Optical Evaluation of Effects of Energy Substrates on PHB Accumulation for Bioplastic Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Microorganisms—Environmental Conditions
2.2. The Laboratory Analyses—Evaluation of the Culture Condition
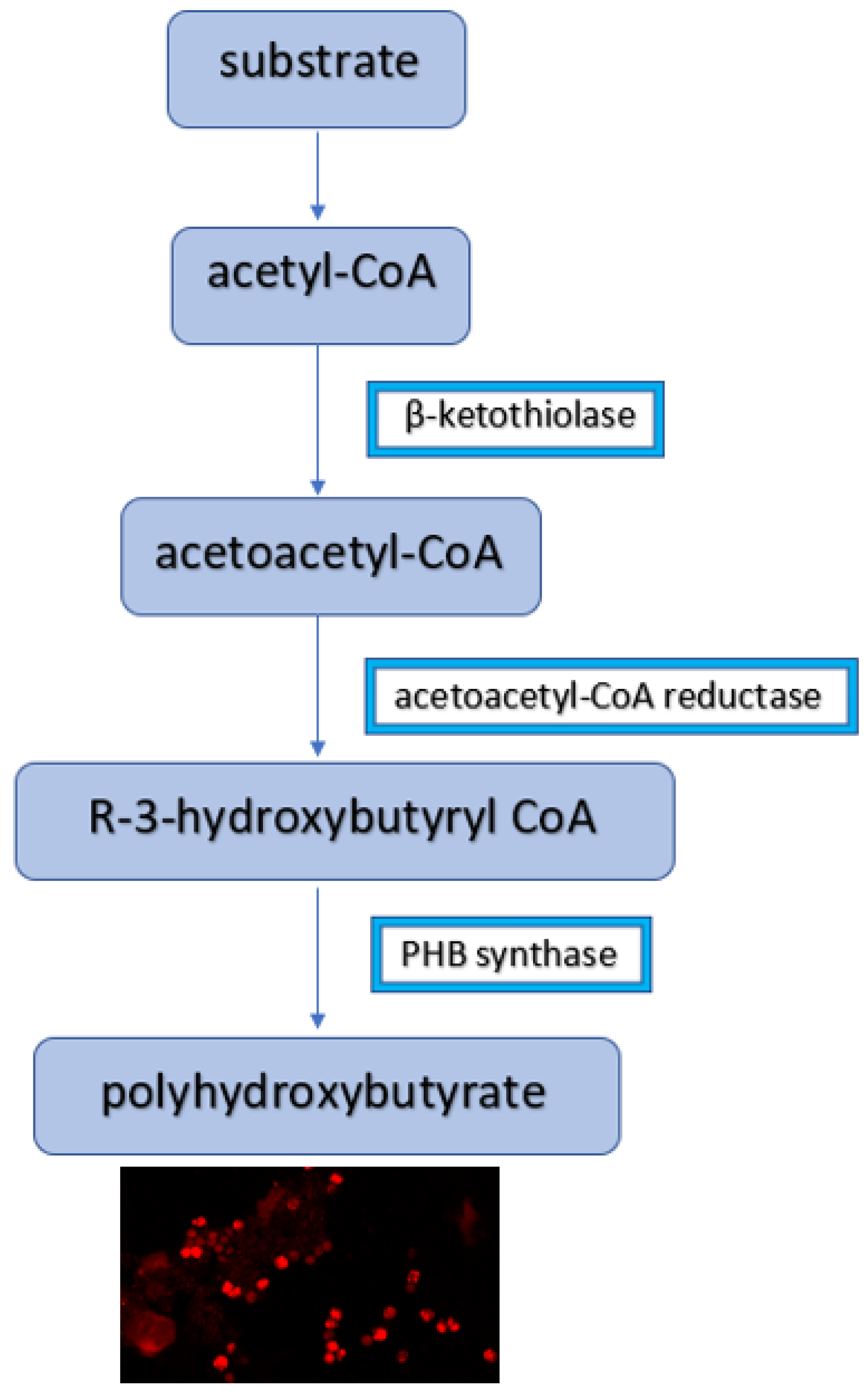
2.3. Analysis of Microscopic Images
3. Results
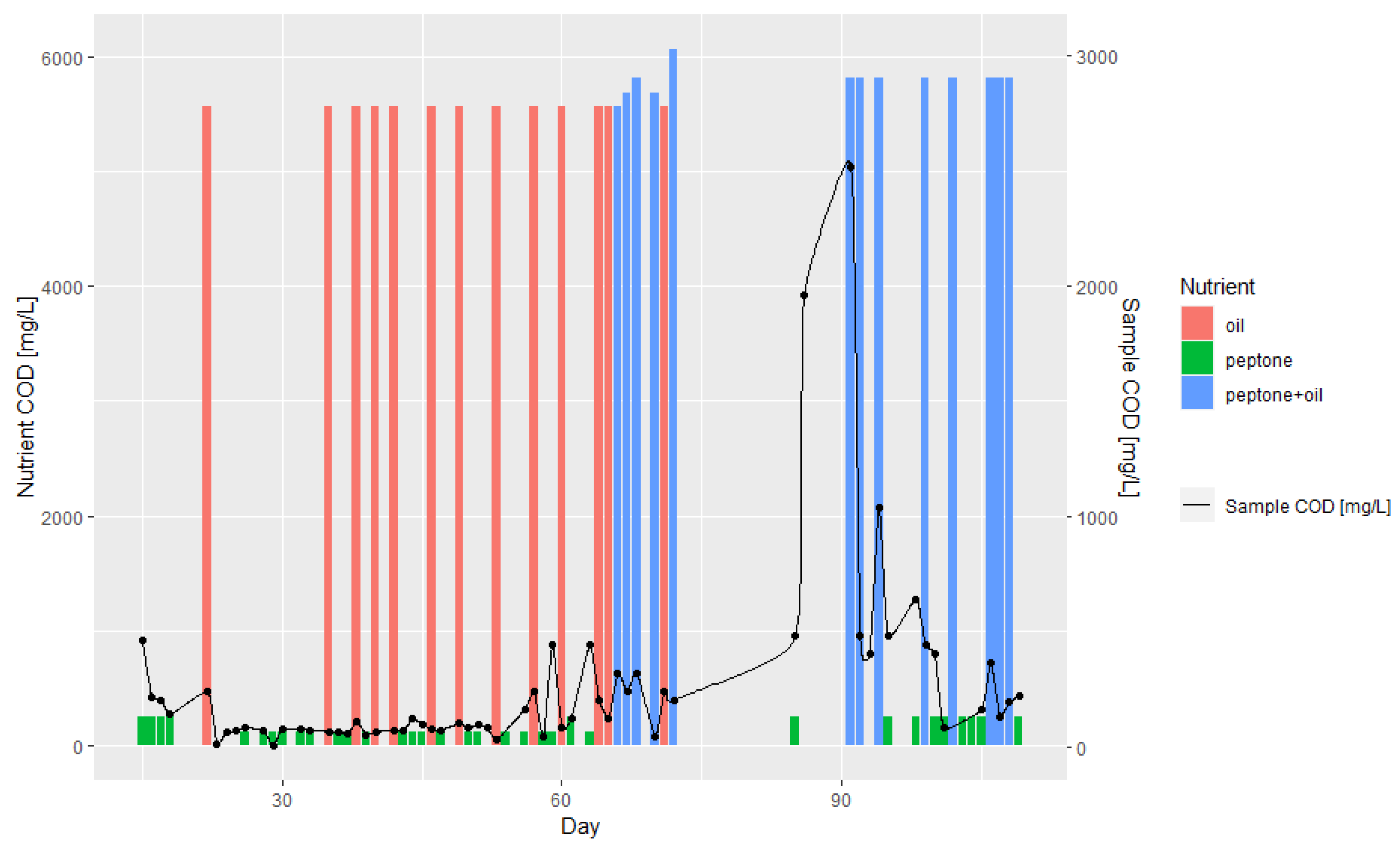
3.1. Batch and Continuous-Flow Bioreactors
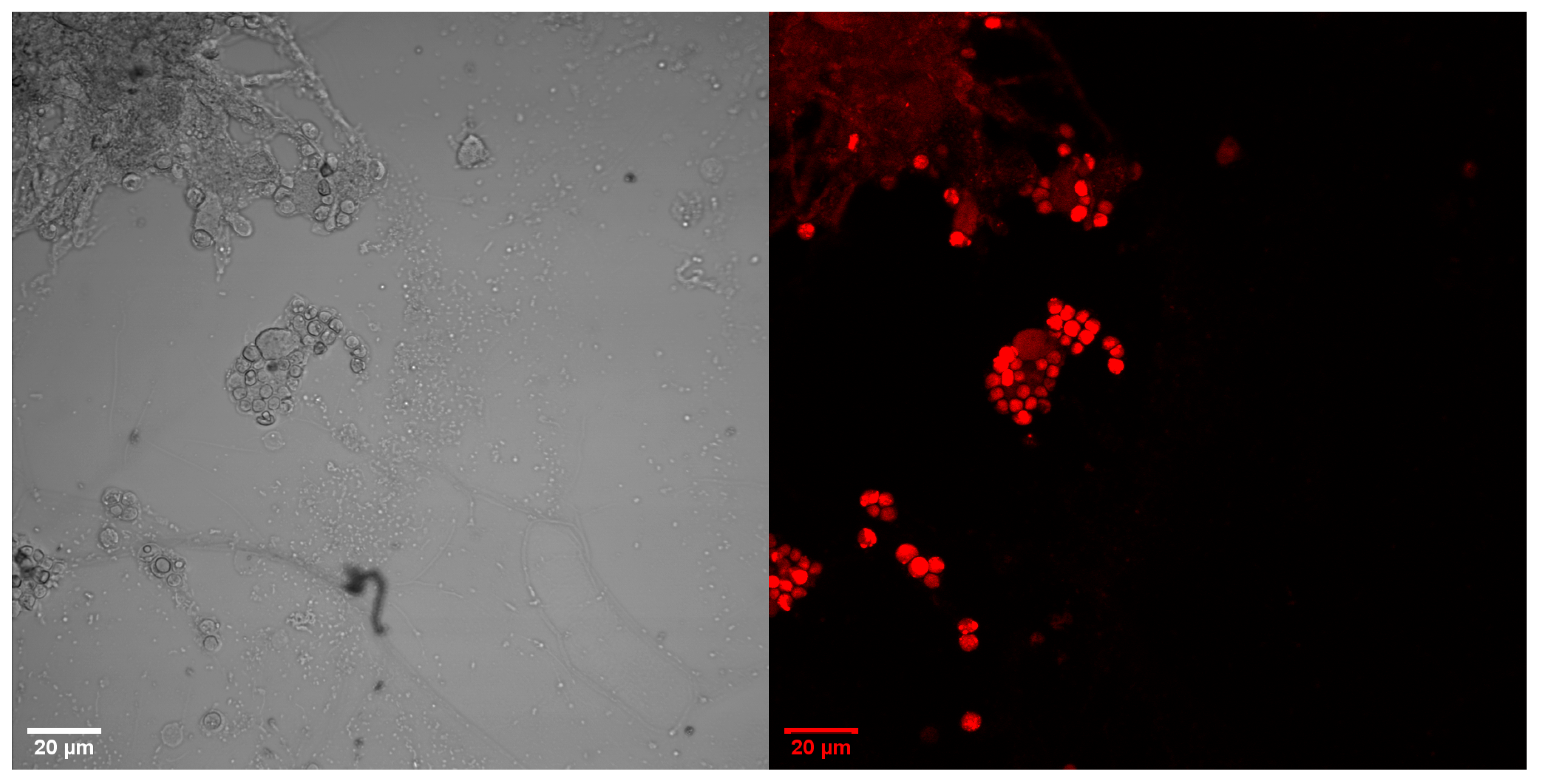
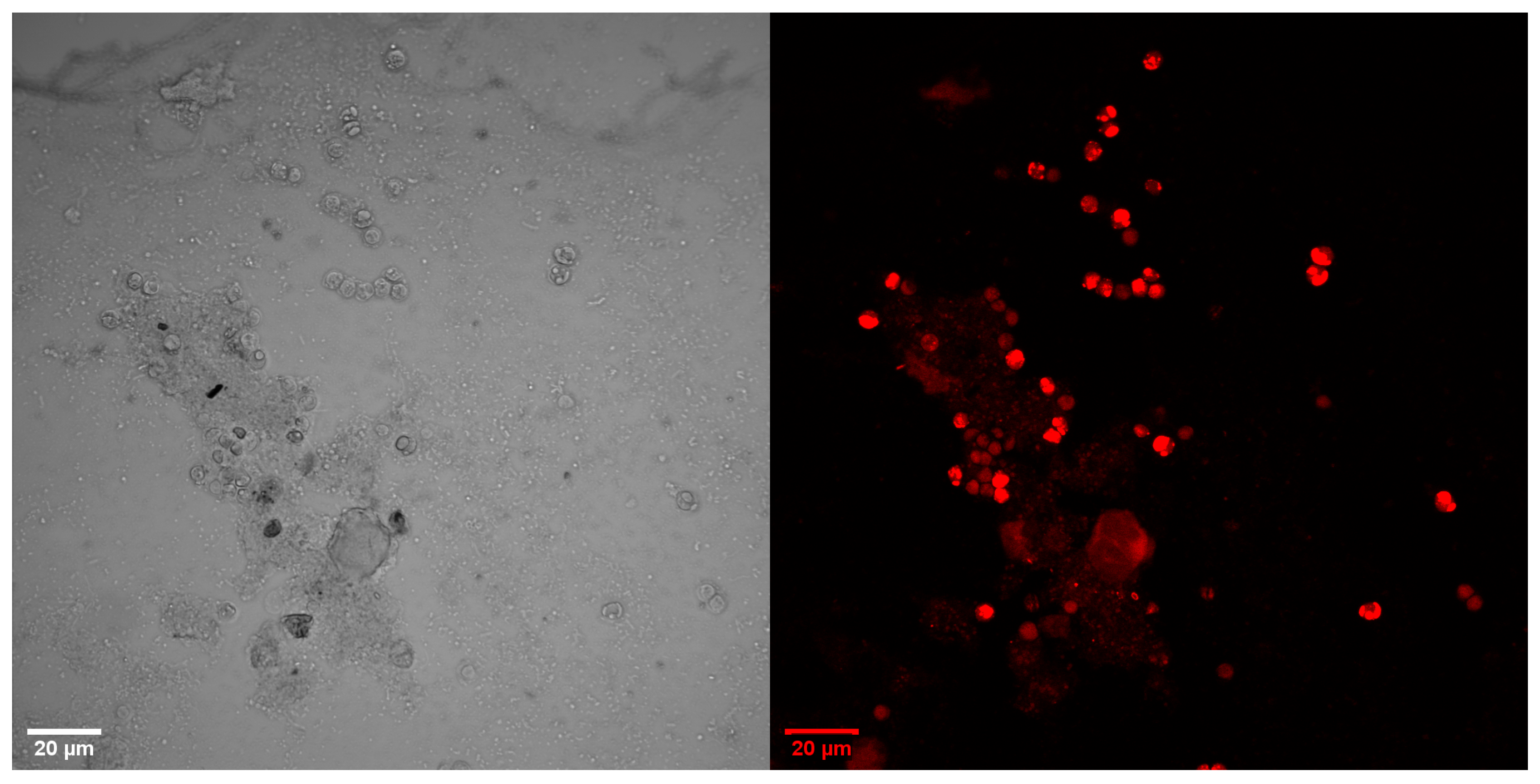
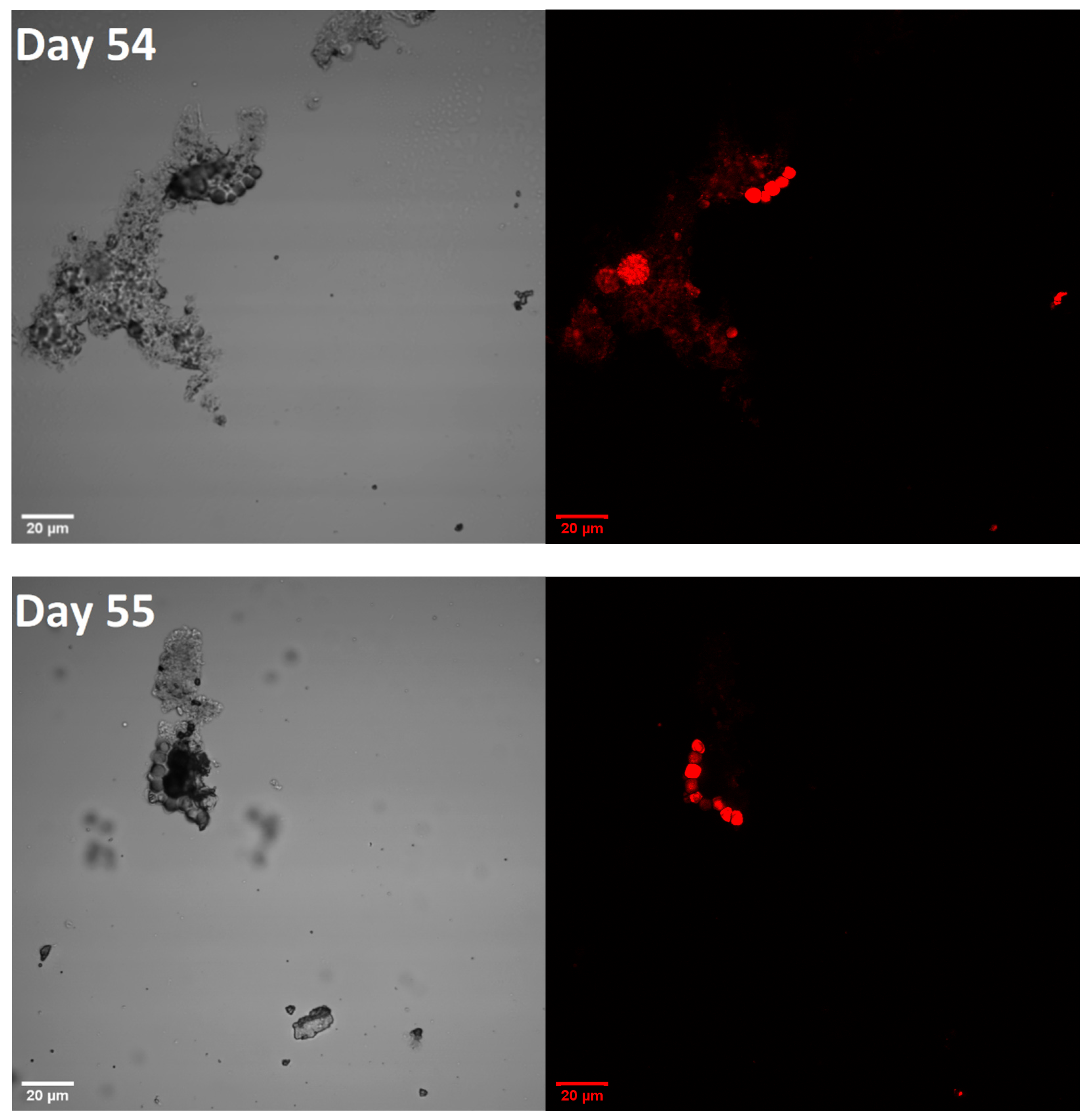
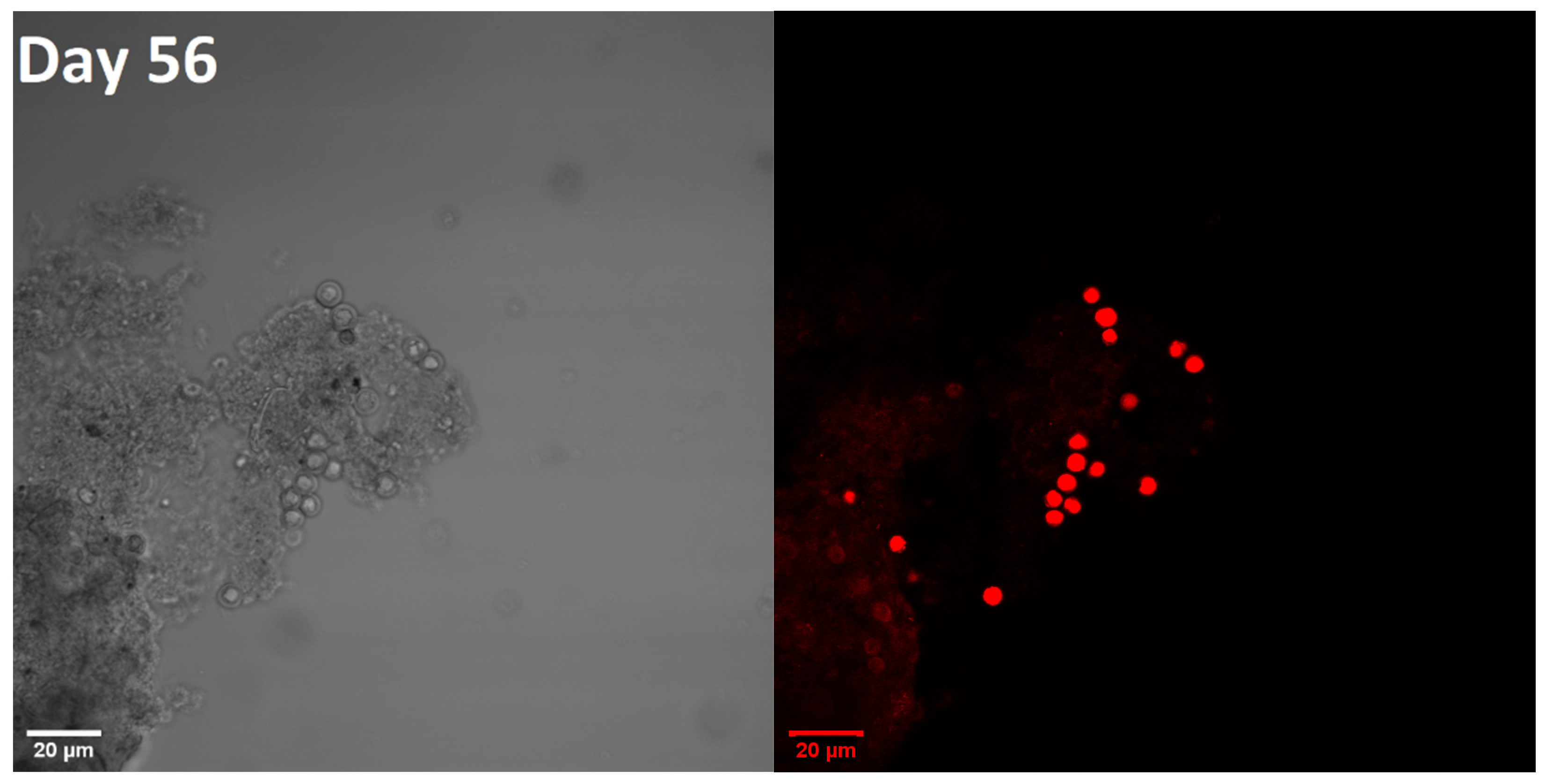
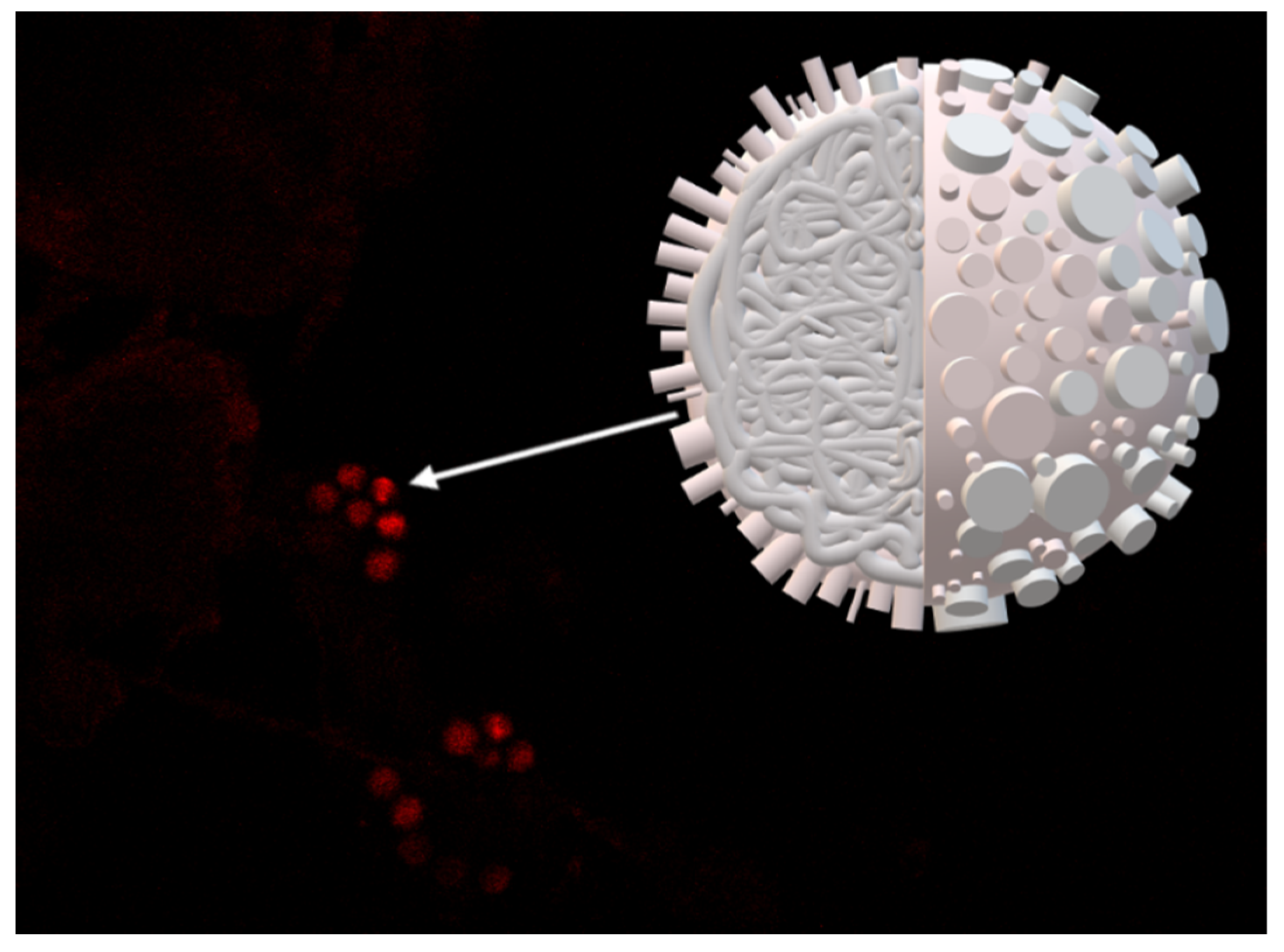
