Biodiesel Synthesis from Milk Thistle (Silybum marianum (L.) Gaertn.) Seed Oil using ZnO Nanoparticles as a Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Extract Preparation
2.2. ZnO Catalyst Synthesis
2.3. Catalyst Characterizations by XRD and SEM
2.4. Oil Extraction from Feedstock
2.5. Free Fatty Acid Content Determination
- A = potassium hydroxide (KOH) volume used in the sample titration;
- B = potassium hydroxide (KOH) volume used in blank titration;
- C = potassium hydroxide (KOH) concentration (g/L);
- V = volume of oil sample.
2.6. Biodiesel Synthesis Process

2.7. Fuel Property Determination
2.8. Chemical Assessment of Biodiesel Synthesis
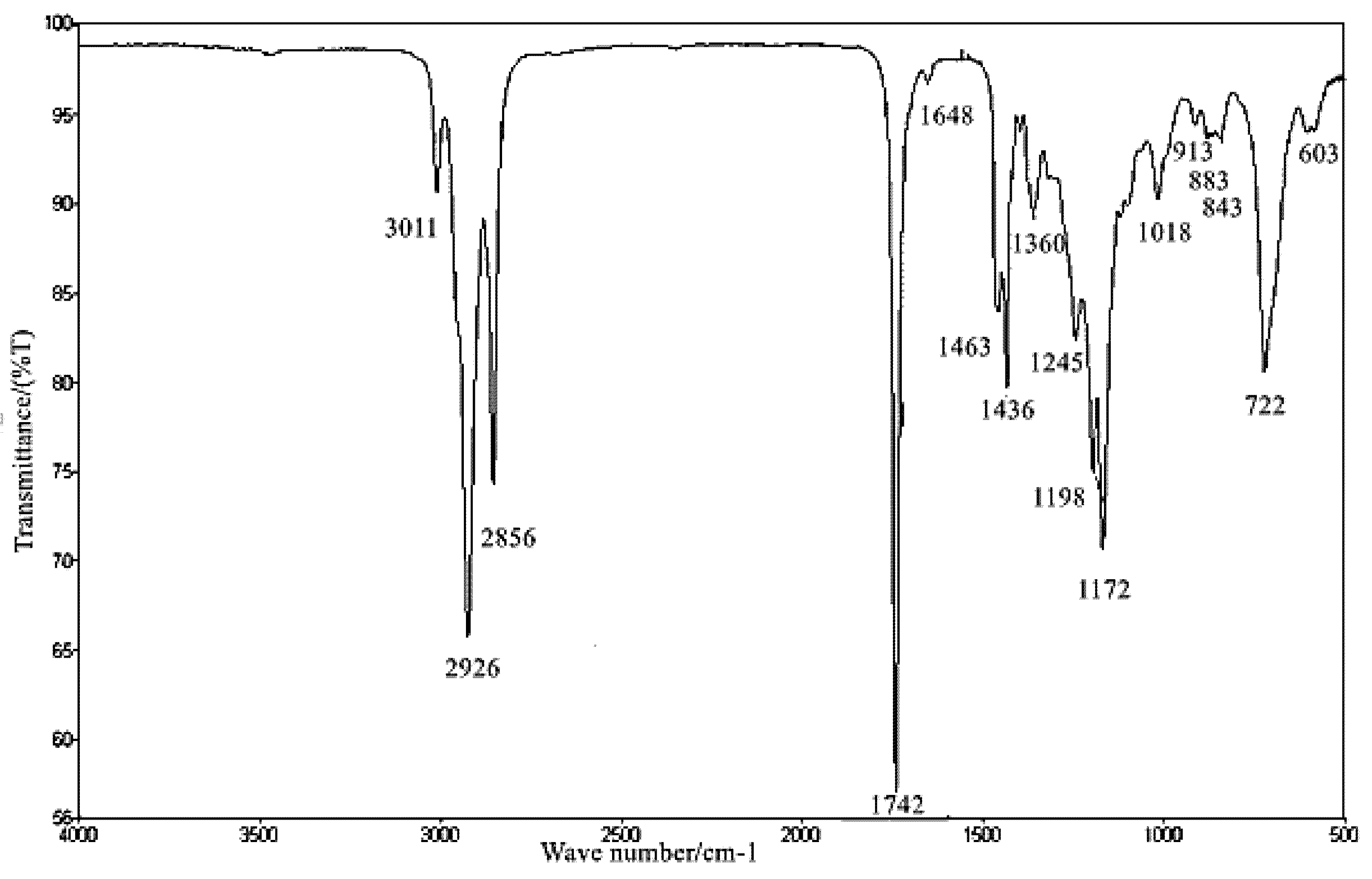
2.9. Fourier Transform Infrared Analysis of SMB
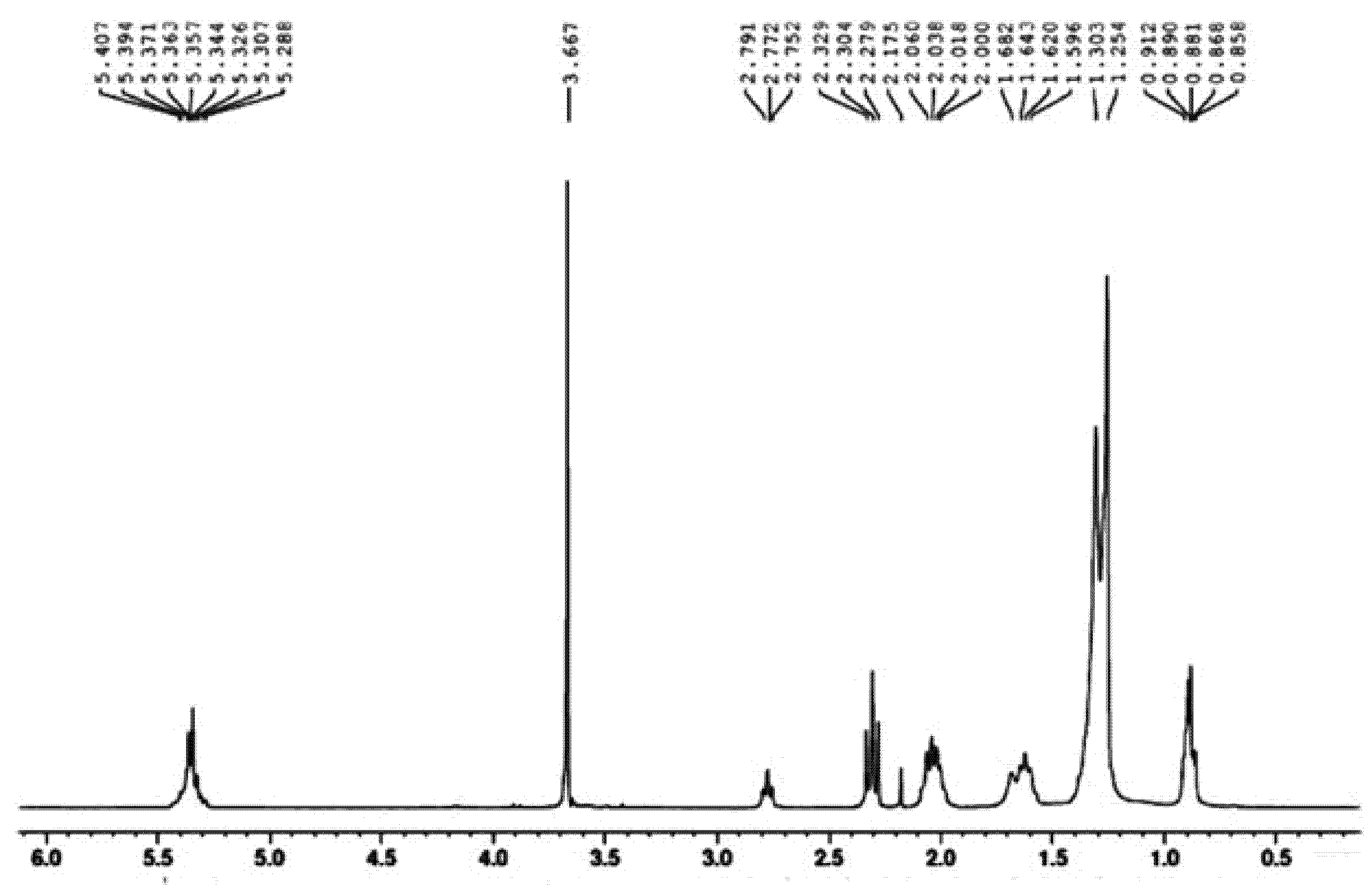
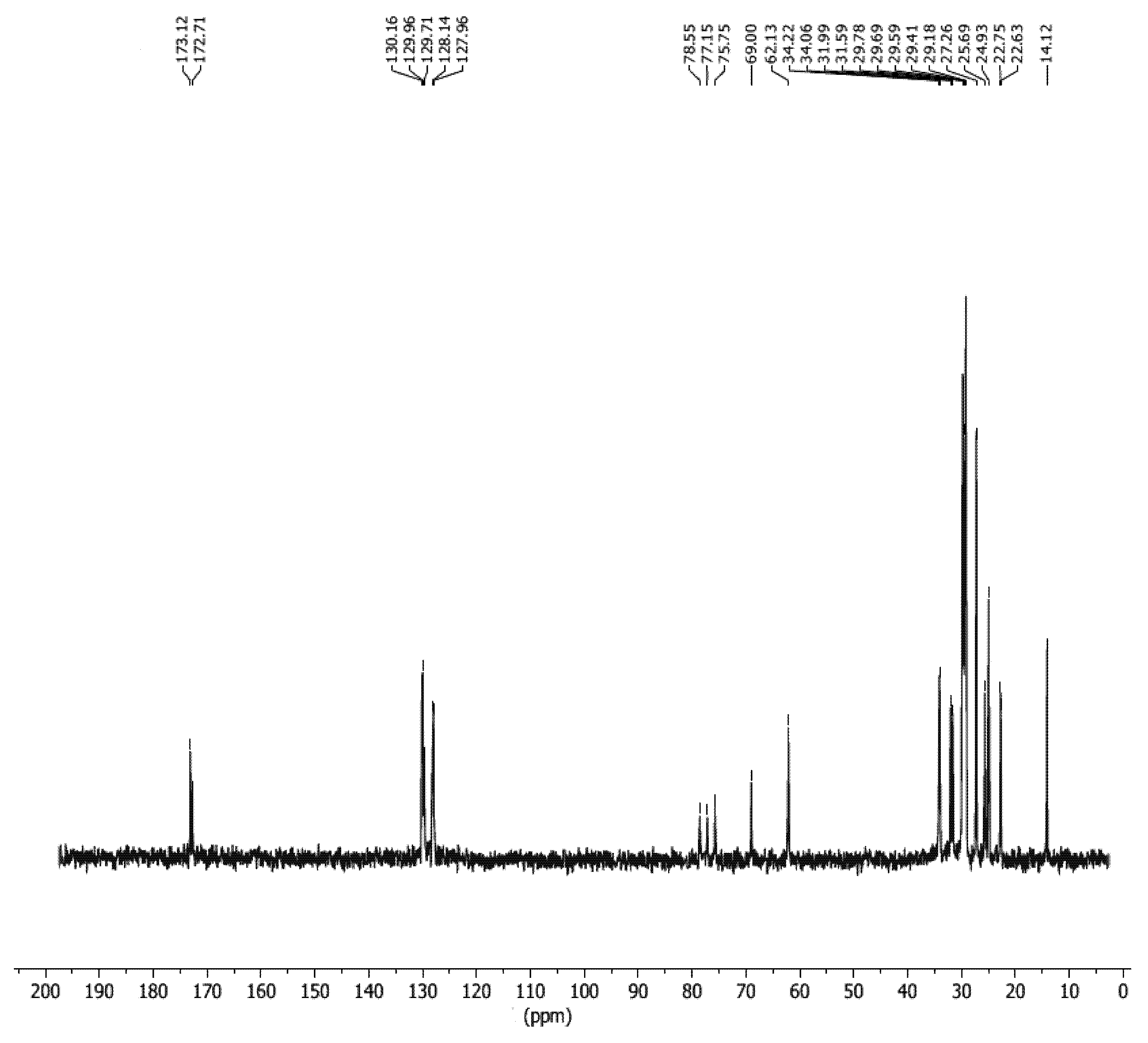
2.10. NMR Spectroscopy of SMB
- C = oil-to-biodiesel conversion percentage;
- AMe = methoxy proton integration value in biodiesel;
- ACH2 = α-methylene proton integration value in biodiesel.
