mmol/g) | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activator | Impregnation ratio (wt/wt) | Temperature (°C) | Heating rate (°C/min) | Time (hour) | Flow rate (mL/min) | (m2/g) | 0 °C | 25 °C | 50 °C | 75 °C | |||
| Wood | 1 | H3PO4 | 2:1 | 450 | 4 | 1 | N/A | 1889 | - | 2.9 | - | - | [132] |
| Palm stone | 1 | 2:1 | 450 | 1 | 2 | 80 | 1320 | 3.1 | - | - | - | [133] | |
| Palm stone | 1 | ZnCl2 | 2:1 | 500 | 1 | 2 | 80 | 924 | 2.7 | - | - | - | [133] |
| Rice | 1 | 1:1 | 501 | 15 | 2 | 101 | 928 | - | 2.3 | - | 0.5 | [134] | |
| Bagasse | 1 | KOH | 1:1 | 500 | 10 | 1 | 100 | 923 | - | 1.7 | - | 0.6 | [134] |
| Ash | 2 | 5:1 | 700 | 5 | 2 | N/A | 161 | - | 0.6 | - | - | [135] | |
| Saw dust | 2 | 4:1 | 700 | 5 | 1 | N/A | 1643 | 8.0 | 4.8 | - | - | [136] | |
| Yeast | 2 | 1:1 | 600, 700, 750 | N/A | 1 | 50 | 1348 | - | 1.3–4.77 | 0.94–3.4 | 0.77–2.4 | [137] | |
| Shell of peanut | 2 | 1:1 | 700 | 5 | 1.5 | 120 | 956 | 5.2 | 4.0 | - | - | [138] | |
| Cellulose | 2 | 2:1/4:1 | 600–800 | 3 | 1 | - | 2370 | 5.8 | 3.5 | 2.2 | - | [139,140] | |
| Starch | 2190 | 5.6 | 3.5 | 1.8 | - | ||||||||
| Microalgae and glucose | 1:1, 2:1 or 4:1 | 650 and 750 | - | 24 | - | 1940 | 5.9–6.4 | 3.5–4.5 | 2.2–2.8 | - | [141] | ||
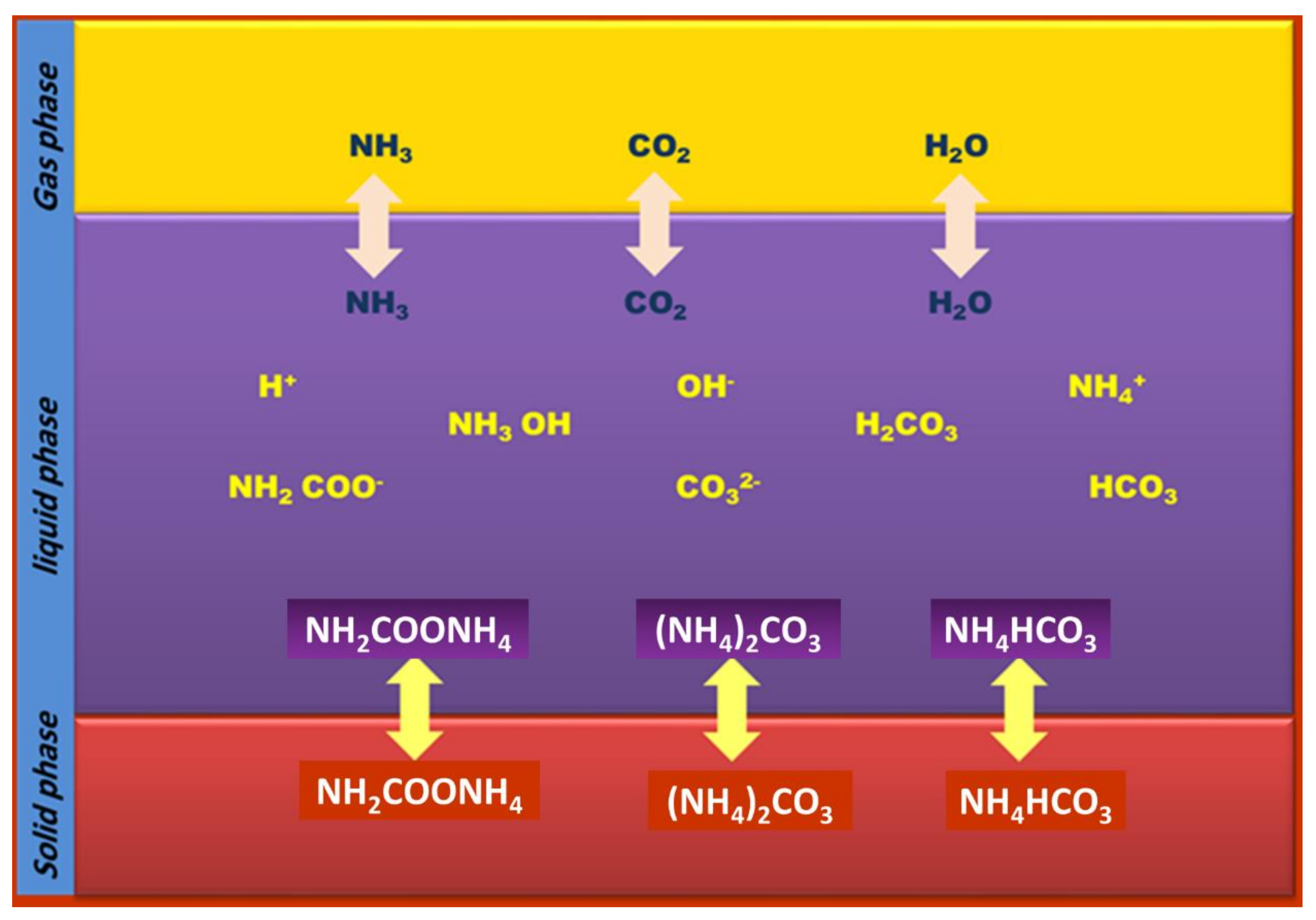
3.1.2. Chemical Absorption Using Aqueous Ammonia
Reaction Leading to the Capture of CO2 Using Aqueous Ammonia
3.1.3. Post-Combustion CO2 Capture Using Nano Materials
Nano Porous Materials
Nano Structured Hollow Materials
Nanocrystalline Particles
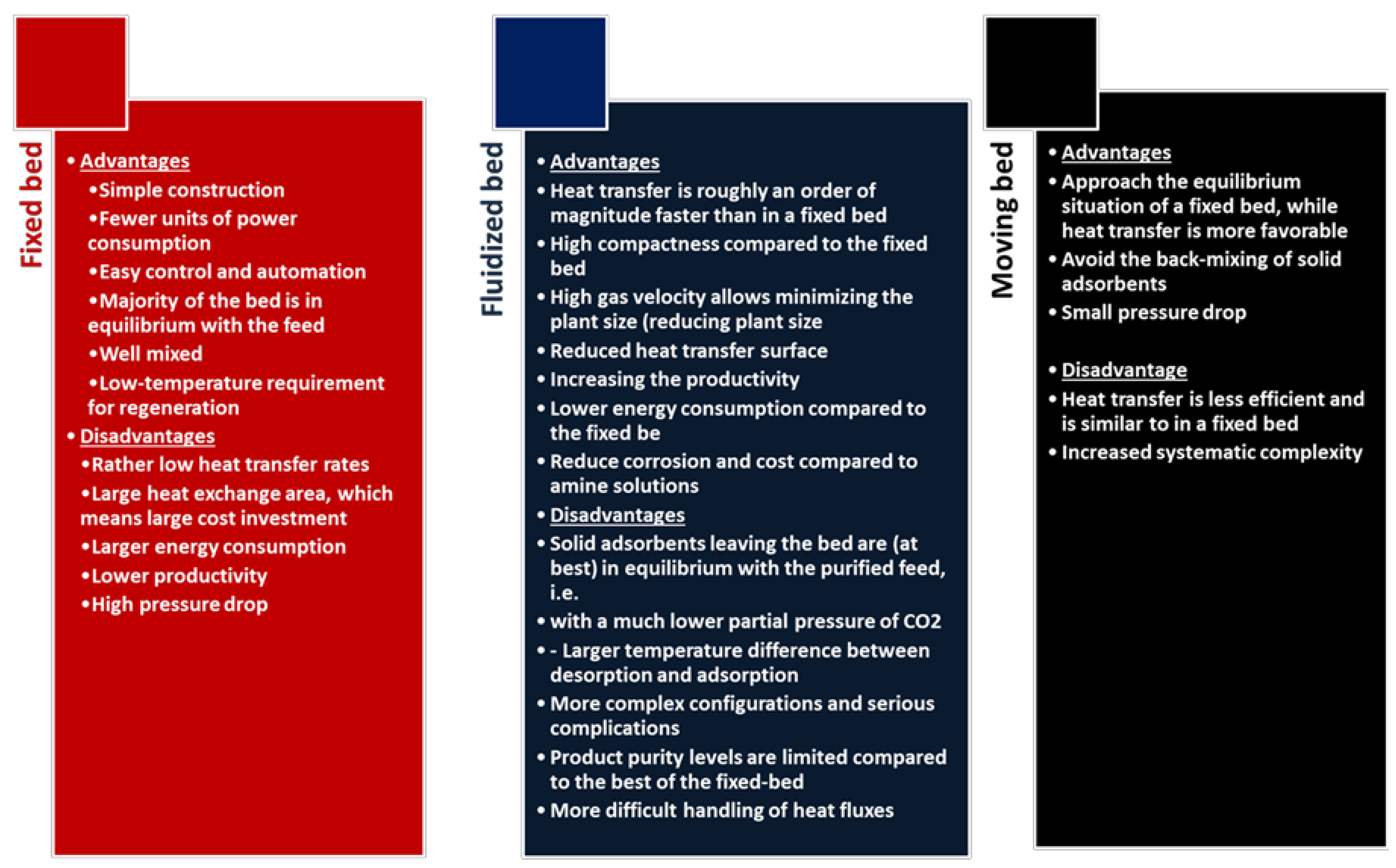
4. Comparing Various Types of Sorbents for Post-Combustion CO2 Capture
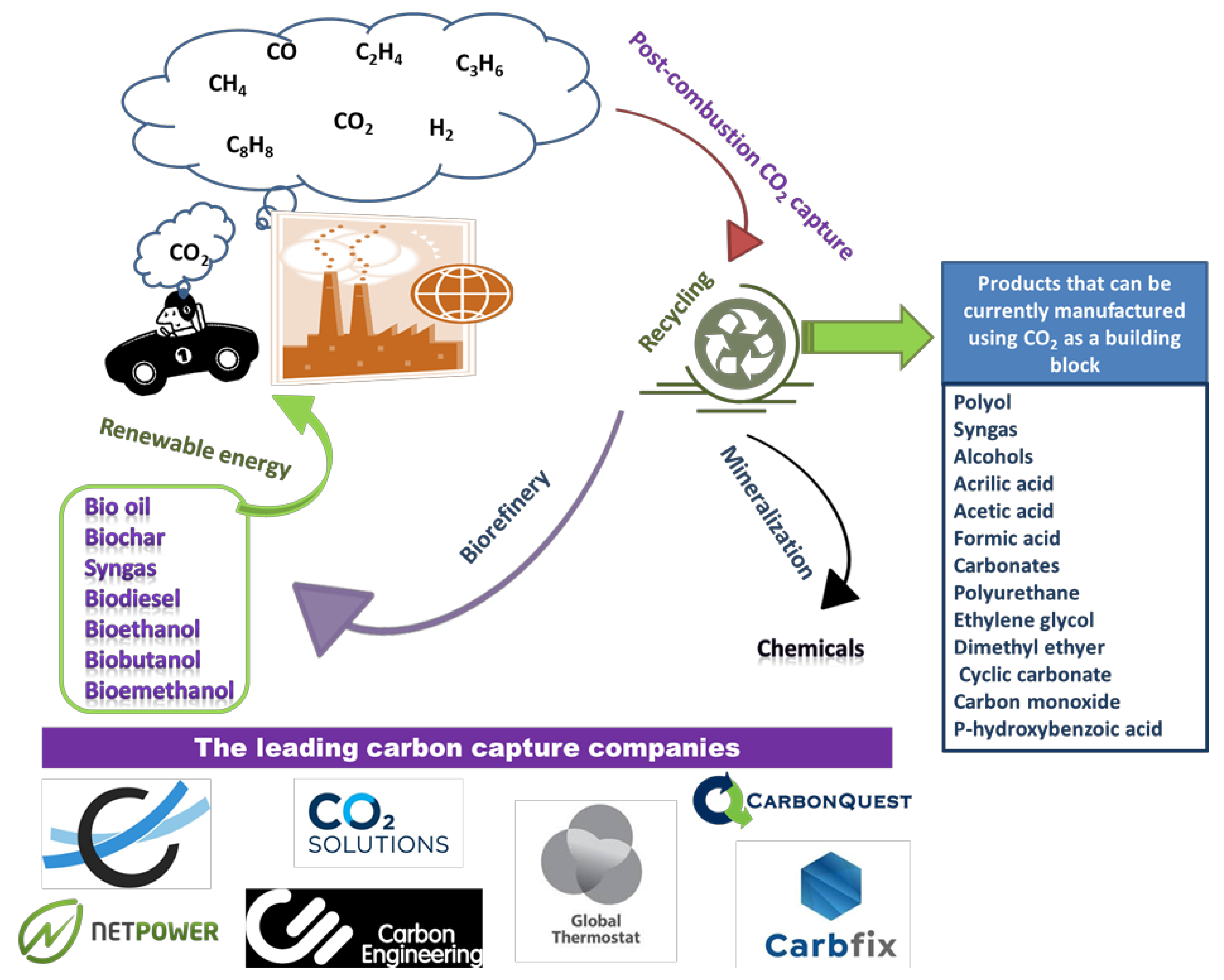