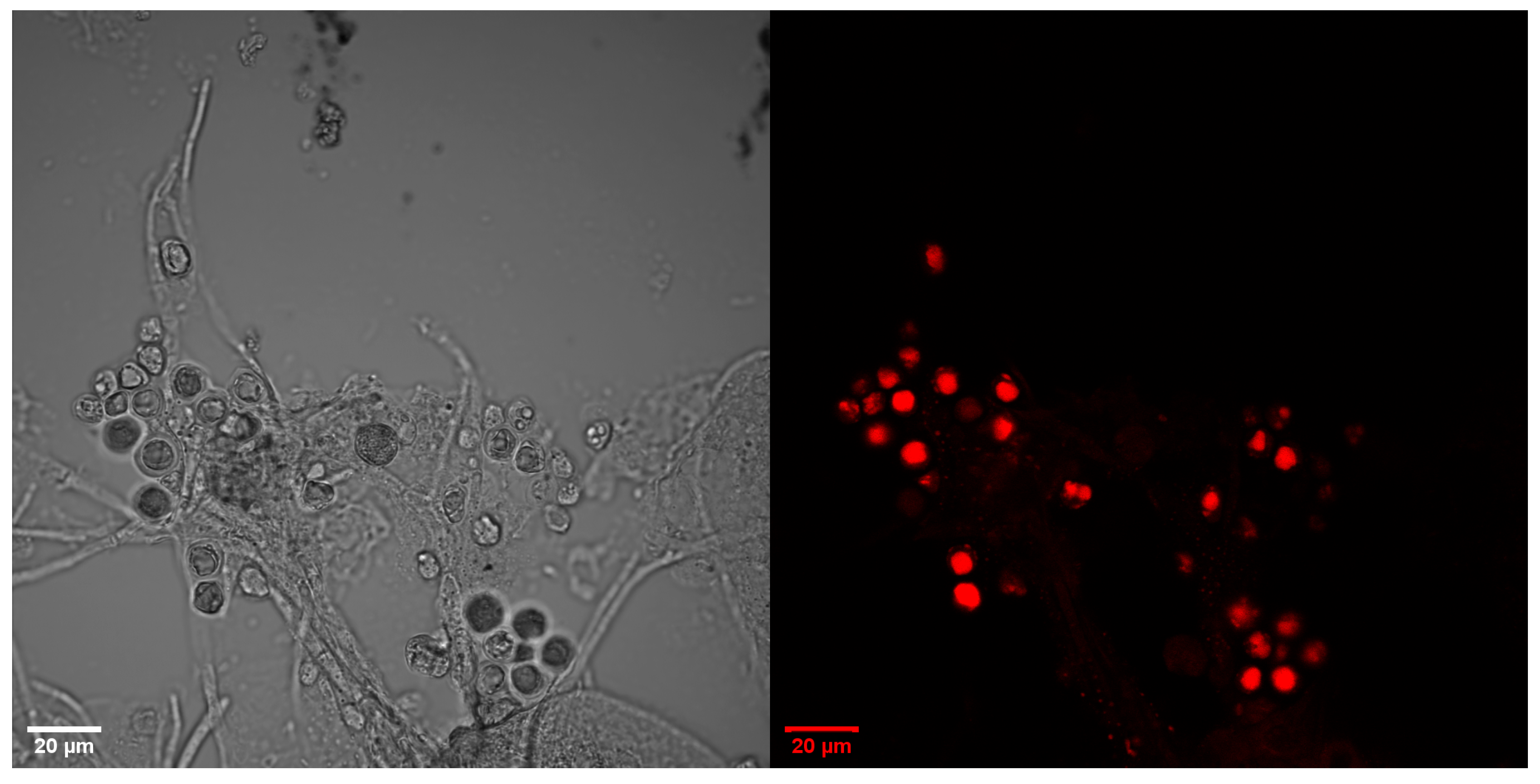
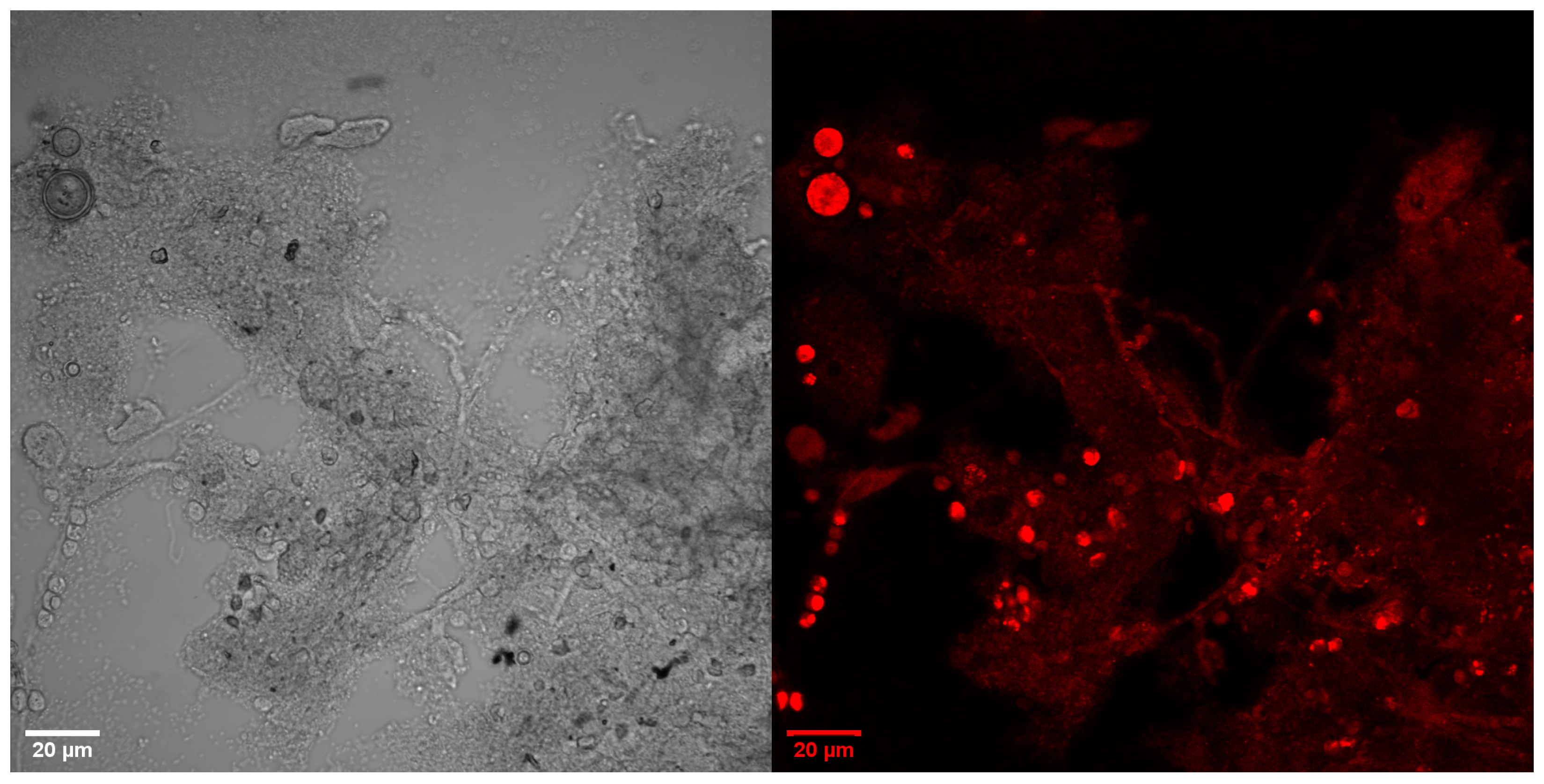
3.2. Microscopic Images
3.3. Optical Analysis and Lipase Activity
4. Discussion and Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Gamal, R.F.; Abdelhady, H.M.; Khodair, T.A.; El-Tayeb, T.S.; Hassan, E.A.; Aboutaleb, K.A. Semi-scale production of PHAs from waste frying oil by Pseudomonas fluorescens S48. Braz. J. Microbiol. 2013, 44, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Roser, M. Plastic pollution. Our World in Data. 2018. Available online: https://ourworldindata.org/plastic-pollution (accessed on 1 September 2022).