2.11. GC-MS Determination of FAMEs
3. Results and Discussion
3.1. X-ray Diffraction of ZnO Nanocatalysts
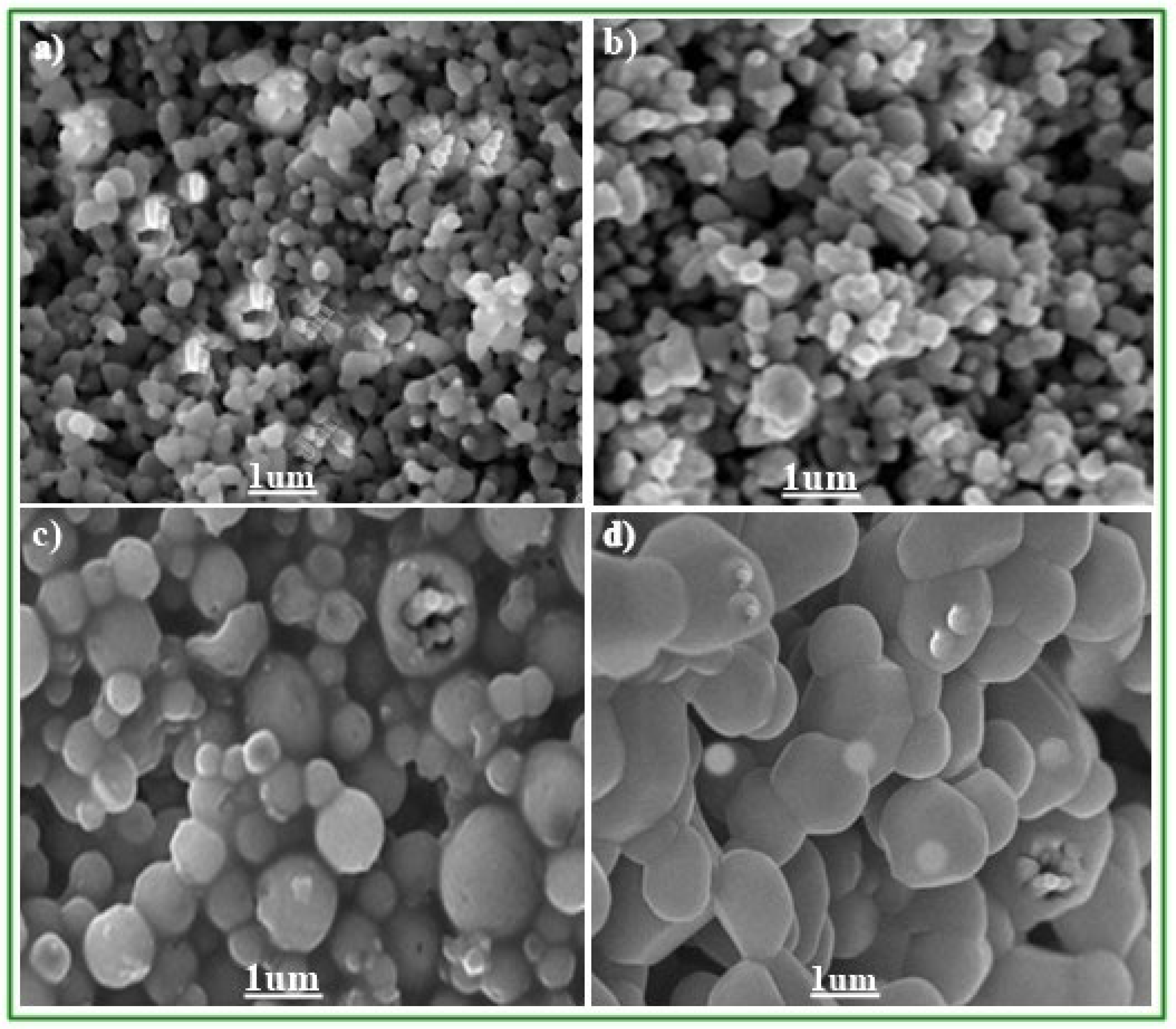
3.2. Scanning Electron Microscopic Study of ZnO
3.3. Oil Extraction and FFA Content Determination
3.4. Biodiesel Synthesis and Optimization
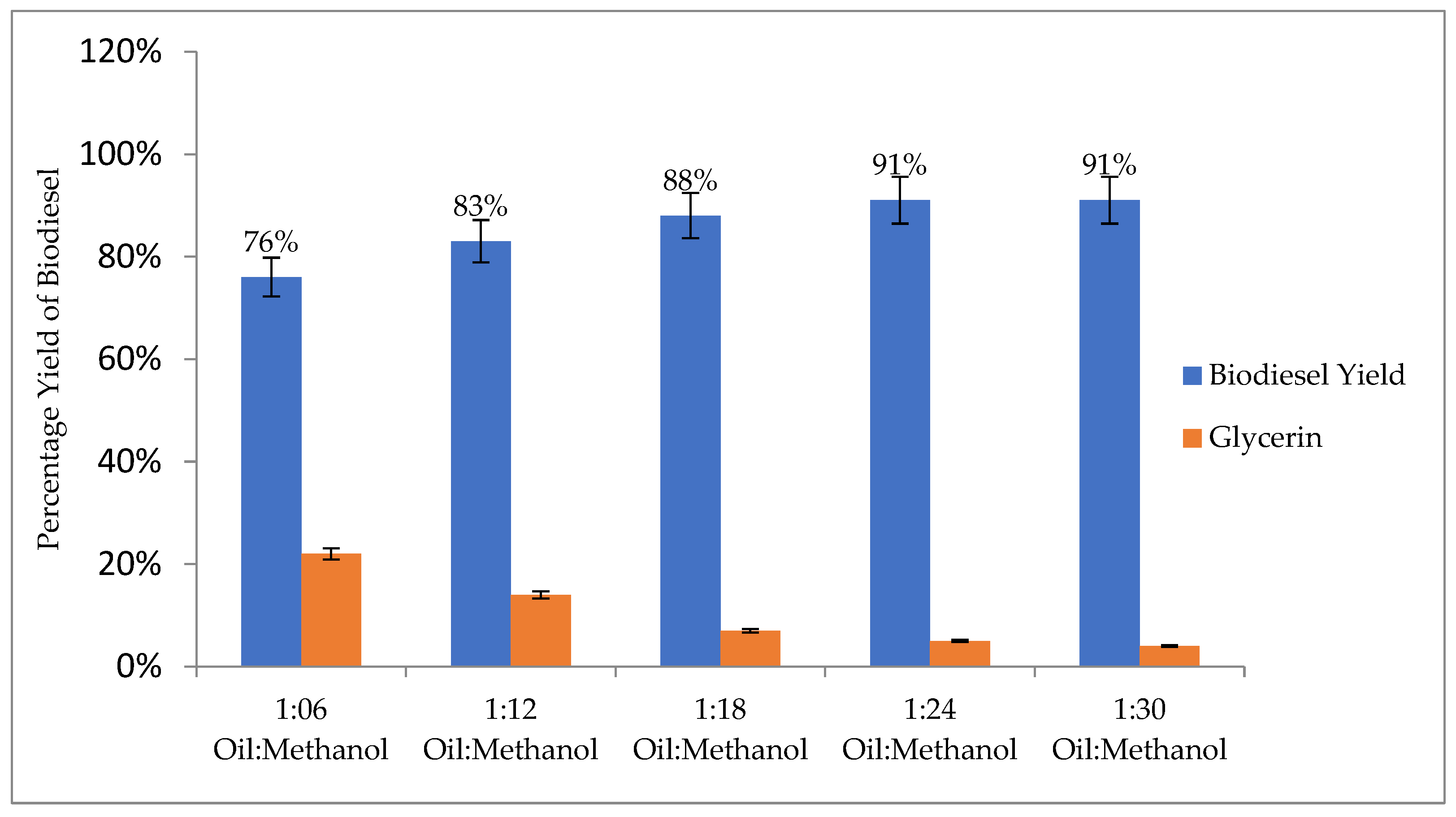
3.4.1. Oil-to-Methanol Ratio
3.4.2. Catalyst Concentration
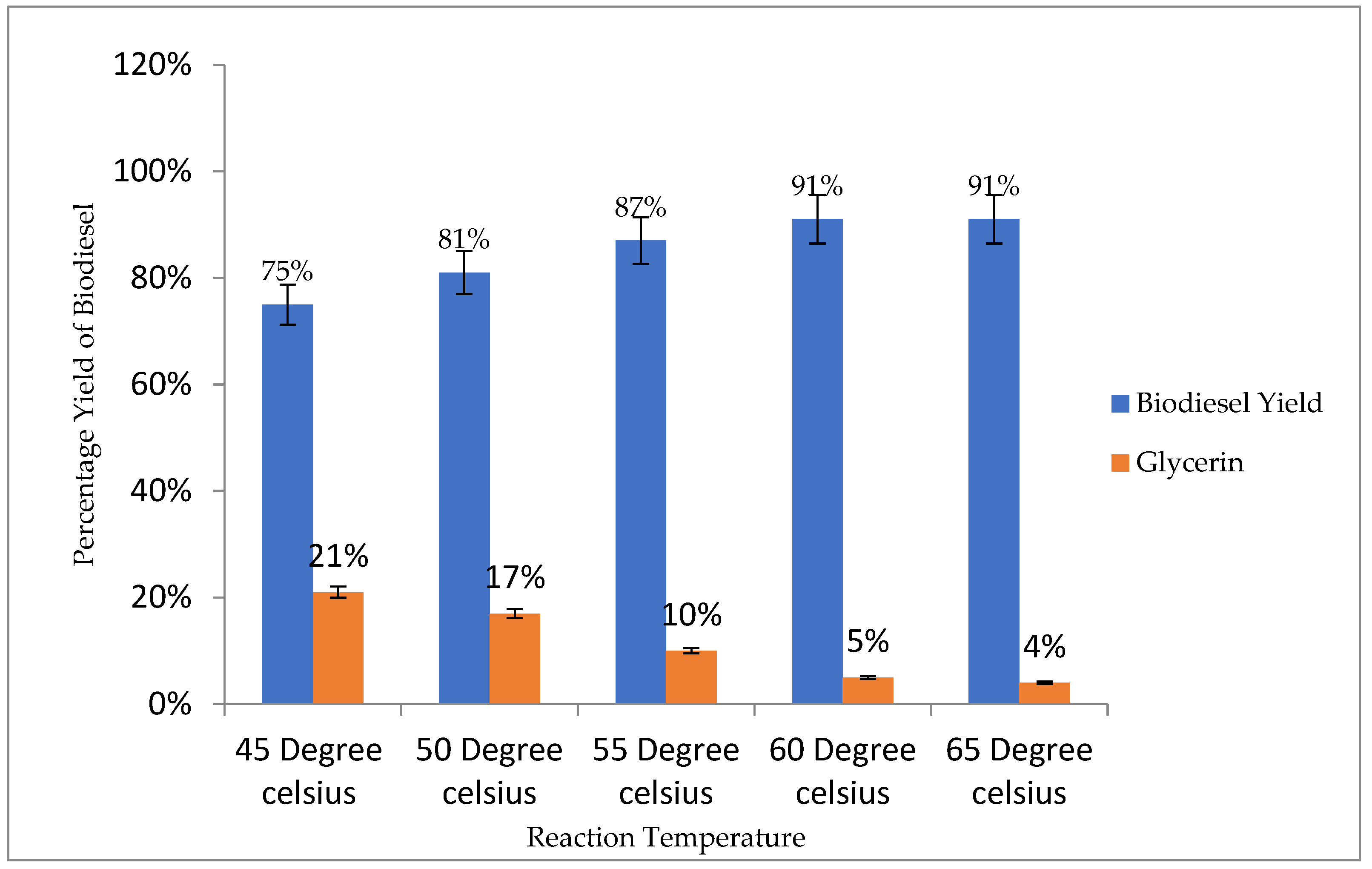
3.4.3. Reaction Temperature
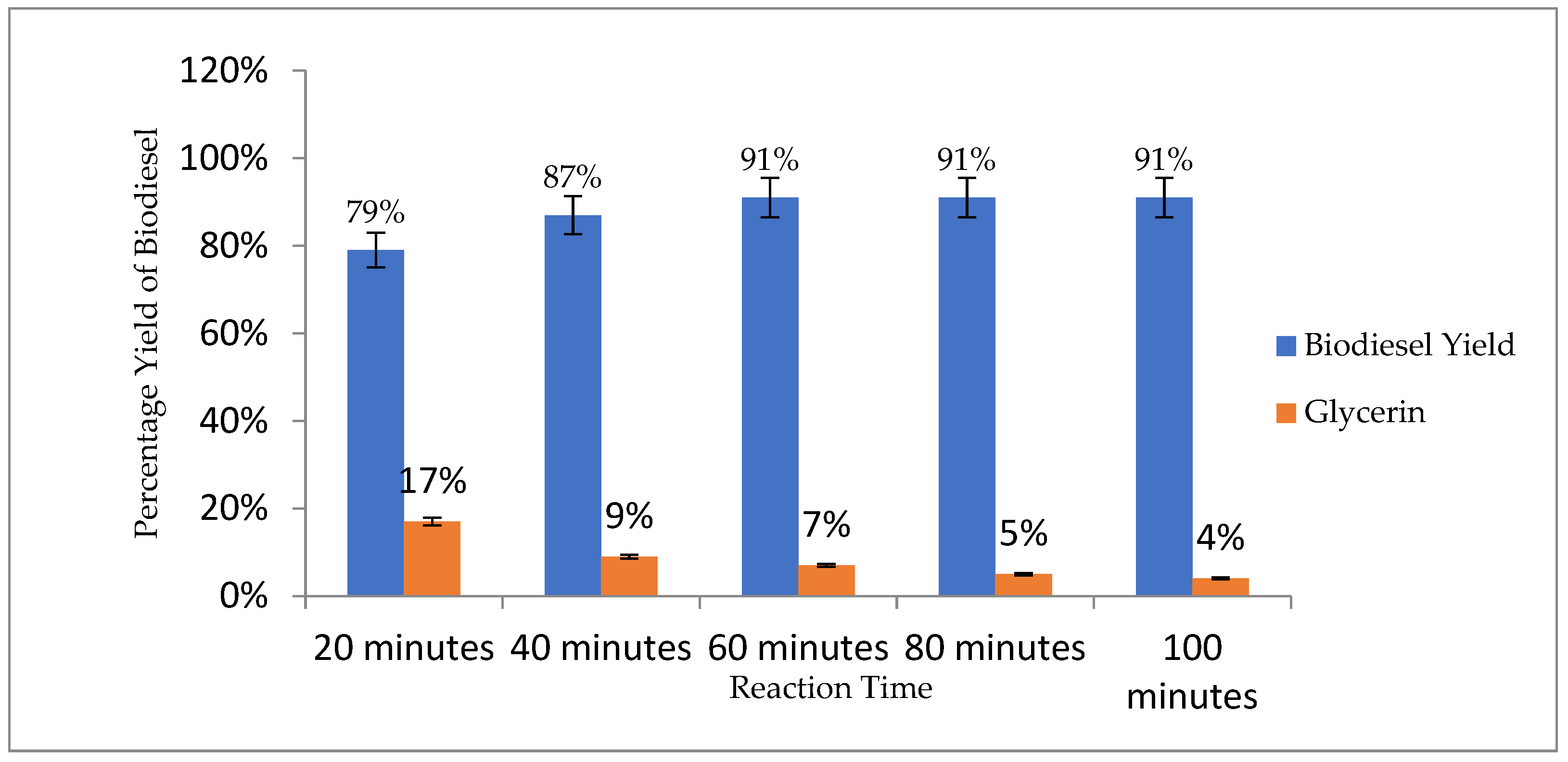
3.4.4. Reaction Time
3.5. Physical and Fuel Properties of Biodiesel
3.6. H-NMR Study of SMB
3.7. 13C-NMR Study of SMB
3.8. Qualitative and Quantitative Analysis of SMB
3.9. FT-IR Analysis of SMB
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| CP | Cloud Point |
| FAME | Fatty Acid Methyl Esters |
| FFA | Free Fatty Acid |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| FT-IR spectroscopy | Fourier Transform Infrared Spectroscopy |
| H and C-NMR | Nuclear Magnetic Resonance |
| HHV | Higher Heating Value |
| PMCC | Flash Point (Pensky-Martens Closed Cup) |
| PP | Pour Point |
| SEM | Scanning Electron Microscopy |
| SMB | Silybum Marianum Biodiesel |
| XRD | X-ray Diffraction |
| ZnO | Zinc Oxide |
References
- Zahed, M.A.; Revayati, M.; Shahcheraghi, N.; Maghsoudi, F.; Tabari, Y. Modeling and optimization of biodiesel synthesis using TiO2–ZnO nanocatalyst and characteristics of biodiesel made from waste sunflower oil. Curr. Res. Green Sustain. Chem. 2021, 4, 100223. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Progress in biodiesel processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Ramakrishnan, V.V.; Dave, D.; Liu, Y.; Routray, W.; Murphy, W. Statistical Optimization of Biodiesel Production from Salmon Oil via Enzymatic Transesterification: Investigation of the Effects of Various Operational Parameters. Processes 2021, 9, 700. [Google Scholar] [CrossRef]
