CO2 Sequestration Overview in Geological Formations: Trapping Mechanisms Matrix Assessment
Abstract
1. Introduction
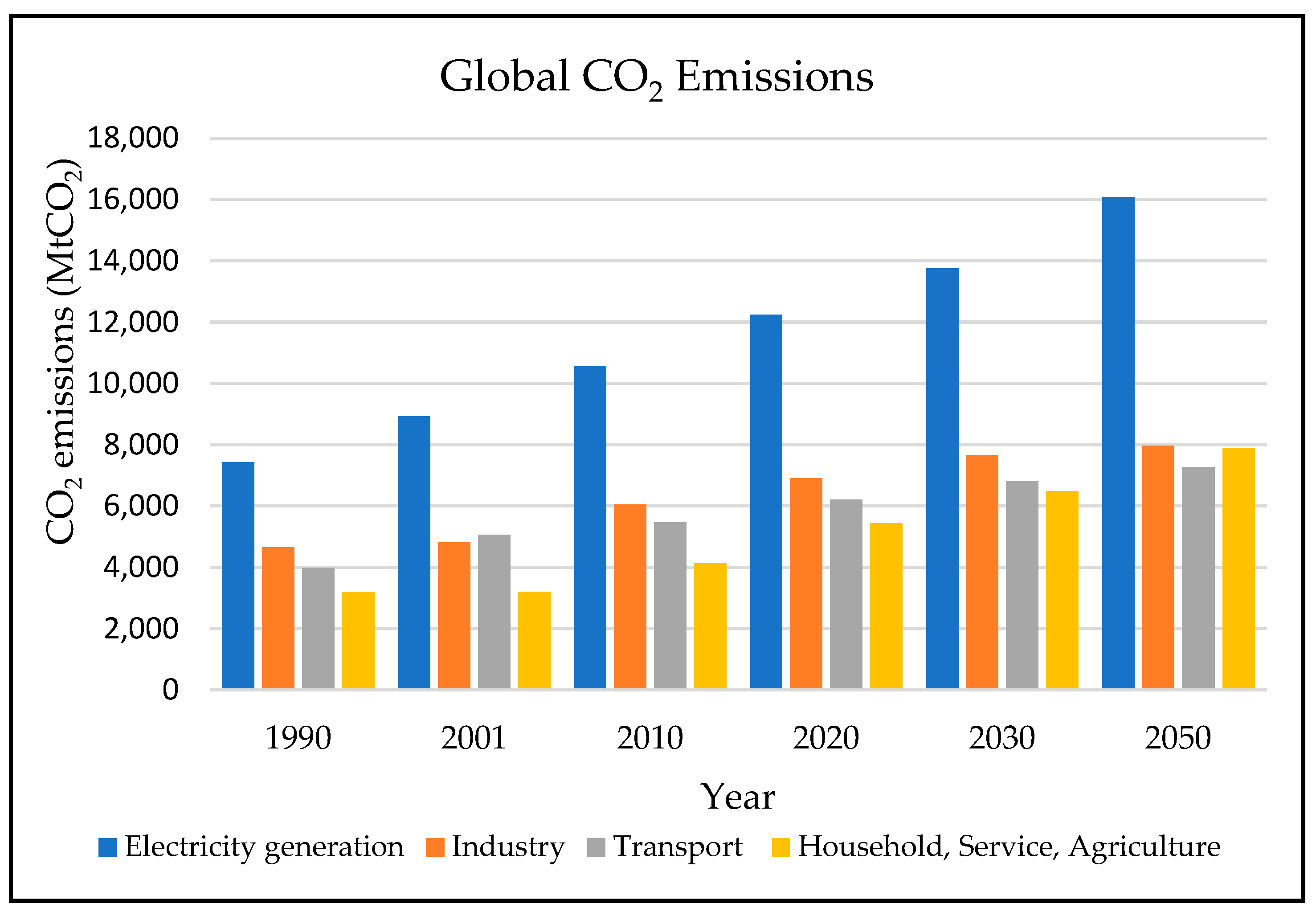
1.1. Global Measures
1.2. Environmental Measures
1.3. Role of CCS
2. Components of the CCS System
2.1. Storage Capacity Assessment
2.2. Carbon Capture
2.3. Carbon Geological Storage
2.3.1. Saline Aquifers
2.3.2. Depleted Oil and Gas Reservoirs
2.3.3. Enhanced Oil Recovery (EOR)
2.3.4. Deep Ocean Storage
3. Carbon Sequestration in Unconventional Reservoirs
3.1. Coalbed Methane
3.2. Shale Reservoirs
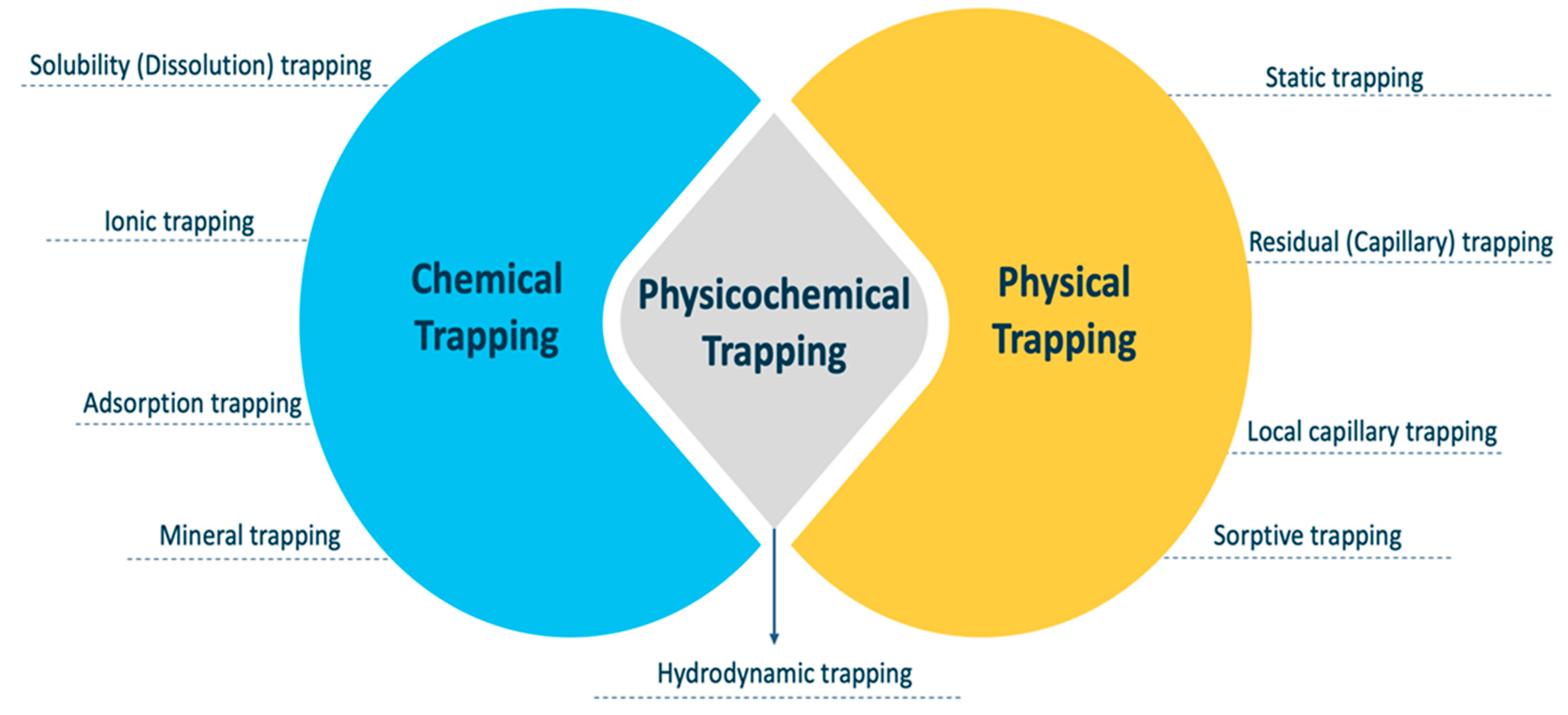
4. Trapping Mechanisms
4.1. Chemical Trapping
- Dissolution (solubility) trapping;
- Ionic trapping;
- Adsorption trapping;
- Mineral trapping.
4.1.1. Solubility (Dissolution) Trapping
4.1.2. Ionic Trapping
4.1.3. Adsorption Trapping
4.1.4. Mineral Trapping
4.2. Physicochemical Trapping
Hydrodynamic Trapping
4.3. Physical Trapping
- Static trapping;
- Residual (capillary) trapping;
- Local capillary trapping;
- Sorption trapping.
4.3.1. Static Trapping
4.3.2. Residual (Capillary) Trapping
4.3.3. Local Capillary Trapping
4.3.4. Sorptive Trapping
5. Conclusions
- Utilizing the science to understand the trapping mechanisms’ contribution to maximize the CO2 storage capacity;