- Coppola, G.; Gaudio, M.T.; Lopresto, C.G.; Calabro, V.; Curcio, S.; Chakraborty, S. Bioplastic from renewable biomass: A facile solution for a greener environment. Earth Syst. Environ. 2021, 5, 231–251. [Google Scholar] [CrossRef]
- Guzik, M. Bioplastiki Zapożyczone ze Świata Mikrobów, Technologiczny Niepokój. 2021. Available online: https://formy.xyz/artykul/bioplastiki-zapozyczone-ze-swiata-mikrobow/ (accessed on 11 October 2022).
- Pasternak, G. Bioreaktory oraz ich zastosowanie w inżynierii i ochronie środowiska. J. Ecol. Health 2011, 15, 121–125. [Google Scholar]
- Donnarumma, G.; Buommino, E.; Fusco, A.; Paoletti, I.; Auricchio, L.; Tufano, M.A. Effect of temperature on the shift of Pseudomonas fluorescens from an environmental microorganism to a potential human pathogen. Int. J. Immunopathol. Pharmacol. 2010, 23, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, E. Biodegradable polyesters-polyhydroxyalkanoic acids (PHA)-synthetized by microorganisms. Biotechnologia 1996, 3, 97–115. [Google Scholar]
- Carpine, R.; Olivieri, G.; Hellingwerf, K.J.; Pollio, A.; Marzocchella, A. Industrial production of poly-β-hydroxybutyrate from CO2: Can cyanobacteria meet this challenge? Processes 2020, 8, 323. [Google Scholar] [CrossRef]
- Du, C.; Webb, C. Engineering Fundamentals of Biotechnology. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 2. [Google Scholar]
- Ushani, U.; Sumayya, A.R.; Archana, G.; Banu, J.R.; Dai, J. Enzymes/biocatalysts and bioreactors for valorization of food wastes. In Food Waste to Valuable Resources; Academic Press: Cambridge, MA, USA, 2020; pp. 211–233. [Google Scholar]
- Polyhydroxybutyrate. Available online: https://en.wikipedia.org/wiki/Polyhydroxybutyrate?fbclid=IwAR3phUlkTepcn6T_juLIGndkUbcFC9XwysgJ3titSdP8SZO4T2au7PHZbUQ (accessed on 11 October 2022).