- Kumar, S.; Shamsuddin, M.R.; Farabi, M.S.A.; Saiman, M.I.; Zainal, Z.; Taufiq-Yap, Y.H. Production of methyl esters from waste cooking oil and chicken fat oil via simultaneous esterification and transesterification using acid catalyst. Energy Convers. Manag. 2020, 226, 113366. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.M.A.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A.K. Experimental evaluation of the catalytic efficiency of calcium based natural and modified catalyst for biodiesel synthesis. Int. J. Green Energy 2017, 14, 878–888. [Google Scholar] [CrossRef]
- Narasimhan, M.; Chandrasekaran, M.; Govindasamy, S.; Aravamudhan, A. Heterogeneous nanocatalysts for sustainable biodiesel production: A review. J. Environ. Chem. Eng. 2021, 9, 104876. [Google Scholar] [CrossRef]
- Toledo Arana, J.; Torres, J.J.; Acevedo, D.F.; Illanes, C.O.; Ochoa, N.A.; Pagliero, C.L. One-step synthesis of CaO-ZnO efficient catalyst for biodiesel production. Inter. J. Chem. Eng. 2019, 2019, 1806017. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Gogate, P.R.; Pandit, A.B. Ultrasound-assisted synthesis of biodiesel from palm fatty acid distillate. Ind. Eng. Chem. Res. 2009, 48, 7923–7927. [Google Scholar] [CrossRef]
- Veljković, V.B.; Banković-Ilić, I.B.; Stamenković, O.S.; Hung, Y.T. Waste Vegetable Oils, Fats, and Cooking Oils in Biodiesel Production. In Integrated Natural Resources Research; Wang, L.K., Wang, M.H.S., Hung, Y.T., Eds.; Handbook of Environmental Engineering; Springer: Cham, Switzerland, 2021; Volume 22. [Google Scholar] [CrossRef]