- Taking a significant step forward in modeling multi-phase flow systems for carbon storage by integrating the chemical, physical, and physiochemical interactions via a thorough investigation of the parameters that optimize storage capacity potential;
- Examining the dynamics of CO2 plumes and their contribution to different trapping mechanisms in unconventional depleted shale reservoirs or coal seams;
- Developing an integrated model that incorporates all of the aforementioned trapping processes and evaluates their trapping storage capability, particularly in tight unconventional formations such as shale or coal seams.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hepple, R.P.; Benson, S.M. Geologic storage of carbon dioxide as a climate change mitigation strategy: Performance requirements and the implications of surface seepage. Environ. Geol. 2005, 47, 576–585. [Google Scholar] [CrossRef]
- Chaves, G. Simulation of CO2 Sequestration in Deep Saline Aquifers; New Mexico Institute of Mining and Technology: Socorro, New Mexico, 2011. [Google Scholar]
- Lapillonne, B.; Chateau, B.; Criqui, P.; Kitous, A.; Menanteau, P.; Mima, S.; Gusbin, D.; Gilis, S.; Soria, A.; Russ, P.; et al. World Energy Technology Outlook-2050-WETO-H2; No. halshs-00121063; Bruxelles: Luxembourg, 2007; Available online: https://halshs.archives-ouvertes.fr/halshs-00121063 (accessed on 12 April 2021).