- Amadu, A.A.; Qiu, S.; Ge, S.; Addico, G.N.D.; Ameka, G.K.; Yu, Z.; Xia, W.; Abbew, A.-W.; Shao, D.; Champagne, P.; et al. A review of biopolymer (Poly-β-hydroxybutyrate) synthesis in microbes cultivated on wastewater. Sci. Total Environ. 2021, 756, 143729. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.L.; Xia, J.L.; Song, Z.W.; Jiang, P.; Xia, Y.; Wan, M.X.; Zhang, R.Y.; Yang, Y.; Liu, K.K. The effect of energy substrates on PHB accumulation of Acidiphilium cryptum DX1-1. Curr. Microbiol. 2013, 67, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes 2016, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Jankiewicz, U. Characteristic and significance of pyoverdines of the genus Pseudomonas. Adv. Microbiol. 2009, 48, 243–254. [Google Scholar]
- Michałkiewicz, M. Mikrobiologia ścieków. Technol. Wody 2018, 6, 58–62. [Google Scholar]
- Tang, M.; Jiang, J.; Lv, Q.; Yang, B.; Zheng, M.; Gao, X.; Han, J.; Zhang, Y.; Yang, Y. Denitrification performance of Pseudomonas fluorescens Z03 immobilized by graphene oxide-modified polyvinyl-alcohol and sodium alginate gel beads at low temperature. R. Soc. Open Sci. 2020, 7, 191542. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, H.; Xia, Y.; Kang, Z.; Qi, Q. Complete PHB mobilization in Escherichia coli enhances the stress tolerance: A potential biotechnological application. Microb. Cell Factories 2009, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Luther, A.K. Ammonia Toxicity in Bacteria and Its Implications for Treatment of and Resource Recovery from Highly Nitrogenous Organic Wastes; Rutgers The State University of New Jersey: New Brunswick, NJ, USA, 2015. [Google Scholar]
- Fiedurek, J.; Trytek, M. The effect of acid and osmotic stress on metabolite production by microorganisms. Post. Mikrobiol. 2016, 55, 195–204. [Google Scholar]
- Jeong, H.; Park, J.; Kim, H. Determination of NH4+ in environmental water with interfering substances using the modified Nessler method. J. Chem. 2013, 2013, 359217. [Google Scholar] [CrossRef]