- Bohlouli, A.; Mahdavian, L. Catalysts used in biodiesel production: A review. Biofuels 2021, 12, 885–898. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; HM Abd El-Azim, M. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra tortuosa and their Cytotoxic Activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, H.; Sher, F.; Sehar, S.; Lima, E.C.; Zhang, S.; Iqbal, H.M.; Nuhanović, M. Green synthesis of ZnO nanoparticles from Syzygium cumini leaves extract with robust photocatalysis applications. J. Mol. Liq. 2021, 335, 116567. [Google Scholar] [CrossRef]
- Dawood, S.; Koyande, A.K.; Ahmad, M.; Mubashir, M.; Asif, S.; Klemeš, J.J.; Bokhari, A.; Saqib, S.; Lee, M.; Qyyum, M.A.; et al. Synthesis of biodiesel from non-edible (Brachychiton populneus) oil in the presence of nickel oxide nanocatalyst: Parametric and optimisation studies. Chemosphere 2021, 278, 130469. [Google Scholar] [CrossRef] [PubMed]
- Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Ahmad, M.; Zafar, M.; Sultana, S.; Azam, A.; Khan, M.A. The Optimization of Biodiesel Production from a Novel Source of Wild Non-Edible Oil Yielding Plant Silybum Marianum. Int. J. Green Energy 2014, 11, 589–594. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef]
- Lahure, P.; Salunke, P.; Soliwal, R.; Yadav, A.; Tripathi, S.; Koser, A.A. X-ray Diffraction Study of ZnO Nanoparticles. Int. J. Sci. Res. Phys. Appl. Sci. 2015, 3, 32–33. [Google Scholar]
- Scherrer, P. Göttinger Nachrichten Gesell. 1918, Volume 2, p. 98. Available online: https://en.wikipedia.org/wiki/Scherrer_equation#cite_ref-1 (accessed on 12 June 2022).