- Zhang, B.; Wang, Z.; Wang, B. Energy production, economic growth and CO2 emission: Evidence from Pakistan. Nat. Hazards 2018, 90, 27–50. [Google Scholar]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R., Meyer, L., Eds.; IPCC: Geneva, Switzerland, 2014; 151p, ISBN 978-92-9169-143-2. [Google Scholar]
- Murshed, M.; Alam, R.; Ansarin, A. The environmental Kuznets curve hypothesis for Bangladesh: The importance of natural gas, liquefied petroleum gas, and hydropower consumption. Environ. Sci. Pollut. Res. 2021, 28, 17208–17227. [Google Scholar] [CrossRef]
- Peridas, G.; Schmidt, B.M. The role of carbon capture and storage in the race to carbon neutrality. Electr. J. 2021, 34, 106996. [Google Scholar] [CrossRef]
- Salvi, B.L.; Jindal, S. Recent developments and challenges ahead in carbon capture and sequestration technologies. SN Appl. Sci. 2019, 1, 1–20. [Google Scholar] [CrossRef]
- Querini, F.; Dagostino, S.; Morel, S.; Rousseaux, P. Greenhouse gas emissions of electric vehicles associated with wind and photovoltaic electricity. Energy Procedia 2012, 20, 391–401. [Google Scholar] [CrossRef]
- Rubin, E.; De Coninck, H. IPCC Special Report on Carbon Dioxide Capture and Storage; TNO (2004): Cost Curves for CO2 Storage, Part 2; Cambridge University Press: Cambridge, UK, 2005; p. 14. [Google Scholar]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The US Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Smit, B.; Reimer, J.A.; Oldenburg, C.M.; Bourg, I.C. Introduction to Carbon Capture and Sequestration; Imperial College Press: London, UK, 2014; Volume 1. [Google Scholar]
- Marchetti, C. On geoengineering and the CO2 problem. Clim. Chang. 1977, 1, 59–68. [Google Scholar] [CrossRef]
- Brewer, P.G. A changing ocean seen with clarity. Proc. Natl. Acad. Sci. USA 2009, 106, 12213–12214. [Google Scholar] [CrossRef] [PubMed]
- Bachu, S.; Adams, J.J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manag. 2003, 44, 3151–3175. [Google Scholar] [CrossRef]
- Bachu, S.; Gunter, W.D. Overview of acid-gas injection operations in western Canada. In Greenhouse Gas Control Technologies; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2005; Volume 7, pp. 443–448. [Google Scholar]
- Vishal, V.; Singh, T. Geologic carbon sequestration. Environ. Geosci. 2016, 16. [Google Scholar] [CrossRef]
- Carter, K.M.; Harper, J.A.; Schmid, K.W.; Kostelnik, J. Unconventional natural gas resources in Pennsylvania: The backstory of the modern Marcellus Shale play. Environ. Geosci. 2011, 18, 217–257. [Google Scholar] [CrossRef]
- Al Hameli, F.; Suboyin, A.; Al Kobaisi, M.; Rahman, M.M.; Haroun, M. Modeling Fracture Propagation in a Dual-Porosity System: Pseudo-3D-Carter-Dual-Porosity Model. Energies 2022, 15, 6779. [Google Scholar] [CrossRef]
- Sha, Z.; Bai, Y.; Li, R.; Lan, H.; Zhang, X.; Li, J.; Liu, X.; Chang, S.; Xie, Y. The global carbon sink potential of terrestrial vegetation can be increased substantially by optimal land management. Commun. Earth Environ. 2022, 3, 8. [Google Scholar] [CrossRef]
- IEA. CO2 Emissions from Energy Combustion and Industrial Processes, 1900–2021; IEA: Paris, France; Available online: https://www.iea.org/data-and-statistics/charts/co2-emissions-from-energy-combustion-and-industrial-processes-1900-2021 (accessed on 12 April 2021).
- Azarabadi, H.; Lackner, K.S. Postcombustion capture or direct air capture in decarbonizing US natural gas power? Environ. Sci. Technol. 2020, 54, 5102–5111. [Google Scholar] [CrossRef]
- Herzog, H.; Golomb, D. Carbon capture and storage from fossil fuel use. Encycl. Energy 2004, 1, 277–287. [Google Scholar]
- Ajayi, T.; Gomes, J.S.; Bera, A. A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches. Pet. Sci. 2019, 16, 1028–1063. [Google Scholar] [CrossRef]
- Herzog, H. Carbon Dioxide Capture and Storage; Typset by SPi: Chennai, India, 2009; pp. 246–283. [Google Scholar]
- CO2 Storage Resource Catalogue Cycle 2-2021, OGCI. Available online: https://www.ogci.com/co2-storage-resource-catalogue/ (accessed on 12 April 2021).