- PanReac AppliChem ITW Reagents, Reagents for COD Analysis. Available online: https://www.itwreagents.com/download_file/info_point/IP-025/en/IP-025_en.pdf (accessed on 11 October 2022).
- Gaca, J.; Duszkiewicz, J.; Michalska, B. Porównanie Metod Oznaczania Chemicznego Zapotrzebowania Tlenu w Obecności Wysokiego Stężenia Chlorków. 1999. Available online: http://repozytorium.ukw.edu.pl/handle/item/3020 (accessed on 28 August 2022).
- Cerk, I.K.; Wechselberger, L.; Oberer, M. Adipose triglyceride lipase regulation: An overview. Curr. Protein Pept. Sci. 2018, 19, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. How Does Sudan III Detect Lipids? Rehabilitationrobotics.net. 2021. Available online: https://rehabilitationrobotics.net/how-does-sudan-iii-detect-lipids/ (accessed on 11 October 2022).
- Iyer, G.; Menon, S.; Gupte, Y.; Phadnis, S.; Pawar, Y. Morpho-physiological characteristics of Botryococcus Braunii (Kutzing, 1849) & its oil production from the species isolated from thane, Maharashtra, India. Asian J. Microbiol. Biotechnol. Environ. Sci. 2012, 14, 523–526. [Google Scholar]
- Aremu, M.O.; Olu-Arotiowa, O.A.; Layokun, S.K.; Solomon, B.O. Growth of Pseudomonas fluorescens on cassava starch hydrolysate for polyhydroxybutyrate production. J. Appl. Sci. Environ. Manag. 2010, 14, 61–66. [Google Scholar] [CrossRef]
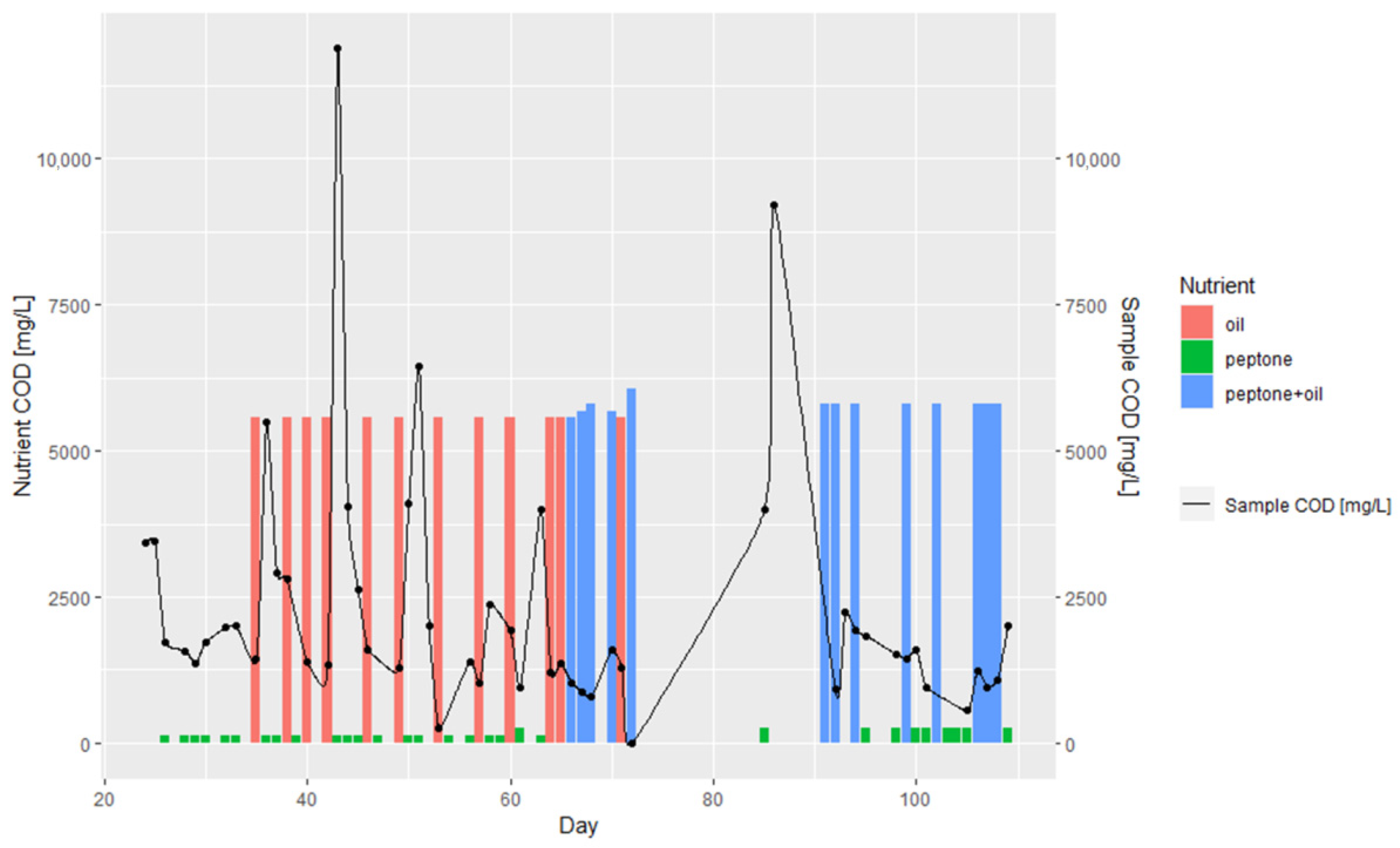
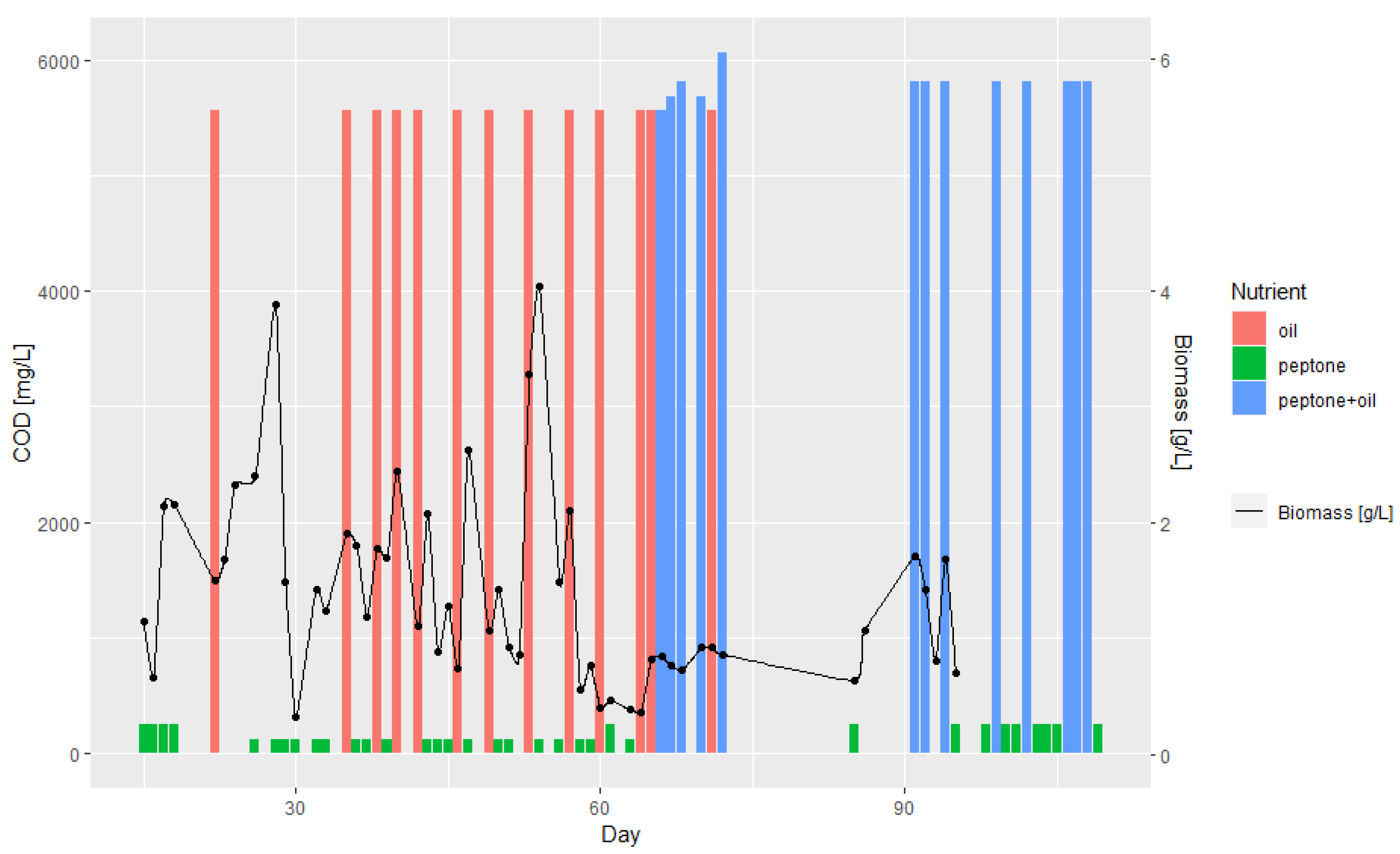
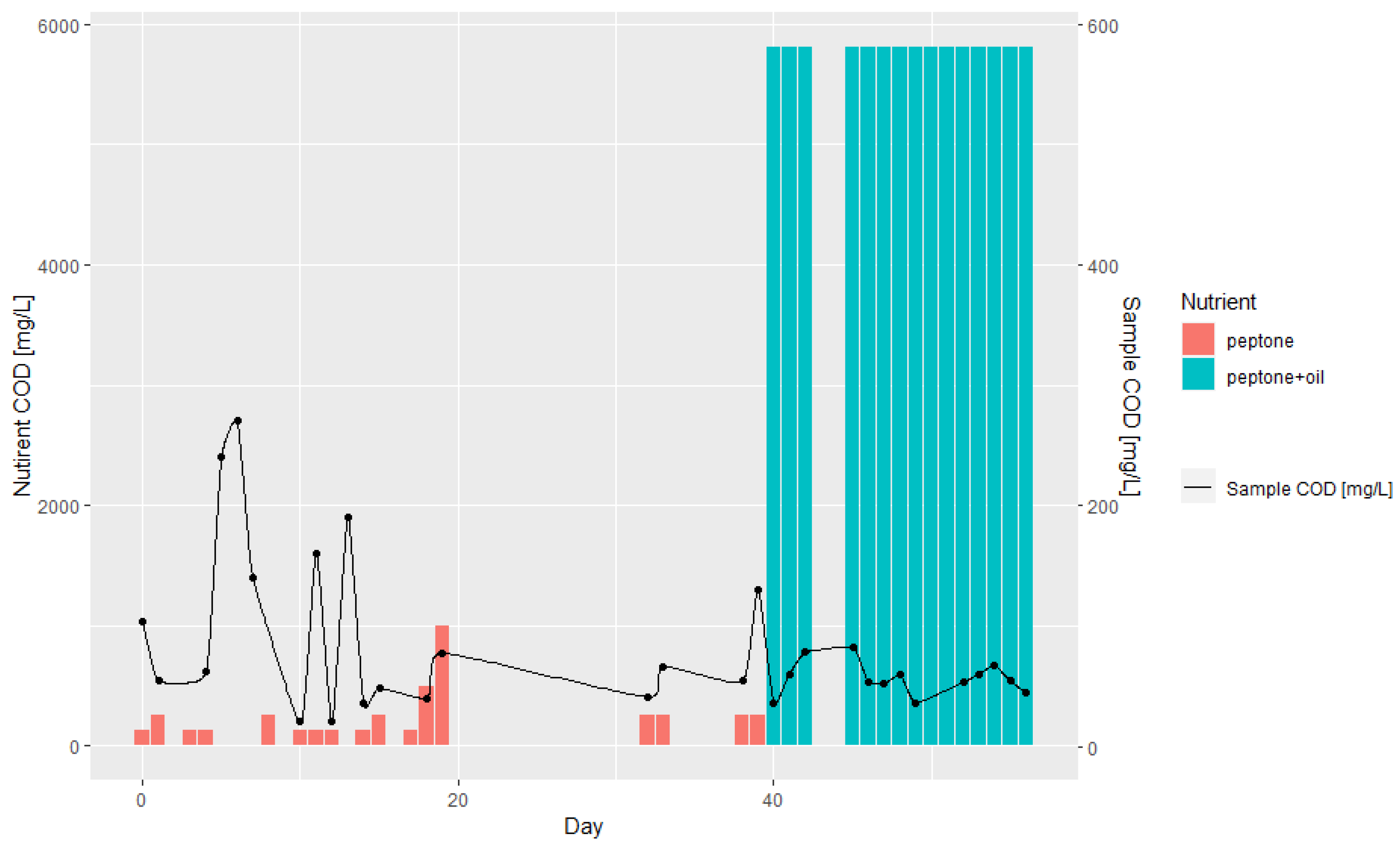
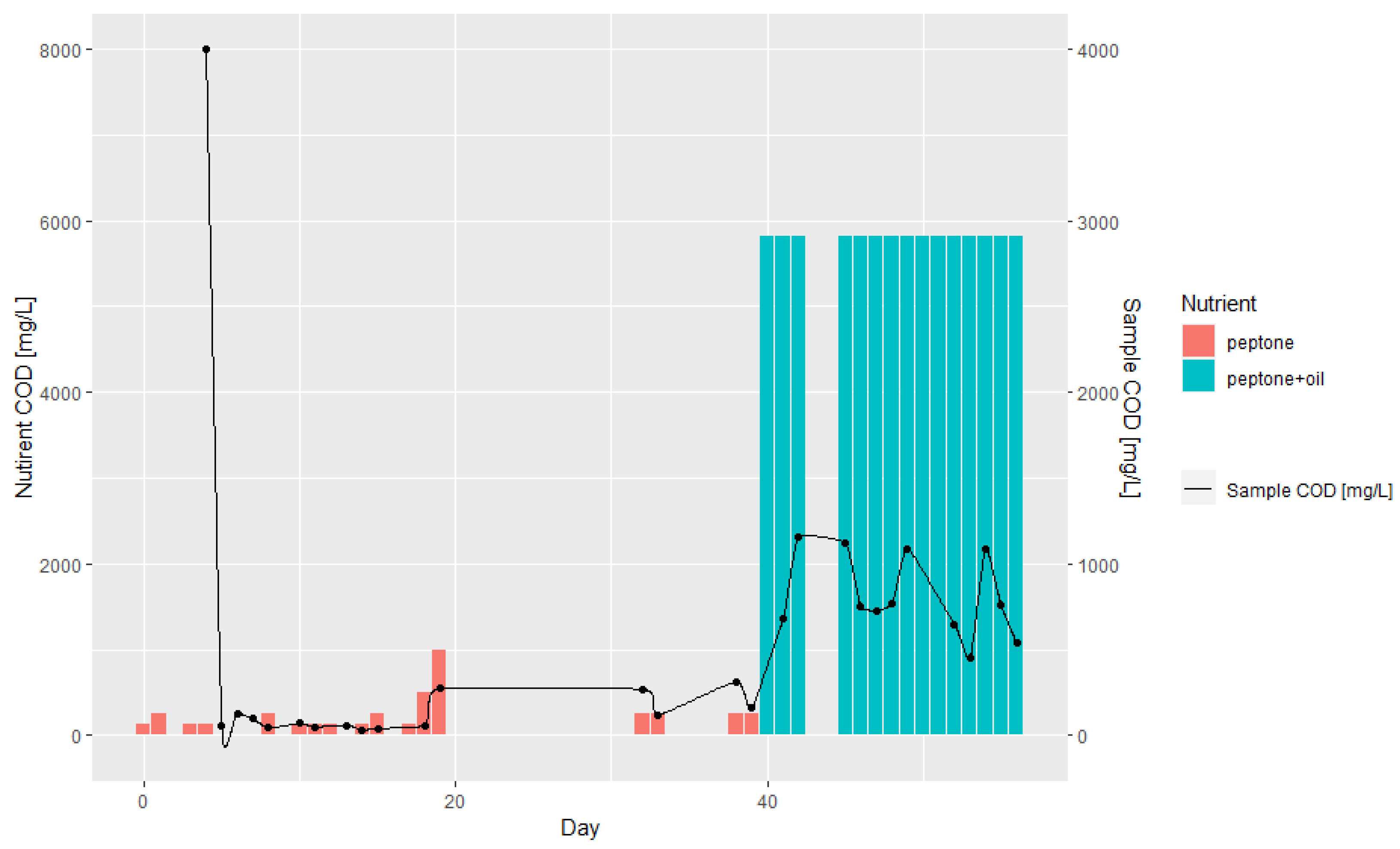



| Day | Lipase Activity, mmol/dm3/s | Nitrogen, mg/L | COD, mg/L | COD (w/o Filtration), mg/L | Biomass, g/L | Oil, mL | Peptone, g |
|---|---|---|---|---|---|---|---|
| 57 | 0.3006 | 4.15 | 160 | 1400 | 1.48 | 0 | 0.25 |
| 58 | 0.2213 | 7.75 | 240 | 1040 | 2.10 | 1 | 0 |
| 59 | 0.1545 | 12.5 | 40 | 2360 | 0.56 | 0 | 0.25 |
| 60 | 0.0083 | 2.75 | 440 | - | 0.76 | 0 | 0.25 |
| 61 | 0.1670 | 15.00 | 80 | 1920 | 0.40 | 1 | 0 |
| 62 | 0.1044 | 7.00 | 120 | 960 | 0.46 | 0 | 0.50 |