- Theivasanthi, T.; Alagar, M. Nano sized copper particles by electrolytic synthesis and characterizations. Int. J. Phys. Sci. 2011, 6, 3662–3671. [Google Scholar] [CrossRef]
- Jan, H.A.; Šurina, I.; Zaman, A.; Al-Fatesh, A.S.; Rahim, F.; Al-Otaibi, R.L. Synthesis of Biodiesel from Ricinus communis L. Seed Oil, a Promising Non-Edible Feedstock Using Calcium Oxide Nanoparticles as a Catalyst. Energies 2022, 15, 6425. [Google Scholar]
- Ullah, K.; Jan, H.A.; Ahmad, M.; Ullah, A. Synthesis and structural characterization of biofuel from Cocklebur sp.; using zinc oxide nano-particle: A novel energy crop for bioenergy industry. Front. Bioeng. Biotech. 2020, 8, 756. [Google Scholar] [CrossRef]
- Birla, A.; Singh, B.; Upadhyay, S.; Sharma, Y. Kinetics studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Bioresour. Technol. 2012, 106, 95–100. [Google Scholar] [CrossRef]
- Takase, M.; Feng, W.; Wang, W.; Gu, X.; Zhu, Y.; Li, T.; Wu, X. Silybum marianum oil as a new potential non-edible feedstock for biodiesel: A comparison of its production using conventional and ultrasonic assisted method. Fuel Process. Technol. 2014, 123, 19–26. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Ahmed, K.M.; Dheyab, M.M. Silybum marianum L. seed oil: A novel feedstock for biodiesel production. Arab. J. Chem. 2017, 10, 683–690. [Google Scholar] [CrossRef]
- Samanta, S.; Sahoo, R.R. Waste cooking (palm) oil as an economical source of biodiesel production for alternative green fuel and efficient lubricant. BioEnergy Res. 2021, 14, 163–174. [Google Scholar] [CrossRef]
- Gowthambabu, V.; Balamurugan, A.; Satheeshkumar, S.; Kanmani, S.S. ZnO nanoparticles as efficient sunlight driven photocatalyst prepared by solution combustion method involved lime juice as biofuel. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 258, 119857. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.K.; Manoharan, K.; Raman, K.; Sundaram, R. Efficient sunlight-driven photocatalytic behavior of zinc sulfide nanorods towards Rose Bengal degradation. J. Mater. Sci. Mater. Electron. 2020, 31, 14795–14809. [Google Scholar] [CrossRef]
- Fan, M.; Liu, Y.; Zhang, P.; Jiang, P. Blocky shapes Ca-Mg mixed oxides as a water-resistant catalyst for effective synthesis of biodiesel by transesterification. Fuel Process. Technol. 2016, 149, 163–168. [Google Scholar] [CrossRef]
- Awual, M.R.; Yaita, T.; Shiwaku, H. Design a novel optical adsorbent for simultaneous ultra-trace cerium (III) detection, sorption and recovery. Chem. Eng. J. 2013, 228, 327–335. [Google Scholar] [CrossRef]
- Takase, M.; Zhang, M.; Feng, W.; Chen, Y.; Zhao, T.; Cobbina, S.J.; Wu, X. Application of zirconia modified with KOH as heterogeneous solid base catalyst to new non-edible oil for biodiesel. Energy Convers. Manag. 2014, 80, 117–125. [Google Scholar] [CrossRef]
- Worapun, I.; Pianthong, K.; Thaiyasuit, P. Two-step biodiesel production from crude Jatropha curcas L. oil using ultrasonic irradiation assisted. J. Oleo Sci. 2012, 61, 165–172. [Google Scholar] [CrossRef]
- Meher, L.; Vidya Sagar, D.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Kumar, K. Standardization of non-edible Pongamia pinnata oil methyl ester conversion using hydroxyl content and GC–MS analysis. J. Taiwan Inst. Chem. Eng. 2013, 45, 1485–1489. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Sabio, E.; Ramiro, M.J. Preparation and properties of biodiesel from Cynara cardunculus L. oil. Ind. Eng. Chem. Res. 1999, 38, 2927–2931. [Google Scholar] [CrossRef]
- Bojan, S.G.; Durairaj, S.K. Producing Biodiesel from High Free Fatty Acid Jatropha Curcas Oil by a Two Step Method—An Indian Case Study. J. Sustain. Energy Environ. 2012, 3, 63–66. [Google Scholar]
- Leung, D.Y.C.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Silva, C.D.; Oliveira, J.V. Biodiesel production through non-catalytic supercritical transesterification: Current state and perspectives. Braz. J. Chem. Eng. 2014, 31, 271–285. [Google Scholar] [CrossRef]
- Barros, S.D.S.; Junior, W.A.P.; Sá, I.S.; Takeno, M.L.; Nobre, F.X.; Pinheiro, W.; de Freitas, F.A. Pineapple (Ananás comosus) leaves ash as a solid base catalyst for biodiesel synthesis. Bioresour. Technol. 2020, 312, 123569. [Google Scholar] [CrossRef]
- Phan, A.N.; Phan, T.M. Biodiesel production from waste cooking oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- Mathiyazhagan, M.; Ganapathi, A. Factors affecting biodiesel production. Res. Plant Biol. 2011, 1, 1–5. [Google Scholar]
- Gebremariam, S.N.; Marchetti, J.M. Economics of biodiesel production. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Karmakar, B.; Ghosh, S.; Halder, G. Parametric optimisation of biodiesel synthesis from waste cooking oil via Taguchi approach. J. Environ. Chem. Eng. 2018, 6, 3971–3980. [Google Scholar] [CrossRef]