- Yadav, S.; Mondal, S.S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- Litynski, J.T.; Klara, S.M.; McIlvried, H.G.; Srivastava, R.D. An overview of terrestrial sequestration of carbon dioxide: The United States Department of Energy’s fossil energy R&D program. Clim. Chang. 2006, 74, 81–95. [Google Scholar]
- Thomson, A.M.; Izaurralde, R.C.; Smith, S.J.; Clarke, L.E. Integrated estimates of global terrestrial carbon sequestration. Glob. Environ. Chang. 2008, 18, 192–203. [Google Scholar] [CrossRef]
- Herzog, H.; Drake, E.; Adams, E. CO Capture, Reuse, and Storage Technologies. In Citeseer1997; DE-AF22-96PC01257; Massachusetts Institute of Technology: Cambridge, MA, USA, 1997. [Google Scholar]
- Metz, B.; Davidson, O.; De Coninck, H.C.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Celia, M.A.; Nordbotten, J.M. Practical modeling approaches for geological storage of carbon dioxide. Groundwater 2009, 47, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Myer, L. Global status of geologic CO2 storage technology development. In United States Carbon Sequestration Council Report July, 2011; 2011; Available online: http://carboncap-cleantech.com/articles/CCS/16%20Global-status-of-geologic-CO2-storage-technology-development.pdf (accessed on 10 April 2021).
- Span, R.; Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Chadwick, R.A.; Noy, D.J.; Holloway, S. Flow processes and pressure evolution in aquifers during the injection of supercritical CO2 as a greenhouse gas mitigation measure. Pet. Geosci. 2009, 15, 59–73. [Google Scholar] [CrossRef]
- Bachu, S. Screening and ranking of sedimentary basins for sequestration of CO2 in geological media in response to climate change. Environ. Geol. 2003, 44, 277–289. [Google Scholar] [CrossRef]
- Kaszuba, J.P.; Janecky, D.R. Geochemical impacts of sequestering carbon dioxide in brine formations. Carbon Sequestration Its Role Glob. Carbon Cycle 2009, 183, 239–248. [Google Scholar]
- Van der Meer, L.G.H. Investigations regarding the storage of carbon dioxide in aquifers in the Netherlands. Energy Convers. Manag. 1992, 33, 611–618. [Google Scholar] [CrossRef]
- Doughty, C.; Freifeld, B.M.; Trautz, R.C. Site characterization for CO2 geologic storage and vice versa: The Frio brine pilot, Texas, USA as a case study. Environ. Geol. 2008, 54, 1635–1656. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Solomon, S.; Carpenter, M.; Flach, T.A. Intermediate storage of carbon dioxide in geological formations: A technical perspective. Int. J. Greenh. Gas Control 2008, 2, 502–510. [Google Scholar] [CrossRef]
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- Pruess, K. Numerical Simulation of Leakage from a Geologic Disposal Reservoir for CO2, with Transitions between Super-and Sub-Critical Conditions; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2003.
- Bandilla, K.W.; Celia, M.A.; Birkholzer, J.T.; Cihan, A.; Leister, E.C. Multiphase modeling of geologic carbon sequestration in saline aquifers. Groundwater 2015, 53, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Michael, K.; Golab, A.; Shulakova, V.; Ennis-King, J.; Allinson, G.; Sharma, S.; Aiken, T. Geological storage of CO2 in saline aquifers—A review of the experience from existing storage operations. Int. J. Greenh. Gas Control 2010, 4, 659–667. [Google Scholar] [CrossRef]
- Herzog, H.; Drake, E.; Tester, J.; Rosenthal, R. A research needs assessment for the capture, utilization, and disposal of carbon dioxide from fossil fuel-fired power plants. In Report to the US Department of Energy; Grant No. DEFG02-92ER30194; MIT Energy Laboratory: Cambridge, UK, 1993. [Google Scholar]
- Davis, L.A.; Graham, A.L.; Parker, H.W.; Abbott, J.R.; Ingber, M.S.; Mammoli, A.A.; Mondy, L.A.; Guo, Q.; Abou-Sayed, A. Maximizing Storage Rate and Capacity and Insuring the Environmental Integrity of Carbon Dioxide Sequestration in Geological Reservoirs; Texas Tech University: Lubbock, TX, USA, 2005. [Google Scholar]
- Johnson, J.W. A solution for carbon dioxide overload. Sci. Technol. Rev. 2000. Available online: http://www.llnl.gov/str/Johnson.html (accessed on 2 May 2021).