5. Impact of Post-Combustion CO2 Capture on the Circular Economy
- Mineralization: it refers to the reaction of alkaline earth oxides –based materials (such as MgO and CaO) with of CO2, yielding valuable carbonate-based products can be developed from industrial wastes;
- Another route for this strategy is the biofixation where CO2 is fixed by microalgae yielding numerous biological organic products, chemicals and biofuels via biorefining technologies. The biofixation has several merits such as the production of lipids, which the main feedstock for the production of green monomers, such as ethylene, as well as it can be transformed into bioethanol via some commercial reaction routes. The process needs large volumes of water, light intensity and land as well as the main nutrients such as carbon, nitrogen and phosphorus at specific concentrations and controlled pH and temperature (<45 °C). Additionally, purification of the flue gas stream from SOx, NOx, and heavy metals is essential to protect the microalgae. Future studies should focus on developing of the biorefineries, i.e., lowering the required area, conserve energy, improving the cell growth, the impact of the flue gas composition and load on the yield of the biomass and reducing the overall cost. Investigating the integration of renewable energy in these biorefineries is preferable;
- The third route is the adsorption of CO2 onto efficient sorbents which are useful for high-pressure applications if the capacities and rates of these sorbents are being enhanced;
- Other routes for the post-combustion CO2 capture such as cryogenic separation are investigated, however, this is process is not economic from circular economy view owning to its high energy demand.