| 64 | 0.0543 | 11.5 | 440 | 4000 | 0.38 | 0 | 0.25 |
| 65 | 0.0835 | 13.25 | 200 | 1200 | 0.36 | 1 | 0 |
| 66 | 0.1336 | 3.75 | 120 | 1360 | 0.82 | 1 | 0 |
| Day | Lipase Activity, mmol/dm3/s | Nitrogen, mg/L | COD, mg/L | COD (w/o Filtration), mg/L | Biomass, g/L | Oil, mL | Peptone, g | H2O, L | NaHCO3, g |
|---|---|---|---|---|---|---|---|---|---|
| 53 | 0.2789 | 0.45 | 53 | 648 | 0.46 | 1 | 0.5 | 0.5 | 0.2 |
| 54 | 0.2755 | 1.05 | 60 | 448 | 0.48 | 1 | 0.5 | 0.5 | 0.2 |
| 55 | 0.2847 | 0.40 | 67 | 648 | 0.68 | 1 | 0.5 | 0.5 | 0.2 |
| 56 | 0.3273 | 0.50 | 54 | 760 | 0.56 | 1 | 0.5 | 0.5 | 0.2 |
| 57 | 0.3373 | 0.20 | 44 | 536 | 0.40 | 1 | 0.5 | 0.5 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staśczak, A.; Langer-Macioł, H.; Widzisz, K.; Śliwińska, W.; Lucińska, K.; Wencel, P.; Strózik, B.; Frąckiewicz, M.; Skupin, P.; Choiński, D.; et al. Optical Evaluation of Effects of Energy Substrates on PHB Accumulation for Bioplastic Production. Energies 2022, 15, 8390. https://doi.org/10.3390/en15228390
Staśczak A, Langer-Macioł H, Widzisz K, Śliwińska W, Lucińska K, Wencel P, Strózik B, Frąckiewicz M, Skupin P, Choiński D, et al. Optical Evaluation of Effects of Energy Substrates on PHB Accumulation for Bioplastic Production. Energies. 2022; 15(22):8390. https://doi.org/10.3390/en15228390
Chicago/Turabian StyleStaśczak, Alicja, Hanna Langer-Macioł, Karolina Widzisz, Wiktoria Śliwińska, Kinga Lucińska, Przemysław Wencel, Barbara Strózik, Mariusz Frąckiewicz, Piotr Skupin, Dariusz Choiński, and et al. 2022. "Optical Evaluation of Effects of Energy Substrates on PHB Accumulation for Bioplastic Production" Energies 15, no. 22: 8390. https://doi.org/10.3390/en15228390
APA StyleStaśczak, A., Langer-Macioł, H., Widzisz, K., Śliwińska, W., Lucińska, K., Wencel, P., Strózik, B., Frąckiewicz, M., Skupin, P., Choiński, D., & Student, S. (2022). Optical Evaluation of Effects of Energy Substrates on PHB Accumulation for Bioplastic Production. Energies, 15(22), 8390. https://doi.org/10.3390/en15228390