- Anderson, J.; Bachu, S.; Nimir, H.B.; Basu, B.; Bradshaw, J.; Deguchi, G.; Gale, J.; Von Goerne, G.; Heidug, W.; Holloway, S.; et al. Underground Geological Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Sharma, S.; Agrawal, V.; McGrath, S.; Hakala, J.A.; Lopano, C.; Goodman, A. Geochemical controls on CO2 interactions with deep subsurface shales: Implications for geologic carbon sequestration. Environ. Sci. Process. Impacts 2021, 23, 1278–1300. [Google Scholar] [CrossRef] [PubMed]
- Winter, E.M.; Bergman, P.D. Availability of depleted oil and gas reservoirs for disposal of carbon dioxide in the United States. Energy Convers. Manag. 1993, 34, 1177–1187. [Google Scholar] [CrossRef]
- Kovscek, A.R. Screening criteria for CO2 storage in oil reservoirs. Pet. Sci. Technol. 2002, 20, 841–866. [Google Scholar] [CrossRef]
- Bossie-Codreanu, D.; Le-Gallo, Y.; Duquerroix, J.P.; Doerler, N.; Le Thiez, P. CO2 sequestration in depleted oil reservoirs. In Proceedings of the 6th International Conference on Greenhouse Gas Control Technologies (GHGT-6), Kyoto, Japan, 1–4 October 2002; Gale, J., Kaya, Y., Eds.; 2003; pp. 403–408. [Google Scholar]
- Holt, T.; Jensen, J.I.; Lindeberg, E. Underground storage of CO2 in aquifers and oil reservoirs. Energy Convers. Manag. 1995, 36, 535–538. [Google Scholar] [CrossRef]
- Bondor, P.L. Applications of carbon dioxide in enhanced oil recovery. Energy Convers. Manag. 1992, 33, 579–586. [Google Scholar] [CrossRef]
- Martin, F.D.; Taber, J.J. Carbon dioxide flooding. J. Pet. Technol. 1992, 44, 396–400. [Google Scholar] [CrossRef]
- Moritis, G. CO2 sequestration adds new dimension to oil, gas production. Oil Gas J. 2003, 101, 71–83. [Google Scholar]
- Klins, M.A.; Farouq Ali, S.M. Heavy oil production by carbon dioxide injection. J. Can. Pet. Technol. 1982, 21, 64–72. [Google Scholar] [CrossRef]
- Klins, M.A. Carbon Dioxide Flooding; D. Reidel Publishing Co.: Boston, MA, USA, 1984; p. 267. [Google Scholar]
- Taber, J.J.; Martin, F.D.; Seright, R.S. EOR screening criteria revisited-part 1: Introduction to screening criteria and enhanced recovery fields projects. SPE Reserv. Eng. 1997, 12, 189–198. [Google Scholar] [CrossRef]
- Shaw, J.C.; Bachu, S. Screening, evaluation and ranking of oil reserves suitable for CO2 flood EOR and carbon dioxide sequestration. J. Can. Pet. Technol. 2002, 41, 51–61. [Google Scholar] [CrossRef]
- Metcalfe, R.S. Effects of impurities on minimum miscibility pressures and minimum enrichment levels for CO2 and rich gas displacements. SPE J. 1982, 22, 219–225. [Google Scholar] [CrossRef]
- Williams, D.J. Greenhouse Gas Control Technologies: Proceedings of the 5th International Conference on Greenhouse Gas Control Technologies; Csiro Publishing: Melbourne, Australia, 2001. [Google Scholar]
- Naik, G.C. Tight gas reservoirs–an unconventional natural energy source for the future. Accessado Em 2003, 1, 2008. [Google Scholar]
- Lan, Y.; Yang, Z.; Wang, P.; Yan, Y.; Zhang, L.; Ran, J. A review of microscopic seepage mechanism for shale gas extracted by supercritical CO2 flooding. Fuel 2019, 238, 412–424. [Google Scholar] [CrossRef]
- Prusty, B.K. Sorption of methane and CO2 for enhanced coalbed methane recovery and carbon dioxide sequestration. J. Nat. Gas Chem. 2008, 17, 29–38. [Google Scholar] [CrossRef]
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Enhanced gas recovery and CO2 storage in gas shales: A summary review of its status and potential. Energy Procedia 2014, 63, 5849–5857. [Google Scholar] [CrossRef]
- Tao, Z.; Clarens, A. Estimating the carbon sequestration capacity of shale formations using methane production rates. Environ. Sci. Technol. 2013, 47, 11318–11325. [Google Scholar] [CrossRef]