6. Prospects of Post Combustion Carbon Capture Technology
| Type of Solvent | Sorbents | Merit | Challenges | Ref. |
|---|---|---|---|---|
| Amine based solvent | Ammonia |
|
| [216,217] |
| Monoethanolamine |
|
| [218,219] | |
| Methyldiethanolamine |
|
| [207,220,221] | |
| Amine mixture | Monoethanolamine + 2–Amino–2–methyl–1–propanol |
|
| [211,222] |
| Methyldiethanolamine + Piperazine |
|
| [209,223] | |
| Ionic liquid | Conventional ILs |
|
| [224] |
| Functionalized ionic liquids |
|
| [212,224,225] | |
| IL-alkanolamine-water mixture |
|
| [224] | |
| Amine based ionic liquid + Methyldiethanolamine |
|
| [226,227] |
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilberforce, T.; Baroutaji, A.; Soudan, B.; Al-Alami, A.H.; Olabi, A.G. Outlook of carbon capture technology and challenges. Sci. Total Environ. 2019, 657, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-S. Chemical Looping Partial Oxidation: Gasification, Reforming, and Chemical Syntheses; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Thiyagarajan, S.; Varuvel, E.G.; Martin, L.J.; Beddhannan, N. Mitigation of carbon footprints through a blend of biofuels and oxygenates, combined with post-combustion capture system in a single cylinder CI engine. Renew. Energy 2019, 130, 1067–1081. [Google Scholar] [CrossRef]
- Interlenghi, S.F.; Raquel de Pádua, F.S.; de Medeiros, J.L.; de Q. Fernandes Araújo, O. Low-emission offshore Gas-To-Wire from natural gas with carbon dioxide: Supersonic separator conditioning and post-combustion decarbonation. Energy Convers. Manag. 2019, 195, 1334–1349. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, L.; Zhao, L.; Deng, S.; Li, S.; Zhang, Y. A comprehensive performance evaluation of temperature swing adsorption for post-combustion carbon dioxide capture. Renew. Sustain. Energy Rev. 2019, 114, 109285. [Google Scholar] [CrossRef]
- Cabral, R.P.; Mac Dowell, N. A novel methodological approach for achieving£/MWh cost reduction of CO2 capture and storage (CCS) processes. Appl. Energy 2017, 205, 529–539. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Fan, L.-S.; Li, F. Chemical Looping Technology and Its Fossil Energy Conversion Applications. Ind. Eng. Chem. Res. 2010, 49, 10200–10211. [Google Scholar] [CrossRef]
- Voshell, S.; Mäkelä, M.; Dahl, O. A review of biomass ash properties towards treatment and recycling. Renew. Sustain. Energy Rev. 2018, 96, 479–486. [Google Scholar] [CrossRef]
- Lackner, K.S.; Brennan, S.; Matter, J.M.; Park, A.-H.A.; Wright, A.; Van Der Zwaan, B. The urgency of the development of CO2 capture from ambient air. Proc. Natl. Acad. Sci. USA 2012, 109, 13156–13162. [Google Scholar] [CrossRef]
- Rau, G.H.; Lackner, K.S. Reversing Excess Atmospheric CO2. Science 2013, 340, 1522–1523. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Li, Y.; Song, Y.; Li, H. Alternative pathways for efficient CO2 capture by hybrid processes—A review. Renew. Sustain. Energy Rev. 2018, 82, 215–231. [Google Scholar] [CrossRef]
- Dods, M.N.; Weston, S.C.; Long, J.R. Prospects for Simultaneously Capturing Carbon Dioxide and Harvesting Water from Air. Adv. Mater. 2022, 34, 2204277. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Li, L.; Wang, Q.; Shi, X.; Fu, L.; Zhang, G.; Luan, C.; Abomohra, A.E.-F. Accelerated carbonation treatment of recycled concrete aggregates using flue gas: A comparative study towards performance improvement. J. CO2 Util. 2021, 43, 101362. [Google Scholar] [CrossRef]
- Hanak, D.P.; Michalski, S.; Manovic, V. From post-combustion carbon capture to sorption-enhanced hydrogen production: A state-of-the-art review of carbonate looping process feasibility. Energy Convers. Manag. 2018, 177, 428–452. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, L.; Song, C.; Zhang, N.; Guo, M.; Zhang, X. Reduction of efficiency penalty for a natural gas combined cycle power plant with post-combustion CO2 capture: Integration of liquid natural gas cold energy. Energy Convers. Manag. 2019, 198, 111852. [Google Scholar] [CrossRef]
- Stephens, J.C.; van der Zwaan, B.; Kennedy, J. CO2 Capture and Storage (CCS): Exploring the Research, Development, Demonstration, and Deployment Continuum; Belfer Center for Science and International Affairs, John F. Kennedy School, Harvard University: Cambridge, MA, USA, 2005. [Google Scholar]
- Gassensmith, J.J.; Furukawa, H.; Smaldone, R.A.; Forgan, R.S.; Botros, Y.Y.; Yaghi, O.M.; Stoddart, J.F. Strong and reversible binding of carbon dioxide in a green metal–organic framework. J. Am. Chem. Soc. 2011, 133, 15312–15315. [Google Scholar] [CrossRef]
- Rabaia, M.K.H.; Abdelkareem, M.A.; Sayed, E.T.; Elsaid, K.; Chae, K.-J.; Wilberforce, T.; Olabi, A.G. Environmental impacts of solar energy systems: A review. Sci. Total Environ. 2021, 754, 141989. [Google Scholar] [CrossRef]
- Sayed, E.T.; Wilberforce, T.; Elsaid, K.; Rabaia, M.K.H.; Abdelkareem, M.A.; Chae, K.-J.; Olabi, A.G. A critical review on environmental impacts of renewable energy systems and mitigation strategies: Wind, hydro, biomass and geothermal. Sci. Total Environ. 2021, 766, 144505. [Google Scholar] [CrossRef]
- Olabi, A.G.; Bahri, A.S.; Abdelghafar, A.A.; Baroutaji, A.; Sayed, E.T.; Alami, A.H.; Rezk, H.; Abdelkareem, M.A. Large-vscale hydrogen production and storage technologies: Current status and future directions. Int. J. Hydrog. Energy 2021, 46, 23498–23528. [Google Scholar] [CrossRef]
- Wilberforce, T.; Sayed, E.T.; Abdelkareem, M.A.; Elsaid, K.; Olabi, A.G. Value added products from wastewater using bioelectrochemical systems: Current trends and perspectives. J. Water Process Eng. 2021, 39, 101737. [Google Scholar] [CrossRef]
- Olabi, A.G.; Elsaid, K.; Sayed, E.T.; Mahmoud, M.S.; Wilberforce, T.; Hassiba, R.J.; Abdelkareem, M.A. Application of nanofluids for enhanced waste heat recovery: A review. Nano Energy 2021, 84, 105871. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Sengupta, S.; Amte, V.; Dongara, R.; Das, A.K.; Bhunia, H.; Bajpai, P.K. Effects of the adsorbent preparation method for CO2 capture from flue gas using K2CO3/Al2O3 adsorbents. Energy Fuels 2015, 29, 287–297. [Google Scholar] [CrossRef]
- Olabi, A.G.; Rezk, H.; Sayed, E.T.; Ghoniem, R.M.; Abdelkareem, M.A. Boosting carbon dioxide adsorption capacity applying Jellyfish optimization and ANFIS-based modelling. Ain Shams Eng. J. 2022, 101931. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Large scale application of carbon capture to process industries—A review. J. Clean. Prod. 2022, 362, 132300. [Google Scholar] [CrossRef]
- Olabi, A.G.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 2022, 153, 111710. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Mohammad, M.; Isaifan, R.J.; Weldu, Y.W.; Rahman, M.A.; Al-Ghamdi, S.G. Progress on carbon dioxide capture, storage and utilisation. Int. J. Glob. Warm. 2020, 20, 124–144. [Google Scholar] [CrossRef]
- Freites, S.G.; Jones, C. A Review of the Role of Fossil Fuel-Based Carbon Capture and Storage in the Energy System; Tyndall Centre: Manchester, UK, 2021. [Google Scholar]
- Lau, H.C.; Ramakrishna, S.; Zhang, K.; Radhamani, A.V. The Role of Carbon Capture and Storage in the Energy Transition. Energy Fuels 2021, 35, 7364–7386. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Qiao, Z.; Wu, H.; Dong, S.; Zhao, S.; Wang, J. Post-combustion CO2 capture with membrane process: Practical membrane performance and appropriate pressure. J. Membr. Sci. 2019, 581, 195–213. [Google Scholar] [CrossRef]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation, review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon dioxide capture using liquid absorption methods: A review. Environ. Chem. Lett. 2021, 19, 77–109. [Google Scholar] [CrossRef]
- Liu, S.-H.; Huang, Y.-Y. Valorization of coffee grounds to biochar-derived adsorbents for CO2 adsorption. J. Clean. Prod. 2018, 175, 354–360. [Google Scholar] [CrossRef]
- Mukherjee, A.; Borugadda, V.B.; Dynes, J.J.; Niu, C.; Dalai, A.K. Carbon dioxide capture from flue gas in biochar produced from spent coffee grounds: Effect of surface chemistry and porous structure. J. Environ. Chem. Eng. 2021, 9, 106049. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef]