- Ávila Vázquez, V.; Díaz Estrada, R.A.; Aguilera Flores, M.M.; Escamilla Alvarado, C.; Correa Aguado, H.C. Transesterification of non-edible castor oil (Ricinus communis L.) from Mexico for biodiesel production: A physicochemical characterization. Biofuels 2020, 11, 753–762. [Google Scholar] [CrossRef]
- Wang, R.; Hanna, M.A.; Zhou, W.W.; Bhadury, P.S.; Chen, Q.; Song, B.A.; Yang, S. Production and selected fuel properties of biodiesel from promising non-edible oils: Euphorbia lathyris L.; Sapium sebiferum L. and Jatropha curcas L. Bioresour. Technol. 2011, 102, 1194–1199. [Google Scholar] [CrossRef]
- Kaisan, M.U.; Anafi, F.O.; Nuszkowski, J.; Kulla, D.M.; Umaru, S. Calorific value, flash point and cetane number of biodiesel from cotton, jatropha and neem binary and multi-blends with diesel. Biofuels 2017, 11, 321–327. [Google Scholar] [CrossRef]
- Refaat, A.A.; Attia, N.K.; Sibak, H.A.; El Sheltawy, S.T.; El Diwani, G.I. Production optimization and quality assessment of biodiesel from waste vegetable oil. Int. J. Environ. Sci. Technol. 2008, 5, 75–82. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.; Almeida, M.F. Comparison of the performance of different homogeneous alkali catalysts during transesterification of waste and virgin oils and evaluation of biodiesel quality. Fuel 2008, 87, 3572–3578. [Google Scholar] [CrossRef]
- Knothe, G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ. Sci. 2009, 2, 759–766. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Rasul, M.G.; Atabani, A.E.; Hazrat, M.A.; Mahmudul, H.M. Effect of biodiesel-diesel blending on physico-chemical properties of biodiesel produced from Moringa oleifera. Procedia Eng. 2015, 105, 665–669. [Google Scholar] [CrossRef]
- Bagher, A.M.; Vahid, A.; Mohsen, M.; Reza, B.M. Effect of Using Renewable Energy in Public Health. Am. J. Energy Sci. 2016, 3, 1–9. [Google Scholar]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Bannister, C.D.; Chuck, C.J.; Bounds, M.; Hawley, J.G. Oxidative stability of biodiesel fuel. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2011, 225, 99–114. [Google Scholar] [CrossRef]
- Fregolente, P.B.L.; Fregolente, L.V.; Wolf Maciel, M.R. Water content in biodiesel, diesel, and biodiesel–diesel blends. J. Chem. Eng. Data 2012, 57, 1817–1821. [Google Scholar] [CrossRef]
- Da Costa Cardoso, L.; de Almeida, F.N.C.; Souza, G.K.; Asanome, I.Y.; Pereira, N.C. Synthesis and optimization of ethyl esters from fish oil waste for biodiesel production. Renew. Energy 2019, 133, 743–748. [Google Scholar] [CrossRef]
- Lugo-Méndez, H.; Sánchez-Domínguez, M.; Sales-Cruz, M.; Olivares-Hernández, R.; Lugo-Leyte, R.; Torres-Aldaco, A. Synthesis of biodiesel from coconut oil and characterization of its blends. Fuel 2021, 295, 120595. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Sivaramakrishnan, K.; Ravikumar, P. Determination of higher heating value of biodiesels. Int. J. Eng. Sci. Technol. 2011, 3, 7981–7987. [Google Scholar]
- Anand, K.; Ranjan, A.; Mehta, P.S. Estimating the viscosity of vegetable oil and biodiesel fuels. Energy Fuels 2010, 24, 664–672. [Google Scholar] [CrossRef]
- Demirbaş, A. Production of biodiesel from algae oils. Energy Sources A Recovery Util. Environ. Eff. 2008, 31, 163–168. [Google Scholar] [CrossRef]
- Knothe, G. Monitoring a progressing transesterification reaction by fiber optic NIR spectroscopy with correlation to 1H NMR spectroscopy. J. Am. Oil Chem. Soc. 2000, 77, 489e93. [Google Scholar] [CrossRef]
- Portela, N.A.; Oliveira, E.C.; Neto, A.C.; Rodrigues, R.R.; Silva, S.R.; Castro, E.V.; Filgueiras, P.R. Quantification of biodiesel in petroleum diesel by 1H NMR: Evaluation of univariate and multivariate approaches. Fuel 2016, 166, 12–18. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Qiu, F. Assessing the experimental investigation of milk thistle oil for biodiesel production using base catalyzed transesterification. Energy 2015, 89, 887–895. [Google Scholar] [CrossRef]
- Asci, F.; Aydin, B.; Akkus, G.U.; Unal, A.; Erdogmus, S.F.; Korcan, S.E.; Jahan, I. Fatty acid methyl ester analysis of Aspergillus fumigatus isolated from fruit pulps for biodiesel production using GC-MS spectrometry. Bioengineered 2020, 11, 408–415. [Google Scholar] [CrossRef]
- O’Donnell, S.; Demshemino, I.; Yahaya, M.; Nwadike, I.; Okoro, L. A review on the spectroscopic analyses of biodiesel. Eur. Int. J. Sci. Tech. 2013, 2, 137–146. [Google Scholar]