- Liu, J.; Xie, L.; Yao, Y.; Gan, Q.; Zhao, P.; Du, L. Preliminary study of influence factors and estimation model of the enhanced gas recovery stimulated by carbon dioxide utilization in shale. ACS Sustain. Chem. Eng. 2019, 7, 20114–20125. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71. [Google Scholar] [CrossRef]
- Mukherjee, M.; Misra, S. A review of experimental research on Enhanced Coal Bed Methane (ECBM) recovery via CO2 sequestration. Earth-Sci. Rev. 2018, 179, 392–410. [Google Scholar] [CrossRef]
- Pan, Z.; Ye, J.; Zhou, F.; Tan, Y.; Connell, L.D.; Fan, J. CO2 storage in coal to enhance coalbed methane recovery: A review of field experiments in China. Int. Geol. Rev. 2018, 60, 754–776. [Google Scholar] [CrossRef]
- Reeves, S.R. Geological sequestration of CO2 in deep, unmineable coalbeds: An integrated research and commerical-scale field demonstration project. In SPE Annual Technical Conference and Exhibition; OnePetro: New Orleans, Louisiana, September 2001. [Google Scholar] [CrossRef]
- Saghafi, A.; Faiz, M.; Roberts, D. CO2 storage and gas diffusivity properties of coals from Sydney Basin, Australia. Int. J. Coal Geol. 2007, 70, 240–254. [Google Scholar] [CrossRef]
- Gunter, W.D.; Gentzis, T.; Rottenfusser, B.A.; Richardson, R.J.H. Deep coalbed methane in Alberta, Canada: A fuel resource with the potential of zero greenhouse gas emissions. Energy Convers. Manag. 1997, 38, S217–S222. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Sun, R.; Liu, X.; Pu, H.; Zhao, J. Study of CO2 Enhancing Shale Gas Recovery Based on Competitive Adsorption Theory. ACS Omega 2020, 5, 23429–23436. [Google Scholar] [CrossRef]
- Khan, C.; Amin, R.; Madden, G. Economic modelling of CO2 injection for enhanced gas recovery and storage: A reservoir simulation study of operational parameters. Energy Environ. Res. 2012, 2, 65–82. [Google Scholar] [CrossRef][Green Version]
- Moinfar, A.; Sepehrnoori, K.; Johns, R.T.; Varavei, A. Coupled geomechanics and flow simulation for an embedded discrete fracture model. In SPE Reservoir Simulation Symposium; OnePetro: The Woodlands, TX, USA, February 2013. [Google Scholar] [CrossRef]
- Sun, H.; Yao, J.; Gao, S.H.; Fan, D.Y.; Wang, C.C.; Sun, Z.X. Numerical study of CO2 enhanced natural gas recovery and sequestration in shale gas reservoirs. Int. J. Greenh. Gas Control 2013, 19, 406–419. [Google Scholar] [CrossRef]
- Liu, F.; Ellett, K.; Xiao, Y.; Rupp, J.A. Assessing the feasibility of CO2 storage in the New Albany Shale (Devonian–Mississippian) with potential enhanced gas recovery using reservoir simulation. Int. J. Greenh. Gas Control 2013, 17, 111–126. [Google Scholar] [CrossRef]
- Li, X.; Elsworth, D. Geomechanics of CO2 enhanced shale gas recovery. J. Nat. Gas Sci. Eng. 2015, 26, 1607–1619. [Google Scholar] [CrossRef]
- Bacon, D.H.; Yonkofski, C.M.; Schaef, H.T.; White, M.D.; McGrail, B.P. CO2 storage by sorption on organic matter and clay in gas shale. J. Unconv. Oil Gas Resour. 2015, 12, 123–133. [Google Scholar] [CrossRef]
- Sang, Q.; Li, Y.; Zhu, C.; Zhang, S.; Dong, M. Experimental investigation of shale gas production with different pressure depletion schemes. Fuel 2016, 186, 293–304. [Google Scholar] [CrossRef]
- Chang, Y.B.; Coats, B.K.; Nolen, J.S. A compositional model for CO2 floods including CO2 solubility in water. In Proceedings of the Permian Basin Oil and Gas Recovery Conference, Midland, TX, USA, 12 March 1981; OnePetro: Midland, TX, USA, March 1996. [Google Scholar] [CrossRef]
- Lindeberg, E.; Wessel-Berg, D. Vertical convection in an aquifer column under a gas cap of CO2. Energy Convers. Manag. 1997, 38, S229–S234. [Google Scholar] [CrossRef]