- Mohamedali, M.; Ibrahim, H.; Henni, A. Incorporation of acetate-based ionic liquids into a zeolitic imidazolate framework (ZIF-8) as efficient sorbents for carbon dioxide capture. Chem. Eng. J. 2018, 334, 817–828. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Ou, C.; Dong, X. Porous Metal–Organic Frameworks for Carbon Dioxide Adsorption and Separation at Low Pressure. ACS Sustain. Chem. Eng. 2020, 8, 15378–15404. [Google Scholar] [CrossRef]
- Dodevski, V.; Janković, B.; Stojmenović, M.; Krstić, S.; Popović, J.; Pagnacco, M.C.; Popović, M.; Pašalić, S. Plane tree seed biomass used for preparation of activated carbons (AC) derived from pyrolysis. Modeling the activation process. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 83–96. [Google Scholar] [CrossRef]
- Song, N.; Wang, T.; Yao, H.; Ma, T.; Shi, K.; Tian, Y.; Zou, Y.; Zhu, S.; Zhang, Y.; Guan, S. Construction and carbon dioxide capture of microporous polymer networks with high surface area based on cross-linkable linear polyimides. Polym. Chem. 2019, 10, 4611–4620. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Kim, J. A review on application of activated carbons for carbon dioxide capture: Present performance, preparation, and surface modification for further improvement. Environ. Sci. Pollut. Res. 2021, 28, 43329–43364. [Google Scholar] [CrossRef] [PubMed]
- Sifat, N.S.; Haseli, Y. A critical review of CO2 capture technologies and prospects for clean power generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef]
- Yadav, S.; Mondal, S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- Bae, J.-S.; Su, S. Macadamia nut shell-derived carbon composites for post combustion CO2 capture. Int. J. Greenh. Gas Control 2013, 19, 174–182. [Google Scholar] [CrossRef]
- Jansen, D.; Gazzani, M.; Manzolini, G.; van Dijk, E.; Carbo, M. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Z.; Xiang, J.; Zhang, L.; Zhang, S.; Luo, C.; Zhao, Y. Fundamental and Technical Challenges for a Compatible Design Scheme of Oxyfuel Combustion Technology. Engineering 2015, 1, 139–149. [Google Scholar] [CrossRef]
- Giordano, L.; Gubis, J.; Bierman, G.; Kapteijn, F. Conceptual design of membrane-based pre-combustion CO2 capture process: Role of permeance and selectivity on performance and costs. J. Membr. Sci. 2019, 575, 229–241. [Google Scholar] [CrossRef]
- Koohestanian, E.; Shahraki, F. Review on principles, recent progress, and future challenges for oxy-fuel combustion CO2 capture using compression and purification unit. J. Environ. Chem. Eng. 2021, 9, 105777. [Google Scholar] [CrossRef]
- Mills, S. Coal-Fired CCS Demonstration Plants, 2012; IEA Clean Coal Centre: London, UK, 2012. [Google Scholar]
- Chen, Z.; Deng, S.; Wei, H.; Wang, B.; Huang, J.; Yu, G. Activated carbons and amine-modified materials for carbon dioxide capture—A review. Front. Environ. Sci. Eng. 2013, 7, 326–340. [Google Scholar] [CrossRef]
- Hornbostel, M.D.; Bao, J.; Krishnan, G.; Nagar, A.; Jayaweera, I.; Kobayashi, T.; Sanjurjo, A.; Sweeney, J.; Carruthers, D.; Petruska, M.A. Characteristics of an advanced carbon sorbent for CO2 capture. Carbon 2013, 56, 77–85. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Sinha, A.; Nair, V.C.; Sharma, T. Enhanced carbon dioxide sequestration by direct injection of flue gas doped with hydrogen into hydrate reservoir: Possibility of natural gas production. Energy 2021, 227, 120521. [Google Scholar] [CrossRef]
- Ebner, A.D.; Ritter, J.A. State-of-the-art adsorption and membrane separation processes for carbon dioxide production from carbon dioxide emitting industries. Sep. Sci. Technol. 2009, 44, 1273–1421. [Google Scholar] [CrossRef]
- Feron, P. Absorption-Based Post-Combustion Capture of Carbon Dioxide; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Zhao, H.; Luo, X.; Zhang, H.; Sun, N.; Wei, W.; Sun, Y. Carbon-based adsorbents for post-combustion capture: A review. Greenh. Gases Sci. Technol. 2018, 8, 11–36. [Google Scholar] [CrossRef]
- Drage, T.C.; Snape, C.E.; Stevens, L.A.; Wood, J.; Wang, J.; Cooper, A.I.; Dawson, R.; Guo, X.; Satterley, C.; Irons, R. Materials challenges for the development of solid sorbents for post-combustion carbon capture. J. Mater. Chem. 2012, 22, 2815–2823. [Google Scholar] [CrossRef]
- Vega, F.; Cano, M.; Camino, S.; Fernández, L.M.G.; Portillo, E.; Navarrete, B. Solvents for carbon dioxide capture. In Carbon Dioxide Chemistry, Capture and Oil Recovery; BoD—Books on Demand: Norderstedt, Germany, 2018; pp. 142–163. [Google Scholar]
- Tontiwachwuthikul, P.; Idem, R. Recent Progress and New Developments in Post-Combustion Carbon-Capture Technology with Reactive Solvents; Future Medicine: London, UK, 2013. [Google Scholar]
- Krzemień, A.; Więckol-Ryk, A.; Duda, A.; Koteras, A. Risk assessment of a post-combustion and amine-based CO2 capture ready process. J. Sustain. Min. 2013, 12, 18–23. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A review of post-combustion CO2 capture technologies from coal-fired power plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Goto, K.; Yogo, K.; Higashii, T. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture. Appl. Energy 2013, 111, 710–720. [Google Scholar] [CrossRef]
- Feron, P.H. Exploring the potential for improvement of the energy performance of coal fired power plants with post-combustion capture of carbon dioxide. Int. J. Greenh. Gas Control 2010, 4, 152–160. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Yeh, C.-Y.; Weng, C.-H. Carbon dioxide adsorption on porous and functionalized activated carbon fibers. Appl. Sci. 2019, 9, 1977. [Google Scholar] [CrossRef]
- Farooq, M.; Saeed, M.; Imran, M.; Uddin, G.; Asim, M.; Bilal, H.; Younas, M.; Andresen, J. CO2 capture through electro-conductive adsorbent using physical adsorption system for sustainable development. Environ. Geochem. Health 2020, 42, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, A.K.; Avila, A.M.; Rajendran, A. Do adsorbent screening metrics predict process performance? A process optimisation based study for post-combustion capture of CO2. Int. J. Greenh. Gas Control 2016, 46, 76–85. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Carbon Capture From Flue Gas and the Atmosphere: A Perspective. Front. Energy Res. 2020, 8, 265. [Google Scholar] [CrossRef]
- Karia, T.; Gurumoorthy, A.V. A Review of Progress in Calcium Looping Technology for CO2 Capture from Power and Cement Plants. Recent Innov. Chem. Eng. (Former. Recent Pat. Chem. Eng.) 2017, 10, 74–87. [Google Scholar] [CrossRef]
- Hu, J.; Hongmanorom, P.; Galvita, V.V.; Li, Z.; Kawi, S. Bifunctional Ni-Ca based material for integrated CO2 capture and conversion via calcium-looping dry reforming. Appl. Catal. B Environ. 2021, 284, 119734. [Google Scholar] [CrossRef]
- Plaza, M.; García, S.; Rubiera, F.; Pis, J.; Pevida, C. Post-combustion CO2 capture with a commercial activated carbon: Comparison of different regeneration strategies. Chem. Eng. J. 2010, 163, 41–47. [Google Scholar] [CrossRef]
- Idrees, M.; Rangari, V.; Jeelani, S. Sustainable packaging waste-derived activated carbon for carbon dioxide capture. J. CO2 Util. 2018, 26, 380–387. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative study of CO2 capture by carbon nanotubes, activated carbons, and zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Shirmohammadi, R.; Aslani, A.; Ghasempour, R.; Romeo, L.M. CO2 utilization via integration of an industrial post-combustion capture process with a urea plant: Process modelling and sensitivity analysis. Processes 2020, 8, 1144. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Lootah, M.A.; Sayed, E.T.; Wilberforce, T.; Alawadhi, H.; Yousef, B.A.; Olabi, A. Fuel cells for carbon capture applications. Sci. Total Environ. 2021, 769, 144243. [Google Scholar] [CrossRef] [PubMed]