- Atabani, A.E.; Shobana, S.; Mohammed, M.N.; Uğuz, G.; Kumar, G.; Arvindnarayan, S.; Ala’a, H. Integrated valorization of waste cooking oil and spent coffee grounds for biodiesel production: Blending with higher alcohols, FT–IR, TGA, DSC and NMR characterizations. Fuel 2019, 244, 419–430. [Google Scholar] [CrossRef]
- Miglio, R.; Palmery, S.; Salvalaggio, M.; Carnelli, L.; Capuano, F.; Borrelli, R. Microalgae triacylglycerols content by FT-IR spectroscopy. J. Appl. Phycol. 2013, 25, 1621–1631. [Google Scholar] [CrossRef]
- Soon, L.B.; Rus, A.Z.M.; Hasan, S. Continuous biodiesel production using ultrasound clamp on tubular reactor. Int. J. Automot. Mech. Eng. 2013, 8, 1396–1405. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Siatis, N.G.; Kimbaris, A.C.; Pappas, C.S.; Tarantilis, P.A.; Polissiou, M.G. Improvement of biodiesel production based on the application of ultrasound: Monitoring of the procedure by FTIR spectroscopy. J. Am. Oil Chem. Soc. 2006, 83, 53–57. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Abdullah, N.F.; Basri, M. Biodiesel production via transesterification of palm oil using NaOH/Al2O3 catalysts. Sains Malays. 2011, 40, 587–594. [Google Scholar]



| Fuel Property | ASTM Methods | 1 ASTM D6751 | 2 SMB |
|---|---|---|---|
| Acid value (KOH mg/kg) | ASTM D-664 | 0.80 | 0.74 |
| Flash Point PMCC (°C) | D-93 | >93 | 87 |
| Density at 15 °C (kg/L) | D-4052 | 0.820–0.900 | 0.857 |
| Kinematic Viscosity at 40 °C (mm2/s) | D-445 | 1.9–6 | 4.69 |
| Pour Point (°C) | D-97-12 | −15 to 16 | −8 |
| Cloud Point (°C) | D-2500-11 | −3 to 12 | −2 |
| Sulphur (wt%) | D-5453 | 0.007 | 0.00018 |
| Calorific Value (kJ/kg) | D-5865 | 35,000 | 26,984 |
| Cetane no. | D-613 | 45 | 49 |
| Oxidative stability at 110 °C (min) | EN-14112 | 3 | 2.8 |
| Water content (mg/kg) | ASTM D-6304 | ≤0.05 | 0.039 |
| Refractive index at 20 °C | ASTM D-1747 | ---- | 1.437 |
| Iodine number (mg I2/100) | ASTM D-4607 | ≤120 | 124 |
| Higher heating value (MJ/kg) | ASTM D-240 | 39–43 | 40.86 |
| Distillation temperature for 90% recovery | ASTM D-1160-06 | 360 | 345 |
| No. | Identified FAMEs | Formula | CAS* | RT* | C* |
|---|---|---|---|---|---|
| 1 | Caprylic acid methyl ester | C9H18O2 | 111-11-5 | 4.739 | 0.16 |
| 2 | Myristic acid methyl ester | C15H30O2 | 124-10-7 | 10.168 | 0.34 |
| 3 | Pentadecanoic acid methyl ester | C16H32O2 | 7132-64-1 | 11.267 | 1.08 |
| 4 | Palmitic acid methyl ester | C17H34O2 | 112-39-0 | 13.424 | 16.11 |
| 5 | Palmitoleic acid methyl ester | C17H32O2 | 1120-25-8 | 13.878 | 0.12 |
| 6 | Margaric acid methyl ester | C18H36O2 | 1731-92-6 | 15.500 | 0.21 |
| 7 | Heptadecenoic acid methyl ester | C18H36O2 | 1731-92-6 | 15.943 | 0.27 |
| 8 | Stearic acid methyl ester | C19H38O2 | 112-61-8 | 17.897 | 2.33 |
| 9 | Oleic acid methyl ester | C19H36O2 | 112-62-9 | 18.375 | 8.52 |
| 10 | Linoleic acid methyl ester | C19H34O2 | 112-63-0 | 19.659 | 62.23 |
| 11 | Octadecenoic acid methyl ester | C19H36O2 | 1937-62-8 | 20.817 | 0.25 |
| 12 | Linolenic acid methyl ester | C19H34O2 | 112-63-0 | 21.743 | 0.31 |
| 13 | Arachidic acid methyl ester | C21H42O2 | 1120-28-1 | 24.661 | 4.47 |
| 14 | 11, 14-Eicosadienoic acid methyl ester | C21H38O2 | 61012-46-2 | 26.948 | 0.39 |
| 15 | 11, 14, 17-Eicosenoic acid methyl ester | C21H40O2 | 1120-28-1 | 29.259 | 0.81 |
| 16 | Behenic acid methyl ester | C23H46O2 | 929-77-1 | 31.994 | 0.83 |
| 17 | Erucic acid methyl ester | C23H44O2 | 1120-34-9 | 33.910 | 0.41 |
| 18 | 13, 16-Docosadienoic acid methyl ester | C23H42O2 | 61012-47-3 | 35.030 | 0.19 |
| 19 | Nervonic acid methyl ester | C25H48O2 | 2733-88-2 | 38.611 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jan, H.A.; Šurina, I.; Al-Fatesh, A.S.; Almutlaq, A.M.; Wali, S.; Lisý, A. Biodiesel Synthesis from Milk Thistle (Silybum marianum (L.) Gaertn.) Seed Oil using ZnO Nanoparticles as a Catalyst. Energies 2022, 15, 7818. https://doi.org/10.3390/en15207818
Jan HA, Šurina I, Al-Fatesh AS, Almutlaq AM, Wali S, Lisý A. Biodiesel Synthesis from Milk Thistle (Silybum marianum (L.) Gaertn.) Seed Oil using ZnO Nanoparticles as a Catalyst. Energies. 2022; 15(20):7818. https://doi.org/10.3390/en15207818
Chicago/Turabian StyleJan, Hammad Ahmad, Igor Šurina, Ahmed S. Al-Fatesh, Abdulaziz M. Almutlaq, Sher Wali, and Anton Lisý. 2022. "Biodiesel Synthesis from Milk Thistle (Silybum marianum (L.) Gaertn.) Seed Oil using ZnO Nanoparticles as a Catalyst" Energies 15, no. 20: 7818. https://doi.org/10.3390/en15207818
APA StyleJan, H. A., Šurina, I., Al-Fatesh, A. S., Almutlaq, A. M., Wali, S., & Lisý, A. (2022). Biodiesel Synthesis from Milk Thistle (Silybum marianum (L.) Gaertn.) Seed Oil using ZnO Nanoparticles as a Catalyst. Energies, 15(20), 7818. https://doi.org/10.3390/en15207818