- Mayer, B.; Shevalier, M.; Nightingale, M.; Kwon, J.S.; Johnson, G.; Raistrick, M.; Hutcheon, I.; Perkins, E. Tracing the movement and the fate of injected CO2 at the IEA GHG Weyburn-Midale CO2 Monitoring and Storage project (Saskatchewan, Canada) using carbon isotope ratios. Int. J. Greenh. Gas Control 2013, 16, S177–S184. [Google Scholar] [CrossRef]
- Merey, Ş.; Sınayuç, Ç. Experimental analysis of adsorption capacities and behaviors of shale samples. In 19th International Petroleum and Natural Gas Congress and Exhibition of Turkey; (cp-380); European Association of Geoscientists & Engineers: Antalya, Turkey, 2013. [Google Scholar]
- Ross, D.J.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Heller, R.J.; Zoback, M.D. Adsorption, swelling and viscous creep of synthetic clay samples. In Proceedings of the 45th US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 26–29 June 2011. [Google Scholar]
- Lancaster, D.E.; Hill, D. A multi-laboratory comparison of isotherm measurements of Antrim shale samples. In 1993 SCA Conference Paper; No. 9303; Otsego County, MI, USA, 1993; pp. 1–16. [Google Scholar]
- Mengal, S.A.; Wattenbarger, R.A. Accounting for adsorbed gas in shale gas reservoirs. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September 2011; OnePetro: Manama, Bahrain, September 2011. [Google Scholar] [CrossRef]
- John Thomas, F.; Crittenden, B. Adsorption Technology and Design; Butterworth-Heinemann, Reed Educational and Professional Publishing: Oxford, UK, 1998. [Google Scholar]
- Mohammad, S.; Fitzgerald, J.; Robinson, R.L., Jr.; Gasem, K.A. Experimental uncertainties in volumetric methods for measuring equilibrium adsorption. Energy Fuels 2009, 23, 2810–2820. [Google Scholar] [CrossRef]
- Merey, Ş. Analysis of the effect of experimental adsorption uncertainty on CH4 production and CO2 sequestration in Dadas shale gas reservoir by numerical simulations. J. Pet. Sci. Eng. 2019, 178, 1051–1066. [Google Scholar] [CrossRef]
- Song, B.; Ehlig-Economides, C.; Economides, M.J. Design of multiple transverse fracture horizontal wells in shale gas reservoirs. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 24–26 January 2011; OnePetro: Manama, Bahrain, 2011. [Google Scholar]
- Zhang, D.; Song, J. Mechanisms for geological carbon sequestration. Procedia IUTAm 2014, 10, 319–327. [Google Scholar] [CrossRef]
- Rochelle, C.A.; Czernichowski-Lauriol, I.; Milodowski, A.E. The impact of chemical reactions on CO2 storage in geological formations: A brief review. Geol. Soc. Lond. Spec. Publ. 2004, 233, 87–106. [Google Scholar] [CrossRef]
- Benson, S.M.; Cole, D.R. CO2 sequestration in deep sedimentary formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Kampman, N.; Bickle, M.; Wigley, M.; Dubacq, B. Fluid flow and CO2–fluid–mineral interactions during CO2-storage in sedimentary basins. Chem. Geol. 2014, 369, 22–50. [Google Scholar] [CrossRef]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443. [Google Scholar] [CrossRef]
- Bachu, S.; Gunter, W.D.; Perkins, E.H. Aquifer disposal of CO2: Hydrodynamic and mineral trapping. Energy Convers. Manag. 1994, 35, 269–279. [Google Scholar] [CrossRef]
- Jiang, X. A review of physical modelling and numerical simulation of long-term geological storage of CO2. Appl. Energy 2011, 88, 3557–3566. [Google Scholar] [CrossRef]
- Ambrose, W.A.; Lakshminarasimhan, S.; Holtz, M.H.; Núñez-López, V.; Hovorka, S.D.; Duncan, I. Geologic factors controlling CO2 storage capacity and permanence: Case studies based on experience with heterogeneity in oil and gas reservoirs applied to CO2 storage. Environ. Geol. 2008, 54, 1619–1633. [Google Scholar] [CrossRef]