- Sharp, C.E.; Urschel, S.; Dong, X.; Brady, A.L.; Slater, G.F.; Strous, M. Robust, high-productivity phototrophic carbon capture at high pH and alkalinity using natural microbial communities. Biotechnol. Biofuels 2017, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- De Haan, A.B.; Eral, H.B.; Schuur, B. Separation Method Selection. In Industrial Separation Processes; De Gruyter: Berlin, Germany, 2020; pp. 385–412. [Google Scholar]
- Castro, M.; Gómez-Díaz, D.; Navaza, J.M.; Rumbo, A. Carbon Dioxide Capture by Chemical Solvents Based on Amino Acids: Absorption and Regeneration. Chem. Eng. Technol. 2021, 44, 248–257. [Google Scholar] [CrossRef]
- Lin, P.-H.; Wong, D.S.H. Carbon dioxide capture and regeneration with amine/alcohol/water blends. Int. J. Greenh. Gas Control 2014, 26, 69–75. [Google Scholar] [CrossRef]
- Lee, U.; Burre, J.; Caspari, A.; Kleinekorte, J.; Schweidtmann, A.M.; Mitsos, A. Techno-economic optimization of a green-field post-combustion CO2 capture process using superstructure and rate-based models. Ind. Eng. Chem. Res. 2016, 55, 12014–12026. [Google Scholar] [CrossRef]
- Salih, H.A.; Pokhrel, J.; Reinalda, D.; AlNashf, I.; Khaleel, M.; Vega, L.F.; Karanikolos, G.N.; Zahra, M.A. Hybrid–Slurry/Nanofluid systems as alternative to conventional chemical absorption for carbon dioxide capture: A review. Int. J. Greenh. Gas Control 2021, 110, 103415. [Google Scholar] [CrossRef]
- Bandehali, S.; Moghadassi, A.; Parvizian, F.; Hosseini, S.M.; Matsuura, T.; Joudaki, E. Advances in high carbon dioxide separation performance of poly (ethylene oxide)-based membranes. J. Energy Chem. 2020, 46, 30–52. [Google Scholar] [CrossRef]
- Lee, W.G.; Kang, S.W. Highly selective poly (ethylene oxide)/ionic liquid electrolyte membranes containing CrO3 for CO2/N2 separation. Chem. Eng. J. 2019, 356, 312–317. [Google Scholar] [CrossRef]
- Sreedhar, I.; Vaidhiswaran, R.; Kamani, B.M.; Venugopal, A. Process and engineering trends in membrane based carbon capture. Renew. Sustain. Energy Rev. 2017, 68, 659–684. [Google Scholar] [CrossRef]
- Godin, J.; Liu, W.; Ren, S.; Xu, C.C. Advances in Recovery and Utilization of Carbon Dioxide: A Brief Review. J. Environ. Chem. Eng. 2021, 9, 105644. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation-A review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.; Bamidele, O.; Basha, M.; Qasem, N.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2020, 138, 110490. [Google Scholar] [CrossRef]
- Subraveti, S.G.; Roussanaly, S.; Anantharaman, R.; Riboldi, L.; Rajendran, A. How much can novel solid sorbents reduce the cost of post-combustion CO2 capture? A techno-economic investigation on the cost limits of pressure–vacuum swing adsorption. Appl. Energy 2022, 306, 117955. [Google Scholar] [CrossRef]
- Calvo-Munoz, E.M.; Garcia-Mateos, F.J.; Rosas, J.M.; Rodriguez-Mirasol, J.; Cordero, T. Biomass waste carbon materials as adsorbents for CO2 capture under post-combustion conditions. Front. Mater. 2016, 3, 23. [Google Scholar] [CrossRef]
- Sharma, A.; Jindal, J.; Mittal, A.; Kumari, K.; Maken, S.; Kumar, N. Carbon materials as CO2 adsorbents: A review. Environ. Chem. Lett. 2021, 19, 875–910. [Google Scholar] [CrossRef]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.A.; Vega, L.F.; Abu-Zahra, M.R. Synthesis and characterization of activated carbon from biomass date seeds for carbon dioxide adsorption. J. Environ. Chem. Eng. 2020, 8, 104257. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Garcia, S.; Pevida, C.; Wong, M.; Maroto-Valer, M. The influence of the precursor and synthesis method on the CO2 capture capacity of carpet waste-based sorbents. J. Environ. Manag. 2011, 92, 2810–2817. [Google Scholar] [CrossRef] [PubMed]
- Nhuchhen, D.R.; Basu, P.; Acharya, B. A comprehensive review on biomass torrefaction. Int. J. Renew. Energy Biofuels 2014, 2014, 506376. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Budinova, T.; Ekinci, E.; Yardim, F.; Grimm, A.; Björnbom, E.; Minkova, V.; Goranova, M. Characterization and application of activated carbon produced by H3PO4 and water vapor activation. Fuel Process. Technol. 2006, 87, 899–905. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K.; Oporto, G.; Dawson-Andoh, B.; McDonald, L.; Sabolsky, E. Effect of one-step and two-step H3PO4 activation on activated carbon characteristics. Bioresour. Technol. Rep. 2019, 8, 100307. [Google Scholar] [CrossRef]
- Hussaro, K. Preparation of activated carbon from palm oil shell by chemical activation with Na2CO3 and ZnCl2 as impregnated agents for H2Sadsorption. Am. J. Environ. Sci. 2014, 10, 336–346. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Y.; Wu, Q.; Liu, B.; Li, D.; Chen, R.; Wang, C.; Li, H.; Zeng, Z.; Li, L. Underlying mechanism of CO2 uptake onto biomass-based porous carbons: Do adsorbents capture CO2 chiefly through narrow micropores? Fuel 2020, 282, 118727. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular Bamboo-Derived Activated Carbon for High CO2 Adsorption: The Dominant Role of Narrow Micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- González, A.S.; Plaza, M.G.; Rubiera, F.; Pevida, C. Sustainable biomass-based carbon adsorbents for post-combustion CO2 capture. Chem. Eng. J. 2013, 230, 456–465. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Martín, C.F.; Fermoso, J.; Pis, J.J.; Rubiera, F. Developing almond shell-derived activated carbons as CO2 adsorbents. Sep. Purif. Technol. 2010, 71, 102–106. [Google Scholar] [CrossRef]
- Ello, A.S.; de Souza, L.K.C.; Trokourey, A.; Jaroniec, M. Coconut shell-based microporous carbons for CO2 capture. Microporous Mesoporous Mater. 2013, 180, 280–283. [Google Scholar] [CrossRef]
- Plaza, M.G.; González, A.S.; Pevida, C.; Pis, J.J.; Rubiera, F. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl. Energy 2012, 99, 272–279. [Google Scholar] [CrossRef]
- Xiong, Z.; Shihong, Z.; Haiping, Y.; Tao, S.; Yingquan, C.; Hanping, C. Influence of NH3/CO2 modification on the characteristic of biochar and the CO2 capture. Bioenergy Res. 2013, 6, 1147–1153. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Fang, M.; Liu, B.; Chen, R.; Shi, R.; Wu, Q.; Zeng, Z.; Li, L. In-situ activated ultramicroporous carbon materials derived from waste biomass for CO2 capture and benzene adsorption. Biomass Bioenergy 2022, 158, 106353. [Google Scholar] [CrossRef]
- Plaza, M.; González, A.; Pis, J.; Rubiera, F.; Pevida, C. Production of microporous biochars by single-step oxidation: Effect of activation conditions on CO2 capture. Appl. Energy 2014, 114, 551–562. [Google Scholar] [CrossRef]
- González, J.F.; Encinar, J.M.; González-García, C.M.; Sabio, E.; Ramiro, A.; Canito, J.L.; Gañán, J. Preparation of activated carbons from used tyres by gasification with steam and carbon dioxide. Appl. Surf. Sci. 2006, 252, 5999–6004. [Google Scholar] [CrossRef]
- Wigmans, T. Industrial aspects of production and use of activated carbons. Carbon 1989, 27, 13–22. [Google Scholar] [CrossRef]
- Katesa, J.; Junpiromand, S.; Tangsathitkulchai, C. Effect of carbonization temperature on properties of char and activated carbon from coconut shell. Suranaree J. Sci. Technol. 2013, 20, 269–278. [Google Scholar]
- Loredo-Cancino, M.; Soto-Regalado, E.; Cerino-Córdova, F.; García-Reyes, R.; García-León, A.; Garza-González, M. Determining optimal conditions to produce activated carbon from barley husks using single or dual optimization. J. Environ. Manag. 2013, 125, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Fatriansyah, J.F.; Matari, T.; Harjanto, S. The preparation of activated carbon from coconut shell charcoal by novel mechano-chemical activation. Mater. Sci. Forum 2018, 929, 50–55. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S.; Borhan, A. Development of novel low-cost activated carbon for carbon dioxide capture. Int. J. Chem. Eng. Appl. 2014, 5, 90. [Google Scholar] [CrossRef]
- Jimenez-Cordero, D.; Heras, F.; Alonso-Morales, N.; Gilarranz, M.A.; Rodriguez, J.J. Development of porosity upon physical activation of grape seeds char by gas phase oxygen chemisorption–desorption cycles. Chem. Eng. J. 2013, 231, 172–181. [Google Scholar] [CrossRef]