- Han, W.S. Evaluation of CO2 Trapping Mechanisms at the SACROC Northern Platform: Site of 35 Years of CO2 Injection. Ph.D. Thesis, The New Mexico Institute of Mining and Technology, Socorro, NM, USA, 2008. [Google Scholar]
- Saadatpoor, E.; Bryant, S.L.; Sepehrnoori, K. New trapping mechanism in carbon sequestration. Transp. Porous Media 2010, 82, 3–17. [Google Scholar] [CrossRef]
- Juanes, R.; Spiteri, E.J.; Orr, F.M., Jr.; Blunt, M.J. Impact of relative permeability hysteresis on geological CO2 storage. Water Resour. Res. 2006, 42. [Google Scholar] [CrossRef]
- Burnside, N.M.; Naylor, M. Review and implications of relative permeability of CO2/brine systems and residual trapping of CO2. Int. J. Greenh. Gas Control 2014, 23, 1–11. [Google Scholar] [CrossRef]
- Lamy, C.; Iglauer, S.; Pentland, C.H.; Blunt, M.J.; Maitland, G. Capillary trapping in carbonate rocks. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Barcelona, Spain, 14–17 June 2010; OnePetro: Barcelona, Spain, June 2010. [Google Scholar] [CrossRef]
- Ren, B. Local capillary trapping in carbon sequestration: Parametric study and implications for leakage assessment. Int. J. Greenh. Gas Control 2018, 78, 135–147. [Google Scholar] [CrossRef]
- Busch, A.; Bertier, P.; Gensterblum, Y.; Rother, G.; Spiers, C.J.; Zhang, M.; Wentinck, H.M. On sorption and swelling of CO2 in clays. Geomech. Geophys. Geo-Energy Geo-Resour. 2016, 2, 111–130. [Google Scholar] [CrossRef]
- Rother, G.; Ilton, E.S.; Wallacher, D.; Hauβ, T.; Schaef, H.T.; Qafoku, O.; Rosso, K.M.; Felmy, A.R.; Krukowski, E.G.; Stack, A.G.; et al. CO2 sorption to subsingle hydration layer montmorillonite clay studied by excess sorption and neutron diffraction measurements. Environ. Sci. Technol. 2013, 47, 205–211. [Google Scholar] [CrossRef] [PubMed]
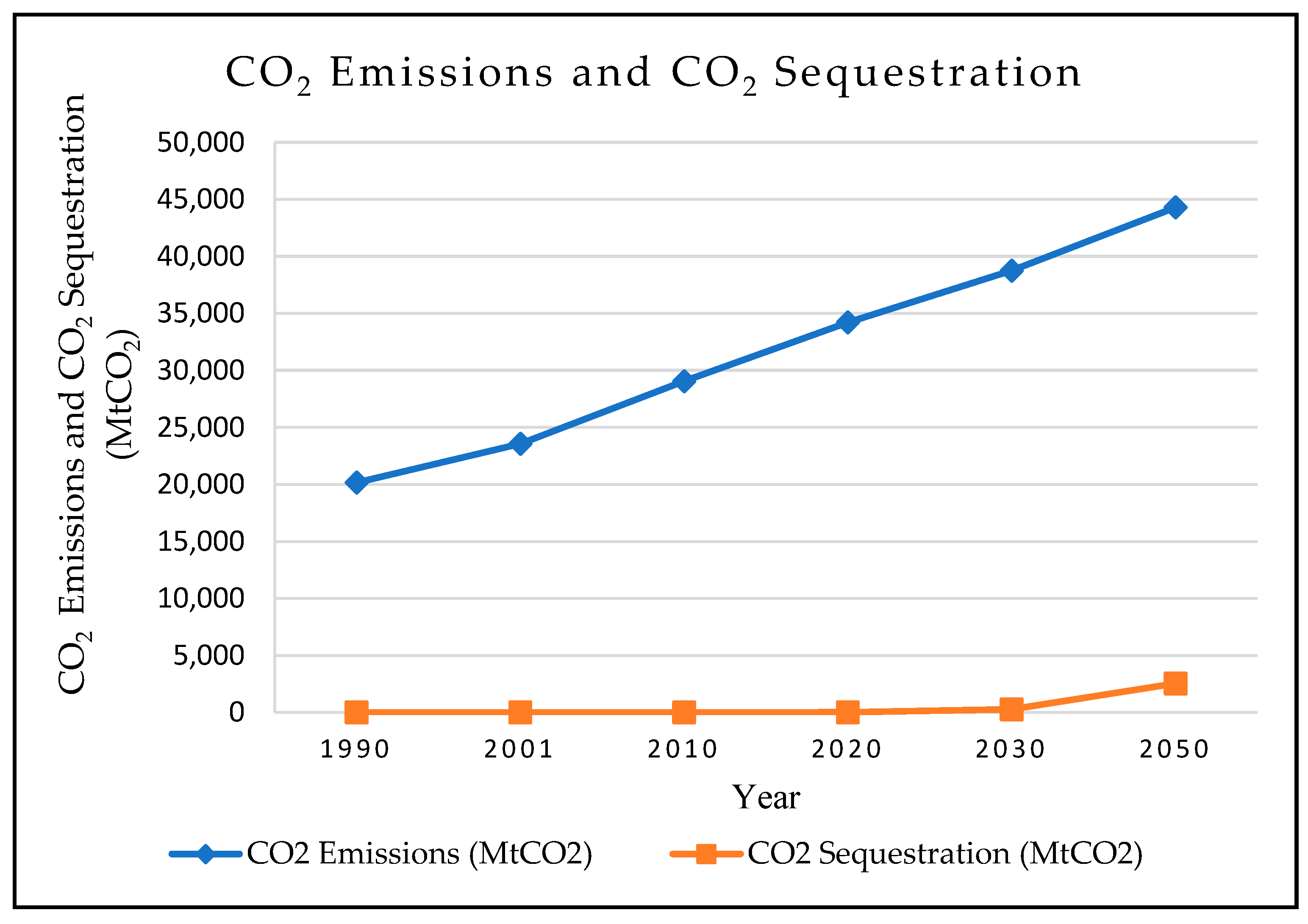

| CO2 Sequestration Options | Storage Capacity * | References |
|---|---|---|
| Geological CO2 Sequestration: | ||
| Ocean | 1000–10,000 + GtC | [17,23] |
| Deep saline formations | 100–10,000 GtC | [17,23] |
| Depleted oil and gas reservoirs | 100–1000 GtC | [17,23] |
| CO2-EOR | 61–123 GtC | [17] |
| Coal seams | 10–1000 GtC | [17,23] |
| Organic-rich shales | 2.5–25 GtC | [18] |
| Biological CO2 Sequestration: | ||
| Terrestrial | 10–100 GtC | [20] |
| Technological CO2 Sequestration: | ||
| Direct air capture (DAC) | <0.1 GtC | [21,22] |
| Classification | CO2 Storage Resource (Gt) | Percentage |
|---|---|---|
| Stored | 0.037 | 0.00029% |
| Capacity | 0.217 | 0.00167% |
| Sub-commercial | 551 | 4.25% |
| Undiscovered | 12,407 | 95.75% |
| Total | 12,958.25 | 100% |
| Storage in saline aquifers | 12,684 | 98% |
| Storage in oil and gas fields | 274 | 2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hameli, F.; Belhaj, H.; Al Dhuhoori, M. CO2 Sequestration Overview in Geological Formations: Trapping Mechanisms Matrix Assessment. Energies 2022, 15, 7805. https://doi.org/10.3390/en15207805
Al Hameli F, Belhaj H, Al Dhuhoori M. CO2 Sequestration Overview in Geological Formations: Trapping Mechanisms Matrix Assessment. Energies. 2022; 15(20):7805. https://doi.org/10.3390/en15207805
Chicago/Turabian StyleAl Hameli, Fatima, Hadi Belhaj, and Mohammed Al Dhuhoori. 2022. "CO2 Sequestration Overview in Geological Formations: Trapping Mechanisms Matrix Assessment" Energies 15, no. 20: 7805. https://doi.org/10.3390/en15207805
APA StyleAl Hameli, F., Belhaj, H., & Al Dhuhoori, M. (2022). CO2 Sequestration Overview in Geological Formations: Trapping Mechanisms Matrix Assessment. Energies, 15(20), 7805. https://doi.org/10.3390/en15207805