- Guardia, L.; Suárez, L.; Querejeta, N.; Pevida, C.; Centeno, T.A. Winery wastes as precursors of sustainable porous carbons for environmental applications. J. Clean. Prod. 2018, 193, 614–624. [Google Scholar] [CrossRef]
- Kilic, M.; Apaydin-Varol, E.; Pütün, A.E. Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues: Equilibrium, kinetics and thermodynamics. J. Hazard. Mater. 2011, 189, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Ateş, F.; Özcan, Ö. Preparation and characterization of activated carbon from poplar sawdust by chemical activation: Comparison of different activating agents and carbonization temperature. Eur. J. Eng. Technol. Res. 2018, 3, 6–11. [Google Scholar]
- Pam, A.A.; Abdullah, A.H.; Tan, Y.P.; Zainal, Z. Optimizing the route for medium temperature-activated carbon derived from agro-based waste material. Biomass Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef]
- Vargas, A.M.; Cazetta, A.L.; Garcia, C.A.; Moraes, J.C.; Nogami, E.M.; Lenzi, E.; Costa, W.F.; Almeida, V.C. Preparation and characterization of activated carbon from a new raw lignocellulosic material: Flamboyant (Delonix regia) pods. J. Environ. Manag. 2011, 92, 178–184. [Google Scholar] [CrossRef]
- Cao, Q.; Xie, K.-C.; Lv, Y.-K.; Bao, W.-R. Process effects on activated carbon with large specific surface area from corn cob. Bioresour. Technol. 2006, 97, 110–115. [Google Scholar] [CrossRef]
- Yagmur, E.; Gokce, Y.; Tekin, S.; Semerci, N.I.; Aktas, Z. Characteristics and comparison of activated carbons prepared from oleaster (Elaeagnus angustifolia L.) fruit using KOH and ZnCl2. Fuel 2020, 267, 117232. [Google Scholar] [CrossRef]
- Zuo, S.-L. A review of the control of pore texture of phosphoric acid-activated carbons. New Carbon Mater. (新型炭材料) 2018, 33, 289–302. [Google Scholar]
- Azmi, A.; Aziz, M. Mesoporous adsorbent for CO2 capture application under mild condition: A review. J. Environ. Chem. Eng. 2019, 7, 103022. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H.; Rashidi, A.; Ghoreyshi, A.A. Evaluation of CO2 adsorption with eucalyptus wood based activated carbon modified by ammonia solution through heat treatment. Chem. Eng. J. 2014, 254, 503–513. [Google Scholar] [CrossRef]
- Vargas, D.; Giraldo, L.; Erto, A.; Moreno-Piraján, J. Chemical modification of activated carbon monoliths for CO2 adsorption. J. Therm. Anal. Calorim. 2013, 114, 1039–1047. [Google Scholar] [CrossRef]
- Boonpoke, A.; Chiarakorn, S.; Laosiripojana, N.; Towprayoon, S.; Chidthaisong, A. Synthesis of activated carbon and MCM-41 from bagasse and rice husk and their carbon dioxide adsorption capacity. J. Sustain. Energy Environ. 2011, 2, 77–81. [Google Scholar]
- Alhamed, Y.A.; Rather, S.U.; El-Shazly, A.H.; Zaman, S.F.; Daous, M.A.; Al-Zahrani, A.A. Preparation of activated carbon from fly ash and its application for CO2 capture. Korean J. Chem. Eng. 2015, 32, 723–730. [Google Scholar] [CrossRef]
- Zhu, X.-L.; Wang, P.-Y.; Peng, C.; Yang, J.; Yan, X.-B. Activated carbon produced from paulownia sawdust for high-performance CO2 sorbents. Chin. Chem. Lett. 2014, 25, 929–932. [Google Scholar] [CrossRef]
- Shen, W.; He, Y.; Zhang, S.; Li, J.; Fan, W. Yeast-based microporous carbon materials for carbon dioxide capture. ChemSusChem 2012, 5, 1274–1279. [Google Scholar] [CrossRef]
- Deng, S.; Hu, B.; Chen, T.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Activated carbons prepared from peanut shell and sunflower seed shell for high CO2 adsorption. Adsorption 2015, 21, 125–133. [Google Scholar] [CrossRef]
- Tong, L.; Yue, T.; Zuo, P.; Zhang, X.; Wang, C.; Gao, J.; Wang, K. Effect of characteristics of KI-impregnated activated carbon and flue gas components on Hg0 removal. Fuel 2017, 197, 1–7. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Sevilla, M.; Falco, C.; Titirici, M.-M.; Fuertes, A.B. High-performance CO2 sorbents from algae. RSC Adv. 2012, 2, 12792–12797. [Google Scholar] [CrossRef]
- Xie, H.; Jiang, W.; Liu, T.; Wu, Y.; Wang, Y.; Chen, B.; Niu, D.; Liang, B. Low-energy electrochemical carbon dioxide capture based on a biological redox proton carrier. Cell Rep. Phys. Sci. 2020, 1, 100046. [Google Scholar] [CrossRef]
- Valluri, S.; Kawatra, S. Use of frothers to improve the absorption efficiency of dilute sodium carbonate slurry for post combustion CO2 capture. Fuel Process. Technol. 2021, 212, 106620. [Google Scholar] [CrossRef]
- Zhang, Z.; Borhani, T.N.; Olabi, A.G. Status and perspective of CO2 absorption process. Energy 2020, 205, 118057. [Google Scholar] [CrossRef]
- Mourad, A.A.H.; Mohammad, A.F.; Altarawneh, M.; Al-Marzouqi, A.H.; El-Naas, M.H.; Al-Marzouqi, M.H. Effects of potassium hydroxide and aluminum oxide on the performance of a modified solvay process for CO2 capture: A comparative study. Int. J. Energy Res. 2021, 45, 13952–13964. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Kaganoi, S.; Rochelle, G.T. CO2 absorption into aqueous mixtures of diglycolamine® and methyldiethanolamine. Chem. Eng. Sci. 2000, 55, 5125–5140. [Google Scholar] [CrossRef]
- Yan, S.; Fang, M.; Zhang, W. Technical analysis and progress of chemical absorption of removing CO2 from combustion flue gas. Chem. Program 2006, 25, 1018–1024. [Google Scholar]
- Zhao, B.; Su, Y.; Tao, W.; Li, L.; Peng, Y. Post-combustion CO2 capture by aqueous ammonia: A state-of-the-art review. Int. J. Greenh. Gas Control 2012, 9, 355–371. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Kenig, E.Y. CO2-alkanolamine reaction kinetics: A review of recent studies. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2007, 30, 1467–1474. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Edwards, T.J.; Newman, J.; Prausnitz, J.M. Thermodynamics of aqueous solutions containing volatile weak electrolytes. AIChE J. 1975, 21, 248–259. [Google Scholar] [CrossRef]
- Ahn, C.; Lee, H.; Lee, M.; Chang, Y.; Han, K.; Rhee, C.; Kim, J.; Chun, H.; Park, J. Determination of ammonium salt/ion speciation in the CO2 absorption process using ammonia solution: Modeling and experimental approaches. Energy Procedia 2011, 4, 541–547. [Google Scholar] [CrossRef]
- Zhao, Y.; Bian, Y.; Li, H.; Guo, H.; Shen, S.; Han, J.; Guo, D. A comparative study of aqueous potassium lysinate and aqueous monoethanolamine for postcombustion CO2 capture. Energy Fuels 2017, 31, 14033–14044. [Google Scholar] [CrossRef]
- Kim, Y.J.; You, J.K.; Hong, W.H.; Yi, K.B.; Ko, C.H.; Kim, J.N. Characteristics of CO2 absorption into aqueous ammonia. Sep. Sci. Technol. 2008, 43, 766–777. [Google Scholar] [CrossRef]
- Bahadori, F.; Esmaeili, N. Simulation of CO2 capture by aqueous solution of ammonia in shallow bubble column reactor. Rend. Lincei 2017, 28, 701–709. [Google Scholar] [CrossRef]
- Valenti, G.; Bonalumi, D. Chemical Absorption by Aqueous Solution of Ammonia. In Carbon Capture, Utilization and Sequestration; BoD—Books on Demand: Norderstedt, Germany, 2018; p. 107. [Google Scholar]
- Park, H.; Jung, Y.M.; You, J.K.; Hong, W.H.; Kim, J.-N. Analysis of the CO2 and NH3 reaction in an aqueous solution by 2D IR COS: Formation of bicarbonate and carbamate. J. Phys. Chem. A 2008, 112, 6558–6562. [Google Scholar] [CrossRef]
- Zhao, M.; Minett, A.I.; Harris, A.T. A review of techno-economic models for the retrofitting of conventional pulverised-coal power plants for post-combustion capture (PCC) of CO2. Energy Environ. Sci. 2013, 6, 25–40. [Google Scholar] [CrossRef]
- Cormos, C.-C. Techno-economic assessment of calcium and magnesium-based sorbents for post-combustion CO2 capture applied in fossil-fueled power plants. Fuel 2021, 298, 120794. [Google Scholar] [CrossRef]
- Savolainen, K.; Pylkkänen, L.; Norppa, H.; Falck, G.; Lindberg, H.; Tuomi, T.; Vippola, M.; Alenius, H.; Hämeri, K.; Koivisto, J. Nanotechnologies, engineered nanomaterials and occupational health and safety—A review. Saf. Sci. 2010, 48, 957–963. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Wu, P.-Y. Adsorption equilibrium of sulfur hexafluoride on multi-walled carbon nanotubes. J. Hazard. Mater. 2010, 178, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.M.; Zhao, X.S. Nanoporous Materials: Science and Engineering; World Scientific: Singapore, 2004; Volume 4. [Google Scholar]
- Zhang, Y.; Li, G.; Wu, Y.; Luo, Y.; Zhang, L. The formation of mesoporous TiO2 spheres via a facile chemical process. J. Phys. Chem. B 2005, 109, 5478–5481. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Franchi, R.S.; Harlick, P.J.; Sayari, A. Applications of pore-expanded mesoporous silica. 2. Development of a high-capacity, water-tolerant adsorbent for CO2. Ind. Eng. Chem. Res. 2005, 44, 8007–8013. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Belmabkhout, Y.; Sayari, A. Further investigations of CO2 capture using triamine-grafted pore-expanded mesoporous silica. Chem. Eng. J. 2010, 158, 513–519. [Google Scholar] [CrossRef]
- Bhagiyalakshmi, M.; Lee, J.Y.; Jang, H.T. Synthesis of mesoporous magnesium oxide: Its application to CO2 chemisorption. Int. J. Greenh. Gas Control 2010, 4, 51–56. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Liu, R.; Zhao, F.; Hu, Y. Synthesis method for silica needle-shaped nano-hollow structure. Mater. Lett. 2008, 62, 3401–3403. [Google Scholar] [CrossRef]
- Tao, F.; Gao, C.; Wen, Z.; Wang, Q.; Li, J.; Xu, Z. Cobalt oxide hollow microspheres with micro-and nano-scale composite structure: Fabrication and electrochemical performance. J. Solid State Chem. 2009, 182, 1055–1060. [Google Scholar] [CrossRef]
- Alexiadis, A.; Kassinos, S. Molecular dynamic simulations of carbon nanotubes in CO2 atmosphere. Chem. Phys. Lett. 2008, 460, 512–516. [Google Scholar] [CrossRef]
- Cinke, M.; Li, J.; Bauschlicher, C.W., Jr.; Ricca, A.; Meyyappan, M. CO2 adsorption in single-walled carbon nanotubes. Chem. Phys. Lett. 2003, 376, 761–766. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Cnen, W.; Bai, H.; Hwang, J.F. Capture of CO2 from flue gas via multiwalled carbon nanotubes. Sci. Total Environ. 2009, 407, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-C.; Lu, C.; Su, F.; Zeng, W.; Chen, W. Thermodynamics and regeneration studies of CO2 adsorption on multiwalled carbon nanotubes. Chem. Eng. Sci. 2010, 65, 1354–1361. [Google Scholar] [CrossRef]
- Florin, N.H.; Harris, A.T. Reactivity of CaO derived from nano-sized CaCO3 particles through multiple CO2 capture-and-release cycles. Chem. Eng. Sci. 2009, 64, 187–191. [Google Scholar] [CrossRef]
- Li, L.; King, D.L.; Nie, Z.; Li, X.S.; Howard, C. MgAl2O4 spinel-stabilized calcium oxide absorbents with improved durability for high-temperature CO2 capture. Energy Fuels 2010, 24, 3698–3703. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, M.; Florin, N.H.; Harris, A.T. Synthesis and characterization of CaO nanopods for high temperature CO2 capture. Ind. Eng. Chem. Res. 2009, 48, 10765–10770. [Google Scholar] [CrossRef]
- Nair, B.N.; Yamaguchi, T.; Kawamura, H.; Nakao, S.I.; Nakagawa, K. Processing of lithium zirconate for applications in carbon dioxide separation: Structure and properties of the powders. J. Am. Ceram. Soc. 2004, 87, 68–74. [Google Scholar] [CrossRef]
- Ochoa-Fernández, E.; Rønning, M.; Grande, T.; Chen, D. Synthesis and CO2 capture properties of nanocrystalline lithium zirconate. Chem. Mater. 2006, 18, 6037–6046. [Google Scholar] [CrossRef]
- Khomane, R.B.; Sharma, B.K.; Saha, S.; Kulkarni, B.D. Reverse microemulsion mediated sol–gel synthesis of lithium silicate nanoparticles under ambient conditions: Scope for CO2 sequestration. Chem. Eng. Sci. 2006, 61, 3415–3418. [Google Scholar] [CrossRef]
- Essaki, K.; Kato, M.; Nakagawa, K. CO2 removal at high temperature using packed bed of lithium silicate pellets. J. Ceram. Soc. Jpn. (日本セラミックス協会学術論文誌) 2006, 114, 739–742. [Google Scholar] [CrossRef]
- Wu, S.F.; Li, Q.H.; Kim, J.N.; Yi, K.B. Properties of a nano CaO/Al2O3 CO2 sorbent. Ind. Eng. Chem. Res. 2008, 47, 180–184. [Google Scholar] [CrossRef]
- Rehman, A.; Park, S.-J. Comparative study of activation methods to design nitrogen-doped ultra-microporous carbons as efficient contenders for CO2 capture. Chem. Eng. J. 2018, 352, 539–548. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Drage, T.; Maroto-Valer, M.M. Novel lithium-based sorbents from fly ashes for CO2 capture at high temperatures. Int. J. Greenh. Gas Control 2010, 4, 623–629. [Google Scholar] [CrossRef]
- Fauth, D.J.; Frommell, E.A.; Hoffman, J.S.; Reasbeck, R.P.; Pennline, H.W. Eutectic salt promoted lithium zirconate: Novel high temperature sorbent for CO2 capture. Fuel Process. Technol. 2005, 86, 1503–1521. [Google Scholar] [CrossRef]
- Ida, J.-i.; Xiong, R.; Lin, Y. Synthesis and CO2 sorption properties of pure and modified lithium zirconate. Sep. Purif. Technol. 2004, 36, 41–51. [Google Scholar] [CrossRef]
- Blamey, J.; Anthony, E.; Wang, J.; Fennell, P. The calcium looping cycle for large-scale CO2 capture. Prog. Energy Combust. Sci. 2010, 36, 260–279. [Google Scholar] [CrossRef]
- Arcenegui-Troya, J.; Sánchez-Jiménez, P.E.; Perejón, A.; Valverde, J.M.; Chacartegui, R.; Pérez-Maqueda, L.A. Calcium-looping performance of biomineralized CaCO3 for CO2 capture and thermochemical energy storage. Ind. Eng. Chem. Res. 2020, 59, 12924–12933. [Google Scholar] [CrossRef]
- Dou, B.; Song, Y.; Liu, Y.; Feng, C. High temperature CO2 capture using calcium oxide sorbent in a fixed-bed reactor. J. Hazard. Mater. 2010, 183, 759–765. [Google Scholar] [CrossRef]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar] [CrossRef]
- Lausselet, C.; Cherubini, F.; Oreggioni, G.D.; del Alamo Serrano, G.; Becidan, M.; Hu, X.; Rørstad, P.K.; Strømman, A.H. Norwegian Waste-to-Energy: Climate change, circular economy and carbon capture and storage. Resour. Conserv. Recycl. 2017, 126, 50–61. [Google Scholar] [CrossRef]
- Pires da Mata Costa, L.; Micheline Vaz de Miranda, D.; Couto de Oliveira, A.C.; Falcon, L.; Stella Silva Pimenta, M.; Guilherme Bessa, I.; Juarez Wouters, S.; Andrade, M.H.S.; Pinto, J.C. Capture and Reuse of Carbon Dioxide (CO2) for a Plastics Circular Economy: A Review. Processes 2021, 9, 759. [Google Scholar] [CrossRef]
- González-Aparicio, I.; Pérez-Fortes, M.; Zucker, A.; Tzimas, E. Opportunities of Integrating CO2 Utilization with RES-E: A Power-to-Methanol Business Model with Wind Power Generation. Energy Procedia 2017, 114, 6905–6918. [Google Scholar] [CrossRef]
- Muthuraj, R.; Mekonnen, T. Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 2018, 145, 348–373. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Viebahn, P.; Vallentin, D.; Höller, S. Integrated Assessment of Carbon Capture and Storage (CCS) in South Africa’s Power Sector. Energies 2015, 8, 14380–14406. [Google Scholar] [CrossRef]
- Viebahn, P.; Vallentin, D.; Höller, S. Prospects of carbon capture and storage (CCS) in China’s power sector—An integrated assessment. Appl. Energy 2015, 157, 229–244. [Google Scholar] [CrossRef]
- Brownsort, P. Worldwide Comparison of CO₂-EOR Conditions: Comparison of Fiscal and Industrial Conditions in Seven Global Regions Where CO₂-EOR Is Active or under Consideration; Scottish Carbon Capture and Storage (SCCS): Edinburgh, UK, 2015. [Google Scholar]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- Tcvetkov, P.; Cherepovitsyn, A.; Fedoseev, S. The Changing Role of CO2 in the Transition to a Circular Economy: Review of Carbon Sequestration Projects. Sustainability 2019, 11, 5834. [Google Scholar] [CrossRef]
- Mangalapally, H.P.; Notz, R.; Hoch, S.; Asprion, N.; Sieder, G.; Garcia, H.; Hasse, H. Pilot plant experimental studies of post combustion CO2 capture by reactive absorption with MEA and new solvents. Energy Procedia 2009, 1, 963–970. [Google Scholar] [CrossRef]
- Bollinger, R.; Muraskin, D.; Hammond, M.; Kozak, F.; Spitznogle, G.; Cage, M.; Sherrick, B.; Varner, M. CCS Project with Alstom’s Chilled Ammonia Process at AEP’s Mountaineer Plant. Alstom Power Systems Technical Report Paper. 2010. Available online: https://www.mcilvainecompany.com/Decision_Tree/subscriber/CO2DescriptionTextLinks/AlstomMountaineer.pdf (accessed on 12 October 2022).
- Darde, V.; Thomsen, K.; van Well, W.J.; Stenby, E.H. Chilled ammonia process for CO2 capture. Energy Procedia 2009, 1, 1035–1042. [Google Scholar] [CrossRef]
- Augustsson, O.; Baburao, B.; Dube, S.; Bedell, S.; Strunz, P.; Balfe, M.; Stallmann, O. Chilled ammonia process scale-up and lessons learned. Energy Procedia 2017, 114, 5593–5615. [Google Scholar] [CrossRef]
- Lu, J.; Wang, L.; Sun, X.; Li, J.; Liu, X. Absorption of CO2 into aqueous solutions of methyldiethanolamine and activated methyldiethanolamine from a gas mixture in a hollow fiber contactor. Ind. Eng. Chem. Res. 2005, 44, 9230–9238. [Google Scholar] [CrossRef]
- Feng, Z.; Fang, C.-G.; Wu, Y.-T.; Wang, Y.-T.; Li, A.-M.; Zhang, Z.-B. Absorption of CO2 in the aqueous solutions of functionalized ionic liquids and MDEA. Chem. Eng. J. 2010, 160, 691–697. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.-F.; Xu, G.-W.; Gao, W.-H.; Wu, Y.-Q. An experimental apparatus to mimic CO2 removal and optimum concentration of MDEA aqueous solution. Ind. Eng. Chem. Res. 2001, 40, 898–901. [Google Scholar] [CrossRef]
- Closmann, F.; Nguyen, T.; Rochelle, G.T. MDEA/Piperazine as a solvent for CO2 capture. Energy Procedia 2009, 1, 1351–1357. [Google Scholar] [CrossRef]
- Amann, J.-M.G.; Bouallou, C. A new aqueous solvent based on a blend of N-methyldiethanolamine and triethylene tetramine for CO2 recovery in post-combustion: Kinetics study. Energy Procedia 2009, 1, 901–908. [Google Scholar] [CrossRef]
- Choi, W.-J.; Seo, J.-B.; Jang, S.-Y.; Jung, J.-H.; Oh, K.-J. Removal characteristics of CO2 using aqueous MEA/AMP solutions in the absorption and regeneration process. J. Environ. Sci. 2009, 21, 907–913. [Google Scholar] [CrossRef]
- Sánchez, L.G.; Meindersma, G.; De Haan, A. Solvent properties of functionalized ionic liquids for CO2 absorption. Chem. Eng. Res. Des. 2007, 85, 31–39. [Google Scholar] [CrossRef]
- Hasib-ur-Rahman, M.; Siaj, M.; Larachi, F. Ionic liquids for CO2 capture—Development and progress. Chem. Eng. Process. Process Intensif. 2010, 49, 313–322. [Google Scholar] [CrossRef]
- Jung, W.; Lee, J. Economic evaluation for four different solid sorbent processes with heat integration for energy-efficient CO2 capture based on PEI-silica sorbent. Energy 2022, 238, 121864. [Google Scholar] [CrossRef]
- Kiani, A.; Jiang, K.; Feron, P. Techno-Economic Assessment for CO Conventional 2 Capture From Liquid-Based Air Using a Absorption Process. In The Role of Carbon Capture and Storage (CCS) Technologies in a Net-Zero Carbon Future; Frontiers in Energy Research: Lausanne, Switzerland, 2021; p. 7. [Google Scholar]
- Bernhardsen, I.M.; Knuutila, H.K. A review of potential amine solvents for CO2 absorption process: Absorption capacity, cyclic capacity and pKa. Int. J. Greenh. Gas Control 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Guo, H.; Li, C.; Shi, X.; Li, H.; Shen, S. Nonaqueous amine-based absorbents for energy efficient CO2 capture. Appl. Energy 2019, 239, 725–734. [Google Scholar] [CrossRef]
- Bonenfant, D.; Mimeault, M.; Hausler, R. Comparative analysis of the carbon dioxide absorption and recuperation capacities in aqueous 2-(2-aminoethylamino) ethanol (AEE) and blends of aqueous AEE and N-methyldiethanolamine solutions. Ind. Eng. Chem. Res. 2005, 44, 3720–3725. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Veawab, A. Characterization and comparison of the CO2 absorption performance into single and blended alkanolamines in a packed column. Ind. Eng. Chem. Res. 2004, 43, 2228–2237. [Google Scholar] [CrossRef]
- Hosseini-Ardali, S.M.; Hazrati-Kalbibaki, M.; Fattahi, M.; Lezsovits, F. Multi-objective optimization of post combustion CO2 capture using methyldiethanolamine (MDEA) and piperazine (PZ) bi-solvent. Energy 2020, 211, 119035. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Duan, D.; Jiang, S.; Nešić, S. Mechanistic modeling of carbon steel corrosion in a methyldiethanolamine (MDEA)-based carbon dioxide capture process. Corrosion 2013, 69, 551–559. [Google Scholar] [CrossRef]
- Mandal, B.; Bandyopadhyay, S. Absorption of carbon dioxide into aqueous blends of 2-amino-2-methyl-1-propanol and monoethanolamine. Chem. Eng. Sci. 2006, 61, 5440–5447. [Google Scholar] [CrossRef]
- Dubois, L.; Thomas, D. CO2 absorption into aqueous solutions of monoethanolamine, methyldiethanolamine, piperazine and their blends. Chem. Eng. Technol. 2009, 32, 710–718. [Google Scholar] [CrossRef]
- Torralba-Calleja, E.; Skinner, J.; Gutiérrez-Tauste, D. CO2 Capture in Ionic Liquids: A Review of Solubilities and Experimental Methods. J. Chem. 2013, 2013, 473584. [Google Scholar] [CrossRef]
- Liu, F.; Huang, K.; Jiang, L. Promoted adsorption of CO2 on amine-impregnated adsorbents by functionalized ionic liquids. AIChE J. 2018, 64, 3671–3680. [Google Scholar] [CrossRef]
- Akinola, T.E.; Oko, E.; Wang, M. Study of CO2 removal in natural gas process using mixture of ionic liquid and MEA through process simulation. Fuel 2019, 236, 135–146. [Google Scholar] [CrossRef]
- Cao, L.; Gao, J.; Zeng, S.; Dong, H.; Gao, H.; Zhang, X.; Huang, J. Feasible ionic liquid-amine hybrid solvents for carbon dioxide capture. Int. J. Greenh. Gas Control 2017, 66, 120–128. [Google Scholar] [CrossRef]
- Drage, T.C.; Arenillas, A.; Smith, K.M.; Pevida, C.; Piippo, S.; Snape, C.E. Preparation of carbon dioxide adsorbents from the chemical activation of urea–formaldehyde and melamine–formaldehyde resins. Fuel 2007, 86, 22–31. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Shehata, N.; Alami, A.H.; Maghrabie, H.M.; Abdelkareem, M.A. Prospect of Post-Combustion Carbon Capture Technology and Its Impact on the Circular Economy. Energies 2022, 15, 8639. https://doi.org/10.3390/en15228639
Olabi AG, Wilberforce T, Sayed ET, Shehata N, Alami AH, Maghrabie HM, Abdelkareem MA. Prospect of Post-Combustion Carbon Capture Technology and Its Impact on the Circular Economy. Energies. 2022; 15(22):8639. https://doi.org/10.3390/en15228639
Chicago/Turabian StyleOlabi, A. G., Tabbi Wilberforce, Enas Taha Sayed, Nabila Shehata, Abdul Hai Alami, Hussein M. Maghrabie, and Mohammad Ali Abdelkareem. 2022. "Prospect of Post-Combustion Carbon Capture Technology and Its Impact on the Circular Economy" Energies 15, no. 22: 8639. https://doi.org/10.3390/en15228639
APA StyleOlabi, A. G., Wilberforce, T., Sayed, E. T., Shehata, N., Alami, A. H., Maghrabie, H. M., & Abdelkareem, M. A. (2022). Prospect of Post-Combustion Carbon Capture Technology and Its Impact on the Circular Economy. Energies, 15(22), 8639. https://doi.org/10.3390/en15228639










